This cohort study examines the indications for and outcomes of expansion sphincter pharyngoplasty for patients with obstructive sleep apnea and lateral pharyngeal wall collapse.
Key Points
Question
Is expansion sphincter pharyngoplasty an effective surgical technique to widen the lateral pharyngeal walls of patients with obstructive sleep apnea, and which patients are likely to benefit most from this surgery?
Findings
In this cohort study of 63 Korean patients who had confirmed moderate or severe obstructive sleep apnea with lateral pharyngeal wall collapse, correction of the collapse by means of expansion sphincter pharyngoplasty was associated with a successful outcome in 67% of the patients, and their objective measures of sleep quality were significantly improved.
Meaning
Expansion sphincter pharyngoplasty may reduce lateral pharyngeal collapse in patients with moderate or severe obstructive sleep apnea and palatal circumferential narrowing with bulky lateral pharyngeal tissue.
Abstract
Importance
The lateral pharyngeal wall is recognized as an important site of upper airway collapse during sleep in patients with obstructive sleep apnea (OSA), and expansion sphincter pharyngoplasty (ESP) may have promising clinical utility in patients with OSA and lateral pharyngeal wall collapse.
Objectives
To evaluate the therapeutic outcomes of ESP in conjunction with other surgical procedures and to investigate indications for ESP in patients with OSA.
Design, Setting, and Participants
Cohort study of 63 patients with OSA diagnosed with lateral pharyngeal collapse under drug-induced sleep endoscopy who underwent ESP combined with tonsillectomy, uvuloplasty, or nasal surgery at Seoul National University Hospital in Seoul, Korea, between March 1, 2015, and December 1, 2016.
Main Outcomes and Measures
The primary outcome measure was the change in the apnea-hypopnea index (AHI) after surgery (AHI represents the number of apnea-hypopnea events per hour). Other outcome measures were differences in the surgical response rates, lowest oxygen saturation, subjective visual analog scale scores for snoring and apnea, and Epworth Sleepiness Scale score.
Results
Fifty of the 63 patients (79%) were male; the mean age was 42.1 (range, 20-54) years, and the mean body mass index (calculated as weight in kilograms divided by height in meters squared) was 27.6 (range, 19.0-32.1). Expansion sphincter pharyngoplasty was performed in patients with OSA with an AHI greater than 15 events per hour, more than 75% retropalatal circumferential narrowing when awake, and narrowed oropharynx due to bulky soft tissue around the lateral pharyngeal wall. In 42 of the 63 patients (67%), ESP was objectively successful in correcting lateral pharyngeal collapse; there was a significant reduction in mean AHI from 35.5 to 17.3 (mean difference, 18.1; 95% CI, 16.3-20.0) and improvement of the lowest mean (SD) oxygen saturation measurement from 78.2% (21.3%) to 86.4% (10.6%) (mean difference, 8.60%; 95% CI, 6.60%-10.60%) 6 months after the operation. The rate of postoperative complications, including pain and bleeding, was minimal after ESP, and a few patients reported an abnormal sensation around the soft palate and swallowing difficulty after ESP.
Conclusions and Relevance
Expansion sphincter pharyngoplasty appears to be a promising surgical technique to reduce lateral pharyngeal collapse in patients with moderate or severe OSA. Clinical data suggest that both severe palatal circumferential narrowing and bulky lateral pharyngeal tissue are favorable surgical indications for ESP in patients with OSA.
Introduction
Obstructive sleep apnea (OSA) is a common sleep disorder characterized by upper airway collapse that causes reduction or cessation of airflow during sleep. In the Starling resistor model, the upper airway is described as a hollow tube. Within this tube, reduced airflow from the nasal cavity and narrowing of the upper airway increase negative pressure in the pharyngeal airway and predispose the pharynx to collapse.1 Both upper airway narrowing and increased airway resistance can contribute to the underlying pathogenesis of OSA and can lead to sleep-related symptoms, such as loud snoring, apnea, and systemic complications, if not properly treated.2,3,4,5 Obstructive sleep apnea occurs because of fixed or dynamic upper airway obstruction caused by anatomical factors or abnormal upper airway motor tone, and upper airway obstruction can be caused by the collapse of single or multiple structures, such as the soft palate, uvula, palatine tonsils, lateral pharyngeal walls, and base of the tongue.6,7 Treatment modalities for OSA, such as behavioral modification, continuous positive airway pressure, surgery, and oral appliances, attempt to widen the upper airway and thereby reduce airway collapsibility. Above all, surgical treatments for OSA aim primarily to remove the pharyngeal tissue that blocks the upper airway, to enhance the stability of the pharyngeal airway, and to enlarge the pharyngeal lumen. Many studies have demonstrated the clinical benefits of sleep surgery, including relief from both subjective symptoms and life-threatening conditions.8,9,10 Diverse surgical techniques have been introduced to correct abnormal upper airway narrowing, and the addition of new surgical options can potentially improve the success rate of OSA treatment.10,11
The lateral pharyngeal wall is a complex structure composed of numerous pharyngeal muscle groups, such as the palatopharyngeus, superior pharyngeal constrictor, and palatoglossus and lymphoid tissue, including the palatine tonsils. The clinical importance of lateral pharyngeal collapse has been documented in the pathogenesis of OSA. The lateral pharyngeal wall is more collapsible in patients with severe OSA than in persons with no OSA or patients with mild OSA, and narrowing of the lateral pharyngeal wall appears to be the sole independent risk factor in patients with OSA.12 However, its clinical significance is still underestimated in patients with OSA who undergo palatal surgery.
According to previously published studies, multiple obstructions at various locations occur at the same time in patients with OSA during sleep, and determining the success rate of sleep surgery depends on precise measurement of anatomical findings for upper airway obstruction, which was shown in several drug-induced sleep endoscopy (DISE) studies.13 Based on DISE findings, the location and type of airway obstructions in patients with OSA can be identified precisely, and the importance of the lateral pharyngeal wall in the severity of OSA has been emphasized.14,15 Greater lateral pharyngeal collapsibility is found in patients with OSA who experience a relapse of snoring or apnea after surgery, and correction of lateral pharyngeal collapse seems to be critical for success of OSA treatment. Diverse surgical techniques to reduce lateral pharyngeal collapse and increase the tension and stability of the lateral pharyngeal wall have been introduced. Lateral pharyngoplasty, relocation pharyngoplasty, and expansion sphincter pharyngoplasty (ESP) have been used to correct lateral pharyngeal collapse in patients with OSA.16,17,18,19 Based on data from these studies, we assumed that ESP is the most innovative technique for creating tension in the lateral pharyngeal walls, preventing collapse, and reducing the number of apneic events. In the present study, we aimed to determine the therapeutic outcomes of ESP in patients with moderate or severe OSA and to suggest favorable surgical indications for ESP using sleep study, DISE, and endoscopic examination.
Methods
Patients and Study Design
Sixty-three patients who had received a diagnosis of OSA in the Department of Otorhinolaryngology at Seoul National University Hospital, Seoul, Korea, from March 1, 2015, to December 1, 2016, were included, and lateral pharyngeal collapse in these patients was confirmed through DISE. Written informed consent was obtained from each participant, and the study complied with the Declaration of Helsinki.20 The study protocol was approved by the institutional review board of Seoul National University Hospital.
All patients underwent ESP, including tonsillectomy, the uvulopalatal (UP) flap procedure, and septoturbinoplasty, to improve their sleep-related symptoms and abnormal results on objective measures of sleep quality. Indications for ESP were (1) apnea-hypopnea index (AHI, number of apnea-hypopnea events per hour) greater than 15 events per hour on polysomnography (PSG); (2) retropalatal circumferential narrowing above DISE grade II (more than 75% narrowing); and (3) narrowed oropharynx due to lateral pharyngeal collapse of the bulky redundant soft tissue around the posterior pillar.
Their medical records, including the results of preoperative and postoperative PSG, were reviewed retrospectively. Total sleep time, AHI (events per hour), and the lowest oxygen saturation measurement were observed before and at 6 months after the operation (eFigure in the Supplement). A successful procedure was defined as 50% reduction in AHI and an AHI less than 20 as described by Sher et al.21 Duration of hospital stay; early complications; primary outcome parameters, such as the Epworth Sleepiness Scale and the visual analog scale for snoring or apnea (observed by the patient’s sleep partner); and late complications were recorded at discharge from the hospital and during follow-up visits.
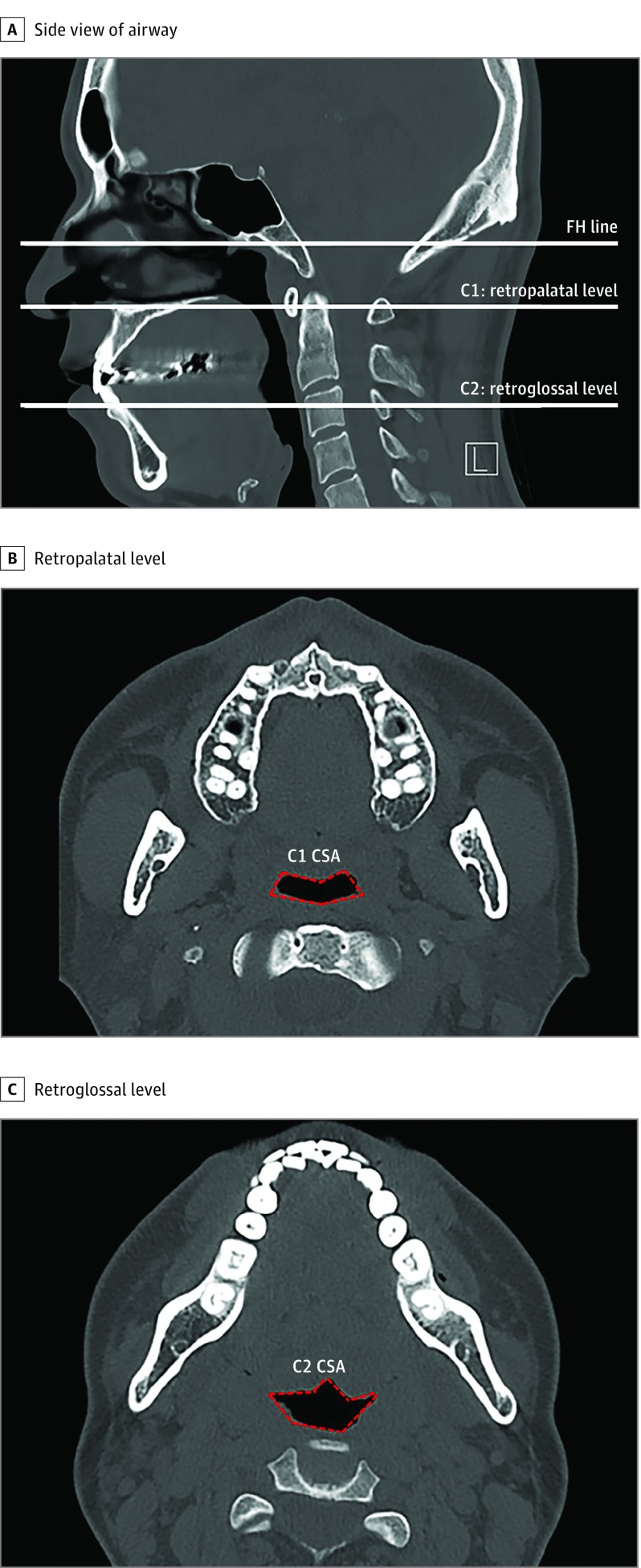
To evaluate changes in the pharyngeal airway at the retropalatal and retroglossal levels, the cross-sectional area (CSA) on each axial plane (C1, C2) was evaluated (Figure 1A). The CSA values of the retropalatal and retroglossal space of 61 of the patients were measured both before and 3 months after the operation by using computed tomographic scans of the paranasal sinuses (Figure 1B and C). To avoid the changes in upper airway lumen during respiration, all participants were asked to hold their breath at the end of expiration for each scan. Changes in the CSA were measured using a method described for the Superimposition module of the Invivo 5 program.22
Figure 1. Measurement of Airway Diameter or Upper Airway Volume Using Computed Tomographic Scan of the Paranasal Sinuses.
A, Sagittal view. The C1 and C2 planes, parallel to the Frankfort horizontal (FH) plane, are tangent to the most caudal medial projections of cervical vertebrae 1 and 2, respectively. B, Axial view. Cross-sectional area (CSA) of the retropharyngeal space (outlined in red). C, Axial view. Cross-sectional area of retroglossal space (outlined in red). L indicates left. All computed tomographic scans were non–contrast enhanced.
Surgical Technique
Patients with OSA were selected for ESP based on the presence of oropharyngeal collapse, as determined by a complete preoperative upper airway examination and DISE with reference to the VOTE (velum, oropharynx, tongue base, and epiglottis) classification.23 The patients did not have obstruction at the tongue base or epiglottis. All patients reported nasal obstruction, and they were scheduled to receive turbinoplasty or septoplasty to obtain better nasal airflow. The surgery was performed under general anesthesia and nasotracheal intubation with the patient in the supine position. Bilateral tonsillectomy and the UP flap procedure were performed. After indicating the design with a marking pen on the soft palate above the uvula, mucosa was removed with sharp pointed scissors or electrosurgery and only uvular mucosa was carefully removed to prevent damage to the uvular muscle. Then the uvular tip was turned over onto the soft palate, and the excess uvula tip was cut properly with electrosurgery. After the UP flap procedure, the palatopharyngeus muscle was identified, and its upper half was transected horizontally (Figure 2A and B). The muscle was isolated, and its posterior surface was left partially attached to the superior pharyngeal constrictor muscle. A mucosal incision was made at the anterior pillar, and a mucosal tunnel was made under the anterior pillar mucosa. The transected palatopharyngeal muscle was rotated superolaterally to mobilize the muscle and allow suturing with polyglactin 910 (Vicryl) suture. The palatopharyngeus muscle was then attached bilaterally to the pterygomandibular raphe with Vicryl 2-0 sutures through the mucosal tunnel on the anterior pillar (Figure 2C), and mucosal closure between the anterior and posterior pillars was done with Vicryl 3-0 sutures (Figure 2D). The tension of the suture on the flap was adjusted to prevent postoperative wound dehiscence. All patients were discharged 2 days after ESP, and the last follow-up visit was 3 months after the surgery.
Figure 2. Main Surgical Steps of Expansion Sphincter Pharyngoplasty (ESP).
A, After completion of tonsillectomy, the palatopharyngeus muscle (asterisk) is identified. B, A horizontal incision is made to divide the inferior end of the palatopharyngeus muscle (asterisk), with care taken to leave its fascia attachments to the deeper horizontal constrictor muscle. C, Absorbable suture is used to fix the palatopharyngeus muscle to the pterygomandibular raphe (white arrowhead) through a mucosal tunnel (yellow arrowhead) at the anterior pillar. D, Mucosal closure is performed, and ESP creates tension in the lateral pharyngeal wall.
Statistical Analysis
Descriptive data are presented as mean (SD). The variables were investigated by using analytical methods (Kolmogorov-Smirnov, Shapiro-Wilk) to determine normality. Postsurgical changes in AHI, lowest oxygen saturation measurement, Epworth Sleepiness Scale score, visual analog scale score for snoring or apnea, and CSA of the retropalatal and retroglossal space were analyzed using a paired t test and the Wilcoxon signed rank test. In all cases, the effect size, measured with Cohen d, and 95% CI around the effect size were used to describe presurgery to postsurgery changes in various measures. Effect size was based on the Cohen d (≤0.2, small; 0.2-0.8, medium; and ≥0.8, large). Significance testing was 2-sided. Statistical calculations were performed using SPSS, version 19.0 (IBM Corp).
Results
We included 63 patients who were diagnosed with OSA and underwent ESP to resolve airway narrowing and reduce lateral pharyngeal collapse. Fifty of the 63 patients (79%) were men; mean (range) age was 42.1 (20.0-54.0) years; and mean (range) body mass index, calculated as weight in kilograms divided by height in meters squared, was 27.6 (19.0-32.1). The severity of OSA was based on AHI; 22 (35%) patients had moderate OSA, and 41 (65%) had severe OSA (Table). We did not recommend ESP to patients with mild OSA even if their DISE findings showed lateral pharyngeal collapse.
Table. Characteristics of 63 Patients Who Underwent ESP for OSA With Pharyngeal Narrowing.
| Characteristic | Values, No. (%) |
|---|---|
| Demographic | |
| Age, mean (range) y | 42.1 (20.0-54.0) |
| BMI, mean (range) | 27.58 (19.00-32.10) |
| Sex | |
| Male | 50 (79.3) |
| Female | 13 (20.7) |
| Clinical | |
| Tonsil sizea | |
| I-II | 0 |
| III | 43 (68.2) |
| IV | 20 (31.8) |
| Modified Mallampati gradeb | |
| I-II | 0 |
| III | 48 (76.2) |
| IV | 15 (23.8) |
| Baseline ESS score, mean | 17.1 |
| AHI, mean (SD), events/h | 35.5 (10.7) |
| CSA, mean (SD), mm2 | |
| Retropalatal | 224.5 (34.8) |
| Retroglossal | 234.6 (26.5) |
| Postoperative treatment | |
| PAP therapy | 14 (22.2) |
| MAD | 2 (3.1) |
| Reoperation | 0 |
Abbreviations: AHI, apnea-hypopnea index; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CSA, cross-sectional area; ESP, expansion sphincter pharyngoplasty; ESS, Epworth Sleepiness Scale; MAD, mandibular advancement device; OSA, obstructive sleep apnea; PAP, positive airway pressure.
Tonsil size: grade I, tonsils just outside of the tonsillar fossa and occupy 25% or less of the oropharyngeal width; grade II, tonsils occupy 26% to 50% of the oropharyngeal width; grade III, tonsils occupy 51% to 75% of the oropharyngeal width; and grade IV, tonsils occupy more than 75% of the oropharyngeal width.
Mallampati grades: assessment performed by 2 trained research nurses with the patient sitting upright with his or her mouth maximally opened and tongue protruded without phonation. In class I, soft palate, fauces, pillars, and uvula are visible; class II, soft palate, fauces, and uvula are visible; class III, soft palate and base of uvula are visible; and class IV: soft palate is not visible at all.
The therapeutic outcome was determined using AHI after ESP; all 63 patients had PSG at 6 months after operation. The mean (SD) AHI for the entire group was 35.5 (10.7) before ESP, which decreased significantly to 17.3 (8.9) (mean difference, 18.1; 95% CI, 16.3-20.0; Cohen d, 1.85) postoperatively in the 63 patients who had PSG after 6 months. Selecting the arbitrary threshold of a 50% reduction in AHI plus an AHI less than 20, the number of responders was 42 for a success rate of sleep surgery (including ESP) of 67% in patients with OSA and lateral pharyngeal collapse (Figure 3A).
Figure 3. Changes in Objective and Subjective Measures of Sleep Among Patients With Obstructive Sleep Apnea (OSA) 6 Months After Expansion Sphincter Pharyngoplasty.
A, Apnea-hypopnea index. B, Lowest oxygen saturation measurement during sleep. C, Epworth Sleepiness Scale for daytime sleepiness. D, Subjective visual analog scale (VAS) score for snoring. E, Apneic events were determined using polysomnography and answers to a VAS questionnaire. The horizontal line represents the mean value.
aIndicates a statistically significant difference after surgery.
Postoperative PSG results also showed that the mean (SD) lowest oxygen saturation measurement improved significantly from 78.2% (21.3%) to 86.4% (10.6%) (MD, 8.60%; 95% CI, 6.60%-10.60%; Cohen d, 1.16) (Figure 3B). In the subjective symptoms, the mean (SD) Epworth Sleepiness Scale score improved from 17.1 (6.2) to 7.2 (5.4) (MD, −9.40; 95% CI, −8.58 to −10.21; Cohen d, 3.61) (Figure 3C), and the visual analog scale score for snoring (MD, 4.25; 95% CI, 3.82-4.69; Cohen d, 2.91) or apnea (MD, 4.54; 95% CI, 4.08-5.00; Cohen d, 3.92) improved significantly in 63 patients after surgery (Figure 3D and E).
As a next step, we measured changes in the diameter of the CSA at the retropalatal and retroglossal areas using peripheral nervous system computed tomographic images of the paranasal sinuses for a more detailed analysis of upper airway narrowing after ESP. Sixty-three patients were included in this part of the study; the mean CSA at the retropalatal and retroglossal levels of the patients who underwent ESP was extended from 224.5 (34.8) to 362.2 (63.5) mm2 (MD, 137.7 mm2; 95% CI, 121.1-154.2 mm2; Cohen d, 2.69) and from 234.6 (26.5) to 251.4 (25.5) mm2 (MD, 16.9 mm2; 95% CI, 12.3-21.5 mm2; Cohen d, 0.65), respectively. The CSA at the retropalatal level was extended from 220.4 (33.3) to 381.6 (56.8) mm2 (MD, 161.2 mm2; 95% CI, 143.0-179.5 mm2) in the responders and 232.7 (37.1) to 323.2 (59.2) mm2 (MD, 90.5 mm2; 95% CI, 65.9-115.1 mm2) in the nonresponders after ESP (Δ of the difference, 70.7 mm2; 95% CI, 31.8-110.0 mm2). The CSA at the retroglossal level was extended from 236.1 (26.2) to 251.8 (25.7) mm2 (MD, 15.7 mm2; 95% CI, 9.7-21.6 mm2) in the responders and 231.4 (27.3) to 250.8 (25.7) mm2 (MD,19.6 mm2; 95% CI, 11.8-26.9 mm2) in the nonresponders after ESP.
Finally, we investigated the complications or adverse effects after ESP: postoperative pain, abnormal sensation, mouth dryness, dysphagia, velopharyngeal insufficiency, and taste loss. We did not observe serious complications related to ESP at 1 week, 1 month, or 3 months after surgery. In total, 52 patients reported pain 1 week after ESP, but 5 patients still experienced oropharyngeal pain 1 month after ESP. Mucosal wound dehiscence was observed in 20 of the 63 patients (32%) after ESP combined with the UP flap procedure. Partial dehiscence was observed in 11 patients (17%), and total dehiscence was observed in 9 patients (14%). We did not suture the mucosal dehiscence, and the occurrence of dehiscence was not correlated with the failure rate of ESP. The mean (SD) hospital stay was 3.8 (0.2) days. No patient experienced postoperative pain 3 months after ESP. In addition, 19 patients (30%) felt an abnormal sensation around the soft palate area 1 week after surgery, but 2 (3%) patients reported it 3 months postoperatively. Some patients reported dysphagia, dry mouth, velopharyngeal insufficiency, and loss of taste after ESP, but those complaints had subsided by 1 month (Figure 4). We did not perform tracheostomy on our OSA participants, nor did we observe any postoperative bleeding at the oropharynx after ESP. No patient was readmitted to the hospital owing to complications after ESP.
Figure 4. Subjective Symptoms or Complications After Expansion Sphincter Pharyngoplasty.
VPI indicates velopharyngeal insufficiency.
Discussion
Through this study, we found that ESP in conjunction with other surgical procedures is associated with reduced upper airway narrowing at the retropalatal level and improved objective measures of OSA for patients with lateral pharyngeal collapse in the upper airway. Our current clinical findings also suggest proper surgical indications for ESP in OSA patients: PSG results, DISE, and endoscopic findings, such as narrowed oropharynx due to lateral bulk of soft tissue around the posterior pillar, as well as circumferential narrowing (DISE grade>II) at the retropalatal level.
In patients with OSA, the lateral pharyngeal wall is more collapsible when pressured by airflow than in healthy persons, and the lateral pharyngeal wall of patients with OSA can be thicker than in persons without OSA, making it a predominant anatomical factor inducing airway narrowing in OSA.14,24 Lateral pharyngeal collapse contributes to the pathogenesis of OSA by increasing airway resistance and causing partial or complete obstruction of the upper airway.25,26 It has been verified that lateral pharyngeal collapse is more closely related to airway resistance and causes aggravated intermittent hypoxia and oxygen desaturation in patients with OSA.26 In addition, patients with OSA with lateral pharyngeal collapse exhibit higher AHI than patients with OSA with anteroposterior upper airway narrowing.27 Therefore, an adequate surgical option is needed to reduce lateral pharyngeal collapse in patients with OSA and to provide a satisfactory therapeutic outcome. The prediction of lateral pharyngeal collapse is important to improve the therapeutic outcome of sleep surgery, and the maintenance of tension or stability of the lateral pharyngeal wall may be critical in patients with OSA who show circumferential narrowing of the upper airway.
Uvulopalatopharyngoplasty (UPPP) is a popular technique in the field of sleep surgery. However, conventional UPPP mainly widens the retropalatal space from anterior to posterior; thus, UPPP seems to be ineffective in patients with OSA with multiple-level obstructions. The therapeutic outcomes of UPPP are especially unsatisfactory in patients with OSA with lateral or circumferential pharyngeal wall collapse.28,29 To date, diverse surgical techniques for replacing UPPP have been introduced to correct lateral pharyngeal collapse in patients with OSA. Lateral pharyngoplasty, relocation pharyngoplasty, and ESP have been suggested as effective surgical procedures to correct lateral pharyngeal collapse. These surgical techniques provide better improvement in AHI than UPPP and more effectively widen the pharyngeal lumen by reducing lateral pharyngeal collapse.16,17,18,19,30
Lateral pharyngoplasty focuses on the muscular properties of the lateral pharyngeal wall and changing the pharyngeal space. As a result, lateral pharyngoplasty produced better clinical and PSG outcomes in patients with OSA than UPPP, although the cross-sectional measurements of the pharyngeal airway did not differ between those treatments.16 However, many patients experience significant dysphagia after lateral pharyngoplasty because it is an invasive process that undermines the superior pharyngeal constrictor muscle and cuts the soft palate muscle. In addition, lateral pharyngoplasty is associated with more complications than UPPP, such as oronasal reflux of liquid and wound dehiscence.16
Relocation pharyngoplasty is a technique similar to lateral pharyngoplasty; both splint the lateral pharyngeal wall, but relocation pharyngoplasty plicates the superior pharyngeal constrictor muscle to prevent scarring contraction of the tonsillar fossa.17 Advancement of the soft palate is achieved by removing the supratonsillar mucosa and adipose tissue to create a space for reconstruction of the velopharynx.17 In addition, relocation pharyngoplasty preserves the palatal muscle by removing only part of the soft palate mucosal layer. Therefore, relocation pharyngoplasty is a favorable surgical option that maintains pharyngeal function in patients with OSA with lateral pharyngeal collapse.
Our study included patients with moderate or severe OSA who showed more than 75% narrowing in their lateral pharyngeal wall, and we expected them to need stronger tension to improve lateral pharyngeal collapse. Although relocation pharyngoplasty has obvious advantages in splinting the lateral pharyngeal wall, we believe that the tension that it provides in the lateral pharyngeal wall is not as powerful as that from ESP. The ESP procedure repositions the underlying muscular structures of the pharynx and palate to widen the lateral pharyngeal airway. After revealing the arching fibers of the palate muscles, isolated palatopharyngeus muscle is attached to the arching fiber of the palate muscle.18 Expansion sphincter pharyngoplasty thus creates more powerful tension in the lateral pharyngeal wall and reduces the bulk of lateral pharyngeal soft tissue by isolating and rotating the palatopharyngeus muscle superoanterolaterally. In the present study, we found ESP combined with uvuloplasty to be an effective surgical option for lateral pharyngeal collapse in patients with moderate or severe OSA. Expansion sphincter pharyngoplasty did not undermine the superior pharyngeal constrictor muscle and preserved the muscle layer of the soft palate. Thus, patients who underwent ESP had fewer complications, such as postoperative pain and dysphagia, than patients who underwent lateral pharyngoplasty.16,18 We modified some of the ESP procedures, including the addition of a mucosal tunnel under the anterior pillar for more effective fixation of the palatopharyngeus muscle to the pterygomandibular raphe. The isolated palatopharyngeus muscle was attached to the adjacent pterygomandibular raphe using absorbable suture in the superolateral direction with limited mucosal incision and was pulled tightly to the soft palate level in the superolateral direction. Our clinical data show that modified ESP combined with the UP flap procedure provided a clinically meaningful improvement for patients with OSA with lateral pharyngeal collapse and effectively reduced lateral pharyngeal bulk, resulting in enhanced soft palate tension or widening of the pharynx without severe complications after surgery. We observed that ESP significantly reduced the AHI scores of patients with moderate or severe OSA, with an overall success rate of approximately 67%. Furthermore, subjective symptoms related to OSA, such as snoring, apnea, and daytime sleepiness, also improved significantly after ESP. The lowest oxygen saturation measurement and valid sleep time also improved, and the extent of the retropalatal area in the upper airway widened significantly after ESP.
Our data show that ESP in conjunction with other surgical procedures led to a significant decrease in AHI for patients with moderate or severe OSA with lateral pharyngeal collapse, providing intensive or stable tension to the lateral pharyngeal wall. We suggest ESP combined with uvuloplasty for patients diagnosed with moderate or severe OSA, lateral pharyngeal collapse of grade II or greater, and narrowed oropharynx due to bulky soft tissue in the lateral pharyngeal wall. Our clinical data also revealed that ESP-induced upper airway extension was evident in both the retropalatal and retroglossal areas in patients with OSA with lateral pharyngeal collapse. The responder group demonstrated a greater extension in CSA of the retropalatal level than the nonresponder group. However, this finding was not evident in the retroglossal level, and ESP provides less expansion around the retroglossal area than in the retropalatal area. Therefore, if retroglossal space narrowing is adversely affecting a patient’s OSA symptoms after ESP, additional therapeutic options, such as a mandibular advancement device or positive airway pressure, should be considered. In addition, DISE provides critical information when deciding whether to perform ESP in patients with OSA to enhance the success rate of sleep surgery.
Limitations
This study possesses numerous limitations. First, it is a retrospective cohort study, and patients’ data did not include patient-reported outcomes such as behavioral, functional, and quality of life information. In addition, the sample size was relatively small and there was no control group in the present study. Therefore, we were unable to estimate the therapeutic effect of ESP precisely, and the design made it difficult to detect clinically meaningful differences between the patients with OSA who underwent ESP.31,32 Second, all patients received a combination of different surgical treatments in addition to ESP, including tonsillectomy, UP flap, and nasal surgery, to improve their sleep-related symptoms and abnormal results on measures of sleep characteristics. It might be hard to determine the independent effect of ESP in patients with OSA through the current clinical findings, and reporting of multiple end points increases the possibility of reporting false-positive results, which limits the ability of the present study to achieve more precise results. Third, duration of follow-up was 6 months, a relatively short time for a chronic condition, and the therapeutic outcome was only evaluated using AHI score. This protocol could lead to uncertain generalizability of results; the results need to be validated in a new cohort.33 Baseline characteristics, outcome measures, and analysis of results will be necessary to enhance study interpretation, and a framework for reporting results from ESP surgical trials will be needed.
Conclusions
Our clinical findings suggest that ESP could be a useful surgical option in patients with OSA who have intensive lateral pharyngeal collapse. The results of this study also suggest reasonable indications for ESP. However, further research, including randomized clinical trials with appropriate control groups, is required to reach definitive conclusions about appropriate indications and the efficacy of the ESP procedure.
eFigure. Schematic Figure of the Study Design and Clinical Evaluation
References
- 1.Bilston LE, Gandevia SC. Biomechanical properties of the human upper airway and their effect on its behavior during breathing and in obstructive sleep apnea. J Appl Physiol (1985). 2014;116(3):314-324. doi: 10.1152/japplphysiol.00539.2013 [DOI] [PubMed] [Google Scholar]
- 2.Chung S, Yoon IY, Shin YK, Lee CH, Kim JW, Ahn HJ. Endothelial dysfunction and inflammatory reactions of elderly and middle-aged men with obstructive sleep apnea syndrome. Sleep Breath. 2009;13(1):11-17. doi: 10.1007/s11325-008-0210-x [DOI] [PubMed] [Google Scholar]
- 3.Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med. 2008;177(4):369-375. doi: 10.1164/rccm.200608-1190PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31(8):1079-1085. [PMC free article] [PubMed] [Google Scholar]
- 5.Parish JM, Somers VK. Obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc. 2004;79(8):1036-1046. doi: 10.4065/79.8.1036 [DOI] [PubMed] [Google Scholar]
- 6.Kushida CA, Littner MR, Hirshkowitz M, et al. ; American Academy of Sleep Medicine . Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29(3):375-380. doi: 10.1093/sleep/29.3.375 [DOI] [PubMed] [Google Scholar]
- 7.Li MX, Yan CY, Wang S. New insights on the role of the insular cortex and habenula in OSA. Sleep Breath. 2015;19(4):1347-1353. doi: 10.1007/s11325-015-1168-0 [DOI] [PubMed] [Google Scholar]
- 8.Choi JH, Lee JY, Cha J, Kim K, Hong SN, Lee SH. Predictive models of objective oropharyngeal OSA surgery outcomes: success rate and AHI reduction ratio. PLoS One. 2017;12(9):e0185201. doi: 10.1371/journal.pone.0185201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li HY. Updated palate surgery for obstructive sleep apnea In: Lin H-C, ed. Sleep-Related Breathing Disorders. Basel, Switzerland: Karger Publishers; 2017:74-80. doi: 10.1159/000470869 [DOI] [PubMed] [Google Scholar]
- 10.Lin HC, Weaver EM, Lin HS, Friedman M. Multilevel obstructive sleep apnea surgery. Adv Otorhinolaryngol. 2017;80:109-115. doi: 10.1159/000470879 [DOI] [PubMed] [Google Scholar]
- 11.Salapatas AM, Bonzelaar LB, Hwang MS, et al. Impact of minimally invasive multilevel surgery on mild/moderate OSA. Otolaryngol Head Neck Surg. 2016;155(4):695-701. doi: 10.1177/0194599816651240 [DOI] [PubMed] [Google Scholar]
- 12.Korhan I, Gode S, Midilli R, Basoglu OK. The influence of the lateral pharyngeal wall anatomy on snoring and sleep apnoea. J Pak Med Assoc. 2015;65(2):125-130. [PubMed] [Google Scholar]
- 13.Kezirian EJ, Hohenhorst W, de Vries N. Drug-induced sleep endoscopy: the VOTE classification. Eur Arch Otorhinolaryngol. 2011;268(8):1233-1236. doi: 10.1007/s00405-011-1633-8 [DOI] [PubMed] [Google Scholar]
- 14.Soares D, Sinawe H, Folbe AJ, et al. Lateral oropharyngeal wall and supraglottic airway collapse associated with failure in sleep apnea surgery. Laryngoscope. 2012;122(2):473-479. doi: 10.1002/lary.22474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee CH, Kim DK, Kim SY, Rhee CS, Won TB. Changes in site of obstruction in obstructive sleep apnea patients according to sleep position: a DISE study. Laryngoscope. 2015;125(1):248-254. doi: 10.1002/lary.24825 [DOI] [PubMed] [Google Scholar]
- 16.Cahali MB. Lateral pharyngoplasty: a new treatment for obstructive sleep apnea hypopnea syndrome. Laryngoscope. 2003;113(11):1961-1968. doi: 10.1097/00005537-200311000-00020 [DOI] [PubMed] [Google Scholar]
- 17.Li HY, Lee LA. Relocation pharyngoplasty for obstructive sleep apnea. Laryngoscope. 2009;119(12):2472-2477. doi: 10.1002/lary.20634 [DOI] [PubMed] [Google Scholar]
- 18.Pang KP, Woodson BT. Expansion sphincter pharyngoplasty: a new technique for the treatment of obstructive sleep apnea. Otolaryngol Head Neck Surg. 2007;137(1):110-114. doi: 10.1016/j.otohns.2007.03.014 [DOI] [PubMed] [Google Scholar]
- 19.Woodson BT, Sitton M, Jacobowitz O. Expansion sphincter pharyngoplasty and palatal advancement pharyngoplasty: airway evaluation and surgical techniques. Oper Tech Otolaryngol. 2012;23:3-10. doi: 10.1016/j.otot.2012.01.002 [DOI] [Google Scholar]
- 20.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 21.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19(2):156-177. doi: 10.1093/sleep/19.2.156 [DOI] [PubMed] [Google Scholar]
- 22.Gokce SM, Gorgulu S, Gokce HS, Bengi AO, Karacayli U, Ors F. Evaluation of pharyngeal airway space changes after bimaxillary orthognathic surgery with a 3-dimensional simulation and modeling program. Am J Orthod Dentofacial Orthop. 2014;146(4):477-492. doi: 10.1016/j.ajodo.2014.06.017 [DOI] [PubMed] [Google Scholar]
- 23.Kezirian EJ. Nonresponders to pharyngeal surgery for obstructive sleep apnea: insights from drug-induced sleep endoscopy. Laryngoscope. 2011;121(6):1320-1326. doi: 10.1002/lary.21749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwab RJ, Gefter WB, Hoffman EA, Gupta KB, Pack AI. Dynamic upper airway imaging during awake respiration in normal subjects and patients with sleep disordered breathing. Am Rev Respir Dis. 1993;148(5):1385-1400. doi: 10.1164/ajrccm/148.5.1385 [DOI] [PubMed] [Google Scholar]
- 25.Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing: significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152(5, pt 1):1673-1689. doi: 10.1164/ajrccm.152.5.7582313 [DOI] [PubMed] [Google Scholar]
- 26.Schellenberg JB, Maislin G, Schwab RJ. Physical findings and the risk for obstructive sleep apnea: the importance of oropharyngeal structures. Am J Respir Crit Care Med. 2000;162(2, pt 1):740-748. doi: 10.1164/ajrccm.162.2.9908123 [DOI] [PubMed] [Google Scholar]
- 27.Genta PR, Sands SA, Butler JP, et al. Airflow shape is associated with the pharyngeal structure causing OSA. Chest. 2017;152(3):537-546. doi: 10.1016/j.chest.2017.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujita S. UPPP for sleep apnea and snoring. Ear Nose Throat J. 1984;63(5):227-235. [PubMed] [Google Scholar]
- 29.Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33(10):1396-1407. doi: 10.1093/sleep/33.10.1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cahali MB, Formigoni GG, Gebrim EM, Miziara ID. Lateral pharyngoplasty versus uvulopalatopharyngoplasty: a clinical, polysomnographic and computed tomography measurement comparison. Sleep. 2004;27(5):942-950. doi: 10.1093/sleep/27.5.942 [DOI] [PubMed] [Google Scholar]
- 31.Schechtman KB, Sher AE, Piccirillo JF. Methodological and statistical problems in sleep apnea research: the literature on uvulopalatopharyngoplasty. Sleep. 1995;18(8):659-666. doi: 10.1093/sleep/18.8.659 [DOI] [PubMed] [Google Scholar]
- 32.Megwalu UC, Piccirillo JF. Methodological and statistical problems in uvulopalatopharyngoplasty research: a follow-up study. Arch Otolaryngol Head Neck Surg. 2008;134(8):805-809. doi: 10.1001/archotol.134.8.805 [DOI] [PubMed] [Google Scholar]
- 33.Kezirian EJ, Weaver EM, Criswell MA, de Vries N, Woodson BT, Piccirillo JF. Reporting results of obstructive sleep apnea syndrome surgery trials. Otolaryngol Head Neck Surg. 2011;144(4):496-499. doi: 10.1177/0194599810396791 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Schematic Figure of the Study Design and Clinical Evaluation