Abstract
Iminodipropionitrile (IDPN) is known to produce axonopathy and vestibular hair cell degeneration. Recent histopathological studies have shown IDPN-induced liver and kidney toxicities in rodents; however, the associated mechanisms are not clearly understood. We investigated the role of proinflammatory cytokines in IDPN-induced liver and kidney toxicities in rats. Rats were treated with saline (control) and IDPN (100 mg/kg, intraperitoneally) daily for 1, 5, and 10 days, respectively. Animals were killed 24 hours after the last dose and liver and kidneys were collected for histopathology and interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α messenger RNA expression analysis. Serum aspartate aminotransferase and alanine aminotransferase activities were significantly increased after 10 doses of IDPN. The level of serum creatinine was initially increased after the first dose of IDPN but subsided on days 5 and 10. Blood urea nitrogen levels were significantly increased on days 5 and 10 following IDPN exposure. Histopathology showed dose-dependent hepatotoxicity in IDPN-treated rats. Iminodipropionitrile-induced expression of proinflammatory cytokines peaked after day 1 in liver and after day 5 in kidneys. In conclusion, repeated exposure of IDPN for 10 days produced significant structural and functional damages in rat liver whereas kidneys showed gradual recovery with time. These findings point toward the role of inflammatory mediators in IDPN-induced toxicity in rats.
Keywords: iminodipropionitrile, toxicity, proinflammatory cytokines, liver, kidney
Introduction
Synthetic nitriles are industrial chemicals that are mainly used as solvent for dyestuffs and pesticides. Iminodipropionitrile (IDPN) is a synthetic nitrile that causes symptoms in rhesus monkeys that typically resemble neurolathyrism, seen in humans. The behavioral syndrome in rodents caused by IDPN is also known as ECC syndrome (excitation with choreiform and circling movements) and characterized by peculiar head movements, hyperactivity, circling, retropulsion, and swimming deficits.1 Iminodipropionitrile is structurally similar to β-aminopropionitrile, an osteolathyrogen, which was isolated from natural seeds.2 It was later observed that several other nitriles of industrial importance such as allylnitrile, acrylonitrile, and crotononitrile also induce motor defects in laboratory animals.3,4 Exposure to nitriles result in 2 different types of behavioral syndromes through either neuronal impairment or vestibular hair cell degeneration.5,6 The ease in quantification and reproducibility of IDPN-induced neurobehavioral symptoms render this prototype nitrile as a suitable compound for validation of functional observational battery and motor deficits for screening of neurotoxic drugs.1,7 Iminodipropionitrile has also been recommended by the US Environmental Protection Agency as a positive control for testing of behavioral toxicity.8 The production and use of IDPN in neurological research may result in its release to the environment through various waste streams while its occupational exposure may occur through inhalation and dermal contact at workplaces where this compound is produced or used.9,10
Exposure of IDPN causes accumulation and swelling of neurofilaments in the proximal axon segments, including those of motor neurons in the spinal cord and sensory neurons in the dorsal root ganglia, and these lesions are the result of a defect in slow axonal transport.11,12 The pathogenesis of IDPN toxicity and motor deficits is complex and multifactorial.13 The nitrile toxicity mainly results from the release of cyanide in the body while the acute toxicity has been shown to vary with different types of nitriles.14 Neuropharmacological, biochemical, and behavioral studies have implicated various neurotransmitters,15-18 neuromodulators,19,20 reactive oxygen species (ROS),21-24 and inflammation25,26 in the development of IDPN-induced ECC syndrome. Llorens and coworkers27 implicated vestibular hair cell degeneration in the development and persistence of IDPN-induced behavioral syndrome, which was later supported by other investigators as well.28,29 Seoane et al30 reported necrosis of hair cells at day 1 after acute high dose IDPN exposure in rats. However, at day 4 with the same dose, and at day 1 and day 4 after repeated medium dose IDPN exposure, they observed DNA fragmentation and apoptosis in vestibular epithelia using the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. Iminodipropionitrile-induced neurotoxicity may be associated with ischemic changes in brain, as the increase in endogenous vasodilator significantly attenuated IDPN-induced behavioral syndrome.31-33
Iminodipropionitrile is also known to damage or impair the function of various sensory systems, including olfactory,34 auditory,35 and visual36 systems. Subsequent researches have shown that IDPN is not only toxic to sensory, vestibular, and nervous systems but also causes reproductive,37 developmental,38 and hepatic39 toxicities. The magnitude of IDPN-induced hepatotoxicity in mice was independent of the severity of vestibular epithelial damage.40 Rats were comparatively less vulnerable to IDPN-induced renal toxicity than mice.39,40 The mechanisms by which IDPN exerts hepatic and renal toxicities are not clear. In this study, we investigated the role of inflammatory mediators in IDPN-induced liver and kidney toxicities in rats. We preferred the use of rats over mice due to ease in behavioral observation as well as in blood collection for liver and renal function tests.
Materials and Methods
Animals and Treatment
Adult male Wistar rats weighing 150 to 200 g were obtained from the animal house of College of Pharmacy, King Saud University, Riyadh, Saudi Arabia. The animals were housed in polycarbonate cages with sawdust bedding and kept in air-conditioned room (23°C ± 1°C) maintained at 12 hours light/dark cycles. The rats were allowed free access to food (Purina rodent chow) and tap water ad libitum. The animals were divided into 4 treatment groups of 8 rats per group and left for 1 week acclimatization period. The experimental protocol was approved by the Institutional Review Board, College of Science, King Saud University, Riyadh, Saudi Arabia.
The rats were injected with IDPN or normal saline through intraperitoneal (IP) route in a volume of 2 mL/kg bodyweight. One group served as control and received normal saline only. The remaining 3 groups were treated with IDPN (100 mg/kg) daily for 1, 5, and 10 days, respectively. This is the standard dose of IDPN to induce dyskinesia in rats.6,16,19,23 The animals were killed at 24 hours after the last injection.
Blood samples were collected in serum separator vacutainer tubes. The tubes were kept at room temperature for 30 minutes and then centrifuged at 1500 g for 10 minutes. Sera were transferred to new clean tubes and stored at −80°C until analyzed. For gene expression analysis, liver and kidney samples were washed with normal saline, weighed, and cut into small pieces before immersing in RNA Later solution (Qiagen, Hilden, Germany) to prevent RNA degradation. These samples were stored at 2°C to 8°C until RNA extraction was performed. For histopathology, whole liver and kidney were promptly fixed in 10% normal buffered formalin.
Behavioral Analysis
The rats were individually examined for the presence or absence of the peculiar signs of ECC syndrome, including dyskinetic head movements, circling and tail hanging, as reported earlier after some modifications.27,28 The animals were observed for a period of 2 minutes to quantify the severity of dyskinetic head movements and circling whereas the tail hanging was tested at least 3 times for grading the severity (0 to 3 scale), as described earlier.16 The maximum severity score was 9, based on 3 behavioral signs.
Serum Alanine Aminotransferase
Serum alanine aminotransferase (ALT) was analyzed using a commercial kit (Cat No 007-050) purchased from United Diagnostic Industry, Riyadh, Saudi Arabia. The analytical protocol provided by the manufacturer was strictly followed for ALT analysis. The test was conducted using 100 µL of sample and the absorbance was recorded using an UV-visible Spectrophotometer (Biochrom, England, United Kingdom). The molar absorptivity of nicotinamide adenine dinucleotide reduced (NADH) at 340 nm (6.22 × 103 L·mole−1cm−1) was used to calculate ALT activity.
Serum Aspartate Aminotransferase
The activity of serum aspartate aminotransferase (AST) was measured using a colorimetric kit (Cat No 015-050; United Diagnostic Industry). The test was performed using 100 µL of sample and the rate of change of absorbance (ΔA/min) was determined and used for calculation of serum AST activity using the molar absorptivity of NADH.
Serum Creatinine
Serum creatinine (SCr) was analyzed using a commercial kit (Cat No 033K-240) supplied by United Diagnostic Industry, Riyadh, Saudi Arabia. A sample (or standard) volume of 100 µL was used for each analysis and the reaction rate (ΔA/min) was determined to compute SCr levels.
Blood Urea Nitrogen
Blood urea nitrogen (BUN) was determined using a colorimetric kit (Cat No. 020-050; United Diagnostic Industry) according to manufacturer’s instructions. A sample (or standard) volume of 100 µL was used for each analysis and the reaction rate (ΔA/min) was used to determine the BUN levels.
Gene Expression Analysis by Real-Time Polymerase Chain Reaction
Total RNA was extracted from approximately 30 mg of liver and kidney tissue samples (preserved in RNA Later solution) by using SV Total RNA Isolation System (Cat No Z3100; Promega, Fitchburg, Wisconsin). The extracted RNA was dissolved in nuclease free distilled water and checked for quality and quantity and then stored at –70°C freezer until analyzed. For real-time polymerase chain reaction (PCR) analysis, we used Power SYBR Green RNA-to-CT one-step kit (Cat No 4389986; Applied Biosystems, Thermo Scientific, Carlsbad, CA, USA) providing a master mix consisting of reagents for both complementary DNA synthesis an PCR amplification. The reaction was carried in a 20 µL reaction mixture containing 0.25 mM of each primer (0.5 µL), 1 µL of RNA template, 10 µL master mix, 0.16 µL reverse transcriptase enzyme, and remaining nuclease free water. The 96-well microplate was incubated at 50°C for 30 minutes and then at 95°C for 10 minutes in the block of a real-time PCR instrument (Stratagene, Agilent Biosciences, Santa Clara, CA, USA). The cycling conditions were as follows: 45 cycles of 95°C for 15 seconds, 60°C for 20 seconds, and 72°C for 60 seconds. The forward and reverse primers sequences for the target (interleukin 1β [IL-1β], IL-6, and tumor necrosis factor α [TNF-α]) as well as housekeeping (GAPDH) genes were as follows: IL-1β, caccttcttttccttcatctttg and gtcgttgcttgtctctccttgta; IL-6, tgatggatccttccaaactg and gagcattggaagttggggta; TNF-α, actgaacttcggggtgattg and gcttggtggtttgctacgac; and GAPDH, gtattgggcgcctggtcacc and cgctcctggaagatggtgatgg.41,42
Histopathology
Liver and kidneys sections from each killed rat dissected out. They were fixed in 10% formalin and were processed for paraffin sectioning by dehydration in different concentration of alcohol, cleared with xylol and embedded in paraffin blocks by a tissue processor. Sections of 3 µm thickness were stained with Harris hematoxylin and eosin automatic stainer for light microscopic evaluation of histopathological changes in liver and kidney.43
Statistics
The data were evaluated by 1-way analysis of variance. The mean values from different treatment groups were compared using Dunnett multiple comparison test. The P values less than .05 were considered as statistically significant. All the statistical analysis was performed using SPSS (version 10) statistical package.
Results
Behavioral Syndrome
There were no behavioral abnormalities in the animals treated with vehicle (control) or a single dose of IDPN (Table 1). The onset of ECC syndrome was noticed after 5 days of daily IDPN treatment, when 4 out of 8 rats showed mild behavioral deficits. Repeated daily exposure of IDPN for 10 days produced severe dyskinetic symptoms in all the animals (Table 1).
Table 1.
Effect of Different IDPN Treatments on Severity of Behavioral Syndrome.a
| Treatment | Severity Index |
|---|---|
| Control (saline) | 0.00 ± 0.00 |
| IDPN (100 mg/kg) 1 dose | 0.00 ± 0.00 |
| IDPN (100 mg/kg) 5 doses | 2.12 ± 0.89b |
| IDPN (100 mg/kg) 10 doses | 6.63 ± 0.37c |
Abbreviation: IDPN, iminodipropionitrile; SEM, standard error of mean.
aValues are mean ± SEM.
b P < .05 versus control group.
c P < .01 versus control group.
Liver and Kidney Weights
Administration of a single dose of IDPN caused significant increase in rat liver (24.46%) and kidney (17.56%) weights, after 24 hours (Table 2). Daily single dose of IDPN for 5 consecutive days (total 5 doses) significantly increased the liver weight (26.59%) but did not affect the kidney weight. The regimen of daily single dose of IDPN for 10 consecutive days did not produce any significant change in liver and kidney weights (Table 2).
Table 2.
Effect of Different IDPN Treatments on Rat Liver and Kidney Weights.a
| Treatment | Liver Weight (g) | Kidney Weight (g) |
|---|---|---|
| Control (saline) | 6.58 ± 0.16 | 0.74 ± 0.02 |
| IDPN (100 mg/kg) 1 dose | 8.19 ± 0.53b | 0.87 ± 0.04c |
| IDPN (100 mg/kg) 5 doses | 8.33 ± 0.58b | 0.72 ± 0.02 |
| IDPN (100 mg/kg) 10 doses | 7.07 ± 0.16 | 0.69 ± 0.03 |
Abbreviation: IDPN, iminodipropionitrile; SEM, standard error of mean.
aValues are mean ± SEM. Kidney weight is the average of left and right kidneys.
b P < .01 versus control group.
c P < .05 versus control group.
Liver Function Test
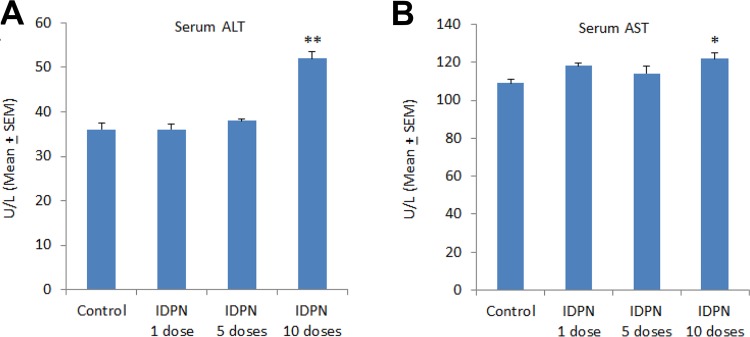
The average serum ALT of the control rats was 36.1 ± 1.39 U/L (Figure 1, A). There were no significant changes in serum ALT activities in rats treated with a single dose (36.3 ± 1.26 U/L) or 5 doses of IDPN (38.4 ± 0.47 U/L). Administration of 10 doses (1 dose per day) of IDPN significantly increased the serum ALT activities (52.2 ± 1.45 U/L; Figure 1, A). There were no significant changes in AST activities in sera of rats treated with a single dose of IDPN (118.2 ± 1.55 U/L) or 5 daily doses of IDPN (114.5 ± 4.11 U/L) as compared to control animals (109.3 ± 1.83 U/L). However, treatment of rats with 10 doses of IDPN significantly increased serum AST activity (122.4 ± 2.81 U/L; Figure 1, B).
Figure 1.
Effect of different daily doses of IDPN on serum markers of liver function: (A) serum ALT and (B) serum AST. *P < .05 and **P < .01 versus control group using Dunnett test. IDPN indicates iminodipropionitrile.
Renal Function Test
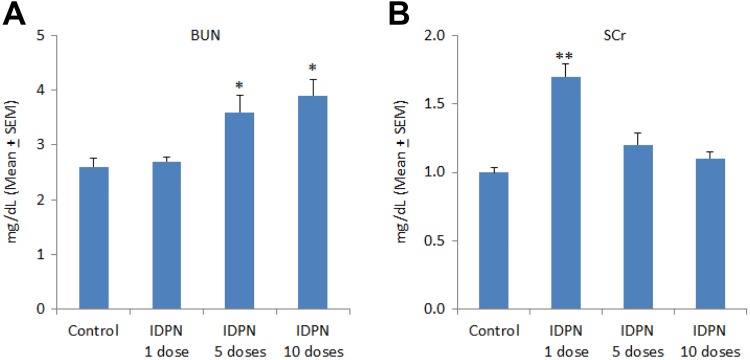
A single dose of IDPN did not affect BUN levels as compared to control values (2.6 ± 0.15 mg/dL). However, daily IDPN treatment for 5 days (3.6 ± 0.29 mg/dL) and 10 days (3.9 ± 0.28 mg/dL) significantly increased BUN levels (Figure 2, A). Serum creatinine was significantly increased after a single dose of IDPN (1.7 ± 0.08 mg/dL) as compared to SCr of control rats (1.0 ± 0.03 mg/dL). Daily injections of IDPN for 5 and 10 days slightly increased SCr levels, which were statistically insignificant (Figure 2, B).
Figure 2.
Effect of different daily doses of IDPN on serum markers of kidney function: (A) blood urea nitrogen and (B) serum creatinine. *P < .05 and **P < .01 versus control group using Dunnett test. IDPN indicates iminodipropionitrile.
Proinflammatory Cytokines Gene Expression
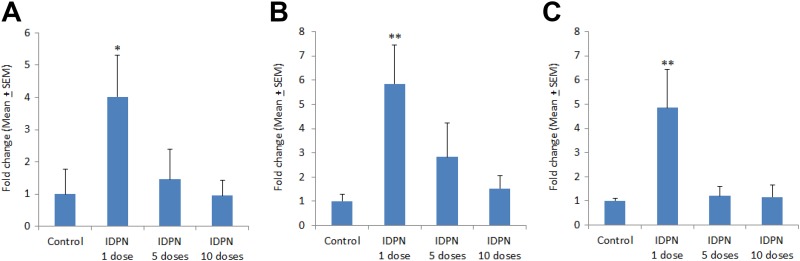
In liver, there was a significant increase in IL-1β gene expression (4.02-fold) at day 1 following a single injection of IDPN (Figure 3A). The IL-1β gene expression in liver was gradually reduced to 1.45-fold and 0.87-fold, following repeated exposure of IDPN for 5 days and 10 days, respectively (Figure 3A). A single dose of IDPN significantly increased IL-6 expression (5.84-fold) as compared to control level (Figure 3B). A gradual decrease in liver IL-6 expression was observed after day 5 (2.83-fold) and day 10 (1.52-fold) following the IDPN exposure (Figure 3B). The expression of TNF-α was also significantly increased (4.85-fold) at day 1, after a single dose of IDPN as compared to control rats (Figure 3C). Repeated daily exposure of IDPN for 5 and 10 days did not cause any significant change in TNF-α expression as compared to controls (Figure 3C).
Figure 3.
Effect of different treatments of IDPN on mRNA expression of (A) IL-1β, (B) IL-6, and (C) TNF-α in rat liver. *P < .05 and **P < .01 versus control group using Dunnett test. IDPN indicates iminodipropionitrile; mRNA, messenger RNA.
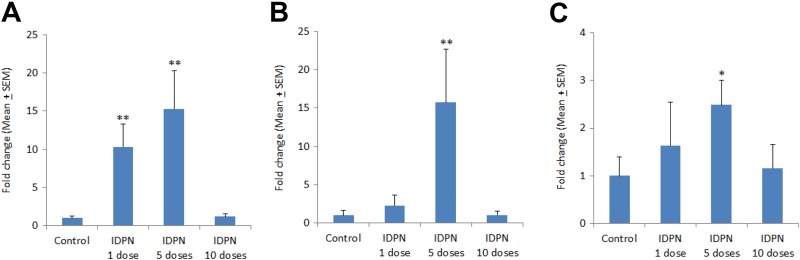
In kidneys, the expression of IL-1β was significantly increased after 1 injection (10.34-fold) and after 5 days daily injections (15.32-fold) as compared to control levels. There was no significant change in IL-1β expression in kidney of rats treated with daily injections of IDPN for 10 days (Figure 4A). The expression of IL-6 in kidney was neither affected by a single dose of IDPN nor 10 days dose regimen. However, IL-6 expression was significantly higher (15.72-fold) after 5 days of IDPN exposure, as compared to control kidney (Figure 4B). The expression of TNF-α was also significantly increased (2.49-fold) after 5 days IDPN treatment whereas the 1 day and 10 days produced no significant changes in TNF-α expression in kidney (Figure 4C).
Figure 4.
Effect of different treatments of IDPN on mRNA expression of (A) IL-1β, (B) IL-6, and (C) TNF-α in rat kidney. *P < .05 and **P < .01 versus control group using Dunnett test. IDPN indicates iminodipropionitrile; mRNA, messenger RNA.
Histopathology
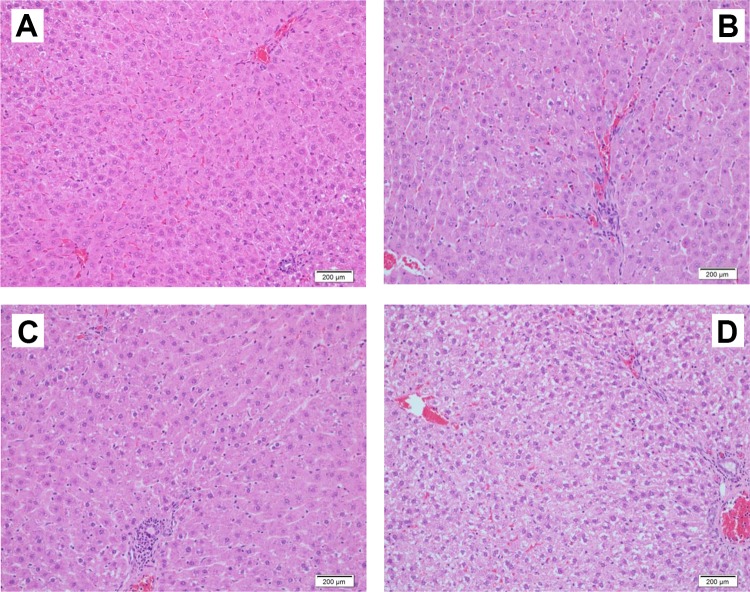
A single dose of IDPN did not show any appreciable change in rat liver histopathology except few mononuclear infiltrations in the portal tract (Figure 5). Administration of IDPN daily for 5 days caused infiltration of mononuclear inflammatory cells as well as mild microsteatosis in hepatocytes. Daily injections of IDPN for 10 days produced massive histopathological changes including infiltration of mononuclear inflammatory cells, microsteatosis, and degeneration of hepatocytes (Figure 5).
Figure 5.
Effect of different daily doses of IDPN on histopathological changes in rat liver. A, Control rat with normal liver cells, portal tract and veins. B, Iminodipropionitrile (1 dose), few mononuclear infiltrations in the portal tract. C, Iminodipropionitrile (5 doses), portal tract with infiltration of mononuclear inflammatory cells and a few hepatocytes with microsteatosis. D, Iminodipropionitrile (10 doses), portal tract with mononuclear inflammatory cell infiltrate, microsteatosis, and degeneration of hepatocytes (H&E staining, magnification 400×). IDPN indicates iminodipropionitrile.
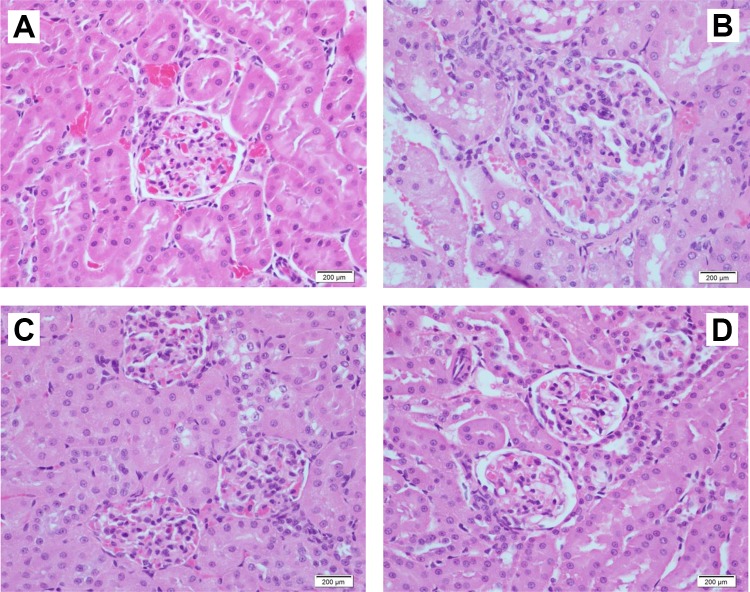
The histopathology of control rat kidney showed normal structures of glomeruli and renal tubules (Figure 6). The glomeruli of rats treated with a single dose of IDPN showed increased mesangial cells and a few neutrophil cells. Administration of IDPN for 5 days also showed increased mesangial cells in glomeruli, however 10 days treatment with IDPN did not show any apparent changes in glomeruli or renal tubules of rats (Figure 6).
Figure 6.
Effect of different daily doses of IDPN on histopathological changes in rat kidney. A, Control rat kidney with normal glomerulus and renal tubules. B, Iminodipropionitrile (1 dose), glomerulus with increased mesangial cells and a few neutrophil cells. C, Iminodipropionitrile (5 doses), glomeruli with increased mesangial cells. D, Iminodipropionitrile (10 doses), renal glomeruli and tubules with no apparent changes (H&E staining, magnification 400×). IDPN indicates iminodipropionitrile.
Discussion
The behavioral analysis showed that the onset of ECC syndrome appeared on day 5 in some rats whereas all the rats became dyskinetic with full-fledged ECC syndrome after 10 days of IDPN treatment. Previous studies have shown similar behavioral deficits in rats treated with either a single high (up to 1000 mg/kg) dose of IDPN27,44 or repeated daily low (100 mg/kg) doses of IDPN.23,28 Aged rats29 and male gender36 were more susceptible to IDPN-induced ECC syndrome. The behavioral effects observed IDPN-treated rats were identical to those observed in knock-out mice lacking vestibular function45 and in rodents with bilateral labyrinthectomy27 Several studies have suggested that IDPN and related nitriles produce behavioral abnormalities through their toxic effects by time- and dose-dependent degeneration of vestibular hair cells, leading to the development of ECC syndrome.16,27,29 Drugs that reduced behavioral abnormalities in IDPN treated rats also prevented vestibular damage in the same animals.22,23,25 On the other hand, drugs that aggravated IDPN-induced behavioral deficits also showed synergistic effects on vestibular toxicity.19,28,31
A single dose of IDPN caused significant increase in liver and kidney weights, which was not observed after subchronic exposure. It has been suggested that great care needs to be taken while evaluating the direct effect of chemicals on organ weight when rapid changes in body weight are also occurring.46 Since this study was conducted in adult rats, the increase in organ weights in 24 hours or 5 days cannot be linked to body growth but is indicative of a short-term edema after IDPN treatment that gradually recovered with time. Schneider et al47 have shown that different organs (brain, heart, liver, spleen) weight to body weight ratios are not influenced by IDPN treatment.
Although there was no significant change in serum AST and ALT after 1 day or 5 days treatments of IDPN, these enzymes were significantly increased after 10 days of IDPN exposure, indicating functional impairment of liver after chronic exposure of IDPN. The results of liver histopathology also confirmed structural damage in livers of these animals. The main pathological alterations indicating liver toxicity were distorted sinusoids, cytoplasmic vacuolization, infiltration of inflammatory cells, and necrosis. It has been observed earlier that the magnitude of liver injury was not associated with the severity of vestibular hair cell degeneration in rats39 and mice.40 The hepatic toxicity caused by IDPN may be attributed to the route of administration as the IP route directly delivers the drug to the close vicinity of liver. Intraperitoneally injected carbon tetrachloride (CCl4) produced liver toxicity as early as 2 hours, peaking at day 1 and then followed by a recovery phase after 3 days.48 The administration of styrene by IP route caused more severe hepatotoxicity than its exposure by inhalation in rats.49 Combined treatment of a hepato-toxicant (CCl4) with IDPN significantly increased the symptoms of IDPN-induced behavioral syndrome, indicating that liver transformation of IDPN to a toxic metabolite may not be required for IDPN-induced behavioral toxicity but rather be involved in the detoxification of IDPN.35,50
There was an acute phase increase in SCr on day 1 (single dose of IDPN) which was subsided after 5 and 10 days of daily IDPN treatment. The increase in SCr on day 1 could be attributed to significant increase in kidney weight in these animals, more likely due to transient edema. Light microscopic observation of kidney sections showed increased mesangial cells with few neutrophils on day 1 and day 5 following IDPN exposure; however, these histopathological changes were absent at 10 days postdosing. In a previous study, histopathological examination of kidney sections showed mild nephrotoxicity in some of the IDPN treaded mice, in the form of mild tubular dilatation and vacuolation in glomeruli.40 Blood urea nitrogen levels were gradually increased and became significantly higher on days 5 and 10, following IDPN exposure. Acute kidney injury is associated with the retention of creatinine, urea, and other metabolic waste products that are normally excreted by the kidney. There is only limited data available on the effect of IDPN on SCr and BUN levels. In a previous study, IDPN treatment did not show any alterations in SCr and BUN levels in rats.28 This discrepancy in results may be attributed to differences in dose regimens and animal strains used in these studies.
The results of real-time PCR analysis showed that exposure of IDPN significantly increased the messenger RNA (mRNA) expressions of IL-1β, IL-6, and TNF-α in rat liver after day 1, which were gradually normalized with time. However, the histopathological changes in liver were more prominent after 10 days of IDPN exposure. A comparative evaluation between real-time PCR and immunostaining showed that the former technique is more sensitive, that could partly be attributed to the amplification strategy used in PCR and partly to the differences in the half lives of cytokines mRNA and their respective proteins.42 The induction, production, stimulation, and inhibition of different cytokines are interlinked in a complex pathway that controls the host response to toxicants. A variety of agents induce the production of IL-1, which is further stimulated by TNF. Both IL-1 and TNF augment the production of other inflammatory mediators including IL-6, indicating that these cytokines work in concert for modulation of proinflammatory cascade. Although an acute phase induction of proinflammatory cytokines is a normal phenomenon of natural defense, it may also trigger excessive generation of potentially toxic ROS, leading to cellular injury.51
Kupffer cells, the resident macrophages present in the hepatic sinusoids, play a significant role in producing the systemic changes in host immune responses through the upregulation and release of proinflammatory cytokines and other mediators. Stephanie and Gao52 have found that IL-1β signaling is required for the development of liver inflammation and injury. Administration of sodium nitrite enhanced oxidative stress with subsequent increases in IL-1β (4-fold) and TNF-α (2-fold) in rat liver; however, the antioxidant and anti-inflammatory drug silymarin significantly ameliorated the impairment of hepatic function.53 Aouey et al54 have suggested that hepatic damages in rats after acute and subchronic exposure to lambda-cyhalothrin (a pyrethroid insecticide with cyano group) is likely due to increased oxidative stress and inflammation in liver. They observed that the gene expressions of IL-1β, IL-6, and TNF-α were significantly increased in the liver of exposed rats compared to controls. Another insecticide, endosulfan, significantly increased the proinflammatory mediators (IL-1β and TNF-α), AST, ALT, SCr, and BUN levels, whereas treatment with antioxidant and anti-inflammatory drug melatonin reversed all these biochemical alterations.55 The hepatic damage in rats by tetrachlorodibenzo-p-dioxin was associated with oxidative stress and increased levels serum IL-1β, and TNF-α, which were normalized by the anti-inflammatory drug, montelukast.56 Carbon tetrachloride-induced acute hepatic injury in rats was accompanied with increased production of inflammatory mediators including IL-1β and TNF-α mRNA expression. Sinapic acid, an anti-inflammatory compound, significantly reduced CCl4-induced abnormalities in liver histology, serum AST and ALT activities, and proinflammatory cytokines.57 Curcumin, an anti-inflammatory and antioxidant compound, significantly protected rats against CCl4-induced acute liver damage by reducing oxidative stress and inhibiting the production of proinflammatory cytokines including IL-1β, IL-6, and TNF-α.58
In kidneys, the mRNA expression of proinflammatory cytokines was significantly increased after day 5 of daily IDPN injections. The delayed immune response of kidneys about the expression of proinflammatory cytokines may be attributed to exposure route and pharmacokinetic profile of IDPN. Increased levels of proinflammatory cytokines gene expression resulted due to the increased number of macrophages infiltration that together with other intrarenal cells elevated the expression of these genes.59 Compared to a normal glomerulus, there is an increase in mesangial size and cellularity associated with inflammatory cells like neutrophils, dendritic cells, macrophages, and T cells in the glomeruli and periglomerular regions.60 The TNF-α mRNA expressed in the kidney tissue probably are produced by the macrophages that begin to accumulate in the kidney before renal injury.59 Methylmalonic acid-induced nephrotoxicity was associated with increased expression of IL-1β and TNF-α in rat kidney.61 Elevations in proinflammatory cytokines (TNF-α, IL-1β, IL-6) have also been demonstrated in humans with acute renal failure.62 Administration of carnitine significantly restored the oxidant/antioxidant balance, decreased SCr and BUN, and inhibited proinflammatory cytokines (IL-1β and TNF-α) and apoptosis in contrast media-induced nephrotoxicity in rats.63 Kayama et al64 have suggested that cadmium-induced IL-6 secretion in the kidney may act to support the regeneration of renal tubular epithelial cells that occurs in the course of cadmium nephrotoxicity. Moro et al65 have suggested that overexpression of proinflammatory cytokines occurring during the first week after ischemia-reperfusion injury may play a role in the adaptive process in the tissue and contribute to remodeling.
Conclusion
Repeated exposure of IDPN for 10 days produced significant structural and functional damages in rat liver whereas kidneys showed gradual recovery with time. Iminodipropionitrile significantly increased the mRNA expression of the proinflammatory cytokines in liver and kidneys of rats, as early as day 1 following the first dose. Unabated inflammation is known to trigger the generation of potentially toxic ROS that may lead to cellular damage. The findings of this study point toward the role of inflammatory mediators and oxidative stress in IDPN-induced toxicity in rats. This is probably the first study reporting the effect of IDPN on proinflammatory cytokines gene expression in liver and kidney. Although this study was conducted in rats, the findings could be of potential relevance to human health, particularly after the observation that IDPN not only causes movement disorder but also produces acute inflammation in vital organs. Based on the findings of this study, it is recommended that environmental or occupational exposure to synthetic nitriles should be carefully monitored and the subjects at risk be evaluated not only for neurological symptoms but also tested for systemic inflammation as well as the liver and renal function tests.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding the Research Group No. RGP-009.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research Group No. RGP-009 received funding from Deanship of Scientific Research at King Saud University.
ORCID iD: Haseeb A. Khan  https://orcid.org/0000-0001-6084-8589
https://orcid.org/0000-0001-6084-8589
References
- 1. Delay P, Pichot P, Thuillier J, Marquiset JP. Action de l’aminodipropionitrilesur le comportementmoteur de la souris blanche. CR Soc Biol. 1952;146:533–534. [PubMed] [Google Scholar]
- 2. McKay GF, Lalich JJ, Schilling ED, Strong FM. A crystalline “lathyrus factor” from Lathyrus odoratus. Arch Biochem Biophys. 1954;52(2):313–322. [DOI] [PubMed] [Google Scholar]
- 3. Gagnaire F, Marignac B, Bonnet P. Relative neurotoxicological properties of five unsaturated aliphatic nitriles in rats. J Appl Toxicol. 1998;18(1): 25–31. [DOI] [PubMed] [Google Scholar]
- 4. Tanii H, Hayashi M, Hashimoto K. Behavioral syndrome induced by allylnitrile, crotononitrile or 2-pentenenitrile in rats. Neuropharmacology. 1991;90:887–892. [DOI] [PubMed] [Google Scholar]
- 5. Boadas-Vaello P, Riera J, Llorens J. Behavioral and pathological effects in the rat define two groups of neurotoxic nitriles. Toxicol Sci. 2005;88(2):456–466. [DOI] [PubMed] [Google Scholar]
- 6. Khan HA, Alhomida AS, Arif IA. Neurovestibular toxicities of acrylonitrile and iminodipropionitrile in rats: a comparative evaluation of putative mechanisms and target sites. Toxicol Sci. 2009;109(1):124–131. [DOI] [PubMed] [Google Scholar]
- 7. Schulze GE, Boysen BG. A neurotoxicity screening battery for use in safety evaluation: effects of acrylamide and 3′,3′-iminodipropionitrile. Fundam Appl Toxicol. 1991;16(3):602–615. [DOI] [PubMed] [Google Scholar]
- 8. U.S. Environmental Protection Agency. Revised Neurotoxicity Testing Guidelines. Springfield, VA: NTIS (National Technical Information Services); 1991; (Publication No. PB 91-154617). [Google Scholar]
- 9. Klaassen CD, Casarett LJ, Doull J, eds. Casarett and Doull’s Toxicology. 5th ed New York, NY: McGraw-Hill; 1996:474–475. [Google Scholar]
- 10.NLM, TOXNET, Toxicology Data Network. Beta, beta'-iminodipropionitrile. National Institute of Health, Bethesda, MD, USA: https://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+5539. Accessed April 24, 2019. [Google Scholar]
- 11. Clark AW, Griffin JW, Price DL. The axonal pathology in chronic IDPN intoxication. J Neuropathol Exp Neurol. 1980;39(1):42–55. [DOI] [PubMed] [Google Scholar]
- 12. Llorens J. Toxic neurofilamentous axonopathies-accumulation of neurofilaments and axonal degeneration. J Intern Med. 2013;273(5):478–489. [DOI] [PubMed] [Google Scholar]
- 13. Khan HA, Alhomida AS, Arif IA. On the mechanism of nitriles toxicity. Toxicol Sci. 2009;110(1):246–248. [Google Scholar]
- 14. Tanii H, Hashimoto K. Studies on the mechanism of acute toxicity of nitriles in mice. Arch Toxicol. 1984;55(1):47–54. [DOI] [PubMed] [Google Scholar]
- 15. Cadet JL. Participation of 5-HT2 serotonin receptor in the IDPN-induced dyskinetic syndrome. Exp Neurol. 1988;99(3):589–593. [DOI] [PubMed] [Google Scholar]
- 16. Khan HA, Al Deeb S, Al Moutaery K, Tariq M. Metoclopramide attenuates iminodipropionitrile-induced oxidative stress and neurobehavioral toxicity in rats. Pharmacol Biochem Behav. 2004;79(3):555–561. [DOI] [PubMed] [Google Scholar]
- 17. Tariq M, Khan HA, Al Moutaery K, Al Deeb S. Protection by 2-deoxy-D-glucose against beta, beta’-iminodipropionitrile-induced neurobehavioral toxicity in mice. Exp Neurol. 1999;158(1):229–233. [DOI] [PubMed] [Google Scholar]
- 18. Wang X, Yan S, Wang A, Li Y, Zhang F. Gastrodin ameliorates memory deficits in 3,3’-iminodipropionitrile-induced rats: possible involvement of dopaminergic system. Neurochem Res. 2014;39(8):1458–1466. [DOI] [PubMed] [Google Scholar]
- 19. Tariq M, Khan HA, Rehana Z, Al Moutaery K, Al Deeb S. Proglumide, a cholecystokinin receptor antagonist, exacerbates beta, beta’-iminodipropionitrile-induced dyskinetic syndrome in rats. Neurotoxicol Teratol. 1998;20(5):571–579. [DOI] [PubMed] [Google Scholar]
- 20. Ogawa N, Haba K, Asanuma M, Mori A. Long lasting effect of ceruletide on dyskinesia and monoaminergic neuronal pathways in rats treated with iminodipropionitrile. Brain Res. 1991;556(2):271–279. [DOI] [PubMed] [Google Scholar]
- 21. Nomoto N. Inhibitory effect of free radical scavenger, MCI-186, in the increase of hydroxyl radical induced by iminodipropionitrile in rats. J Neurol Sci. 2004;219(1-2):41–44. [DOI] [PubMed] [Google Scholar]
- 22. Tariq M, Al Deeb S, Al Moutaery K, Khan HA. Cysteamine attenuates iminodipropionitrile (IDPN) induced dyskinesia in rats. Int J Neurosci. 1995;83(3-4):165–175. [DOI] [PubMed] [Google Scholar]
- 23. Tariq M, Khan HA, Al Moutaery K, Al Deeb S. Sodium benzoate attenuates iminodipropionitrile-induced behavioral syndrome in rats. Behav Pharmacol. 2004;15(8):585–588. [DOI] [PubMed] [Google Scholar]
- 24. Wakata N, Araki Y, Sugimoto H, Iguchi H, Kinoshita M. IDPN-induced monoamine and hydroxyl radical changes in the rat brain. Neurochem Res. 2000;25(3):401–404. [DOI] [PubMed] [Google Scholar]
- 25. Tariq M, Khan HA, Al Moutaery K, Al Deeb S. Attenuation of iminodipropionitrile induced behavioral syndrome by sodium salicylate in rats. Pharmacol Biochem Behav. 2002;73(3):647–654. [DOI] [PubMed] [Google Scholar]
- 26. Tariq M, Khan HA, Siddiquei MM, Al Moutaery K, Al Deeb S. Protective effect of hydrocortisone on iminodipropionitrile-induced neurotoxicity in rats. Basic Clin Pharmacol Toxicol. 2007;100(3):176–181. [DOI] [PubMed] [Google Scholar]
- 27. Llorens J, Demêmes D, Sans A. The behavioral syndrome caused by 3,3’-iminodipropionitrile and related nitriles in the rat is associated with degeneration of the vestibular sensory hair cells. Toxicol Appl Pharmacol. 1993;123(2):199–210. [DOI] [PubMed] [Google Scholar]
- 28. Al Deeb S, Al Moutaery K, Khan HA, Tariq M. Exacerbation of iminodipropionitrile-induced behavioral toxicity, oxidative stress, and vestibular hair cell degeneration by gentamicin in rats. Neurotoxicol Teratol. 2000;22(2):213–220. [DOI] [PubMed] [Google Scholar]
- 29. Khan HA, Al Deeb S, Al Moutaery K, Tariq M. Influence of age on iminodipropionitrile-induced vestibular and neurobehavioral toxicities in rats. Exp Toxicol Pathol. 2003;55(2-3):181–186. [DOI] [PubMed] [Google Scholar]
- 30. Seoane A, Demêmes D, Llorens J. Relationship between insult intensity and mode of hair cell loss in the vestibular system of rats exposed to 3,3’-iminodipropionitrile. J Comp Neurol. 2001;439(4):385–399. [DOI] [PubMed] [Google Scholar]
- 31. Tariq M, Khan HA, Al Deeb S, Al Moutaery K. Nitric oxide synthase inhibitor aminoguanidine potentiates iminodipropionitrile-induced neurotoxicity in rats. Neurosci Lett. 1999;276:49–52. [DOI] [PubMed] [Google Scholar]
- 32. Al Kadasah S, Al Mutairy A, Siddiquei M, et al. Pentoxifylline attenuates iminodipropionitrile-induced behavioral abnormalities in rats. Behav Pharmacol. 2009;20(4):356–360. [DOI] [PubMed] [Google Scholar]
- 33. Khan HA. N-nitro-L-arginine, a nitric oxide synthase inhibitor, aggravates iminodipropionitrile-induced neurobehavioral and vestibular toxicities in rats. Exp Toxicol Pathol. 2012;64(7-8):791–796. [DOI] [PubMed] [Google Scholar]
- 34. Genter MB, Llorens J, O’Callaghan JP, Peele DB, Morgan KT, Crofton KM. Olfactory toxicity of beta, beta’-iminodipropionitrile in the rat. J Pharmacol Exp Ther. 1992;263(3):1432–1439. [PubMed] [Google Scholar]
- 35. Llorens J, Crofton KM. Enhanced neurotoxicity of 3,3’-iminodipropionitrile following carbon tetrachloride pretreatment in the rat. Neurotoxicology. 1991;12(3):583–594. [PubMed] [Google Scholar]
- 36. Moser VC, Boyes WK. Prolonged neurobehavioral and visual effects of short-term exposure to 3,3’-iminodipropionitrile (IDPN) in rats. Fundam Appl Toxicol. 1993;21(3):277–290. [DOI] [PubMed] [Google Scholar]
- 37. Takahashi N, Tarumi W, Ishizuka B. Acute reproductive toxicity of 3,3’-iminodipropionitrile in female rats. Reprod Toxicol. 2012;33(1):27–34. [DOI] [PubMed] [Google Scholar]
- 38. Itahashi M, Abe H, Tanaka T, et al. Maternal exposure to 3,3’-iminodipropionitrile targets late-stage differentiation of hippocampal granule cell lineages to affect brain-derived neurotrophic factor signaling and interneuron subpopulations in rat offspring. J Appl Toxicol. 2015;35(8):884–894. [DOI] [PubMed] [Google Scholar]
- 39. Ibrahim KE, Khan HA, Omer FA. Histological insights iminodipropionitrile-induced toxicity in rats. Exp Toxicol Pathol. 2014;66(2-3):89–96. [DOI] [PubMed] [Google Scholar]
- 40. Khan HA, Ibrahim KE. Pattern of neurobehavioral and organ-specific toxicities of β, β’-iminodipropionitrile in mice. Arch Med Sci. 2015;11(5):1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khan HA, Abdelhalim MA, Alhomida AS, Al-Ayed MS. Effects of naked gold nanoparticles on proinflammatory cytokines mRNA expression in rat liver and kidney. Biomed Res Int. 2013;2013:590730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Khan HA, Ibrahim KE, Khan A, Alrokayan SH, Alhomida AS, Lee YK. Comparative evaluation of immunohistochemistry and real-time PCR for measuring proinflammatory cytokines gene expression in livers of rats treated with gold nanoparticles. Exp Toxicol Pathol. 2016;68(7):381–390. [DOI] [PubMed] [Google Scholar]
- 43. Ibrahim KE, Al-Mutary MG, Bakhiet AO, Khan HA. Histopathology of the liver, kidney, and spleen of mice exposed to gold nanoparticles. Molecules. 2018;23(8):pii: E1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nace CG, Genter MB, Sayre LM, Crofton KM. Effect of methimazole, an FMO substrate and competitive inhibitor, on the neurotoxicity of 3,3’-iminodipropionitrile in male rats. Fundam Appl Toxicol. 1997;37(2):131–140. [DOI] [PubMed] [Google Scholar]
- 45. Akil O, Lustig LR. Severe vestibular dysfunction and altered vestibular innervation in mice lacking prosaposin. Neurosci Res. 2012;72(4):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Uemitsu N, Nakayoshi H. Evaluation of liver weight changes following a single oral administration of carbon tetrachloride in rats. Toxicol Appl Pharmacol. 1984;75(1):1–7. [DOI] [PubMed] [Google Scholar]
- 47. Schneider G, Oepen H, Klapproth A. The effect of the neurolathyrogenic substance beta, beta’-iminodipropionitrile (IDPN) on some biological parameters in rats and mice. Gen Pharmacol. 1981;12(2):109–114. [DOI] [PubMed] [Google Scholar]
- 48. Janakat S, Al-Merie H. Optimization of the dose and route of injection, and characterisation of the time course of carbon tetrachloride-induced hepatotoxicity in the rat. J Pharmacol Toxicol Methods. 2002;48(1):41–44. [DOI] [PubMed] [Google Scholar]
- 49. De Piceis Polver P, Fenoglio C, Nano R, et al. Styrene hepatotoxicity in rats treated by inhalation or intraperitoneally: a structural investigation. Histol Histopathol. 2003;18(1):49–54. [DOI] [PubMed] [Google Scholar]
- 50. Denlinger RH, Anthony DC, Amarnath K, Amarnath V, Graham DG. Metabolism of beta, beta′-iminodipropionitrile and deuterium-substituted analogs: potential mechanisms of detoxification and activation. Toxicol Appl Pharmacol. 1994;124(1):59–66. [DOI] [PubMed] [Google Scholar]
- 51. Khan HA, Ibrahim KE, Khan A, Alrokayan SH, Alhomida AS. Immunostaining of proinflammatory cytokines in renal cortex and medulla of rats exposed to gold nanoparticles. Histol Histopathol. 2017;32(6):597–607. [DOI] [PubMed] [Google Scholar]
- 52. Stephanie M, Gao B. Therapeutic potential of interleukin 1 inhibitors in the treatment of alcoholic liver disease. Hepatology. 2013;57(5):2078–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sherif IO, Al-Gayyar MM. Antioxidant, anti-inflammatory and hepatoprotective effects of silymarin on hepatic dysfunction induced by sodium nitrite. Eur Cytokine Netw. 2013;24(3):114–121. [DOI] [PubMed] [Google Scholar]
- 54. Aouey B, Derbali M, Chtourou Y, Bouchard M, Khabir A, Fetoui H. Pyrethroid insecticide lambda-cyhalothrin and its metabolites induce liver injury through the activation of oxidative stress and proinflammatory gene expression in rats following acute and subchronic exposure. Environ Sci Pollut Res Int. 2017;24(6):5841–5856. [DOI] [PubMed] [Google Scholar]
- 55. Omurtag GZ, Tozan A, Sehirli AO, Sener G. Melatonin protects against endosulfan-induced oxidative tissue damage in rats. J Pineal Res. 2008;44(4):432–438. [DOI] [PubMed] [Google Scholar]
- 56. Bentli R, Ciftci O, Cetin A, Otlu A. Anti-inflammatory Montelukast prevents toxic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin: oxidative stress, histological alterations in liver, and serum cytokine levels. Toxicol Ind Health. 2016;32(5):769–776. [DOI] [PubMed] [Google Scholar]
- 57. Shin DS, Kim KW, Chung HY, Yoon S, Moon JO. Effect of sinapic acid against carbon tetrachloride-induced acute hepatic injury in rats. Arch Pharm Res. 2013;36(5):626–633. [DOI] [PubMed] [Google Scholar]
- 58. Reyes-Gordillo K, Segovia J, Shibayama M, Vergara P, Moreno MG, Muriel P. Curcumin protects against acute liver damage in the rat by inhibiting NF-kappaB, proinflammatory cytokines production and oxidative stress. Biochim Biophys Acta. 2007;1770(6):989–996. [DOI] [PubMed] [Google Scholar]
- 59. Boswell JM, Yui MA, Burt DW, Kelley VE. Increased tumor necrosis factor and IL-1 beta gene expression in the kidneys of mice with lupus nephritis. J Immunol. 1988;141(9):3050–3054. [PubMed] [Google Scholar]
- 60. Scindia YM, Deshmukh US, Bagavant H. Mesangial pathology in glomerular disease: targets for therapeutic intervention. Adv Drug Deliv Rev. 2010;62(14):1337–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Goyenechea E, Andrade F, de Las Heras J, et al. Expression of proinflammatory factors in renal cortex induced by methylmalonic acid. Ren Fail. 2012;34(7):885–891. [DOI] [PubMed] [Google Scholar]
- 62. Ramesh G, Reeves WB. Inflammatory cytokines in acute renal failure. Kidney Int Suppl. 2004;(91):56–61. [DOI] [PubMed] [Google Scholar]
- 63. Kunak CS, Ugan RA, Cadirci E, et al. Nephroprotective potential of carnitine against glycerol and contrast-induced kidney injury in rats through modulation of oxidative stress, proinflammatory cytokines, and apoptosis. Br J Radiol. 2016;89(1058):20140724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kayama F, Yoshida T, Elwell MR, Luster MI. Cadmium-induced renal damage and proinflammatory cytokines: possible role of IL-6 in tubular epithelial cell regeneration. Toxicol Appl Pharmacol. 1995;134(1):26–34. [DOI] [PubMed] [Google Scholar]
- 65. Moro C, Jouan MG, Rakotovao A, et al. Delayed expression of cytokines after reperfused myocardial infarction: possible trigger for cardiac dysfunction and ventricular remodeling. Am J Physiol Heart Circ Physiol. 2007;293(5):H3014–9. [DOI] [PubMed] [Google Scholar]