Key Points
Question
Do outcomes of bariatric surgery differ between black and white patients?
Findings
This study of 14 000 propensity-matched black and white patients undergoing bariatric surgery in Michigan demonstrated that black patients had higher overall rates of complications and health care resource utilization within 30 days of surgery. While weight loss at 1 year was slightly lower in black patients, comorbidity remission was similar between patient cohorts.
Meaning
This study suggests that short-term and long-term outcomes after bariatric surgery differ by race, and these differences should be considered when developing strategies to optimize results in patients undergoing weight-loss procedures.
This study of 14 000 propensity-matched black and white patients undergoing bariatric surgery assesses outcomes, including weight loss and complications, in subgroups of different races.
Abstract
Importance
The outcomes of bariatric surgery vary considerably across patients, but the association of race with these measures remains unclear.
Objective
To examine the association of race on perioperative and 1-year outcomes of bariatric surgery.
Design, Setting, and Participants
Propensity score matching was used to assemble cohorts of black and white patients from the Michigan Bariatric Surgery Collaborative who underwent a primary bariatric operation (Roux-en-Y gastric bypass, sleeve gastrectomy, or adjustable gastric banding) between June 2006 and January 2017. Cohorts were balanced on baseline characteristics and procedure. Conditional fixed-effects models were used to evaluate the association of race on outcomes within hospitals and surgeons. Data analysis occurred from June 2006 through August 2018.
Main Outcomes and Measures
Thirty-day complications and health care resource utilization measures, as well as 1-year weight loss, comorbidity remission, quality of life, and satisfaction.
Results
In each group, 7105 patients were included. Black patients had a higher rate of any complication (628 [8.8%] vs 481 [6.8%]; adjusted odds ratio, 1.33 [95% CI, 1.17-1.51]; P = .02), but there were no significant differences in the rates of serious complications (178 [2.5%] vs 135 [1.9%]; adjusted odds ratio, 1.32 [95% CI, 1.05-1.66]; P = .29) or mortality (5 [0.10%] vs 7 [0.10%]; adjusted odds ratio, 0.73 [95% CI, 0.23-2.31]; P = .54). Black patients had a greater length of stay (mean [SD], 2.2 [3.0] days vs 1.9 [1.7] days; adjusted odds ratio, 0.30 [95% CI, 0.20-0.40]; P < .001), as well as a higher rate of emergency department visits (541 [11.6%] vs 826 [7.6%]; adjusted odds ratio, 1.60 [95% CI, 1.43-1.79]; P < .001) and readmissions (414 [5.8%] vs 245 [3.5%]; adjusted odds ratio, 1.73 [95% CI, 1.47-2.03]; P < .001). At 1 year, black patients had lower mean total body weight loss and as a percentage of weight (32.0 kg [26%]; vs 38.3 kg [29%]; P < .001) and this held true across procedures. Remission of hypertension was lower for black patients (564 [40.0%] vs 1096 [56.0%]; P < .001), but the rate of sleep apnea remission (467 [62.6%] vs 615 [56.1%]; P = .005) and gastroesophageal reflux disease (309 [78.6%] vs 453 [75.4%]; P = .049) were higher. There were no significant differences in remission of diabetes with insulin dependence, diabetes without insulin dependence,or hyperlipidemia hyperlipidemia. Fewer black patients than white patients reported a good or very good quality of life (1379 [87.2%] vs 2133 [90.4%]; P = .002) and being very satisfied with surgery (1908 [78.4%] vs 2895 [84.2%]; P < .001) at 1 year.
Conclusions and Relevance
Black patients undergoing bariatric surgery in Michigan had significantly higher rates of 30-day complications and resource utilization and experienced lower weight loss at 1 year than a matched cohort of white patients. While sleep apnea and gastroesophageal reflux disease remission were higher and hypertension remission lower in black patients, comorbidity remission was otherwise similar between matched cohorts. Racial and cultural differences among patients should be considered when designing strategies to optimize outcomes with bariatric surgery.
Introduction
Bariatric surgery has proven to be the most effective treatment for patients with morbid obesity.1 These procedures lead not only to substantial weight loss but also to a substantial reduction in many obesity-associated comorbidities, as well as long-term mortality.2,3,4 Furthermore, with improvements in technique over the past decade, these operations are now performed with very low morbidity and mortality.5,6 The remarkable safety and effectiveness of bariatric procedures have led to their becoming some of the most commonly performed operations in the United States.7
Despite the overall success of bariatric surgery, weight loss and comorbidity remission appear to vary considerably across patients and procedures.8,9,10 A number of studies, including a recent meta-analysis,11 have suggested that race is an important factor associated with weight loss and possibly comorbidity remission after bariatric surgery.12,13,14,15,16,17 However, many of these reports represent single-center series with small numbers of patients. Furthermore, few of these studies have data on sleeve gastrectomy, comorbidity remission, or the effect of other socioeconomic variables. Finally, there have been conflicting results, highlighting the need for further evaluation of the association of race with outcomes after bariatric surgery.
The objective of this study was to better understand the association of race with the safety and effectiveness of bariatric surgery. To this end, we analyzed data from a prospective statewide clinical registry, comparing 30-day safety outcomes and health care resource utilization, as well as 1-year clinical and quality of life outcomes, between matched cohorts of black and white patients undergoing bariatric surgery in Michigan.
Methods
Study Population
The Michigan Bariatric Surgery Collaborative (MBSC)5 is a consortium of hospitals and surgeons in Michigan whose goal is to improve the quality of care for patients undergoing bariatric surgery. Participating hospitals submit data to a clinical outcomes registry. The group meets regularly to examine registry data, design changes in care, and assess the outcomes of these improvement efforts. The project is funded by Blue Cross and Blue Shield of Michigan/Blue Care Network and coordinated by the Center for Healthcare Outcomes and Policy at the University of Michigan.
Analysis of data from the MBSC registry has been approved by the institutional review boards of all participating hospitals. Written consent was obtained from participants.
With patient enrollment beginning in 2006, the MBSC now enjoys the participation of all 42 bariatric programs in Michigan and enters the data of approximately 6000 patients per year into its clinical registry. Participating hospitals submit data from a review of the medical records for all of their bariatric procedures at 30 days after surgery. The information collected includes sociodemographic data, clinical characteristics, and comorbid conditions, as well as perioperative care processes and clinical outcomes.
Study Outcomes
The primary outcomes included 30-day complications and measures of resource utilization, as well as 1-year outcomes. The definition of serious complications in this study encompassed abdominal abscess (requiring percutaneous drainage or reoperation), bowel obstruction (requiring reoperation), leak (requiring percutaneous drainage or reoperation), bleeding (requiring transfusion of >4 units, reoperation, or splenectomy), respiratory failure (requiring 2 or more days of intubation or tracheostomy), renal failure (requiring dialysis), wound infection/dehiscence (requiring reoperation), venous thromboembolism (ie, deep vein thrombosis or pulmonary embolism), myocardial infarction or cardiac arrest, and death. Resource utilization outcomes included length of hospital stay, reoperation, interfacility transfer, readmission, and emergency department visits.
One-year outcomes were derived from patient surveys, administered preoperatively and annually after bariatric procedures to obtain information on weight, comorbidity remission, and measures of quality of life and satisfaction. Weight at 1 year was obtained from the medical records of patients who did not complete surveys, and weight loss was determined using the most recent preoperative weight. Notably, analyses based on weight at program start were not significantly different. Comorbidity remission was defined as discontinuation of treatment for the condition at 1 year in patients who had received treatment for that condition at baseline. For example, remission of hypertension was defined as the discontinuation of antihypertensive medications. Patients were also asked to rate their overall quality of life using a 5-response Likert scale ranging from very good to very bad and were asked about their satisfaction with the results of bariatric surgery using a similar 5-item scale.
Statistical Analysis
We used propensity score matching to assemble cohorts of black and white patients who had undergone a primary laparoscopic bariatric operation, including gastric bypass (Roux-en-Y), vertical sleeve gastrectomy, or adjustable gastric band, between June 2006 and January 2017. The selection of black and white patients was balanced on baseline characteristics. The probability of black race was estimated for each patient using a nonparsimonious multivariate logistic regression model in which black race was the dependent variable and all of the demographic, weight, medical history, and weight-associated comorbidity variables in the data set were included as covariates. Black patients were matched to white patients using a protocol of 1-to-1 matching without replacement, resulting in cohorts that were well balanced on all baseline characteristics.
Baseline characteristics and outcomes were then compared among the cohorts using χ2 and t tests as appropriate. We used fixed-effects linear and logistic models to evaluate whether observed differences in outcomes among black and white patients could be attributed to surgeon or hospital effects. These models were stratified by income and procedure type, both of which remained significantly different between matched cohorts. One-year outcomes were further adjusted for baseline values.
Propensity score matching was done in Stata version 13.1 (StataCorp), and statistical analyses were performed using SAS version 9.4 (SAS Institute). Data analysis took place from June 2006 to August 2018.
Results
Matching resulted in cohorts of black and white patients (n = 7105 per cohort) that were well balanced on baseline characteristics (Table 1). The only significant differences between study cohorts were income brackets (<$10 000: black patients, 899 [12.7%]; white patients, 951 [13.4%]; $10 000-$24 999: black patients, 1405 [19.8%]; white patients, 1493 [21.0%]; $25 000-$44 999: black patients, 2000 [28.1%]; white patients, 1900 [26.7%]; $45 000-$75 999: black patients, 1739 [24.5%]; white patients, 1635 [23.0%]; >$75 000: black patients, 1062 [14.9%]; white patients, 1126 [15.7%]; P = .02) and procedure type (gastric bypass: black patients, 2418 [34.0%]; white patients, 2350 [33.1%]; sleeve gastrectomy: black patients, 3886 [54.7%]; white patients, 3801 [53.5%]; adjustable gastric band: black patients, 801 [11.3%]; white patients, 954 [13.4%]; P < .001), but the difference within any income or procedure stratum was never more than 1 or 2 percentage points.
Table 1. Baseline Clinical and Sociodemographic Characteristics in the Propensity-Matched Study Cohorts.
| Variable | No. (%) | P Value | |
|---|---|---|---|
| Black | White | ||
| No. | 7105 | 7105 | NA |
| Age, mean (SD), y | 44 (10.4) | 43 (12.6) | .28 |
| BMI, mean (SD) | 50 (9.0) | 49 (9.1) | .53 |
| Male | 990 (13.9) | 1010 (14.2) | .63 |
| Private insurance | 5158 (72.6) | 5152 (72.5) | .91 |
| Current or past smoking | 2091 (29.4) | 2065 (29.1) | .63 |
| Mobility limitations | 477 (6.7) | 486 (6.8) | .76 |
| Married or living with significant other | 2928 (41.2) | 2957 (41.6) | .62 |
| Working full time or part time | 4596 (64.7) | 4582 (64.5) | .81 |
| Education | |||
| <High school | 1359 (19.1) | 1327 (18.7) | .79 |
| College | 4894 (68.9) | 4918 (69.2) | |
| Graduate school | 852 (12.0) | 860 (12.1) | |
| Annual income level, $ | |||
| <10 000 | 899 (12.7) | 951 (13.4) | .02 |
| 10 000-24 999 | 1405 (19.8) | 1493 (21.0) | |
| 25 000-44 999 | 2000 (28.1) | 1900 (26.7) | |
| 45 000-75 999 | 1739 (24.5) | 1635 (23.0) | |
| >75 000 | 1062 (14.9) | 1126 (15.7) | |
| Asthma | 1796 (25.3) | 1779 (25.0) | .74 |
| Other obstructive lung disease | 515 (7.2) | 518 (7.3) | .92 |
| Cardiovascular or peripheral vascular disease | 4239 (59.7) | 4182 (58.5) | .33 |
| Hypertension | 4142 (58.3) | 4103 (57.7) | .51 |
| Hyperlipidemia | 2931 (41.2) | 2888 (40.6) | .46 |
| Gastroesophageal reflux disease | 3310 (46.6) | 3347 (47.1) | .53 |
| Peptic ulcer disease | 259 (3.6) | 264 (3.7) | .82 |
| Cholelithiasis | 1359 (19.1) | 1336 (18.8) | .62 |
| Urinary incontinence | 1207 (17.0) | 1174 (16.5) | .46 |
| Renal failure | 6 (0.1) | 3 (0.0) | .32 |
| Non–insulin-dependent diabetes | 2323 (32.7) | 2297 (32.3) | .64 |
| Insulin-dependent diabetes | 708 (10.0) | 694 (9.8) | .69 |
| Liver disorder | 356 (5.0) | 362 (5.1) | .82 |
| Venous thromboembolism | 309 (4.3) | 297 (4.2) | .62 |
| Sleep apnea | 3174 (44.7) | 3183 (44.8) | .88 |
| Musculoskeletal disorder | 5447 (76.7) | 5434 (76.5) | .80 |
| History of hernia repair | 121 (1.7) | 122 (1.7) | .95 |
| Psychological disorder | 2709 (38.1) | 2735 (38.5) | .65 |
| Procedure type | |||
| Gastric bypass | 2418 (34.0) | 2350 (33.1) | <.001 |
| Sleeve gastrectomy | 3886 (54.7) | 3801 (53.5) | |
| Adjustable gastric band | 801 (11.3) | 954 (13.4) | |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable.
Thirty-day outcomes by cohort are detailed in Table 2. The rates of serious complications were significantly higher for black patients (178 [2.5%]) than white patients (135 [1.9%]) in the models adjusted for patient characteristics only (adjusted odds ratio, 1.32 [95% CI, 1.05-1.66]) but were no longer significant after also accounting for surgeon and hospital effects (adjusted odds ratio, 1.16 [95% CI, 0.89-1.52]). A similar finding was seen with respect to reoperation (black patients, 126 [1.8%]; white patients, 82 [1.2%]; adjusted odds ratio, 1.54 [95% CI, 1.16-2.04] after adjustment for patient characteristics; 1.31 [95% CI, 0.94-1.83] after further adjustment).
Table 2. Thirty-Day Outcome Comparison Between Propensity-Matched Study Cohorts.
| Outcome | No. (%) | Adjusted Odds Ratio (95% CI) | ||
|---|---|---|---|---|
| Black | White | Adjusted for Patient Characteristicsa | Adjusted for Patient Characteristics, Surgeon, and Hospitalb | |
| Any complication | 628 (8.8) | 481 (6.8) | 1.33 (1.17-1.51) | 1.20 (1.03-1.39) |
| Serious complication | 178 (2.5) | 135 (1.9) | 1.32 (1.05-1.66) | 1.16 (0.89-1.52) |
| Death | 5 (0.1) | 7 (0.1) | 0.73 (0.23-2.31) | 0.66 (0.18-2.46) |
| Length of stay, mean (SD), d | 2.2 (3.0) | 1.9 (1.7) | 0.30 (0.24-0.40)c | 0.29 (0.21-0.38)c |
| Reoperation | 126 (1.8) | 82 (1.2) | 1.54 (1.16-2.04) | 1.31 (0.94-1.83) |
| Readmission | 414 (5.8) | 245 (3.5) | 1.73 (1.47-2.03) | 1.56 (1.28-1.89) |
| Transfer | 24 (0.3) | 13 (0.2) | 1.88 (0.96-3.70) | 1.91 (0.87-4.16) |
| Emergency department visit | 826 (11.6) | 541 (7.6) | 1.60 (1.43-1.79) | 1.61 (1.40-1.85) |
A logistic regression model adjusted for patient characteristics and stratified by procedure type and income level.
A conditional fixed-effects logistic model, adjusted for patient characteristics, surgeon, and hospital and stratified by income level and procedure type.
Regression coefficient.
The length of stay (mean [SD]: black patients, 2.2 [3.0] days; white patients, 1.9 [1.7] days; adjusted odds ratio, 0.30 [95% CI, 0.24-0.40] after adjustment for patient characteristics; adjusted odds ratio, 0.29 [95% CI, 0.21-0.38] after further adjustment), as well as the rate of overall complications (black patients, 628 [8.8%]; white patients, 481 [6.8%]; adjusted odd ratio, 1.33 [95% CI, 1.17-1.51] after adjustment for patient characteristics; 1.20 [95% CI, 1.03-1.39] after further adjustment), readmissions (black patients, 414 [5.8%]; white patients, 245 [3.5%]; 1.73 [95% CI, 1.47-2.03] after adjustment for patient characteristics; 1.56 [95% CI, 1.28-1.89] after further adjustment), and emergency department visits (black patients, 826 [11.6%]; white patients, 541 [7.6%]; 1.60 [95% CI, 1.43-1.79] after adjustment for patient characteristics; 1.61 [95% CI, 1.40-1.85] after further adjustment) were significantly higher for black patients. There was little attenuation of these odds ratios or regression coefficients in the fixed-effects models, suggesting that the racial differences were independent of surgeon and hospital.
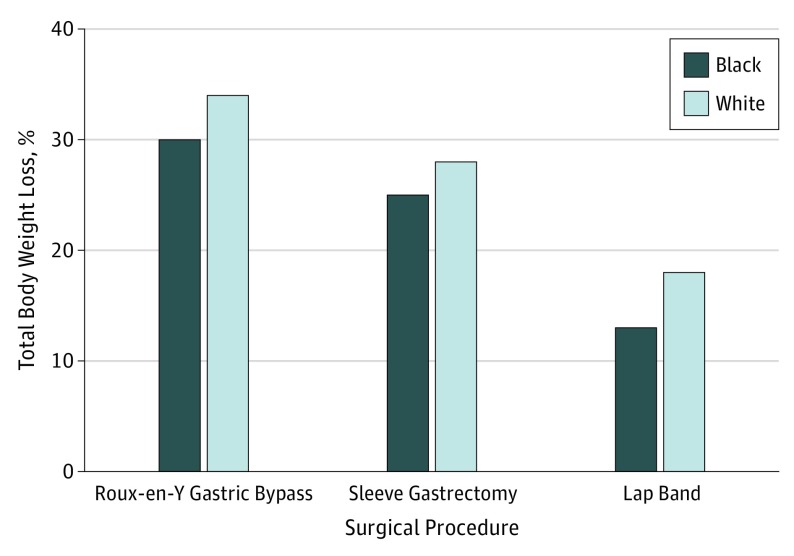
Rates of 1-year follow-up were lower for black patients (2462 [41.6%] vs 3461 [58.4%], P < .001). Black patients had lower mean weight loss (32.0 kg vs 38.3 kg; P < .001) and percentage of total weight loss (26% vs 29%; P < .001) than their matched cohort at 1 year across all procedures (Figure 1) and was not significantly altered in the fixed-effects models (β, 9.82 [SE, 0.94] after adjustment for patient characteristics; β, 9.75 [SE, 1.06] after additional adjustment for surgeon and hospital).
Figure 1. Mean Percentage of Total Body Weight Loss at 1 Year After Bariatric Surgery by Race Category and Procedure Type.
At 1 year after surgery, black patients experienced significantly lower remission of hypertension (564 [40.0%] vs 1096 [56.0%]; P < .001); higher remission of sleep apnea (467 [62.6%] vs 615 [56.1%]; P = .005) and gastroesophageal reflux disease (309 [78.6%] vs 453 [75.4%]; P = .049); and no difference in remission of insulin-dependent diabetes (188 [64.8%] vs 231 [60.8%]; P = .43) or hyperlipidemia (359 [57.1%] vs 505 [53.2%], P = .13). Notably, black patients were more likely than white patients to report good or very good quality of life at baseline (2672 [49.5%] vs 2354 [41.4%]; P < .001) but less likely to do so at 1 year after surgery (1379 [87.2%] vs 2133 [90.4%]; P = .002). Black patients were also less likely to report feeling very satisfied with surgery at 1 year (1908 [78.4%] vs 2895 [84.2%]; P < .001).
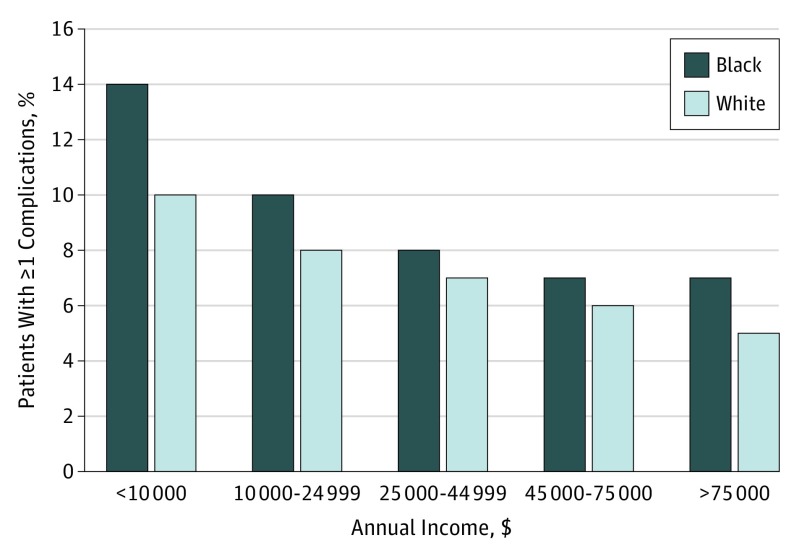
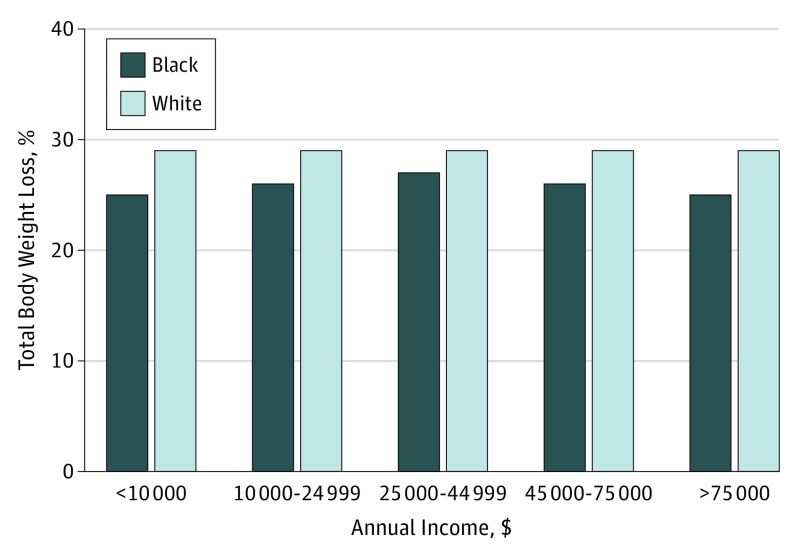
To better understand the interplay of race and socioeconomic status on outcomes, the matched cohorts were stratified by annual income category. Income level was associated with changes in both the overall rates of 30-day outcomes and the differences between cohorts. As Figure 2 shows, the rates of overall complications declined for both black and white patients with rising income status. Furthermore, differences between the cohorts were most pronounced in the lowest income strata. We found very similar results with readmissions. On the other hand, overall weight loss at 1 year did not vary across income strata, nor did the differences between cohorts (Figure 3).
Figure 2. Mean Rate of Overall Complications at 30 Days After Bariatric Surgery by Race Category and Income Level.
Figure 3. Mean Percentage of Total Body Weight Loss at 1 Year After Bariatric Surgery by Race Category and Income Level.
Discussion
In this retrospective analysis of a prospectively maintained population-based clinical registry, we compared safety and effectiveness data between matched cohorts of black and white patients undergoing bariatric surgery. We found that black patients had a significantly higher rate of overall complications at 30 days than white patients, while rates of serious complications and mortality were not significantly different. Furthermore, measures of resource utilization (emergency department visits, readmissions, and lengths of hospital stay) were significantly higher for black patients. Weight loss at 1 year was also modestly lower for black patients across all procedures. On the other hand, there was no difference in remission of diabetes or hyperlipidemia between cohorts, and black patients experienced higher rates of remission with gastroesophageal reflux disease and sleep apnea. Only remission of hypertension was lower for black patients. Finally, black patients reported lower overall quality of life and had less satisfaction with surgery at 1 year than white patients.
Although we found a higher rate of overall complications for black patients, the rate of serious complications and mortality were not significantly different. Adjustment for surgeon and hospital did not significantly attenuate the differences between cohorts for most 30-day outcomes. However, differences in the rates of serious complications and reoperations lost significance in the fixed-effects models, which suggests that the surgeon and site of care may explain some of the difference between cohorts for more severe adverse events.
Previous studies have shown mixed results with regard to the association of race with adverse events after bariatric surgery. For example, an analysis of 108 333 patients undergoing Roux-en-Y gastric bypass at Bariatric Centers of Excellence identified a higher rate of serious and overall complications in black patients but no difference in mortality.18 On the other hand, Nguyen and Patel 19 reported higher mortality rates among black patients undergoing bariatric surgery in an analysis of the Nationwide Inpatient Sample. Data from the Longitudinal Assessment of Bariatric Surgery (LABS), however, suggested that race was not a significant risk factor for adverse outcomes.6 Similarly, race was not associated with risk of serious complications after bariatric surgery in an analysis of New York State discharge data.20 Unlike these other reports, however, this study is based on a large, population-based, robust clinical registry and includes data on vertical sleeve gastrectomy.
This study also demonstrated higher rates of resource utilization after bariatric surgery among black patients, including length of stay, emergency department visits, and readmission rates. The differences were not significantly attenuated in the fixed-effects models, suggesting that they were independent of surgeon and hospital. Furthermore, the findings are consistent with those of other studies. For example, Dorman et al21 examined data from more than 50 000 patients undergoing bariatric surgery and found that the odds of readmission were 34% higher for black patients than white patients after Roux-en-Y gastric bypass. Additionally, in an analysis of New York state hospital discharge data, Patterson et al22 reported that black patients were at significantly greater risk for a potentially preventable readmission after bariatric surgery than white patients. Furthermore, several studies have reported higher rates of readmission and greater length of stay for black patients after a wide range of nonbariatric operations as well.23,24,25,26,27
We found that both adverse events and resource utilization declined across the board with rising income status and racial differences were most pronounced at the lower income levels. Indeed, previous studies have reported that at least some of the variation in postdischarge resource utilization can be explained by racial differences in socioeconomic factors, such as social support, transportation issues, insurance, and access to a primary care physician.28,29,30 Other potential contributing factors include unmeasured differences in the severity of underlying comorbid diseases and/or functional status between black and white patients. There is also evidence of racial differences in tolerance to pain, which may contribute to increased length of stay and emergency department visits.31,32 Finally, racial differences in the quality of patient-clinician communication may also contribute to higher resource use by black patients in the perioperative period.33,34
In this study, we found that black patients lost less weight after bariatric surgery than white patients, although the difference is modest and may not be associated with comorbidity remission. These results are echoed in previous studies of Roux-en-Y and adjustable gastric band surgeries. A recent meta-analysis, for example, reported that black patients lost significantly less weight than white patients, with a mean deficit in the percentage of excess weight loss of 8.4%.11 Furthermore, in a study of more than 100 000 patients undergoing Roux-en-Y gastric bypass, Sudan et al18 reported that the mean percentage change in body mass index at 1 year was 30.2% in black patients and 33.6% for white patients. Moreover, results from the LABS consortium revealed that black patients lost 2.7% less weight than white patients after Roux-en-Y gastric bypass. Racial differences in weight loss are similarly reflected in several single-center studies as well.15,35,36,37
Fewer studies have examined racial differences in weight loss after vertical sleeve gastrectomy and results have been mixed. As with our study, 2 single-center reports demonstrated lower weight loss in black patients than white patients after both Roux-en-Y gastric bypass and vertical sleeve gastrectomy.16,35 However, in an analysis of more than 20 000 patients in a large, integrated health care system, excess weight loss was significantly lower for black patients after Roux-en-Y procedures, but there were no racial differences in weight loss after vertical sleeve gastrectomy.12
The reasons for racial disparity in weight loss after bariatric surgery remain unclear, although these results suggest that it is independent of surgeon and site. Biological determinants may play a role; previous studies have demonstrated racial differences in the postprandial responses of incretins associated with appetite, such as ghrelin and glucagon-like peptide 1.38,39,40 Furthermore, total and resting energy expenditure appear to be lower in black individuals than white individuals, even after accounting for fat-free mass. Similarly, resting energy expenditure and aerobic capacity have been shown to be significantly lower in black participants after weight loss.41,42,43 Thus, there may be important metabolic differences that predispose black patients toward lower weight loss and greater weight regain after bariatric surgery.
However, socioenvironmental factors may also contribute to differences in weight loss after bariatric surgery. For instance, studies looking at the spatial distribution of fast-food restaurants and supermarkets have demonstrated that predominantly black areas, regardless of income, were less likely than predominantly white, higher-income communities to have access to foods meeting recommended dietary standards.44,45Other factors, such as access to transportation and availability of recreation centers also appear to contribute to the obesogenicity of different living environments.46
Interestingly, these weight loss results were largely independent of income level, and it may be that social and behavioral factors are more important drivers of weight loss after bariatric surgery. A number of studies have shown substantial racial differences in preferences for body size. In 1 survey of more than 500 morbidly obese individuals seeking Roux-en-Y gastric bypass, white women reported the highest impairment in quality of life, despite having lower body mass index values than the other race and sex groups.47 By contrast, black men reported the least social impairment with obesity. Other surveys have shown that ideal body size for oneself and the opposite sex was larger for black individuals than white individuals.48,49,50 Furthermore, black women with obesity are more likely than white women to consider themselves attractive.51,52 Another study involving focus groups of black women with obesity revealed that weight loss sometimes elicited negative feelings and comments from family and friends, suggesting that black individuals not only feel less social pressure to lose weight but may feel some pressure to limit weight loss.53
Previous studies examining differences in comorbidity remission after bariatric surgery have had conflicting results. As with our study, Sugerman et al37 reported lower remission of hypertension in black patients after Roux-en-Y gastric bypass, although diabetes remission was higher. A more recent meta-analysis11 identified a nonsignificant trend toward greater diabetes remission in black patients after bariatric surgery. By contrast, Ng et al15 found no ethnic differences in remission of diabetes, hyperlipidemia, hypertension, or sleep apnea in their single-center series of more than 1900 patients undergoing Roux-en-Y or adjustable gastric band procedures. Some of these discrepancies between studies may be associated with differences in determining remission. We expect, however, that hypertension resolution actually is lower in black patients, given the higher rate of essential hypertension in this population, which may not resolve with weight loss.54
This study demonstrated lower scores at 1 year after surgery on overall quality of life and satisfaction with surgery among black patients, even though their baseline quality of life scores were better than white patients. While the reasons for this disparity are not entirely clear, it may be associated with differences in weight loss. We have found that in previous surveys of MBSC patients, less than expected weight loss is the most commonly listed reason for dissatisfaction with surgery for both black patients (84%) and white patients (76%) (data not shown). As with differences in resource use, disparities in patient-clinician communication may contribute to lower satisfaction with surgery among black patients, perhaps especially when there is patient-clinician discordance in racial identity.55
This study benefits from a population-based registry with data that are clinically rich and audited regularly. Furthermore, this is, to our knowledge, one of very few recent studies that have assessed racial differences after vertical sleeve gastrectomy and the only one to examine racial differences in quality of life and patient satisfaction after bariatric surgery.
Limitations
There are a number of limitations to consider when interpreting the results of this study. To minimize confounding, we used propensity matching to create study cohorts that were well balanced on all measured baseline variables. However, unmeasured factors, such as disease duration and/or severity, may have been greater in black patients, thereby altering the results. Furthermore, the MBSC registry captures adverse events and resource use occurring within 30 days of the index operation. It is possible that a wider time horizon could have altered the results. However, serious adverse events and resource use tend to be highest within the first month after surgery, and we anticipate that this effect would have been negligible. Additionally, comorbidity remission in the MBSC is defined as discontinuation of treatment for the specified condition. These definitions may lead to less accuracy in determining remission than other, more objective measures, such as hemoglobin A1c levels and can be affected by factors like patient compliance and access to care. Finally, the quality of life and satisfaction scores were each based on a single Likert-scale question and not validated survey instruments, which may have yielded different results.
Conclusions
Per this analysis, there are significant racial disparities in perioperative outcomes, weight loss, and quality of life after bariatric surgery. While biological differences may explain some of the disparity in outcomes, environmental, social, and behavioral factors are likely play a role. Efforts to reduce unnecessary health care use after surgery should focus on improving predischarge education and ensuring that patients have adequate access to the surgical team and their primary care physician after discharge from the hospital. Strategies to improve postsurgical weight loss in black patients may need to prioritize education that emphasizes finding the best food sources in a given neighborhood and highlights the health benefits of weight loss.
References
- 1.Steinbrook R. Surgery for severe obesity. N Engl J Med. 2004;350(11):-. doi: 10.1056/NEJMp048029 [DOI] [PubMed] [Google Scholar]
- 2.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753-761. doi: 10.1056/NEJMoa066603 [DOI] [PubMed] [Google Scholar]
- 3.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724-1737. doi: 10.1001/jama.292.14.1724 [DOI] [PubMed] [Google Scholar]
- 4.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567-1576. doi: 10.1056/NEJMoa1200225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birkmeyer NJ, Dimick JB, Share D, et al. ; Michigan Bariatric Surgery Collaborative . Hospital complication rates with bariatric surgery in Michigan. JAMA. 2010;304(4):435-442. doi: 10.1001/jama.2010.1034 [DOI] [PubMed] [Google Scholar]
- 6.Flum DR, Belle SH, King WC, et al. ; Longitudinal Assessment of Bariatric Surgery (LABS) Consortium . Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361(5):445-454. doi: 10.1056/NEJMoa0901836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen NT, Masoomi H, Magno CP, Nguyen XM, Laugenour K, Lane J. Trends in use of bariatric surgery, 2003-2008. J Am Coll Surg. 2011;213(2):261-266. doi: 10.1016/j.jamcollsurg.2011.04.030 [DOI] [PubMed] [Google Scholar]
- 8.Carlin AM, Zeni TM, English WJ, et al. ; Michigan Bariatric Surgery Collaborative . The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg. 2013;257(5):791-797. doi: 10.1097/SLA.0b013e3182879ded [DOI] [PubMed] [Google Scholar]
- 9.Courcoulas AP, Christian NJ, Belle SH, et al. ; Longitudinal Assessment of Bariatric Surgery (LABS) Consortium . Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310(22):2416-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Still CD, Wood GC, Chu X, et al. Clinical factors associated with weight loss outcomes after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring). 2014;22(3):888-894. doi: 10.1002/oby.20529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Admiraal WM, Celik F, Gerdes VE, Dallal RM, Hoekstra JB, Holleman F. Ethnic differences in weight loss and diabetes remission after bariatric surgery: a meta-analysis. Diabetes Care. 2012;35(9):1951-1958. doi: 10.2337/dc12-0260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman KJ, Huang YC, Hendee F, Watson HL, Casillas RA, Brookey J. Three-year weight outcomes from a bariatric surgery registry in a large integrated healthcare system. Surg Obes Relat Dis. 2014;10(3):396-403. doi: 10.1016/j.soard.2014.02.044 [DOI] [PubMed] [Google Scholar]
- 13.Harvin G, DeLegge M, Garrow DA. The impact of race on weight loss after Roux-en-Y gastric bypass surgery. Obes Surg. 2008;18(1):39-42. doi: 10.1007/s11695-007-9278-9 [DOI] [PubMed] [Google Scholar]
- 14.Lutfi R, Torquati A, Sekhar N, Richards WO. Predictors of success after laparoscopic gastric bypass: a multivariate analysis of socioeconomic factors. Surg Endosc. 2006;20(6):864-867. doi: 10.1007/s00464-005-0115-8 [DOI] [PubMed] [Google Scholar]
- 15.Ng J, Seip R, Stone A, Ruano G, Tishler D, Papasavas P. Ethnic variation in weight loss, but not co-morbidity remission, after laparoscopic gastric banding and Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2015;11(1):94-100. doi: 10.1016/j.soard.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 16.Elli EF, Gonzalez-Heredia R, Patel N, et al. Bariatric surgery outcomes in ethnic minorities. Surgery. 2016;160(3):805-812. doi: 10.1016/j.surg.2016.02.023 [DOI] [PubMed] [Google Scholar]
- 17.Istfan N, Anderson WA, Apovian C, Ruth M, Carmine B, Hess D. Racial differences in weight loss, hemoglobin A1C, and blood lipid profiles after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2016;12(7):1329-1336. doi: 10.1016/j.soard.2015.12.028 [DOI] [PubMed] [Google Scholar]
- 18.Sudan R, Winegar D, Thomas S, Morton J. Influence of ethnicity on the efficacy and utilization of bariatric surgery in the USA. J Gastrointest Surg. 2014;18(1):130-136. doi: 10.1007/s11605-013-2368-1 [DOI] [PubMed] [Google Scholar]
- 19.Nguyen GC, Patel AM. Racial disparities in mortality in patients undergoing bariatric surgery in the U.S.A. Obes Surg. 2013;23(10):1508-1514. doi: 10.1007/s11695-013-0957-4 [DOI] [PubMed] [Google Scholar]
- 20.Weller WE, Rosati C, Hannan EL. Predictors of in-hospital postoperative complications among adults undergoing bariatric procedures in New York state, 2003. Obes Surg. 2006;16(6):702-708. doi: 10.1381/096089206777346790 [DOI] [PubMed] [Google Scholar]
- 21.Dorman RB, Miller CJ, Leslie DB, et al. Risk for hospital readmission following bariatric surgery. PLoS One. 2012;7(3):e32506. doi: 10.1371/journal.pone.0032506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patterson WL, Peoples BD, Gesten FC. Predicting potentially preventable hospital readmissions following bariatric surgery. Surg Obes Relat Dis. 2015;11(4):866-872. [DOI] [PubMed] [Google Scholar]
- 23.Girotti ME, Shih T, Revels S, Dimick JB. Racial disparities in readmissions and site of care for major surgery. J Am Coll Surg. 2014;218(3):423-430. doi: 10.1016/j.jamcollsurg.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 24.Parsons HM, Habermann EB, Stain SC, Vickers SM, Al-Refaie WB. What happens to racial and ethnic minorities after cancer surgery at American College of Surgeons National Surgical Quality Improvement Program hospitals? J Am Coll Surg. 2012;214(4):539-547. doi: 10.1016/j.jamcollsurg.2011.12.024 [DOI] [PubMed] [Google Scholar]
- 25.Sosa JA, Mehta PJ, Wang TS, Yeo HL, Roman SA. Racial disparities in clinical and economic outcomes from thyroidectomy. Ann Surg. 2007;246(6):1083-1091. doi: 10.1097/SLA.0b013e31812eecc4 [DOI] [PubMed] [Google Scholar]
- 26.Taub DA, Hollenbeck BK, Cooper KL, et al. Racial disparities in resource utilization for cystectomy. Urology. 2006;67(2):288-293. doi: 10.1016/j.urology.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 27.Tsai TC, Orav EJ, Joynt KE. Disparities in surgical 30-day readmission rates for Medicare beneficiaries by race and site of care. Ann Surg. 2014;259(6):1086-1090. doi: 10.1097/SLA.0000000000000326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arbaje AI, Wolff JL, Yu Q, Powe NR, Anderson GF, Boult C. Postdischarge environmental and socioeconomic factors and the likelihood of early hospital readmission among community-dwelling Medicare beneficiaries. Gerontologist. 2008;48(4):495-504. doi: 10.1093/geront/48.4.495 [DOI] [PubMed] [Google Scholar]
- 29.Joynt KE, Jha AK. A path forward on Medicare readmissions. N Engl J Med. 2013;368(13):1175-1177. doi: 10.1056/NEJMp1300122 [DOI] [PubMed] [Google Scholar]
- 30.Shi L, Lebrun-Harris LA, Daly CA, et al. Reducing disparities in access to primary care and patient satisfaction with care: the role of health centers. J Health Care Poor Underserved. 2013;24(1):56-66. doi: 10.1353/hpu.2013.0022 [DOI] [PubMed] [Google Scholar]
- 31.Mechlin MB, Maixner W, Light KC, Fisher JM, Girdler SS. African Americans show alterations in endogenous pain regulatory mechanisms and reduced pain tolerance to experimental pain procedures. Psychosom Med. 2005;67(6):948-956. doi: 10.1097/01.psy.0000188466.14546.68 [DOI] [PubMed] [Google Scholar]
- 32.Rahim-Williams FB, Riley JL III, Herrera D, Campbell CM, Hastie BA, Fillingim RB. Ethnic identity predicts experimental pain sensitivity in African Americans and Hispanics. Pain. 2007;129(1-2):177-184. doi: 10.1016/j.pain.2006.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White-Means SI, Osmani AR. Racial and ethnic disparities in patient-provider communication with breast cancer patients: evidence from 2011 MEPS and experiences with cancer supplement. Inquiry. 2017;54:46958017727104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong MS, Gudzune KA, Bleich SN. Provider communication quality: influence of patients’ weight and race. Patient Educ Couns. 2015;98(4):492-498. doi: 10.1016/j.pec.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bayham BE, Bellanger DE, Hargroder AG, Johnson WD, Greenway FL. Racial differences in weight loss, payment method, and complications following Roux-en-Y gastric bypass and sleeve gastrectomy. Adv Ther. 2012;29(11):970-978. doi: 10.1007/s12325-012-0062-4 [DOI] [PubMed] [Google Scholar]
- 36.Limbach KE, Ashton K, Merrell J, Heinberg LJ. Relative contribution of modifiable versus non-modifiable factors as predictors of racial variance in roux-en-Y gastric bypass weight loss outcomes. Obes Surg. 2014;24(8):1379-1385. doi: 10.1007/s11695-014-1213-2 [DOI] [PubMed] [Google Scholar]
- 37.Sugerman HJ, Wolfe LG, Sica DA, Clore JN. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg. 2003;237(6):751-756. doi: 10.1097/01.SLA.0000071560.76194.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bacha F, Arslanian SA. Ghrelin and peptide YY in youth: are there race-related differences? J Clin Endocrinol Metab. 2006;91(8):3117-3122. doi: 10.1210/jc.2005-2448 [DOI] [PubMed] [Google Scholar]
- 39.Brownley KA, Heymen S, Hinderliter AL, Galanko J, Macintosh B. Low-glycemic load decreases postprandial insulin and glucose and increases postprandial ghrelin in white but not black women. J Nutr. 2012;142(7):1240-1245. doi: 10.3945/jn.111.146365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Velásquez-Mieyer PA, Cowan PA, Pérez-Faustinelli S, et al. Racial disparity in glucagon-like peptide 1 and inflammation markers among severely obese adolescents. Diabetes Care. 2008;31(4):770-775. doi: 10.2337/dc07-1525 [DOI] [PubMed] [Google Scholar]
- 41.Foster GD, Wadden TA, Swain RM, Anderson DA, Vogt RA. Changes in resting energy expenditure after weight loss in obese African American and white women. Am J Clin Nutr. 1999;69(1):13-17. doi: 10.1093/ajcn/69.1.13 [DOI] [PubMed] [Google Scholar]
- 42.Staiano AE, Harrington DM, Johannsen NM, et al. Uncovering physiological mechanisms for health disparities in type 2 diabetes. Ethn Dis. 2015;25(1):31-37. [PMC free article] [PubMed] [Google Scholar]
- 43.Weinsier RL, Hunter GR, Schutz Y, Zuckerman PA, Darnell BE. Physical activity in free-living, overweight white and black women: divergent responses by race to diet-induced weight loss. Am J Clin Nutr. 2002;76(4):736-742. doi: 10.1093/ajcn/76.4.736 [DOI] [PubMed] [Google Scholar]
- 44.Baker EA, Schootman M, Barnidge E, Kelly C. The role of race and poverty in access to foods that enable individuals to adhere to dietary guidelines. Prev Chronic Dis. 2006;3(3):A76. [PMC free article] [PubMed] [Google Scholar]
- 45.Morland K, Wing S, Diez Roux A, Poole C. Neighborhood characteristics associated with the location of food stores and food service places. Am J Prev Med. 2002;22(1):23-29. doi: 10.1016/S0749-3797(01)00403-2 [DOI] [PubMed] [Google Scholar]
- 46.Swinburn B, Egger G, Raza F. Dissecting obesogenic environments: the development and application of a framework for identifying and prioritizing environmental interventions for obesity. Prev Med. 1999;29(6, pt 1):563-570. doi: 10.1006/pmed.1999.0585 [DOI] [PubMed] [Google Scholar]
- 47.White MA, O’Neil PM, Kolotkin RL, Byrne TK. Gender, race, and obesity-related quality of life at extreme levels of obesity. Obes Res. 2004;12(6):949-955. doi: 10.1038/oby.2004.116 [DOI] [PubMed] [Google Scholar]
- 48.Becker DM, Yanek LR, Koffman DM, Bronner YC. Body image preferences among urban African Americans and whites from low income communities. Ethn Dis. 1999;9(3):377-386. [PubMed] [Google Scholar]
- 49.Greenberg DR, LaPorte DJ. Racial differences in body type preferences of men for women. Int J Eat Disord. 1996;19(3):275-278. doi: [DOI] [PubMed] [Google Scholar]
- 50.Powell AD, Kahn AS. Racial differences in women’s desires to be thin. Int J Eat Disord. 1995;17(2):191-195. doi: [DOI] [PubMed] [Google Scholar]
- 51.Allan JD, Mayo K, Michel Y. Body size values of white and black women. Res Nurs Health. 1993;16(5):323-333. doi: 10.1002/nur.4770160503 [DOI] [PubMed] [Google Scholar]
- 52.Stevens J, Kumanyika SK, Keil JE. Attitudes toward body size and dieting: differences between elderly black and white women. Am J Public Health. 1994;84(8):1322-1325. doi: 10.2105/AJPH.84.8.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lynch CS, Chang JC, Ford AF, Ibrahim SA. Obese African-American women’s perspectives on weight loss and bariatric surgery. J Gen Intern Med. 2007;22(7):908-914. doi: 10.1007/s11606-007-0218-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Minor DS, Wofford MR, Jones DW. Racial and ethnic differences in hypertension. Curr Atheroscler Rep. 2008;10(2):121-127. doi: 10.1007/s11883-008-0018-y [DOI] [PubMed] [Google Scholar]
- 55.Cooper LA, Roter DL, Johnson RL, Ford DE, Steinwachs DM, Powe NR. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139(11):907-915. doi: 10.7326/0003-4819-139-11-200312020-00009 [DOI] [PubMed] [Google Scholar]