Abstract
Background:
Chronic plantar ulcers are common problems for leprosy patients with numb feet due to their prolonged healing time. Chronic plantar ulcers affect the quality of life of patients and can lead to more serious complications, such as disability and deformity, if not handled appropriately. Wound-care products in the market, however, give unsatisfactory results. One factor in the delayed healing of chronic plantar ulcers due to leprosy is the lack of growth factors and cytokines in the wound due to reduced blood supply. We speculated that application of human amniotic membrane stem cell (hAMSC) secretome, which contains growth factors and cytokines, could improve wound healing.
Aim:
To evaluate the effect of topical application of a hAMSC secretome gel on wound healing of chronic plantar ulcers due to leprosy.
Materials and Methods:
We recruited 11 patients after leprosy treatment with chronic plantar ulcers due to leprosy. hAMSC secretome gel was applied topically to ulcers every 3 days for up to 2 months. Ulcer size and possible side effects or complications from gel application were evaluated weekly.
Results:
The ulcers of 8 of 11 patients (72.7%) completely healed, the ulcers of 2 patients (18.2%) partially healed, and the ulcers of 1 patient (9.1%) persisted. No ulcers became worse.
Conclusion:
hAMSC secretome was found to be an efficacious and well-tolerated alternative therapy for chronic plantar ulcers due to leprosy.
KEY WORDS: Human amniotic membrane stem cell secretome, leprosy chronic plantar ulcers, wound healing
Introduction
Plantar ulcers are a serious complication of leprosy and are categorized by the World Health Organization as a grade 2 disability, that is, “visible deformity or damage present.”[1] Plantar ulcers are a serious health problem because healing is usually slow, often leading to chronic ulceration. Chronic ulcers result from repeated trauma due to sensory impairment, muscular paralysis, autonomic nerve impairment, callus formation, and superinfection.[2,3] Slow healing of chronic ulcers is likely exacerbated by the lack of growth factors and blood supply in the wound due to autonomic nerve impairment.[4,5] Wound healing is a dynamic process that requires coordination between cells, growth factors, cytokines, and the extracellular matrix.[6,7] Wound care products in the market and daily practice regarding wound management seem to give unsatisfactory results because they do not supply growth factors and cytokines.
Stem cell therapy has recently gained substantial interest. In stem cell therapy, stem cells grow into the injured tissue and differentiate into specialized cells, secreting various growth factors and cytokines; these secreted factors are referred to as the stem cell secretome. In vitro, stem cell secretomes can be harvested from used stem cell growth medium. Stem cell secretomes alone are now being applied in areas of regenerative medicine including chronic wound therapy.[8,9]
Human amniotic membrane stem cells (hAMSCs) have high proliferative and immunomodulatory activities. Both fresh and freeze-dried human amniotic membrane have been effectively used to assist wound healing, likely driven by the regenerative growth factors and cytokines produced by hAMSC. These growth factors and cytokines are also found in the hAMSC secretome, suggesting that the secretome alone could be used as a treatment modality for wound healing.[10,11,12]
Here, we describe a work to evaluate the contribution of application of a hAMSC secretome gel for healing of leprosy chronic plantar ulcer wounds.
Materials and Methods
Study design and participants
Our descriptive study monitored the effect of a 2-month hAMSC secretome gel treatment course on 11 subjects in the Dermato-Venereology outpatient clinic of Dr. Soetomo General Hospital, Surabaya between November 2015 and April 2016. The study was approved by the Ethical Committee Board of Dr. Soetomo General Hospital Surabaya, and informed consent was obtained from all subjects.
Eleven subjects with plantar ulcers due to leprosy after leprosy treatment were recruited based on the following criteria:
Inclusion criteria
Patients who had a chronic plantar ulcer resulting from leprosy for at least 6 weeks
Ulcer no larger than 9 cm2 and no deeper than 0.5 cm
Ulcer which lacked complications; with no surrounding erythema, no swelling, did not cause acute pain, the surrounding skin was not warm upon examination by palpation, ulcer which lacked wound sinuses, and no tendons or bones were exposed
Patients who were in otherwise good general health
Patients willing to sign their informed consent.
Exclusion criteria
Patients who had used a systemic corticosteroid in the previous 2 weeks.
Patient with a history of hemophilia, blood clotting disorders, or antiplatelet consumption.
Patient with a history of hypersensitivity to adhesive bandage.
Preparation of hAMSC secretome gel
The Stem Cell Laboratory at the Institute of Tropical Disease, Airlangga University, Indonesia, provided hAMSC secretome samples. Donor procedures and stem cells were handled according to the international standard for human stem cell culture.
Because the hAMSC secretome was obtained in liquid form, effective application to a wound required that we first made it viscous. We made a hAMSC secretome gel by adding alginate acid sodium from brown algae (Phaeophyceae), choosing alginate because of its hypoallergenicity. To make a hAMSC secretome gel of appropriate viscosity, 0.08 g of alginate acid sodium was added per milliliter of secretome medium.[13] The resulting hAMSC secretome gel was stored refrigerated for up to 1 month, avoiding exposure to direct sunlight and temperatures exceeding 37°C.
Study procedures
At the beginning of the study, all ulcers underwent surgical debridement, had their baseline widths and depths recorded, and were photographed. Secretome gel was applied to ulcers every 3 days for up to 2 months. After gel application, ulcers were covered with transparent film dressing followed by an adhesive bandage. Ulcers were evaluated every week: the width and depth of the ulcer were measured, direct or indirect side effects or complications due to gel application assessed, and photographs taken of the ulcer.
We anticipated allergic contact dermatitis resulting in ulcer deterioration as a possible complication of our study. It was decided that in case such events were to occur, gel application would be stopped and complications managed. To preemptively minimize complication risk, study participants were asked to avoid long periods of standing or walking and were not given tasks involving carrying or unloading heavy weights.
Results
We recruited more male subjects than female subjects, with a mean age of 49.64 ± 8.31 years. Subjects on average had ulcers for 2.22 ± 1.83 years, and the average width of ulcers at the start of the study was 1.64 ± 0.84 cm2. All subjects were present for the entire duration of the study. Patient demographics and ulcer characteristics at the start of the study are listed in Table 1.
Table 1.
Patient demographics and ulcer baseline characteristics
| Variable | Subjects (n=11), n (%) |
|---|---|
| Sex | |
| Male | 5 (45.5) |
| Female | 6 (54.5) |
| Age (years) | |
| 25-50 | 6 (54.5) |
| >50 | 5 (45.5) |
| Duration of ulcer (years) | |
| <1 | 3 (27.3) |
| 1-5 | 7 (63.6) |
| >5 | 1 (9.1) |
| Width of ulcers at baseline (cm2) | |
| <1 | 1 (9.1) |
| 1-4 | 10 (90.9) |
| Location of plantar ulcers | |
| Forefoot | 10 (90.9) |
| Midfoot | 1 (9.1) |
| Body weight of subjects (kg) | |
| 50-60 | 4 (36.4) |
| 61-70 | 6 (54.5) |
| >70 | 1 (9.1) |
At the end of the study, the ulcers of eight patients (72.7%) had completely healed, those of two patients (18.2%) had improved, and those of one patient persisted; no ulcers got worse. The percentage of healing, measured by the percentage size reduction of ulcers, increased every week [Figure 1]. The average reduction of the width and depth of ulcers at the end of the study was 78.64% ± 39.63% and 86.82% ± 30.19%, respectively. Over the course of the study, the mean reduction in the width of ulcers was 1.45 ± 0.84 cm2, and the mean reduction in the depth of ulcers was 0.35 ± 0.16 cm.
Figure 1.
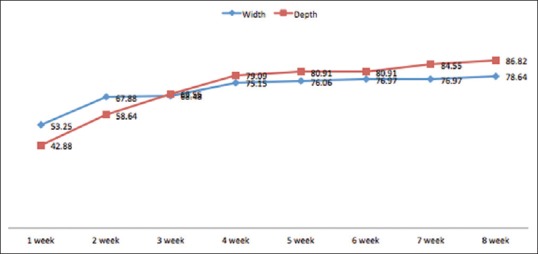
The mean percentage of wound healing every week
The characteristics of participants and the degree of ulcer healing are tabulated in Table 2. None of the participants experienced adverse effects or complications from the study. The progression of ulcers before and after treatment is shown in Figure 2.
Table 2.
Characteristics of participants whose ulcer completely healed, partially healed, and persisted
| Variable | Completely healed (n=8) | Partially healed (n=2) | Persisted (n=1) |
|---|---|---|---|
| Sex | |||
| Male | 3 | 2 | 0 |
| Female | 5 | 0 | 1 |
| Age (years) | |||
| 21-50 | 4 | 1 | 1 |
| >50 | 4 | 1 | 0 |
| Duration of ulcer (years) | |||
| <1 | 3 | 0 | 0 |
| 1-5 | 5 | 1 | 1 |
| >5 | 0 | 1 | 0 |
| Width of ulcers at baseline (cm2) | |||
| <1 | 1 | 0 | 0 |
| 1-4 | 7 | 2 | 1 |
| Location of the ulcers | |||
| Forefoot | 7 | 2 | 1 |
| Midfoot | 1 | 0 | 0 |
| Body weight of participants (kg) | |||
| 50-60 | 4 | 0 | 0 |
| 61-70 | 3 | 2 | 1 |
| >70 | 1 | 0 | 0 |
Figure 2.
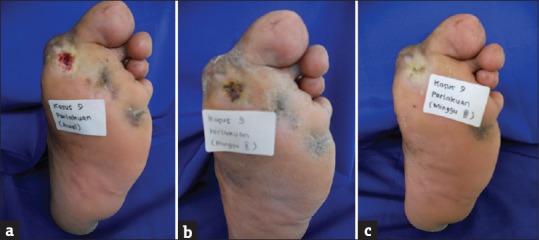
Figure 2: (a) Before treatment. (b) Second week after treatment. (c) Ulcer completely healed on third week after treatment
Discussion
Management of chronic plantar ulcers due to leprosy is challenging because ulcers take a long time to heal, the treatment affects patients and their families socioeconomically, and it is frustrating to patients. Many kinds of wound management have been developed to tackle these challenges, including the application of stem cell secretome as described in this study.
Our results showed that hAMSC secretome gel improved healing rates of chronic plantar ulcers. In the majority of subjects (72.7%), ulcers completely healed; in 18.2% subjects, ulcers partially healed; and in one subject, ulcer persisted. In no case did ulcers worsen. Additionally, none of the subjects experienced side effects or complications.
There are many causes of chronic ulcers, including prolonged inflammatory conditions, elevated protease activity, and decreased growth factor concentrations. We note that hAMSC secretome gel contains various paracrine factors, including growth factors and cytokines. Application of this secretome gel may function by improving the conditions of the wound microenvironment and thus encouraging healing. Cytokines in the hAMSC secretome gel will act on the inflammatory phase of wound healing proinflammatory cytokines will treat any infection in the wound, while antiinflammatory cytokines will reduce the inflammation process. Simultaneously, growth factors in the hAMSC secretome gel will contribute to the proliferation phase of wound healing by inducing angiogenesis and stimulating cell proliferation for the epithelialization process of wound healing.[9,14,15,16]
To remove calluses and necrotic tissues, surgical debridement was performed on all patients before application of the gel. Removing calluses and necrotic tissues is important for management of wound healing because they could be a source of infection that might inhibit the healing process. Calluses also exert continuous pressure on the ulcer and inhibit epithelialization during wound healing.[3]
In conclusion, hAMSC secretome gel was beneficial to treatment of chronic plantar ulcers due to leprosy. Additional controlled prospective clinical trials, however, will be necessary to definitively demonstrate its efficacy.
Financial support and sponsorship
Stem Cell Research and Development Center of Universitas Airlangga and Faculty of Medicine Universitas Airlangga.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors thank all the patients who participated in this study.
References
- 1.Brandsma JW, Van Brakel WH. WHO disability grading: operational definitions. Lepr Rev. 2003;74:366–73. [PubMed] [Google Scholar]
- 2.Sehgal VN, Prasad PVS, Kaviarasan PK, Rajan D. Trophic skin ulceration in leprosy: evaluation of the efficacy of topical phenytoin sodium zinc oxide paste. Int J Dermatol. 2014;53:873–8. doi: 10.1111/ijd.12457. [DOI] [PubMed] [Google Scholar]
- 3.Puri V, Venkateshwaran N, Khare N. Trophic ulcers – Practical management guidelines. Indian J Plast Surg. 2012;45:340–51. doi: 10.4103/0970-0358.101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braund R, Hook S, Medlicott J. The role of topical growth factors in chronic wounds. Curr Drug Deliv. 2007;4:195–204. doi: 10.2174/156720107781023857. [DOI] [PubMed] [Google Scholar]
- 5.Suryanarayan S, Budamakuntia L, Khadri SIS, Sarvajnamurthy S. Efficacy of autologous platelet-rich plasma in the treatment of chronic nonhealing leg ulcers. Plast Aesthet Res. 2014;1:65–9. [Google Scholar]
- 6.Lee DE, Ayoub N, Agrawal DK. Mesenchymal stem cells and cutaneous wound healing: Novel methods to increase cell delivery and therapeutic efficacy. Stem Cells Res Ther. 2016;7:1–8. doi: 10.1186/s13287-016-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menandez YM, Alvarez MV, Ferrero AG, Perez MB, Perez SL, Escudero D, et al. Adult stem cell therapy in chronic wound healing. J Stem Cell Res Ther. 2014;4:1–6. [Google Scholar]
- 8.Pawitan JA. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int. 2014;2014:1–14. doi: 10.1155/2014/965849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: Toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18:1–24. doi: 10.3390/ijms18091852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kagan S, Sood A, Granick MS. Amniotic membrane adjuncts and clinical applications in wound healing: A review of the literature. Wounds. 2018;30:168–73. [PubMed] [Google Scholar]
- 11.Murri MS, Moshirfar M, Birdsong OC, Ronquillo YC, Ding Y, Hoopes PC. Amniotic membrane extract and eye drops: A review of literature and clinical application. Clin Ophthalmol. 2018;12:1105–12. doi: 10.2147/OPTH.S165553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharya N, Sengupta P, Bhattacharyaa A. Application of freshly collected amniotic membrane and amniotic fluid in arthritis and wound healing. Madridge J AID. 2018;1:38–41. [Google Scholar]
- 13.Aderibigbe BA, Buyana B. Alginate in wound dressings. Pharmaceutics. 2018;10:1–19. doi: 10.3390/pharmaceutics10020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nuschke A. Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis. 2014;10:29–37. doi: 10.4161/org.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JW, Hwang SR, Yoon I. Advanced growth factor delivery system in wound management and skin regeneration. Molecules. 2017;22:1–20. doi: 10.3390/molecules22081259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anandan V, Jameela WA, Saraswathy P, Sarankumar S. Platelet rich plasma: Efficacy in treating trophic ulcers in leprosy. J Clin Diagn Res. 2016;10:1–8. doi: 10.7860/JCDR/2016/21899.8758. [DOI] [PMC free article] [PubMed] [Google Scholar]


