Key Points
Question
What and when is the earliest sign of treatment-induced immunosuppression associated with survival in patients with human papillomavirus–negative oropharyngeal cancer?
Findings
In this cohort study of 108 patients undergoing treatment for oropharyngeal cancer, 19 of 65 (29.2%) had grades 3 to 4 lymphopenia 3 months after radiotherapy start, which was associated with worse recurrence-free and overall survival.
Meaning
Postradiotherapy immunosuppression may be used to identify patients at risk of recurrence.
This cohort study assessed whether posttreatment lymphopenia and an elevated ratio of neutrophils to lymphocytes are associated with clinical outcomes in patients with human papillomavirus–negative oropharyngeal cancer after radiotherapy.
Abstract
Importance
Better biomarkers are needed for human papillomavirus (HPV)–negative oropharyngeal cancer (OPC) to identify patients at risk of recurrence. Lymphopenia and an elevated ratio of neutrophils to lymphocytes (NLR) have been associated with poor disease outcomes in a number of solid tumors.
Objective
To test the hypothesis that postradiotherapy lymphopenia and elevated NLR are associated with poor clinical outcomes.
Design, Setting, and Participants
This single-institution retrospective analysis included patients with HPV-negative OPC treated from January 1, 1997, through January 4, 2017. Median follow-up was 37 months (range, 2-197 months). A total of 108 patients with HPV-negative OPC and at least 1 complete blood cell count 2 to 12 months after the start of radiotherapy were included. Data were analyzed from August 26 to September 7, 2017.
Interventions
Surgery followed by radiotherapy vs definitive radiotherapy, with or without chemotherapy.
Main Outcomes and Measures
Absolute lymphocyte (ALC) and absolute neutrophil (ANC) counts were tested as variables affecting locoregional control, recurrence-free survival, and overall survival.
Results
Of a total of 108 patients included in the analysis (87.0% male; mean age, 56 years [range, 35-84 years]), 57 received surgery followed by postoperative radiotherapy and 51 received definitive radiotherapy. During treatment, 67 of 79 patients (84.8%) had grades 3 to 4 lymphopenia and 17 of 79 (21.5%) had grade 4 lymphopenia. The ANC recovered by 6 months after radiotherapy, but ALC remained depressed to 1 year after radiotherapy. Posttreatment lymphopenia and elevated NLR were associated with worse recurrence-free and overall survival. The estimated 3-year LRC in patients with and without grades 3 to 4 lymphopenia at 3 months after radiotherapy start was 73% vs 82% (hazard ratio [HR], 0.58; 95% CI, 0.19-1.8); estimated 3-year recurrence-free survival, 36% vs 63% (HR, 0.45; 95% CI, 0.23-0.87); and estimated 3-year overall survival, 34% vs 64% (HR, 0.45; 95% CI, 0.23-0.88). In multivariable analysis, an association with worse overall survival was found for definitive radiotherapy (HR, 3.3; 95% CI, 1.6-7.1) and grades 3 to 4 lymphopenia (HR, 2.6; 95% CI, 1.3-5.5) at 3 months after radiotherapy.
Conclusions and Relevance
Lymphopenia and NLR as early as 3 months after treatment start may serve as biomarkers of clinical outcomes in patients with HPV-negative OPC. These patients may benefit from adjuvant treatment intensification or closer surveillance.
Introduction
In the past several decades, the role of the host immune response in cancer progression has been increasingly recognized.1 Numerous studies have investigated neutrophils as key mediators in promoting tumor growth and metastasis in patients with cancer.2,3,4 In vivo and in vitro laboratory studies have demonstrated that the tumor microenvironment promotes neutrophil release from the bone marrow and recruitment to the tumor site through cytokine mediators.5 At the tumor site, neutrophils then release cytokines that promote angiogenesis, tumorigenesis, and metastasis.6 In addition, the increased number of circulating neutrophils has been shown to downregulate the cytotoxic activity of other leukocyte cell types—including lymphocytes and natural killer cells—and thereby compromises the host’s antitumor response.7 Taking advantage of this pathophysiological process, an elevated ratio of peripheral blood neutrophils to lymphocytes (NLR) has been examined as a cost-effective biomarker and has been found to be associated with adverse overall survival in a number of solid tumor sites.8
Despite emerging data on the prognostic value of NLR in several types of head and neck cancers, its association with mortality in human papillomavirus (HPV)–negative oropharyngeal cancers (OPCs) has been conflicting. Initial data from Huang et al9 showed that pretreatment circulating lymphocyte and neutrophil counts were not independently associated with increased mortality risk in patients with HPV-negative cancer. In contrast, Rachidi et al10 concluded that an elevation of the pretreatment NLR in patients with HPV-negative cancer resulted in a statistically significant increase in the risk of death, while surprisingly finding no statistically significant association for NLR in patients with HPV-positive tumors. In the face of these conflicting findings, the prognostic value of pretreatment NLR in HPV-negative OPCs has not yet been confirmed satisfactorily. In addition, treatment-induced lymphopenia has been associated with worse survival in patients with HPV-negative OPCs.11 Therefore, we sought to investigate the clinical importance of pretreatment compared with posttreatment NLR in patients with HPV-negative OPCs.
Methods
Study Design and Patient Selection
The institutional review board of Washington University School of Medicine in Saint Louis, St Louis, Missouri, approved this retrospective review of patients with head and neck cancer, with waiver of consent, who underwent radiotherapy at a single institution. Patients with HPV-negative OPC were included. Data were collected retrospectively for patients treated from January 1, 1997, through December 31, 2004, and prospectively gathered from January 1, 2005, through January 4, 2017. Median follow-up was 3.1 years (0.2-16.4 years). Human papillomavirus status was routinely checked in oropharyngeal squamous cell cancers since the secondary analysis of Radiation Therapy Oncology Group study 0129 in 2010.12 Before 2010, some patients had HPV status checked on archived pathology samples and were included in the analysis. Positive p16INK4A (p16) immunohistochemistry staining (>75% of tumor cells) was used as a surrogate marker for HPV.13 Patients were excluded if they did not have a complete blood cell count 2 months before treatment to 1 year after radiotherapy ended.
Treatment
All patients were cared for in a multidisciplinary setting with surgeons (R.S.J., R.P., J.R., and J.Z.), medical oncologists (P.O. and D.A.), and radiation oncologists (MD, H.G., and W.T.). Patients underwent staging by the American Joint Committee on Cancer Staging Manual 7th edition criteria. All patients diagnosed and receiving their first course of treatment at our institution underwent prospective evaluation for comorbidities using the Adult Comorbidity Evaluation 27 index.14 Patients were treated with definitive chemoradiotherapy with or without induction chemotherapy or were treated with surgery followed by adjuvant radiotherapy with or without chemotherapy.
Follow-up
Patients were evaluated with a physical examination and computed tomography of the neck at 6 to 8 weeks after radiotherapy. Starting in 2000, patients also received fluordeoxyglucose F 18–labeled positron emission tomography and computed tomography at 10 to 16 weeks. Subsequently, patients underwent evaluation with physical examination every 3 to 4 months and additional imaging if indicated. After 4 years, examinations occurred annually. Computed tomography or radiography of the chest was obtained annually.
Assessment of Hematologic Toxic Effects
Absolute lymphocyte counts (ALC) were evaluated before treatment, at the lymphocyte count nadir during treatment (ALC nadir) and 3 months (±1 month), 6 months (±2 months), and 12 months (±2 months) after the start of radiotherapy. Absolute neutrophil counts (ANC) were analyzed at the same points to calculate the NLR (ANC/ALC). Lymphopenia was assessed by Common Terminology Criteria for Adverse Events (version 4.03) as grades 3 to 4 (ALC <500/μL) and grade 4 (ALC <200/μL) (to convert to ×109 per liter, multiply by 0.001).
Statistical Analysis
Data were analyzed from August 26 through September 7, 2017. Clinically meaningful end points included grades 3 to 4 lymphopenia at any assessed point, locoregional control, recurrence-free survival, and overall survival. Time 0 for time to event analysis was defined as the end of radiotherapy. The effect size of ANC, ALC, and NLR change with treatment, compared with pretreatment levels, was reported with analysis of variance η2 statistics.15 A clinically meaningful effect size was defined as η2>0.3. Hematologic variables were dichotomized at grades 3 to 4 lymphopenia, or the median value and their association with locoregional control, recurrence-free survival, and overall survival was explored using Kaplan-Meier and Cox proportional hazards regression analyses. Variables that were associated with the outcomes on the univariable analysis were entered into a stepwise regression analysis using the backward-conditional method. Logistic regression was used to determine clinical and treatment-related risk factors associated with grades 3 to 4 lymphopenia during and after treatment. Adjusted odds ratios (ORs), hazard ratios (HRs), and 95% CIs were reported. All statistical analyses were performed using SPSS software (version 23; IBM Corp).
Results
Baseline Patient and Tumor Characteristics
Of 475 patients with OPC and known HPV and/or p16 status, tumors in 120 (25.3%) were HPV negative. Twelve patients did not have laboratory values or had no follow-up and were excluded. The baseline demographic and tumor characteristics of the remaining 108 patients (94 male [87.0%] and 14 female [13.0%]; mean age, 56 years [range, 35-84 years]) are summarized in Table 1. Patients receiving postoperative radiotherapy (PORT) (n = 57) were more likely to be male (OR, 5.0; 95% CI, 1.3-19.0) with T1 to T2 disease (OR, 2.9; 95% CI, 1.3-6.4). Patients receiving PORT did not have significantly better Adult Comorbidity Evaluation 27 scores (moderate to severe vs none to mild: OR, 0.6; 95% CI, 0.3-1.4). Patients receiving definitive radiotherapy (n = 51) were more likely to receive concurrent chemotherapy with or without induction chemotherapy (OR, 7.8; 95% CI, 2.9-21). Median radiotherapy dose was 70 Gy (range, 66-75 Gy) for definitive radiotherapy and 66 Gy (range, 60-70 Gy) for PORT. Most of the surgical procedures were transoral with concurrent neck dissections. Intensity-modulated radiotherapy planning was used for all patients receiving radiotherapy. Chemotherapy typically included 3 cycles of intravenous bolus cisplatin (100 mg/m2) if given concurrently with radiotherapy. Concurrent intravenous cetuximab (400 mg/m2 for induction; 250 mg/m2 weekly) was given to 7 patients. Induction chemotherapy generally included 3 cycles of a docetaxel-cisplatin-fluorouracil–based or an abraxane-based regimen.
Table 1. Baseline Patient and Tumor Characteristics.
| Characteristic | Treatment Group | ||
|---|---|---|---|
| Total (n = 108) | Surgery-PORT (n = 57) | Definitive Radiotherapy (n = 51) | |
| Age, mean (range), y | 56 (35-84) | 56 (37-74) | 58 (35-84) |
| Sex, No. (%) | |||
| Male | 94/108 (87.0) | 54/57 (94.7) | 40/51 (78.4) |
| Female | 14/108 (13.0) | 3/57 (5.3) | 11/51 (21.6) |
| Race, No. (%) | |||
| White | 78/108 (72.2) | 49/57 (86.0) | 29/51 (56.9) |
| Black | 28/108 (25.9) | 7/57 (12.3) | 21/51 (41.2) |
| Other | 2/108 (1.9) | 1/57 (1.8) | 1/51 (2.0) |
| Smoking, No. (%) | 83/92 (90.2) | 39/45 (86.7) | 44/47 (93.6) |
| Pack-years, median (range) | 40 (6-200) | 43 (6-200) | 40 (10-90) |
| ACE-27 comorbidity score, No. (%) | |||
| None | 26/101 (25.5) | 18/53 (34.0) | 8/47 (17.0) |
| Mild | 40/101 (39.5) | 20/53 (37.7) | 20/47 (42.6) |
| Moderate | 21/101 (21.0) | 11/53 (20.8) | 10/47 (21.2) |
| Severe | 14/101 (25.5) | 5/53 (9.4) | 9/47 (19.1) |
| Location, No. (%) | |||
| Tonsil | 63/108 (58.3) | 32/57 (56.1) | 31/51 (60.8) |
| Base of tongue | 38/108 (35.2) | 21/57 (36.8) | 17/51 (33.3) |
| Soft palate | 7/108 (6.5) | 4/57 (7.0) | 3/51 (5.9) |
| Tumor staging, No. (%) | |||
| T1 | 17/107 (15.9) | 14/57 (24.6) | 3/50 (6.0) |
| T2 | 35/107 (32.7) | 21/57 (36.8) | 14/50 (28.0) |
| T3 | 19/107 (17.8) | 10/57 (17.5) | 9/50 (18.0) |
| T4a | 31/107 (29.0) | 11/57 (19.3) | 20/50 (40.0) |
| T4b | 5/107 (4.7) | 1/57 (1.8) | 4/50 (8.0) |
| Node staging, No. (%) | |||
| N0 | 17/108 (15.7) | 11/57 (19.3) | 6/51 (11.8) |
| N1 | 21/108 (19.4) | 9/57 (15.8) | 12/51 (23.5) |
| N2a | 8/108 (7.4) | 3/57 (5.3) | 5/51 (9.8) |
| N2b | 28/108 (25.9) | 18/57 (31.6) | 10/51 (19.6) |
| N2c | 25/108 (23.1) | 14/57 (24.6) | 11/51 (21.6) |
| N3 | 9/108 (8.3) | 2/57 (3.5) | 7/51 (13.7) |
| Poorly differentiated, No. (%) | 50/106 (47.2) | 31/57 (54.4) | 19/49 (38.8) |
| Moderately differentiated, No. (%) | 54/106 (50.9) | 26/57 (45.6) | 28/49 (57.1) |
| Well differentiated, No. (%) | 2/106 (1.9) | 0/57 | 2/49 (4.1) |
| Neck dissection, No. (%) | |||
| Unilateral | 36/56 (64.3) | 36/56 (64.3) | NA |
| Bilateral | 19/56 (33.9) | 19/56 (33.9) | NA |
| None | 1/56 (1.8) | 1/56 (1.8) | NA |
| Nodal extracapsular extension, No. (%) | 40/55 (72.73) | 40/55 (72.7) | NA |
| Positive margin, No. (%) | 9/55 (16.4) | 9/55 (16.4) | NA |
| Nerve involvement, No. (%) | 14/50 (28.0) | 14/50 (28.0) | NA |
| Prescription dose, median (range), Gy | 70 (60-75) | 66 (60-70) | 70 (66-75) |
| Dose per fraction, median (range), Gy | 2.0 (1.7-2.14) | 2.0 (1.9-2.14) | 2.0 (1.7-2.14) |
| No chemotherapy, No. (%) | 34/108 (31.5) | 29/57 (50.9) | 5/51 (9.8) |
| Concurrent chemotherapy, No. (%) | 46/108 (39.8) | 28/57 (49.1) | 18/51 (35.3) |
| No. of cycles, median (range) | 3 (1-14) | 2 (1-8) | 3 (1-14) |
| Induction and concurrent chemotherapy, No. (%) | 28/108 (28.7) | 0/57 | 28/51 (54.9) |
| No. of induction cycles, median (range) | 3 (1-4) | NA | 3 (1-4) |
Abbreviation: NA, not applicable.
Hematologic Toxic Effects
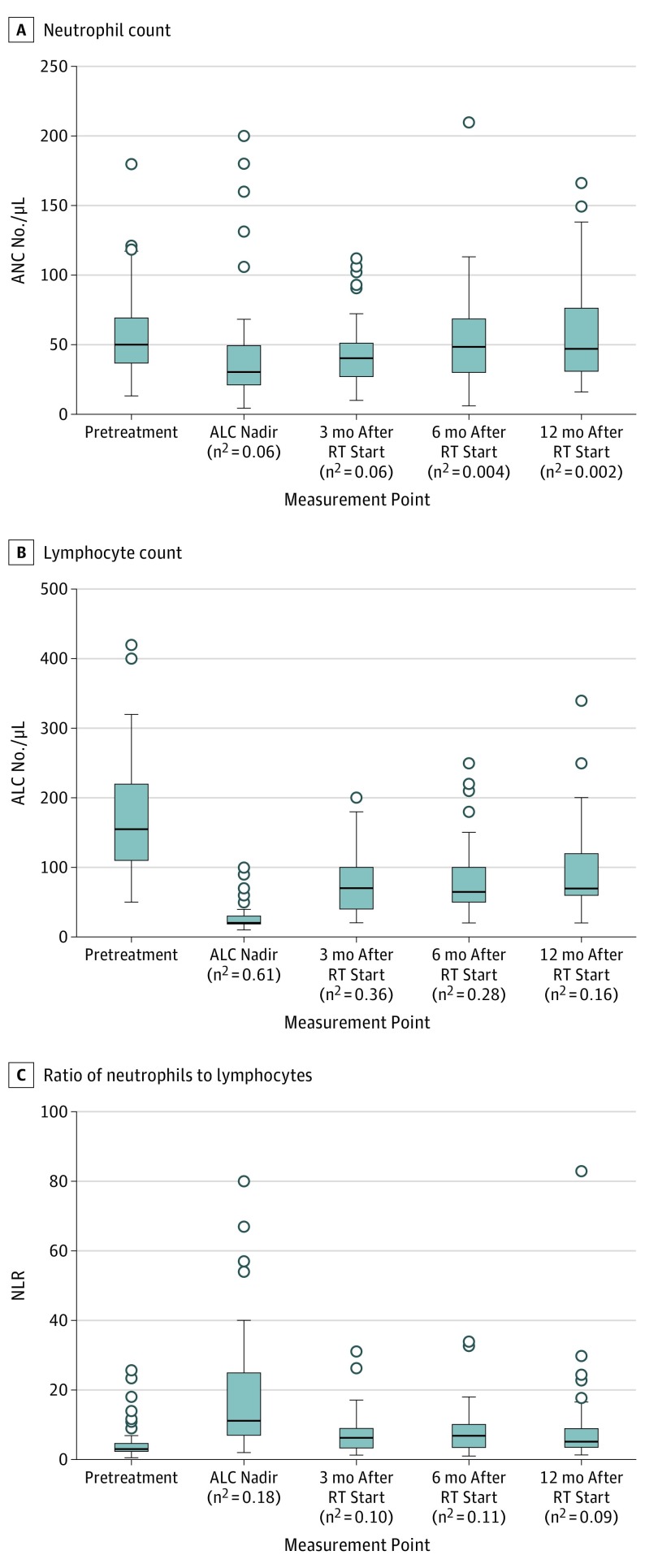
Complete blood cell counts were available for analysis in 92 patients at before treatment, 79 patients at the ALC nadir, 65 patients at 3 months after radiotherapy start, 54 patients at 6 months after radiotherapy start, and 35 patients at 12 months after radiotherapy start. After radiotherapy start, the ALC nadir occurred and remained depressed at the 3-month point (Figure 1). The ANC did not meaningfully change with treatment. At the ALC nadir, 67 of 79 patients (84.8%) had grades 3 to 4 lymphopenia and 17 of 79 (21.5%) had grade 4 lymphopenia. Grades 3 to 4 lymphopenia occurred in 19 of 65 patients (29.2%) at 3 months, 8 of 54 (14.8%) at 6 months, and 3 of 35 (8.6%) at 12 months after radiotherapy start. No differences in grade 3 to 4 lymphopenia rates at 3 months after radiotherapy start were seen between patients receiving PORT and those receiving definitive radiotherapy (PORT OR, 1.1; 95% CI, 0.4-3.4).
Figure 1. Box and Whisker Plots of Outcomes.
Box and whisker plots of absolute neutrophil count (ANC), absolute lymphocyte count (ALC), and ratio of neutrophils to lymphocytes (NLR) at pretreatment, the ALC nadir during treatment and 3, 6, and 12 months after radiotherapy (RT) start. Median is denoted by the line inside the box; interquartile range, box. The whiskers include 95% of a normally distributed range around the median. Open circles indicate outliers (to convert ALC and ANC to ×109 per liter, multiply by 0.001).
Survival Analyses
Median follow-up was 37 months (range, 2-197 months) after radiotherapy start. Median overall survival was 57 months (95% CI, 27-88 months). In Cox proportional hazards regression, grades 3 to 4 lymphopenia (HR, 2.2; 95% CI, 1.1-4.3) and NLR (HR, 1.1; 1.0-1.1) at 3 months after radiotherapy start were associated with death. Baseline and nadir ALC, ANC, and NLR were not significantly associated with death. In multivariable analysis, the earliest hematologic toxic effect to be associated with death was grades 3 to 4 lymphopenia at 3 months after radiotherapy start (HR, 2.5; 95% CI, 1.3-5.0). Patients who were selected for surgery or PORT had increased survival over patients selected for definitive radiotherapy (HR, 0.3; 95% CI, 0.1-0.6) (Table 2).
Table 2. Cox Proportional Hazards Regression of Clinicopathologic Factors Associated With Mortality.
| Factor | HR (95% CI) | |
|---|---|---|
| Univariable | Multivariable | |
| Age | 1.0 (1.00-1.04) | NA |
| Female vs male | 0.8 (0.4-2.0) | NA |
| Smoking >20 pack-years | 1.1 (0.5-2.3) | NA |
| ACE-27 score | ||
| None to mild | 1 [Reference] | Not included in final model |
| Moderate to severe | 1.8 (1.1-3.1) | |
| Location | ||
| Tonsil | 1 [Reference] | NA |
| Base of tongue | 1.1 (0.6-1.8) | NA |
| Soft palate | 2.5 (0.9-7.2) | NA |
| T3-T4 vs T1-T2 | 2.4 (1.4-4.0)a | Not included in final model |
| N0-N1 | 1 [Reference] | NA |
| N2a-N2b | 0.7 (0.4-1.3) | NA |
| N2c-N3 | 1.4 (0.8-2.6) | NA |
| Poorly differentiated vs well or moderately differentiated | 0.5 (0.3-0.9)a | Not included in final model |
| Surgery-PORT vs definitive CRT | 0.6 (0.4-1.0)a | 0.3 (0.1-0.6)a |
| Chemotherapy | ||
| None | 1 [Reference] | NA |
| Concurrent | 1.1 (0.6-1.9) | NA |
| Induction and concurrent | 1.7 (0.9-3.3) | NA |
| Baseline ALC | 0.9 (0.7-1.3) | NA |
| Nadir ALC | 0.9 (0.1-6.3) | NA |
| Nadir grade 3-4 lymphopenia | 0.8 (0.3-1.9) | NA |
| 3-mo post-RT ALC | 0.5 (0.2-1.2) | NA |
| 3-mo post-RT grades 3-4 lymphopenia | 2.2 (1.1-4.3)a | 2.6 (1.3-5.5)a |
| 6-mo post-RT ALC | 0.3 (0.1-0.9)a | Not included in final model |
| 6-mo post-RT grades 3-4 lymphopenia | 1.6 (0.6-4.3) | NA |
| 12-mo post-RT ALC | 0.2 (0.1-0.7)a | Not included in final model |
| 12-mo post-RT grades 3-4 lymphopenia | 1.4 (0.3-6.3) | NA |
| Pretreatment NLR | 1.0 (1.00-1.08) | NA |
| ALC nadir NLR | 1.00 (0.99-1.01) | NA |
| 3-mo post-RT NLR | 1.07 (1.01-1.13)a | Not included in final model |
| 6-mo post-RT NLR | 1.02 (0.97-1.07) | NA |
| 12-mo post-RT NLR | 1.06 (1.02-1.09)a | Not included in final model |
| Second cancer after RT | 0.8 (0.4-1.5) | NA |
Abbreviations: ACE, Adult Comorbidity Evaluation 27; ALC, absolute lymphocyte count; CRT, chemoradiotherapy; NA, not applicable; NLR, ratio of neutrophils to lymphocytes; PORT, postoperative radiotherapy; RT, radiotherapy.
Indicates significance.
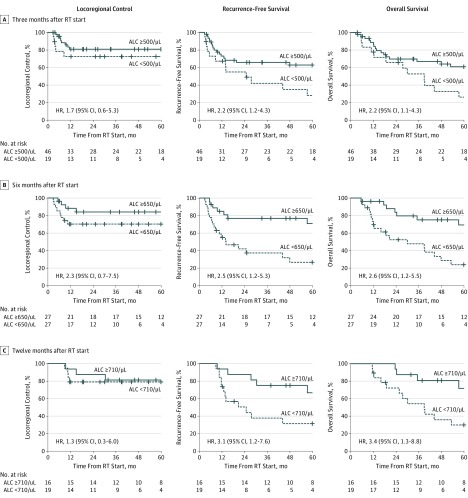
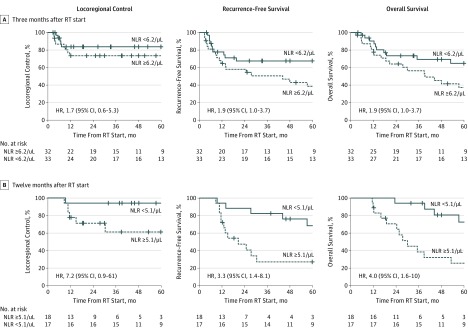
The estimated 3-year rate of locoregional control in patients with grades 3 to 4 lymphopenia at 3 months after radiotherapy start was 73% compared with 82% for those without lymphopenia (HR, 1.7; 95% CI, 0.56-5.3); estimated recurrence-free survival, 43% vs 66% (HR, 2.2; 95% CI, 1.2-4.3); and estimated overall survival, 54% vs 67% (HR, 2.2; 95% CI, 1.1-4.3), respectively (Figure 2A). Patients with prolonged lymphopenia at 6 and 12 months after radiotherapy start also had worse recurrence-free survival (6-month HR, 2.5 [95% CI, 1.2-5.3]; 12-month HR, 3.1 [95% CI, 1.2-7.6]) and overall survival (6-month HR, 2.6 [95% CI, 1.2-5.5]; 12-month HR, 3.4 [95% CI, 1.3-8.8]), but not locoregional control (6-month HR, 2.3 [95% CI, 0.7-7.5]; 12-month HR, 1.3 [95% CI, 0.3-6.0]) (Figure 2B and C). The NLR, which inversely correlated with ALC at each time point, also separated recurrence-free survival (3-month HR, 1.9 [95% CI, 1.0-3.7]; 12-month HR, 3.3 [95% CI, 1.4-8.1]) and overall survival (3-month HR, 1.9 [95% CI, 1.0-3.7]; 12-month HR, 4.0 [95% CI, 1.6-10.0]) curves after radiotherapy start (Figure 3).
Figure 2. Kaplan-Meier Curves for Outcomes by Absolute Lymphocyte Count (ALC).
Kaplan-Meier curves of locoregional control, recurrence-free survival, and overall survival for patients stratified by designated points for measurement of ALC after radiotherapy (RT) start. Grades 3 to 4 lymphopenia was used for the 3-month RT cutpoint. The median ALC was used for the 6- and 12-month RT cutpoints (to convert ALC to ×109 per liter, multiply by 0.001). HR indicates hazard ratio.
Figure 3. Kaplan-Meier Curves for Outcomes by Ratio of Neutrophils to Lymphocytes (NLR).
Kaplan-Meier curves of locoregional control, recurrence-free survival, and overall survival for patients stratified by designated points for measurement of NLR after radiotherapy (RT) start. The median NLR was used for the 3- and 12-month RT cutpoints. HR indicates hazard ratio.
Patients who received surgery were less likely to have locoregional recurrence compared with patients treated with definitive radiotherapy (HR, 0.31; 95% CI, 0.12-0.79). In the definitive radiotherapy subset, lymphopenia at 3 months after radiotherapy start was not significantly associated with locoregional recurrence (HR, 2.2; 95% CI, 0.7-6.8), but this could be owing to limited patient numbers.
The patients with postradiotherapy lymphopenia were more likely to have distant recurrence (HR for 3 months after radiotherapy start, 2.0 [95% CI, 0.7-5.9]; HR for 6 months after radiotherapy start, 5.2 [95% CI, 1.4-19]; HR for 12 months after radiotherapy start, 9.6 [95% CI, 1.2-79.0]). The lung was the sole site of metastasis in 14 of 23 patients (60.9%) with distant disease. Bone was the next most common site, found in 6 of 23 patients (26.1%) with distant metastasis. The other 3 patients (13.0%) had soft tissue metastases.
Modifiers of Grades 3 to 4 Lymphopenia
The only clinical or treatment factors associated with developing grade 4 lymphopenia at the ALC nadir were T3 to T4 vs T1 to T2 tumors (OR, 5.3; 95% CI, 1.4-20.0). Tumor stage, patient age, sex, smoking history, comorbidities, induction and concurrent chemotherapy, concurrent chemotherapy alone, and surgery were not significantly associated with grades 3 to 4 lymphopenia at any other point (all 95% CIs crossed 1.0).
Discussion
The clinical significance of circulating blood cell counts in risk stratification for HPV-negative OPCs remains controversial. In this study of patients with HPV-negative OPC, we evaluated the significance of ALC and NLR as risk factors and found no correlation of pretreatment or nadir hematologic markers with patient mortality after treatment. This study supports the findings of Huang et al,9 who found that pretreatment ALC and NLR were not prognostic in patients with HPV-negative OPCs treated with definitive chemoradiotherapy. A growing body of evidence suggests that although pretreatment complete blood cell counts are ubiquitously available to physicians, an elevated baseline NLR may not be an accurate metric in estimating treatment response to definitive chemoradiotherapy or surgery.
Our study has pointed to posttreatment ALC and NLR as effective biomarkers associated with outcomes in OPCs. Notably, the ALC and NLR were found to be associated with recurrence-free and overall survival as early as 3 months after radiotherapy start. Our findings are consistent with those of a recent report in which patients with HPV-negative OPC and severe lymphopenia at 2 months after beginning chemoradiotherapy had significantly higher rates of disease progression and early tumor recurrence.11 Moreover, in a study of 104 patients with head and neck cancer, Kim et al16 found posttreatment NLR to be superior to pretreatment NLR in estimating recurrence and cancer-specific survival. Elevated posttreatment NLR values have been associated with decreased disease-free and overall survival in other disease sites as well, including esophageal17 and locally advanced lung cancer.18 Together these studies indicate a potential need for rigorous posttreatment survelliance to identify patient cohorts at risk for recurrence. Patients with elevated NLR or lymphopenia after radiotherapy may be selected for clinical trials testing circulating tumor DNA19 and/or repeated imaging for salvage treatment to be initiated earlier.
An intriguing finding in our study is that the elevated NLR was primarily driven by ALC, implying that posttreatment lymphopenia alone may be adequate for estimating disease outcomes. Although radiotherapy resulted in comparable decreases of ALC and ANC initially, ANC returned to baseline by 6 months after radiotherapy start, while ALC remained persistently low. The mechanism underlying prolonged radiotherapy-induced lymphopenia remains unclear. Irradiation to the circulating blood likely plays a major role: lymphocytes are highly radiosensitive and even low-dose total body radiotherapy has been observed to result in lymphopenia that persists for years after initial radiotherapy exposures.20 Some recent animal studies have also observed that radiotherapy results in the release of in vivo soluble factors, such as galectin-1, to promote a state of chronic inflammation.21 This inflammation then results in downstream direct destruction of circulating lymphocytes and indirect effects on bone marrow hematopoietic stem-cell populations.21,22,23 Stages T3 to T4 disease were associated with grade 4 lymphopenia in this study. Indeed, previous work has shown that large radiotherapy target volume sizes result in irradiation of circulating blood and subsequent reduction of the circulating lymphocyte count.24,25 When radiated volumes have been reduced in patients with lateralized tonsil cancer and glioblastoma, rates of acute grades 3 to 4 lymphopenia also dropped.26,27 With this finding in mind, clinical trials may be suggested to limit radiation exposure to the major neck vessels, such as sparing contralateral neck radiotherapy in patients who had negative findings of neck dissection28 or well-lateralized disease.29 We hypothesize that shortening radiotherapy courses and using higher dose rates may also limit radiotherapy-induced lymphopenia.
Chemotherapy was not associated with lymphopenia or elevated NLR in our study of patients with HPV-negative OPC. In contrast, previous glioma studies30 have found concurrent and adjuvant chemotherapy to be dominant factors in acute and prolonged lymphopenia, respectively. This finding may be partly attributed to toxic effects associated with particular chemotherapeutic agents. Temozolomide and a combination of procarbazine hydrochloride, carmustine, and vincristine sulfate, commonly used for gliomas, are frequently associated with drug-induced cytopenias and bone marrow toxic effects.31 On the other hand, cisplatin, the most common chemotherapy used in treatment of OPCs, is more likely to result in nephrotoxic effects or peripheral neuropathy.31 We also did not account for patients with neutropenia who received granulocyte colony-stimulating factor, which may partially explain the recovery of ANC from the nadir. Granulocyte colony-stimulating factor does not significantly change ALC,32 and no analogous recombinant growth factor has been approved by the US Food and Drug Administration to stimulate production of lymphocytes. Results of an on-going clinical trial33 using interleukin-7 to increase ALC after chemoradiotherapy for high-grade gliomas will be informative.
How posttreatment lymphopenia affects adjuvant immunotherapy strategies remains to be seen. The recent success of adjuvant durvalumab, a programmed cell death ligand 1 inhibitor, in stage III lung cancer34 treated with definitive chemoradiotherapy bodes well for adjuvant immunotherapy trials in head and neck cancers.35,36 Both nivolumab and pembrolizumab have been safe with signals of activity in recurrent and/or metastatic head and neck squamous cell carcinoma.37,38 However, recent abstracts have indicated that patients’ response rate to programmed cell death receptor 1 inhibitors such as nivolumab and pembrolizumab are significantly reduced in the setting of persistent lymphopenia39 and corticosteroid use.40 Radiotherapy-induced severe lymphopenia at the initiation of therapy with checkpoint inhibitors was associated with worse survival in patients with metastatic cancer.41 Although radiotherapy in patients with nonmetastatic disease has not been associated with prolonged lymphopenia,26,30 older or sicker patients may not have the bone marrow reserve to recover from acute hematologic injury due to radiotherapy and/or chemotherapy. Consequently, preemptive strategies to reduce treatment-induced lymphopenia should still remain at the forefront of patient treatment plans.
Limitations
This retrospective study has several limitations, including selection bias and incomplete laboratory follow-up. In our heterogeneously treated cohort, Cox proportional hazards regression analysis revealed that patients who were selected for surgery and PORT had better outcomes compared with patients selected for definitive chemoradiotherapy. Patients receiving surgery were more likely to have smaller T1 to T2 tumors, and we were not able to separate these confounding factors out in our analysis. Given these limitations, future prospective trials in homogenously treated patients are critical to further evaluate the utility of posttreatment NLR in patients with HPV-negative OPCs.
Conclusions
In summary, we add to the growing body of evidence that treatment-induced lymphopenia and elevated NLR are associated with survival outcomes in HPV-negative OPCs as early as 3 months after radiotherapy start. We hypothesize that modifying radiotherapy volumes, dose, and fractionation for select patients as well as trials looking at lymphocyte-stimulating factors are potential strategies to mitigate recurrence risk. Finally, early monitoring specifically for treatment-induced lymphopenia may stratify high-risk patients who could not only benefit from treatment intensification (whether adjuvant checkpoint inhibitors or cytokine infusions), but who would also warrant closer surveillance imaging.
References
- 1.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883-899. doi: 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cools-Lartigue J, Spicer J, McDonald B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123(8):3446-3458. doi: 10.1172/JCI67484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Larco JE, Wuertz BRK, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res. 2004;10(15):4895-4900. doi: 10.1158/1078-0432.CCR-03-0760 [DOI] [PubMed] [Google Scholar]
- 4.Brandau S, Dumitru CA, Lang S. Protumor and antitumor functions of neutrophil granulocytes. Semin Immunopathol. 2013;35(2):163-176. doi: 10.1007/s00281-012-0344-6 [DOI] [PubMed] [Google Scholar]
- 5.Dumitru CA, Lang S, Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. Semin Cancer Biol. 2013;23(3):141-148. doi: 10.1016/j.semcancer.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 6.Tazzyman S, Lewis CE, Murdoch C. Neutrophils: key mediators of tumour angiogenesis. Int J Exp Pathol. 2009;90(3):222-231. doi: 10.1111/j.1365-2613.2009.00641.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Treffers LW, Hiemstra IH, Kuijpers TW, van den Berg TK, Matlung HL. Neutrophils in cancer. Immunol Rev. 2016;273(1):312-328. doi: 10.1111/imr.12444 [DOI] [PubMed] [Google Scholar]
- 8.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 9.Huang SH, Waldron JN, Milosevic M, et al. Prognostic value of pretreatment circulating neutrophils, monocytes, and lymphocytes in oropharyngeal cancer stratified by human papillomavirus status. Cancer. 2015;121(4):545-555. doi: 10.1002/cncr.29100 [DOI] [PubMed] [Google Scholar]
- 10.Rachidi S, Wallace K, Wrangle JM, Day TA, Alberg AJ, Li Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck. 2016;38(suppl 1):E1068-E1074. doi: 10.1002/hed.24159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campian JL, Sarai G, Ye X, Marur S, Grossman SA. Association between severe treatment-related lymphopenia and progression-free survival in patients with newly diagnosed squamous cell head and neck cancer. Head Neck. 2014;36(12):1747-1753. doi: 10.1002/hed.23535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. doi: 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis JS., Jr p16 Immunohistochemistry as a standalone test for risk stratification in oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2012;6(suppl 1):S75-S82. doi: 10.1007/s12105-012-0369-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441-2447. doi: 10.1001/jama.291.20.2441 [DOI] [PubMed] [Google Scholar]
- 15.Ellis P. The Essential Guide to Effect Sizes: Statistical Power, Meta-Analysis, and the Interpretation of Research Results. Cambridge, UK: Cambridge University Press; 2015. [Google Scholar]
- 16.Kim DY, Kim IS, Park SG, Kim H, Choi YJ, Seol YM. Prognostic value of posttreatment neutrophil-lymphocyte ratio in head and neck squamous cell carcinoma treated by chemoradiotherapy. Auris Nasus Larynx. 2017;44(2):199-204. doi: 10.1016/j.anl.2016.05.013 [DOI] [PubMed] [Google Scholar]
- 17.Hyder J, Boggs DH, Hanna A, Suntharalingam M, Chuong MD. Changes in neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios during chemoradiation predict for survival and pathologic complete response in trimodality esophageal cancer patients. J Gastrointest Oncol. 2016;7(2):189-195. doi: 10.3978/j.issn.2078-6891.2015.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Contreras JA, Lin AJ, Weiner A, et al. Cardiac dose is associated with immunosuppression and poor survival in locally advanced non–small cell lung cancer. Radiother Oncol. 2018;128(3):498-504. doi: 10.1016/j.radonc.2018.05.017 [DOI] [PubMed] [Google Scholar]
- 19.Payne K, Spruce R, Beggs A, et al. Circulating tumor DNA as a biomarker and liquid biopsy in head and neck squamous cell carcinoma. Head Neck. 2018;40(7):1598-1604. doi: 10.1002/hed.25140 [DOI] [PubMed] [Google Scholar]
- 20.Brooks AL. Biomarkers of exposure, sensitivity and disease. Int J Radiat Biol. 1999;75(12):1481-1503. doi: 10.1080/095530099139106 [DOI] [PubMed] [Google Scholar]
- 21.Norling LV, Perretti M, Cooper D. Endogenous galectins and the control of the host inflammatory response. J Endocrinol. 2009;201(2):169-184. doi: 10.1677/JOE-08-0512 [DOI] [PubMed] [Google Scholar]
- 22.Kuo P, Bratman SV, Shultz DB, et al. Galectin-1 mediates radiation-related lymphopenia and attenuates NSCLC radiation response. Clin Cancer Res. 2014;20(21):5558-5569. doi: 10.1158/1078-0432.CCR-14-1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapoor V, Khudanyan A, de la Puente P, et al. Stem cell transfusion restores immune function in radiation-induced lymphopenic C57BL/6 mice. Cancer Res. 2015;75(17):3442-3445. doi: 10.1158/0008-5472.CAN-15-1412 [DOI] [PubMed] [Google Scholar]
- 24.Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31(2):140-144. doi: 10.3109/07357907.2012.762780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang J, DeWees TA, Badiyan SN, et al. Clinical and dosimetric predictors of acute severe lymphopenia during radiation therapy and concurrent temozolomide for high-grade glioma. Int J Radiat Oncol Biol Phys. 2015;92(5):1000-1007. doi: 10.1016/j.ijrobp.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 26.Lin AJ, Rao YJ, Chin RI, et al. Post-operative radiation effects on lymphopenia, neutrophil to lymphocyte ratio, and clinical outcomes in palatine tonsil cancers. Oral Oncol. 2018;86(August):1-7. doi: 10.1016/j.oraloncology.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 27.Rudra S, Hui C, Rao YJ, et al. Effect of radiation treatment volume reduction on lymphopenia in patients receiving chemoradiation for glioblastoma. Int J Radiat Oncol Biol Phys. 2018;101(1):217-225. doi: 10.1016/j.ijrobp.2018.01.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spencer CR, Chin RI, Pierro MJ, et al. Eliminating radiation therapy to the pathologic node-negative neck does not increase the risk of regional recurrence: a prospective phase 2 trial. Int J Radiat Oncol Biol Phys. 2015;93(3):S70. doi: 10.1016/j.ijrobp.2015.07.168 [DOI] [Google Scholar]
- 29.Chin RI, Rao YJ, Hwang MY, et al. Comparison of unilateral versus bilateral intensity-modulated radiotherapy for surgically treated squamous cell carcinoma of the palatine tonsil. Cancer. 2017;123(23):4594-4607. doi: 10.1002/cncr.30931 [DOI] [PubMed] [Google Scholar]
- 30.Lin AJ, Campian JL, Hui C, et al. Impact of concurrent versus adjuvant chemotherapy on the severity and duration of lymphopenia in glioma patients treated with radiation therapy. J Neurooncol. 2018;136(2):403-411. doi: 10.1007/s11060-017-2668-5 [DOI] [PubMed] [Google Scholar]
- 31.DeVita VT, Lawrence TS, Rosenbery SA. DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology. 10th ed Philadelphia, PA: Wolters Kluwer Health Adis; 2015. [Google Scholar]
- 32.Pollmächer T, Korth C, Mullington J, et al. Effects of granulocyte colony-stimulating factor on plasma cytokine and cytokine receptor levels and on the in vivo host response to endotoxin in healthy men. Blood. 1996;87(3):900-905. [PubMed] [Google Scholar]
- 33.ClinicalTrials.gov Study of the effect IL-7/NT-I7 on CD4 counts in patients with high grade gliomas. NCT02659800. https://clinicaltrials.gov/ct2/show/NCT02659800. Accessed December 31, 2018.
- 34.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med. 2017;377:1919-1929. doi: 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 35.ClinicalTrials.gov Study to compare avelumab in combination with standard of care chemoradiotherapy (SoC CRT) versus SoC CRT for definitive treatment in patients with locally advanced squamous cell carcinoma of the head and neck (JAVELIN HEAD AND NECK 100). NCT02952586. https://clinicaltrials.gov/ct2/show/NCT02952586. Accessed December 31, 2018.
- 36.ClinicalTrials.gov Randomized trial of avelumab-cetuximab-radiotherapy versus SOCs in LA SCCHN (REACH). NCT02999087. https://clinicaltrials.gov/ct2/show/NCT02999087. Accessed December 31, 2018.
- 37.Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856-1867. doi: 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chow LQM, Haddad R, Gupta S, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34(32):3838-3845. doi: 10.1200/JCO.2016.68.1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diehl A, Yarchoan M, Hopkins A, et al. Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. J Clin Oncol. 2017;8:114268-114280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arbour KC, Mezquita LM, Long N, et al. Deleterious effect of baseline steroids on efficacy of PD-(L)1 blockade in patients with NSCLC. J Clin Oncol. June 5, 2018:suppl; abstr 9003. https://meetinglibrary.asco.org/record/160292/abstract. [DOI] [PubMed]
- 41.Pike LRG, Bang A, Mahal BA, et al. The impact of radiation therapy on lymphocyte count and survival in metastatic cancer patients receiving PD-1 immune checkpoint inhibitors. Int J Radiat Oncol Biol Phys. 2019;103(1):142-151. doi: 10.1016/j.ijrobp.2018.09.010 [DOI] [PubMed] [Google Scholar]