Key Points
Question
What is the association between treatment guided by ambulatory hemodynamic monitoring and clinical outcomes in patients with heart failure?
Findings
In this matched cohort study, 1087 patients receiving a wireless pulmonary artery pressure sensor had a significantly lower rate of heart failure hospitalization at 12 months than a cohort of concurrently treated, propensity-matched control patients.
Meaning
In patients with chronic heart failure, ambulatory hemodynamic monitoring is associated with reduced rates of heart failure hospitalization.
This matched cohort study of US Medicare recipients assesses the association between ambulatory hemodynamic monitoring via a pulmonary artery pressure sensor and rates of heart failure hospitalization at 12 months after implant.
Abstract
Importance
In a randomized clinical trial, heart failure (HF) hospitalizations were lower in patients managed with guidance from an implantable pulmonary artery pressure sensor compared with usual care. It remains unclear if ambulatory monitoring could also improve long-term clinical outcomes in real-world practice.
Objective
To determine the association between ambulatory hemodynamic monitoring and rates of HF hospitalization at 12 months in clinical practice.
Design, Setting, and Participants
This matched cohort study of Medicare beneficiaries used claims data collected between June 1, 2014, and March 31, 2016. Medicare patients who received implants of a pulmonary artery pressure sensor were identified from the 100% Medicare claims database. Each patient who received an implant was matched to a control patient by demographic features, history of HF hospitalization, and number of all-cause hospitalizations. Propensity scoring based on comorbidities (arrhythmia, hypertension, diabetes, pulmonary disease, and renal disease) was used for additional matching. Data analysis was completed from July 2017 through January 2019.
Exposures
Implantable pulmonary artery pressure monitoring system.
Main Outcomes and Measures
The rates of HF hospitalization were compared using the Andersen-Gill method. Days lost owing to events were compared using a nonparametric bootstrap method.
Results
The study cohort consisted of 1087 patients who received an implantable pulmonary artery pressure sensors and 1087 matched control patients. The treatment and control cohorts were well matched by age (mean [SD], 72.7 [10.2] years vs 72.9 [10.1] years) and sex (381 of 1087 female patients [35.1%] in each group), medical history, comorbidities, and timing of preimplant HF hospitalization. At 12 months postimplant, 616 HF hospitalizations occurred in the treatment cohort compared with 784 HF hospitalizations in the control cohort. The rate of HF hospitalization was lower in the treatment cohort at 12 months postimplant (hazard ratio [HR], 0.76 [95% CI, 0.65-0.89]; P < .001). The percentage of days lost to HF hospitalizations or death were lower in the treatment group (HR, 0.73 [95% CI, 0.64-0.84]; P < .001) and the percentage of days lost owing to all-cause hospitalization or death were also lower (HR, 0.77 [95% CI, 0.68-0.88]; P < .001).
Conclusions and Relevance
Patients with HF who were implanted with a pulmonary artery pressure sensor had lower rates of HF hospitalization than matched controls and spent more time alive out of hospital. Ambulatory hemodynamic monitoring may improve outcomes in patients with chronic HF.
Introduction
Despite therapeutic advances, chronic heart failure (HF) remains a progressive disease, with advanced stages characterized by worsening symptoms, recurrent hospitalizations, and eventual death. Regardless of ejection fraction, elevations of intracardiac and pulmonary artery pressures (PAP) are a defining hemodynamic disturbance in the progression of HF. Because pressure elevations precede clinical signs and symptoms in statistically and clinically significant ways,1,2,3 longitudinal monitoring of central hemodynamics can facilitate timely interventions to reduce pressures, improve symptoms, and avoid costly hospitalizations.
Clinical trials of ambulatory hemodynamic monitoring systems have shown that lower cardiac-filling pressures are associated with lower rates of HF hospitalization3,4 and mortality.5 Moreover, real-world experience with an approved, implantable PAP sensor has shown that clinically significant reductions in PAP6 and reduced rates of HF7 are associated with sensor implants.
Uncertainty remains about the utility of ambulatory hemodynamic monitoring and its association with long-term outcomes.8 We hypothesized that improved hemodynamic management could reduce hospitalizations and increase days alive out of hospital. We therefore analyzed Medicare claims data to explore rates of HF hospitalization and death at 12 months in a cohort of patients implanted with a PAP sensor compared with matched control patients without a sensor.
Methods
Data Source
This matched cohort study using Centers for Medicare and Medicaid Services (CMS) administrative claims data from the Standard Analytic File was designed to evaluate clinical outcomes in beneficiaries of fee-for-service Medicare programs who received a PAP sensor implant after US Food and Drug Administration approval of the device for commercial use (from June 1, 2014, onward) compared with matched control patients who did not receive a sensor. Claims data include Part A inpatient claims, Part B outpatient claims, and the associated denominator files.9 Inpatient and outpatient files contain institutional claims with International Classification of Diseases, Ninth Revision, Clinical Modification and Tenth Revision, Clinical Modification diagnosis codes, procedure codes, and reimbursement associated with inpatient stays or ambulatory visits. The denominator files include unique deidentified patient identification numbers, age, sex, geographic location, race/ethnicity, date of death, and information about program eligibility and Medicare insurance enrollment.
The study was a retrospective analysis of a deidentified database and thus exempt from institutional review board approval. Deidentified health information can be used without authorization or any other permission specified in the Health Insurance Portability and Accountability Act Privacy Rule, and this study was therefore exempt from informed consent procedures.
Identification of the Treatment Cohort
We identified patients implanted with a PAP sensor using inpatient claims associated with the procedure codes 38.26, 02HQ30Z, or 02HR30Z and outpatient claims associated with Current Procedural Terminology codes C9741 and C2624 (eTable 1 in the Supplement). Because Medicare data were available through March 31, 2017, only patients who received implants on or prior to March 31, 2016, were included, to ensure a minimum of 12 months of potential follow-up time. The cohort was limited to patients with continuous, fee-for-service Medicare insurance enrollment (Medicare parts A and B) for at least 12 months before and after sensor implant. Health maintenance organization paid claims are excluded from the CMS data set; accordingly, health maintenance organization–insured patients were excluded to avoid incomplete data. The claims data extraction and statistical analyses were conducted using RStudio version 1.0.143 (R Consortium) and R version 3.4.0 (R Foundation for Statistical Computing).
Identification of Matched Controls
Using the full Medicare data set, a matched control was identified for each patient that received the CardioMEMS hemodynamic sensor (Abbott). Potential control patients were first identified based on the presence of at least 1 HF hospitalization between July 1, 2013, and March 31, 2016, yielding a population of 1.5 million patients with HF. The date of implant for each patient undergoing PAP sensor implant was used as an anchor point to identify the matched control patients. For example, if the patient in the treatment cohort was implanted with a sensor on January 1, 2016, this date served as a temporal reference point to find a matched control patient with an identical clinical profile in the 12 months prior to that date. All patient characteristics used for matching were retrieved in the 12 months preceding the anchor date.
A stepwise, iterative algorithm was then used to identify the closest match between a patient who received an implant and a patient who did not receive an implant. In each iterative run of the algorithm, matched pairs were withdrawn from further consideration. After 3 steps of the iterative algorithm, matched controls were identified for 1087 of the 1185 patients who received implants. An identical match was defined in each iterative run based on sequential matching of demographic attributes (sex, race, history of implantable cardioverter defibrillator or cardiac resynchronization therapy implant, end-stage renal disease status, and age within 5 years), then comorbidities (diabetes, hypertension, renal disorders, pulmonary disorders, and arrhythmia), followed by the closest propensity score (derived using logistic regression). Additionally, patients were matched if they incurred an identical number of HF and non-HF hospitalizations and if the timing of each HF hospitalization was within 4 months. This method was used to provide a close approximation of HF severity between patients who received implants and control patients. Finally, after the matching procedure, we compared the severity of HF hospitalization using length of stay for each HF hospitalization and cumulative number of hospitalized days per patient.
Comorbidities for each patient were determined from outpatient and inpatient encounters in the 12 months prior to the anchoring point. Detailed diagnoses codes for the comorbidities of diabetes, hypertension, renal disorders, pulmonary disorders, and arrhythmia10,11 are provided in eTable 2 in the Supplement. A hospitalization was deemed to be HF-associated based on the presence of diagnoses codes attributed to HF as defined by CMS methods (eTable 3 in the Supplement).12 In the treatment cohort, we identified preimplant HF events as those for which a diagnosis code of HF was recorded anywhere in the hospitalization claim; after sensor implant, we used only HF events that were the primary reason for admission. In the control cohort, we counted all HF events in which an HF diagnosis code was the primary reason for hospitalization to ensure that this cohort included only patients who met the approved indication (namely, an HF hospitalization in the preceding 12 months). For both treatment and control cohorts, all other hospitalizations that did not have a HF diagnosis were classified as non-HF events.
Statistical Analysis
The primary outcomes were HF hospitalization and cumulative days lost because of HF hospitalization or death at the 12 months after the anchor date. Rates of HF hospitalization were compared using the Andersen-Gill model for recurrent events with censoring at the time of death, ventricular assist device (VAD) implant, or heart transplant. Heart transplant or VAD implantation was identified using a Medicare Severity Diagnosis Related Groups assignment of 001 or 002 (heart transplant or implant of heart assist system without major comorbidity or complication). A robust variance estimate was used in the Andersen-Gill model to account for possible within-participant dependence from recurrent hospitalizations. Results in relevant subgroups defined by age, sex, comorbidities, and history of implantable cardioverter defibrillator and/or cardiac resynchronization therapy implant were also analyzed. Crude mortality rates were compared between treatment and control populations using Kaplan-Meier survival analysis. The comparison of the days lost owing to events was performed using a nonparametric bootstrap model; events were defined as HF hospitalization, all-cause hospitalization, and death.
Sensitivity Analysis
The primary outcomes were assessed in a 1:1 matched cohort based on the algorithm described in eFigure 1 in the Supplement. The matching algorithm was designed to minimize the mean difference between hospitalization timing within pairs of patients in the treatment group and control patients. A sensitivity analysis was conducted to characterize the variability in outcomes using a nonparametric bootstrap model using a 1:X matched cohort that satisfied the matching criteria of the algorithm. To assess for variations in care provided at implanting centers vs nonimplanting centers, we further analyzed outcomes in a subgroup of treatment-control pairs that received inpatient care at the same hospital, as determined by the 6-digit Medicare provider identification code.
All tests were 2-tailed, and P values of .05 or less were considered significant. Data analysis was completed from July 2017 to January 2019. Claims data were extracted with RStudio version 1.0.143 (RStudio Inc) and R version 3.4.0 (R Foundation for Statistical Computing), and the statistical analyses were conducted using R version 3.1.1 (Revolution Analytics).
Results
Of 1451 Medicare patients who underwent a PAP sensor implant procedure from June 1, 2014, to March 31, 2016, and had a hospitalization in the previous 12 months, 107 patients were excluded owing to discontinuous enrollment in Medicare insurance and 159 owing to their health maintenance organization insurance recipient status or receiving a VAD implant prior to sensor implant (eFigure 2 in the Supplement). The remaining 1185 patients were continuously enrolled in Medicare and had available data regarding clinical outcomes for at least 12 months before and after implantation. Of the 1.5 million potential control patients with HF from the full Medicare data set, 1087 were matched to this treatment cohort. All outcomes are reported in these 1087 matched treatment and control pairs.
Baseline Patient Characteristics
The patient characteristics for the 2 groups are reported in Table 1. The treatment and control cohorts were well matched for age (mean [SD], 72.7 [10.2] years vs 72.9 [10.1] years), demographic characteristics, and comorbidity profiles (Table 1). Chronic ischemic heart disease was not a matching criteria yet was similarly prevalent between the treatment and control cohorts (785 of 1087 [72.2%] vs 761 of 1087 [70.0%]; P = .26). Propensity score density plots show close matching between cohorts based on the 5 comorbidities of interest used in the matching algorithm (eFigure 3 in the Supplement). Standardized mean difference estimates were lower than 10% for all match parameters.
Table 1. Patient Characteristics at Baseline.
| Characteristic | Cohort, No. (%) | P Value | |
|---|---|---|---|
| Treatment (n = 1087) | Control (n = 1087) | ||
| Demographic factors | |||
| Age, median (IQR), y | 73 (67-80) | 74 (67-80) | .60a |
| Female | 381 (35.1) | 381 (35.1) | >.99 |
| Race/ethnicity | |||
| White | 910 (83.7) | 910 (83.7) | >.99 |
| Black | 137 (12.6) | 137 (12.6) | >.99 |
| Hispanic | 11 (1.0) | 11 (1.0) | >.99 |
| Asian | 13 (1.2) | 13 (1.2) | >.99 |
| Other | 16 (1.5) | 16 (1.5) | >.99 |
| History of ICD or CRT device | 494 (45.4) | 494 (45.4) | >.99 |
| Comorbidities | |||
| Arrhythmia | 810 (74.5) | 824 (75.8) | .52 |
| Hypertension | 945 (86.9) | 963 (88.6) | .27 |
| Diabetes | 577 (53.1) | 564 (51.9) | .61 |
| Pulmonary disease | 608 (55.9) | 638 (58.7) | .21 |
| Renal disease | 540 (49.7) | 580 (53.4) | .09 |
| Without end-stage renal disease | 1068 (98.3) | 1068 (98.3) | >.99 |
Abbreviations: CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; IQR, interquartile range.
P value from Wilcoxon rank sum test; all other P values for all comorbidities χ2 test.
The Elixhauser Comorbidity Index variables consist of 30 conditions. Although 5 comorbidities of interest were used to match control patients, there were no statistical differences between the treatment and control cohorts among the remaining 25 comorbid conditions (eTable 4 in the Supplement).
Heart Failure Characteristics
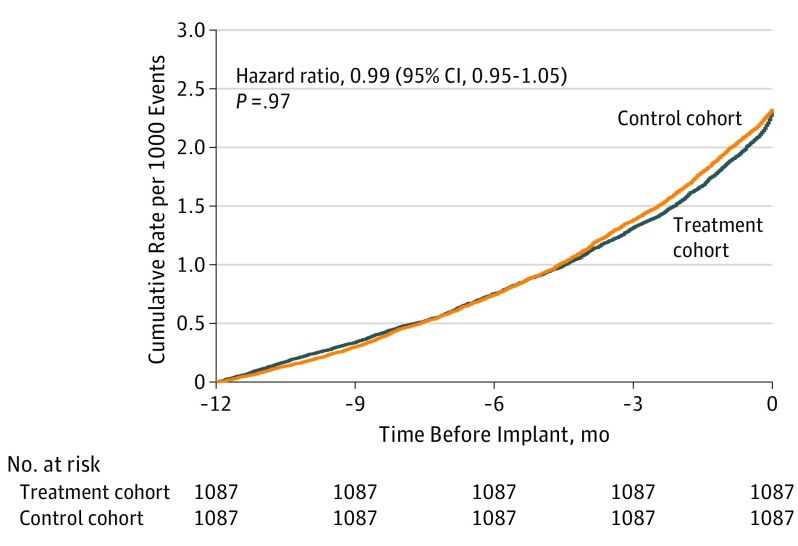
Heart failure hospitalizations were matched temporally for the 2 groups. Figure 1 represents the time series of the heart failure hospitalizations in the 12 months prior to the anchor point for treatment and control pairs. These recurrent events were compared between the 2 groups using the Andersen-Gill model (hazard ratio [HR], 0.99 [95% CI, 0.95-1.05]; P = .97), showing a closely matched temporal profile. The mean (SD) difference between hospitalization timing was 39.5 (38.5) days, and the mean (SD) cumulative days in hospital for the year prior to implant were 12.7 (12.7) days per patient for the treatment cohort and 11.4 (11.4) days per patient for the control group.
Figure 1. Time Series of Heart Failure Hospitalizations in the 12 Months Before Pulmonary Artery Pressure Sensor Implant.
Data from prior to the pulmonary artery pressure sensor implant include heart failure events (treatment cohort, 2532; control cohort, 2532; P > .99); length of stay per heart failure hospitalization (mean [SD]: treatment cohort, 5.5 [4.9] days; control cohort, 4.9 [4.1] days; P = .003); time in hospital per patient (mean [SD]: 12.7 [12.7] days; 11.4 [11.4] days; P = .05); and total hospital time (treatment cohort, 13 857 days; control cohort, 12 442 days; P > .99).
Clinical Outcomes
Over the 12-month follow-up period, the 1087 treatment cohort patients had 616 HF hospitalizations, 241 deaths, and 20 VAD implants or heart transplants; over the same period, the control cohort had 784 HF hospitalizations, 325 deaths, and 13 VAD implants or heart transplants. The clinical events over 12 months’ follow-up are reported in Table 2; follow-up was censored at death, VAD implant, or heart transplant. The mean (SD) length of hospital stay was 6.6 (6.5) days in the treatment cohort and 6.5 (5.8) days in the control cohort (P = .70). The mean (SD) total time in hospital for HF was 3.7 (9.5) days per patient for the treatment group and 4.4 (10.3) days per patient for the control group.
Table 2. Clinical Outcomes During Follow-up Period.
| Outcome | Patients, No. (%) | |
|---|---|---|
| Treatment Cohort (n = 1087) | Control Cohort (n = 1087) | |
| Clinical events | ||
| Heart failure hospitalization, No. of eventsa | 616 | 784 |
| Death | 241 (22.2) | 325 (29.9) |
| Ventricular assist device or transplant | 20 (1.8) | 13 (1.2) |
| Hospitalization for any cause, No. of events | 1846 | 1818 |
| Patients with ≥1 clinical event | ||
| Heart failure hospitalizationa | 345 (31.7) | 422 (38.8) |
| Heart failure hospitalization or deatha | 469 (43.1) | 597 (54.9) |
| Hospitalization for any cause | 695 (63.9) | 695 (63.9) |
| Hospitalization for any cause or death | 735 (67.6) | 783 (72.0) |
Inpatient heart failure hospitalization; identified in primary diagnosis code per Centers for Medicare and Medicaid Services.
Hospitalization for HF or death occurred in 469 patients (43.1%) in the treatment cohort and 597 (54.9%) of the control cohort. The percentage of days lost owing to HF hospitalization or death was reduced in the treatment cohort (13.7% [95% CI, 12.1%-15.3%) compared with the control cohort (18.8% [95% CI, 16.9%-20.7%]; risk ratio, 0.73 [95% CI, 0.63-0.85]; P < .001). The treatment cohort lost a mean (SD) of 50.0 (98.3) days per year, vs 68.4 (116.0) days per year lost in the control cohort (Table 3).
Table 3. Comparative Effectiveness of Pulmonary Artery Pressure Sensor-Based Management on Clinical Event Rates and Days Lost.
| Outcome | Mean (SD) | Hazard Ratioa or Risk Ratiob (95% CI) | Absolute Difference, d | P Value | |
|---|---|---|---|---|---|
| Treatment Cohort (n = 1087) | Control Cohort (n = 1087) | ||||
| Clinical event rates per patient per y | |||||
| Heart failure hospitalizationc | 0.65 | 0.88 | 0.76 (0.65-0.89) | NA | <.001 |
| Mortality, death per y | 0.23 | 0.30 | 0.70 (0.59-0.83) | NA | <.001 |
| Heart failure or death | 0.90 | 1.23 | 0.73 (0.64-0.84) | NA | <.001 |
| Days lost per patienta | |||||
| To death | 46.2 | 64.2 | 0.72 (0.62-0.84) | −17.9 | <.001 |
| To heart failure hospitalization or death | 50.0 | 68.4 | 0.73 (0.63-0.85) | −18.5 | <.001 |
| To any-cause hospitalization or death | 56.9 | 74.5 | 0.77 (0.68-0.88) | −17.5 | <.001 |
Abbreviation: NA, not applicable.
Hazard ratios, 95% CIs, and P values for events were derived using the Andersen-Gill extension of the Cox proportional hazards model. Mortality rates are the Kaplan-Meier estimates of mortality.
Mean, 95% CI, hazard ratio, and P values for comparing days lost were derived from nonparametric bootstrap model.
Inpatient heart failure hospitalization; identified in primary diagnosis code per Centers for Medicare and Medicaid Services.
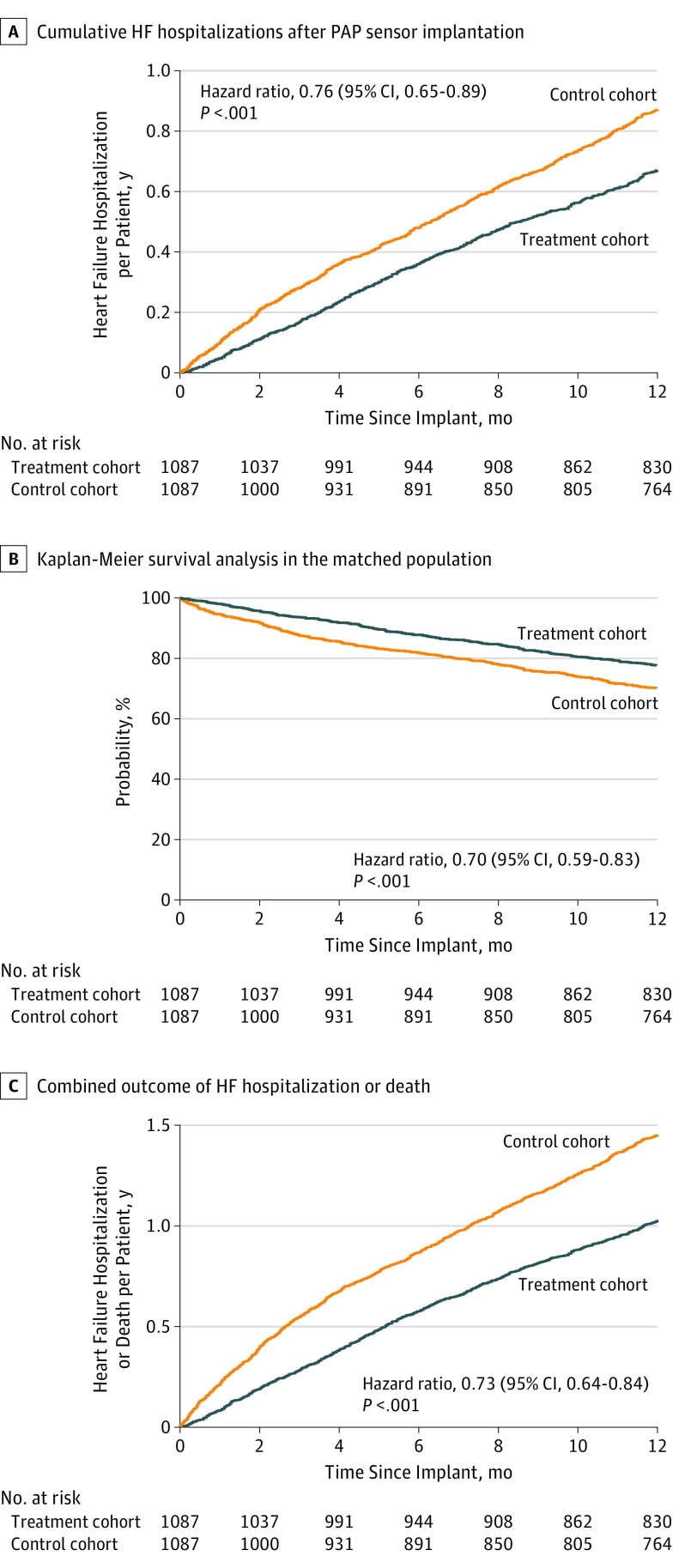
Heart failure hospitalizations occurred at a lower rate in the treatment cohort than the control cohort (0.65 vs 0.88; HR, 0.76 [95% CI, 0.65-0.89]; P < .001). The cumulative incidence of HF hospitalization is shown in Figure 2A. The mortality rate at 12 months was also significantly lower in the treatment cohort than the control cohort (0.23 deaths per year vs 0.30 deaths per year; HR, 0.70 [95% CI, 0.59-0.83]; P < .001; Table 3). The Kaplan-Meier survival curve for all-cause mortality is shown in Figure 2B. A combined end point of heart failure hospitalization and mortality was lower in the treatment cohort than in the control cohort (0.90 events per patient per year vs 1.23 events per patient per year; HR, 0.73 [95% CI, 0.64-0.84]; P < .001). Days lost to death (46.2 vs 64.2 days; absolute difference, −17.9 days; HR, 0.72 [95% CI, 0.62-0.84]; P < .001), HF hospitalization or death (50.0 vs 68.4 days; absolute difference, −18.5 days; HR, 0.73 [95% CI, 0.63-0.85]; P < .001), and all-cause hospitalization or death (56.9 vs 74.5 days; absolute difference, −17.5 days; HR, 0.77 [95% CI, 0.68-0.88]; P < .001) were significantly lower in the treatment cohort than the control cohort. Sensor implant was associated with a difference of 17 more days alive and out of the hospital.
Figure 2. Cumulative Events After Pulmonary Artery Pressure (PAP) Sensor Implant.
A, Heart failure (HF) hospitalizations. B, Deaths. C, Combined heart failure hospitalizations and death.
The relative reduction in HF hospitalization, mortality, and the combined end point of HF hospitalization and death was consistent across subgroups defined by age (<75 years and ≥75 years), sex, comorbidities, and history of implantable cardioverter defibrillator and/or cardiac resynchronization therapy implant (eFigure 4 in the Supplement).
Sensitivity Analysis
The primary analysis was based on a 1:1 matched cohort fulfilling all prespecified match criteria and minimizing the mean difference between hospitalization timing. To characterize variation in clinical outcomes, we used a nonparametric bootstrap model based on data from 41 347 control patients who were matched to the 1087 patients who received implants. The HF hospitalization rate of the control cohort was 0.93 (interquartile range [IQR], 0.90-0.95), and relative risk for HF hospitalization was 0.70 (IQR, 0.68-0.72, P < .001). The mortality rate of the control cohort was 0.31 (IQR, 0.30-0.32). The relative risk for mortality was 0.71 (IQR, 0.69-0.73; P < .001). eFigure 5 in the Supplement shows the cumulative probability distribution for HF hospitalization and mortality outcomes from the model (× 10 000).
Because implanting centers may have unmeasured, systemic differences from nonimplanting centers that could influence patient outcomes, we further compared outcomes of treatment-control pairs that had received care at the same implanting center. The patients in the treatment arm were implanted at 255 unique facilities.13 From the treatment arm, 774 patients were matched with 8973 control patients treated at the same centers. The HF hospitalization rate in this subgroup of the treatment cohort was lower than that of control patients (0.52 vs 0.81 [IQR, 0.80-0.84]; P < .001; relative risk, 0.64 [IQR, 0.63-0.66]; P < .001). The mortality rate of this subset of patients with implants was also lower than the matched control patients (0.22 vs 0.28 [IQR, 0.27-0.29]; relative risk, 0.76 [IQR, 0.74-0.79]; P < .01]). The cumulative probability distribution for HF hospitalization and mortality outcomes from the model (× 10 000) is shown in eFigure 6 in the Supplement.
Discussion
In this retrospective Medicare claims analysis, we observed lower rates of HF hospitalization among patients implanted with a PAP sensor compared with a contemporary cohort of propensity-matched controls. We also observed a lower rate of all-cause mortality among patients implanted with a PAP sensor, although this finding is considered exploratory because claims data may be insufficiently detailed to adjust adequately for HF severity. This study substantially extends the findings of a prior analysis of ambulatory hemodynamic monitoring in a smaller population of Medicare recipients that did not have a separate control cohort.7
We used a novel matching algorithm to construct a propensity-matched control cohort that allows for a pseudorandomized comparison. Treatment and control cohorts were matched for 5 relevant comorbidities, and in this large patient sample, the matching algorithm yielded a similar comorbidity profile for diverse conditions not specified by the algorithm. Bootstrap analysis shows that the resulting differences in clinical outcomes between treatment and control cohorts are robust and relatively insensitive to the choice of matched control patient. Further analysis of treatment-control pairs that had received care at the same implanting center indicates that outcome differences between treatment and control cohorts could be in part because of practice variations. After accounting for these differences, however, the clinical outcomes remain significantly different in all settings.
The reduced rates of HF hospitalization and mortality associated with sensor implant in this study are similar to the outcomes of the randomized CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association Functional Class III Heart Failure Patients (CHAMPION) trial.4,14 Whereas differences in HF hospitalization rates became evident at 3 months in CHAMPION, we observed lowered HF hospitalization rates as early as 1 month after sensor implant. One potential explanation for this difference is that, in CHAMPION, both arms underwent right-heart catheterization. Knowledge of baseline hemodynamic data could favorably influence treatment of control patients and thereby delay the benefit of ambulatory pressure monitoring. Additionally, the patients who received PAP sensors in the Medicare population in this study are significantly older, with a higher proportion of female patients than in CHAMPION. Real-world experience with the implantable PAP monitoring system in a demographically similar population showed higher baseline PAP and greater pressure changes than in CHAMPION.6
The composite outcome of days alive and out of hospital is an important, patient-centered measure that summarizes the treatment effect of an intervention on both hospitalization and mortality.15 Compared with conventional time-to-event end points, this measure accounts for multiple events occurring during follow-up. In a prospectively randomized study of a structured remote patient management intervention vs usual care,16,17 the telemedicine intervention resulted in a mean difference of 6 days per year lost owing to cardiovascular hospitalization or death. This difference was deemed to be clinically meaningful for patients, physicians, and payers. In the present study, implant of a hemodynamic sensor was associated with 17 fewer days lost for all-cause hospitalization or death within the year of follow-up.
Episodes of worsening HF requiring intensification of therapy are associated with increased risks of hospital readmission and death.18,19 Remote monitoring of nonhemodynamic clinical surrogates, including body weight,20,21 intrathoracic impedance,22 natriuretic peptides,23 and device-based telemonitoring16,24,25 have produced mixed results in prospective randomized clinical trials. Although CHAMPION demonstrated possible improved survival outcomes, it was not statistically powered to assess mortality.4 A prospective, randomized study (Hemodynamic-Guided Management of Heart Failure [GUIDE-HF]; NCT03387813) is underway to assess the effect of ambulatory PAP monitoring on long-term survival and hospitalization in chronic HF, coupled with quality of life and functional capacity.
Limitations
The limitations of the study are those inherent to an observational analysis using Medicare claims data. Important clinical information, such as ejection fraction, natriuretic peptide levels, and renal function, are not known. Medical therapy, indications for PAP sensor implant, and complications of sensor implant are similarly not available. Residual confounding by unmeasured covariates remains possible. We are unable to assess medication changes or link outcomes to PAP sensor data; thus, we cannot definitely exclude the possibilities that selection bias or heightened involvement of the health care team after sensor implant, rather than interventions triggered by hemodynamic data per se, are partially responsible for differences in outcomes. Finally, CMS methods were used to define the study end points, and clinical events were not formally adjudicated. We attempted to reduce the inherent limitations of an observational, claims-based design by cohort matching based on demographic factors, comorbidity profiles, and HF hospitalization frequency and timing. While randomization remains the evidentiary gold standard for experimental design, the novel matching procedure allows for a more rigorous outcomes comparison between cohorts. Several pitfalls of using big data based on claims reporting are reduced using this method.26,27
Conclusions
Hemodynamic, guided HF management was associated with improved long-term clinical outcomes, including significant reduction in days lost owing to hospitalization or death. These findings are being prospectively evaluated in an ongoing randomized clinical trial (GUIDE-HF).
eTable 1. Billing Procedure Codes Used to Identify PAP Sensor Implants
eTable 2. Diagnoses Codes Used to Identify Comorbidities
eTable 3. Billing Codes Used to Identify HF Hospitalization
eTable 4. Baseline Patient Characteristics: All Elixhauser Comorbidities that were not used for Matching criteria
eFigure 1. Algorithm for Matched Patient Identification
eFigure 2. Patient Cohort Identification
eFigure 3. Propensity score Match comparison
eFigure 4. Clinical outcomes (Heart Failure Hospitalizations and Mortality) in Subgroups of Interest in the 12-months after PA pressure sensor implant
eFigure 5. Distribution of outcomes amongst all matches
eFigure 6. Distribution of outcomes in patients treated at CMEM implanting sites
References
- 1.Zile MR, Bennett TD, St John Sutton M, et al. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118(14):1433-1441. doi: 10.1161/CIRCULATIONAHA.108.783910 [DOI] [PubMed] [Google Scholar]
- 2.Adamson PB, Zile MR, Cho YK, et al. Hemodynamic factors associated with acute decompensated heart failure: part 2—use in automated detection. J Card Fail. 2011;17(5):366-373. doi: 10.1016/j.cardfail.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 3.Stevenson LW, Zile M, Bennett TD, et al. Chronic ambulatory intracardiac pressures and future heart failure events. Circ Heart Fail. 2010;3(5):580-587. doi: 10.1161/CIRCHEARTFAILURE.109.923300 [DOI] [PubMed] [Google Scholar]
- 4.Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB; CHAMPION Trial Study Group . Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet. 2016;387(10017):453-461. doi: 10.1016/S0140-6736(15)00723-0 [DOI] [PubMed] [Google Scholar]
- 5.Zile MR, Bennett TD, El Hajj S, et al. Intracardiac pressures measured using an implantable hemodynamic monitor: relationship to mortality in patients with chronic heart failure. Circ Heart Fail. 2017;10(1):e003594. doi: 10.1161/CIRCHEARTFAILURE.116.003594 [DOI] [PubMed] [Google Scholar]
- 6.Heywood JT, Jermyn R, Shavelle D, et al. Impact of practice-based management of pulmonary artery pressures in 2000 patients implanted with the CardioMEMS sensor. Circulation. 2017;135(16):1509-1517. doi: 10.1161/CIRCULATIONAHA.116.026184 [DOI] [PubMed] [Google Scholar]
- 7.Desai AS, Bhimaraj A, Bharmi R, et al. Ambulatory hemodynamic monitoring reduces heart failure hospitalizations in “real-world” clinical practice. J Am Coll Cardiol. 2017;69(19):2357-2365. doi: 10.1016/j.jacc.2017.03.009 [DOI] [PubMed] [Google Scholar]
- 8.Krumholz HM, Dhruva SS. Real-world data on heart failure readmission reduction: real or real uncertain? J Am Coll Cardiol. 2017;69(19):2366-2368. doi: 10.1016/j.jacc.2017.03.019 [DOI] [PubMed] [Google Scholar]
- 9.DeVore AD, Hammill BG, Hardy NC, Eapen ZJ, Peterson ED, Hernandez AF. Has public reporting of hospital readmission rates affected patient outcomes?: analysis of Medicare claims data. J Am Coll Cardiol. 2016;67(8):963-972. doi: 10.1016/j.jacc.2015.12.037 [DOI] [PubMed] [Google Scholar]
- 10.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 11.Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55(7):698-705. doi: 10.1097/MLR.0000000000000735 [DOI] [PubMed] [Google Scholar]
- 12.Centers for Medicare & Medicaid Services Hospital inpatient quality reporting program measures International Classification of Diseases, 10th Edition, Clinical Modification System (ICD-10-CM) draft code set. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/HIQR-ICD9-to-ICD10-Tables.pdf. Published 2013. Accessed April 11, 2019.
- 13.Data.Medicare.gov Hospital general information. https://data.medicare.gov/widgets/xubh-q36u. Accessed April 11, 2019.
- 14.Abraham WT, Adamson PB, Bourge RC, et al. ; CHAMPION Trial Study Group . Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377(9766):658-666. doi: 10.1016/S0140-6736(11)60101-3 [DOI] [PubMed] [Google Scholar]
- 15.Ariti CA, Cleland JG, Pocock SJ, et al. Days alive and out of hospital and the patient journey in patients with heart failure: insights from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Am Heart J. 2011;162(5):900-906. doi: 10.1016/j.ahj.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 16.Koehler F, Koehler K, Deckwart O, et al. Telemedical Interventional Management in Heart Failure II (TIM-HF2), a randomised, controlled trial investigating the impact of telemedicine on unplanned cardiovascular hospitalisations and mortality in heart failure patients: study design and description of the intervention. Eur J Heart Fail. 2018;20(10):1485-1493. doi: 10.1002/ejhf.1300 [DOI] [PubMed] [Google Scholar]
- 17.Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392(10152):1047-1057. doi: 10.1016/S0140-6736(18)31880-4 [DOI] [PubMed] [Google Scholar]
- 18.Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260-266. doi: 10.1016/j.ahj.2007.01.041 [DOI] [PubMed] [Google Scholar]
- 19.Okumura N, Jhund PS, Gong J, et al. ; PARADIGM-HF Investigators and Committees* . Importance of clinical worsening of heart failure treated in the outpatient setting: evidence from the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial (PARADIGM-HF). Circulation. 2016;133(23):2254-2262. doi: 10.1161/CIRCULATIONAHA.115.020729 [DOI] [PubMed] [Google Scholar]
- 20.Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363(24):2301-2309. doi: 10.1056/NEJMoa1010029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ong MK, Romano PS, Edgington S, et al. ; Better Effectiveness After Transition–Heart Failure (BEAT-HF) Research Group . Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the Better Effectiveness After Transition—Heart Failure (BEAT-HF) randomized clinical trial. JAMA Intern Med. 2016;176(3):310-318. doi: 10.1001/jamainternmed.2015.7712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Veldhuisen DJ, Braunschweig F, Conraads V, et al. ; DOT-HF Investigators . Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation. 2011;124(16):1719-1726. doi: 10.1161/CIRCULATIONAHA.111.043042 [DOI] [PubMed] [Google Scholar]
- 23.Felker GM, Anstrom KJ, Adams KF, et al. Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2017;318(8):713-720. doi: 10.1001/jama.2017.10565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cleland JG, Louis AA, Rigby AS, Janssens U, Balk AH; TEN-HMS Investigators . Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: the Trans-European Network-Home-Care Management System (TEN-HMS) study. J Am Coll Cardiol. 2005;45(10):1654-1664. doi: 10.1016/j.jacc.2005.01.050 [DOI] [PubMed] [Google Scholar]
- 25.Inglis SC, Clark RA, Dierckx R, Prieto-Merino D, Cleland JG. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev. 2015;(10):CD007228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iacus SM, King G, Porro G Matching for causal inference without balance checking: coarsened exact matching. https://gking.harvard.edu/files/abs/cem-plus-abs.shtml. Published 2008. Accessed April 8, 2019.
- 27.Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25(1):1-21. doi: 10.1214/09-STS313 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Billing Procedure Codes Used to Identify PAP Sensor Implants
eTable 2. Diagnoses Codes Used to Identify Comorbidities
eTable 3. Billing Codes Used to Identify HF Hospitalization
eTable 4. Baseline Patient Characteristics: All Elixhauser Comorbidities that were not used for Matching criteria
eFigure 1. Algorithm for Matched Patient Identification
eFigure 2. Patient Cohort Identification
eFigure 3. Propensity score Match comparison
eFigure 4. Clinical outcomes (Heart Failure Hospitalizations and Mortality) in Subgroups of Interest in the 12-months after PA pressure sensor implant
eFigure 5. Distribution of outcomes amongst all matches
eFigure 6. Distribution of outcomes in patients treated at CMEM implanting sites