Abstract
Background:
Copper and zinc are important trace elements involved in the development of psoriasis. However, reports regarding changes in serum copper and zinc levels in patients with psoriasis have been inconsistent.
Aims:
This meta-analysis was designed to analyze changes in serum copper and zinc levels between patients with psoriasis and a healthy population.
Materials and Methods:
English and Chinese literature from international and national electronic databases from 1988 to May 2016 was analyzed. Studies that performed a comparative analysis of serum copper and zinc levels between patients with psoriasis and healthy controls were included in the meta-analysis. The random-effects model was used to calculate the overall combined estimates of serum copper and zinc levels between patients with psoriasis and healthy individuals. Results: Fifteen references were included in this study, including 1324 patients with psoriasis and 1324 healthy controls. Compared with healthy controls, serum copper levels were significantly increased (Z = 4.02, P < 0.0001; standardized mean difference [SMD], 1.23; 95% confidence interval [CI], 0.63 to 1.82), and serum zinc levels were significantly decreased (Z = 2.95, P < 0.0001; SMD, −1.35; 95% CI, −2.25 to − 0.45) in patients with psoriasis.
Conclusions:
In conclusion, increased serum copper and decreased serum zinc levels were generally observed in patients with psoriasis. Treatments to normalize the serum copper and zinc levels may improve the outcome of psoriasis patients.
KEY WORDS: Meta-analysis, psoriasis, serum copper, serum zinc
Introduction
Psoriasis is a common chronic inflammatory skin disease and is primarily characterized by localized or generalized scaly erythematous plaques. The rapid proliferation of epidermal keratinocytes in the basal layer is the primary pathological characteristic of psoriasis.[1] Psoriasis is a disease of the immune system. Not only it can affect the skin and joints, but it is also associated with metabolic syndrome, obesity, cardiovascular disease, and mental diseases.[2,3,4] Furthermore, psoriasis can result in bodily harm to the patient as well as impacts patients’ appearance, social activities, and quality of life.
The worldwide prevalence of psoriasis varies in different countries, ranging from 0.6% to 4.8%.[5] Europe and the United States have higher prevalence at approximately 1%–3%, whereas Asia has the lowest prevalence at approximately 0.3%. Psoriasis can develop at any age, but incidence peaks between 15 and 30 years of age. There is no significant difference in psoriasis incidence between men and women.[5] Individuals with a family history of psoriasis are more likely to develop psoriasis.[6]
The pathogenesis of psoriasis is not fully understood. At present, psoriasis is considered as an immune-mediated, polygenic, and genetic disease. A number of environmental factors can trigger or worsen psoriasis such as infection, drugs, stress, trauma, obesity, alcohol consumption, and smoking.[7]
Copper (Cu) and zinc (Zn) are essential trace elements. The balance of trace elements plays an important role in maintaining overall health. Numerous studies have shown that abnormal serum Cu and Zn levels are important mechanisms underlying the occurrence and development of skin diseases. Cu and Zn deficiencies reduce the activity of enzymes related to melanin synthesis, thereby causing a reduction in melanin production and aggravating white spots or patches of vitiligo.[8] Cu and Zn abnormalities are also involved in the pathophysiology of psoriasis.[9] Serum Cu binds to α2 globulin and promotes the formation of ceruloplasmin protein, which is involved in the clearance of excessive free radicals in patients with psoriasis.[10] Zn serves as a coenzyme for DNA and RNA polymerases and plays an important role in excessive keratinocyte proliferation in psoriatic lesional skin.[11] Studies have reported that serum Cu levels were increased in patients with psoriasis,[12,13,14] whereas serum Zn levels were decreased.[5,15] However, these results have been inconsistent.[16,17,18] Given the inconsistency of the existing literature and the insufficient statistical power of primary studies, we conducted a meta-analysis to investigate changes in serum Cu and Zn levels in patients with psoriasis.
Materials and Methods
Data sources
We searched PubMed, Cochrane Central, OVID, SpringerLink, Clinical Evidence, Google scholar, the Chinese National Knowledge Infrastructure, Wanfang Med Online, Chinese Biology Medical Literature Database, and VIP Database for articles published between 1988 and May 2016 in Chinese or English. The search words included psoriasis or skin disease, trace elements, copper, and zinc. References of the included studies were retrieved to identify additional relevant articles.
Inclusion criteria
Studies were included in the meta-analysis if they met the following criteria: studies reported data on patients with psoriasis; studies used a case-control or cohort design; serum Cu or Zn levels were detected; and the sample size, mean, and standard deviation were presented in the results. If there were duplicates or overlapping publications, the first study was included.
Data extraction
Two investigators independently extracted relevant data from the included studies, including the first author name, publication year, country, sample size, and serum Cu or Zn level means and SD values. Any discrepancies in data extraction between the two investigators were resolved by consensus with a third investigator or by re-evaluation of the data from the studies.
Statistical analysis
Data were analyzed with Review Manager 5.3 software (Cochrane Collaboration, Oxford, England). The standardized mean difference (SMD) and 95% confidence intervals (CI) were calculated based on the fixed-effects models or random-effects model. The inconsistency index (I2) test was also calculated to measure the inconsistency across studies. When I2 <50%, a fixed-effect model was used to calculate the combined effect value. When I2 >50%, a random-effects model was applied. The significance of combined SMD was assessed by Z test, and P < 0.05 was considered statistically significant. The Begg and Egger tests were used to evaluate the possible existence of publication bias, with P > 0.05 indicative of no publication bias. Influence analysis was used to analyze the impact of the signal study on the overall results of meta-analysis. The Begg test, Egger test, and influence analysis were performed using Stata version 12.0 software. When the mean serum Cu or Zn levels and SD values were not calculated in the included study, but the outcomes in several subgroups were analyzed, the means and SD values across several subgroups were combined and included in the final analysis.
Results
Results of literature retrieval
According to the retrieval strategy, 3874 references were primarily screened, and 15 studies that met the inclusion criteria were ultimately included. A flow chart of the screening is displayed in Figure 1. The characteristics of the included studies are indicated in Table 1.
Figure 1.
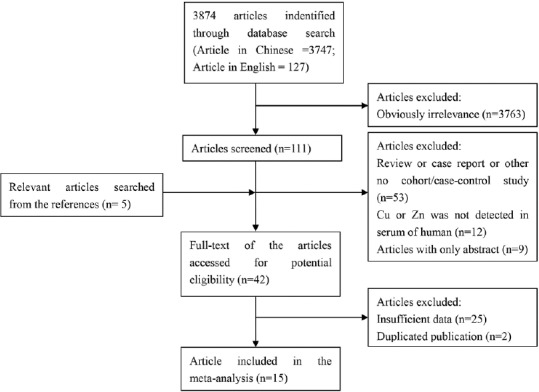
Flow chart of study selection in the meta-analysis
Table 1.
The characteristics of included studies in the meta-analysis
| First author | Year | Location | Total Cases (n) | Psoriasis Group (n) | Healthy Group (n) | Detected in serum | ||
|---|---|---|---|---|---|---|---|---|
| Cu | Zn | Both | ||||||
| Sheikh G[5] | 2015 | India | 200 | 100 | 100 | √ | ||
| Ala S[14] | 2013 | Iran | 50 | 25 | 25 | √ | ||
| Afridi HI[15] | 2011 | Pakistan | 659 | 418 | 241 | √ | ||
| Rashmi R[19] | 2010 | India | 127 | 82 | 45 | √ | ||
| Basavaraj KH[9] | 2009 | India | 75 | 50 | 25 | √ | ||
| Lei TB[20] | 2008 | China | 150 | 120 | 30 | √ | ||
| Cao JQ[21] | 2005 | China | 114 | 84 | 30 | √ | ||
| Nigam PK[12] | 2005 | India | 80 | 60 | 20 | √ | ||
| Wang GZ[11] | 2004 | China | 98 | 52 | 46 | √ | ||
| Yan ZD[17] | 2004 | China | 270 | 120 | 150 | √ | ||
| Ding ZY[18] | 2003 | China | 64 | 32 | 32 | √ | ||
| Halevy S[16] | 2001 | Israel | 36 | 23 | 13 | √ | ||
| Tasaki M[13] | 1993 | Japan | 76 | 28 | 48 | √ | ||
| Yang WL[10] | 1993 | China | 90 | 70 | 20 | √ | ||
| Doğan P[22] | 1989 | Turkey | 93 | 60 | 33 | √ | ||
Symbol of “√” means the Cu or Zn was studied in the serum
Changes in serum Cu levels in patients with psoriasis
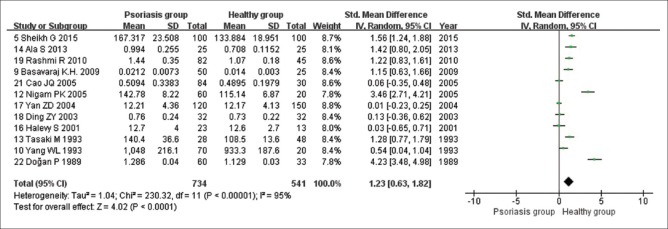
Twelve studies analyzed the changes in serum Cu levels in patients with psoriasis. Of these, seven studies reported that serum Cu levels were significantly increased in patients with psoriasis. In contrast, five studies reported that there was no statistically significant difference in serum Cu levels between patients with psoriasis and healthy controls. The heterogeneity test results indicated high heterogeneity of the included studies (I2 = 95%; Tau2 = 1.04; Chi-square = 230.32; df = 11; P < 0.00001). Thus, we used a random-effects model for pooled analysis of the results. According to the pooled analysis, we determined that compared with those of healthy controls, serum Cu levels were significantly increased in patients with psoriasis (Z = 4.02, P < 0.0001; SMD, 1.23; 95% CI, 0.63 to 1.82; Figure 2).
Figure 2.
Random-effects meta-analysis of studies that examined serum Cu levels
Changes in serum Zn levels in patients with psoriasis
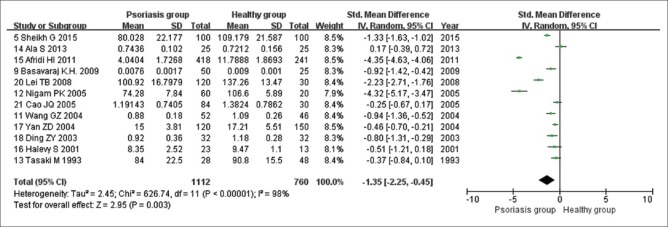
Twelve studies analyzed changes in serum Zn levels in patients with psoriasis. Among these, eight studies concluded that serum Zn levels were significantly decreased in patients with psoriasis compared with those of healthy controls, whereas four references identified no statistically significant differences. Heterogeneity test results indicated that the heterogeneity of the included references was relatively high (I2 = 98%; Tau2 = 2.45; Chi-square = 626.74; df = 11; P < 0.00001). Therefore, a random-effects model was selected for pooled estimate. Pooled analysis revealed that serum Zn levels were significantly lower in patients with psoriasis than those in healthy controls (Z = 2.95, P < 0.0001; SMD, −1.35; 95% CI, −2.25 to -0.45; Figure 3).
Figure 3.
Random-effects meta-analysis of studies that examined serum Zn levels
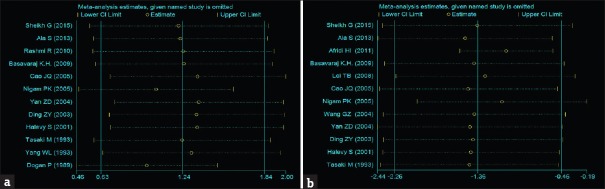
Influence analysis
The influence analysis results are displayed in Figure 4. After rejecting the studies indicated with strikethrough lines, the combined effect value of the remaining studies was calculated. The influence analysis indicated that no single study produced obvious effects on the total combined effect value.
Figure 4.
Influence analysis of serum Cu (a) and serum Zn (b) levels from 12 articles
Publication bias
The possible existence of publication bias was assessed using the Begg and Egger tests. The results indicated that there was no obvious publication bias regarding the pooled analysis of serum Cu and Zn levels in patients with psoriasis (Begg P = 0.304, Egger P = 0.258; Begg P = 0.732, Egger P = 0.738, respectively).
Discussion
Zn is one of the most important trace elements and plays an important role in protein synthesis, DNA and RNA repair, biofilm stability, enzyme activity regulation, and free radical scavenging activity.[8,14] Studies have determined that Zn impacts psoriasis by regulating keratinization in epithelium, immune function, and enzyme functions.[23] However, the relationship between serum Zn levels and psoriasis remains controversial. Butnaru et al.[24] reported that serum Zn levels were increased in patients with psoriasis. Ala et al.,[14] Cao et al.,[21] Halevy et al.,[16] and Tasaki et al.[13] concluded that no significant changes in serum Zn levels existed in patients with psoriasis compared with those of healthy controls. However, other studies determined that serum Zn levels were lower in patients with psoriasis.[5,9,11,12,15,17,18,20]
In this meta-analysis, we included 12 references that analyzed changes in serum Zn levels in patients with psoriasis. We determined that compared with healthy controls, serum Zn levels were significantly lower in patients with psoriasis. The mean value for serum Zn levels in patients with psoriasis and the correlation with gender, severity, duration, and disease stage are displayed in Table 2. There were no differences in serum Zn levels between male and female patients with psoriasis.[9] As psoriasis worsened, serum Zn levels gradually decreased,[9,15] indicating that levels may be closely associated with psoriasis outcome. Yan et al.[17] demonstrated that serum Zn levels were negatively correlated with psoriasis duration, but Cao et al.[21] and Lei et al.[20] reached opposite conclusions.
Table 2.
Serum levels of Zn in different types of psoriasis
| Study | Gender | Severity | Stage | Disease period | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Mild | Moderate | Severe | More severe | Progressing | Quiescent | Regression | ||
| 14 | 0.73±0.16 | 0.71±0.15 | ||||||||
| 15 | 4.32±1.70 | 4.04±1.73 | 5.8±1.67 | 4.42±0.97 | 3.54±0.82 | 2.24±0.51 | ||||
| 9 | 0.008±0.002 | 0.007±0.001 | ||||||||
| 20 | 116.91±16.66 | 97.31±9.17 | 88.54±7.93 | |||||||
| 21 | 1.35±0.82 | 1.03±0.60 | 0.98±0.57 | |||||||
| 11 | 0.84±0.19 | 0.97±0.15 | ||||||||
| 17 | Y=11.5750.342X | |||||||||
Serum levels of Zn is presented in the form of mean ± SD
The mechanism underlying the reduction in Zn levels in patients with psoriasis is not fully understood. Zn is an essential component of DNA and RNA polymerases. The excessive keratinocyte proliferation associated with psoriasis consumes large amounts of Zn, which may lead to a reduction in serum Zn levels.[5] In patients with severe psoriasis, skin lesions become more serious, which makes keratinocytes even more exfoliate. Thus, the low serum Zn levels were more obvious in patients with severe psoriasis than those in patients with mild psoriasis. With prolonged disease duration, Zn consumption is increased. Therefore, serum Zn levels are negatively correlated with disease duration.[20] However, the results of studies investigating the relationship between Zn levels and the different stages of psoriasis were inconsistent. In addition, only three references were included, which was insufficient to perform a pooled analysis of changing serum Zn level trends. Additional studies are required to clarify this relationship.
Zn is an essential component of antioxidant-related enzymes, such as superoxide dismutase (SOD), and plays an important role in free radical scavenging and lipid peroxidation.[8] In addition, Zn is an essential element for maintenance of T cells and other immune cells and functions and plays a role in T cell differentiation, maturation, activation, and signal transduction.[11] Low serum Zn levels lead to decreased antioxidant-related enzyme activities and cellular immune dysfunction. Patients with low serum Zn levels are more susceptible to viruses and bacterial infections, which can cause abnormal changes in the skin and trigger psoriasis. Therefore, oral Zn supplementation may be an adjuvant therapy for psoriasis. However, one study reported that oral Zn supplementation was not effective in reducing the psoriasis area and severity index.[25]
This meta-analysis demonstrated that, in contrast to the low serum Zn levels, serum Cu levels were increased in patients with psoriasis. Cu, another trace element and an important component of enzymes and other functional proteins, has critical biological functions.[26] With the rapid proliferation of epidermal cells, it is reasonable to expect that Cu consumption should be increased and serum Cu levels should be decreased. However, consistent with previous studies,[5,9,12,13,14,22] we observed that serum Cu levels were higher in patients with psoriasis than in healthy controls. Furthermore, serum Cu levels were further elevated in patients with psoriasis that was more serious, more active, and with a longer duration [Table 3]. Serum Cu is primarily bound to ceruloplasmin in serum.[5,14] Ceruloplasmin is a multifunctional enzyme that maintains Cu homeostasis. It was recently determined that ceruloplasmin is an acute inflammatory reaction protein that has the ability to scavenge free radicals, thereby removing free radicals released into the extracellular matrix and protecting cell lipid membranes from free radical damage.[10,27] Psoriasis is a chronic inflammatory skin disease with a relatively higher level of oxidative stress. As a result, ceruloplasmin activity increased.[5] Serum Cu levels are positively correlated with ceruloplasmin levels.[10] Thus, the high serum Cu levels observed in patients with psoriasis might be caused by the increased ceruloplasmin levels.
Table 3.
Serum levels of Cu in different types of psoriasis
| Study | Gender | Severity | Stage | Disease duration | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Mild | Moderate | Severe | Progressing | Quiescent | Regressing | <5 year | 5-10 year | >10 year | |
| 14 | 0.73±0.15 | 0.68±0.01 | |||||||||
| 19 | 1.24±0.22 | 1.48±0.40 | 1.51±0.3 | ||||||||
| 9 | 0.022±0.008 | 0.02±0.006 | |||||||||
| 21 | 0.60±0.41 | 0.40±0.19 | 0.42±0.18 | ||||||||
| 10 | 1097.6±228.7 | 992.4±189.3 | 986.8±213.3 | 1057.8±244.9 | 1082.9±240.2 | ||||||
| 17 | Y=1.508+0.403X | ||||||||||
Serum levels of Cu is presented in the form of mean ± SD
A publication bias analysis was performed on studies that reported Cu and Zn serum levels. We demonstrated that there was no significant publication bias. However, heterogeneity test results indicated greater homogeneity among the included studies. Therefore, we performed an influence analysis and determined that the exclusion of any single article did not affect the overall results, indicating that the results were reliable.
We first assessed changes in serum Cu and Zn using evidence-based medicine. We determined that serum Zn levels were reduced, and serum Cu levels were elevated in patients with psoriasis. However, the role of serum Cu and Zn in the pathogenesis and development of psoriasis and the exact mechanism underlying this phenomenon remains unclear and requires further investigation. Furthermore, the therapeutic value of Zn supplementation needs to be confirmed in clinical studies.
Financial support and sponsorship
This work was supported by Grant No.81430075 and No.81830096 from the key project of the National Science Foundation and supported Grant No.81572679 and 81773341 by National Natural Science Foundation of China.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Deng Y, Chang C, Lu Q. The inflammatory response in psoriasis: A comprehensive review. Clin Rev Allergy Immunol. 2016;50:377–89. doi: 10.1007/s12016-016-8535-x. [DOI] [PubMed] [Google Scholar]
- 2.Brito-Luna MJ, Villanueva-Quintero DG, Sandoval-Talamantes AK, Fafutis-Morris M, Graciano-Machuca O, Sanchez-Hernandez PE, et al. Correlation of IL-12, IL-22, and IL-23 in patients with psoriasis and metabolic syndrome. Preliminary report. Cytokine. 2016;85:130–6. doi: 10.1016/j.cyto.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 3.Ergun T, Seckin Gencosmanoglu D, Karakoc-Aydiner E, Salman A, Tekin B, Bulbul-Baskan E, et al. Prevalence of obesity in paediatric psoriasis and its impact on disease severity and progression. Australas J Dermatol. 2017;58:e182–7. doi: 10.1111/ajd.12491. [DOI] [PubMed] [Google Scholar]
- 4.Ku SH, Kwon WJ, Cho EB, Park EJ, Kim KH, Kim KJ. The Association between psoriasis area and severity index and cardiovascular risk factor in Korean psoriasis patients. Ann Dermatol. 2016;28:360–3. doi: 10.5021/ad.2016.28.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheikh G, Masood Q, Majeed S, Hassan I. Comparison of levels of serum copper, zinc, albumin, globulin and alkaline phosphatase in psoriatic patients and controls: A hospital based case-control study. Indian Dermatol Online J. 2015;6:81–3. doi: 10.4103/2229-5178.153006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burden-Teh E, Thomas KS, Ratib S, Grindlay D, Adaji E, Murphy R. The epidemiology of childhood psoriasis: A scoping review. Br J Dermatol. 2016;174:1242–57. doi: 10.1111/bjd.14507. [DOI] [PubMed] [Google Scholar]
- 7.Zhu W, Li J, Su J, Li J, Li J, Deng B, et al. FOS-like antigen 1 is highly expressed in human psoriasis tissues and promotes the growth of HaCaT cells in vitro . Mol Med Rep. 2014;10:2489–94. doi: 10.3892/mmr.2014.2509. [DOI] [PubMed] [Google Scholar]
- 8.Zeng Q, Yin J, Fan F, Chen J, Zuo C, Xiang Y, et al. Decreased copper and zinc in sera of Chinese vitiligo patients: A meta-analysis. J Dermatol. 2014;41:245–51. doi: 10.1111/1346-8138.12392. [DOI] [PubMed] [Google Scholar]
- 9.Basavaraj KH, Darshan MS, Shanmugavelu P, Rashmi R, Mhatre AY, Dhanabal SP, et al. Study on the levels of trace elements in mild and severe psoriasis. Clin Chim Acta. 2009;405:66–70. doi: 10.1016/j.cca.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Yang WL, Wang RL, Zhang YC. Serum ceruloplasmin and copper levels in psoriasis patients. Zhongguo Pifu Xingbinxue Zazhi. 1993:85–6+135. [Google Scholar]
- 11.Wang GZ, Yu XJ, Xiao JG. Changes of serum level of zinc in children with psoriasis vulgaris. Zhongguo Zhongxiyi Jiehe Pifu Xingbingxue Zazhi. 2004;3:144–6. [Google Scholar]
- 12.Nigam PK. Serum zinc and copper levels and Cu:Zn ratio in psoriasis. Indian J Dermatol Venereol Leprol. 2005;71:205–6. doi: 10.4103/0378-6323.16242. [DOI] [PubMed] [Google Scholar]
- 13.Tasaki M, Hanada K, Hashimoto I. Analyses of serum copper and zinc levels and copper/zinc ratios in skin diseases. J Dermatol. 1993;20:21–4. doi: 10.1111/j.1346-8138.1993.tb03823.x. [DOI] [PubMed] [Google Scholar]
- 14.Ala S, Shokrzadeh M, Golpour M, Salehifar E, Alami M, Ahmadi A. Zinc and copper levels in Iranian patients with psoriasis: A case control study. Biol Trace Elem Res. 2013;153:22–7. doi: 10.1007/s12011-013-9643-6. [DOI] [PubMed] [Google Scholar]
- 15.Afridi HI, Kazi TG, Kazi N, Kandhro GA, Baig JA, Shah AQ, et al. Evaluation of cadmium, chromium, nickel, and zinc in biological samples of psoriasis patients living in Pakistani cement factory area. Biol Trace Elem Res. 2011;142:284–301. doi: 10.1007/s12011-010-8778-y. [DOI] [PubMed] [Google Scholar]
- 16.Halevy S, Giryes H, Friger M, Grossman N, Karpas Z, Sarov B, et al. The role of trace elements in psoriatic patients undergoing balneotherapy with Dead Sea bath salt. Isr Med Assoc J. 2001;3:828–32. [PubMed] [Google Scholar]
- 17.Yang ZD. A dynamic observation on serum copper and zinc in patients with psoriasis. Huaihai Yiyao. 2004;22:365–6. [Google Scholar]
- 18.Ding ZY, Sun XJ, Gao SQ, Laing SY, Zheng FL, Xu SQ. Detection of serum trace elements in patients with psoriasis. Linchuang Pifuke Zazhi. 2003;32:525–5. [Google Scholar]
- 19.Rashmi R, Yuti AM, Basavaraj KH. Relevance of copper and ceruloplasmin in psoriasis. Clin Chim Acta. 2010;411:1390–2. doi: 10.1016/j.cca.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 20.Lei TB, Chen DY, Du Y, Zheng YY, Luo SQ, Yang Y. Analysis of linear correlation between zn and PASI in 40 patients with psoriasis vulgaris. Luzhou Yixueyuan Xuebao. 2008;31:50–2. [Google Scholar]
- 21.Cao JQ, Cui R, Zhang ZX, Xu HQ, Feng J. Determination of serum trace elements in patients with psoriasis. Zhongguo Mafen Pifubing Zazhi. 2005;21:617–9. [Google Scholar]
- 22.Dogan P, Soyuer U, Tanrikulu G. Superoxide dismutase and myeloperoxidase activity in polymorphonuclear leukocytes, and serum ceruloplasmin and copper levels, in psoriasis. Br J Dermatol. 1989;120:239–44. doi: 10.1111/j.1365-2133.1989.tb07788.x. [DOI] [PubMed] [Google Scholar]
- 23.Yin LL, Zhang Y, Guo DM, An K, Yin MS, Cui X. Effects of zinc on interleukins and antioxidant enzyme values in psoriasis-induced mice. Biol Trace Elem Res. 2013;155:411–5. doi: 10.1007/s12011-013-9799-0. [DOI] [PubMed] [Google Scholar]
- 24.Butnaru C, Pascu M, Mircea C, Agoroaei L, Solovastru L, Vata D, et al. Serum zinc and copper levels in some dermatological diseases. Rev Med Chir Soc Med Nat Iasi. 2008;112:253–7. [PubMed] [Google Scholar]
- 25.Burrows NP, Turnbull AJ, Punchard NA, Thompson RP, Jones RR. A trial of oral zinc supplementation in psoriasis. Cutis. 1994;54:117–8. [PubMed] [Google Scholar]
- 26.Grubman A, White AR. Copper as a key regulator of cell signalling pathways. Expert Rev Mol Med. 2014;16:e11. doi: 10.1017/erm.2014.11. [DOI] [PubMed] [Google Scholar]
- 27.Nikolic A, Cabarkapa V, Novakov Mikic A, Jakovljevic A, Stosic Z. Ceruloplasmin and antioxidative enzymes in pre-eclampsia. J Matern Fetal Neonatal Med. 2015;29:1–7. doi: 10.3109/14767058.2015.1111333. [DOI] [PubMed] [Google Scholar]