Abstract
We report on the application of radon therapy to relieve the suffering of 2 patients with autoimmune diseases, one with pemphigus with an old myocardial infarction and diabetes mellitus and the other with type I diabetes. We include a lengthy discussion of the biological mechanisms that we believe produced the observed benefits. During the 6 to 9 months of the treatments, the marker values decreased to the upper limit of their normal ranges and the symptoms of the diseases were alleviated. Disorders of Th1/Th2 balance are implicated in the onset of many diseases, including autoimmune diseases. Our decision to give radon (222Rn) therapy to these patients was based on the results of 2 similar case reports and our earlier mouse experiments, which indicated that low doses of radiation induce regulatory T cells. Regulatory T cells regulate the T helper 1 cell and the T helper 2 cell balance. There are more than 80 different autoimmune diseases that are treated with anti-inflammatory agents or immune-suppressing drugs because the exact causes of these diseases and the cures are unknown. These and other case reports indicate that proper radon therapy is an effective treatment. We urge physicians to consider radon as a standard therapy for refractory autoimmune diseases.
Keywords: radon therapy, autoimmune diseases, pemphigus, diabetes, regulatory T cells, Th1/Th2 balance
Introduction
Autoimmune disease is a disorder of the immune system. Normally, it can distinguish between foreign cells and cells of the body; however, more than 80 different diseases are caused when a failure in the immune system causes it to release antibody proteins that attack healthy cells in an organ or in the whole body.1
Helper T cells of the cluster of differentiation 4 positive (CD4+) cells are classified into the T helper 1 (Th1) cell and the T helper 2 (Th2) cell, depending on the difference of cytokines produced by each cell.2–4 Disorders of the Th1/Th2 balance are considered to be involved in the onset of many diseases, including autoimmune diseases.5–10 The pattern of cytokine production of Th1 and Th2 cells correlates with functional differences of both cells and regulates each response. T helper 1 cells mainly produce interferon-γ (IFN-γ) and interleukin-12 (IL-12) and induce cellular immunity typified by delayed-type hypersensitivity. On the other hand, Th2 cells mainly produce IL-4, IL-5, IL-6, IL-10, and IL-13 and assist in producing antibodies such as immunoglobulin E and immunoglobulin G1 by B-cell activation and by class switching, to induce humoral immunity. Cytokines of Th1 cells inhibit humoral immunity, while cytokines of Th2 cells suppress cellular immunity. Both Th1 and Th2 cells regulate each other and maintain balance by the cytokine network.
Regulatory T (Tregs) cells, 5% to 10% of the peripheral helper T cells, exert potent immunosuppressive action. They inhibit activation and proliferation of other T cells and prevent autoimmune diseases by suppressing cytokine production.11 It is believed that Treg reduction and functional impairment leads to the development of various immune diseases.12–15 Thus, attempts are actively being made to develop a method of treating immune diseases by focusing on Tregs.
T helper 17 has been found to be a helper T cell that elicits a strong immune response through IL-17 production. Even though both Treg and Th17 differentiate from naïve helper T cells (Th0) in the presence of transforming growth factor-β (TGF-β), the former differentiates from Th0 in the presence of IL-2 and the latter from Th0 in the presence of IL-6.16 These T-cell differentiations are important when studying autoimmune disease pathology.
We previously examined the effects of a small dose of γ-radiation on autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, experimental allergic encephalomyelitis (EAE), and so on using a mouse model of each. We discovered that these diseases are improved by low-dose γ-irradiation and that Tregs are involved in the mechanism.17–22 In these experiments, mice were given whole-body γ-radiation at 500 mGy, 5 times per week for 4 weeks. Recently, we reported the case of a patient with ulcerative colitis and another with rheumatoid arthritis, both autoimmune diseases.23,24 They were given low doses of ionizing radiation by radon therapy and showed remarkable recoveries. When the patients with pemphigus and type I diabetes arrived, we realized that treatments with low doses of ionizing would likely relieve the symptoms because these are considered to be autoimmune diseases.
Pemphigus is thought to be inclined toward the Th2 side.25–28 It is common in people older than 60 years, especially in those 70 to 90 years old. An autoantibody (antiepidermal–dermal junction antibody) is formed against the “basement membrane zone” (hemidesmosome constituent components, BP 230 and BP 180), which forms the boundary between the epidermis and the dermis in the skin. It binds to the autoantigen, present in the basement membrane of the epidermis. Adhesion between the epidermis and the dermis deteriorates, and blisters develop. The symptoms are red spots (erythema) accompanied by itching and water blisters and large abrasion-resistant blisters and erosions all over the body. Sometimes erosions are found in the oral cavity. It is not clear whether autoantibodies are formed, but a relationship with visceral disease is suggested. The treatment administered is mainly oral corticosteroid hormone (steroid) therapy.
Although type I diabetes is not necessarily a Th1-dominant autoimmune disease, it is very likely that a disturbance of the Th1/Th2 balance is involved, at least partly.5,29–31
Since we previously found that the Th1/Th2 balance was restored to the normal level in the splenocytes derived from dinitrofluorobenzene-ascaris immunized BALB/c mice that had been fed radon (222Rn) water,22 we recommended radon inhalation therapy to the 2 patients who were suffering with pemphigus and diabetes.
Methods to Deliver Radon Inhalation Therapy
Radon (222Rn) Room
As previously described,24,32 the radon therapy room had been designed to reproduce the conditions of a natural radon health spa, that is, a warm, moist atmosphere of low-level ionizing radiation, like those at the Bad Gastein Hot Springs in Austria. Supplied by Lead & Company, Yokohama, Japan, the radon room has walls that contain natural uranium ore. The average γ-radiation dose rate inside is 11 μGy/h, and the average concentration of radon in the air is 200 000 Bq/m3, as measured using a TRACERLAB Alpha-Scint-1 monitor.
α-Radiorespiro-Rn Generator
As previously described, this radon (222Rn) generator is made from very simple parts and housed in a small cabinet.32 A layer of high-grade uranium ore particles, about 4 mm in size, is covered by 2.5 L of water in a 16-L tank. The concentration of radon in the air above the water can be adjusted over the range from about 1 to 10 MBq/m3, as prescribed by the therapist. The patient inhales radon through the suction tube using a special respirator for the time specified.
Defining the Radiation Exposure From Radon Therapy
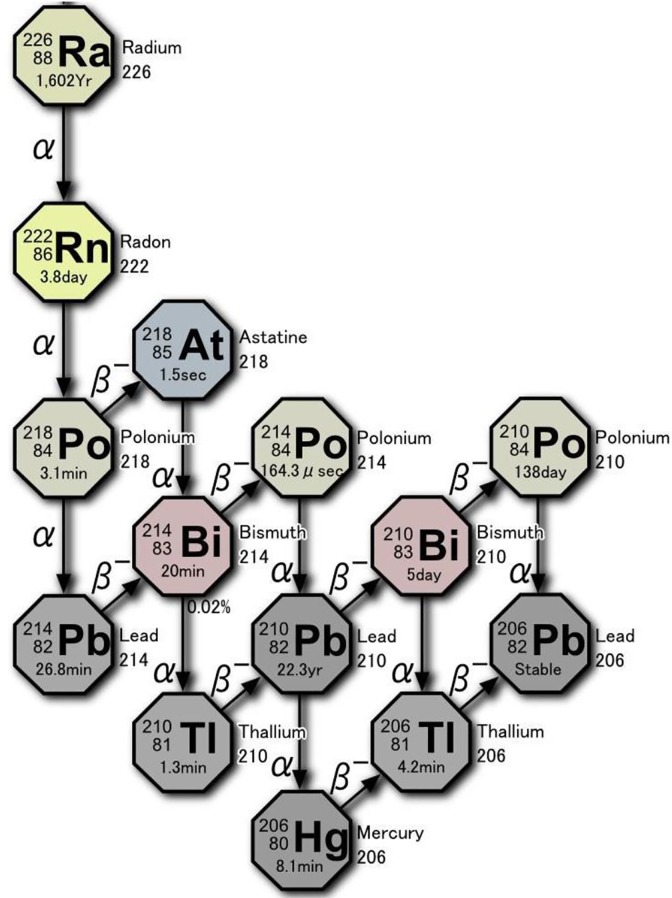
As shown in Figure 1, the radioactive decay of radium-226 (present in natural uranium ore) is the source of radon-222 gas. There are 4 α-particles (each with energy of about 5 MeV) that primarily contribute to the internal exposure to inhaled radon. Also released are 4 β-particles and their associated γ-rays. Most of inhaled radon is exhaled, but a small amount of gas and its decay products (progeny) adhere to the mucosa of the trachea and the lung surface. Some are taken up by alveolar epithelial cells and transferred into the bloodstream together with oxygen. After 2 weeks, the gas (3.8-day half-life) almost disappears. There is no reported evidence of adverse health effects from this therapy and no significant long-term accumulation of radionuclides in any specific tissue. The patient also receives an exposure due to the γ-rays emitted from the walls of the radon room.
Figure 1.
Radioactive decay chain of 222Rn.
The absorbed dose (Gy) from a treatment is complicated to calculate, and the mechanisms by which the different radiations produce health effects are very complex. There are direct hits on biomolecules (including DNA) in the lungs and throughout the body. Water molecules are ionized and various reactive oxygen species are formed, mainly hydroxyl radicals and hydrogen peroxide. These events send signals, which stimulate many of the body’s natural protection systems (>150 genes) to remedy the radiation-induced damage.
These systems, which normally cope with endogenous oxidative stress and the effects of toxins, injuries, diseases, and so on, begin to function much more intensively after an exposure to inhaled radon. This results in very important beneficial effects. In this article, we express the dose received as simply the radon concentration and duration of each treatment, recognizing that each patient inhales air at a different rate (L/min).
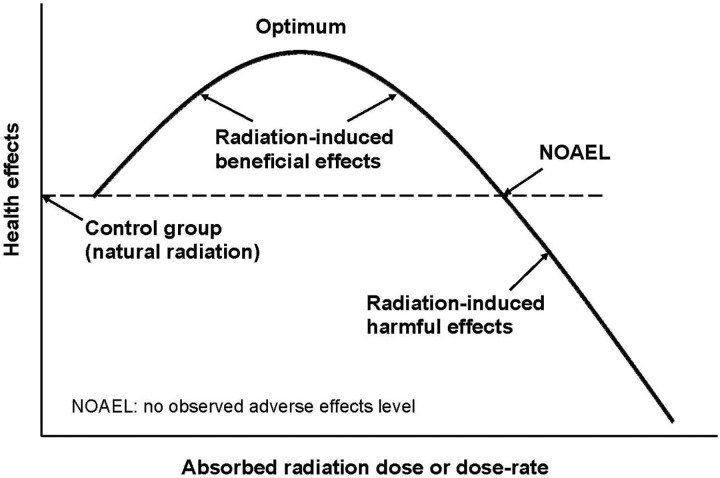
Generally, there are thresholds and optimum levels of radiation dose and dose rate, specific to each individual, for observing beneficial effects (Figure 2). The treatments are repeated, at a frequency and for a period of time, until lasting relief from the symptoms of the disease is achieved. The number of weeks of radon therapy necessary to reach a recovery will depend on the genetics of the patient, the disease, its severity, the radon dose (concentration and duration of a treatment), and the number of treatments per week.
Figure 2.
Hormetic dose–response model.
The extreme health scare about radiation-induced cancer that was started in the late 1950s has persisted for more than 60 years. The authorities that regulate the uses of ionizing radiation have been ignoring the many successful medical treatments to cure diseases with moderate doses of radiation.33 However, the recent evidence of a rather high-dose threshold for onset of radiation-induced leukemia in humans and a high-dose rate threshold for lifelong exposure of dogs to radiation suggests that this low-dose radon therapy does not present health risks.34,35
Case of Patient A With Pemphigus
Patient A is a 69-year-old female who was diagnosed on October 4, 2017 with “old myocardial infarction,” hypertrophied in 2 places. She came for treatment to an outpatient clinic about once every 2 months. Months later, bleeding erythema accompanied by itchiness appeared on her whole body. On March 29, 2018, she was diagnosed, additionally, as having “bullous pemphigoid.” She began taking 20 mg of prednisolone (Predonine) and applied the external preparation for adrenal cortex at the same time. However, there was no improvement in the symptoms of pemphigus. The itchiness, swelling, and bleeding that occurred throughout her body became severe. At the peak of the disease, she found it necessary to exchange the gauze coverings and her underwear frequently, while at work.
On April 11, she stopped taking Predonine on her own judgment and began to receive radon-room treatments, 0.2 MBq/m3 for 40 minutes. This was followed by inhalation of radon, 1 MBq/m3 for 1 hour, from the α-Radiorespiro-Rn generator. This continued for 2 months at a frequency of once or twice a week. From June 5 to July 31, 2018, she received radon therapy for just 1 hour each week from the α-Radiorespiro-Rn generator, installed in a radon room. By the end of July, the symptoms of pemphigus had largely subsided; however, some “bubble pemphigoid” itchiness remained (Figure 3). The treatments, once or twice a week, have continued from August 1 onward at the patient’s request.
Figure 3.
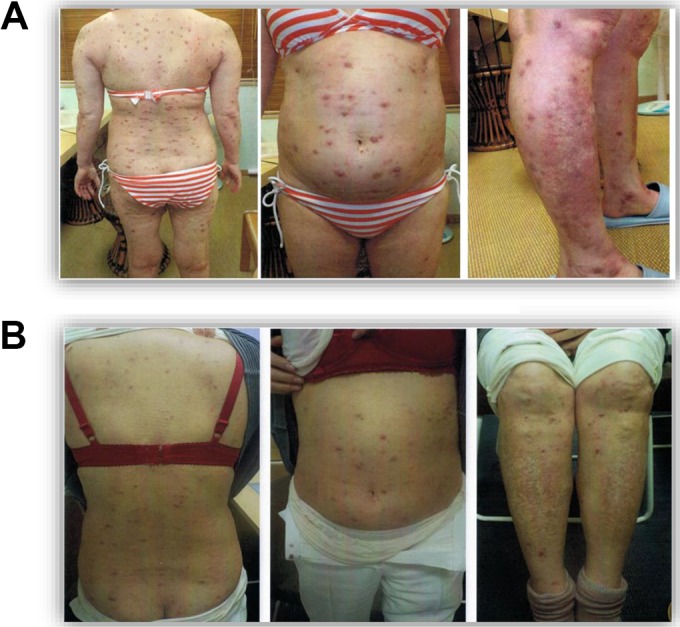
A, Patient A on March 27, 2018. B, Patient A on August 1, 2018, after radon therapy.
Regarding the old myocardial infarction, the patient’s condition improved with respect to the stent-untreated blood vessel. This myocardial infarction improvement was confirmed by computed tomography diagnostic imaging. She did not receive any treatment with myocardial infarction drugs during the radon treatment, and no side effects associated with radon therapy were seen.
On March 28, before radon therapy, lactate dehydrogenase, blood sugar, and glycated hemoglobin (HbA1c) were 342 IU/L, 196 mg/L, and 9.8%, respectively. The decreases to 223 IU/L, 126 mg/L, and 7.1%, respectively, were recorded on October 25, 2018 (Table 1).
Table 1.
Change of Marker During Radon Therapy for Patient With Pemphigus.
| Marker | Normal Valuea | March 28, 2018 | May 10, 2018 | June 28, 2018 | July 18, 2018 | September 6, 2018 | October 25, 2018 |
|---|---|---|---|---|---|---|---|
| LDH (IU/L) | 106-211 | 342 | 305 | 261 | 267 | 221 | 223 |
| Glucose (mg/L) | 60-110 | 196 | 159 | 100 | 169 | 124 | 126 |
| HbA1c (%) | 4.6-6.2 | 9.8 | 8.8 | 7.8 | – | 7.2 | 7.1 |
Abbreviations: LDH, lactate dehydrogenase; HbA1c, glycated hemoglobin.
aAs recommended by the Japanese medical community.
These health improvements have been confirmed regarding pemphigus and hyperglycemia in high-glucose pemphigus, which is an autoimmune disease. There is a recent finding that pemphigus may develop if the drug “Teneligliptin” (dipeptidyl peptidase-4 inhibitor) is taken for diabetes.36 However, this patient had no history of taking this medicine.
Case of Patient B With Diabetes
Patient B is a 70-year-old male who had mild fever since December 2017. On January 29, 2018, he visited a hospital in Tokyo for a detailed examination because of hyperglycemia. His HbA1c value was 11.2%. Soon after, he began to receive a daily intramuscular injection of a long-acting insulin drug for type I diabetes before going to bed.
Because no improvement was seen at all, he stopped taking the insulin drug. Radon-room treatments started from March 9, 2018. The radon concentration was 0.2 MBq/m3, and the exposure time was 40 minutes. He received a treatment twice daily, once a week, for the first 2 months and twice daily, 4 times a week, from May 2 onward. The value of HbA1c, which is one of the markers for type I diabetes, began to improve after August 6 and decreased to the upper limit of the normal range, as recorded on November 7, 2018 (Table 2).
Table 2.
Change of Marker During Radon Therapy for Patient B With Hyperglycemia.
| Marker | Normal Valuea | January 29, 2018 | March 14, 2018 | April 4, 2018 | August 6, 2018 | September 26, 2018 | October 17, 2018 | November 7, 2018 |
|---|---|---|---|---|---|---|---|---|
| HbA1c (%) | 4.6-6.2 | 11.2 | 8.4 | 7.3 | 6.5 | 6.3 | 6.3 | 6.2 |
Abbreviation: HbA1c, glycated hemoglobin.
aAs recommended by the Japanese medical community.
Discussion
We provided radon therapy to 2 patients, one with pemphigus and the other with diabetes, both of which occurred due to failure to maintain Th1/Th2 balance. Our decision to offer radon therapy to these patients was based on our experimental results that indicated low doses of γ-radiation induce Tregs, which regulate the Th1/Th2 balance, bringing it to normality. This effect was involved in the mechanism that produced health improvements in mice that modeled 3 types of autoimmune disease. These mice were exposed to γ-rays and α-particles.20–22 We also had the evidence from our radon therapy treatments of a patient with ulcerative colitis and a patient with rheumatoid arthritis.23,24
Patient A
Patient A suffers from pemphigus with old myocardial infarction and hyperglycemia. During the 5 months, from March 25 to July 31, 2018, she received a total 25 treatments of radon therapy. The myocardial infarction was alleviated with respect to the stent-untreated blood vessel, and the redness and edema disappeared.
On March 28, at the beginning of radon treatment, her blood glucose level and HbA1c value, which are markers of diabetes, were 196 mg/L and 9.8%, respectively. They decreased to 126 mg/L and 7.1%, respectively, as measured on October 25, 2018. This evidence confirms that a health benefit resulted from the radon treatments for the pemphigus patient, who also has diabetes.
The improvement induced in this patient by radon therapy can be understood from the fact that this pathological condition is due to the Th1/Th2 unbalance. So what is the mechanism that caused this recovery of this patient from myocardial infarction? Several reports have examined the effect of low-dose radiation on diabetic complications.37–39 One of the most serious complications is diabetic cardiomyopathy, characterized by cardiac remodeling that includes cardiac hypertrophy and prefrontal changes. It is related to cardiac dysfunction. Zhang and colleagues examined the effect of low-dose x-rays on cardiac dysfunction using the streptozotocin-induced type I diabetes mouse model. They showed that an x-ray dose of 25 to 50 mGy suppresses this diabetic cardiomyopathy. The mechanism that they suggested is suppression of diabetogenic apoptosis and oxidative stress via the Akt-mediated MDM2/P53 pathway and the Nrf2/keap1 pathway.37
Another research group compared the effect of repeated, combination whole-body low-dose x-ray (LDR) irradiation with local treatment of basic fibroblast growth factor-zinc (bFGF-Zn) with each individual treatment (LDR, bFGF, or zinc) in a type I diabetic rat model. A superior effect on wound healing in the LDR–bFGF–zinc combination group was observed in any of these independent treatment groups.38 As a mechanism of this effect, they found that repeated irradiations of 75 mGy X-rays (LDR) increased the proportion of CD31+/CD3+ stem cells in the bone marrow and circulation, and induced vascular regeneration, cell proliferation, and the expression of matrix metalloproteinase-2 (MMP-2). It is presumed that the expression of MMP-9 was promoted, and the skin wound healing in diabetes was significantly promoted.
Furthermore, a study was carried out on mice modeling type II diabetes mellitus that received whole-body γ-irradiation at low doses, 50 to 75 mGy. It indicated that LDR improves kidney abnormal hypertrophy, dysfunction, or pathological changes that accompany diabetes.39 Although there are many unresolved points about the mechanism, radon therapy is postulated to cause the suppression of insulin resistance and of subsequent lipotoxicity, inflammation, and further oxidative stress induced by lipid abnormalities. These mechanisms may be ways of understanding the improvement against myocardial infarction in patients with pemphigus.
Patient B
The treatment of patient B, who has diabetes mellitus, confirmed the improvement potential of radon therapy for type I diabetes. Starting on March 9, 2018, he received a radon-room treatment (0.2 MBq/m3 for 40 minutes) twice daily once a week for the first 2 months and twice daily 4 times a week from May 2 until August 6. His HbA1C value, which was 11.2% on January 29, decreased progressively to 6.2%, the upper limit of the normal range by November 7, 2018.
Other Discussion
The prevention of type I diabetes by IL-4, via Treg cells, in nonobese diabetic mice has been reported.40 The authors concluded that IL-4 treatment favors the expansion of Tregs in vivo and prevents the onset of insulitis and noninsulin-dependent diabetes mellitus, mediated by autoreactive Th1 cells. In addition, the activation of antioxidants in animal organs by radon inhalation has also been reported.41,42 Thus, it was expected that the in vivo antioxidant capacity will increase also in diabetic patients during this radon therapy.
Even though failure to maintain the balance of Th1/Th2 cells is very much implicated in the pathogenesis of immune diseases so far, this balanced disorder-induced disease model is under review, since the variety and the plasticity of the CD4 T cell became clear, as a result of recent research. Besides Th1 cells and Th2 cells that were identified in the 1980s, CD4 T cells have recently been divided into various subsets, such as Th17 cells, Th9 cells, follicular helper T cells, and Treg cells.7,43,44 Here, we will evaluate our results, focusing on the well-established subsets, such as Th1, Th2, Th17, and Tregs.
As mentioned above, involvement of Tregs has been reported on the pathophysiological improvement effect of low-dose γ-irradiation for various autoimmune diseases.19–21 Since pemphigus is a Th2-dominant autoimmune disease, which is different from Th1-dominant EAE, the mechanisms that restore Th1/Th2 balance to the normal state, induced by low-dose radiation, seem to be common. The details from induction of Tregs to normalization of Th1/Th2 by low-dose radiation, which we have elucidated so far in EAE, are described below.21 Experimental allergic encephalomyelitis is characterized by inflammation of the central nervous system, accompanied by destruction of myelin sheath and infiltration of inflammatory cells such as neutrophils and myelin constituent protein-specific CD4+ T cells into the central nervous system. These inflammatory immune cells produce cytokines (IFN-γ, TNF-α, IL-6, IL-17, etc) that induce inflammation and demyelination. The TNF-α has been shown to play an important role in the pathogenesis of inflammatory demyelinating diseases in the central nervous system in EAE.45 Interleukin-6 activates B lymphocytes and produces antibodies from plasma cells. Excess antibody production is involved in the EAE pathology. In addition, cytotoxic T cells activated by a Th1-dominant immune response also directly damage target cells. Furthermore, IL-17 produced from Th17 induces transcription of genes encoding inflammatory cytokines and chemokines. It is considered that IL-17 and IFN-γ mutually regulate the development of the cytokine producing cells during immune responses, and IL-17 plays a far more important role than IFN-γ on the development of EAE pathology.46
On the other hand, Treg strongly suppresses the autoimmune reaction and is involved in the control of the disease states.11,47 In our previous experiment, the EAE model mice were prepared by immunizing SJL/J mice (6 weeks old, female) with an emulsion prepared by suspending myelin basic protein in complete Freund’s adjuvant. The mice were given whole-body irradiation with γ-rays (0.5 Gy) once a week for 4 weeks. As a result, the incidence rate, pathological condition score, suppression of body weight loss, and delay in the progression of the disease state were observed in γ-ray irradiation group compared with the nonirradiation diseases group, suggesting improvement of the disease condition by irradiation.21 The inflammatory cytokines and CD8+ cytotoxic T cells play an important role in the pathogenesis. The increased level of the cytokine in the nonirradiated disease group was suppressed by irradiation. The content ratio of CD8+ cytotoxic T cells was lowered by the irradiation. The Th1/Th2 balance (IFN-γ/IL-4 ratio), which was Th1 dominant in the nonirradiated disease group, was normalized as the decreased amount of IFN-γ produced by the irradiation. The elevated level of autoantibody production in the nonirradiated group was significantly suppressed by the irradiation as well. This is thought to be due to a decrease in the production amount of IL-6, which is deeply involved in the differentiation from B cells to antibody-producing cells, whereas the production of IL-17 was significantly suppressed by irradiation.
Since the suppressive action of Tregs in excessive immune reaction has been reported in the pathological condition control of EAE, the content ratio of CD4+CD25+Foxp3+Treg in the spleen lymphocytes was assayed, resulting in a significant increase in the irradiated group. It is well established that Th17 and Treg differentiate from naïve CD4+T cells and that IL-6 suppresses differentiation into Tregs.16,48 Thus, IL-6 is one of the extremely important factors controlling the differentiation pathway from naïve CD4+T cells to Th17 cell and Treg. However, it is thought that these differentiated cells perform the opposite function: Th17 cells cause autoimmunity and inflammation, whereas Tregs inhibit these phenomena and maintain immunostatic homeostasis. Therefore, elucidating the mechanisms affecting Th17/Treg cell balance is important for a better understanding of autoimmunity and tolerance.49,50 In our previous study, IL-6 production was suppressed by γ-radiation and graded toward differentiation toward Treg, leading to decreasing Th17 cell. We had demonstrated that low-dose γ-radiation induces suppression of the inflammatory cytokine production, normalization of Th1/Th2 balance, reduction of cytotoxic T cells, and encephalitogenic autoantibody production, leading to the improvement of EAE pathology via Treg induction in EAE model mice. Besides Th1/Th2 balance, Th17/Treg cell balance may contribute to the improvement of EAE pathology.
To sum up, we believe that Tregs in the pemphigus and diabetic patients were upregulated by the radon therapy, eliminating the Th1/Th2 balance disorder. Moreover, these results suggest that radon therapy may be effective, not only for Th1 dominant autoimmune diseases including rheumatism, but also for Th2-dominant autoimmune diseases. Detailed studies on these mechanisms in the clinic would be difficult to perform; however, this subject is very important and urgent.
Because of the radiation health scare that was introduced in the late 1950s and sustained for the past 60 years, we recognize that many radiobiologists and medical practitioners will be very skeptical about the efficacy of radon therapy for remediating autoimmune diseases. More research will be required to understand and confirm the biological mechanisms responsible for the observed benefits.
Conclusions
Radiotherapy has been employed mainly to treat cancer by destroying tumors with high doses of targeted radiation. In this article, we provided case reports of 2 patients with pemphigus and type I diabetes who benefited significantly from radiotherapy that employed low doses of nontargeted α-radiation and its associated β- and γ-radiations, delivered by the inhalation of radon. We also discussed biological processes that we believe shed light on the mechanisms through which low doses of ionizing radiation affect these 2 incurable autoimmune diseases and others, such as rheumatism. Radon therapy, properly delivered, has been shown to induce very important remedies that provide relief from severe suffering and the possibility of return to normal living. No adverse “side effects” have been observed in any of our patients.
Optimum protocols of radon therapy for different individuals and different diseases remain to be determined. The radon room employed to treat these patients was designed to simulate the conditions of a typical radon spa. Our radon generator has been designed to provide a much higher concentration of radon in air (1-10 MBq/m3), which can be adjusted for treating various types of cancer. We recently discovered that a concentration of about 6 MBq/m3 was required in order to treat of a patient with advanced hepatocellular cancer.51
The duration of a treatment is usually 40 minutes up to 1 hour, for patient convenience. The frequency ranges from once daily to 1 per week. The therapy may extend from several months to more than a year, until a satisfactory outcome is achieved. It is important to record the values of 1 or more disease markers during the course of the therapy to assess whether or not the treatments are effective and to predict when they should end.
Autoimmune diseases are currently treated symptomatically, mainly with anti-inflammatory agents, because there is no other accepted treatment that provides lasting relief and effective cures. Since Tregs can be induced by low doses of external or internal irradiation, this kind of treatment is expected to be effective, not only for Th1-dominant autoimmune diseases including rheumatism, but also for Th2-dominant autoimmune diseases.
Although more research will be required to understand the biological mechanisms responsible for the observed benefits, radon therapy should be evaluated urgently by medical practitioners, in view of the very important remedies it appears to provide. We are not aware of any evidence of harmful side effects, and therefore, we recommend that it be approved expeditiously for treatment of refractory autoimmune diseases.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Shuji Kojima  https://orcid.org/0000-0001-6944-9250
https://orcid.org/0000-0001-6944-9250
References
- 1. US National Library of Medicine. Autoimmune disorders. MedlinePlus. January 28, 2019. https://medlineplus.gov/ency/article/000816.htm.
- 2. Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Ann Rev Immunol. 1989;7:145–173. [DOI] [PubMed] [Google Scholar]
- 3. Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2(12):933–944. [DOI] [PubMed] [Google Scholar]
- 4. Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Christen U, von Herrath MG. Manipulating the type 1 vs type 2 balance in type 1 diabetes. Immunol Res. 2004;30(3):309–325. [DOI] [PubMed] [Google Scholar]
- 6. Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. [DOI] [PubMed] [Google Scholar]
- 7. Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–190. [DOI] [PubMed] [Google Scholar]
- 8. Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. [DOI] [PubMed] [Google Scholar]
- 9. Umetsu DT, DeKruyff RH. The regulation of allergy and asthma. Immunol Rev. 2006;212:238–255. [DOI] [PubMed] [Google Scholar]
- 10. Hirahara K, Yamashita M, Iwamura C, et al. Repressor of GATA regulates TH2-driven allergic airway inflammation and airway hyperresponsiveness. J Allergy Clin Immunol. 2008;122(3):512–520.e11. [DOI] [PubMed] [Google Scholar]
- 11. Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockage. J Exp Med. 2001;193(11):1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. [DOI] [PubMed] [Google Scholar]
- 13. Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune diseases. Immunol Rev. 2006;212:203–216. [DOI] [PubMed] [Google Scholar]
- 14. Dominguez-Villar M, Hafler DA. Regulatory T cells in autoimmune disease. Nat Immunol. 2018;19(7):665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Belge K, Brück J, Ghorechi K. Advances in treating psoriasis. F1000Prime Rep. 2014;6:4 doi:10.12703/P6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng SG. Regulatory T cells vs Th17: differentiation of Th17 versus Treg, are the mutually exclusive? Am J Clin Exp Immunol. 2013;2(1):94–106. [PMC free article] [PubMed] [Google Scholar]
- 17. Tanaka T, Tago F, Fang SP, Shimura N, Kojima S. Repeated 0.5 Gy gamma-ray irradiation attenuates autoimmune manifestation in MRL-lpr/lpr mice. Int J Radait Biol. 2005;81(10):731–740. [DOI] [PubMed] [Google Scholar]
- 18. Tago F, Tsukimoto M, Nakatsukasa H, Kojima S. Repeated 0.5-Gy gamma irradiation attenuates autoimmune disease in MRL-lpr/lpr mice with suppression of CD3+CD4−CD8−B220+ T-cell proliferation and with up-regulation of CD4+CD25+Foxp3+ regulatory T cells. Radiat Res. 2008;169(1):59–66. [DOI] [PubMed] [Google Scholar]
- 19. Nakatsukasa H, Tsukimoto M, Ohshima Y, Tago F, Masada A, Kojima S. Suppressing effect of low-dose gamma-ray irradiation on collagen-induced arthritis. J Radiat Res. 2008;49(4):381–389. [DOI] [PubMed] [Google Scholar]
- 20. Nakatsukasa H, Tsukimoto M, Tokunaga A, Kojima S. Repeated gamma irradiation attenuates collagen-induced arthritis via up-regulation of regulatory T cells but not by damaging lymphocytes directly. Radiat Res. 2010;174(3):313–324. [DOI] [PubMed] [Google Scholar]
- 21. Tsukimoto M, Nakatsukasa K, Sugawara K, Yamashita Y, Kojima S. Repeated 0.5-Gy gamma irradiation attenuates experimental autoimmune encephalomyelitis with up-regulation of regulatory T cells and suppression of IL-17 production. Radiat Res. 2008;170(4):429–436. [DOI] [PubMed] [Google Scholar]
- 22. Takahashi M, Kojima S. Suppression of atopic dermatitis and tumor metastatsis in mice by small amounts of radon. Radiat Res. 2006;165(3):337–342. [DOI] [PubMed] [Google Scholar]
- 23. Kojima S, Tsukimoto M, Shimura N, Koga H, Murata A, Takara T. Treatment of cancer and inflammation with low-dose ionizing radiation: three case reports. Dose-Response. 2017;15(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kojima S, Thukimoto M, Cuttler JM, et al. Recovery from rheumatoid arthritis following 15 months of therapy with low doses of ionizing radiation; a case report. Dose-Response. 2018;16(3):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takahashi H, Kuwana M, Amagai M. A single helper T-cell clone is sufficient to commit polyclonal naive B-cells to produce pathogenic IgG in experimental pemphigus vulgaris. J Immunol. 2000;182(3):1740–1745. [DOI] [PubMed] [Google Scholar]
- 26. Amagai M, Tsunoda K, Suzuki H, Nishifuji K, Koyasu S, Nishikawa T. Use of autoantigen knockout mice in developing an active autoimmune disease model for pemphigus. J Clin Invest. 2000;105(5):625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ujiie H, Shibaki A, Shinkuma S, Moriuchi R, Qiao H, Shimizu H. Noncollagenous 16A domain of type XVII collagen-reactive CD4+T cells play a pivotal role in the development of active disease in experimental bullous pemphigoid model. Clin Immunol. 2012;142(2):167–175. [DOI] [PubMed] [Google Scholar]
- 28. Lee SH, Hong WJ, Kim SC. Analysis of serum cytokine profile in pemphigus. Ann Dermatol. 2017;29(4):438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Azar S, Tamim H, Beyhum HN, Habbal Z, Almawi WY. Type I (insulin-dependent) diabetes is a Th1- and Th2-mediated autoimmune. Clin Diagn Lab Immunol. 1999;6(3):306–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rabinovitch A. Immunoregulatory and cytokine imbalances in the pathogenesis of IDDM. Therapeutic intervention by immunostimulation? Diabetes. 1994;43(5):613–621. [DOI] [PubMed] [Google Scholar]
- 31. Huaang X, Yuang J, Golddard A, et al. Interferon expression in the pancreases of patients with type 1 diabetes. Diabetes.1995;44(6):658–664. [DOI] [PubMed] [Google Scholar]
- 32. Kojima S, Cuttler JM, Shimura N, Koga H, Murata A, Kawashima A. Present and future prospects of radiation therapy using α-emitting nuclides. Dose-Response. 2018;16(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Calabrese EJ, Dhawan G, Kapoor R, Kozumbo WJ. Radiotherapy treatment of human inflammatory diseases and conditions: optimal dose. Hum Exper Toxicol. 2019;38(5):1–11. [DOI] [PubMed] [Google Scholar]
- 34. Cuttler JM. Evidence of dose threshold for radiation-induced leukemia: absorbed dose and uncertainty. Dose-Response. 2019;17(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cuttler JM, Feinendegen LE, Socol Y. Evidence of a dose-rate threshold for life span reduction of dogs exposed lifelong to γ-radiation. Dose-Response. 2018;16(4):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aouidad I, Fite C, Marinho E, Deschamps L, Crickx B, Descamps V. A case report of bullous pemphigoid induced by dipeptidyl peptidase-4 inhibitors. JAMA Dermatol. 2013;149(2):243–524. [DOI] [PubMed] [Google Scholar]
- 37. Zhang F, Lin X, Yu L, et al. Low-dose radiation prevents type 1 diabetes-induced cardiomyopathy via activation ofAKT mediated anti-apoptotic and anti-oxidant effects. J Cell Mol Med. 2016;20(7):1352–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang H, Cheng J, Lv Y, et al. Repeated whole-body exposure to low-dose radiation combined with topical application of basic fibroblast growth factor and zinc accelerates wound healing in diabetic rats. Dose-Response 2018;16(3). doi:10.1177/1559325818789845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shao M, Lu X, Cong W, et al. Multiple low-dose radiation prevents type II diabetes-induced renal damage through attenuation of dyslipidemia and insulin resistance and subsequent renal inflammation and oxidative stress. PLoS One. 2014;9(3):e92574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cameron MJ, Arreaza GA, Zucker P, Chensue SW, Strieter RM, Chakrabarti S, Delovitch TL. IL-4 prevents insulitis and insulin-dependent diabetes mellitus in nonobese diabetes mice by potentiation of regulatory T helper T cell function. J Immunol. 1997;159(10):4686–4692. [PubMed] [Google Scholar]
- 41. Ma J, Yonehara H, Ikebuchi M, Aoyama T. Effect of radon exposure on superoxide dismutase (SOD) activity in rats. J Radit Res. 1996;37(1):12–19. [DOI] [PubMed] [Google Scholar]
- 42. Kataoka T. Study of antioxidative effects and ant-inflammatory effects in mice due to low-dose X-irradiation or radon inhalation. J Radit Res. 2013;54(4):587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Steinman L. A brief history of Th17, the first major revision in the Th1/Th2 hypothesis of T cell-mediated tissue damage. Nature Med. 2008;13(2):139–145. [DOI] [PubMed] [Google Scholar]
- 44. Hirahara K, Nakayama T. CD4+T-cell subsets in inflammatory diseases: beyond the Th1/Th2 paradigm. Int Immunol. 2016;28(4):163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Villarroya H, Marie Y, Ouallet JC, Le Saux F, Tehelingerian JL, Baumann N. Expression of TNF alpha in central neurons of Lewis rat spinal cord after EAE induction. J Neurosci Res. 1997;49(5):529–599. [DOI] [PubMed] [Google Scholar]
- 46. Komiyama Y, Nakae S, Matsuki T, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177(1):566–573. [DOI] [PubMed] [Google Scholar]
- 47. Mimran A, Cohen IR. Regulatory T cells in autoimmune diseases: anti-ergotypic T cells. Int Rev Immunol. 2005;24:159–179. [DOI] [PubMed] [Google Scholar]
- 48. Gaffen SL, Hajishengallis G. A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 Cells and IL-17. J Dent Res. 2008;87(9):817–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. [DOI] [PubMed] [Google Scholar]
- 50. Lee GR. The balance of Th17 versus Treg cells in autoimmunity. Int J Mol Sci. 2018;19(3):730–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kojima S, Cuttler JM, Inoguchi K, et al. Radon therapy is very promising as a primary or an adjuvant treatment for different types of cancers: 4 case reports. Dose-Response. 2019;17(2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]