Abstract
Background
The primary objective of this study was to assess the cumulative incidence of cause-specific mortality (CSM) and other causes of mortality (OCM) for patients with metastatic pancreatic duct adenocarcinoma (mPDAC). The secondary objective was to calculate the probability of CSM and build a competing risk nomogram to predict CSM for mPDAC.
Material/Methods
We identified patients with mPDAC between 2010 and 2015 from the Surveillance, Epidemiology, and End Results (SEER) database. We assessed the cumulative incidence function (CIF) for cause-specific mortality and other causes of mortality. We used Gray’s test to investigate the differences. The Fine and Gray proportional subdistribution hazard model was applied to model CIF. And a competing risk nomogram was built to predict the probability of CSM for mPDAC.
Results
There were 10 527 eligible patients diagnosed with mPDAC from 2010 to 2015 who were included in our formal analysis. The 6-month cumulative incidence of CSM was 60.3% and 5.9% for other causes. Predictors of SCM for mPDAC included surgery, age, tumor size, chemotherapy, radiation therapy, bone metastasis, and liver metastasis. The nomogram was proven to be well calibrated, and had good model discriminative ability.
Conclusions
We assessed the CIF of CSM and competing risk mortality in patients with mPDAC using the SEER database. The Fine and Gray proportional subdistribution hazard model performance was good, with a concordance index of 0.74, and the competing-risks nomogram was built, which can be a helpful predictive tool for cases with mPDAC. However, a validation sample data set and further verification are still needed to assess a profile for prognostic use in a prospective study.
MeSH Keywords: Nomograms, Pancreatic Neoplasms, SEER Program
Background
With substantial advances in diagnosis and treatment, many ordinary cancers have achieved steady improvements in survival rates during the last decades, whereas survival rates remain unoptimistic for pancreatic cancer. Pancreatic cancer has been the fourth leading cause of cancer-related death in America, with its mortality (n=44 330) estimated to approach its incidence rate (n=55 440) in 2018 [1]. Owing to the lack of effective screening protocols, approximately half of the cases of pancreatic cancer present with metastatic disease at diagnosis, for which the 5-year survival rate is a dismal 2% [2]. Meanwhile, over 85% of pancreatic cancer cases are pancreatic duct adenocarcinoma (PDAC) in pathology which unfortunately has the worst prognosis [3].
Because of the disappointing survival rate for metastatic PDAC (mPDAC), most published studies have mainly focused on overall survival (OS) or cancer-specific survival (CSS) using the Kaplan-Meier method and Cox proportional approach [4–6]. These traditional statistical approaches ignore other competing events and might lead to unreliable results [7]. Thus, it might be necessary to take the competing risks into consideration when evaluating the prognosis of patients with mPDAC.
In this study, we conducted a competing risk analysis to assess the prognosis of mPDAC patients using data from the US Surveillance, Epidemiology and End Result (SEER) registry. In addition, we built a convenient competing risk nomogram to evaluate the probability of mPDAC-specific mortality.
Material and Methods
Data collection and patient selection
Data about patients with metastatic pancreatic adenocarcinoma were extracted from the publicly available SEER-18 registry of the US National Cancer Institute (1973–2015). Primary cancer site and histology were coded by using the International Classification of Diseases for Oncology, Third Edition (ICD-O-3). The analysis was restricted to pancreatic adenocarcinoma, according to the ICD-O-3 histology codes 8140/3 and the site codes: C250–C254 and C257–C259.
We limited our study in 2010–2015 as information on distant metastatic sites is only available for 2010+ diagnoses. In addition, only patients who met the following criteria were included in the current analysis: 1) older than 30 years at diagnosis; 2) clear information available about survival time, tumor size, surgery, TNM stage, and metastatic sites; 3) pancreatic adenocarcinoma as the first primary tumor if there were 2 or more. Of the 32 763 adults with metastatic pancreatic adenocarcinoma from 2010 to 2015 included into the SEER registry, 10 527 people were eligible for the present study. As the patient information is de-identified in the SEER registry, this study was exempted from institutional review board oversight.
Statistical analysis
Considering the influence of tumor characteristics and psychological mentation on prognosis, we presented the cumulative incidence function (CIF) by tumor location, gender, age at diagnosis, race, marital status at diagnosis, tumor size, sex, radiation therapy, chemotherapy, T classification at presentation, N classification at presentation, distant metastasis and surgery for cause-specific mortality and other causes of mortality (2 competing events). The diversities in CIF among variables were estimated by using Gray’s test [8]. In addition, we made the proportional subdistribution hazard model by Fine and Gray to predict cause-specific mortality (CSM) and a competing-risk nomogram to predict prognosis for patients with mPDAC [9]. A bootstrap approach with 200 resamples was conducted to evaluate the model performance. An R function was used to calculate the c-index of the competing risk model to assess discrimination [10,11] and calibration was assessed using a calibration curve [12].
Statistical analysis was conducted by R software (version 3.3.1). The R packages cmprsk, rms, and mstate were used to build the model and nomogram [12–15] and package pec was used for assessing model performance. Statistical significance was considered if a 2-tailed P value was lower than 0.05.
Results
Patient characteristics
Our study extracted 10 527 eligible patients diagnosed with mPDAC from 2010 to 2015 in the SEER program. Table 1 summarizes the baseline patients’ characteristics. Of these, 1690 patients (16.1%) were diagnosed before aged 55 years old, 3156 patients (30.0%) were between the ages of 56 years and 65 years old, 3287 patients (31.2%) were aged 66 years to 75 years old, and 2394 patients (22.7%) were aged >75 years old. The majority were male (5680 patients; 54.0%), white (8283 patients; 78.7%), and married (5887 patients; 55.9%). Body/tail (41.5%) was the most common site, followed by the head (39.8%), and other (18,7%). Distribution of T stage was 3.6, 38.3, 46.2, and 21.3% for T1, T2, T3, and T4, respectively. Meanwhile, the proportion of N stage was 59.3 and 40.7 for N0 and N1, respectively. Liver (77.9%) was the most common metastatic site, followed by lung (20.2%), bone (6.4%), and brain (0.5%). Most patients (59.0%) were treated with chemotherapy.
Table 1.
Six-month cumulative incidences of mortality among patients with mPDAC.
| Characteristics | n | (%) | Event | (%) | Cause-specific mortality | Mortality from other causes | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6-month (%) | SE(10−5) | P | 6-month (%) | SE(10−5) | P | |||||
| Total | 10527 | 9187 | 60.3 | 2.4 | 5.9 | 0.54 | ||||
| Gender | 0.83 | 0.11 | ||||||||
| Male | 5680 | (54.0) | 4998 | (54.4) | 60.2 | 4.4 | 6.4 | 1.1 | ||
| Female | 4847 | (46.0) | 4189 | (45.6) | 60.3 | 5.3 | 5.3 | 1.1 | ||
| Age (years) | <0.001 | <0.001 | ||||||||
| Up to 55 | 1690 | (16.1) | 1420 | (15.5) | 49.1 | 15.7 | 4.5 | 2.7 | ||
| 56–65 | 3156 | (30.0) | 2716 | (29.6) | 56.1 | 8.2 | 5.1 | 1.6 | ||
| 66–75 | 3287 | (31.2) | 2835 | (30.9) | 60.2 | 7.8 | 5.7 | 1.7 | ||
| 76+ | 2394 | (22.7) | 2216 | (24.1) | 73.7 | 8.3 | 8.1 | 3.2 | ||
| Race/ethnicity | 0.33 | <0.001 | ||||||||
| White | 8283 | (78.7) | 7243 | (78.8) | 60.3 | 3.0 | 5.4 | 0.6 | ||
| Black | 1441 | (13.7) | 1258 | (13.7) | 60.7 | 17.5 | 8.3 | 5.4 | ||
| Others | 803 | (7.6) | 686 | (7.5) | 59.5 | 32.2 | 6.0 | 7.4 | ||
| Marital status | <0.001 | <0.001 | ||||||||
| Single | 1539 | (14.6) | 1338 | (14.6) | 61.7 | 16.3 | 7.9 | 4.9 | ||
| Married | 5887 | (55.9) | 5068 | (55.2) | 56.5 | 4.4 | 4.9 | 0.8 | ||
| Divorced | 1098 | (10.4) | 967 | (10.5) | 63.7 | 22.2 | 6.0 | 5.3 | ||
| Widowed | 1452 | (13.8) | 1339 | (14.6) | 71.2 | 14.6 | 6.9 | 4.5 | ||
| Others | 551 | (5.2) | 475 | (5.2) | 60.3 | 46.3 | 7.7 | 13.4 | ||
| Tumor location | 0.009 | 0.44 | ||||||||
| Body/tail | 4368 | (41.5) | 3814 | (41.5) | 61.1 | 5.8 | 5.8 | 1.3 | ||
| Head | 4194 | (39.8) | 3649 | (39.7) | 58.3 | 6.1 | 5.5 | 1.3 | ||
| Others | 1965 | (18.7) | 1724 | (18.8) | 62.6 | 12.5 | 6.9 | 3.3 | ||
| T stage, AJCC 7th | <0.001 | 0.1 | ||||||||
| T1 | 382 | (3.6) | 330 | (3.6) | 56.6 | 67.9 | 8.6 | 21.1 | ||
| T2 | 4036 | (38.3) | 3568 | (38.8) | 64.7 | 6.0 | 6.1 | 1.5 | ||
| T3 | 4865 | (46.2) | 3334 | (36.3) | 58.5 | 6.7 | 5.5 | 1.4 | ||
| T4 | 2244 | (21.3) | 1955 | (21.3) | 56.0 | 11.5 | 5.5 | 2.4 | ||
| N stage, AJCC 7th | 0.20 | 0.65 | ||||||||
| N0 | 6241 | (59.3) | 5464 | (59.5) | 61.0 | 4.0 | 5.9 | 0.9 | ||
| N1 | 4286 | (40.7) | 3723 | (40.5) | 59.2 | 6.0 | 5.8 | 1.3 | ||
| Tumor size (mm) | <0.001 | 0.9 | ||||||||
| ≤30 | 2559 | (24.3) | 2219 | (24.2) | 55.8 | 10.1 | 5.7 | 2.2 | ||
| 31–40 | 2731 | (25.9) | 2388 | (26.0) | 59.6 | 9.3 | 5.4 | 1.9 | ||
| 41–50 | 2209 | (21.0) | 1913 | (20.8) | 60.2 | 11.5 | 5.7 | 2.5 | ||
| 51–60 | 1388 | (13.2) | 1222 | (13.3) | 65.0 | 17.4 | 6.5 | 4.6 | ||
| >60 | 1640 | (15.6) | 1445 | (15.7) | 64.5 | 14.6 | 6.5 | 3.8 | ||
| Chemotherapy | <0.001 | <0.001 | ||||||||
| None/unknown | 4321 | (41.0) | 4050 | (44.1) | 81.3 | 3.5 | 9.9 | 2.1 | ||
| Yes | 6206 | (59.0) | 5137 | (55.9) | 45.9 | 4.3 | 3.1 | 0.5 | ||
| Radiation therapy | <0.001 | 0.2 | ||||||||
| None/unknown | 9953 | (94.5) | 8688 | (94.6) | 61.0 | 2.5 | 5.9 | 0.6 | ||
| Yes | 574 | (5.5) | 499 | (5.4) | 46.8 | 45.2 | 4.5 | 7.7 | ||
| Bone metastasis | 0.004 | 0.04 | ||||||||
| No | 9855 | (93.6) | 8574 | (93.3) | 59.9 | 2.6 | 5.7 | 0.6 | ||
| Yes | 672 | (6.4) | 613 | (6.7) | 65.9 | 34.7 | 7.9 | 11.1 | ||
| Brain metastasis | 0.015 | 0.45 | ||||||||
| No | 10472 | (99.5) | 9137 | (99.5) | 60.2 | 2.4 | 5.8 | 0.5 | ||
| Yes | 55 | (0.5) | 50 | (0.5) | 76.6 | 347.5 | 9.6 | 168.3 | ||
| Liver metastasis | <0.001 | 0.24 | ||||||||
| No | 2324 | (22.1) | 2001 | (21.8) | 49.3 | 11.3 | 5.7 | 2.4 | ||
| Yes | 8203 | (77.9) | 7186 | (78.2) | 63.4 | 3.0 | 5.9 | 0.7 | ||
| Lung metastasis | 0.009 | 0.27 | ||||||||
| No | 8400 | (79.8) | 7302 | (79.5) | 59.8 | 3.0 | 5.6 | 0.6 | ||
| Yes | 2127 | (20.2) | 1885 | (20.5) | 62.0 | 11.5 | 7.0 | 3.1 | ||
| Surgery | <0.001 | 0.3 | ||||||||
| No | 10313 | (98.0) | 9021 | (98.2) | 60.9 | 2.4 | 5.9 | 0.6 | ||
| Yes | 214 | (2.0) | 166 | (1.8) | 31.1 | 105.3 | 3.4 | 15.7 | ||
mPDAC – metastatic pancreatic duct adenocarcinoma; SE – standard error; AJCC – American Joint Committee on Cancer.
Cause-specific mortality in mPDAC
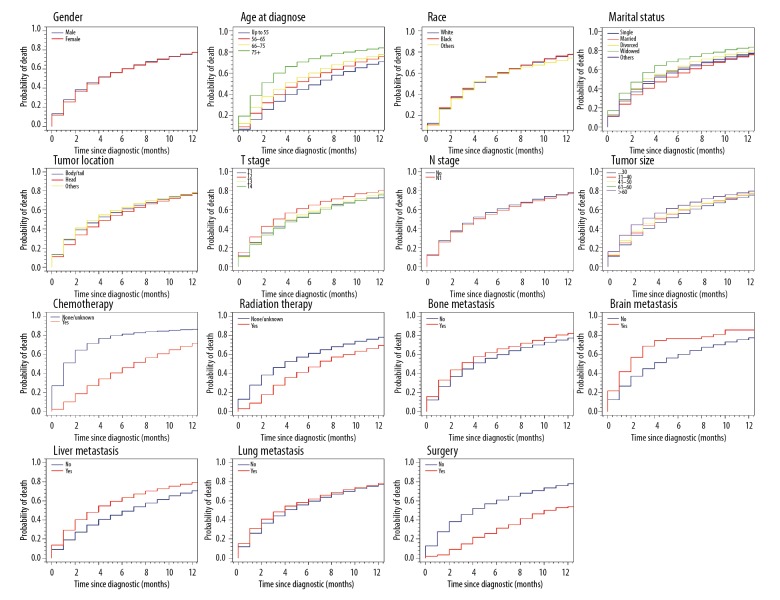
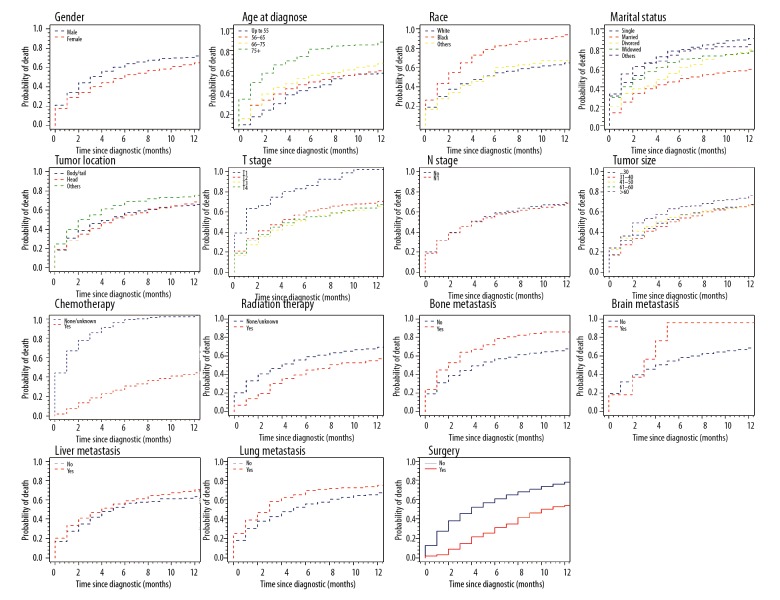
Over a median follow-up of 3 months, mortality occurred in 8459 patients (80.36%): 8459 from mPDAC and 728 from other causes. Median cancer-specific survival remained steady at 2 months from 2010 to 2015. Table 1 summarizes the 6-month estimates of the cumulative incidence of CSM, and OCM according to gender, sex, age, race, tumor size, tumor location, marital status, TNM stage, chemotherapy, radiation therapy, bone metastasis, brain metastasis, liver metastasis, lung metastasis, and surgery. Six-month cumulative incidence of cause-specific mortality for mPDAC was 60.3% and 5.9% for other causes. Gender, race, tumor location, N stage and lung metastasis didn’t markedly affect cumulative incidence of cause-specific mortality, whereas patients with older age, larger tumor size, widowed, T2 stage, bone metastasis, brain metastasis and liver metastasis had a high one. Chemotherapy subdistribution hazard ratio (sdHR)was 0.54 (95% CI: 0.51–0.57), radiation therapy sdHR was 0.89 (95% CI: 0.83–0.97); and surgery sdHR was 0.65 (95% CI: 0.57–0.75), which resulted in decreased probability of cause-specific mortality. Figures 1 and 2 show the CIF curves of CSM and other causes, respectively.
Figure 1.
Cumulative incidence curves of cause-specific mortality based on patient characteristics.
Figure 2.
Cumulative incidence curves of other cause of mortality based on patient characteristics.
Table 2 shows coefficients and sdHRs according to the results of the competing-risk model for cause-specific mortality of mPDAC. Age and tumor size could forcefully predict cause-specific mortality. Patients who received chemotherapy, radiation therapy, or surgery had a lower rate of CSM, with sdHR of 0.54 (95% CI: 0.51–0.57), 0.89 (95% CI: 0.83–0.97) and 0.65 (95% CI 0.57–0.75), respectively. Liver metastasis (sdHR 1.24, 95% CI: 1.17–1.30) was the most dangerous among these 4 sites. Marital status, tumor location, and N stage almost had no influence on cause-specific mortality.
Table 2.
Proportional subdistribution hazard models of probabilities of cancer-specific mortality for patients with mPDAC.
| Characteristic | Coefficient | sdHR (95% CI) | p Value |
|---|---|---|---|
| Female | −0.03 | 0.98 (0.93–1.02) | 0.28 |
| Age (years) | |||
| 56–65 | 0.08 | 1.01 (1.01–1.15) | 0.018 |
| 66–75 | 0.17 | 1.14 (1.07–1.21) | <0.001 |
| 76+ | −0.02 | 1.22 (1.13–1.32) | <0.001 |
| Race/ethnicity | |||
| Black | −0.07 | 0.93 (0.87–0.99) | 0.042 |
| Others | −0.06 | 0.94 (0.86–1.02) | 0.14 |
| Marital status | |||
| Married | 0.01 | 1.01 (0.94–1.08) | 0.85 |
| Divorced | 0.01 | 1.07 (0.97–1.17) | 0.17 |
| Widowed | 0.10 | 1.10 (0.99–1.20) | 0.05 |
| Others | −0.06 | 1.00 (0.84–1.01) | 0.34 |
| Tumor location | |||
| Head | −0.04 | 0.96 (0.92–1.01) | 0.13 |
| Others | −0.04 | 0.96 (0.90–1.02) | 0.24 |
| T stage | |||
| T2 | 0.21 | 1.23 (1.01–1.41) | 0.003 |
| T3 | 0.12 | 1.13 (0.98–1.30) | 0.08 |
| T4 | 0.11 | 1.12 (0.97–1.29) | 0.12 |
| N1 | 0.02 | 1.02 (0.98–1.07) | 0.31 |
| Tumor size(mm) | |||
| 31–40 | 0.05 | 1.05 (1.00–1.12) | 0.08 |
| 41–50 | 0.06 | 1.06 (1.00–1.13) | 0.07 |
| 51–60 | 0.16 | 1.17 (1.08–1.26) | <0.001 |
| >60 | 0.15 | 1.16 (1.07–1.25) | <0.001 |
| Chemotherapy | −0.61 | 0.54 (0.51–0.57) | <0.001 |
| Radiation therapy | −0.11 | 0.89 (0.83–0.97) | 0.005 |
| Bone metastasis | 0.13 | 1.14 (1.04–1.25) | 0.007 |
| Brain metastasis | 0.19 | 1.21 (0.82–1.78) | 0.35 |
| Liver metastasis | 0.21 | 1.24 (1.17–1.30) | <0.001 |
| Lung metastasis | 0.05 | 1.05 (1.00–1.11) | 0.13 |
| Surgery | −0.43 | 0.65 (0.57–0.75) | <0.001 |
mPDAC – metastatic pancreatic duct adenocarcinoma; sdHR – subdistribution hazard ratios.
A nomogram predicting the probability of CSM is shown in Figure 3, which was based on the Fine and Gray’s model we built. With the help of this useful tool, we can individually predict the probability of half-year CSM for patients with mPDAC, by calculating the total points of patient’s characteristics. The c-index for CSM of our model was 0.74, which suggests acceptable model discriminative ability. The calibration curve is displayed in Figure 4. The calibration plot shows that the nomogram was well calibrated.
Figure 3.
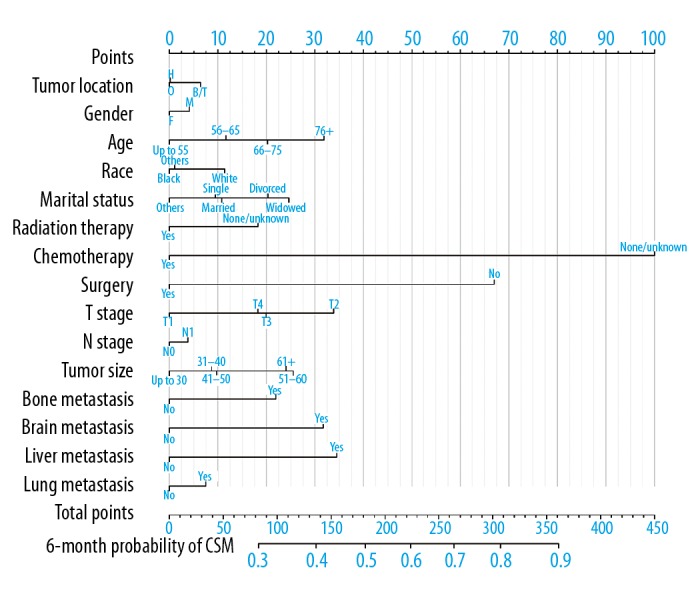
Nomogram for predicting six-month probabilities of CSM in patients with mPDAC. CSM – cause-specific mortality; mPDAC – metastatic pancreatic duct adenocarcinoma; H – head; B/T – body/tail; O – others; M – Male; F – Female.
Figure 4.
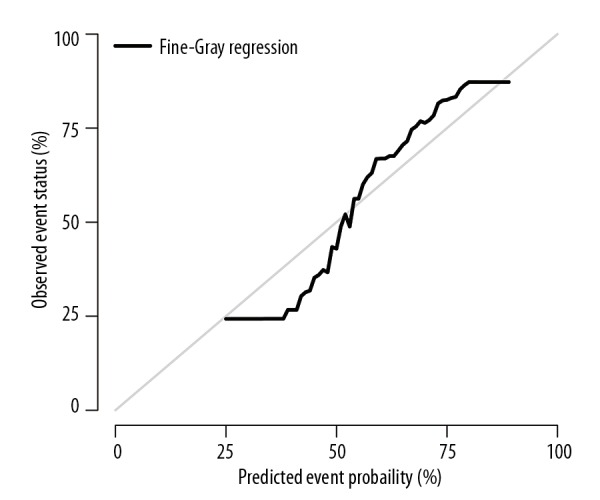
Calibration plot. The gray line represents equality between the predicted and observed probability.
Discussion
In the present study, we assessed the mortality for patients with mPDAC diagnosed from 2010 to 2015 in the SEER registry. Six-month cumulative incidence for cause-specific mortality and other causes of mortality was 60.3% and 5.9%, respectively. Moreover, we built a convenient nomogram to predict the probability of cause-specific mortality for this cancer, however, this was all correlative not causative information.
In our current study, gender had no influence on cause-specific mortality (sdHR 0.98, 95%CI: 0.93–1.02). While another study on the prognosis of metastatic pancreatic cancer patients had a different point of view, indicating that female patients had better outcomes [16]. The difference between our study results might be from the discrepancy of statistical approaches, selection principles, and sample sizes.
As for the treatment of mPDAC, chemotherapy has been the first-line therapy for many decades, with proven efficacy in the area of meaningfully increased survival. Recently, FOLFIRINOX (fluorouracil, leucovorin, irinotecan, and oxaliplatin) and gemcitabine+nab-paclitaxel have shown a modest improvement in survival of mPDAC patients [2]. Similar to this result, about 60% of patients in our study were confirmed to have received chemotherapy which resulted in significant reduction of 6-month mortality (sdHR 0.54, 95% CI: 0.51–0.57). While 574 patients (5.5%) received radiation therapy, and they benefited a little from it (sdHR 0.89, 95% CI: 0.83–0.97). Consistent with our finding, Wang et al. [17] previously demonstrated that the overall survival for locally advanced and metastatic pancreatic cancer with acceptable toxicities can be improved in concurrent chemoradiotherapy use.
The benefit of local tumor resection has been proven for many solid tumors, including metastatic hepatocellular carcinoma [18], metastatic breast cancer [19], and metastatic non-functioning pancreatic neuroendocrine tumors [20,21]. In a recent study, Tao et al. revealed that surgical resection of the primary tumor benefits both cancer-specific survival and overall survival in metastatic pancreatic cancer patients. However, their study was limited by a small surgical cohort [22]. Our observations also implied that surgery was associated with good survival (sdHR 0.65, 95% CI: 0.57–0.75), but only a tiny minority of patients (2%) had resection performed. Surgery might play a role in the treatment of a highly selected subset of mPDAC patients.
One of our study advantages was the large cohort size and the precision of the competing risk model. The SEER database offers a large-scale sample. Unlike studies from single-institutions, we can drastically reduce potential selection bias [23] using the SEER database. In addition, with the help of the nomogram we built, clinicians can estimate individualized prognosis for patients with mPDAC.
Undeniably, our analysis has several potential limitations. First, some useful clinicopathological factors (such as C-reactive protein, pain, neutrophil-to-lymphocyte ratio) were not included in our analysis, without the needed corresponding records in the SEER program [24,25]. Second, as the information on metastatic sites was unavailable before 2010, we only chose patients diagnosed from 2010 to 2015. The accuracy of this model might improve with longer follow-up. Finally, a bootstrap approach was used to assess model performance. Although it showed good performance, external validation based on other cohorts is still needed to estimate model accuracy.
Conclusions
In our current study, we assessed the CIF of CSM and OCM mortality for patients with mPDAC using the SEER database. We built a proportional subdistribution model to calculate the probability of 6-month CSM for patients with mPDAC. In addition, an easy nomogram was offered as a predictive tool for prognosis. However, a prospective study with a validation sample data set and further verification are needed to assess a profile for prognostic use.
Acknowledgments
The authors would like to express appreciation for the SEER program for providing open access to the database.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Sohal DP, Mangu PB, Khorana AA, et al. Metastatic pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(23):2784–96. doi: 10.1200/JCO.2016.67.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(22):2140–41. doi: 10.1056/NEJMc1412266. [DOI] [PubMed] [Google Scholar]
- 4.Golan T, Sella T, Margalit O, et al. Short- and long-term survival in metastatic pancreatic adenocarcinoma, 1993–2013. J Natl Comp Cancer Netw. 2017;15(8):1022–27. doi: 10.6004/jnccn.2017.0138. [DOI] [PubMed] [Google Scholar]
- 5.Kalra S, Atkinson BJ, Matrana MR, et al. Prognosis of patients with metastatic renal cell carcinoma and pancreatic metastases. BJU Int. 2016;117(5):761–65. doi: 10.1111/bju.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crippa S, Bittoni A, Sebastiani E, et al. Is there a role for surgical resection in patients with pancreatic cancer with liver metastases responding to chemotherapy? Eur J Surg Oncol. 2016;42(10):1533–39. doi: 10.1016/j.ejso.2016.06.398. [DOI] [PubMed] [Google Scholar]
- 7.Noordzij M, Leffondre K, van Stralen KJ, et al. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant. 2013;28(11):2670–77. doi: 10.1093/ndt/gft355. [DOI] [PubMed] [Google Scholar]
- 8.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. [Google Scholar]
- 9.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 10.Wolbers MKM, Witteman JC, Steyerberg EW. Prognostic models with competing risks: Methods and application to coronary risk prediction. Epidemiology. 2009;20(4):555–61. doi: 10.1097/EDE.0b013e3181a39056. [DOI] [PubMed] [Google Scholar]
- 11.Harrell FE., Jr . Regression modeling strategies. Springer-Verlag; Berlin, Heidelberg: 2015. [Google Scholar]
- 12.Regression modeling strategies. R package version 43-1. 2015. http://CRAN.R-project.org.
- 13.Subdistribution analysis of competing risks. R package version 22-7. 2014. http://CRAN.R-project.org.
- 14.Zhang Z, Geskus RB, Kattan MW, et al. Nomogram for survival analysis in the presence of competing risks. Ann Transl Med. 2017;5(20):403. doi: 10.21037/atm.2017.07.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: Competing risks and multi-state models. Stat Med. 2007;26(11):2389–430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 16.Oweira H, Petrausch U, Helbling D, et al. Prognostic value of site-specific metastases in pancreatic adenocarcinoma: A Surveillance Epidemiology and End Results database analysis. World J Gastroenterol. 2017;23(10):1872–80. doi: 10.3748/wjg.v23.i10.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Ren ZG, Ma NY, et al. Intensity modulated radiotherapy for locally advanced and metastatic pancreatic cancer: A mono-institutional retrospective analysis. Radiat Oncol. 2015;10:14. doi: 10.1186/s13014-014-0312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdel-Rahman O. Role of liver-directed local tumor therapy in the management of hepatocellular carcinoma with extrahepatic metastases: A SEER database analysis. Expert Rev Gastroenterol Hepatol. 2017;11(2):183–89. doi: 10.1080/17474124.2017.1259563. [DOI] [PubMed] [Google Scholar]
- 19.Warschkow R, Guller U, Tarantino I, et al. Improved survival after primary tumor surgery in metastatic breast cancer: A propensity-adjusted, population-based SEER trend analysis. Ann Surg. 2016;263(6):1188–98. doi: 10.1097/SLA.0000000000001302. [DOI] [PubMed] [Google Scholar]
- 20.Keutgen XM, Nilubol N, Glanville J, et al. Resection of primary tumor site is associated with prolonged survival in metastatic nonfunctioning pancreatic neuroendocrine tumors. Surgery. 2016;159(1):311–18. doi: 10.1016/j.surg.2015.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huttner FJ, Schneider L, Tarantino I, et al. Palliative resection of the primary tumor in 442 metastasized neuroendocrine tumors of the pancreas: A population-based, propensity score-matched survival analysis. Langenbecks Arch Surg. 2015;400(6):715–23. doi: 10.1007/s00423-015-1323-x. [DOI] [PubMed] [Google Scholar]
- 22.Tao L, Yuan C, Ma Z, et al. Surgical resection of a primary tumor improves survival of metastatic pancreatic cancer: A population-based study. Cancer Manag Res. 2017;9:471–79. doi: 10.2147/CMAR.S145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez SR, Tseng WH, Young SE. Outcomes for lymph node-positive cutaneous melanoma over two decades. World J Surg. 2011;35(7):1567–72. doi: 10.1007/s00268-010-0903-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asari S, Matsumoto I, Toyama H, et al. Preoperative independent prognostic factors in patients with borderline resectable pancreatic ductal adenocarcinoma following curative resection: the neutrophil-lymphocyte and platelet-lymphocyte ratios. Surg Today. 2016;46(5):583–92. doi: 10.1007/s00595-015-1206-3. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z, Jin K, Guo M, et al. Prognostic value of the CRP/Alb ratio, a novel inflammation-based score in pancreatic cancer. Ann Surg Oncol. 2017;24(2):561–68. doi: 10.1245/s10434-016-5579-3. [DOI] [PubMed] [Google Scholar]