Abstract
Background
Prior to introducing pneumococcal conjugate vaccines (PCVs), Streptococcus pneumoniae was most commonly isolated from middle ear fluid of children with acute otitis media (AOM). Reducing nasopharyngeal colonisation of this bacterium by PCVs may lead to a decline in AOM. The effects of PCVs deserve ongoing monitoring since studies from the post‐PCV era report a shift in causative otopathogens towards non‐vaccine serotypes and other bacteria. This updated Cochrane Review was first published in 2002 and updated in 2004, 2009, and 2014. The review title was changed (to include the population, i.e. children) for this update.
Objectives
To assess the effect of PCVs in preventing AOM in children up to 12 years of age.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL, LILACS, Web of Science, and trials registers (ClinicalTrials.gov and WHO ICTRP) to 29 March 2019.
Selection criteria
Randomised controlled trials of PCV versus placebo or control vaccine.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane. The primary outcomes were frequency of all‐cause AOM and adverse effects. Secondary outcomes included frequency of pneumococcal AOM and frequency of recurrent AOM (defined as three or more AOM episodes in six months or four or more in one year). We used GRADE to assess the quality of the evidence.
Main results
We included 14 publications of 11 trials (60,733 children, range 74 to 37,868 per trial) of 7‐ to 11‐valent PCVs versus control vaccines (meningococcus type C vaccine in three trials, and hepatitis A or B vaccine in eight trials). We included two additional trials for this update. We did not find any relevant trials with the newer 13‐valent PCV. Most studies were funded by pharmaceutical companies. Overall, risk of bias was low. In seven trials (59,415 children) PCVs were administered in early infancy, while four trials (1318 children) included children aged one year and over who were either healthy or had a history of respiratory illness. There was considerable clinical heterogeneity across studies, therefore we did not perform meta‐analyses.
Adverse events
Nine trials reported on adverse effects (77,389 children; high‐quality evidence). Mild local reactions and fever were common in both groups, and occurred more frequently in PCV than in control vaccine groups: redness (< 2.5 cm): 5% to 20% versus 0% to 16%; swelling (< 2.5 cm): 5% to 12% versus 0% to 8%; and fever (< 39 °C): 15% to 44% versus 8% to 25%. More severe redness (> 2.5 cm), swelling (> 2.5 cm), and fever (> 39 °C) occurred less frequently (0% to 0.9%, 0.1% to 1.3%, and 0.4% to 2.5%, respectively in children receiving PCV) and did not differ significantly between PCV and control vaccine groups. Pain or tenderness, or both was reported more frequently in PCV than in control vaccine groups: 3% to 38% versus 0% to 8%. Serious adverse events judged causally related to vaccination were rare and did not differ significantly between groups, and no fatal serious adverse event judged causally related to vaccination was reported.
PCV administered in early infancy
PCV7
The effect of a licenced 7‐valent PCV with CRM197 as carrier protein (CRM197‐PCV7) on all‐cause AOM varied from −5% (95% confidence interval (CI) −25% to 12%) relative risk reduction (RRR) in high‐risk infants (1 trial; 944 children; moderate‐quality evidence) to 6% (95% CI −4% to 16%; 1 trial; 1662 children) and 6% (95% CI 4% to 9%; 1 trial; 37,868 children) RRR in low‐risk infants (high‐quality evidence). PCV7 with the outer membrane protein complex of Neisseria meningitidis serogroup B as carrier protein (OMPC‐PCV7), was not associated with a reduction in all‐cause AOM (RRR −1%, 95% CI −12% to 10%; 1 trial; 1666 children; high‐quality evidence).
CRM197‐PCV7 and OMPC‐PCV7 were associated with 20% (95% CI 7% to 31%) and 25% (95% CI 11% to 37%) RRR in pneumococcal AOM, respectively (2 trials; 3328 children; high‐quality evidence) and CRM197‐PCV7 with 9% (95% CI −12% to 27%) to 10% (95% CI 7% to 13%) RRR in recurrent AOM (2 trials; 39,530 children; high‐quality evidence).
PHiD‐CV10/11
The effect of a licenced 10‐valent PCV conjugated to protein D, a surface lipoprotein of Haemophilus influenzae, (PHiD‐CV10) on all‐cause AOM varied from 6% (95% CI −6% to 17%; 1 trial; 5095 children) to 15% (95% CI −1% to 28%; 1 trial; 7359 children) RRR in healthy infants (moderate‐quality evidence). PHiD‐CV11 was associated with 34% (95% CI 21% to 44%) RRR in all‐cause AOM (1 trial; 4968 children; high‐quality evidence).
PHiD‐CV10 and PHiD‐CV11 were associated with 53% (95% CI 16% to 74%) and 52% (95% CI 37% to 63%) RRR in pneumococcal AOM (2 trials; 12,327 children; high‐quality evidence) and PHiD‐CV11 with 56% (95% CI −2% to 80%) RRR in recurrent AOM (1 trial; 4968 children; moderate‐quality evidence).
PCV administered at later age
PCV7
We found no evidence of a beneficial effect on all‐cause AOM of administering CRM197‐PCV7 in children aged 1 to 7 years with a history of respiratory illness or frequent AOM (2 trials; 457 children; high‐quality evidence) and CRM197‐PCV7 combined with a trivalent influenza vaccine in children aged 18 to 72 months with a history of respiratory tract infections (1 trial; 597 children; high‐quality evidence).
CRM197‐PCV9
In 1 trial including 264 healthy day‐care attendees aged 1 to 3 years, CRM197‐PCV9 was associated with 17% (95% CI −2% to 33%) RRR in parent‐reported all‐cause OM (low‐quality evidence).
Authors' conclusions
Administration of the licenced CRM197‐PCV7 and PHiD‐CV10 during early infancy is associated with large relative risk reductions in pneumococcal AOM. However, the effects of these vaccines on all‐cause AOM is far more uncertain. We found no evidence of a beneficial effect on all‐cause AOM of administering PCVs in high‐risk infants, after early infancy (i.e. in children one year and above), and in older children with a history of respiratory illness. Compared to control vaccines, PCVs were associated with an increase in mild local reactions (redness, swelling), fever, and pain and/or tenderness. We found no evidence of a difference in more severe local reactions, fever, or serious adverse events judged causally related to vaccination.
Plain language summary
Pneumococcal vaccination for preventing acute middle ear infections in children
Review question
We reviewed the evidence about the effect of vaccination against Streptococcus pneumoniae (pneumococcus, a type of bacterium) for preventing acute middle ear infections in children.
Background
Before nationwide implementation of vaccination against Streptococcus pneumoniae with pneumococcal conjugate vaccines (PCVs), pneumococcus was the most frequent cause of acute middle ear infections in children. Vaccination against this bacterium with PCVs may therefore lead to fewer acute middle ear infections in children. However, ongoing monitoring of the effects of PCVs on acute middle ear infections is warranted since recent studies report a shift in bacteria causing acute middle ear infections towards pneumococcal types not included in the vaccines and other bacteria.
Study characteristics
The evidence is current up to 29 March 2019. We included 11 trials of PCVs versus control vaccines (meningococcus type C conjugate vaccine in three trials, and hepatitis A or B vaccine in eight trials) involving a total of 60,733 children. The PCVs used in the trials contained 7 to 11 different types of pneumococcus. None of the trials used the newer PCV containing 13 different types. Most trials were funded by pharmaceutical companies. Overall, risk of bias was low. In seven trials (59,415 children), children received PCVs in early infancy, and four trials included 1318 children aged one year and over who were either healthy or who had previous respiratory illness or frequent acute middle ear infections.
Key results
When a licenced vaccine containing seven different types of pneumococcus (CRM197‐PCV7) was given during early infancy, the risk of experiencing acute middle ear infections either increased by 5% in high‐risk infants or decreased by 6% in low‐risk infants. When administrating a licenced vaccine containing 10 types of pneumococcus together with a carrier protein from another bacterium called Haemophilus influenzae (PHiD‐CV10), the risk of experiencing acute middle ear infections decreased by 6% to 15%, however neither of these estimates reached significance.
Giving PCV7 after early infancy (to children aged one year and above), and in older children with a history of respiratory illness or frequent acute middle ear infections, was not associated with reductions in acute middle ear infections.
Mild local reactions (redness, swelling), fever, and pain/tenderness were common and occurred more frequently in children receiving PCV than in those receiving control vaccines. More severe local reactions (redness and swelling > 2.5 cm) and fever (> 39 °C) occurred far less frequently and did not differ between vaccine groups. Serious adverse events judged causally related to vaccination were rare and did not differ significantly between vaccine groups.
Quality of the evidence
We assessed the quality of the evidence for PCV7 in early infancy to be high (further research is very unlikely to change our confidence in the estimate of effect). We judged the quality of the evidence for PHiD‐CV10 to be moderate (further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate). We judged the quality of the evidence for PCV7 in older children with or without a history of respiratory illness to be high.
Summary of findings
Background
Description of the condition
Acute otitis media (AOM), defined as the presence of middle ear fluid together with one or more signs or symptoms of acute middle ear inflammation such as otalgia, otorrhoea, fever, or irritability, is one of the most common diseases in childhood and imposes a large burden on public health (Lieberthal 2013). Global AOM incidence rates are highest in children one to four years of age, with a peak incidence in six‐ to 11‐month‐old infants (Monasta 2012). By the age of two years, up to 5% of all children have experienced recurrent AOM, defined as three or more AOM episodes in six months or four or more in one year (Kvaerner 1997; Lieberthal 2013). The three main bacterial pathogens isolated from the middle ear fluid of children with AOM collected before the widespread use of pneumococcal conjugate vaccines (PCVs) were Streptococcus pneumoniae (25% to 39%), (non‐typeable) Haemophilus influenzae (12% to 23%), and Moraxella catarrhalis (4% to 15%) (Bluestone 1992; Heikkinen 1999; Jacobs 1998; Luotonen 1981). Recent studies have shown that nationwide implementation of PCVs may have changed the frequency of the causative otopathogens involved in AOM towards pneumococcal serotypes not included in the vaccines and other bacteria including non‐typeable H influenzae (Allemann 2017; Barenkamp 2017; Casey 2013; Coker 2010; Kaur 2017; Somech 2011; Tamir 2015; Wiertsema 2011).
Description of the intervention
The marginal benefits of antibiotics for AOM in low‐risk populations (Rovers 2006; Venekamp 2015); the increasing problem of bacterial resistance against antibiotics (Laxminarayan 2013); and the high estimated direct and indirect annual costs associated with AOM have prompted a search for effective vaccines to prevent this condition (Ahmed 2014; Boonacker 2011). With S pneumoniae (pneumococcus) being a common causative pathogen in childhood AOM and pneumonia, and one of the most frequent causes of invasive bacterial disease such as bacteraemia and meningitis, research has focused on the prevention of pneumococcal infections by pneumococcal vaccines. Pneumococcal polysaccharide vaccines (PPVs) have been available for decades, but have been shown to be poorly immunogenic in children aged up to two years, who are most prone to pneumococcal infections. In the most recent versions of this review, no further attention has been paid to the effect of PPVs, which were described in prior versions of this review (Straetemans 2003).
The first pneumococcal conjugate vaccines (PCVs), in which the pneumococcal capsular serotypes are covalently conjugated to carrier proteins, were developed in the 1990s and proved to be adequately immunogenic in infants and toddlers (Dagan 1997; Eskola 1999; Shinefield 1999). Over the past decades, various PCVs have been developed for use in children including:
licenced 7‐valent PCV containing the polysaccharides of seven serotypes (4, 6B, 9V, 14, 18C, 19F, and 23F) conjugated to the diphtheria‐derived carrier protein CRM197 (CRM197‐PCV7);
7‐valent PCV with the outer membrane complex of Neisseria meningitidis serogroup B as carrier protein (OMPC‐PCV7);
9‐valent PCV containing the capsular polysaccharides of serotypes 1 and 5 in addition to those included in PCV7, conjugated to CRM197 (CRM197‐PCV9);
licenced 10‐valent PCV containing the capsular polysaccharides of 10 serotypes (1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F) mostly conjugated to protein D, which is a surface lipoprotein of H influenzae (PHiD‐CV10);
11‐valent containing the capsular polysaccharides of serotype 3 as well as those included in PHiD‐CV10 (PHiD‐CV11); and
licenced 13‐valent PCV containing the capsular polysaccharides of 13 serotypes (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F) conjugated to CRM197 (CRM197‐PCV13).
How the intervention might work
Early and dense colonisation of the nasopharynx with bacterial otopathogens, including S pneumoniae, increases the risk of AOM substantially (Faden 1997; Leach 1994; Schilder 2016). As a consequence, reducing or eliminating nasopharyngeal colonisation of S pneumoniae by PCVs may lead to reductions in AOM incidence. In recent years, evidence has accumulated that PCVs might also disrupt the continuum of evolution from pneumococcal‐associated otitis media (OM) towards chronic/recurrent OM by prevention of early vaccine‐serotype AOM and thereby reducing subsequent and more complex disease caused by non‐vaccine serotypes and non‐typeable H influenzae (Ben‐Shimol 2014; Dagan 2016).
Why it is important to do this review
With AOM amongst the most common diseases in early childhood, the need for a vaccine to effectively prevent AOM is high. Over the past decades various randomised controlled trials have been performed to assess the effects of pneumococcal vaccination to prevent AOM. From 2009 onwards, two multivalent PCVs (PHiD‐CV10 and CRM197‐PCV13) have been licenced and are being implemented in nationwide immunisation programmes worldwide (WHO 2012). These new vaccines may have an increased benefit in preventing AOM (Marom 2014; O'Brien 2009). As such, it was important to provide an up‐to‐date systematic review on the effects of PCVs on preventing AOM. This review is an update of a Cochrane Review first published in 2002 (Straetemans 2002), and updated in 2004 (Straetemans 2004), 2009 (Jansen 2009), and 2014 (Fortanier 2014).
Objectives
To assess the effect of PCVs in preventing AOM in children up to 12 years of age.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), irrespective of type, assessing the effect of pneumococcal conjugate vaccines (PCV) versus placebo or control vaccine in preventing acute otitis media (AOM) with a minimum follow‐up duration of six months. As per previous versions of this review, we excluded studies that did not report outcome data relevant for this review.
Types of participants
Children aged up to 12 years.
Types of interventions
PCV versus placebo or control vaccine.
Types of outcome measures
Primary outcomes
Frequency of all‐cause AOM episodes defined as AOM irrespective of causative pathogen. We considered this to be the most relevant outcome for children, parents, and clinicians.
Adverse effects including local (redness, swelling) and systemic reactions (fever), pain/tenderness, and serious adverse events (SAEs) judged causally related to vaccination.
Secondary outcomes
Frequency of pneumococcal AOM.
Frequency of pneumococcal serotype‐specific AOM (including vaccine serotype, non‐vaccine serotype, and cross‐reactive serotypes which are non‐vaccine serotypes with a serogroup that is included in the vaccine).
Frequency of recurrent AOM (defined as three or more episodes in the last six months or four or more in the last year).
Search methods for identification of studies
The Cochrane Acute Respiratory Infections Group (2018 search update) and Cochrane Infectious Disease Group (2019 search update) Information Specialists conducted systematic searches for RCTs and controlled clinical trials. There were no language, publication year, or publication status restrictions. The date of latest search was 29 March 2019.
Electronic searches
For the 2014 review update, we used the search strategy presented in Appendix 1.
For this 2019 update, we searched the Cochrane Central Register of Controlled Trials (CENTRAL; Issue 3, 2019), which contains the Cochrane Acute Respiratory Infections Specialised Register; MEDLINE (Ovid) (1995 to 29 March 2019); Embase (Elsevier) (1995 to 29 March 2019); CINAHL (EBSCO) (Cumulative Index to Nursing and Allied Health Literature) (2007 to 28 March 2019); LILACS (BIREME) (Latin American and Caribbean Health Science Information database) (2007 to 28 March 2019), and Web of Science (Clarivate Analytics) (2007 to 28 March 2019).
We used the search strategy presented in Appendix 2 to search CENTRAL and MEDLINE. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search strategy to search Embase (Appendix 3), CINAHL (Appendix 4), LILACS (Appendix 5), and Web of Science (Appendix 6).
Searching other resources
To increase the yield of relevant studies, two review authors (ACF, RPV) reviewed the reference lists of all relevant studies and review articles retrieved. We searched the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov/) (Appendix 7) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/trialsearch) (Appendix 8) on 29 March 2019 for completed and ongoing trials. We furthermore searched the internet (via Google using the search terms 'pneumococcal conjugate vaccination for acute otitis media trial') and the extended abstracts published in the Recent Advances in Otitis Media (grey literature) on 29 March 2019 for any additional trials.
Data collection and analysis
Selection of studies
Two review authors (ACF, RPV) independently screened titles and abstracts obtained from the database searches and reviewed the full text of the potentially relevant titles and abstracts against the inclusion criteria. Any disagreements were resolved by discussion.
Data extraction and management
Two review authors (ACF, RPV) independently extracted data from the included studies. Any disagreements were resolved by discussion.
Assessment of risk of bias in included studies
Two review authors (ACF, RPV) independently assessed the methodological quality of the included trials. Any disagreements were resolved by discussion. We assessed the methodological quality of included studies using the 'Risk of bias' tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We judged the following domains as high, low, or unclear risk of bias: random sequence generation (selection bias), concealment of allocation (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other sources of bias.
Measures of treatment effect
We expressed estimates of treatment effects as relative risks/hazard ratios with accompanying 95% confidence intervals (CIs). Vaccine efficacy was estimated as 1 minus the relative risk/hazard ratio (relative risk reduction (RRR)).
Unit of analysis issues
We included all types of RCTs. In the case of cluster‐randomised trials, we considered potential differences between the intervention effects being estimated and checked whether clustering was taken into account in the analysis of the individual trials.
Dealing with missing data
For each trial, we determined the number of missing data and whether the authors took duration of follow‐up (and censoring) of individual participants into account in their statistical analyses.
Assessment of heterogeneity
We first assessed clinical heterogeneity across trials by reviewing the differences in the types of participants recruited, interventions used, and outcomes measured. We did not pool studies where clinical heterogeneity made it unuseful to do so. Where studies were sufficiently homogeneous, we proposed to assess statistical heterogeneity for each outcome by visually inspecting the forest plots and by using the Chi² test and the I² statistic.
Assessment of reporting biases
We proposed to assess reporting bias as within‐study (outcome reporting) and between‐study reporting (publication) bias (Higgins 2011).
Outcome reporting bias
We searched the internet, ClinicalTrials.gov, and the WHO ICTRP for available study protocols to determine whether outcomes reported were predefined and whether all outcomes listed in the study protocol were reported in the trial publications. Where information was insufficient to judge the risk of bias, we classified the risk of bias as unclear (Higgins 2011).
Publication bias
We proposed a more formal method of assessing reporting bias, that is by creating funnel plots, if sufficient trials (10 or more) were available for an outcome.
Data synthesis
We primarily analysed the available data according to the intention‐to‐treat principle, that is by analysing all participants in the groups to which they were originally randomised. As a secondary analysis, we presented data based on a per‐protocol analysis.
Where possible, we proposed conducting meta‐analyses using Review Manager 5 by calculating treatment effects with the Mantel‐Haenszel method, using a fixed‐effect model where no substantial statistical heterogeneity was present (I² < 50%) (Review Manager 2014). If substantial statistical heterogeneity was detected and unresolved by sensitivity analysis, we proposed to calculate treatment effects using a random‐effects (DerSimonian and Laird) model to provide more conservative effect estimates. Where clinical heterogeneity precluded meta‐analyses, we reported the effect estimates as presented by the individual trials. If possible, we reported the incidences of the various outcomes in the study arms together with the vaccine efficacy estimates, with 95% CIs.
We proposed the following methods to conduct meta‐analyses. The generalised Cox proportional hazard method proposed by Andersen 1982 is regarded as the most appropriate to assess the effect of PCVs on AOM (Jahn‐Eimermacher 2007). Under the assumption that the hazard rate is proportional between both groups over time, and that the risk of AOM is not affected by previous episodes (although this is untrue), this model takes all available information into account, that is all episodes (including recurrences), differences in individual patient follow‐up time, and time until a case of AOM (Jahn‐Eimermacher 2007). However, information on individual follow‐up time until the first, second, third, etc. case of AOM is difficult to obtain for each study to be included in the meta‐analysis. Poisson regression is based on the assumption of a constant risk of AOM over time and that this risk is not affected by previous episodes of AOM. This method only requires the total follow‐up time and total number of episodes, and therefore appears to be a more feasible method for meta‐analysis. Furthermore, Poisson regression seems not to be affected by the deviation from a constant risk over time, having very similar results for the effect of PCVs on AOM to the Andersen‐Gill approach (Jahn‐Eimermacher 2007). For Poisson regression, the treatment effect is measured as a rate ratio defined as follows: (total AOM episodes in pneumococcal vaccination group divided by the number of children in the pneumococcal vaccination group multiplied by the follow‐up time in months) divided by (total AOM episodes in control group divided by the number of children in the control group multiplied by the follow‐up time in months) (McCullagh 1989).
GRADE and 'Summary of findings' table
We created Table 1 for PCVs administered in early infancy using the following outcomes: frequency of all‐cause AOM episodes (primary outcome), frequency of pneumococcal AOM, and frequency of recurrent AOM (defined as three or more AOM episodes in six months or four or more in one year). We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We judged the quality of the evidence as high, moderate, low, or very low. We judged evidence from RCTs that did not have serious limitations as high quality. However, we downgraded the quality of evidence to moderate, low, or very low based on the following factors: study limitations (risk of bias), inconsistency (consistency of results), imprecision (precision of results), indirectness of evidence (directness of evidence), and publication bias (existence of publication bias). We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), employing GRADEpro GDT software (GRADEpro GDT 2015). We justified all decisions to down‐ or upgrade the quality of studies using footnotes, and made comments to aid the reader's understanding of the review where necessary.
Summary of findings for the main comparison. Pneumococcal conjugate vaccine versus control vaccine for preventing acute otitis media.
| Pneumococcal conjugate vaccine versus control vaccine for preventing acute otitis media | ||||
|
Patient or population: infants (predominantly < 6 months of age) and older children (aged 1 to 7 years) Settings: community (Finland, the Netherlands, Czech Republic and Slovakia, Israel, USA, Argentina, Colombia and Panama) Intervention: multivalent PCVs Comparison: control vaccine | ||||
| PCV type | VE ‐ relative effect (95% CI)* | No. of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Frequency of all‐cause AOM (co‐primary outcome) | ||||
| CRM197‐PCV7in low‐risk infants | RRR: 6% (−4% to 16%) to 6% (4% to 9%)# | 39,530 (2) | ⊕⊕⊕⊕ high | Results are derived from 1 very large trial including 37,868 infants, Black 2000/Fireman 2003, and 1 smaller trial including 1662 infants, Eskola 2001/Palmu 2009, with low risk of bias. |
| CRM197‐PCV7in high‐risk infants | RRR: −5% (−25% to 12%) | 944 (1) | ⊕⊕⊕⊝ moderate1 | Results are derived from 1 relatively small trial with low risk of bias (O'Brien 2008). |
| OMPC‐PCV7in low‐risk infants | RRR: −1% (−12% to 10%) | 1666 (1) | ⊕⊕⊕⊕ high | Results are derived from 1 trial with low risk of bias (Kilpi 2003). |
| PHiD‐CV10in low‐risk infants | RRR: 6% (−6% to 17%) to 15% (−1% to 28%) | 12,454 (2) | ⊕⊕⊕⊝ moderate2 | Results are derived from 2 trials with low, Tregnaghi 2014/Sáez‐Llorens 2017, and unclear risk of bias (Vesikari 2016). AOM incidence rate in control group in 1 of the trials, Tregnaghi 2014/Sáez‐Llorens 2017, was low compared to the other studies (Table 2). |
| PHiD‐CV11in low‐risk infants | RRR: 34% (21% to 44%) | 4968 (1) | ⊕⊕⊕⊕ high | Results are derived from 1 trial with low risk of bias (Prymula 2006). AOM incidence rate in control group was low compared to other studies (Table 2). |
| Adverse effects (co‐primary outcome) | ||||
|
CRM197‐PCV7in low‐risk infants OMPC‐PCV7in low‐risk infants PHiD‐PC10/11 in low‐risk infants CRM197‐PCV7/9 and CRM197‐PCV7 plus TIVin older children |
Mild local reactions and fever were common in both groups. These adverse events occurred more frequently in the PCV than in the control vaccine groups: redness (< 2.5 cm): 5% to 20% versus 0% to 16%, swelling (< 2.5 cm): 5% to 12% versus 0% to 8%, and fever (< 39 °C): 15% to 44% versus 8% to 25%. More severe redness (> 2.5 cm), swelling (> 2.5 cm), and fever (> 39 °C) occurred less frequently (0% to 0.9%, 0.1% to 1.3%, and 0.4% to 2.5%, respectively in children receiving PCV) and did not differ significantly between PCV and control vaccine groups. Pain/tenderness was reported more frequently in children receiving PCV than in those receiving control vaccines: 3% to 38% versus 0% to 8%. Serious adverse events judged causally related to vaccination were rare and did not differ significantly between vaccine groups. No fatal serious adverse event judged causally related to vaccination was reported. |
77,389 (9) | ⊕⊕⊕⊕ high | Results are derived from 9 trials with low risk of bias. |
| Frequency of pneumococcal AOM | ||||
| CRM197‐PCV7in low‐risk infants | RRR: 20% (7% to 31) to 34% (21% to 45%) | 1662 (1) | ⊕⊕⊕⊕ high | Results are derived from 1 trial with low risk of bias (Eskola 2001/Palmu 2009). |
| OMPC‐PCV7in low‐risk infants | RRR: 25% (11% to 37%) | 1666 (1) | ⊕⊕⊕⊕ high | Results are derived from 1 trial with low risk of bias (Kilpi 2003). |
| PHiD‐CV10in low‐risk infants | RRR: 53% (16% to 74%) | 7359 (1) | ⊕⊕⊕⊕ high | Results are derived from 1 trial with low risk of bias (Tregnaghi 2014/Sáez‐Llorens 2017). |
| PHiD‐CV11in low‐risk infants | RRR: 52% (37% to 63%) | 4968 (1) | ⊕⊕⊕⊕ high | Results are derived from 1 trial with low risk of bias (Prymula 2006). |
| Frequency of recurrent AOM (defined as 3 or more AOM episodes in 6 months or 4 or more in 1 year) | ||||
| CRM197‐PCV7in low‐risk infants | RRR: 9% (−12% to 27%) to 10% (7% to 13%) | 39,530 (2) | ⊕⊕⊕⊕ high | Results are derived from 1 very large trial including 37,868 infants, Black 2000/Fireman 2003, and 1 smaller trial including 1662 infants, Eskola 2001/Palmu 2009, with low risk of bias. |
| PHiD‐CV11in low‐risk infants | RRR: 56% (−2% to 80%) | 4968 (1) | ⊕⊕⊕⊝ moderate3 | Results are derived from 1 trial with low risk of bias (Prymula 2006). |
|
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate. *For readability purposes, absolute rates (episodes/person‐year and incidence rate differences) are displayed in Table 1. # Depending on whether the outcome was assessed by a composite of positive culture or positive pneumolysin polymerase chain reaction (PCR) or by positive culture only or whether ITT or per‐protocol analysis was performed. | ||||
1We downgraded the quality of the evidence from high to moderate due to imprecise effect estimate (only one trial with relatively small sample size). 2We downgraded the quality of the evidence from high to moderate due to study limitations (risk of bias) and imprecise effect estimates. 3We downgraded the quality of the evidence from high to moderate due to the imprecise effect estimate.
AOM: acute otitis media; CI: confidence interval; CRM197‐PCV7: 7‐valent pneumococcal conjugate vaccine conjugated to carrier protein CRM197; OMPC‐PCV7: 7‐valent pneumococcal conjugate vaccine conjugated to the outer membrane protein complex of Neisseria meningitidis serogroup B; PCV: pneumococcal conjugate vaccine; PHiD‐CV10: 10‐valent pneumococcal conjugate vaccine conjugated to protein D (surface lipoprotein of non‐typeable Haemophilus influenzae); PHiD‐CV11: 11‐valent pneumococcal conjugate vaccine conjugated to protein D (surface lipoprotein of non‐typeable Haemophilus influenzae); RRR: relative risk reduction; TIV: trivalent influenza vaccine; VE: vaccine efficacy
Subgroup analysis and investigation of heterogeneity
Because the effect of PCVs on AOM may be influenced by the age at which the PCV was administered, occurrence of previous AOM or respiratory tract infection episodes, and by the type of PCV used, we described the studies accordingly, that is we stratified those with vaccination in early infancy versus those with vaccination later in childhood by type of PCV used.
Sensitivity analysis
We planned to carry out sensitivity analyses for risk of bias of included studies to assess the robustness of review findings by excluding studies with high risk of bias (defined as high risk of allocation concealment bias and attrition bias (overall loss to follow‐up of more than 20% or differential follow‐up observed, or both)) from meta‐analysis.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
This review is an update of a Cochrane Review first published in 2002 (Straetemans 2002), and updated in 2004 (Straetemans 2004), 2009 (Jansen 2009), and 2014 (Fortanier 2014). In the 2014 review, which included studies up to December 2013, we included nine RCTs, which were reported in 11 publications (Black 2000/Fireman 2003; Dagan 2001; Eskola 2001/Palmu 2009; Jansen 2008; Kilpi 2003; O'Brien 2008; Prymula 2006; van Kempen 2006; Veenhoven 2003).
For this update we searched electronic databases (December 2013 to March 2019) and retrieved 374 records. After removal of duplicates, we assessed 225 records by title and abstract, and identified eight potentially eligible studies which we obtained in full text. After reviewing the full texts, we excluded two publications that were additional analyses of the Eskola 2001 study but did not include new outcome data useful to this review (Palmu 2015a; Sarasoja 2013), and three publications that were secondary analyses of the Finnish invasive pneumococcal disease (FinIP) vaccine trial but did not report on any of our outcomes of interest (Palmu 2014; Palmu 2015b; Palmu 2018). This left three publications, Sáez‐Llorens 2017; Tregnaghi 2014; Vesikari 2016, that related to two RCTs, Tregnaghi 2014; Vesikari 2016, that were suitable for inclusion. Sáez‐Llorens 2017 was a further analysis of Tregnaghi 2014. See Figure 1.
1.
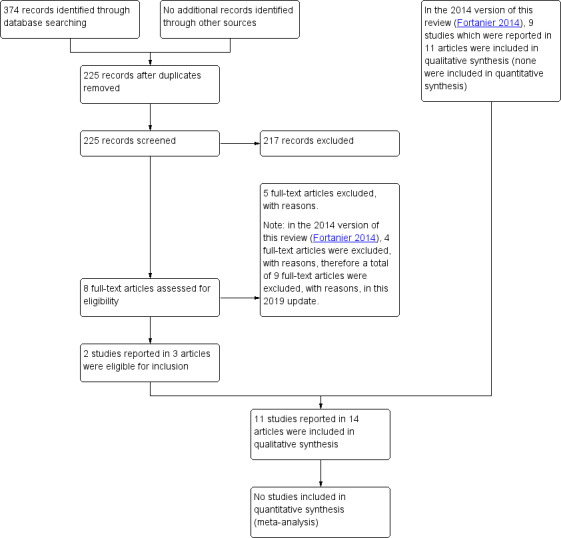
Study flow diagram.
We did not identify additional relevant completed trials or any ongoing studies by scanning the reference lists of relevant systematic reviews or by searching the internet, the grey literature, and the trial registries ClinicalTrials.gov and WHO ICTRP.
Included studies
See Characteristics of included studies.
We included 11 RCTs reported in 14 publications (Black 2000/Fireman 2003; Dagan 2001; Eskola 2001/Palmu 2009; Jansen 2008; Kilpi 2003; O'Brien 2008; Prymula 2006; Tregnaghi 2014/Sáez‐Llorens 2017; van Kempen 2006; Veenhoven 2003; Vesikari 2016). We added two RCTs (reported in three publications) for this update (Tregnaghi 2014/Sáez‐Llorens 2017; Vesikari 2016). The included trials involved a total of 60,733 children.
Study designs
Of the 11 included studies, nine were standard, individually randomised trials, and two were cluster‐RCTs (O'Brien 2008; Vesikari 2016). Both cluster‐RCTs took the cluster effect into account in their analyses.
Study populations (early infancy versus later in life)
In seven trials (Black 2000/Fireman 2003; Eskola 2001/Palmu 2009; Kilpi 2003; O'Brien 2008; Prymula 2006; Tregnaghi 2014/Sáez‐Llorens 2017; Vesikari 2016), PCVs were predominantly administered in children's first six months of life. Four trials, Dagan 2001; Jansen 2008; van Kempen 2006; Veenhoven 2003, assessed the effects of PCVs administered at a later age on AOM in either healthy infants, Dagan 2001, or in children with a history of respiratory illness or frequent AOM (Jansen 2008; van Kempen 2006; Veenhoven 2003). Three trials were performed in Finland (Eskola 2001/Palmu 2009; Kilpi 2003; Vesikari 2016), two in the USA (Black 2000/Fireman 2003; O'Brien 2008), two in the Netherlands (Jansen 2008; Veenhoven 2003), and the remaining in Belgium (van Kempen 2006), Israel (Dagan 2001), Czech Republic and Slovakia (Prymula 2006), and Argentina, Colombia, and Panama (Tregnaghi 2014/Sáez‐Llorens 2017). Most of these countries had AOM diagnosis and management guidelines at the time of the study (Tamir 2017).
Interventions
Type of PCV used and co‐administration of other vaccines
In six trials, CRM197‐PCV7 was used as the intervention (Black 2000/Fireman 2003; Eskola 2001/Palmu 2009; Jansen 2008; O'Brien 2008; van Kempen 2006; Veenhoven 2003). In two studies, a booster dose with 23‐valent PPV (containing capsular polysaccharides of the serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F) was given to all children (van Kempen 2006; Veenhoven 2003). In one trial, CRM197‐PCV7 was administered together with a trivalent inactivated influenza vaccine (TIV) (Jansen 2008).
Four different interventions were used in five trials: OMPC‐PCV7 in Kilpi 2003 (a subset of these children received PPV23 as a booster dose); CRM197‐PCV9 in Dagan 2001; PHiD‐CV10 in Tregnaghi 2014/Sáez‐Llorens 2017 and Vesikari 2016; and PHiD‐CV11 in Prymula 2006.
Comparator
Control vaccines were used as comparators in all trials. Comparator vaccines included meningococcus type C conjugate vaccine (10 µg of group C oligosaccharide conjugated to carrier protein CRM197; MenC) in three trials (Black 2000/Fireman 2003; Dagan 2001; O'Brien 2008), whilst hepatitis A or B vaccine was used in the remaining eight trials.
Outcome measures
Adverse effects were reported in nine trials including a total of 77,389 children (Black 2000/Fireman 2003; Dagan 2001; Eskola 2001/Palmu 2009; Jansen 2008; Kilpi 2003; Prymula 2006; Tregnaghi 2014/Sáez‐Llorens 2017; Veenhoven 2003; Vesikari 2016). Tregnaghi 2014/Sáez‐Llorens 2017 was part of the Clinical Otitis Media and Pneumonia Study (COMPAS; clinicaltrials.gov/show/NCT00466947), which assessed the efficacy and safety of PHiD‐CV10 against invasive pneumococcal disease, community‐acquired pneumonia, and AOM in 23,821 young Latin American children. Acute otitis media was studied in the Panama cohort only, which included 7357 children, whereas safety data were available for all 23,821 children.
Six trials applied a standardised diagnosis of AOM (Eskola 2001/Palmu 2009; Kilpi 2003; Prymula 2006; Tregnaghi 2014/Sáez‐Llorens 2017; van Kempen 2006; Veenhoven 2003), and one trial used standardised AOM registration forms to be completed by general practitioners (Jansen 2008). In two trials, AOM episodes were extracted from a computerised data source containing all visits registered by physicians (Black 2000/Fireman 2003; O'Brien 2008). Two trials relied on parent‐reported AOM episodes (Dagan 2001; Vesikari 2016); Vesikari 2016 used parent‐reported, physician‐confirmed AOM as the outcome of interest. Two trials assessed outcomes during influenza seasons (Jansen 2008; van Kempen 2006).
Seven trials also assessed the effect of PCVs on (serotype‐specific) pneumococcal AOM (Black 2000/Fireman 2003; Eskola 2001; Kilpi 2003; O'Brien 2008; Prymula 2006; Tregnaghi 2014/Sáez‐Llorens 2017; Veenhoven 2003). Three studies cultured middle ear fluid from all AOM episodes (Eskola 2001; Kilpi 2003; Prymula 2006), and one trial cultured middle ear fluid by tympanocentesis when fluid was suspected in the middle ear (Tregnaghi 2014/Sáez‐Llorens 2017). One trial only cultured middle ear fluid from the first AOM episode by tympanocentesis or from spontaneously draining ears (Veenhoven 2003). Two trials assessed the effect on reported cultures that were obtained from spontaneously draining ears (Black 2000/Fireman 2003; O'Brien 2008).
Three trials reported the effects of PCVs on recurrent AOM (Black 2000/Fireman 2003; Eskola 2001; Prymula 2006). Three studies included all types of OM, including but not exclusively AOM, as an outcome (Black 2000/Fireman 2003; Dagan 2001; O'Brien 2008).
Funding and conflicts of interest
Six trials were funded by pharmaceutical companies (Black 2000/Fireman; Eskola 2001/Palmu 2009; Kilpi 2003; Prymula 2006; Tregnaghi 2014/Sáez‐Llorens 2017; Vesikari 2016). Three trials reported receiving support from non‐commercial (governmental) sources, but study vaccines were supplied by pharmaceutical companies (Jansen 2008; van Kempen 2006; Veenhoven 2003). One trial was supported both by a pharmaceutical company and governmental funding (O'Brien 2008). One trial reported that study vaccines were supplied by a pharmaceutical company (Dagan 2001).
Brief overview of clinical heterogeneity across included studies
There was considerable clinical heterogeneity across the included trials. There were differences in the timing of PCV administration, that is trials administering PCV during infancy and trials administering PCV later in life. As such, study populations varied from healthy infants to those at high risk of AOM. Secondly, the number of pneumococcal serotypes present in the vaccines, the type of conjugate method used, and co‐administration of other vaccines differed substantially across trials. Study designs also varied, including both individually randomised controlled trials and cluster‐RCTs. Finally, large differences in outcome assessments and AOM definitions were observed, varying from 'passive' (chart review at the end of the trial) to 'active' (parents were instructed to visit a physician in case of AOM symptoms) outcome assessments and physician‐confirmed AOM episodes versus parent‐reported AOM episodes. Consequently, AOM incidence in the control groups varied widely across the studies administering PCV during infancy, that is from 0.13 to 1.3 episodes per person‐year. We therefore did not perform meta‐analyses.
Excluded studies
In the 2014 version of this review (Fortanier 2014), four studies were excluded since they (i) did not include a control vaccine (Gisselsson‐Solen 2011); (ii) did not report outcome data relevant for this review (Jokinen 2012); (iii) assessed the effect of PCV on otitis media with effusion rather than AOM (Le 2007); and (iv) reported the effect of PCV on suppurative otitis media in an abstract of a conference meeting (Roy 2011). In this 2019 update, a further five studies were excluded that did not report outcome data relevant for this review (Palmu 2014; Palmu 2015a; Palmu 2015b; Palmu 2018; Sarasoja 2013). See Characteristics of excluded studies.
Ongoing studies
We did not identify any ongoing studies.
Risk of bias in included studies
We judged the methodological quality of the included studies to be moderate to high. We presented the 'Risk of bias' assessment graphically in Figure 2 and Figure 3.
2.
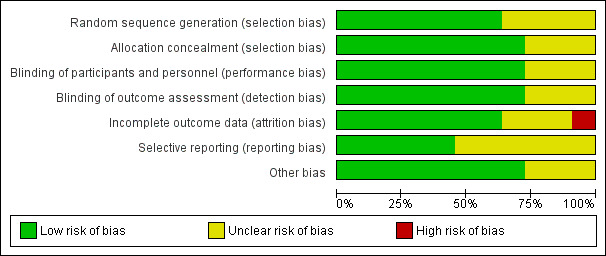
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
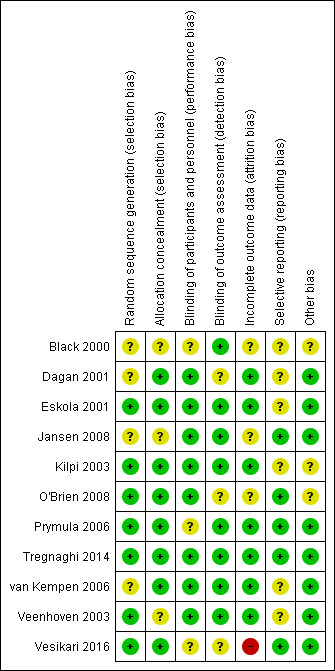
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Eight included trials described concealment of allocation adequately, whilst this domain was assessed as unclear for three trials due to insufficient information (Black 2000/Fireman 2003; Jansen 2008; Veenhoven 2003). We judged random sequence generation to be adequate in seven trials, whilst four trials provided insufficient information on methods of random sequence generation used (Black 2000/Fireman 2003; Dagan 2001; Jansen 2008; van Kempen 2006).
Blinding
Although all studies indicated that trials were double‐blinded, three trials provided insufficient information about how blinding was performed (Black 2000/Fireman 2003; Prymula 2006; Vesikari 2016).
Incomplete outcome data
We judged risk of attrition bias to be high in one trial (Vesikari 2016), unclear in three trials (Black 2000/Fireman 2003; Jansen 2008; O'Brien 2008), and low in seven trials.
Selective reporting
We judged risk of reporting bias to be unclear in six trials, Black 2000/Fireman 2003; Dagan 2001; Eskola 2001/Palmu 2009; Kilpi 2003; van Kempen 2006; Veenhoven 2003, and low in five trials.
Other potential sources of bias
We judged risk of bias due to other sources (including balances in baseline characteristics, use of co‐intervention across groups, presence of formal sample size calculations, and (prespecified) interim analyses) as unclear in three trials, Black 2000/Fireman 2003; Kilpi 2003; O'Brien 2008, and low in the remaining eight trials.
Effects of interventions
See: Table 1
Effect estimates of the various PCV types, stratified by the age at which PCVs were administered and the occurrence of previous AOM/respiratory tract infection (RTI) episodes (i.e. administration in early infancy versus later in life), on frequency of all‐cause AOM, (vaccine‐type) frequency of pneumococcal AOM, and frequency of recurrent AOM (defined as three or more AOM episodes in six months or four or more in one year), are summarised in Table 2, Table 3, and Table 4, respectively. The main results for PCVs administered in early infancy are described in Table 1.
1. Effect of pneumococcal conjugate vaccination on frequency of all‐cause acute otitis media episodes.
| Intention‐to‐treat | Per‐protocol | |||||||
| Episodes/person‐year | Incidence rate difference ‐ episodes per person‐year (95% CI) | VE expressed as relative reduction in risk (95% CI)a | Episodes/person‐year | Incidence rate difference ‐ episodes per person‐year (95% CI) | VE expressed as relative reduction in risk (95% CI)a | |||
| Treatment | Control | Treatment | Control | |||||
| PCV administered in early infancy | ||||||||
| CRM197‐PCV7 | ||||||||
|
Black 2000 Fireman 2003 |
‐ ‐ |
‐ ‐ |
‐ ‐ |
6% (4% to 9%) 6% (4% to 8%) |
‐ ‐ |
‐ ‐ |
‐ ‐ |
7% (4% to 10%) 7% (4% to 9%) |
| Eskola 2001 | ‐ | ‐ | ‐ | ‐ | 1.16 | 1.24 | −0.08d | 6% (−4% to 16%) |
| O'Brien 2008b | 1.43 | 1.36 | 0.07 (−0.05 to 0.18) | −5% (−25% to 12%)c | 1.35 | 1.35 | 0.00 (−0.13 to 0.14) | 0% (−21% to 17%) |
| OMPC‐PCV7 | ||||||||
| Kilpi 2003 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | −1% (−12% to 10%) |
| PHiD‐PC10/11 | ||||||||
|
Tregnaghi 2014 Sáez‐Llorens 2017 |
0.03 | 0.04 | −0.01 (−0.01 to 0.00) | 15% (−1% to 28%) | ‐ | ‐ | ‐ | 13% (−5% to 28%) |
| Vesikari 2016b | ‐ | ‐ | ‐ | ‐ | 0.99 | 1.01 | −0.02d | 6% (−6% to 17%) |
| Prymula 2006 | ‐ | ‐ | ‐ | ‐ | 0.08 | 0.13 | −0.04d | 34% (21% to 44%) |
| PCV administered at a later age | ||||||||
| CRM197‐PCV7 followed by PPV23 | ||||||||
| Veenhoven 2003 | ‐ | ‐ | ‐ | −25% (−57% to 1%) | 1.1 | 0.83 | −0.27d | −29% (−62% to −2%) |
| van Kempen 2006 | ‐ | ‐ | ‐ | ‐ | 0.78 | 0.67 | −0.11d | −16% (−96% to 31%) |
| CRM197‐PCV7/TIV | ||||||||
| Jansen 2008 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 57% (6% to 80%)e |
| CRM197‐PCV9 | ||||||||
| Dagan 2001 | ‐ | ‐ | ‐ | ‐ | 0.66 | 0.79 | −0.14 (−0.29 to 0.02) | 17% (−2% to 33%) |
AOM: acute otitis media; CI: confidence interval; CRM197‐PCV7: 7‐valent pneumococcal conjugate vaccine conjugated to carrier protein CRM197; CRM197‐PCV7/TIV: trivalent influenza vaccine plus 7‐valent pneumococcal conjugate vaccine conjugated to carrier protein CRM197; CRM197‐PCV9: 9‐valent pneumococcal conjugate vaccine conjugated to carrier protein CRM197; HBV: hepatitis B virus; OMPC‐PCV7: 7‐valent pneumococcal conjugate vaccine conjugated to the outer membrane protein complex of Neisseria meningitidis serogroup B; PCV: pneumococcal conjugate vaccine; PHiD‐CV10: 10‐valent pneumococcal conjugate vaccine conjugated to protein D (surface lipoprotein of non‐typeable Haemophilus influenzae); PHiD‐CV11: 11‐valent pneumococcal conjugate vaccine conjugated to protein D (surface lipoprotein of non‐typeable Haemophilus influenzae); PPV23: 23‐valent pneumococcal polysaccharide vaccine; TIV: trivalent influenza vaccine; VE: vaccine efficacy
aPositive effect estimates indicates a relative reduction in the risk (e.g. 6% means that the vaccine reduces the risk by 6%); negative effect estimates indicates a relative increase in the risk (e.g. −5% means that the vaccine increases the risk by 5%). bCluster‐randomised controlled trial. cDefined as primary efficacy analysis. Analysis not entirely according to intention‐to‐treat principle, as 88/944 children were not included in analysis due to not meeting strict chart review criteria. d95% CI could not be calculated as person‐time across treatment groups was not reported. eIndex group: CRM197‐PCV7/TIV, control: HBV/placebo; VE placebo/TIV versus HBV/placebo: 71% (95% CI 30% to 88%), i.e. larger VE placebo/TIV versus HBV/placebo than CRM197‐PCV7/TIV versus HBV/placebo.
Note: negative values for VE expressed as relative reduction in risk represent an increase in the risk for AOM.
2. Effect of pneumococcal conjugate vaccination on frequency of pneumococcal acute otitis media episodes.
| Intention‐to‐treat | Per‐protocol | |||||||
| VE expressed as relative reduction in risk (95% CI) | VE expressed as relative reduction in risk (95% CI) | |||||||
|
Pneumococcal AOM |
Vaccine‐type AOM |
Cross‐reactive‐type AOM | Non‐vaccine‐type AOM |
Pneumococcal AOM |
Vaccine‐type AOM |
Cross‐reactive‐type AOM | Non‐vaccine‐type AOM | |
| PCV administered in infancy | ||||||||
| CRM197‐PCV7 | ||||||||
|
Black 2000a Fireman 2003 |
‐ ‐ |
65% P = 0.04 ‐ |
‐ ‐ |
‐ ‐ |
‐ ‐ |
67% P = 0.08 ‐ |
‐ ‐ |
‐ ‐ |
|
Eskola 2001 Palmu 2009b |
‐ ‐ |
54% (41% to 64%) ‐ |
‐ ‐ |
‐ ‐ |
34% (21% to 45%) 20% (7% to 31%) |
57% (44% to 67%) ‐ |
51% (27% to 67%) ‐ |
−33% (−80% to 1%) ‐ |
| O'Brien 2008a,c | ‐ | 64% (−34% to 90%) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| OMPC‐PCV7 | ||||||||
| Kilpi 2003 | ‐ | ‐ | ‐ | ‐ | 25% (11% to 37%) | 56% (44% to 66%) | −5% (−47% to 25%) | −27% (−70% to 6%) |
| PHiD‐PC10/11 | ||||||||
|
Tregnaghi 2014 Sáez‐Llorens 2017 |
53% (16% to 74%) | 70% (30% to 87%) | 29% (−123% to 77%) | 15% (−153% to 71%) | 56% (13% to 78%) | 67% (17% to 87%) | 26% (−232% to 83%) | 26% (−231% to 83%) |
| Vesikari 2016c | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Prymula 2006 | ‐ | ‐ | ‐ | ‐ | 52% (37% to 63%) | 58% (41% to 69%) | 66% (22% to 85%) | 9% (−64% to 49%) |
| PCV administered at a later age | ||||||||
| CRM197‐PCV7 followed by PPV23 | ||||||||
| Veenhoven 2003 | ‐ | ‐ | ‐ | ‐ | 34% P = 0.22 | 52% P = 0.21 | ‐ | 21% P = 0.44 |
| van Kempen 2006 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| CRM197‐PCV7/TIV | ||||||||
| Jansen 2008 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| CRM197‐PCV9 | ||||||||
| Dagan 2001 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
AOM: acute otitis media CI: confidence interval CRM197‐PCV7: 7‐valent pneumococcal conjugate vaccine conjugated to carrier protein CRM197 CRM197‐PCV7/TIV: trivalent influenza vaccine plus 7‐valent pneumococcal conjugate vaccine conjugated to carrier protein CRM197 CRM197‐PCV9: 9‐valent pneumococcal conjugate vaccine conjugated to carrier protein CRM197 MEF: middle ear fluid OMPC‐PCV7: 7‐valent pneumococcal conjugate vaccine conjugated to the outer membrane protein complex of Neisseria meningitidis serogroup B PCR: polymerase chain reaction PCV: pneumococcal conjugate vaccine PHiD‐CV10: 10‐valent pneumococcal conjugate vaccine conjugated to protein D (surface lipoprotein of non‐typeable Haemophilus influenzae) PHiD‐CV11: 11‐valent pneumococcal conjugate vaccine conjugated to protein D (surface lipoprotein of non‐typeable Haemophilus influenzae) PPV23: 23‐valent pneumococcal polysaccharide vaccine TIV: trivalent influenza vaccine VE: vaccine efficacy aMEF collected from spontaneous draining ears; in the other studies MEF was routinely collected during AOM episodes through paracentesis. bAdditional analysis of Eskola 2001 including pneumococcal AOM by a positive culture or PCR. cCluster‐randomised controlled trial. Note: negative values represent an increase in the risk of AOM.
3. Effect of pneumococcal conjugate vaccination on frequency of recurrent acute otitis media.
| Intention‐to‐treat | Per‐protocol | |
| VE expressed as relative reduction in risk (95% CI) | VE expressed as relative reduction in risk (95% CI) | |
| PCV administered in infancy | ||
| CRM197‐PCV7 | ||
|
Black 2000 Fireman 2003 |
9% (4 to 14) 10% (7 to 13) |
9% (3 to 15) ‐ |
| Eskola 2001 | 9% (−12 to 27) | 16% (−6 to 35) |
| O'Brien 2008a | ‐ | ‐ |
| OMPC‐PCV7 | ||
| Kilpi 2003 | ‐ | ‐ |
| PHiD‐PC10/11 | ||
|
Tregnaghi 2014 Sáez‐Llorens 2017 |
‐ | ‐ |
| Vesikari 2016a | ‐ | ‐ |
| Prymula 2006 | ‐ | 56% (−2 to 81) |
| PCV administered at a later age | ||
| CRM197‐PCV7 followed by PPV23 | ||
| Veenhoven 2003 | ‐ | ‐ |
| van Kempen 2006 | ‐ | ‐ |
| CRM197‐PCV7/TIV | ||
| Jansen 2008 | ‐ | ‐ |
| CRM197‐PCV9 | ||
| Dagan 2001 | ‐ | ‐ |
CI: confidence interval CRM197‐PCV7: 7‐valent pneumococcal conjugate vaccine conjugated to carrier protein CRM197 CRM197‐PCV7/TIV: trivalent influenza vaccine plus 7‐valent pneumococcal conjugate vaccine conjugated to carrier protein CRM197 CRM197‐PCV9: 9‐valent pneumococcal conjugate vaccine conjugated to carrier protein CRM197 OMPC‐PCV7: 7‐valent pneumococcal conjugate vaccine conjugated to the outer membrane protein complex of Neisseria meningitidis serogroup B PCV: pneumococcal conjugate vaccine PHiD‐CV10: 10‐valent pneumococcal conjugate vaccine conjugated to protein D (surface lipoprotein of non‐typeable Haemophilus influenzae) PHiD‐CV11: 11‐valent pneumococcal conjugate vaccine conjugated to protein D (surface lipoprotein of non‐typeable Haemophilus influenzae) PPV23: 23‐valent pneumococcal polysaccharide vaccine TIV: trivalent influenza vaccine VE: vaccine efficacy aCluster‐randomised controlled trial Note: negative values represent an increase in the risk of recurrent AOM
We included a total of 14 publications of 11 RCTs (60,733 children, range 74 to 37,868 per trial) of 7‐ to 11‐valent PCVs versus control vaccines. Seven trials included infants who predominantly received primary vaccinations before six months of age (59,415 children in total) (Black 2000/Fireman 2003; Eskola 2001/Palmu 2009; Kilpi 2003; O'Brien 2008; Prymula 2006; Tregnaghi 2014/Sáez‐Llorens 2017; Vesikari 2016). One study included day‐care attendees aged from 12 to 35 months (264 children) (Dagan 2001). Two trials included children aged from one to seven years with a history of AOM (457 children) (van Kempen 2006; Veenhoven 2003). One trial included children aged from 18 to 72 months with a previously diagnosed RTI (597 children) (Jansen 2008).
We have presented the results of individual trials as reported in the published papers; meta‐analysis was inappropriate due to substantial differences among studies. We have assessed the statistical methods used to analyse data in each study.
Adverse effects (co‐primary outcome)
An overview of adverse effects reported in the individual studies can be found in Table 5.
4. Adverse effects.
| Study ID | No. of participants | PCV type | Redness | Swelling | Pain/tenderness | Fever | Serious adverse events |
| Black 2000/Fireman 2003 | 37,868 | CRM197‐PCV7 | Depending on timing of dose, redness occurred in around 10% to 14% of children receiving CRM197‐PCV7 versus 5% to 9% of children receiving MenC vaccination. More severe redness (> 3 cm) occurred in 0% to 0.6% of children receiving CRM197‐PCV7 and did not differ significantly from those receiving MenC vaccination. |
Depending on timing of dose, swelling occurred in around 10% to 12% of children receiving CRM197‐PCV7 versus 3% to 8% of children receiving MenC vaccination. More severe swelling (> 3 cm) occurred in 0.1% to 0.6% of children receiving CRM197‐PCV7 and did not differ significantly from those receiving MenC vaccination. |
Depending on timing of dose, tenderness was reported in 15% to 23% of children receiving CRM197‐PCV7 and did not differ significantly from those receiving MenC vaccination. | Depending on timing of dose, fever > 38 °C occurred in around 15% to 24% of children receiving CRM197‐PCV7 versus 9% to 17% of children receiving MenC vaccination. Fever (> 39 °C) occurred in 0.9% to 2.5% of children receiving CRM197‐PCV7 and did not differ significantly from those receiving MenC vaccination. |
No severe adverse events related to vaccination resulting in hospitalisation, emergency, or clinic visits were reported. |
| Dagan 2001 | 264 | CRM197‐PCV9 | Depending on timing of dose, redness occurred in 5% to 6% of children receiving CRM197‐PCV9 versus 0% to 5% of children receiving MenC vaccination. | Depending on timing of dose, swelling occurred in 7% to 12% of children receiving CRM197‐PCV9 versus 0% to 5% of children receiving MenC vaccination. | Depending on timing of dose, tenderness was reported in 25% to 38% of children receiving CRM197‐PCV9 versus 0% to 8% of children receiving MenC vaccination. | Depending on timing of dose, fever > 38 °C occurred in around 15% to 44% of children receiving CRM197‐PCV9 versus 8% to 25% of children receiving MenC vaccination. Fever (> 39.5 °C) occurred in only 1 child receiving CRM197‐PCV9 versus 3 children receiving MenC vaccination. |
Not reported |
| Eskola 2001/Palmu 2009 | 1662 | CRM197‐PCV7 | Depending on timing of dose, redness occurred in 14% to 20% of children receiving CRM197‐PCV7 versus 9% to 16% of children receiving hepatitis vaccines. More severe redness (> 2.5 cm) occurred in 0% to 0.9% of children receiving CRM197‐PCV7 and did not differ significantly from those receiving hepatitis vaccines. |
Depending on timing of dose, swelling occurred in 5% to 6% of children receiving CRM197‐PCV7 versus 2% to 6% of children receiving hepatitis vaccines. More severe swelling (> 2.5 cm) occurred in 0.5% to 1.3% of children receiving CRM197‐PCV7 and did not differ significantly from those receiving hepatitis vaccines. |
Depending on timing of dose, pain was reported in 3% to 8% of children receiving CRM197‐PCV7 versus 2% to 3% of children receiving hepatitis vaccines. | Fever (> 39 °C) occurred in 0.4% to 2.0% of children receiving CRM197‐PCV7 versus 0.2% to 1.7% of children receiving hepatitis vaccines. | No significant differences between vaccine groups were observed for unexpected events (6 versus 4 events). 1 child in the CRM197‐PCV7 group died from bowel obstruction, necrosis, and shock at the age of 8 months (85 days after administration of third dose), but death was assessed as unrelated to study vaccine (autopsy revealed mesenteric defects with volvulus and other congenital abnormalities). |
| Jansen 2008 | 579 | CRM197‐PCV7/TIV | ‐ | ‐ | ‐ | ‐ | Quote: “In general, the vaccinations were well‐tolerated, and no immediate or severe adverse events were recorded.” |
| Kilpi 2003 | 1666 | OMPC‐PCV7 | OMPC‐PCV7 caused local reactions within 3 days of each dose more often than the hepB vaccine (data not shown). | OMPC‐PCV7 caused local reactions within 3 days of each dose more often than the hepB vaccine (data not shown). | Not reported | Not reported | There were no statistically significant differences in the occurrence of any diagnosis among individuals who experienced serious adverse events between the 2 vaccine groups. 1 child in the OMPC‐PCV7 group died from volvulus due to bowel obstruction. Death was assessed as unrelated to study vaccine. |
| Prymula 2006 | 4968 | PHiD‐CV11 | Not reported | Not reported | Not reported | Not reported | The percentages of infants with unsolicited symptoms that were judged to be causally related to vaccination were similar in the PHiD‐CV11 and hepA group (2.5% versus 3.0%). 14 serious adverse events were judged to be causally related to vaccination: 8 occurred in children receiving PHiD‐CV11 vaccination (7 after co‐administration with Infanrix hexa and 1 after PHiD‐CV11 booster) versus 6 in children receiving hepatitis A control vaccine (7 after co‐administration with Infanrix hexa and 1 after hepatitis A booster with Infanrix hexa). All events, apart from 1 case of epilepsy in the hepatitis A group, resolved without sequelae. 4 children died during the study, 1 of which occurred in the PHiD‐CV11 group (8 months after third dose, diagnosis of epilepsy was made; 25 months after the third dose the child had grand mal epilepsy and died from suffocation). None of the deaths were regarded by the investigators as related to the study vaccine. |
| Tregnaghi 2014/Sáez‐Llorens 2017 | 23,821 | PHiD‐CV10 | Not reported | Not reported | Not reported | Not reported | Serious adverse events did not differ significantly between PHiD‐CV10 and hepatitis control vaccines (21.5% versus 22.6%). Only 1 event (in the control group) was judged to be causally related to vaccination by the investigator, and it resolved without sequelae. 19 children died in the PHiD‐CV10 group (0.16%) versus 26 in the control group (0.22%). None of the deaths were considered by the investigator to be causally related to vaccination. |
| Veenhoven 2003 | 383 | CRM197‐PCV7 | Not reported | Not reported | Not reported | Not reported | No serious adverse events were noted after administration of CRM197‐PCV7 or hepatitis control vaccines. |
| Vesikari 2016 | 6178 | PHiD‐CV10 | Not reported | Not reported | Not reported | Not reported | Serious adverse events considered by the investigator to be causally related to vaccination were reported for 4 infants in the PHiD‐CV10 group (all in 3 + 1 group: sepsis with non‐specified aetiology in 1 infant, pyrexia in 1 infant, convulsion in 2 infants) and for 2 infants in HepB group (petit mal epilepsy in 1 infant and pyrexia in 1 infant). 1 fatal serious adverse event (sudden infant death, not considered to be vaccination related) was reported in the PHiD‐CV10 (2 + 1) group. |
C: Celcius CRM197‐PCV7: 7‐valent pneumococcal conjugate vaccine conjugated to carrier protein CRM197 CRM197‐PCV7/TIV: trivalent influenza vaccine plus 7‐valent pneumococcal conjugate vaccine conjugated to carrier protein CRM197 CRM197‐PCV9: 9‐valent pneumococcal conjugate vaccine conjugated to carrier protein CRM197 hepB: hepatitis B MenC: meningococcus type C OMPC‐PCV7: 7‐valent pneumococcal conjugate vaccine conjugated to the outer membrane protein complex of Neisseria meningitidis serogroup B PHiD‐CV10: 10‐valent pneumococcal conjugate vaccine conjugated to protein D (surface lipoprotein of non‐typeable Haemophilus influenzae) PHiD‐CV11: 11‐valent pneumococcal conjugate vaccine conjugated to protein D (surface lipoprotein of non‐typeable Haemophilus influenzae) TIV: trivalent influenza vaccine
Mild local reactions and fever were common in both groups, occurring more frequently in the PCV than in the control vaccine groups: redness (< 2.5 cm): 5% to 20% versus 0% to 16%; swelling (< 2.5 cm): 5% to 12% versus 0% to 8%; and fever (< 39 °C): 15% to 44% versus 8% to 25%. More severe redness (> 2.5 cm), swelling (> 2.5 cm), and fever (> 39° C) occurred less frequently (0% to 0.9%, 0.1% to 1.3%, and 0.4% to 2.5%, respectively, in children receiving PCV) and did not differ significantly between PCV and control vaccine groups. Pain or tenderness, or both was reported more frequently in children receiving PCV than in those receiving control vaccines: 3% to 38% versus 0% to 8%. Serious adverse events (SAEs) judged causally related to vaccination were rare and did not differ significantly between vaccine groups. No fatal SAE judged causally related to vaccination was reported.
The evidence for this outcome was of high quality.
Acute otitis media outcomes (co‐primary outcome and secondary outcomes)
Seven studies used the generalised Cox proportional hazard method proposed by Andersen 1982, currently regarded as the most optimal for analysing this kind of data (Black 2000/Fireman 2003; Eskola 2001/Palmu 2009; Kilpi 2003; Prymula 2006; Tregnaghi 2014/Sáez‐Llorens 2017; van Kempen 2006; Veenhoven 2003).
Dagan 2001 compared rates of AOM, but rather than comparing them by Poisson or negative binomial regression analysis (which would presumably yield results similar to those obtained with the Andersen approach), the Chi² test was used, which is suboptimal for comparing rates.
Jansen 2008 used Poisson, and Vesikari 2016 used negative binomial regression analysis to compare rates of AOM between groups, accounting for the potential dependency of observations between individuals.
O'Brien 2008 was a cluster‐randomised trial that calculated incidence rate ratios with a Poisson regression with sandwich variance estimation to account for within‐community correlation.
Effect of PCV administered in early infancy (predominantly < 6 months of age)
Seven trials (59,415 children) included infants who predominantly received various types of PCV before six months of age (Black 2000/Fireman 2003; Eskola 2001/Palmu 2009; Kilpi 2003; O'Brien 2008; Prymula 2006; Tregnaghi 2014/Sáez‐Llorens 2017; Vesikari 2016)
PCV7
In two trials (39,530 children), CRM197‐PCV7 was the intervention for healthy infants aged two months (Black 2000/Fireman 2003; Eskola 2001/Palmu 2009). The same vaccine was used as the intervention in one trial including 944 Navajo and White Mountain Apache children aged up to two years. These children carry one of the highest risks of developing AOM in the world (O'Brien 2008).
In one trial (1666 children), OMPC‐PCV7, with a subset of children receiving PPV23 as a booster dose, was used as the intervention in healthy infants aged two months (Kilpi 2003).
Primary outcome
Frequency of all‐cause AOM episodes
In one trial including 37,868 healthy infants aged two months (Black 2000/Fireman 2003), CRM197‐PCV7 was associated with a 6% (95% confidence interval (CI) 4% to 9%) relative risk reduction (RRR) in all‐cause AOM episodes in an intention‐to‐treat (ITT) analysis. Per‐protocol analysis of a trial including 1662 healthy infants aged two months showed that this same vaccine was associated with a non‐significant 6% (95% CI −4% to 16%) RRR in all‐cause AOM episodes (Eskola 2001/Palmu 2009).
In young children who carry a high baseline risk of developing AOM, CRM197‐PCV7 was not associated with a reduction in all‐cause AOM episodes (1 trial; 944 children; RRR −5%, 95% CI −25% to 12%; ITT analysis) (O'Brien 2008).
In one trial including 1666 healthy infants aged two months (Kilpi 2003), OMPC‐PCV7 was not associated with a reduction in all‐cause AOM episodes in per‐protocol analysis (RRR −1%, 95% CI −12% to 10%).
The evidence for the use of CRM197‐PCV7 and OMPC‐PCV7 in low‐risk infants for this outcome was of high quality. However, the evidence for use of CRM197‐PCV7 in young children with high baseline risk of developing AOM for this outcome was of moderate quality; the evidence quality was downgraded one level due to imprecise effect estimate (one trial with a relatively small sample size).
Secondary outcomes
Frequency of pneumococcal AOM
In one trial including 1662 healthy infants aged two months (Eskola 2001/Palmu 2009), CRM197‐PCV7 was associated with a 20% (95% CI 7% to 31%) to 34% (95% CI 21% to 45%) RRR in pneumococcal AOM episodes in per‐protocol analysis, depending on whether this outcome was assessed by a composite of positive culture or positive pneumolysin polymerase chain reaction (PCR) or by positive culture only.
In one trial including 1666 healthy infants aged two months (Kilpi 2003), OMPC‐PCV7 was associated with a 25% (95% CI 11% to 37%) RRR in pneumococcal AOM episodes in per‐protocol analysis.
The evidence for this outcome was of high quality.
Frequency of pneumococcal serotype‐specific AOM
In two trials (39,530 healthy infants aged two months) (Black 2000/Fireman 2003; Eskola 2001/Palmu 2009), administration of CRM197‐PCV7 was associated with a 54% (95% CI 41% to 64%) to 65% (P = 0.04) RRR in vaccine‐type pneumococcal AOM episodes in ITT analysis.
In one of these trials (Eskola 2001/Palmu 2009), CRM197‐PCV7 was associated with a 51% (95% CI 27% to 67%) RRR in AOM episodes caused by cross‐reactive serotypes and a non‐significant 33% (95% CI −80% to 1%) relative increase in the risk of non‐vaccine type AOM episodes in per‐protocol analyses.
In one trial (944 children) (O'Brien 2008), administration of CRM197‐PCV7 in young children who carry a high baseline risk of developing AOM was associated with a non‐significant 64% (95% CI −34% to 90%) RRR in vaccine‐type pneumococcal AOM episodes in ITT analysis.
In one trial including 1666 healthy infants aged two months (Kilpi 2003), OMPC‐PCV7 was associated with a 56% (95% CI 44% to 66%) RRR in vaccine‐type pneumococcal AOM episodes in per‐protocol analysis. In the same trial (Kilpi 2003), OMPC‐PCV7 failed to show cross‐protection (RRR −5%, 95% CI −47% to 25%), and this vaccine was associated with a non‐significant 27% (95% CI −70% to 6%) relative increase in the risk of non‐vaccine‐type AOM episodes in per‐protocol analyses.
The evidence for use of CRM197‐PCV7 and OMPC‐PCV7 in healthy infants for this outcome was of high quality. However, evidence for the use of CRM197‐PCV7 in young children with high baseline risk of developing AOM for this outcome was of moderate quality; the evidence quality was downgraded one level due to study limitations (risk of bias) and imprecise effect estimate.
Frequency of recurrent AOM
In two trials (39,530 children) (Black 2000/Fireman 2003; Eskola 2001/Palmu 2009), administration of CRM197‐PCV7 in healthy infants aged two months was associated with a 9% (95% CI −12% to 27%) to 10% (95% CI 7% to 13%) RRR in developing recurrent AOM.
The evidence for this outcome was of high quality.
PHiD‐CV10/11
PHiD‐CV10 was used as the intervention in two trials (12,307 children) (Tregnaghi 2014/Sáez‐Llorens 2017; Vesikari 2016). PHiD‐CV11 was used in one trial (4968 children) (Prymula 2006).
Primary outcome
Frequency of all‐cause AOM episodes
In one trial including 7359 healthy infants aged from 6 to 16 weeks (Tregnaghi 2014/Sáez‐Llorens 2017), PHiD‐CV10 was associated with a non‐significant 15% (95% CI −1% to 28%) RRR in all‐cause AOM episodes in ITT analysis. Per‐protocol analysis of a trial including 5095 healthy infants aged from 6 weeks to 18 months showed that this same vaccine was associated with a non‐significant 6% (95% CI −6% to 17%) RRR in all‐cause AOM episodes (Vesikari 2016).
In one trial including 4968 healthy infants aged from 6 weeks to 5 months, PHiD‐CV11 was associated with a 34% (95% CI 21% to 44%) RRR in all‐cause AOM episodes in per‐protocol analysis (Prymula 2006).
However, it should be noted that the AOM incidence rates in the two trials with the largest point estimates, Tregnaghi 2014/Sáez‐Llorens 2017; Prymula 2006, were low (Table 2). Consequently, the absolute risk differences in these trials were rather small.
The evidence for the use of PHiD‐CV11 for this outcome was of high quality. The evidence for the use of PHiD‐CV10 was of moderate quality; the evidence quality was downgraded one level due to study limitations (risk of bias) and imprecise effect estimates.
Secondary outcomes
Frequency of pneumococcal AOM
In one trial including 7359 healthy infants aged from 6 to 16 weeks (Tregnaghi 2014/Sáez‐Llorens 2017), PHiD‐CV10 was associated with a 53% (95% CI 16% to 74%) RRR in pneumococcal AOM episodes in ITT analysis.
In one trial including 4968 healthy infants aged from 6 weeks to 5 months, PHiD‐CV11 was associated with a 52% (95% CI 37% to 63%) RRR in pneumococcal AOM episodes in per‐protocol analysis (Prymula 2006).
The evidence for pneumococcal AOM episodes was of high quality.
Frequency of pneumococcal serotype‐specific AOM
In one trial including 7359 healthy infants aged from 6 to 16 weeks (Tregnaghi 2014/Sáez‐Llorens 2017), PHiD‐CV10 was associated with a 70% (95% CI 30% to 87%) RRR in vaccine‐type pneumococcal AOM episodes in ITT analysis. In the same trial (Tregnaghi 2014/Sáez‐Llorens 2017), PHiD‐CV10 was associated with a non‐significant 29% (95% CI −123% to 77%) RRR in AOM episodes caused by cross‐reactive serotypes and a non‐significant 15% (95% CI −153% to 71%) RRR in non‐vaccine‐type AOM episodes in ITT analyses.
In one trial including 4968 healthy infants aged from 6 weeks to 5 months, PHiD‐CV11 was associated with a 58% (95% CI 41% to 69%) RRR in vaccine‐type pneumococcal AOM episodes in per‐protocol analysis (Prymula 2006). In the same trial (Prymula 2006), PHiD‐CV11 was associated with a 66% (95% CI 22% to 85%) RRR in AOM episodes caused by cross‐reactive serotypes and a non‐significant 9% (95% CI −64% to 49%) RRR in non‐vaccine‐type AOM episodes in per‐protocol analyses.
The evidence for vaccine‐type pneumococcal AOM episodes was of high quality. The evidence for cross‐reactive serotypes and non‐vaccine‐type AOM episodes was of moderate quality; the evidence quality was downgraded one level due to imprecise effect estimates.
Frequency of recurrent AOM
In one trial including 4968 healthy infants aged from 6 weeks to 5 months, PHiD‐CV11 was associated with a non‐significant 56% (95% CI −2% to 80%) RRR in developing recurrent AOM in per‐protocol analysis (Prymula 2006).
The evidence for this outcome was of moderate quality; the evidence quality was downgraded one level due to the imprecise effect estimate.
Effect of PCV administered at a later age (one year and above)
In three trials, various types of PCV7 were administered in children with a history of either RTI (597 participants), Jansen 2008, or AOM (457 participants in total) (van Kempen 2006; Veenhoven 2003).
CRM197‐PCV7
Primary outcome
Frequency of all‐cause AOM episodes
In two trials (457 children) (van Kempen 2006; Veenhoven 2003), CRM197‐PCV7 followed by PPV23 in children aged from one to seven years with a history of AOM was not associated with further reductions in AOM episodes (1 trial; 383 children; RRR −25%, 95% CI −57% to 1%; ITT‐analysis (Veenhoven 2003); 1 trial; 74 children; RRR −16%, 95% CI −96% to 31%; per‐protocol analysis (van Kempen 2006)).
In one trial including 597 children with a history of RTI (Jansen 2008), CRM197‐PCV7 administered together with a trivalent influenza vaccine (CRM197‐PCV7/TIV) was associated a 57% (95% CI 6% to 80%) RRR in all‐cause AOM episodes compared to hepatitis B/placebo vaccination in per‐protocol analysis. However, the effect of TIV/placebo compared to hepatitis B/placebo vaccination on all‐cause AOM episodes appeared to be even larger (RRR 71%, 95% CI 30% to 88%) (Jansen 2008).
The evidence for this outcome was of high quality.
Secondary outcomes
Frequency of pneumococcal AOM
In per‐protocol analysis of one trial including 383 children with a history of AOM (Veenhoven 2003), CRM197‐PCV7 followed by PPV23 was associated with a non‐significant 34% (P = 0.22) RRR in pneumococcal AOM episodes.
The evidence for this outcome was of moderate quality; the evidence quality was downgraded one level due to imprecise effect estimates (one study with a relatively small sample size).
Frequency of pneumococcal serotype‐specific AOM
In a per‐protocol analysis of one trial including 383 children with a history of AOM (Veenhoven 2003), CRM197‐PCV7 followed by PPV23 was associated with a non‐significant 52% (P = 0.21) and 21% (P = 0.21) RRR in pneumococcal serotype‐specific AOM and non‐vaccine‐type AOM episodes.
The evidence for this outcome was of moderate quality; the evidence quality was downgraded one level due to imprecise effect estimates (one study with a relatively small sample size).
Frequency of recurrent AOM
None of the three trials in older children reported the effect of PCV7 on recurrent AOM.
CRM197‐PCV9
In one trial (264 children) (Dagan 2001), CRM197‐PCV9 was administered in healthy day‐care attendees aged from 12 to 35 months.
Primary outcome
Frequency of all‐cause AOM episodes
In a per‐protocol analysis, CRM197‐PCV9 was associated with a non‐significant 17% (95% CI −2% to 33%) RRR in all‐cause OM episodes (Dagan 2001).
The evidence for this outcome was of low quality; the evidence quality was downgraded two levels due to study limitations (risk of bias and questions about outcome assessment) and imprecise effect estimate (one study with a relatively small sample size).
Secondary outcomes
Dagan 2001 did not report on any of our secondary outcomes of interest.
Discussion
Summary of main results
The current evidence base for the effects of PCVs for preventing AOM in children comes from 11 RCTs (60,733 children) of 7‐ to 11‐valent PCVs versus control vaccines (meningococcus type C conjugate vaccine in three trials, and hepatitis A or B vaccine in eight trials) with a generally low risk of bias. No relevant RCTs with the newer 13‐valent PCV were available. In seven trials (59,415 children), PCVs were predominantly administered in children's first months of life, whilst four trials (1318 children) included children aged one year and over who were either healthy or who had a history of respiratory illness or frequent AOM. There was considerable clinical heterogeneity across studies in terms of design, study population, type of PCV used, and outcome measures, therefore we did not perform meta‐analyses.
The licenced CRM197‐PCV7 and PHiD‐CV10 vaccines, when administered during early infancy (< 6 months of age), were associated with substantial RRR in pneumococcal AOM (high‐quality evidence). However, their effects on all‐cause AOM are far more uncertain, as most trials failed to demonstrate statistical significant differences for this outcome. Relative risk reductions for CRM197‐PCV7 varied from −5% (95% CI −25% to 12%) in high‐risk infants (moderate‐quality evidence) to 6% (95% CI −4% to 16%) and 6% (95% CI 4% to 9%) in low‐risk infants (high‐quality evidence); whereas RRRs for PHiD‐CV10 varied from 6% (95% CI −6% to 17%) to 15% (95% CI −1% to 28%) in healthy infants (moderate‐quality evidence).
Administration of PCVs in high‐risk infants, after early infancy, and in older children with a history of a history of respiratory illness or frequent AOM was not associated with reductions in all‐cause AOM.
Local redness, swelling, fever, and tenderness/pain were commonly reported and occurred more frequently in children receiving PCV than in those receiving control vaccines, but these adverse effects were mostly mild. More severe redness (> 2.5 cm), swelling (> 2.5 cm), and fever (> 39 °C) occurred far less frequently and did not differ between vaccine groups. Serious adverse events judged causally related to vaccination were rare and did not differ significantly between vaccine groups. No fatal SAE judged causally related to vaccination was reported.
Overall completeness and applicability of evidence
The 11 RCTs included in this review differed substantially in terms of RCT type, study population (age of PCV administration and AOM baseline risk), PCV type (vaccine valency, carrier protein, and booster regimen), co‐administration of other vaccines, and AOM assessment and definition used. Furthermore, in the infant studies focusing on AOM bacteriology (Eskola 2001; Kilpi 2003; Prymula 2006; Tregnaghi 2014), the control groups varied markedly in the proportions of S pneumoniae, H influenzae, and M catarrhalis found in the middle ear fluid. This might be related to time and geographic region as well as case definition, and has important implications for the effects of PCV on preventing all‐cause AOM episodes. Additionally, three studies included older otitis‐prone children, so the intervention was aimed at secondary or even tertiary prevention, and not primary prevention (Jansen 2008; van Kempen 2006; Veenhoven 2003). The reduced efficacy of CRM197‐PCV7 in children with a history of AOM may be explained by an increased susceptibility to subsequent infections, not only with non‐vaccine‐type pneumococci, but also other nasopharyngeal colonisers, due to 'damage' already suffered by the middle ear mucosa caused by prior AOM (Veenhoven 2003). Another explanation, although debated, could be the non‐protective, impaired antibody responses of children who are otitis‐prone (Pichichero 2013; Wiertsema 2012). It thus appears that the age at which PCV is administered, a history of AOM episodes, or both, modifies the effect of PCV on AOM, despite the fact that age alone could not be identified as a statistically significant effect modifier (Black 2000/Fireman 2003; Veenhoven 2003). Our review did not focus on the effects of PCVs on shifts in serotypes over time. Further research into the impact of PCVs on (serotype) replacement is warranted since a shift in causative pathogens may have considerable implications on both AOM burden and vaccine effectiveness.
Quality of the evidence
We judged the methodological quality of the included studies to be generally high. For most outcomes, the quality of the evidence varied from high (further research is very unlikely to change our confidence in the estimate of effect) to moderate (further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate). The evidence for the effect of CRM197‐PCV9 administered in healthy day‐care attendees aged 12 to 35 months on all‐cause AOM episodes was of low quality (further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate).
Potential biases in the review process
We adhered to the prespecified review protocol. In this 2019 update, two review authors (ACF and RPV) independently searched all relevant electronic databases using a search syntax comprising all relevant synonyms for PCV and AOM. We also performed a broad internet search to identify potentially relevant articles. To increase the yield of relevant studies, we reviewed the reference lists of all identified studies and systematic reviews or meta‐analyses. We searched ClinicalTrials.gov and the WHO ICTRP for completed and ongoing trials.
Agreements and disagreements with other studies or reviews
Our main findings are in agreement with two other systematic reviews on the effect of PCV in children, indicating that PCVs provide substantial protection against pneumococcal AOM, but that their effects on all‐cause AOM are more uncertain and far less pronounced (Ewald 2016; Pavia 2009; Taylor 2012).
In AOM, there is a high potential for replacement by other bacterial pathogens that are common colonisers of the nasopharynx. CRM197‐PCV7 is known to affect nasopharyngeal carriage of pneumococci, with a shift from vaccine‐type pneumococci to non‐vaccine‐type pneumococci and other otopathogens including non‐typeable H influenza and Staphylococcus aureus (Biesbroek 2014; Block 2006; Casey 2013; Coker 2010; Eskola 2001; Obaro 1996; Somech 2011; van Gils 2011; Wiertsema 2011). Nasopharyngeal carriage results from a recent RCT on PHiD‐CV10 showed similar bacterial colonisation patterns as observed in CRM197‐PCV7 among healthy Dutch children aged up to two years (van den Bergh 2013). The middle ear is directly connected to the nasopharynx, and by lowering the carriage of vaccine‐type pneumococci, a niche may be created for other bacteria with pathogenic potential (Block 2006; Veenhoven 2003; Veenhoven 2004). Recent studies have shown that nationwide implementation of PCVs may have changed the frequency of the causative otopathogens involved in AOM towards pneumococcal serotypes not included in the vaccines and non‐typeable H influenzae (Casey 2013; Coker 2010; Kaur 2017; Somech 2011; Wiertsema 2011).
Although RCT data failed to demonstrate a convincing beneficial effect of CRM197‐PCV7 and PHiD‐CV10 on all‐cause AOM, various global postmarketing studies with these licenced vaccines, as well as the newer CRM197‐PCV13, suggest that the impact of PCVs on AOM may be substantial (Eythorsson 2018; Gisselsson‐Solen 2017; Kawai 2018; Lau 2015; Magnus 2012; Marom 2014; Poehling 2007; Sigurðsson 2018; Zhou 2008), which may be attributable to indirect (herd) effects of vaccination. However, it should be noted that findings from observational studies warrant careful interpretation, as variability in baseline incidence, study population, and case definition, as well as fluctuations in risk factors for AOM such as breastfeeding, household smoking, day‐care attendance rates, and implementation of AOM clinical practice guidelines, may affect the AOM incidences reported. For example, results from Boston (USA) showed that the decline in uncomplicated AOM, treatment failure, and AOM relapse was at least as large in the 2000 to 2004 period compared to the 1996 to 2000 period, leaving the 'true' contribution of PCV in reducing AOM incidence uncertain (Sox 2008). Furthermore, reduced exposure to household smoking, among other factors such as PCV7 coverage since 2002, may have contributed to the steady decline in USA paediatric ambulatory visits for OM over the period of 1993 to 2006 (Alpert 2011).
The impact of PHiD‐CVs may expand beyond their effects on pneumococcal AOM to AOM caused by non‐typeableH influenzae due to the carrier protein D (Forsgren 2008). A recent review including pre‐clinical, clinical, and postmarketing studies concluded that PHiD‐CVs may decrease AOM caused by non‐typeableH influenzae, but that more evidence including pathogen‐specific outcomes is clearly warranted (Clarke 2017). The diversity of non‐typeableH influenzae, with some strains lacking protein D, may limit the effect of PHiD‐CVs on non‐typeableH influenzae AOM. In our review, administration of the licenced PHiD‐CV10 in healthy infants was associated with non‐significant 6% (95% CI −6% to 17%) and 15% (95% CI −1% to 28%) relative reductions in the risk of all‐cause AOM. The added benefit of PHiD‐CV10 over the previously licenced CRM197‐PCV7 on all‐cause AOM therefore remains uncertain.
We found limited evidence that administration of PCVs during infancy may reduce the risk of recurrent AOM. This is in line with accumulating evidence that PCVs might disrupt the continuum of evolution from pneumococcal‐associated OM towards chronic/recurrent OM by prevention of early vaccine‐serotype AOM, thereby reducing subsequent and more complex disease caused by non‐vaccine serotypes and non‐typeable H influenzae (Ben‐Shimol 2014; Dagan 2016). These findings are further supported by secondary analyses of some of the trials included in our review indicating that licenced CRM197‐PCV7 and PHiD‐PCV10 lead to fewer ventilation tubes insertions for chronic/recurrent OM (Black 2000; Palmu 2015b; Sarasoja 2013).
Authors' conclusions
Implications for practice.
Current evidence from randomised controlled trials indicates that, albeit associated with large relative risk reductions in pneumococcal acute otitis media (AOM), the effect of administration of the licenced CRM197‐PCV7 and PHiD‐CV10 in healthy, low‐risk infants on AOM is uncertain and modest at best. However, global postmarketing studies of these vaccines, as well as the licenced CRM197‐PCV13, suggest that the impact (i.e. both direct and indirect effects) of pneumococcal conjugate vaccines (PCVs) on AOM may be substantial. Furthermore, it should be noted that the decision whether or not to implement PCV should not come from just AOM studies, but also from studies that see and show the big picture, including data on invasive pneumococcal disease such as pneumonia, bacteraemia, and meningitis.
Compared to control vaccines, PCVs were associated with an increase in mild local reactions (redness, swelling), fever, and pain/tenderness. However, we found no evidence of a difference in (far less frequently occurring) more severe local reactions, fever, or serious adverse events judged to be causally related to vaccination.
Implications for research.
Since most countries across the world have implemented PCV in nationwide immunisation programmes, future randomised controlled trials comparing PCVs versus control vaccines are unlikely to be performed. Whilst there is some observational evidence of a difference in effects on AOM between PHiD‐CV10 and the newer CRM197‐PCV13 (Gisselsson‐Solen 2017), future trials may compare the efficacy of various types of PCVs. More importantly, future research will likely shed a light on the effects of other vaccines to prevent AOM, including (protein‐based) vaccines directed at Streptococcus pneumoniae (NCT01545375), as well as other pathogens including non‐typeable Haemophilus influenzae and Moraxella catarrhalis (Pettigrew 2017).
Whether any decline in AOM will continue or wane over time due to replacement is relevant and deserves ongoing monitoring. Besides a reduction of nasopharyngeal vaccine‐type serotypes, which is presumed to induce herd effects, replacing pneumococcal serotypes may not only lead to replacement disease in vaccines, but also in the population. Continuing surveillance of nasopharyngeal carriage and pneumococcal disease in both the short and long term (Spijkerman 2012), in different settings and geographic locations, is therefore of utmost importance.
What's new
| Date | Event | Description |
|---|---|---|
| 9 March 2020 | Amended | The authors' Declarations of interest have been updated to reflect the review's compliance with the Cochrane conflict of interest policy, which includes the relevant parts of the Cochrane Commercial Sponsorship Policy. |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 2, 2002
| Date | Event | Description |
|---|---|---|
| 29 March 2019 | New citation required and conclusions have changed | With this 2019 update, further information on the effect of PHiD‐CV10/11 for the prevention of acute otitis media (AOM) has become available. Administration of the licenced CRM197‐PCV7 and PHiD‐CV10 during early infancy is associated with large relative risk reductions in pneumococcal AOM. However, the effects of these vaccines on all‐cause AOM is far more uncertain. We found no evidence of a beneficial effect on all‐cause AOM of administering pneumococcal conjugate vaccines (PCVs) in high‐risk infants, after early infancy (i.e. in children aged one year and above) and in older children with a history of respiratory illness. Compared to control vaccines, PCVs were associated with an increase in mild local reactions (redness, swelling), fever, and pain/tenderness, but we found no evidence of a difference in more severe local reactions, fever, or serious adverse events judged to be causally related to vaccination. |
| 29 March 2019 | New search has been performed | We updated the searches and included three new publications: two new randomised controlled trials (RCTs) (Tregnaghi 2014; Vesikari 2016), and one new trial publication (Sáez‐Llorens 2017), which reported outcome data relevant for this review as part of a secondary analysis of Tregnaghi 2014. We excluded five new trial publications (secondary analyses of previously included RCTs) (Palmu 2014; Palmu 2015a; Palmu 2015b; Palmu 2018; Sarasoja 2013), since they did not report outcome data relevant to this review. We did not identify any ongoing studies. The 14 included publications in this review originate from 11 RCTs (60,733 children) in total: Black 2000/Fireman 2003; Dagan 2001; Eskola 2001/Palmu 2009; Jansen 2008; Kilpi 2003; O'Brien 2008; Prymula 2006; Tregnaghi 2014/Sáez‐Llorens 2017; van Kempen 2006; Veenhoven 2003; Vesikari 2016. Seven trials (59,415 children), Black 2000/Fireman 2003; Eskola 2001/Palmu 2009; Kilpi 2003; O'Brien 2008; Prymula 2006; Tregnaghi 2014/Sáez‐Llorens 2017; Vesikari 2016, included infants who predominantly received primary vaccinations before six months of age (Black 2000/Fireman 2003; Eskola 2001/Palmu 2009; Kilpi 2003; O'Brien 2008; Prymula 2006; Tregnaghi 2014/Sáez‐Llorens 2017; Vesikari 2016), while the other four trials (1318 children), Dagan 2001; Jansen 2008; van Kempen 2006; Veenhoven 2003, assessed the effects of PCVs administered at a later age on AOM in either healthy infants, Dagan 2001, or in children with a history of respiratory illness or frequent AOM (Jansen 2008; van Kempen 2006; Veenhoven 2003). We added 'adverse effects' as co‐primary outcome. |
| 21 February 2014 | New search has been performed | With this update, more precise information on the effect of PCV7 for the prevention of otitis media has become available. We judged the quality of the evidence for PCV7 in both early infancy and older children to be high, with further research very unlikely to change our confidence in the estimate of effect. Based on current evidence of the effects of pneumococcal conjugate vaccines (PCVs) for preventing acute otitis media (AOM), the licenced 7‐valent PCV has modest beneficial effects in healthy infants with a low baseline risk of AOM. Administering PCV7 in high‐risk infants, after early infancy, and in older children with a history of AOM appears to have no benefit in preventing further episodes. Several randomised controlled trials (RCTs) with different (newly licensed, multivalent) PCVs administered during early infancy to establish their effects on AOM are currently ongoing. The results of these studies may provide a better understanding of the role of the newly licenced, multivalent PCVs in preventing AOM. Also, the impact of the carrier protein D, as used in certain pneumococcal vaccines for AOM, needs to be further established. |
| 3 December 2013 | New search has been performed | Three new review authors joined the team to update this review. The updated search (November 2007 to December 2013) retrieved 171 records. After removal of duplicates 165 records remained. After full‐text review, three new publications (Palmu 2009; Prymula 2006; van Kempen 2006) remained for inclusion. One study was an additional analysis of the previous included Eskola 2001 study. We identified five ongoing RCTs (NCT00466947; NCT00861380; NCT01545375; NCT01735084; NCT01174849). The 11 included studies in this review concerned a total of nine RCTs: (1) Black 2000/Fireman 2003; (2) Dagan 2001; (3) Eskola 2001/Palmu 2009; (4) Kilpi 2003; (5) Prymula 2006; (6) van Kempen 2006; (7) Veenhoven 2003; (8) Jansen 2008; and (9) O'Brien 2008. Five trials (Black 2000/Fireman 2003; Eskola 2001/Palmu 2009; Kilpi 2003; O'Brien 2008; Prymula 2006) (n = 47,108) included healthy infants and studied the effect of PCV administered in early infancy on otitis media (OM), while the other four trials (n = 1318), Dagan 2001; Jansen 2008; van Kempen 2006; Veenhoven 2003, assessed the effects of PCV administered at a later age on OM in either healthy infants, Dagan 2001, or in children with a known history of respiratory disease including OM (Jansen 2008; van Kempen 2006; Veenhoven 2003). |
| 29 April 2008 | New citation required but conclusions have not changed | New review authors |
| 28 April 2008 | Amended | Converted to new review format |
| 15 November 2007 | New search has been performed | Searches conducted |
| 26 November 2003 | New citation required and conclusions have changed | Substantive amendment |
| 29 June 2003 | New search has been performed | Searches conducted |
| 19 August 2000 | New search has been performed | Searches conducted |
Notes
The focus in research has shifted from the use of pneumococcal polysaccharide vaccines (PPVs) to pneumococcal conjugate vaccines (PCVs) in children, and the role of PPVs in the prevention of AOM in children is not relevant anymore as PPVs are no longer used as primary intervention in children since the introduction of PCVs. The focus of the current review has therefore shifted from the effect of PPVs to the effect of PCVs on acute otitis media. No further attention will be paid to the effects of PPVs, which were described in prior versions of this review (Straetemans 2003).
Acknowledgements
The authors are indebted to Reinier Veenhoven (in memoriam) for the invaluable collaboration and his contributions to prior versions of the review.
We would like to thank Angelique Jansen, Masja Straetemans, and Gerhard Zielhuis for their contributions to prior versions of this review. We would also like to thank David Honeyman and Vittoria Lutje for their support with the searches, and the following people for commenting on this review: Barbara Loe Fisher, Morio Aihara, Mark Jones, Paul Glasziou, Tal Moram, Janet Wale, and Susan Smith.
Appendices
Appendix 1. Previous search details
For the 2014 version of the review we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 11), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register; MEDLINE (1995 to November week 3, 2013); Embase (1995 to December 2013); CINAHL (2007 to December 2013); LILACS (2007 to December 2013) and Web of Science (2007 to December 2013).
We used the following search strategy to search CENTRAL and MEDLINE. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search strategy to search Embase, CINAHL, LILACS and Web of Science.
MEDLINE (Ovid)
1 exp Otitis Media/ 2 otitis media.tw. 3 aom.tw. 4 or/1‐3 5 Pneumococcal Vaccines/ 6 Vaccines, Conjugate/ 7 Bacterial Vaccines/ 8 (pneumococc* adj5 (vaccin* or conjugat* or immuni*)).tw,nm. 9 pcv*.tw,nm. 10 or/5‐9 11 4 and 10
For the 2009 version of the review we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2007, issue 2), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register; MEDLINE (January 1995 to November 2007); and EMBASE (January 1995 to November 2007).
We used the following search strategy for searching MEDLINE and CENTRAL and modified terms for searching EMBASE.
MEDLINE
#1 explode ‘bacterial‐vaccine’ / all subheadings #2 explode ‘bacterial AND vaccine’ / all subheadings #3 explode ‘Pneumococcus‐vaccine’ / all subheadings #4 pneumococc* near immunity* #5 pneumococc* near vaccin* #6 #1 or #2 or #3 or #4 or #5 #7 explode ‘otitis media’ / all subheadings #8 (otitis media in ti) or (otitis media in ab) #9 #7 or #8 #10 #6 or #9 #11 explode ‘randomised‐controlled‐trial’ / all subheadings #12 explode ‘controlled‐study’ / all subheadings #13 explode ‘randomisation’ / all subheadings #14 explode ‘single‐blind‐procedure’ / all subheadings #15 explode ‘double‐blind‐procedure’ / all subheadings #16 explode ‘crossover‐procedure’ / all subheadings #17 explode ‘phase‐3‐clinical‐trial’ / all subheadings #18 (control* near trial*) in ti) #19 (control* near trial*) in ab) #20 (singl* or doubl* or trebl* or tripl*) near ((blind* or mask*) in ti) #21 (singl* or doubl* or trebl* or tripl*) near ((blind* or mask*) in ab) #22 random* near ((allocat* or assign*) in ti) #23 random* near ((alsocat* or assign*) in ab) #24 #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 #25 #10 and #24
Appendix 2. MEDLINE (Ovid) search strategy
1 exp Otitis Media/ 2 otitis media.tw. 3 (aom or om or ome or csom).tw. 4 glue ear.tw. 5 (otorrhea* or otorrhoea*).tw. 6 (middle ear adj5 (infect* or inflam* or effus*)).tw. 7 or/1‐6 8 exp Pneumococcal Vaccines/ 9 Vaccines, Conjugate/ 10 Bacterial Vaccines/ 11 (pneumococc* adj5 (vaccin* or conjugat* or immuni*)).tw,nm. 12 (pcv* or 5PPV* or 7PCV* or 9PCV* or 10PCV* or 11PCV* or 13PCV* or 15PCV* or synflorix).tw,nm. 13 or/8‐12 14 7 and 13
Appendix 3. Embase (Elsevier) search strategy
#17. #12 AND #16 #16. #13 OR #14 OR #15 #15. random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR 'cross‐over':ab,ti OR volunteer*:ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR ((singl* OR doubl*) NEAR/1 blind*):ab,ti #14. 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp #13. 'randomized controlled trial'/exp #12. #6 AND #11 #11. #7 OR #8 OR #9 OR #10 #10. (pcv* or 5PPV* or 7PCV* or 9PCV* or 10PCV* or 11PCV* or 13PCV* or 15PCV* or synflorix):ab,ti,tn #9. (pneumococc* NEAR/5 (vaccin* OR conjugat* OR immuni*)):ab,ti,tn #8. 'bacterial vaccine'/de #7. 'pneumococcus vaccine'/de #6. #1 OR #2 OR #3 OR #4 OR #5 #5. ("middle ear" NEAR/5 (infect* OR inflam* OR effus*)):ab,ti #4. otorrhea*:ab,ti OR otorrhoea*:ab,ti #3. 'glue ear':ab,ti #2. 'otitis media':ab,ti OR aom:ab,ti OR om:ab,ti OR ome:ab,ti OR csom:ab,ti #1. 'otitis media'/exp
Appendix 4. CINAHL (EBSCO) search strategy
S24 S14 and S23 S23 S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 S22 (MH "Quantitative Studies") S21 (MH "Placebos") S20 TI placebo* OR AB placebo* S19 TI random* OR AB random* S18 TI ( singl* blind* or doubl* blind* or tripl* blind* or trebl* blind* or singl* mask* or doubl* mask* or tripl* mask* or trebl* mask* ) OR AB (singl* blind* or doubl* blind* or tripl* blind* or trebl* blind* or singl* mask* or doubl* mask* or tripl* mask* or trebl* mask* ) S17 TI clinic* trial* OR AB clinic* trial* S16 PT clinical trial S15 (MH "Clinical Trials+") S14 S6 and S13 S13 S7 or S8 or S9 or S10 or S11 or S12 S12 (TI pcv* OR AB pcv* OR TI 5PPV* OR AB 5PPV* OR TI 7PCV* OR AB 7PCV* OR TI 9PCV* OR AB 9PCV* OR TI 10PCV* OR AB 10PCV* OR TI 11PCV* OR AB 11PCV* OR TI 13PCV* OR AB 13PCV* OR TI 15PCV* OR AB 15PCV* OR TI synflorix OR AB synflorix) S11 TI pneumococc* N5 immuni* OR AB pneumococc* N5 immuni* S10 TI pneumococc* N5 conjugat* OR AB pneumococc* N5 conjugat* S9 TI pneumococc* N5 vaccin* OR AB pneumococc* N5 vaccin* S8 (MH "Bacterial Vaccines") S7 (MH "Pneumococcal Vaccine") S6 S1 or S2 or S3 or S4 or S5 S5 ((TI "middle ear" OR AB "middle ear") N5 (TI infect* OR AB infect* OR TI inflam* OR AB inflam* OR TI effus* OR AB effus*)) S4 TI (otorrhea* or otorrhoea*) OR AB (otorrhea* or otorrhoea*) S3 TI glue ear OR AB glue ear S2 TI ( otitis media or aom or ome or csom) OR AB ( otitis media or aom or ome or csom) S1 (MH "Otitis Media+")
Appendix 5. LILACS (BIREME) search strategy
tw:((mh:"otitis media" OR "otitis media" OR "Otite Média" OR mh:c09.218.705.663* OR aom OR om OR ome OR csom OR "glue ear" OR otorrhea* OR otorrhoea* OR (middle ear AND (infect* OR inflam* OR effus*))) AND (mh:"Pneumococcal Vaccines" OR "Vacunas Neumococicas" OR "Vacinas Pneumocócicas" OR mh:"Vaccines, Conjugate" OR "Vacunas Conjugadas" OR "Vacinas Conjugadas" OR mh:"Bacterial Vaccines" OR "Vacunas Bacterianas" OR "Vacinas Bacterianas" OR "pneumococcal vaccine" OR "pneumococcal vaccines" OR "conjugate vaccines" OR "conjugate vaccine" OR pcv* OR 5ppv* OR 7pcv* OR 9pcv* OR 10pcv* OR 11pcv* OR 13pcv* OR 15pcv* OR synflorix)) AND (instance:"regional") AND ( db:("LILACS"))
Appendix 6. Web of Science (Clarivate Analytics) search strategy
| # 5 | 113 | #4 AND #3 Indexes=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED Timespan=2013‐2018 |
| # 4 | 719,817 |
TOPIC: ((random* or placebo* or allocat* or crossover* or "cross over" or ((doubl* or singl*) NEAR/1 (blind* or mask*)))) ORTITLE: (trial) Indexes=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED Timespan=2013‐2018 |
| # 3 | 498 | #2 AND #1 Indexes=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED Timespan=2013‐2018 |
| # 2 | 6,232 |
TOPIC: ((pneumococc* NEAR/5 (vaccin* or conjugat* or immuni*))) ORTOPIC: ((pcv* or 5PPV* or 7PCV* or 9PCV* or 10PCV* or 11PCV* or 13PCV* or 15PCV* or synflorix)) Indexes=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED Timespan=2013‐2018 |
| # 1 | 15,363 |
TOPIC: ("otitis media" or aom or om or ome or csom or "glue ear" or otorrhea* or otorrhoea* or ("middle ear" NEAR/5 (infect* or inflam* or effus*))) Indexes=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED Timespan=2013‐2018 |
Appendix 7. ClinicalTrials.gov search strategy
("otitis media" OR aom OR om OR ome OR csom OR "glue ear" OR otorrhea OR otorrhoea OR "middle ear infection" OR "middle ear infections") AND (pneumococcal OR pneumococcus) AND (vaccine OR vaccines OR vaccinating OR vaccination OR vaccinations)
("otitis media" OR aom OR om OR ome OR csom OR "glue ear" OR otorrhea OR otorrhoea OR "middle ear infection" OR "middle ear infections") AND (pneumococcal OR pneumococcus) AND (conjugate OR conjugated OR immunisation)
("otitis media" OR aom OR om OR ome OR csom OR "glue ear" OR otorrhea OR otorrhoea OR "middle ear infection" OR "middle ear infections") AND (pneumococcal OR pneumococcus) AND (immunisations OR immunised OR immunising)
(Search has been split into 3 to work better on the ClinicalTrials.gov search portal)
Appendix 8. WHO ICTRP search strategy
pneumococc* AND vaccin* AND otitis media OR pneumococc* AND conjugat* AND otitis media OR pneumococc* AND immuni* AND otitis media OR pcv* AND otitis media OR pneumococc* AND vaccin* AND aom OR pneumococc* AND conjugat* AND aom OR pneumococc* AND immuni* AND aom OR pcv* AND aom OR pneumococc* AND vaccin* AND middle ear infection* OR pneumococc* AND conjugat* AND middle ear infection* OR pneumococc* AND immuni* AND middle ear infection* OR pcv* AND middle ear infection*
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Black 2000.
| Methods |
Randomised: yes, at individual level Design: standard parallel‐group design Intention‐to‐treat: yes Follow‐up: 6 to 31 months |
|
| Participants |
N: 37,868 healthy infants
Age: 2 months Setting: 23 medical centres within Northern California Kaiser Permanente (NCKP), USA Inclusion criteria: healthy children aged 2 months Exclusion criteria: children with sickle cell disease, known immunodeficiency, any serious chronic or progressive disease, a history of seizures, or a history of either pneumococcal or meningococcal disease Baseline characteristics: not described |
|
| Interventions | Children were randomly allocated to either CRM197‐PCV7 or a meningococcus type C conjugate vaccine (10 µg of group C oligosaccharide conjugated to carrier protein CRM197; MenC) at 2, 4, 6 and 12 to 15 months of age Tx: CRM197‐PCV7; N = 18,927 received 1 dose or more of the vaccine (unclear how many children were included in otitis media analyses) C: MenC; N = 18,941 received 1 dose or more of the vaccine (unclear how many children were included in otitis media analyses) Additional vaccines: routine childhood vaccines were administered at the recommended ages: DTwP or DTaP; oral poliovirus vaccine or inactivated poliovirus vaccine; Hib; hepatitis B; measles‐mumps‐rubella vaccine; varicella. Initially all participants received a vaccine combining Haemophilus b conjugate and DTwP into the opposite leg and oral poliovirus vaccine concurrently. When recommendations changed, the protocol was amended to allow administration of DTaP and inactivated poliovirus vaccine. Vaccines not given concomitantly were given at least 2 weeks apart from study vaccine. |
|
| Outcomes |
Primary outcome: invasive pneumococcal disease caused by vaccine serotypes Secondary outcomes: number of otitis media episodes in fully vaccinated per protocol; number of otitis media visits; time to recurrent otitis media (defined as 3 or more episodes in 6 months or 4 or more in 12 months); number of tympanostomy tubes placements; number of cases of spontaneously draining ruptured tympanic membranes with culture of a vaccine serotype pneumococcus; safety (local and systemic reactions at 48 to 72 hours after vaccination, uncommon events requiring medical attention 30 days and 60 days after vaccination, and mortality) Clinical diagnoses of AOM were obtained from computerised data sources using diagnoses registered by emergency physicians and paediatricians in the NCKP population. Each clinic visit constituted a new episode unless it was classified as a follow‐up visit. A visit < 21 days after another otitis media visit was always considered a follow‐up visit. A visit 42 days or more after the most recent otitis media visit was considered a new episode. Visits occurring between 21 and 42 days, if the appointment was made < 3 days in advance, were considered new episodes. |
|
| Funding sources | The study was supported by an unrestricted grant from Wyeth(‐Ayerst); authors were employed at Kaiser Permanente Vaccine Study Center (Oakland), Wyeth Lederle Vaccines and Pediatrics (Pearl River), University of Pennsylvania (Philadelphia), and Vanderbilt University Medical Center (Nashville). | |
| Declarations of interest | Not described; authors were employed at Kaiser Permanente Vaccine Study Center (Oakland), Wyeth Lederle Vaccines and Pediatrics (Pearl River), University of Pennsylvania (Philadelphia), and Vanderbilt University Medical Center (Nashville) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Indicated as a double‐blind study, but insufficient details provided to ensure blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinical diagnoses of AOM were obtained from computerised data sources using diagnoses registered by emergency physicians and paediatricians (non‐trialists). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how many children were included in otitis media analyses |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available. Otitis media endpoint (efficacy against otitis media episodes) is reported as a secondary endpoint. |
| Other bias | Unclear risk | Study enrolment was stopped as a result of prespecified interim analysis. |
Dagan 2001.
| Methods |
Randomised: yes, at individual level Design: standard parallel‐group design Intention‐to‐treat: no, per‐protocol analysis Follow‐up: 2 years starting 1 month after complete immunisation |
|
| Participants |
N: 264 healthy infants (261 children were included in clinical follow‐up)
Age: 12 to 35 months Setting: 8 day‐care centres in Beer‐Sheva, Israel Inclusion criteria: healthy children aged 12 to 35 months Exclusion criteria: children who had received any vaccine within the previous 4‐week period, or who were scheduled to receive any vaccine during the 4 weeks after the administration of the study vaccines, or who had received immunoglobulin within 8 weeks of study vaccination; known or suspected impairment of immunologic functions; major congenital malformation or serious chronic disease; known hypersensitivity to any components of the study vaccine; previous severe vaccine‐associated adverse reaction; previous vaccination with any pneumococcal or meningococcal vaccine; febrile illness (rectal temperature 38 °C) within 72 h before vaccination Baseline characteristics: described and balanced (Table 1 of trial publication) |
|
| Interventions | Children were randomly allocated to either CRM197‐PCV9 or MenC. Children aged 12 to 17 months at time of enrolment received 2 intramuscular injections 2 to 3 months apart, and those 18 to 35 months at time of enrolment received 1 intramuscular injection. Tx: CRM‐197‐PCV9; N = 131 C: MenC; N = 130 Additional vaccines: not described |
|
| Outcomes |
Primary outcome: nasopharyngeal carriage of Streptococcus pneumoniae of the serotypes found in the vaccines in general and antibiotic‐resistant S pneumoniae in particular Secondary outcomes: parent‐reported respiratory infections including otitis media, tolerance (tenderness, local and systemic reactions after vaccination including erythema, induration, and fever) 18 encounters were planned for each child during the 2‐year follow‐up period. Encounters were planned to take place monthly during the first year and bimonthly during the second year. At each visit the parents were questioned about illness and antibiotic use since the last visit. Illness episodes were divided into 4 categories: (1) upper respiratory infections; (2) lower respiratory problems; (3) otitis media; and (4) other illnesses. Only episodes starting 1 month after complete immunisation were counted. |
|
| Funding sources | The study vaccines were provided by Wyeth‐Lederle Vaccines and Pediatrics (Pearl River, NY). | |
| Declarations of interest | Not described | |
| Notes |
Participants lost to follow‐up during first 12 months:total: 32/261 (12.3%) Participants lost to follow‐up during first 12 months:Tx: 16/131 (12.2%) Participants lost to follow‐up during first 12 months:C: 16/130 (12.3%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not described. Block randomisation (n = 6) stratified by DCC and age. |
| Allocation concealment (selection bias) | Low risk | Randomisation list provided in a sealed envelope by Wyeth‐Lederle Vaccines. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | PCV9 and MenC vaccines dissimilar in appearance. 2 nurses not belonging to the study team injected the vaccines. They were not allowed to reveal the child's allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Parental interview. A positive report of OM was defined as an episode. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up rates reported in Table 1. 12% of children followed up for < 12 months. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available. |
| Other bias | Low risk | No other sources of bias identified. |
Eskola 2001.
| Methods | This trial was part of a study including Kilpi 2003 (FinOM Vaccine Trial). Both Eskola 2001 and Kilpi 2003 used the same control group (hepatitis B vaccine containing 5 μg of recombinant hepatitis B surface protein) but a different treatment group, each with a different PCV7 type. Eskola 2001 used CRM197‐PCV7, while Kilpi 2003 used OMPC‐PCV7. Randomised: yes, at individual level Design: standard parallel‐group design Intention‐to‐treat: yes, both ITT and per‐protocol analysis described Follow‐up: 22 consecutive months (children were followed up to 24 months of age) |
|
| Participants |
N: 1662 healthy infants
Age: 2 months Setting: 8 study clinics in the communities of Tampere, Kangsala, and Nokia, Finland Inclusion criteria: healthy children aged 2 months Exclusion criteria: not described Baseline characteristics: described and balanced (Table 1 of trial publication) |
|
| Interventions | Children were randomly allocated to either CRM197‐PCV or a hepatitis B vaccine at 2, 4, 6 and 12 to 15 months of age. Tx: CRM197‐PCV; N = 831 (N = 786 completed the follow‐up as specified in the protocol) C: hepatitis B vaccine; N = 831 (N = 794 completed the follow‐up as specified in the protocol) Additional vaccines: a combination vaccine containing whole‐cell DTP and Hib was given in the child's opposite thigh at the same visit as the pneumococcal vaccine at 2, 4, and 6 months of age. In half of the study clinics, the carrier protein in the DTP and Haemophilus influenzae vaccine was CRM197, and in the other half it was tetanus toxoid. Inactivated poliovirus vaccine was given at 7 months of age and again at the same time as the fourth dose of the study vaccine at 12 months of age. Measles–mumps–rubella vaccine was administered at 18 months. |
|
| Outcomes |
Primary outcome: number of AOM episodes due to the pneumococcal serotypes included in the vaccine Secondary outcomes: number of all‐cause AOM episodes, culture‐confirmed and pathogen‐specific AOM episodes; preventing first and subsequent AOM episodes; number of children with recurrent AOM episodes (defined as 3 or more AOM episodes in the last 6 months or 4 or more in the last 12 months); serious adverse events, safety (pain, local and systemic reactions within 3 days after vaccination, unexpected events after vaccination and mortality) All children attended 1 of the study clinics for enrolment at 2 months of age and thereafter at 4, 6, 7, 12, 13, 18, and 24 months. Parents were encouraged to bring their child to the study clinic for evaluation of symptoms suggesting respiratory infection or AOM. AOM was diagnosed by otoscopy (visibly abnormal tympanic membrane in terms of colour, position, or mobility, suggesting middle ear effusion) and the presence of at least 1 of the following symptoms or signs of acute infection: fever, earache, irritability, diarrhoea, vomiting, acute otorrhoea not caused by otitis externa, and other symptoms of respiratory infection. For the overall and pathogen‐specific AOM episodes, a new episode was considered to have started if at least 30 days had elapsed since the beginning of the previous episode. For AOM episodes according to serotype, a new episode was considered to have started if 30 days had elapsed since the beginning of an episode due to the same serotype, or if any interval had elapsed since the beginning of an episode due to a different serotype. If more than 1 serotype was recovered from the middle ear fluid at the same time, only 1 episode was considered to have started. |
|
| Funding sources | Supported by Merck, Pasteur Mérieux Connaught, and Wyeth‐Lederle Vaccines and Pediatrics | |
| Declarations of interest | Dr Eskola and Dr Kilpi have served as consultants to Wyeth‐Lederle Vaccines. | |
| Notes |
Participants lost to follow‐up:total: 82/1662 (4.9%) did not complete the follow‐up period specified in the protocol Participants lost to follow‐up:Tx: 45/831 (5.4%) did not complete the follow‐up period specified in the protocol Participants lost to follow‐up:C: 37/831 (4.5%) did not complete the follow‐up period specified in the protocol |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 6 letters corresponding to the 3 treatment options were randomly allocated to consecutive participant identification numbers, using an allocation of 1:1:1 and a block size of 12 (see Kilpi 2003). |
| Allocation concealment (selection bias) | Low risk | Individual treatment assignments were kept in sealed envelopes until vaccination (see Kilpi 2003). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of vaccinators who were not otherwise involved in the trial follow‐up. Letter code was destroyed immediately after vaccination (see Kilpi 2003). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment of the outcome was done according to a strict definition of AOM. Assessment was done by personnel other than those who vaccinated the children (vaccinators were not otherwise involved in the trial follow‐up) (see Kilpi 2003). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for dropout or lost to follow‐up, or both not reported. Not expected to have major impact on outcome since 94.6% in the CRM197‐PCV7 and 95.5% in the control group completed the follow‐up as specified in the protocol. |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes (primary and secondary) are listed in ClinicalTrials.gov (although uploaded after study end). |
| Other bias | Low risk | No other sources of bias identified. |
Jansen 2008.
| Methods |
Randomised: yes, at individual level Design: standard parallel‐group design Intention‐to‐treat: yes Follow‐up: follow‐up started 14 days after the second set of vaccinations and continued for 6 to 18 months, depending on the year of inclusion |
|
| Participants |
N: 597 children with a previously diagnosed RTI
Age: 18 to 72 months Setting: GPs in the centre of the Netherlands selected children Inclusion criteria: children aged 18 to 72 months with a previously diagnosed RTI registered according to the ICPC, i.e. AOM; cough (with fever); acute upper RTI; acute laryngitis/tracheitis; acute bronchitis/bronchiolitis; influenza; pneumonia; pleurisy/pleural effusion Exclusion criteria: children with chronic asthma or recurrent wheezing (for longer than 3 months) treated with corticosteroids; craniofacial abnormalities; clinically significant hypersensitivity to eggs; previous serious adverse reactions to vaccines; previous influenza, pneumococcal, or hepatitis B vaccinations and those with conditions for which these vaccinations are already recommended, such as chronic cardiac and respiratory conditions Baseline characteristics: described and balanced (Table 1 of trial publication) |
|
| Interventions | Children were randomly allocated to either TIV/PCV7, TIV/placebo (TIV plus standard diluent (0.9% phosphate buffered NaCl)) or HBV/placebo (recombinant HBV vaccine‐Engerix B Junior plus placebo vaccine). Strains in the TIV 2003‐2004 formulation were H1N1, H3N2, and B/HongKong/330/01; strains in the TIV 2004‐2005 formulation were H1N1, H3N2, and B/Shanghai/361/2002; strains in the TIV 2005‐2006 formulation included H1N1, H3N2, and B/Shanghai/361/2002. Children received 2 vaccinations 4 to 8 weeks apart in the first year of inclusion, and the first 2 cohorts of children received a subsequent vaccination in the subsequent year. Tx: TIV/CRM197‐PCV7; N = 197 (N = 163 completed; 67,867 person‐days analysed, 14% missing) C1: TIV/placebo; N = 187 (N = 148 completed; 60,515 person‐days analysed, 20% missing) C2: HBV/placebo; N = 195 (N = 160 completed; 67,679 person‐days analysed, 15% missing) Additional vaccines: not described |
|
| Outcomes |
Primary outcome: febrile RTI, defined as fever (tympanic temperature 38.0 °C) for at least 2 consecutive days accompanied by 1 or more of the aforementioned signs or symptoms of RTI with a moderate or severe severity score Secondary outcomes: febrile RTI–related PCR‐confirmed influenza, GP visits, antibiotic prescriptions, or a physician‐diagnosed episode of AOM, tolerability and safety Each parent was instructed to keep a daily diary, recording any clinical signs or symptoms associated with RTI and to characterise their severity on a scale of 1 (mild) to 3 (severe). The parent was also instructed to measure the child's body temperature using a validated electronic tympanic thermometer, and was asked to record all GP visits due to their child's RTI‐related complaints. For each such visit, the GP was instructed to complete a form including information on the diagnosis and possible antibiotic prescriptions. During influenza seasons, the parent was instructed to contact the trial centre for evaluation for influenza if the child had fever (tympanic temperature 38.0 °C) for more than 1 day accompanied by at least 1 RTI‐associated sign or symptom of severity score 2. A trained research assistant obtained a nasopharyngeal swab for viral determination within 4 days of onset of fever and symptoms. Each sample was analysed by real‐time PCR for the presence of influenza A and B viruses. |
|
| Funding sources | The study was funded by the Netherlands Organisation for Health Research and Development (ZonMw). The funding agency played no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript. Influenza vaccines were provided by Solvay, Weesp, the Netherlands. Pneumococcal vaccines were provided by Wyeth Vaccines Research, Berkshire, UK. Hepatitis B vaccines were provided by GlaxoSmithKline BV, Rixensart, Belgium. |
|
| Declarations of interest | The authors declared no conflicts of interest. | |
| Notes |
Participants lost to follow‐up:total: 108/579 (18.7%) completely (N = 41) or partially (N = 67) lost to follow‐up Participants lost to follow‐up:Tx: 34/197 (17.3%) completely (N = 8) or partially (N = 26) lost to follow‐up; 67,867 person‐days analysed, 14% missing Participants lost to follow‐up:C1: 39/187 (20.8%) completely (N = 19) or partially (N = 20) lost to follow‐up; 60,515 person‐days analysed, 20% missing Participants lost to follow‐up:C2: 35/195 (17.9%) completely (N = 14) or partially (N = 21) lost to follow‐up; 67,679 person‐days analysed, 15% missing 2 of the 3 treatment arms received an additional vaccination in the second year of the study. To evaluate blinding, parents of these cohorts of children were asked which vaccinations that they thought their child had received just after the vaccinations were given and at the end of the study. Just after the vaccination, 87% of the parents either did not know or identified the wrong set of vaccinations; at the end of the study, this percentage was 80%, indicating successful blinding. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not described; children were randomly assigned in blocks of 3 in a 1:1:1 ratio. |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment was described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The injections were administered by non‐blinded research nurses who were not involved in subsequent follow‐up and who were instructed not to reveal the intervention allocation. The treatment group assignments were not revealed to parents, investigators, research personnel conducting the follow‐up, or healthcare providers, all of whom remained blinded throughout the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The parents were asked to record all GP visits due to their child's RTI‐related complaints. For each such visit, the GP was instructed to complete a form including information on the diagnosis and possible antibiotic prescriptions. The treatment group assignments were not revealed to parents, investigators, and research personnel conducting the follow‐up or healthcare providers, all of whom remained blinded throughout the study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Substantial loss to follow‐up (< 14% in both groups) |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes (primary and secondary) are listed in ClinicalTrials.gov. |
| Other bias | Low risk | No other sources of bias identified. |
Kilpi 2003.
| Methods | This trial was part of a study including Eskola 2001 (FinOM Vaccine Trial). Both Eskola 2001 and Kilpi 2003 used the same control group (hepatitis B vaccine containing 5 μg of recombinant hepatitis B surface protein) but a different PCV7 type. Eskola 2001 used CRM197‐PCV7, while Kilpi 2003 used OMPC‐PCV7. Randomised: yes, at individual level Design: standard parallel‐group design Intention‐to‐treat: no, per‐protocol analysis Follow‐up: 22 consecutive months (children were followed up to 24 months of age) |
|
| Participants |
N: 1666 healthy infants
Age: 2 months Setting: 8 study clinics in the communities of Tampere, Kangsala, and Nokia, Finland Inclusion criteria: healthy children aged 2 months Exclusion criteria: not described Baseline characteristics: described and balanced (Table 1) |
|
| Interventions | Children were randomly allocated to either OMPC‐PCV7 or a hepatitis B vaccine at 2, 4, 6 and 12 to 15 months of age. From 3 November 1997 onwards, for the children randomised to receive OMPC‐PCV7, the fourth dose of the conjugate vaccine was replaced by PPV23, Pneumovax23 including serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F. Tx: OMPC‐PCV7; N = 835 (N = 805 completed the follow‐up as specified in the protocol) C: hepatitis B vaccine; N = 831 (N = 794 completed the follow‐up as specified in the protocol) Additional vaccines: a diphtheria–tetanus toxoids–pertussis vaccine with a whole‐cell pertussis component, combined with a Haemophilus influenzae type b conjugate vaccine (DTP‐Hib), was administered concomitantly with the first 3 doses of the study vaccine, and an inactivated poliovirus vaccine was administered with the fourth dose. In 4 study clinics, the carrier protein in the DTP‐Hib conjugate combination was CRM197, and in the other 4 it was tetanus toxoid. |
|
| Outcomes | See Eskola 2001. | |
| Funding sources | Supported by Aventis Pasteur, Merck, and Wyeth‐Lederle Vaccines and Pediatrics | |
| Declarations of interest | Not described; from Eskola 2001: Dr Eskola and Dr Kilpi have served as consultants to Wyeth‐Lederle Vaccines | |
| Notes |
Participants lost to follow‐up:total: 67/1666 (4.0%) did not complete the follow‐up period specified in the protocol Participants lost to follow‐up:Tx: 30/835 (3.6%) did not complete the follow‐up period specified in the protocol Participants lost to follow‐up:C: 37/831 (4.5%) did not complete the follow‐up period specified in the protocol |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 6 letters corresponding to the 3 treatment options were randomly allocated to consecutive participant identification numbers, using an allocation of 1:1:1 and a block size of 12. |
| Allocation concealment (selection bias) | Low risk | Individual treatment assignments were kept in sealed envelopes until vaccination. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of vaccinators who were not otherwise involved in the trial follow‐up. Letter code was destroyed immediately after vaccination. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment of the outcome was done according to a strict definition of AOM. Assessment was done by personnel other than those who vaccinated the children (vaccinators were not otherwise involved in the trial follow‐up). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reporting of reasons for dropout or loss to follow‐up, or both. Not expected to have a major impact on outcome since 96.0% in the OMPC‐PCV7 and 95.5% in the control group completed the follow‐up as specified in the protocol |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes (primary and secondary) are listed in ClinicalTrials.gov (although uploaded after study end). |
| Other bias | Unclear risk | Mixed schedule with 187 children boosted with PPV23. Unclear how researchers identified those allocated to OMPC‐PCV7 to receive PPV23 after November 1997 |
O'Brien 2008.
| Methods | The design of this cluster‐randomised trial has been described extensively in Moulton 2001, while the findings on IPD (main outcome of the trial) have been published in O'Brien 2003. Randomised: yes, at group level Design: cluster‐randomised design Intention‐to‐treat: no, per‐protocol analysis Follow‐up: depending on time of inclusion, maximum duration of follow‐up 40 months |
|
| Participants |
N: 944 (944 of the 4476 children were randomly selected for chart review. This sample size was determined by logistic feasibility and expected frequency of healthcare events. Of these 944 children, 856 were found to have strictly met the chart review criteria.)
Age: below 2 years of age Setting: Navajo and White Mountain Apache region, USA Inclusion criteria: Navajo and White Mountain Apache children below 2 years of age Exclusion criteria: no exclusion criteria described Baseline characteristics: balanced but data not shown |
|
| Interventions | Children were randomly allocated to either CRM197‐PCV7 or MenC (10 µg of group C oligosaccharide conjugated to carrier protein CRM197). For each of the study and control vaccines, 3 immunisation schedules were designed according to age of entry into the trial: 6 weeks to 6 months (3 doses, ideally at 2, 4, and 6 months of age and a booster at 12 to 15 months of age), 7 months to 11 months (2 doses 1 month apart and a booster at 12 to 15 months of age), and 12 months to 23 months (2 doses separated by at least 2 months). Over the course of the trial, the great majority of new enrollees are in the first group, which is referred to as the primary efficacy cohort. Tx: CRM197‐PCV7; N = unknown (N = 424 analysed in primary efficacy group) C: MenC; N = unknown (N = 432 analysed in primary efficacy group) Additional vaccines: not described |
|
| Outcomes |
Primary outcome: clinically diagnosed episodes of OM Every medical visit made by study children was evaluated through 2 years of age. OM visits, as documented by the patients' treating physician, were recorded. A new OM episode was counted if any of the following were recorded as the diagnosis: OM, AOM, bilateral OM, chronic OM, OM with perforation, otorrhoea, pressure‐equalising tube placement, perforated tympanic membrane, serous OM and bullous myringitis. An episode of AOM was categorised as either AOM or bilateral AOM. An OM episode was categorised as severe if there were 3 or more OM visits for the episode. A child's first medical visit for OM was considered their first episode. OM visits occurring less than 21 days after the immediately prior otitis‐related visit and visits noted as a follow‐up to a previous otitis‐related visit were counted as follow‐up visits, not as OM episodes. |
|
| Funding sources | Financial support for the American Indian PnCRM7 Efficacy Trial was from Wyeth Vaccines, National Institutes of Health, World Health Organization, The National Vaccine Program Office, and the Centers for Disease Control and Prevention. | |
| Declarations of interest | Dr O'Brien and Dr Santosham participated in Wyeth Scientific Advisory Boards. Dr O'Brien, Dr Moulton, Dr Reid, Dr Weatherholtz, and Dr Santosham received research funding from Wyeth Vaccines. | |
| Notes |
Participants lost to follow‐up:total: 88/944 (9.3%) not included in primary efficacy analysis Participants lost to follow‐up:Tx: unknown Participants lost to follow‐up:C: unknown |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation using 38 independent randomisation units, stratified using 3 blocks of 4 units and 13 blocks of 2 units |
| Allocation concealment (selection bias) | Low risk | 6 labels were assigned to the vaccines (B, F, H, M, T, U), with 3 labels for CRM197‐PCV7 and 3 for MenC. The grouping of these codes was known only to a statistician employed by the manufacturer (who had no other responsibilities with respect to the trial other than handling treatment allocation and randomisation issues). No loss of clusters |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Masked treatment assignment (vaccines were labelled). In addition, field staff were blinded as to serotype of the invasive disease cases, and thus did not know which ones would be likely to be prevented by an effective vaccine. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Every medical visit made by study children was evaluated through 2 years of age. OM visits, as documented by the patients' treating physician, were recorded. Treating physicians were blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 88 of the 944 children (9.3%) not included in primary efficacy analysis; no information provided on the distribution across treatment groups |
| Selective reporting (reporting bias) | Low risk | Study design was described extensively in Moulton 2001 and O'Brien 2003. |
| Other bias | Unclear risk | Study enrolment was stopped as a result of prespecified interim analysis. |
Prymula 2006.
| Methods |
Randomised: yes, at individual level Design: standard parallel‐group design Intention‐to‐treat: yes, both ITT and per‐protocol analysis described Follow‐up: efficacy follow‐up started on the day of the first dose of study vaccine (for ITT analysis) or 2 weeks after the third vaccine dose (for the per‐protocol analysis) and continued until 24 to 27 months of age |
|
| Participants |
N: 4968 healthy infants
Age: between 6 weeks and 5 months Setting: 27 paediatric centres in the Czech Republic and 23 in Slovakia Inclusion criteria: healthy children aged between 6 weeks and 5 months with no acute illness Exclusion criteria: use of any investigational or non‐registered drug or vaccine other than the study vaccines within 30 days preceding first dose of the study vaccines; previous vaccination against Streptococcus pneumoniae; fever (defined as a rectal temperature of 38 °C or higher or temperature by other routes of 37.5 °C or higher); history of allergic disease or reactions likely to be exacerbated by any component of the study vaccines; other conditions that might have potentially interfered with the interpretation of study outcomes according to the investigator Baseline characteristics: described and balanced (Table 1 of trial publication) |
|
| Interventions | Children were randomly allocated to either PHiD‐CV11 or a hepatitis A vaccine (containing 720 ELISA units of inactivated hepatitis A virus antigen (strain HM 175)) at about 3, 4, 5 and 12 to 15 months of age. Tx: PHiD‐CV11; N = 2489 (N = 2455 included in per‐protocol cohort for efficacy) C: hepatitis A vaccine; N = 2479 (N = 2452 included in per‐protocol cohort for efficacy) Additional vaccines: a concomitant hexavalent diphtheria‐tetanus‐3‐component acellular pertussis‐hepatitis B‐inactivated poliovirus types 1, 2, and 3 Haemophilus influenzae type b (DTPa‐HBV‐IPV/Hib) vaccine was offered to all study participants, followed by a booster dose at age 15 to 18 months |
|
| Outcomes |
Primary outcome: first episode of AOM caused by vaccine pneumococcal serotypes Secondary outcomes: first episode of AOM caused by non‐typeable Haemophilus influenzae, any all‐cause AOM episodes, any vaccine‐type AOM episodes, any cross‐reactive serotypes AOM, any non‐vaccine‐type AOM, safety (adverse events arising within 31 days of vaccination and serious adverse events occurring throughout the study period) There was no active surveillance. Unscheduled doctor visits could take place any time during follow‐up according to standard local practice (parents consulting their local paediatrician in case of illness of their child). Parents were advised to consult their paediatrician if their child was sick, had ear pain, or had spontaneous ear discharge. Children with suspected AOM were immediately referred to ENT surgeons. AOM was defined as either abnormal findings of the tympanic membrane at otoscopy (i.e. redness, bulging, loss of light reflex) or the presence of middle ear effusion as shown by simple or pneumatic otoscopy or by microscopy together with at least 2 of the following signs or symptoms: ear pain, ear discharge, hearing loss, fever, lethargy, irritability, anorexia, vomiting, or diarrhoea. These signs or symptoms had to be present for a maximum of 14 days. For patients with repeated doctor visits, a new episode of AOM was judged to have started if more than 30 days had elapsed since the beginning of the previous episode. Additionally, for categories defined according to bacterial pathogen or serotype, a new episode was judged to have started if any interval had elapsed since the beginning of an episode caused by a different bacterial pathogen or serotype. Recurrent AOM was defined as 3 or more AOM episodes in the last 6 months or 4 or more in the last 12 months. |
|
| Funding sources | The study was supported by GlaxoSmithKline Biologicals, Rixensart, Belgium. | |
| Declarations of interest | Dr Prymula is a consultant to GlaxoSmithKline and other pharmaceutical companies, and has received travel grants or honoraria paid by healthcare companies within the past 3 years. 7 co‐authors are employees of GlaxoSmithKline Biologicals, of which 4 own shares in GlaxoSmithKline. | |
| Notes |
Participants lost to follow‐up:total: 61/4968 (1.2%) did not complete the follow‐up period specified in the protocol Participants lost to follow‐up:Tx: 34/2489 (1.4%) did not complete the follow‐up period specified in the protocol Participants lost to follow‐up:C: 27/2479 (1.1%) did not complete the follow‐up period specified in the protocol |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random list |
| Allocation concealment (selection bias) | Low risk | Randomisation (1:1) was done with a study‐specific central randomisation system via the internet which, on receipt of the infant's initials and birth date, determined the vaccine number to be used. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Indicated as a double‐blinded study. The sponsor numbered the vaccine supplies. However, it is unknown whether the appearance of the vaccines was similar at the time of administration. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Visits during efficacy follow‐up were according to standard local clinical practice. When AOM was suspected, children were referred to ENT surgeons. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reporting of reasons for dropout or loss to follow‐up, or both. Not expected to have a major impact on outcome since 98.6% in the PHiD‐CV11 and 98.9% in the control group completed the follow‐up as specified in the protocol. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes (primary and secondary) are listed in ClinicalTrials.gov. |
| Other bias | Low risk | Study enrolment was stopped as a result of prespecified interim analysis. No other sources of bias identified. |
Tregnaghi 2014.
| Methods | This trial was part of Clinical Otitis Media and Pneumonia Study (COMPAS; clinicaltrials.gov/show/NCT00466947) to assess the efficacy of PHiD‐CV10 against IPD, CAP, and AOM in young Latin American children. Randomised: yes, at individual level Design: standard parallel‐group design Intention‐to‐treat: yes, both ITT and per‐protocol analysis described Follow‐up: total follow‐up duration 4 years |
|
| Participants |
N: 23,821 healthy infants (for Panama, AOM cohort: 7359)
Age: mean age 9 months Setting: well‐baby clinics at 5 sites (3 in Argentina, 1 in Colombia, and 1 in Panama); all countries are classified as upper‐middle economies Inclusion criteria: healthy children aged 6 to 16 weeks Exclusion criteria – use or planned use of any investigational or unregistered drug or vaccine other than the study vaccines; previous vaccination against diphtheria, tetanus, pertussis, Haemophilus influenzae type b, hepatitis A, and/or Streptococcus pneumoniae; history of allergic disease or reactions likely to be exacerbated by any components of the study vaccines; acute disease at time of enrolment; low birthweight (< 2500 g) not permitted for Colombia Baseline characteristics: described and balanced (Table 3 of trial publication) |
|
| Interventions | Children were randomly allocated either to PHiD‐CV10 or a hepatitis B vaccine at 2, 4, and 6 months followed by 1 dose of PHiD‐CV10 or hepatitis A vaccine at 15 to 18 months of age. Tx: PHiD‐CV10; N = 11,875 (N = 10,295 completed the follow‐up as specified in the protocol); for AOM cohort N = 3602 (N = 3010 completed the follow‐up as specified in the protocol) C: hepatitis B vaccine; N = 11,863 (N = 10,201) completed the follow‐up as specified in the protocol); for AOM cohort N = 3612 (N = 2979 completed the follow‐up as specified in the protocol) Additional vaccines: a combination vaccine containing diphtheria‐tetanus‐acellular pertussis‐inactivated polio and Haemophilus influenzae type b (DTPa‐IPV/Hib) was given in the child's opposite thigh at the same visit as the pneumococcal vaccine at 2, 4, 6 and 15 to 18 months |
|
| Outcomes |
Primary outcome – likely bacterial CAP Secondary outcomes: other CAP outcomes, first episode of clinically confirmed AOM, first episode of pathogen‐specific AOM, serious adverse events and mortality occurring throughout the study period The AOM outcome was studied in Panama only (7357 of the 23,821 randomised children). Initially, AOM cases were captured only when parents sought medical attention for children with AOM symptoms. However, because of a lower‐than‐expected AOM rate, the surveillance was enhanced in July 2009 (2 years after start of enrolment) through regular telephone calls or home visits by study personnel who advised parents to visit the clinic if their child had symptoms suggestive of AOM. If the physician suspected AOM, the child was referred to one of the ENT surgeons involved in the trial. Clinically confirmed AOM was defined as either altered visual appearance of the tympanic membrane (e.g. redness, bulging, loss of light reflex) or the presence of middle ear effusion (by pneumatic otoscopy or otomicroscopy). A recent onset (duration less than 5 days) of at least 2 of the following clinical symptoms was also required: ear pain, ear discharge, hearing loss, fever, lethargy, irritability, anorexia, vomiting, or diarrhoea. The severity of each AOM episode (mild, moderate, severe) was assessed by combining objective elements of the Friedman scale (the Ear Treatment Group‐five items (ETG‐5) and otoscopy scale with eight grades of severity (OS‐8)) with subjective elements in a clinical otologic scale. When middle ear fluid was suspected, tympanocentesis was performed and bacterial presence was assessed by culture. |
|
| Funding sources | Sponsored by GlaxoSmithKline Biologicals, the vaccine developer and manufacturer. The data generated in the trial are subject to a confidentiality agreement between the investigators and the sponsor that allowed the investigators full access to the study data and included an obligation for GlaxoSmithKline Biologicals to permit publication without excessive delay. | |
| Declarations of interest | Dr Sáez‐Llorens declares having received financial support from the study sponsor for travel to meetings, and his institution received grants from Health Research International. Drs López and Calvo declare their institutions received support for travel to meetings and grants from the study sponsor. Dr Calvo declares her institution received consulting fee/honorary from the study sponsor. Dr Hausdorff is a patent coholder of PCV13 (no royalties). 9 co‐authors are employees of GlaxoSmithKline companies and own stock/stock options from the GlaxoSmithKline group of companies. | |
| Notes |
Participants lost to follow‐up:total: 592/3602 (16.4%) did not complete the follow‐up period specified in the protocol Participants lost to follow‐up:Tx: 633/3612 (17.5%) did not complete the follow‐up period specified in the protocol Participants lost to follow‐up:C: 1225/7214 (17.0%) did not complete the follow‐up period specified in the protocol |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization list was generated by the sponsor using a standard SAS (SAS Institute) program and was used to number the vaccines. A randomization blocking scheme was used to ensure that balance between treatment groups was maintained" |
| Allocation concealment (selection bias) | Low risk | Quote: "Vaccine allocation at each site was performed using a central randomization system on the Internet (SBIR, GlaxoSmithKline Vaccines), and treatment was concealed from all study personnel" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Vaccines were numbered by the sponsor, and treatment allocation was concealed from study personnel. There were minor differences in vaccine appearance, but vaccines were prepared and administered by personnel who took no further part in the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Vaccines were prepared and administered by personnel who took no further part in the study. Parents/guardians of participating children and study personnel involved in data gathering, processing, and analysis and safety assessment were blind to vaccine allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Almost all randomised children were included in ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes listed in clinicaltrials.gov/show/NCT00466947. |
| Other bias | Low risk | No other sources of bias identified. |
van Kempen 2006.
| Methods | This study was performed in parallel with Veenhoven 2003 (OMAVAX‐trial), but analysed separately due to differences in study population. Randomised: yes, at individual level Design: standard parallel‐group design Intention‐to‐treat: unclear Follow‐up: 26 months |
|
| Participants |
N: 74 children with a history of AOM
Age: between 1 and 7 years Setting: ENT department of the Ghent University Hospital in Belgium Inclusion criteria: children aged 1 to 7 years with a history of AOM defined as at least 2 separate clinically diagnosed AOM episodes in the past year Exclusion criteria: children with any underlying illnesses including immunocompromising conditions other than partial serum IgA and IgG2 deficiencies, craniofacial abnormalities, previous pneumococcal vaccination, or documented hypersensitivity to any of the vaccine components Baseline characteristics: described and balanced (Table 1 of trial publication) |
|
| Interventions | Children were randomly allocated to either a CRM197‐PCV7 or a hepatitis A vaccine (containing 720 units of inactivated hepatitis A virus). Children aged 12 to 24 months received 2 intramuscular injections with a 1‐month interval, and those aged over 2 years received 1 intramuscular injection. Those allocated to CRM197‐PCV7 additionally received PPV23 6 months (in children aged 12 to 24 months) or 7 months (in those aged over 2 years) later. Tx: CRM197‐PCV7 plus PPV23; N = 38 (N = 35 completed the vaccination scheme) C: hepatitis A vaccine; N = 36 (N = 33 completed the vaccination scheme) Additional vaccines: not described |
|
| Outcomes |
Primary outcome: number of AOM episodes during 18‐month follow‐up Secondary outcomes: immunogenicity; nasopharyngeal carriage of conjugate vaccine‐related serotypes; and antibiotic‐resistant pneumococci At scheduled hospital visits at 7, 14, 20, and 26 months after randomisation, a medical history was taken, antibiotic usage noted, and an otomicroscopic examination performed. When, at least 1 month following complete vaccination, a new AOM episode was suspected, parents were asked to bring their sick child within 24 hours to the study centre for otoscopic diagnosis. In case of all other AOM episodes during follow‐up, participants were allowed to visit the study centre, their family physician, or a paediatrician, who was asked to report otoscopic findings, diagnosis, and treatment on an AOM registration form. AOM was defined by an abnormal tympanic membrane on otomicroscopy (red, dull, or bulging) plus at least 1 of the following symptoms or signs of acute infection: earache, acute otorrhoea, fever (> 38.5 °C rectally), or irritability. |
|
| Funding sources | The study was supported by the Netherlands Organisation for Health Research and Development (ZonMw) and the Dutch Health Insurance Company Zilveren Kruis‐Achmea as part of the OMAVAX‐trial. Wyeth‐Lederle Vaccines and Pediatrics provided the pneumococcal vaccines, and GlaxoSmithKline provided the hepatitis A vaccines. | |
| Declarations of interest | The authors declared no conflicts of interest. | |
| Notes |
Participants lost to follow‐up:total: 6/74 (8.1%) did not complete the follow‐up period specified in the protocol Participants lost to follow‐up:Tx: 3/38 (7.9%) did not complete the follow‐up period specified in the protocol Participants lost to follow‐up:C: 3/36 (8.3%) did not complete the follow‐up period specified in the protocol |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not described, randomisation stratified according to age (12 to 24 months versus 25 to 84 months) and number of previous AOM episodes per year (2 to 3 versus 4 or more episodes). |
| Allocation concealment (selection bias) | Low risk | 2 study nurses immunised all children according to a randomisation list provided to them in a sealed envelope by a third party (the Julius Center for Health Sciences, Utrecht, the Netherlands). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The nurses that vaccinated children were not allowed to reveal the child's allocation to either the study team or the parents. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | When a new AOM episode was suspected, parents were asked to bring their sick child within 24 hours to the study centre for otoscopic diagnosis. In case of all other AOM episodes during follow‐up, participants were allowed to visit the study centre, their family physician, or a paediatrician, who was asked to report otoscopic findings, diagnosis, and treatment on an AOM registration form. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In total, 6 of the 74 children (8.1%) did not complete the follow‐up period specified in the protocol (equally distributed across groups). Reasons for withdrawals are described in the Results section of the article. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Low risk | No other sources of bias identified. |
Veenhoven 2003.
| Methods | This study was performed in parallel with van Kempen 2006, but analysed separately due to differences in study population. Randomised: yes, at individual level Design: standard parallel‐group design Intention‐to‐treat: yes Follow‐up: 18 months, starting 1 month after completion of the vaccination scheme |
|
| Participants |
N: 383 children with a history of AOM
Age: between 1 and 7 years Setting: a general hospital (Spaarne Hospital, Haarlem) and a tertiary care hospital (Wilhelmina Children's Hospital of the University Medical Centre Utrecht) in the Netherlands Inclusion criteria: children aged 1 to 7 years with a history of AOM defined as 2 or more AOM episodes in the year before study entry. The number of previous AOM episodes was based on parental report and on clinical confirmation of the diagnosis by a physician. Exclusion criteria: children with immunodeficiency, cystic fibrosis, immotile cilia syndrome, craniofacial abnormalities, chromosomal abnormalities such as Down's syndrome, and severe adverse events during previous vaccinations Baseline characteristics: described and balanced (Table 1 of trial publication) |
|
| Interventions | Children were randomly allocated to either CRM197‐PCV7 followed by a PPV23 or a hepatitis A or B vaccine. Children aged 12 to 24 months in the pneumococcal vaccination group received PCV7 twice with a 1‐month interval, followed 6 months later by PPV23. The control vaccine group received 3 hepatitis B vaccinations (Engerix‐B) according to a similar time schedule. Children aged 25 to 84 months in the pneumococcal vaccine group received 1 dose of PCV7 followed 7 months later by PPV23. The control group received hepatitis A vaccine (Havrix) twice. Tx: CRM197‐PCV7 plus PPV23; N = 190 (N = 190 included in ITT analysis) C: hepatitis A or hepatitis B vaccine; N = 193 (N = 193 included in ITT analysis) Additional vaccines: not described |
|
| Outcomes |
Primary outcome: number of clinical AOM episodes during 18‐month follow‐up Secondary outcomes: number of AOM episodes due to the 7 pneumococcal serotypes included in the PCV7 vaccine and nasopharyngeal carriage of conjugate vaccine serotypes, serious adverse events Parents were instructed to visit the study clinics or their GP, ENT surgeon, or paediatrician to assess symptoms suggesting AOM. Physicians registered signs and symptoms of every AOM episode on standard registration forms and were unaware of treatment allocation. AOM was defined according to the guideline issued by the Dutch College of General Practitioners, i.e. presence of an abnormal tympanic membrane on otoscopy (red, dull, or bulging) or otorrhoea and at least 1 of the following signs or symptoms of acute infection: acute earache, new‐onset otorrhoea, irritability, or fever greater than 38.5 °C rectally or 38.0 °C axillary. |
|
| Funding sources | The study was supported by the Netherlands Organisation for Health Research and Development (ZonMw) and the Dutch Health Insurance Company Zilveren Kruis‐Achmea as part of the OMAVAX‐trial. Wyeth‐Lederle Vaccines and Pediatrics provided the pneumococcal vaccines, and GlaxoSmithKline provided the hepatitis A vaccines. | |
| Declarations of interest | The authors declared no conflicts of interest. | |
| Notes |
Participants lost to follow‐up:total: 1/383 (0.3%); all children included in ITT analysis Participants lost to follow‐up:Tx: 0/190 (0%) Participants lost to follow‐up:C: 1/193 (0.5%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers that identified the vaccine scheme, randomisation stratified according to age (12 to 24 months versus 25 to 84 months) and number of previous AOM episodes per year (2 to 3 versus 4 or more episodes) |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment was described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Vaccine was administered to the child by a study nurse, so that parents and physicians were unaware of treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Parents were instructed to visit the study clinics or their family physician, otolaryngologist, or paediatrician to assess symptoms suggesting AOM. Physicians registered signs and symptoms of every AOM episode on standard registration forms and were unaware of treatment allocation. AOM was defined according to the guideline issued by the Dutch College of General Practitioners. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised children were included in ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Low risk | No other sources of bias identified. |
Vesikari 2016.
| Methods | This trial was nested within the Finnish invasive pneumococcal disease vaccine trial (FinIP; clinicaltrials.gov/show/NCT00861380; Palmu 2013), a cluster‐randomised double‐blind trial to assess the efficacy of PHiD‐CV10 against IPD, all‐cause antibiotic‐purchases, tympanostomy tube placements, and vaccine‐preventable diseases. Randomised: yes, at cluster level Design: parallel‐group (4 groups) design Intention‐to‐treat: no, per‐protocol analysis Follow‐up – mean duration of follow‐up 18 months |
|
| Participants |
N:– 5095 healthy infants Age: mean age at first dose 2.3 months Setting: 15 study centres in Finland Inclusion criteria: healthy children aged 6 weeks to 18 months Exclusion criteria: prior administration of pneumococcal vaccine, hepatitis A or B vaccine, any investigational or non‐registered product, contraindication to immunisation Baseline characteristics: described and balanced (Suppl. Table 1) |
|
| Interventions | Children were randomly allocated (2:2:1:1) to either PHiD‐CV10 3 + 1, 2 + 1, control 3 + 1, control 2 + 1 (control vaccine was hepatitis B for children aged < 12 months or hepatitis A for those aged 12 months or above). Tx1: PHiD‐CV10 3 + 1; N = 1846 (N = 1846 completed the follow‐up as specified in the protocol) Tx2: PHiD‐CV10 2 + 1; N = 1313 (N = 942 completed the follow‐up as specified in the protocol) C1: hepatitis A or hepatitis B vaccine 3 + 1; N = 1073 (N = 468 completed the follow‐up as specified in the protocol) C2: hepatitis A or hepatitis B vaccine 2 + 1; N = 861 (N = 861 completed the follow‐up as specified in the protocol) Additional vaccines: a combination vaccine containing diphtheria‐tetanus‐acellular pertussis‐inactivated polio and Haemophilus influenzae type b (DTPa‐IPV/Hib) and human rotavirus vaccine were given at the same visit as the pneumococcal vaccine at 3 and 5 months. The DTPa‐IPV/Hib vaccine was also co‐administered at 11 to 12 months of age. |
|
| Outcomes |
Primary outcome – parent‐reported, physician‐confirmed all‐cause AOM (stratified to 1 or more AOM episodes and overall AOM) Secondary outcomes: parent‐reported, physician‐confirmed all‐cause AOM with antibiotic prescription (stratified to 1 or more AOM episodes and overall AOM), serious adverse events occurring throughout the study period Parents were asked by automatic text message every 2 weeks if their child had had a physician‐confirmed AOM diagnosis. If no contact could be made, AOM status was checked at the next study visit. |
|
| Funding sources | GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also took responsibility for all costs associated with the development and publishing of this article. | |
| Declarations of interest | Dr Vesikari declares that he received payment from the GlaxoSmithKline group of companies and other vaccine manufacturers for board membership, consultancy, and attending meetings; the institution of Dr Kaijalainen received grants from the GlaxoSmithKline group of companies. 7 co‐authors are employees of the GlaxoSmithKline group of companies. Dr Hezareh is a consultant for Chiltern International for the GlaxoSmithKline group of companies. Dr Puumalainen was a GlaxoSmithKline group of companies employee during the study. 4 co‐authors declare stock and stock options ownership in the GlaxoSmithKline group of companies, and 1 co‐author declares shares ownership in the GlaxoSmithKline group of companies. Dr Forsten and Dr Seppä declare no conflicts of interest. | |
| Notes |
Participants lost to follow‐up:total: 976/5093 (19.2%) did not complete the follow‐up period specified in the protocol Participants lost to follow‐up:Tx1: 0/1846 (0%) did not complete the follow‐up period specified in the protocol Participants lost to follow‐up:Tx2: 371/1313 (28.3%) did not complete the follow‐up period specified in the protocol Participants lost to follow‐up:C1: 605/1073 (56.4%) did not complete the follow‐up period specified in the protocol Participants lost to follow‐up:C2: 0/861 (0%) did not complete the follow‐up period specified in the protocol |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Clusters were randomized (2:2:1:1: PHiD‐CV 3+1, PHiD‐CV 2+1, control 3+1, control 2+1) using a blocking scheme, stratified according to cluster size (below/above average), urbanity (urban/rural), and Tampere University Vaccine Research Centre trial enrolment" |
| Allocation concealment (selection bias) | Low risk | Quote: "For nested study participants, individual randomization codes were used, aligned with cluster randomization based on place of residence" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Stated that this was a double‐blind trial, but no further details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Stated that this was a double‐blind trial, but no further details provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Substantial number of participants not included in analysis due to “randomization error” |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes listed in clinicaltrials.gov/show/NCT00839254. |
| Other bias | Low risk | No other sources of bias identified. |
Ab: antibiotics AOM: acute otitis media C: control CAP: community‐acquired pneumonia CRM197‐PCV7: 7‐valent pneumococcal conjugate vaccine conjugated to carrier protein CRM197 CRM197‐PCV9: 9‐valent pneumococcal conjugate vaccine conjugated to carrier protein CRM197 DCC: day‐care centre DTaP: diphtheria‐tetanus toxoid‐acellular pertussis vaccine DTP: diphtheria‐tetanus toxoid‐pertussis vaccine DTwP: diphtheria‐tetanus toxoid‐whole cell pertussis vaccine ELISA: enzyme‐linked immunosorbent assay
ENT: ear, nose, and throat GP: general practitioner HBV/placebo: hepatitis B virus vaccination plus placebo vaccine Hib: Haemophilus influenzae type b ICPC: International Classification of Primary Care IgA: immunoglobulin A IgG: immunoglobulin G IPD: invasive pneumococcal disease ITT: intention‐to‐treat MenC: meningococcus type C conjugate vaccine N: number NaCl: sodium chloride OM: otitis media OMPC‐PCV7: 7‐valent pneumococcal conjugate vaccine conjugated to the outer membrane protein complex of Neisseria meningitidis serogroup B PCR: polymerase chain reaction PCV: pneumococcal conjugate vaccine PHiD‐CV10: 10‐valent pneumococcal conjugate vaccine conjugated to protein D (surface lipoprotein of non‐typeable Haemophilus influenzae) PHiD‐CV11: 11‐valent pneumococcal conjugate vaccine conjugated to protein D (surface lipoprotein of non‐typeable Haemophilus influenzae) PPV23: 23‐valent pneumococcal polysaccharide vaccine RTI: respiratory tract infection TIV: trivalent influenza vaccine TIV/CRM197‐PCV7: trivalent influenza vaccine plus 7‐valent pneumococcal conjugate vaccine conjugated to carrier protein CRM197 TIV/placebo: trivalent influenza vaccine plus placebo vaccine Tx: treatment
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gisselsson‐Solen 2011 | No control vaccination |
| Jokinen 2012 | Re‐analysis of the Eskola 2001 study without new outcome data that could be used for our review |
| Le 2007 | RCT studying the effect of PCV on OME |
| Palmu 2014 | Secondary analysis of the FinIP trial, Palmu 2013, without outcome data that could be used for our review |
| Palmu 2015a | Re‐analysis of the Eskola 2001 study without new outcome data that could be used for our review |
| Palmu 2015b | Secondary analysis of the FinIP trial, Palmu 2013, without outcome data that could be used for our review |
| Palmu 2018 | Secondary analysis of the FinIP trial, Palmu 2013, without outcome data that could be used for our review |
| Roy 2011 | RCT studying the effect of PCV on suppurative otitis media (abstract of conference meeting) |
| Sarasoja 2013 | Re‐analysis of the Eskola 2001 study without new outcome data that could be used for our review |
OME: otitis media with effusion PCV: pneumococcal conjugate vaccine RCT: randomised controlled trial
Differences between protocol and review
Following feedback from a peer reviewer and editorial advice, we changed the title of this 2019 update to include the population (children). We also added 'adverse effects' as the co‐primary outcome. We created a 'Summary of findings' table for PCVs administered in early infancy using the following outcomes: frequency of all‐cause AOM episodes (co‐primary outcome), adverse effects (co‐primary outcome), frequency of pneumococcal AOM, and frequency of recurrent AOM (defined as three or more AOM episodes in six months or four or more in one year). Furthermore, we used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data to the prespecified outcomes. This was not proposed in the protocol. Also, some (other) Cochrane methods have evolved over time. The revised methods have been applied in the 2019 update where required.
Contributions of authors
Alexandre C Fortanier and Roderick P Venekamp co‐ordinated the review, were involved in data collection, and performed the 'Risk of bias' assessment and analysis and interpretation of the data. All review authors reviewed the manuscript and approved the final version of the review.
Sources of support
Internal sources
Department of Pediatric Immunology and Infectious Diseases, UMC Utrecht, Wilhelmina Children's Hospital Utrecht, Netherlands.
Julius Center for Health Sciences and Primary Care, UMC Utrecht, the Netherlands, Netherlands.
University Center for Pharmacy, PharmacoEpidemiology & PharmacoEconomics, University of Groningen, Netherlands.
The National Institute for Public Health and the Environment, Biltoven, The Netherlands, Netherlands.
External sources
No sources of support supplied
Declarations of interest
Alexandre C Fortanier: in 2012, Alexandre C Fortanier was appointed as PhD student at the Julius Center for Health Sciences and Primary Care, University Medical Center, University Utrecht, the Netherlands. This review is an integral part of his PhD programme. Until 2017, Alexandre C Fortanier was employed by Janssen Vaccines & Prevention, Leiden, the Netherlands (formerly Crucell Holland B.V.). Alexandre C Fortanier is currently an employee of Seqirus Netherlands B.V., Amsterdam, the Netherlands. Neither company was involved in any aspect of the submitted work, nor did they act as a commercial sponsor of the review or his PhD programme. His employer has no commercial or vested interest in the findings of this review and does not hold any patent relevant to the review.
Roderick P Venekamp is an Editor for Cochrane Acute Respiratory Infections and Cochrane ENT, but had no role in the editorial process of this review.
Chantal WB Boonacker: none known.
Eelko Hak has authored a paper on the design of the CAPITA study (Netherlands Journal of Medicine), but was not involved in the actual conduct of that study, nor does it pose a conflict of interest to the current work.
Anne GM Schilder: the evidENT team at University College London is supported in part by the National Institute for Health Research (NIHR) University College London Hospitals Biomedical Research Centre. Our research is funded by the NIHR and EU Horizon2020. I am the national chair of the NIHR Clinical Research Network ENT Specialty. I am the Surgical Specialty Lead for ENT for the Royal College of Surgeons of England's Clinical Trials Initiative. I am co‐investigator on the NIHR PGfAR grant 'Defining best Management for Adults with Chronic RhinOsinusitis: the MACRO Programme'. In my role as director of the NIHR UCLH BRC Deafness and Hearing Problems Theme, I act as an adviser on clinical trial design and delivery to a range of biotech companies.
Elisabeth AM Sanders: for research on pneumococcal vaccines, carriage and surveillance studies, Elisabeth AM Sanders received money paid by governmental agencies and pharmaceutical companies GSK and Pfizer, and paid to the institution or collaborating institutions. Furthermore, Elisabeth AM Sanders has participated in Independent Data Monitoring Committees and Advisory Boards for pharmaceutical companies for vaccine studies and/or respiratory tract infections with fees paid to the institution before 2014. In general, fees were always paid to the institution and used for research purposes.
Roger AMJ Damoiseaux: none known.
Elisabeth AM Sanders and Eelko Hak are authors of studies included in this review (Elisabeth AM Sanders: Jansen 2008; van Kempen 2006; Veenhoven 2003; Eelko Hak: Jansen 2008). To avoid any potential conflicts of interest, other review authors reviewed eligibility and performed 'Risk of bias' assessment and data extraction for these studies.
Clarification statement added from the Co‐ordinating Editor, Mark Jones on 9 March, 2020: this review was found by the Cochrane Funding Arbiters, post‐publication, to be non‐compliant with the Cochrane conflict of interest policy, which includes the relevant parts of the Cochrane Commercial Sponsorship Policy. The next update to the review will have a majority of authors and the lead author free of conflicts of interest. The update will be published in nine months.
Edited (no change to conclusions)
References
References to studies included in this review
Black 2000 {published data only}
- Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatric Infectious Disease Journal 2000;19(3):187‐95. [DOI] [PubMed] [Google Scholar]
- Fireman B, Black SB, Shinefield HR, Lee J, Lewis E, Ray P. Impact of the pneumococcal conjugate vaccine on otitis media. Pediatric Infectious Disease Journal 2003;22:10‐6. [DOI] [PubMed] [Google Scholar]
Dagan 2001 {published data only}
- Dagan R, Sikuler‐Cohen M, Zamir O, Janco J, Givon‐Lavi N, Fraser D. Effect of a conjugate pneumococcal vaccine on the occurrence of respiratory infections and antibiotic use in day‐care center attendees. Pediatric Infectious Disease Journal 2001;20(10):951‐8. [DOI] [PubMed] [Google Scholar]
Eskola 2001 {published data only}
- Eskola J, Kilpi T, Palmu A, Jokinen J, Haapakoski J, Herva E, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. New England Journal of Medicine 2001;344(6):403‐9. [DOI] [PubMed] [Google Scholar]
- Palmu AA, Saukkoriipi A, Jokinen J, Leinonen M, Kilpi TM. Efficacy of pneumococcal conjugate vaccine against PCR‐positive acute otitis media. Vaccine 2009;27:1490‐1. [DOI] [PubMed] [Google Scholar]
Jansen 2008 {published data only}
- Jansen AG, Sanders EA, Hoes AW, Loon AM, Hak E. Effects of influenza plus pneumococcal conjugate vaccination versus influenza vaccination alone in preventing respiratory tract infections in children: a randomized, double‐blind, placebo‐controlled trial. Journal of Pediatrics 2008;153:764‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kilpi 2003 {published data only}
- Kilpi T, Ahman H, Jokinen J, Lankinen KS, Palmu A, Savolainen H, et al. Protective efficacy of a second pneumococcal conjugate vaccine against pneumococcal acute otitis media in infants and children: randomized, controlled trial of a 7‐valent pneumococcal polysaccharide‐meningococcal outer membrane protein complex conjugate vaccine in 1666 children. Clinical Infectious Diseases 2003;37(9):1155‐64. [DOI] [PubMed] [Google Scholar]
O'Brien 2008 {published data only}
- O'Brien KL, David AB, Chandran A, Moulton LH, Reid R, Weatherholtz R, et al. Randomized, controlled trial efficacy of pneumococcal conjugate vaccine against otitis media among Navajo and White Mountain Apache infants. Pediatric Infectious Disease Journal 2008;27:71‐3. [DOI] [PubMed] [Google Scholar]
Prymula 2006 {published data only}
- Prymula R, Peeters P, Chrobok V, Kriz P, Novakova E, Kaliskova E, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non‐typeable Haemophilus influenzae: a randomised double‐blind efficacy study. Lancet 2006;367(9512):740‐8. [DOI] [PubMed] [Google Scholar]
Tregnaghi 2014 {published data only}
- Sáez‐Llorens X, Rowley S, Wong D, Rodríguez M, Calvo A, Troitiño M, et al. Efficacy of 10‐valent pneumococcal non‐typeable Haemophilus influenzae protein D conjugate vaccine against acute otitis media and nasopharyngeal carriage in Panamanian children ‐ a randomized controlled trial. Human Vaccines and Immunotherapeutics 2017;13(6):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregnaghi MW, Sáez‐Llorens X, López P, Abate H, Smith E, Pósleman A, et al. Efficacy of pneumococcal nontypable Haemophilus influenzae protein D conjugate vaccine (PHiD‐CV) in young Latin American children: a double‐blind randomized controlled trial. PLOS Medicine 2014;11(6):e1001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Kempen 2006 {published data only}
- Kempen MJ, Vermeiren JS, Vaneechoutte M, Claeys G, Veenhoven RH, Rijkers GT, et al. Pneumococcal conjugate vaccination in children with recurrent acute otitis media: a therapeutic alternative?. International Journal of Pediatric Otorhinolaryngology 2006;70(2):275‐85. [DOI] [PubMed] [Google Scholar]
Veenhoven 2003 {published data only}
- Veenhoven R, Bogaert D, Uiterwaal C, Brouwer C, Kiezebrink H, Bruin J, et al. Effect of conjugate pneumococcal vaccine followed by polysaccharide pneumococcal vaccine on recurrent acute otitis media: a randomised study. Lancet 2003;361(9376):2189‐95. [DOI] [PubMed] [Google Scholar]
Vesikari 2016 {published data only}
- Vesikari T, Forsten A, Seppä I, Kaijalainen T, Puumalainen T, Soininen A, et al. Effectiveness of the 10‐valent pneumococcal nontypeable Haemophilus influenzae protein D‐conjugated vaccine (PHiD‐CV) against carriage and acute otitis media ‐ a double‐blind randomized clinical trial in Finland. Journal of the Pediatric Infectious Disease Society 2016;5(3):237‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Gisselsson‐Solen 2011 {published data only}
- Gisselsson‐Solén M, Melhus A, Hermansson A. Pneumococcal vaccination in children at risk of developing recurrent acute otitis media ‐ a randomized study. Acta Paediatrica 2011;100:1354‐8. [DOI] [PubMed] [Google Scholar]
Jokinen 2012 {published data only}
- Jokinen J, Palmu AA, Kilpi T. Acute otitis media replacement and recurrence in the Finnish otitis media vaccine trial. Clinical Infectious Diseases 2012;55:1673‐6. [DOI] [PubMed] [Google Scholar]
Le 2007 {published data only}
- Le TM, Rovers MM, Veenhoven RH, Sanders EA, Schilder AG. Effect of pneumococcal vaccination on otitis media with effusion in children older than 1 year. European Journal of Pediatrics 2007;166:1049‐52. [DOI] [PubMed] [Google Scholar]
Palmu 2014 {published data only}
- Palmu AA, Jokinen J, Nieminen H, Rinta‐Kokko H, Ruokokoski E, Puumalainen T, et al. Effect of pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD‐CV10) on outpatient antimicrobial purchases: a double‐blind, cluster randomised phase 3‐4 trial. Lancet Infectious Diseases 2014;14(3):205‐12. [DOI] [PubMed] [Google Scholar]
Palmu 2015a {published data only}
- Palmu AA, Kaijalainen T, Jokinen J, Kilpi TM. Efficacy of the 7‐valent pneumococcal conjugate vaccine against acute otitis media caused by serotype 6C pneumococcus. Pediatric Infectious Disease Journal 2015;34(7):796‐7. [DOI] [PubMed] [Google Scholar]
Palmu 2015b {published data only}
- Palmu AA, Jokinen J, Nieminen H, Rinta‐Kokko H, Ruokokoski E, Puumalainen T, et al. Effectiveness of the ten‐valent pneumococcal conjugate vaccine against tympanostomy tube placements in a cluster‐randomized trial. Pediatric Infectious Disease Journal 2015;34(11):1230‐5. [DOI] [PubMed] [Google Scholar]
Palmu 2018 {published data only}
- Palmu AA, Jokinen J, Nieminen H, Rinta‐Kokko H, Ruokokoski E, Puumalainen T, et al. Vaccine‐preventable disease incidence of pneumococcal conjugate vaccine in the Finnish invasive pneumococcal disease vaccine trial. Vaccine 2018;36(14):1816‐22. [DOI] [PubMed] [Google Scholar]
Roy 2011 {unpublished data only}
- Roy E, Steinhoff MC, Omer SB, Arifeen SE, Raqib R, Breiman R, et al. Clinical effectiveness of pneumococcal conjugate vaccine in suppurative otitis media: a randomized controlled trial in Bangladeshi infants. Pediatric Academic Societies Annual Meeting. Boston (MA), USA, April 28‐May 1 2011.
Sarasoja 2013 {published data only}
- Sarasoja I, Jokinen J, Lahdenkari M, Kilpi T, Palmu AA. Long‐term effect of pneumococcal conjugate vaccines on tympanostomy tube placements. Pediatric Infectious Disease Journal 2013;32(5):517‐20. [DOI] [PubMed] [Google Scholar]
Additional references
Ahmed 2014
- Ahmed S, Shapiro NL, Bhattacharyya N. Incremental health care utilization and costs for acute otitis media in children. Laryngoscope 2014;124(1):301‐5. [DOI] [PubMed] [Google Scholar]
Allemann 2017
- Allemann A, Frey PM, Brugger SD, Hilty M. Pneumococcal carriage and serotype variation before and after introduction of pneumococcal conjugate vaccines in patients with acute otitis media in Switzerland. Vaccine 2017;35(15):1946‐53. [DOI] [PubMed] [Google Scholar]
Alpert 2011
- Alpert HR, Behm I, Connolly GN, Kabir Z. Smoke‐free households with children and decreasing rates of paediatric clinical encounters for otitis media in the United States. Tobacco Control 2011;20(3):207‐11. [DOI] [PubMed] [Google Scholar]
Andersen 1982
- Andersen PK, Gill RD. Cox regression model for counting processes: a large sample study. Annals of Statistics 1982;10(4):1100‐20. [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barenkamp 2017
- Barenkamp SJ, Chonmaitree T, Hakansson AP, Heikkinen T, King S, Nokso‐Koivisto J, et al. Panel 4: Report of the Microbiology Panel. Otolaryngology Head and Neck Surgery 2017;156(Suppl 4):S51‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ben‐Shimol 2014
- Ben‐Shimol S, Givon‐Lavi N, Leibovitz E, Raiz S, Greenberg D, Dagan R. Near‐elimination of otitis media caused by 13‐valent pneumococcal conjugate vaccine (PCV) serotypes in southern Israel shortly after sequential introduction of 7‐valent/13‐valent PCV. Clinical Infectious Diseases 2014;59(12):1724‐32. [DOI] [PubMed] [Google Scholar]
Biesbroek 2014
- Biesbroek G, Wang X, Keijser BJ, Eijkemans RM, Trzciński K, Rots NY, et al. Seven‐valent pneumococcal conjugate vaccine and nasopharyngeal microbiota in healthy children. Emerging Infectious Diseases Journal 2014;20(2):201‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Block 2006
- Block SL. Searching for the Holy Grail of acute otitis media. Archives of Disease in Childhood 2006;91(12):959‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bluestone 1992
- Bluestone CD, Stephenson JS, Martin LM. Ten‐year review of otitis media pathogens. Pediatric Infectious Disease Journal 1992;11(8 Suppl):7‐11. [DOI] [PubMed] [Google Scholar]
Boonacker 2011
- Boonacker CW, Broos PH, Sanders EA, Schilder AG, Rovers MM. Cost effectiveness of pneumococcal conjugate vaccination against acute otitis media in children: a review. Pharmacoeconomics 2011;29(3):199‐211. [DOI] [PubMed] [Google Scholar]
Casey 2013
- Casey JR, Kaur R, Friedel VC, Pichichero ME. Acute otitis media otopathogens during 2008 to 2010 in Rochester, New York. Pediatric Infectious Disease Journal 2013;32:805‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke 2017
- Clarke C, Bakaletz LO, Ruiz‐Guiñazú J, Borys D, Mrkvan T. Impact of protein D‐containing pneumococcal conjugate vaccines on non‐typeable Haemophilus influenzae acute otitis media and carriage. Expert Review of Vaccines 2017;16(7):1‐14. [DOI] [PubMed] [Google Scholar]
Coker 2010
- Coker TR, Chan LS, Newberry SJ, Limbos MA, Suttorp MJ, Shekelle PG, et al. Diagnosis, microbial epidemiology, and antibiotic treatment of acute otitis media in children: a systematic review. JAMA 2010;304:2161‐9. [DOI] [PubMed] [Google Scholar]
Dagan 1997
- Dagan R, Melamed R, Zamir O, Leroy O. Safety and immunogenicity of tetravalent pneumococcal vaccines containing 6B, 14, 19F and 23F polysaccharides conjugated to either tetanus toxoid or diphtheria toxoid in young infants and their boosterability by native polysaccharide antigens. Pediatric Infectious Disease Journal 1997;16(11):1053‐9. [DOI] [PubMed] [Google Scholar]
Dagan 2016
- Dagan R, Pelton S, Bakaletz L, Cohen R. Prevention of early episodes of otitis media by pneumococcal vaccines might reduce progression to complex disease. Lancet Infectious Diseases 2016;16(4):480‐92. [DOI] [PubMed] [Google Scholar]
Eskola 1999
- Eskola J, Anttila M. Pneumococcal conjugate vaccines. Pediatric Infectious Disease Journal 1999;18(6):543‐51. [DOI] [PubMed] [Google Scholar]
Ewald 2016
- Ewald H, Briel M, Vuichard D, Kreutle V, Zhydkov A, Gloy V. The clinical effectiveness of pneumococcal conjugate vaccines: a systematic review and meta‐analysis of randomized controlled trials. Deutsches Ärzteblatt International 2016;113(9):139‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eythorsson 2018
- Eythorsson E, Hrafnkelsson B, Erlendsdóttir H, Gudmundsson SA, Kristinsson KG, Haraldsson Á. Decreased acute otitis media with treatment failure after introduction of the ten‐valent pneumococcal Haemophilus influenzae protein D conjugate vaccine. Pediatric Infectious Disease Journal 2018;37(4):361‐6. [DOI] [PubMed] [Google Scholar]
Faden 1997
- Faden H, Duffy L, Wasielewski R, Wolf J, Krystofik D, Tung Y. Relationship between nasopharyngeal colonization and the development of otitis media in children. Tonawanda/Williamsville Pediatrics. Journal of Infectious Diseases 1997;175(6):1440‐5. [DOI] [PubMed] [Google Scholar]
Forsgren 2008
- Forsgren A, Riesbeck K, Janson H. Protein D of Haemophilus influenzae: a protective nontypeable H. influenzae antigen and a carrier for pneumococcal conjugate vaccines. Clinical Infectious Diseases 2008;46(5):726‐31. [DOI] [PubMed] [Google Scholar]
Gisselsson‐Solen 2017
- Gisselsson‐Solen M. Trends in otitis media incidence after conjugate pneumococcal vaccination: a national observational study. Pediatric Infectious Disease Journal 2017;36(11):1027‐31. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 16 August 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Heikkinen 1999
- Heikkinen T, Thint M, Chonmaitree T. Prevalence of various respiratory viruses in the middle ear during acute otitis media. New England Journal of Medicine 1999;340(4):260‐4. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jacobs 1998
- Jacobs MR, Dagan R, Appelbaum PC, Burch DJ. Prevalence of antimicrobial‐resistant pathogens in middle ear fluid: multinational study of 917 children with acute otitis media. Antimicrobial Agents and Chemotherapy 1998;42(3):589‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jahn‐Eimermacher 2007
- Jahn‐Eimermacher A, du Prel JB, Schmitt HJ. Assessing vaccine efficacy for the prevention of acute otitis media by pneumococcal vaccination in children: a methodological overview of statistical practice in randomized controlled clinical trials. Vaccine 2007;25(33):6237‐44. [DOI] [PubMed] [Google Scholar]
Kaur 2017
- Kaur R, Morris M, Pichichero M. Epidemiology of acute otitis media in the postpneumococcal conjugate vaccine era. Pediatrics 2017;140(3):e20170181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kawai 2018
- Kawai K, Adil EA, Barrett D, Manganella J, Kenna MA. Ambulatory visits for otitis media before and after the introduction of pneumococcal conjugate vaccination. Journal of Pediatrics 2018;201:122‐7. [DOI] [PubMed] [Google Scholar]
Kvaerner 1997
- Kvaerner KJ, Nafstad P, Hagen JA, Mair IW, Jaakkola JJ. Recurrent acute otitis media: the significance of age at onset. Acta Oto‐Laryngologica 1997;117(4):578‐84. [DOI] [PubMed] [Google Scholar]
Lau 2015
- Lau WC, Murray M, El‐Turki A, Saxena S, Ladhani S, Long P, et al. Impact of pneumococcal conjugate vaccines on childhood otitis media in the United Kingdom. Vaccine 2015;33(39):5072‐9. [DOI] [PubMed] [Google Scholar]
Laxminarayan 2013
- Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. Antibiotic resistance ‐ the need for global solutions. Lancet Infectious Diseases 2013;13(12):1057‐98. [DOI] [PubMed] [Google Scholar]
Leach 1994
- Leach AJ, Boswell JB, Asche V, Nienhuys TG, Mathews JD. Bacterial colonization of the nasopharynx predicts very early onset and persistence of otitis media in Australian aboriginal infants. Pediatric Infectious Disease Journal 1994;13(11):983‐9. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lieberthal 2013
- Lieberthal AS, Carroll AE, Chonmaitree T, Ganiats TG, Hoberman A, Jackson MA, et al. The diagnosis and management of acute otitis media. Pediatrics 2013;131(3):e964‐99. [DOI] [PubMed] [Google Scholar]
Luotonen 1981
- Luotonen J, Herva E, Karma P, Timonen M, Leinonen M, Makela PH. The bacteriology of acute otitis media in children with special reference to Streptococcus pneumoniae as studied by bacteriological and antigen detection methods. Scandinavian Journal of Infectious Diseases 1981;13(3):177‐83. [DOI] [PubMed] [Google Scholar]
Magnus 2012
- Magnus MC, Vestrheim DF, Nystad W, Håberg SE, Stigum H. Decline in early childhood respiratory tract infections in the Norwegian mother and child cohort study after introduction of pneumococcal conjugate vaccination. Pediatric Infectious Disease Journal 2012;31(9):951‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marom 2014
- Marom T, Tan A, Wilkinson GS, Pierson KS, Freeman JL, Chonmaitree T. Trends in otitis media‐related health care use in the United States, 2001‐2011. JAMA Pediatrics 2014;168(1):68‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCullagh 1989
- McCullagh P, Nelder JA. Generalized Linear Models. London: Chapman and Hall, 1989. [Google Scholar]
Monasta 2012
- Monasta L, Ronfani L, Marchetti F, Montico M, Vecchi Brumatti L, Bavcar A, et al. Burden of disease caused by otitis media: systematic review and global estimates. PLOS ONE 2012;7(4):e36226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moulton 2001
- Moulton LH, O'Brien KL, Kohberger R, Chang I, Reid R, Weatherholtz R, et al. Design of a group‐randomized Streptococcus pneumoniae vaccine trial. Controlled Clinical Trials 2001;22:438‐52. [DOI] [PubMed] [Google Scholar]
NCT01545375
- NCT01545375. Evaluation of a vaccine for reducing ear and lung infections in children. clinicaltrials.gov/ct2/show/study/NCT01545375 (first received 6 March 2012).
O'Brien 2003
- O'Brien KL, Moulton LH, Reid R, Weatherholtz R, Oski J, Brown L, et al. Efficacy and safety of a seven‐valent conjugate pneumococcal vaccine in American Indian children: group randomized trial. Lancet 2003;362(9381):255‐61. [DOI] [PubMed] [Google Scholar]
O'Brien 2009
- O'Brien MA, Prosser LA, Paradise JL, Ray GT, Kulldorff M, Kurs‐Lasky M. New vaccines against otitis media: projected benefits and cost‐effectiveness. Pediatrics 2009;123:1452‐63. [DOI] [PubMed] [Google Scholar]
Obaro 1996
- Obaro SK, Adegbola RA, Banya WA, Greenwood BM. Carriage of pneumococci after pneumococcal vaccination. Lancet 1996;348(9022):271‐2. [DOI] [PubMed] [Google Scholar]
Palmu 2013
- Palmu AA, Jokinen J, Borys D, Nieminen H, Ruokokoski E, Siira L, et al. Effectiveness of the ten‐valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD‐CV10) against invasive pneumococcal disease: a cluster randomised trial. Lancet 2013;381(9862):214‐22. [DOI] [PubMed] [Google Scholar]
Pavia 2009
- Pavia M, Bianco A, Nobile CG, Marinelli P, Angelillo IF. Efficacy of pneumococcal vaccination in children younger than 24 months: a meta‐analysis. Pediatrics 2009;123(6):e1103‐10. [DOI] [PubMed] [Google Scholar]
Pettigrew 2017
- Pettigrew MM, Alderson MR, Bakaletz LO, Barenkamp SJ, Hakansson AP, Mason KM. Panel 6: Vaccines. Otolaryngology Head and Neck Surgery 2017;156(Suppl 4):76‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pichichero 2013
- Pichichero ME, Casey JR, Almudevar A. Nonprotective responses to pediatric vaccines occur in children who are otitis prone. Pediatric Infectious Disease Journal 2013;32(11):1163‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poehling 2007
- Poehling KA, Szilagyi PG, Grijalva CG, Martin SW, LaFleur B, Mitchel E, et al. Reduction of frequent otitis media and pressure‐equalizing tube insertions in children after introduction of pneumococcal conjugate vaccine. Pediatrics 2007;119(4):707‐15. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2015.
Rovers 2006
- Rovers MM, Glasziou P, Appelman CL, Burke P, McCormick DP, Damoiseaux RA, et al. Antibiotics for acute otitis media: a meta‐analysis with individual patient data. Lancet 2006;368(9545):1429‐35. [DOI] [PubMed] [Google Scholar]
Schilder 2016
- Schilder AG, Chonmaitree T, Cripps AW, Rosenfeld RM, Casselbrant ML, Haggard MP, et al. Otitis media. Nature Reviews Disease Primer 2016;2:16063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shinefield 1999
- Shinefield HR, Black S, Ray P, Chang I, Lewis N, Fireman B, et al. Safety and immunogenicity of heptavalent pneumococcal CRM197 conjugate vaccine in infants and toddlers. Pediatric Infectious Disease Journal 1999;18(9):757‐63. [DOI] [PubMed] [Google Scholar]
Sigurðsson 2018
- Sigurðsson S, Eythorsson E, Hrafnkelsson B, Erlendsdóttir H, Kristinsson KG, Haraldsson Á. Reduction in all‐cause acute otitis media in children less than three years of age in primary care following pneumococcal vaccination with PHiD‐CV10: a whole population study. Clinical Infectious Diseases 2018 March 30 [Epub ahead of print]. [DOI: 10.1093/cid/ciy233] [DOI] [PubMed]
Somech 2011
- Somech I, Dagan R, Givon‐Lavi N, Porat N, Raiz S, Leiberman A, et al. Distribution, dynamics and antibiotic resistance patterns of Streptococcus pneumoniae serotypes causing acute otitis media in children in southern Israel during the 10 year‐period before the introduction of the 7‐valent pneumococcal conjugate vaccine. Vaccine 2011;29:4202‐9. [DOI] [PubMed] [Google Scholar]
Sox 2008
- Sox CM, Finkelstein JA, Yin R, Kleinman K, Lieu TA. Trends in otitis media treatment failure and relapse. Pediatrics 2008;121:674‐9. [DOI] [PubMed] [Google Scholar]
Spijkerman 2012
- Spijkerman J, Prevaes SM, Gils EJ, Veenhoven RH, Bruin JP, Bogaert D, et al. Long‐term effects of pneumococcal conjugate vaccine on nasopharyngeal carriage of S. pneumoniae,S. aureus,H. influenzae and M. catarrhalis. PLOS ONE 2012;7(6):e39730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tamir 2015
- Tamir SO, Roth Y, Dalal I, Goldfarb A, Grotto I, Marom T. Changing trends of acute otitis media bacteriology in central Israel in the pneumococcal conjugate vaccines era. Pediatric Infectious Disease Journal 2015;34(2):195‐9. [DOI] [PubMed] [Google Scholar]
Tamir 2017
- Tamir SO, Shemesh S, Oron Y, Marom T. Acute otitis media guidelines in selected developed and developing countries: uniformity and diversity. Archives of Disease in Childhood 2017;102(5):450‐7. [DOI] [PubMed] [Google Scholar]
Taylor 2012
- Taylor S, Marchisio P, Vergison A, Harriague J, Hausdorff WP, Haggard M. Impact of pneumococcal conjugate vaccination on otitis media: a systematic review. Clinical Infectious Diseases 2012;54(12):1765‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
van den Bergh 2013
- Bergh MR, Spijkerman J, Swinnen KM, François NA, Pascal TG, Borys D, et al. Effects of the 10‐valent pneumococcal nontypeable Haemophilus influenzae protein D‐conjugate vaccine on nasopharyngeal bacterial colonization in young children: a randomized controlled trial. Clinical Infectious Diseases 2013;56(3):e30‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Gils 2011
- Gils EJ, Hak E, Veenhoven RH, Rodenburg GD, Bogaert D, Bruin JP, et al. Effect of seven‐valent pneumococcal conjugate vaccine on Staphylococcus aureus colonisation in a randomised controlled trial. PLOS ONE 2011;6(6):e20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Veenhoven 2004
- Veenhoven RH, Bogaert D, Schilder AG, Rijkers GT, Uiterwaal CS, Kiezebrink HH, et al. Nasopharyngeal pneumococcal carriage after combined pneumococcal conjugate and polysaccharide vaccination in children with a history of recurrent acute otitis media. Clinical Infectious Diseases 2004;39(7):911‐9. [DOI] [PubMed] [Google Scholar]
Venekamp 2015
- Venekamp RP, Sanders SL, Glasziou PP, Mar CB, Rovers MM. Antibiotics for acute otitis media in children. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD000219.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2012
- World Health Organization. Pneumococcal vaccines WHO position paper – 2012 – Recommendations. Vaccine 2012;30(32):4717‐8. [DOI] [PubMed] [Google Scholar]
Wiertsema 2011
- Wiertsema SP, Kirkham LA, Corscadden KJ, Mowe EN, Bowman JM, Jacoby P, et al. Predominance of nontypeable Haemophilus influenzae in children with otitis media following introduction of a 3+0 pneumococcal conjugate vaccine schedule. Vaccine 2011;29(32):5163‐70. [DOI] [PubMed] [Google Scholar]
Wiertsema 2012
- Wiertsema SP, Corscadden KJ, Mowe EN, Zhang G, Vijayasekaran S, Coates HL, et al. IgG responses to pneumococcal and Haemophilus influenzae protein antigens are not impaired in children with a history of recurrent acute otitis media. PLOS ONE 2012;7(11):e49061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2008
- Zhou F, Shefer A, Kong Y, Nuorti JP. Trends in acute otitis media‐related health care utilization by privately insured young children in the United States, 1997‐2004. Pediatrics 2008;121:253‐60. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Fortanier 2014
- Fortanier AC, Venekamp RP, Boonacker CW, Hak E, Schilder AG, Sanders EA, et al. Pneumococcal conjugate vaccines for preventing otitis media. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD001480.pub4] [DOI] [PubMed] [Google Scholar]
Jansen 2009
- Jansen AG, Hak E, Veenhoven RH, Damoiseaux RA, Schilder AG, Sanders EA. Pneumococcal conjugate vaccines for preventing otitis media. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD001480.pub3] [DOI] [PubMed] [Google Scholar]
Straetemans 2002
- Straetemans M, Sanders EA, Veenhoven RH, Schilder AG, Damoiseaux RA, Zielhuis GA. Pneumococcal vaccines for preventing otitis media. Cochrane Database of Systematic Reviews 2002, Issue 2. Art, No.: CD001480. [DOI: 10.1002/14651858.CD001480] [DOI] [PubMed] [Google Scholar]
Straetemans 2003
- Straetemans M, Sanders EAM, Veenhoven RH, Schilder AGM, Damoiseaux RAMJ, Zielhuis GA. Review of randomized controlled trials on pneumococcal vaccination for prevention of otitis media. Pediatric Infectious Disease Journal 2003;22(6):515‐24. [DOI] [PubMed] [Google Scholar]
Straetemans 2004
- Straetemans M, Sanders EAM, Veenhoven RH, Schilder AGM, Damoiseaux RAMJ, Zielhuis GA. Pneumococcal vaccines for preventing otitis media. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD001480.pub2] [DOI] [PubMed] [Google Scholar]


