Key Points
Question
What are the incidence and risk factors for posttransplant keratinocyte carcinoma in Canada?
Findings
This population-based cohort study of 10 198 transplant recipients in Ontario, Canada, found a 6.6-times increased risk of keratinocyte carcinoma in transplant recipients compared with the general population. The strongest risk factors for keratinocyte carcinoma were older age at transplant, white race, pretransplant invasive skin cancer, and posttransplant precancerous skin lesions.
Meaning
More frequent skin cancer screening, education, and early use of chemopreventive interventions may be warranted in the high-risk patient subsets identified by key risk factors.
Abstract
Importance
Keratinocyte carcinoma (KC), also known as nonmelanoma skin cancer, is the most common malignancy after solid organ transplant. Epidemiologic data on posttransplant KC in North America are limited by a lack of KC capture in cancer and transplant registries.
Objective
To estimate the incidence and identify risk factors for posttransplant KC.
Design, Setting, and Participants
This population-based inception cohort study in Ontario, Canada, used linked administrative databases and a health insurance claims–based algorithm. Participants were adult recipients of a first kidney, liver, heart, or lung transplant from January 1, 1994, to December 31, 2012. The cohort (n = 10 198) was followed up to December 31, 2013. Data were analyzed from May 31, 2016, to April 21, 2017.
Exposures
Solid organ transplant with functioning graft.
Main Outcomes and Measures
Age- and sex-adjusted standardized incidence ratio for KC in the transplant cohort was compared with that in the general population. Cumulative incidence of posttransplant KC was estimated using cumulative incidence functions, accounting for the competing risks of death or kidney graft loss. The association between KC and patient-, transplant-, and health services–related factors was evaluated with a multivariable cause-specific hazards model.
Results
A total of 10 198 transplant recipients were included in the study. The median (interquartile range [IQR]) age at transplant was 51 (41-59) years, with most recipients being male (6608 [64.8%]) and white (5964 [58.5%]). Posttransplant KC was diagnosed in 1690 patients (16.6%) after a median (IQR) of 3.96 (1.94-7.09) years, with an incidence rate of 2.63 per 100 patient-years (95% CI, 2.51-2.76). The rate of KC was significantly higher after transplant compared with the general population (standardized incidence ratio, 6.61; 95% CI, 6.31-6.93). The highest 10-year cumulative incidence was in the subsets of patients with a history of pretransplant skin cancer (66.5%), older than 50 years at transplant (27.5% for 51-65 years; 40.5% for >65 years), and of the white race (24.1%). The strongest independent risk factors for KC included older age at transplant (adjusted hazard ratio [aHR], 9.27; 95% CI, 7.08-12.14 for >65 years vs 18-35 years), white vs black race (aHR, 8.50; 95% CI, 4.03-17.91), pretransplant invasive skin cancer (aHR, 4.30; 95% CI, 3.72-4.98), and posttransplant precancerous skin lesions (aHR, 4.32; 95% CI, 3.77-4.95).
Conclusions and Relevance
The incidence of KC appeared to be substantially increased after transplant, particularly in patients who were older at transplant, were white, and had a history of cancerous or precancerous skin tumors; intensified skin cancer screening, education, and early use of chemopreventive interventions may be warranted for these high-risk patient subsets.
This population-based cohort study examines insurance claims data and linked province-wide health databases to identify the presence or likelihood of keratinocyte carcinoma among residents of Ontario, Canada, who had undergone a solid organ transplant.
Introduction
Keratinocyte carcinoma (KC), also known as nonmelanoma skin cancer (squamous and basal cell carcinoma), is by far the most frequent malignancy after solid organ transplant. Relative incidence estimates in Europe range from 16 to 40 compared with the general population.1,2,3,4 Despite rarely causing death in the general population, KC is associated with substantially higher morbidity rates and 30- to 52-fold higher standardized mortality rates in the transplant population.5,6,7
Owing to the markedly elevated risk of KC, posttransplant care guidelines recommend at least annual dermatologic screening to enable early KC detection and reduce morbidity and mortality.8,9 Although specific subsets of transplant recipients who are at particularly high risk for KC may find intensified screening and early chemopreventive interventions advantageous, existing clinical practice guidelines provide little guidance on risk stratification.10,11,12,13,14
Robust population-based data are required to ascertain the incidence of KC and identify the major risk factors associated with posttransplant KC. Such data are not readily available in North America because of the routine exclusion of KC from cancer and transplant registries.15 Using a novel health insurance claims–based algorithm and linked administrative health databases, we conducted a population-based inception cohort study to estimate the incidence and risk factors for KC in transplant recipients in Ontario, Canada.
Methods
Using the Canadian Organ Replacement Register (CORR), a national database of transplant recipients with more than 98% completeness,16 we identified adult residents (aged ≥18 years) in the province of Ontario, Canada, who received their first solid organ (kidney, liver, heart, or lung) transplant from January 1, 1994, to December 31, 2012, and survived beyond 6 months after transplant. The cohort was followed up to December 31, 2013. This study was approved by the Women's College Hospital Research Ethics Board, which granted a consent waiver because of the use of deidentified administrative databases held securely at the Institute for Clinical Evaluative Sciences (ICES) in Toronto, Ontario, Canada.
Data on KC incidence and potential risk factors were extracted from population-based administrative databases that were linked and analyzed using unique encoded identifiers at ICES. The primary outcome of KC was defined as the first incident cutaneous basal cell carcinoma or invasive squamous cell carcinoma diagnosis at least 3 months after transplant until the end of follow-up. The 3-month criterion was intended to exclude cancers that existed before transplant but were detected during the intense health surveillance period immediately after transplant. The KC diagnoses were ascertained using a validated Ontario Health Insurance Plan (OHIP) claims–based algorithm.17 The OHIP provides universal health coverage for medically necessary care to all Ontario residents.
Demographic data (birthdate, sex, and neighborhood income quintile derived from the residential postal code) were extracted from the Registered Persons Database, a registry maintained by the Ministry of Health and Long-Term Care that contains demographic and vital status information on Ontario residents. Race data were ascertained from CORR; the Organ Transplant Tracking Record at University Health Network; the Canadian Cystic Fibrosis Patient Data Registry; the Ontario Renal Reporting System for patients on predialysis or dialysis care; a validated surname algorithm applied to the Registered Persons Database18; and Immigration, Refugees and Citizenship Canada. We used OHIP to identify pretransplant KC and history of precancerous skin lesions, and we used the Ontario Cancer Registry to identify pretransplant melanoma. Transplant-associated factors (organ type, multiorgan transplant, subsequent transplants, and transplant year) were extracted from CORR. We ascertained health services factors from CORR (transplant center), ICES Physician Database (number of dermatologists practicing at the transplant center at time of transplant), Registered Persons Database (rural vs urban residence), and OHIP (number of outpatient dermatologist and nondermatologist visits in the previous 12 months; inpatient hospitalization in the previous 90 days).
Statistical Analysis
Data were analyzed from May 31, 2016, to April 21, 2017. The standardized incidence ratio was calculated by dividing the observed number of patients by the expected number of patients with KC in the transplant population, adjusted by age, sex, and calendar year using indirect standardization methods. The expected number was calculated by multiplying the population incidence rate by the corresponding person-years of follow-up in transplant recipients. We found the age- and sex-specific population incidence rate by applying the OHIP claims–based algorithm to the Ontario general population identified in the Registered Persons Database in each year from 1994 to 2012. We assumed that the entire population aged 18 years or older was at risk. The adult population size was based on the Ontario census data with linear interpolation between census years. The 95% CI was calculated assuming a Poisson error distribution.
Cumulative incidence of KC at 2, 5, and 10 years was estimated using cumulative incidence functions to account for the competing risks of death and kidney graft loss (ie, no longer immunosuppressed). This approach prevented the overestimation of KC incidence.19
A multivariable, cause-specific hazards model was used to identify the factors associated with the incidence rate of KC while accounting for the competing risks of death and kidney graft loss.19,20 Each patient was followed up from the transplant date until the date of first KC diagnosis, death, kidney graft loss, outmigration from Ontario, or last medical contact up to December 31, 2013. To account for surveillance bias, we adjusted for health services use (number of outpatient physician visits in the previous 12 months; hospitalization in the previous 3 months) as time-varying covariates as well as the number of dermatologists at the transplant center. Other factors that could change over the follow-up period were also treated as time-varying covariates, including income quintile, rurality of residence, precancerous skin lesions (actinic keratosis or carcinoma in situ) after transplant, and subsequent transplant of any organ. Associations were estimated as adjusted hazard ratios (aHRs) and 95% CIs, with 2-sided P < .05 considered statistically significant.
Results
A total of 14 604 patients received a solid organ transplant from January 1, 1994, to December 31, 2012. The reasons for exclusion included non–Ontario residence (n = 1059), younger than 18 years (n = 874), bowel transplant alone (n = 9), non-first organ transplant (n = 1411), death within 6 months (n = 612), and kidney graft failure within 6 months (n = 441). The remaining 10 198 transplant recipients were included in the analysis, with a total of 64 156 person-years of follow-up time (median [interquartile range (IQR)] of 5.15 [2.64-8.96] years). Patients exited the observation period because of KC (1690 [16.6%]), end of study (5781 [56.7%]), death unrelated to KC (1771 [17.4%]), kidney graft failure (795 [7.8%]), and outmigration from Ontario (161 [1.6%]).
Overall, the median (IQR) age at transplant was 51 (41-59) years, with most recipients being male (6608 [64.8%]) and white (5964 [58.5%]) (Table 1). Kidney transplant (6296 [61.7%]) was the most common graft type, followed by liver (2413 [23.7%]), lung (805 [7.9%]), and heart (684 [6.7%]). Pretransplant KC or melanoma was seen in 467 patients (4.6%).
Table 1. Characteristics of First-Time Adult Recipients of Transplants Residing in Ontario From 1994 to 2012.
| Variable | No. (%) | |||||
|---|---|---|---|---|---|---|
| Kidney (n = 6296) | Liver (n = 2413) | Heart (n = 684) | Lung (n = 805) | Total (n = 10 198) | ||
| Age at transplant | ||||||
| Median (IQR), y | 49 (39-59) | 53 (46-59) | 53 (44-59) | 53 (39-60) | 51 (41-59) | |
| 18-35 | 1182 (18.8) | 214 (8.9) | 86 (12.6) | 168 (20.9) | 1650 (16.2) | |
| 36-50 | 2175 (34.5) | 739 (30.6) | 204 (29.8) | 183 (22.7) | 3301 (32.4) | |
| 51-65 | 2267 (36.0) | 1317 (54.6) | 362 (52.9) | 381 (47.3) | 4327 (42.4) | |
| >65 | 672 (10.7) | 143 (5.9) | 32 (4.7) | 73 (9.1) | 920 (9.0) | |
| Sex | ||||||
| Male | 3997 (63.5) | 1655 (68.6) | 530 (77.5) | 426 (52.9) | 6608 (64.8) | |
| Race | ||||||
| White | 4368 (69.4) | 634 (26.3) | 361 (52.8) | 601 (74.7) | 5964 (58.5) | |
| Black | 374 (5.9) | 19 (0.8) | 14 (2.0) | 11 (1.4) | 418 (4.1) | |
| Asian/mixed/other | 1101 (17.5) | 288 (11.9) | 41 (6.0) | 54 (6.7) | 1484 (14.6) | |
| Unknown | 453 (7.2) | 1472 (61.0) | 268 (39.2) | 139 (17.3) | 2332 (22.9) | |
| Income quintile: 4th or 5th | 2377 (37.8) | 964 (40.0) | 286 (41.8) | 331 (41.1) | 3958 (38.8) | |
| Urban residence | 5562 (88.3) | 2161 (89.6) | 575 (84.1) | 710 (88.2) | 9008 (88.3) | |
| Pretransplant skin cancer: keratinocyte carcinoma or melanoma | 287 (4.6) | 103 (4.3) | 22 (3.2) | 55 (6.8) | 467 (4.6) | |
| Pretransplant precancerous lesions: actinic keratosis or carcinoma in situ | 75 (1.2) | 32 (1.3) | 6 (0.9) | 10 (1.2) | 123 (1.2) | |
| Multiorgan transplant at initial treatmenta | 289 (4.6) | 18 (0.7) | 4 (0.6) | 23 (2.9) | 334 (3.3) | |
| Subsequent transplant | 99 (1.6) | 111 (4.6) | 13 (1.9) | 23 (2.9) | 246 (2.4) | |
| Year of transplant | ||||||
| 1994-2000 | 1884 (29.9) | 666 (27.6) | 249 (36.4) | 161 (20.0) | 2960 (29.0) | |
| 2001-2006 | 1858 (29.5) | 784 (32.5) | 199 (29.1) | 241 (29.9) | 3082 (30.2) | |
| 2007-2012 | 2554 (40.6) | 963 (39.9) | 236 (34.5) | 403 (50.1) | 4156 (40.8) | |
Abbreviation: IQR, interquartile range.
Classified under the single organ listed in parentheses: kidney-pancreas (kidney), liver-kidney (liver), lung-heart or lung-liver (lung), and heart-liver or heart-kidney (heart).
Incidence of Posttransplant KC
Posttransplant KC was diagnosed in 1690 patients (16.6%) after a median (IQR) of 3.96 (1.94-7.09) years, with an incidence rate of 2.63 per 100 patient-years (95% CI, 2.51-2.76). The age-, sex-, and calendar year–adjusted standardized incidence ratio was 6.61 (95% CI, 6.31-6.93) for transplant recipients compared with the general population.
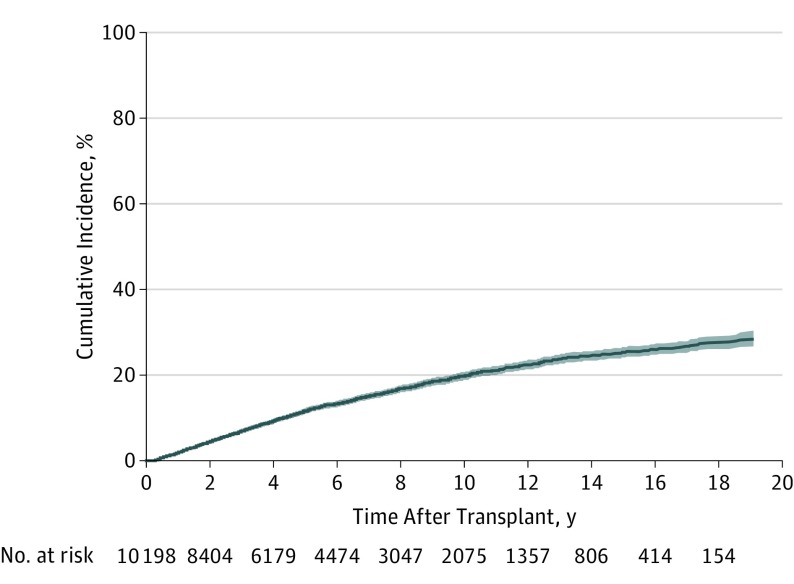
Overall, the cumulative incidence of KC ranged from 4.4% at 2 years to 19.8% at 10 years after transplant (Table 2 and Figure). The 10-year cumulative incidence was highest in patients with pretransplant skin cancer (66.5%), older than 50 years at transplant (27.5% for 51-65 years; 40.5% for >65 years), and of the white race (24.1%). Less than 2% of patients of non-white race or aged 18 to 35 years at transplant developed KC within 5 years, and less than 5% developed KC within 10 years.
Table 2. Cumulative Incidence of Keratinocyte Carcinoma After Solid Organ Transplant, Overall and Stratified by Key Risk Factors.
| Risk Factor | % Cumulative Incidence (95% CI) | ||||
|---|---|---|---|---|---|
| 2-y | 5-y | 10-y | 15-y | 19-ya | |
| Overall | 4.40 (4.01-4.81) | 11.54 (10.88-12.22) | 19.79 (18.85-20.74) | 25.23 (24.04-26.43) | 28.50 (26.76-30.28) |
| Age, y | |||||
| 18-35 | 0.24 (0.08-0.60) | 1.64 (1.07-2.42) | 4.19 (3.12-5.50) | 7.03 (5.47-8.84) | 9.70 (6.96-12.96) |
| 36-50 | 1.97 (1.54-2.50) | 6.52 (5.66-7.47) | 13.93 (12.53-15.40) | 21.03 (19.05-23.08) | 26.19 (22.59-29.93) |
| 51-65 | 5.87 (5.18-6.60) | 15.96 (14.79-17.17) | 27.46 (25.77-29.17) | 33.77 (31.60-35.95) | 36.31 (33.56-39.06) |
| >65 | 13.81 (11.63-16.17) | 28.66 (25.43-31.95) | 40.46 (36.20-44.67) | 43.96 (39.11-48.70) | 46.48 (39.55-53.11) |
| Sex | |||||
| Male | 5.30 (4.77-5.86) | 13.33 (12.46-14.22) | 22.30 (21.09-23.54) | 28.31 (26.77-29.87) | 31.61 (29.28-33.96) |
| Female | 2.74 (2.24-3.32) | 8.24 (7.30-9.25) | 15.18 (13.76-16.66) | 19.62 (17.83-21.49) | 22.93 (20.27-25.69) |
| Race | |||||
| White | 5.66 (5.09-6.28) | 14.45 (13.51-15.42) | 24.06 (22.78-25.35) | 30.23 (28.69-31.78) | 33.37 (31.31-35.44) |
| Black | 0.24 (0.02-1.30) | 1.18 (0.39-2.84) | 2.58 (1.10-5.14) | 2.58 (1.10-5.14) | 2.58 (1.10-5.14) |
| Asian/mixed/other | 0.42 (0.18-0.88) | 1.72 (1.10-2.59) | 2.57 (1.70-3.72) | 3.77 (2.44-5.54) | 3.77 (2.44-5.54) |
| Unknown | 4.39 (3.60-5.29) | 11.76 (10.40-13.22) | 21.20 (19.12-23.35) | 26.95 (24.08-29.89) | 32.84 (27.18-38.61) |
| Pretransplant skin cancer: keratinocyte carcinoma or melanoma | 36.88 (32.45-41.32) | 55.77 (50.73-60.50) | 66.46 (60.55-71.70) | 73.74 (65.79-80.13) | 73.74 (65.79-80.13) |
| Organ type | |||||
| Kidney | 4.11 (3.64-4.63) | 11.20 (10.37-12.06) | 19.17 (17.99-20.37) | 24.75 (23.25-26.29) | 27.30 (25.18-29.47) |
| Lung | 8.10 (6.33-10.14) | 14.67 (12.15-17.41) | 23.61 (20.05-27.35) | 24.95 (21.16-28.91) | 24.95 (21.16-28.91) |
| Liver | 3.54 (2.84-4.34) | 10.39 (9.12-11.75) | 18.24 (16.39-20.17) | 23.72 (21.33-26.19) | 29.86 (25.42-34.42) |
| Heart | 5.68 (4.10-7.61) | 15.04 (12.32-18.02) | 26.67 (22.73-30.76) | 34.27 (29.37-39.23) | 37.18 (31.48-42.87) |
End of study.
Figure. Cumulative Incidence Curve With 95% Confidence Band for Keratinocyte Carcinoma After First Solid Organ Transplant.
Factors Associated With Posttransplant KC
The strongest independent risk factors for KC were age, race, and a history of cancerous or precancerous skin lesions. The rate of KC increased with older age at transplant, starting from an aHR of 3.07 (95% CI, 2.40-3.95) for ages 36 to 50 years to an aHR of 9.27 (95% CI, 7.08-12.14) for older than 65 years compared with 18 to 35 years of age (Table 3). White race was associated with higher rates of KC compared with black race (aHR, 8.50; 95% CI, 4.03-17.91) and Asian, mixed, and other races (aHR, 7.77; 95% CI, 5.43-11.13). Pretransplant skin cancer (aHR, 4.30; 95% CI, 3.72-4.98) and posttransplant precancerous skin lesions (aHR, 4.32; 95% CI, 3.77-4.95) were also strongly associated with the rate of posttransplant KC.
Table 3. Association Between Potential Risk Factors and Rate of Keratinocyte Carcinoma After Solid Organ Transplant.
| Variable | Unadjusted Hazard Ratio (95% CI) | P Value | Adjusted Hazard Ratio (95% CI)a | P Value |
|---|---|---|---|---|
| Patient Characteristics | ||||
| Age at transplant, reference: 18-35 y | ||||
| 36-50 | 3.28 (2.56-4.21) | <.001 | 3.07 (2.40-3.95) | <.001 |
| 51-65 | 7.13 (5.62-9.05) | <.001 | 5.91 (4.63-7.55) | <.001 |
| >65 | 13.45 (10.39-17.42) | <.001 | 9.27 (7.08-12.14) | <.001 |
| Sex: male vs female | 1.60 (1.44-1.78) | <.001 | 1.53 (1.37-1.70) | <.001 |
| Race | ||||
| White vs black | 12.51 (5.95-26.30) | <.001 | 8.50 (4.03-17.91) | <.001 |
| White vs Asian/mixed/other | 10.29 (7.20-14.69) | <.001 | 7.77 (5.43-11.13) | <.001 |
| White vs unknown | 1.21 (1.07-1.35) | .001 | 1.06 (0.93-1.21) | .41 |
| Income quintile: high (4-5) vs low (1-3)b | 1.41 (1.28-1.55) | <.001 | 1.22 (1.11-1.34) | <.001 |
| Skin Cancer Factors | ||||
| Pretransplant skin cancer: keratinocyte carcinoma or melanoma | 8.56 (7.50-9.76) | <.001 | 4.30 (3.72-4.98) | <.001 |
| Pretransplant precancerous lesions: actinic keratosis or carcinoma in situ | 5.78 (4.49-7.43) | <.001 | 1.06 (0.80-1.41) | .66 |
| Posttransplant precancerous lesions: actinic keratosis or carcinoma in situb | 8.16 (7.23-9.21) | <.001 | 4.32 (3.77-4.95) | <.001 |
| Transplant Factors | ||||
| Organ type, reference: kidney | ||||
| Lung | 1.60 (1.34-1.90) | <.001 | 1.29 (1.06-1.57) | .009 |
| Liver | 0.94 (0.84-1.06) | .32 | 0.72 (0.62-0.84) | <.001 |
| Heart | 1.41 (1.19-1.67) | <.001 | 0.99 (0.83-1.19) | .92 |
| Multiorgan transplant at initial treatment | 0.61 (0.44-0.84) | .002 | 0.94 (0.67-1.30) | .69 |
| Subsequent transplantb | 1.40 (1.02-1.94) | .04 | 1.72 (1.23-2.41) | .001 |
| Year of transplant | ||||
| 1994-2000 vs 2001-2006 | 1.08 (0.94-1.22) | .26 | 1.32 (1.12-1.52) | .001 |
| 1994-2000 vs 2007-2012 | 1.05 (0.89-1.23) | .55 | 1.49 (1.18-1.89) | .001 |
| Health Services Factors | ||||
| Treatment center, reference: center 1c | ||||
| Center 2 | 1.08 (0.92-1.26) | .34 | 0.96 (0.80-1.15) | .67 |
| Center 3 | 0.98 (0.62-1.57) | .94 | 0.85 (0.53-1.37) | .49 |
| Center 4 | 1.09 (0.93-1.28) | .29 | 0.93 (0.75-1.15) | .49 |
| Center 5 | 1.27 (1.09-1.48) | .002 | 0.84 (0.70-0.99) | .04 |
| Center 6 | 0.73 (0.63-0.86) | .002 | 0.94 (0.78-1.13) | .53 |
| Rural residenceb | 1.59 (1.39-1.79) | <.001 | 1.28 (1.12-1.45) | <.001 |
| No. of dermatologists practicing at transplant center, year- and center-specific | 0.97 (0.96-0.99) | .009 | 0.97 (0.94-1.00) | .08 |
Multivariable cause-specific hazards regression model accounting for competing risks of death or graft loss.
Time-varying covariate.
Centers were anonymized.
To a lesser magnitude, male sex (aHR, 1.53; 95% CI, 1.37-1.70), higher socioeconomic status (aHR, 1.22; 95% CI, 1.11-1.34), and rural residence (aHR, 1.28; 95% CI, 1.12-1.45) were associated with higher rates of KC (Table 3). Compared with kidney transplant, lung transplant was associated with an increased rate of KC (aHR, 1.29; 95% CI, 1.06-1.57), whereas liver transplant was associated with a reduced rate of KC (aHR, 0.72; 95% CI, 0.62-0.84). Subsequent transplants (aHR, 1.72; 95% CI, 1.23-2.41) and earlier years of transplant (1994-2000 vs 2001-2006 aHR, 1.32; 95% CI, 1.12-1.52; 1994-2000 vs 2007-2012 aHR 1.49; 95% CI, 1.18-1.89) were also statistically significant risk factors.
Discussion
Greater susceptibility to cancer development in immunosuppressed transplant recipients has been associated with decreased host immunosurveillance, oncogenic virus infections, increased photosensitivity, and carcinogenicity of immunosuppressants.21,22 In this population-based study, we found a 7-fold higher age- and sex-standardized incidence ratio of KC after solid organ transplant compared with the general population.
To our knowledge, this result represents the first population-based estimate of the standardized incidence ratio in North America. Using a validated insurance claims–based algorithm, we were able to comprehensively capture KC diagnoses in a Canadian population despite their exclusion from cancer and transplant registries. A population-based study in the United States identified KC diagnoses using a transplant database and found an incidence rate that was about half of that observed in this Canadian cohort.23 This underestimation can be attributed to the incomplete, voluntary KC reporting by transplant centers to the Organ Procurement Transplant Network, a US transplant database found in a validation study to be missing more than half of KC diagnoses.15
We also identified key demographic and transplant-related risk factors associated with posttransplant KC while accounting for the competing risks of death and graft loss. The risk factors identified in this large population-based study supported findings from previous cohort studies, most of which were conducted in smaller or less racially diverse settings.24,25,26 A unique strength of the present study’s data sources and methods was the availability of socioeconomic and geographic data as well as our ability to account for surveillance bias by adjusting for health services use.
The standardized incidence ratio of 6.61 was lower than the ratios of 16 to 40 reported in population-based cohort studies conducted in Europe.1,2,3,4 One explanation was that the present cohort included patients with more recent transplants than patients in previous studies. As this study and others have shown, the incidence of posttransplant KC tended to be lower in patients whose transplant was performed in more recent years,27 possibly owing to evolving immunosuppressant regimens or improving sun-protective behaviors. Another potential explanation for the lower standardized incidence ratio in this cohort was the differing methods of KC ascertainment between studies. We used health insurance data to identify KC diagnoses according to physician billings. Previous studies relied on data from cancer registries, which may be more likely to capture KC diagnosed or treated in the hospital than in the community setting.28,29 Because transplant recipients may be more likely to be seen in hospitals and undergo surgical excision for advanced tumors, KC data in registries could be subject to surveillance bias, leading to overestimation of the relative risk of posttransplant KC.
The large burden of skin cancer highlighted the importance of early detection and sun-protective measures. Increased adherence to annual dermatologist visits has been associated with improved KC-related outcomes.9 Although current posttransplant care guidelines universally recommend annual skin examination, they provide limited guidance on risk stratification.8 We identified key clinical risk factors for KC that can be applied to individualize posttransplant dermatologic care: older than 50 years at transplant, white race, and history of pretransplant skin cancer.
Transplant recipients with these risk factors had a particularly high 10-year cumulative incidence of KC (24% to 66%) and may find advantageous an increased frequency of follow-up and early use of topical or systemic chemopreventive interventions. In contrast, patients with darker skin types (non-white race) or younger than 36 years at transplant had a low risk of KC (<5%) within 10 years after transplant, suggesting that frequent screening within this time frame may not be routinely necessary in the absence of other risk factors. Given that the limited accessibility to a dermatologist can be a health services barrier to annual skin examination after transplant, the ability to risk-stratify individual patients would help prioritize dermatologic follow-up for those who need it the most.
The major risk factors identified in the present cohort were consistent with those in previous studies in the transplant population.2,4,23,24,25,26 Age at transplant reflected the number of years of cumulative UV radiation exposure, whereas white race was a surrogate measure for fair skin pigmentation. Transplant recipients with a history of KC had an inherent risk of developing additional skin cancers.26 The association between male sex and KC may be attributed to differences between the sexes in sun-protective measures and higher occupational exposure to UV light.30,31,32
The multivariable analysis we conducted showed that lung transplant was associated with an increased rate of KC, whereas liver transplant recipients had a lower rate of KC compared with kidney transplant recipients, supporting the findings of other population-based studies.26,33 A multicenter American study reported that thoracic organ transplant was associated with an increased rate of squamous cell carcinoma compared with abdominal organ transplant.23 As did the present study, an Italian population-based cohort study found similar risks of KC for recipients of heart and kidney transplant.24 The variation in KC risk between graft types likely reflected the differing levels of immunosuppression required to prevent rejection34: lung transplant recipients were generally the most immunosuppressed individuals and liver transplant recipients the least immunosuppressed among all organ recipients. Similarly, the higher rate of KC observed in patients undergoing subsequent transplants may also be attributable to the surge in immunosuppressant exposure with each new transplant.
Higher socioeconomic status was another risk factor for posttransplant KC in this cohort, potentially because of the increased financial capacity for holiday travel to sunny destinations.35,36 A single-center study in white recipients of kidney transplant in the United Kingdom found that the odds of squamous cell carcinoma increased with the number of weeks per year of holidays abroad.36 An additional KC risk factor in the present study was rural residence, in which access to health care services was more limited and cumulative sun exposure was potentially greater than in urban areas.35
The development of a clinical prediction model that incorporates these risk factors would be helpful. Such a model would provide individualized risk estimates that identify patients for whom rigorous surveillance, education, and early preventive interventions would be valuable.37
Limitations
This study has some limitations. As with previous population-based studies, the administrative databases lacked data on immunosuppressive drugs and sun-protective behaviors. However, our use of time-to-event analyses accounted for the duration of immunosuppression, a key measure of exposure, and the type of transplant organ, which corresponded clinically with differing levels of immunosuppression. Furthermore, the insurance claims–based algorithm we used to identify KC could not distinguish squamous and basal cell carcinomas, although most posttransplant KC is known to consist of squamous cell carcinoma.
Conclusions
We identified a substantially higher incidence of KC in the solid organ transplant population. We also ascertained the specific risk factors associated with developing KC. A clinical prediction model that incorporates these risk factors would help identify patients at risk as well as who may need increased surveillance, education, and early preventive interventions.
References
- 1.Bannon FJ, McCaughan JA, Traynor C, et al. . Surveillance of nonmelanoma skin cancer incidence rates in kidney transplant recipients in Ireland. Transplantation. 2014;98(6):646-652. doi: 10.1097/TP.0000000000000115 [DOI] [PubMed] [Google Scholar]
- 2.Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010;10(8):1889-1896. doi: 10.1111/j.1600-6143.2010.03181.x [DOI] [PubMed] [Google Scholar]
- 3.Kyllönen L, Salmela K, Pukkala E. Cancer incidence in a kidney-transplanted population. Transpl Int. 2000;13(suppl 1):S394-S398. doi: 10.1111/j.1432-2277.2000.tb02068.x [DOI] [PubMed] [Google Scholar]
- 4.Moloney FJ, Comber H, O’Lorcain P, O’Kelly P, Conlon PJ, Murphy GM. A population-based study of skin cancer incidence and prevalence in renal transplant recipients. Br J Dermatol. 2006;154(3):498-504. doi: 10.1111/j.1365-2133.2005.07021.x [DOI] [PubMed] [Google Scholar]
- 5.Acuna SA, Fernandes KA, Daly C, et al. . Cancer mortality among recipients of solid-organ transplantation in Ontario, Canada. JAMA Oncol. 2016;2(4):463-469. doi: 10.1001/jamaoncol.2015.5137 [DOI] [PubMed] [Google Scholar]
- 6.Lindelöf B, Jarnvik J, Ternesten-Bratel A, Granath F, Hedblad MA. Mortality and clinicopathological features of cutaneous squamous cell carcinoma in organ transplant recipients: a study of the Swedish cohort. Acta Derm Venereol. 2006;86(3):219-222. doi: 10.2340/00015555-0069 [DOI] [PubMed] [Google Scholar]
- 7.Martinez J-C, Otley CC, Stasko T, et al. ; Transplant-Skin Cancer Collaborative . Defining the clinical course of metastatic skin cancer in organ transplant recipients: a multicenter collaborative study. Arch Dermatol. 2003;139(3):301-306. http://www.ncbi.nlm.nih.gov/pubmed/12622621. doi: 10.1001/archderm.139.3.301 [DOI] [PubMed] [Google Scholar]
- 8.Acuna SA, Huang JW, Scott AL, et al. . Cancer screening recommendations for solid organ transplant recipients: a systematic review of clinical practice guidelines. Am J Transplant. 2017;17(1):103-114. doi: 10.1111/ajt.13978 [DOI] [PubMed] [Google Scholar]
- 9.Chan A-W, Fung K, Austin PC, et al. . Improved keratinocyte carcinoma outcomes with annual dermatology assessment after solid organ transplantation: population-based cohort study. Am J Transplant. 2019;19(2):522-531. doi: 10.1111/ajt.14966 [DOI] [PubMed] [Google Scholar]
- 10.Kasiske BL, Vazquez MA, Harmon WE, et al. ; American Society of Transplantation . Recommendations for the outpatient surveillance of renal transplant recipients. J Am Soc Nephrol. 2000;11(suppl 15):S1-S86. [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(November)(suppl 3):S1-S155. doi: 10.1111/j.1600-6143.2009.02834.x [DOI] [PubMed] [Google Scholar]
- 12.Baker R, Jardine A, Andrews P. Renal association clinical practice guideline on post-operative care of the kidney transplant recipient. Nephron Clin Pract. 2011;118(suppl 1):c311-c347. doi: 10.1159/000328074 [DOI] [PubMed] [Google Scholar]
- 13.McGuire BM, Rosenthal P, Brown CC, et al. ; American Society of Transplantation . Long-term management of the liver transplant patient: recommendations for the primary care doctor. Am J Transplant. 2009;9(9):1988-2003. doi: 10.1111/j.1600-6143.2009.02733.x [DOI] [PubMed] [Google Scholar]
- 14.Costanzo MR, Dipchand A, Starling R, et al. ; International Society of Heart and Lung Transplantation Guidelines . The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29(8):914-956. doi: 10.1016/j.healun.2010.05.034 [DOI] [PubMed] [Google Scholar]
- 15.Garrett GL, Yuan JT, Shin TM, Arron ST; Transplant Skin Cancer Network (TSCN) . Validity of skin cancer malignancy reporting to the Organ Procurement Transplant Network: a cohort study. J Am Acad Dermatol. 2018;78(2):264-269. doi: 10.1016/j.jaad.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 16.Canadian Institute for Health Information Data Quality Study on the Canadian Organ Replacement Register. Ottawa, ON: Canadian Institute for Health Information; 2009. [Google Scholar]
- 17.Chan A-W, Fung K, Tran JM, et al. . Application of recursive partitioning to derive and validate a claims-based algorithm for identifying keratinocyte carcinoma (nonmelanoma skin cancer). JAMA Dermatol. 2016;152(10):1122-1127. doi: 10.1001/jamadermatol.2016.2609 [DOI] [PubMed] [Google Scholar]
- 18.Shah BR, Chiu M, Amin S, Ramani M, Sadry S, Tu JV. Surname lists to identify South Asian and Chinese ethnicity from secondary data in Ontario, Canada: a validation study. BMC Med Res Methodol. 2010;10:42. doi: 10.1186/1471-2288-10-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601-609. doi: 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244-256. doi: 10.1093/aje/kwp107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutierrez-Dalmau A, Campistol JM. Immunosuppressive therapy and malignancy in organ transplant recipients: a systematic review. Drugs. 2007;67(8):1167-1198. doi: 10.2165/00003495-200767080-00006 [DOI] [PubMed] [Google Scholar]
- 22.Hofbauer GFL, Bouwes Bavinck JN, Euvrard S. Organ transplantation and skin cancer: basic problems and new perspectives. Exp Dermatol. 2010;19(6):473-482. doi: 10.1111/j.1600-0625.2010.01086.x [DOI] [PubMed] [Google Scholar]
- 23.Garrett GL, Blanc PD, Boscardin J, et al. . Incidence of and risk factors for skin cancer in organ transplant recipients in the United States. JAMA Dermatol. 2017;153(3):296-303. doi: 10.1001/jamadermatol.2016.4920 [DOI] [PubMed] [Google Scholar]
- 24.Naldi L, Fortina AB, Lovati S, et al. . Risk of nonmelanoma skin cancer in Italian organ transplant recipients: a registry-based study. Transplantation. 2000;70(10):1479-1484. http://www.ncbi.nlm.nih.gov/pubmed/11118094. doi: 10.1097/00007890-200011270-00015 [DOI] [PubMed] [Google Scholar]
- 25.Molina BD, Leiro MGC, Pulpón LA, et al. . Incidence and risk factors for nonmelanoma skin cancer after heart transplantation. Transplant Proc. 2010;42(8):3001-3005. doi: 10.1016/j.transproceed.2010.08.003 [DOI] [PubMed] [Google Scholar]
- 26.Krynitz B, Edgren G, Lindelöf B, et al. . Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008–a Swedish population-based study. Int J Cancer. 2013;132(6):1429-1438. doi: 10.1002/ijc.27765 [DOI] [PubMed] [Google Scholar]
- 27.Rizvi SMH, Aagnes B, Holdaas H, et al. . Long-term change in the risk of skin cancer after organ transplantation: a population-based nationwide cohort study. JAMA Dermatol. 2017;153(12):1270-1277. doi: 10.1001/jamadermatol.2017.2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoy WE. Nonmelanoma skin carcinoma in Albuquerque, New Mexico: experience of a major health care provider. Cancer. 1996;77(12):2489-2495. doi: [DOI] [PubMed] [Google Scholar]
- 29.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30(5 Pt 1):774-778. http://www.ncbi.nlm.nih.gov/pubmed/8176018. doi: 10.1016/S0190-9622(08)81509-5 [DOI] [PubMed] [Google Scholar]
- 30.Ferreira FR, Ogawa MM, Nascimento LFC, Tomimori J. Risk factors for nonmelanoma skin cancer in renal transplant recipients: a case-control study from a reference outpatient clinic in Southeast Brazil. Int J Dermatol. 2017;56(2):154-160. doi: 10.1111/ijd.13508 [DOI] [PubMed] [Google Scholar]
- 31.Carey RN, Glass DC, Peters S, et al. . Occupational exposure to solar radiation in Australia: who is exposed and what protection do they use? Aust N Z J Public Health. 2014;38(1):54-59. doi: 10.1111/1753-6405.12174 [DOI] [PubMed] [Google Scholar]
- 32.Haluza D, Simic S, Höltge J, Cervinka R, Moshammer H. Gender aspects of recreational sun-protective behavior: results of a representative, population-based survey among Austrian residents. Photodermatol Photoimmunol Photomed. 2016;32(1):11-21. doi: 10.1111/phpp.12213 [DOI] [PubMed] [Google Scholar]
- 33.Krynitz B, Olsson H, Lundh Rozell B, Lindelöf B, Edgren G, Smedby KE. Risk of basal cell carcinoma in Swedish organ transplant recipients: a population-based study. Br J Dermatol. 2016;174(1):95-103. doi: 10.1111/bjd.14153 [DOI] [PubMed] [Google Scholar]
- 34.Madariaga MLL, Kreisel D, Madsen JC. Organ-specific differences in achieving tolerance. Curr Opin Organ Transplant. 2015;20(4):392-399. doi: 10.1097/MOT.0000000000000206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steding-Jessen M, Birch-Johansen F, Jensen A, Schüz J, Kjær SK, Dalton SO. Socioeconomic status and non-melanoma skin cancer: a nationwide cohort study of incidence and survival in Denmark. Cancer Epidemiol. 2010;34(6):689-695. doi: 10.1016/j.canep.2010.06.011 [DOI] [PubMed] [Google Scholar]
- 36.Ramsay HM, Fryer AA, Reece S, Smith AG, Harden PN. Clinical risk factors associated with nonmelanoma skin cancer in renal transplant recipients. Am J Kidney Dis. 2000;36(1):167-176. doi: 10.1053/ajkd.2000.8290 [DOI] [PubMed] [Google Scholar]
- 37.Lowenstein SE, Garrett G, Toland AE, et al. ; National Cancer Institute Keratinocyte Carcinoma Consortium . Risk prediction tools for keratinocyte carcinoma after solid organ transplantation: a review of the literature. Br J Dermatol. 2017;177(5):1202-1207. doi: 10.1111/bjd.15889 [DOI] [PubMed] [Google Scholar]