Key Points
Question
What are the dosing patterns and outcomes of onabotulinum toxin A therapy for patients with facial synkinesis?
Findings
In this cohort study of 99 patients, 6 facial muscles were commonly injected and the dose progressively increased until a steady state was achieved after 3 consecutive treatment sessions. Younger patients, females, and those with oral symptoms and greater overall disease severity responded significantly and most substantially.
Meaning
In facial synkinesis, onabotulinum toxin A injections begin with the treatment of a group of core facial muscles at lower doses and progressively increase until a steady state is achieved, resulting in improved patient-reported outcomes.
Abstract
Importance
In the last decade, there has been a significant increase in the number of practitioners administering botulinum toxin for facial synkinesis. However, there are few resources available to guide treatment patterns, and little is known about how these patterns are associated with functional outcomes and quality of life.
Objective
To evaluate botulinum treatment patterns, including the dosing and frequency of muscle targeting, for treatment of facial synkinesis and to quantify patient outcomes.
Design, Setting, and Participants
This prospective cohort study of 99 patients treated for facial synkinesis was conducted from January 2016 through December 2018 at the Vanderbilt Bill Wilkerson Center in Nashville, Tennessee, a tertiary referral center.
Intervention
Onabotulinum toxin A treatment of facial synkinesis.
Main Outcomes and Measures
Patient-reported outcomes on the Synkinesis Assessment Questionnaire and botulinum treatment patterns, including the dosages and frequency of injection for each facial muscle, were compared at the initiation of treatment and at the end of recorded treatment.
Results
In total, 99 patients (80 female patients [81%]) underwent botulinum injections for treatment of facial synkinesis. The median (interquartile range) age was 54.0 (43.5-61.5) years, and the median (interquartile range) follow-up was 27.1 (8.9-59.7) months. Most patients underwent injections after receiving a diagnosis of Bell palsy (41 patients, 41%) or after resection of vestibular schwannoma (36 patients [36%]). The patients received a total of 441 treatment injections, and 369 pretreatment and posttreatment Synkinesis Assessment Questionnaire scores were analyzed. The mean botulinum dose was 2 to 3 U for each facial muscle and 9 to 10 U for the platysma muscle. The dose increased over time for the majority of all muscles, with steady state achieved after a median of 3 treatments (interquartile range, 2-3). Linear regression analysis for cluster data of the mean total questionnaire score difference was −14.2 (95% CI, −17.0 to −11.5; P < .001). There was a significant association of postinjection questionnaire score with younger patients, female sex, total dose, and synkinesis severity. Oculo-oral synkinesis may respond more to treatment compared with oro-ocular synkinesis.
Conclusion and Relevance
Patients with facial synkinesis responded significantly to botulinum treatment. Treatment began with 6 core facial muscles that were injected during most treatment sessions, and dosages increased after the first injection until steady state was achieved. Those with a greater degree of morbidity, younger patients, and females showed significant improvement, and the larger the dose administered, the greater the response. Oculo-oral synkinesis may be more responsive than oro-ocular synkinesis.
Level of Evidence
3.
This cohort study assesses the treatment patterns (dosages and frequency) and the association of treatment with self-reported functional outcomes and quality of life among patients receiving onabotulinum toxin A for treatment of facial synkinesis.
Introduction
Coinnervation of facial musculature by misdirected axonal guidance can result in static and dynamic aberrancy and hypertonicity. Facial synkinesis and hyperkinesis, which have been reported to occur in as many as 50% of those with longstanding facial weakness, results in psychological, aesthetic, and functional consequences.1,2,3,4,5,6,7,8,9 Although this manifests as various functional challenges, the impairment to social interaction, personal appearance, and perception may contribute more significantly to the morbidity of disease.10
Given the variability of the involved mimetic musculature and resulting symptomology, the treatment of synkinesis is multimodal and tailored to individual patient concerns. Treatment options include musculature retraining with physical therapy, targeted chemodenervation, and select surgical procedures.9,11,12,13,14,15,16,17,18 Onabotulinum toxin type A (BT) is the standard for chemodenervation and has the capacity to produce objective and subjective results, including improved facial symmetry, reduced involuntary muscle contractions, and enhanced quality of life.16,17,19,20,21,22,23,24,25
Current literature, however, fails to elucidate patient- and injection-specific factors that predict BT responses, and many health care professionals administer BT with irregular or capricious dosing. The intent of the present study was, therefore, to (1) describe the systematic use and experience with BT injections at a tertiary referral center that has been using electromyography (EMG)–guided muscle targeting for 18 years to help guide other clinicians; (2) describe BT treatment patterns over time; (3) evaluate BT outcomes using the validated Synkinesis Assessment Questionnaire (SAQ); and (4) identify factors contributing to patient-reported outcomes.26,27
Methods
Patients were included if they were treated with BT for facial synkinesis and had completed both pretreatment and posttreatment SAQs between January 2016 and February 2018. Patients were excluded if they received BT for reasons other than facial synkinesis or were treated with other botulinum formulations. Institutional review board approval was obtained from Vanderbilt University Medical Center to perform a prospective cohort study in conjunction with a retrospective database review of patients with facial synkinesis who were treated at the Bill Wilkerson Center Pi Beta Phi Rehabilitation Institute and the Department of Otolaryngology at Vanderbilt University Medical Center (Nashville, Tennessee). Written informed consent was obtained from patients for botulinum injections at each visit; the SAQ questionnaires were used in the routine care of patients as a clinical tool and for treatment decision making (not solely for research).
Clinic Setup and Procedure
Patients are traditionally offered chemodenervation 3 to 6 months after initiation of rigorous facial physical therapy under the supervision of a therapist who specializes in facial rehabilitation. In our clinic in general, including for the present study, BT treatments are performed every 3 months on designated treatment days. Topical anesthetic containing benzocaine, 20%, lidocaine, 6%, and tetracaine, 4%, is applied to potential injection sites, and EMG leads are placed while awaiting appropriate anesthetic time. Patients are taken to a treatment room where they are examined by both the treating physician (W.R. or S.S.) and a facial physical therapist (C.N.). Injections are performed with a 1.5-inch × 27-gauge hypodermic needle electrode having a Luer lock hub and a BT dilution of 2.5 U/0.1 mL. A 1-mL syringe is used to facilitate precise dosing. During the injection, a portable EMG system (Nicolet VikingQuest; Natus Neurology Inc) monitors the presence of muscle hypertonicity and synkinesis at rest and with volitional movements.
Data Collection
Routine collection of SAQ data was introduced in 2016, and patients complete the survey on the day of treatment and 2 weeks after treatment through RedCap, a secure, Health Insurance Portability and Accountability Act–compliant, online database. The cause of facial paralysis, muscles treated, reported duration of response, and dosages for patients completing an SAQ were retrospectively collected.
Given the evolving nature of facial synkinesis and the need to titrate dosing in the early phases of treatment, it was expected that patients would reach a stable treatment course after several visits. A preliminary analysis was therefore performed to evaluate when steady state was achieved (Table 1). The novel algorithm is sum = (|Δdose|) + (|ΔCMs|) + (|ΔOMs|) = [|Δdose|/(CMs × 2) + OMs] = [(ΔCMs × 4)/10)] + [(|ΔOMs|)/10], where changes in dose are in units of botulinum, CMs represent core muscles, and OMs represent other muscles, was generated to compare the intertreatment changes in dosing and muscles targeted with the following 2 considerations. First, several muscles were treated considerably more frequently at our center (termed herein core muscles), and changes in these muscles were more heavily weighted than those of the other muscles. Second, dosing and facial muscle treatments were weighted using a correctional denominator to balance the expressions within the algorithm. A value less than 1 was classified as minimal fluctuation between treatment sessions, whereas a value of 1 or greater was considered substantial fluctuation. Steady state was defined as the first visit after at least 3 consecutive visits of algorithmic outputs less than 1. Steady state deviance was defined as a substantial change in BT pattern (≥1) after achieving steady state. To understand how treatment patterns evolved with time, we compared initial mean injection doses and muscles targeted with those of the last 3 treatments. This comparison was performed only in patients with pretreatment and posttreatment SAQ data who had received at least 5 injections. The study design and the analyses performed are illustrated in eFigure 1 in the Supplement.
Table 1. Steady State Outcomes.
| Steady State Outcome | Mean Value (Range) |
|---|---|
| Injections | 13.7 (5.0-29.0) |
| Injections until steady state | 3.7 (2.0-12.0) |
| Never reaching steady state, No. (%) | 6 (8.5) |
| Injections in those never reaching steady state | 5.5 (5.0-6.0) |
| Mean deviation from steady state | 1.0 (0.0-4.0) |
Statistical Analysis
Patient demographics and characteristics of treatment are summarized using medians and interquartile ranges (IQRs) for continuous variables and using frequencies for categorical variables. Wilcoxon signed rank tests were used to compare the paired preinjection and 2-week postinjection scores, treating multiple treatments and injections independently. The median of the difference and 95% CI were estimated nonparametrically. To account for the difference of multiple injections within a single patient, linear regression for clustered data without covariate (R package “miceadds”) using the calculated preinjection and postinjection score difference of each injection was performed to estimate the mean and 95% CI of the difference. Further subgroup analysis was performed using SAQ questions 1 through 3 as a surrogate for oro-ocular synkinesis (ie, inappropriate eye closure in synchrony with volitional perioral movement) and questions 5 through 7 for oculo-oral synkinesis (inappropriate facial or neck movement with volitional eye closure). The postinjection score associations with preinjection score, age, sex, dose, and time since first injection were studied using multivariable linear regression with robust standard errors (to account for multiple injections within a patient). Bonferroni correction for multiple comparisons was applied when appropriate, and a 2-sided P < .05 was considered statistically significant. All analysis was carried out using R, version 3.5.0 (The R Project for Statistical Computing).
Results
Of 103 patients completing the SAQ, 99 patients (80 female [80%]; median [IQR] age, 54 [43.5-61.5] years) met the inclusion criteria. The median (IQR) follow-up, was 27.1 (8.9-59.7) months. Baseline characteristics and a summary of the 441 treatments and injections are given in Table 2. Specific considerations and the treatment paradigm for each facial muscle is described in the eAppendix in the Supplement.
Table 2. Patient Demographics and Injection Data.
| Characteristic | No. (%) |
|---|---|
| Total patients, No. | 99 |
| Age, median (IQR) | 54.0 (43.5-61.5) |
| Follow-up, median (IQR), mo | 27.1 (8.9-59.7) |
| Sex | |
| Male | 19 (19) |
| Female | 80 (81) |
| Race/ethnicity | |
| White | 85 (86) |
| Other | 14 (14) |
| Cause | |
| Bell palsy | 41 (41) |
| Vestibular schwannoma | 36 (36) |
| Trauma | 4 (4) |
| Ramsay Hunt | 9 (9) |
| Other | 9 (9) |
| Total injections, No. | 441 |
| Laterality | |
| Left | 171 (46) |
| Right | 158 (43) |
| Bilateral | 40 (11) |
| Time between injections, median (IQR), d | 91 (84-98) |
| Total dose, median (IQR) | 20.0 (12.5-32.5) |
| Duration of response, median (IQR), d | 69 (62-76) |
Abbreviation: IQR, interquartile range.
Steady State
Steady state (defined above) data are shown in Table 1. For 71 patients who received at least 5 injections, the median number of treatments was 13.5 (IQR, 9.3-17) and steady state was achieved in a median of 3 treatment sessions (IQR, 2-3). One patient had 29 total treatment sessions, and 1 patient required 12 sessions prior to achieving steady state. The mean number of steady state deviations was 1 (range, 0-4).
Treatment Patterns
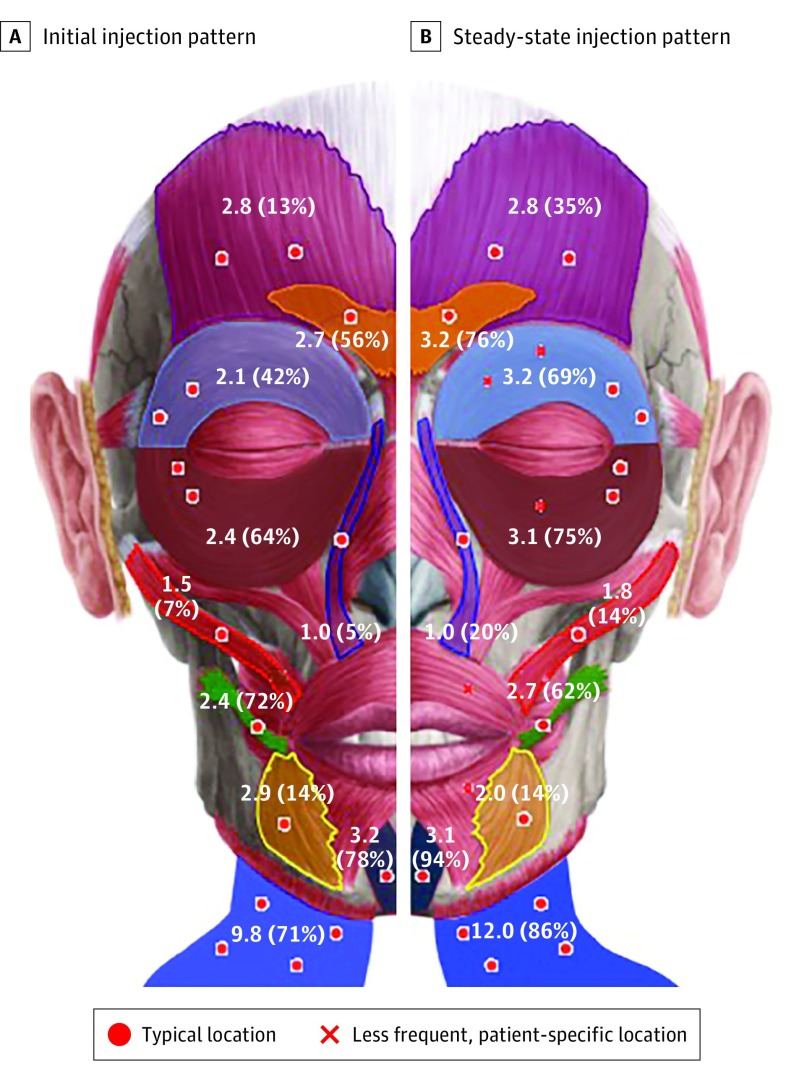
The 71 patients who underwent at least 5 injections were included in the evaluation of the initial and final treatment patterns. The 6 muscles most frequently targeted both at initial and final treatments were the corrugator, orbicularis oculi superioris, orbicularis oculi inferioris, risorius, mentalis, and platysma (Figure and eFigure 2 in the Supplement). Each muscle was targeted more commonly at each treatment session. The mean botulinum dose was 2 to 3 U for each facial muscle, and 9 to 10 U for the platysma muscle. Increases in mean (range) doses between initial and final treatments were observed in the upper face (5.2 [1.3-17.5] vs 8.1 [1.3-35.0] U), midface (2.8 [0.8-8.3] vs 4.5 [0.8-37.5] U), and neck (9.8 [2.5-30.0] vs 12.0 [2.5-32.5] U), while dosing in the lower face remained stable (3.3 [1.3-10.0] vs 3.3 [1.3-12.5] U).
Figure. Initial and Steady-State Treatment Patterns.
Color transparency reflects the frequency a muscle is injected in each new patient (A) and after the botulinum toxin dose is titrated (B). The mean dose in units of botulinum is shown, along with the frequency a muscle is injected (shown in parentheses).
Of the 71 patients undergoing at least 5 injections, 53 completed paired pretreatment and posttreatment SAQ surveys at their most recent treatment session. Therefore, the correlation analysis with SAQ outcomes at latter phases of treatment was limited to these 53 patients. Most patients began their treatment prior to the SAQ collection period (January 2016); therefore, analysis of initial SAQ outcomes was limited to the 27 patients who completed pretreatment and posttreatment SAQs for their first treatment visit. The SAQ score improved by a mean of 13.3 points (range, −44.4 to 13.3 points) at initial visits and by a mean of 15.6 points (range, −37.8 to 17.8 points) at final treatments (P = .38).
Overall Outcomes
Mean pretreatment and posttreatment total SAQ scores were 57.9 and 46.9, respectively (P < .001). Overall and individual patient SAQ scores are summarized in Table 3, and eTables 1 and 2 in the Supplement. The results of Wilcoxon signed rank tests revealed significant improvement in overall, (median difference, −15.3; 95% CI, −16.7 to −12.5), oro-ocular (median difference, −16.7; 95% CI, −16.7 to −12.5) and oculo-oral (median difference, −20.8; 95% CI, −25.0 to −16.7) synkinesis scores after BT treatment (all P < .001). When stratified by each of the 9 individual survey questions, similar results were found. After accounting for the dependence of multiple injections for a patient, linear regression analyses indicated a significantly improved total score (mean difference, −14.2; 95% CI, −17.0 to −11.5) as well individual oro-ocular (mean difference, −11.3; 95% CI, −14.6 to −7.9) and oculo-oral (mean difference, −15.7; 95% CI, −19.4 to −12.1) scores; significance was maintained after Bonferroni correction (all P < .001) (Table 3).
Table 3. Wilcoxon Signed Rank Test and Simple Linear Regression Results.
| Item | Patient Outcome | |||
|---|---|---|---|---|
| Wilcoxon Signed Rank | Linear Regression | |||
| Median Difference (95% CI)a | P Value | Mean Difference (95% CI)b | P Value | |
| Total survey score | −15.3 (−16.7 to −12.5) | <.001 | −14.2 (−17.0 to −11.5) | <.001 |
| Oro-ocular synkinesis (Q1-Q3) | −16.7 (−16.7 to −12.5) | <.001 | −11.3 (−14.6 to −7.9) | <.001 |
| Oculo-oral synkinesis (Q5-Q7) | −20.8 (−25.0 to −16.7) | <.001 | −15.7 (−19.4 to −12.1) | <.001 |
Abbreviation: Q, question.
Derived total score median of difference (postscore minus prescore).
Estimated mean difference (postscore minus prescore) of derived total score.
Table 4 summarizes the results of the multivariable regression analysis. In these regression models, besides the main effect, the interactions between preinjection score and other covariates were included. Total postinjection SAQ–derived scores were significantly associated with baseline severity (P < .001), younger patients (P = .03), female sex (P = .03), and total dose administered (P = .01). For 2 groups of patients with the same synkinesis severity (baseline score of 50), the estimated mean postinjection score decreased 2.2 points (95% CI, 0.2-4.2 points) for the group that was 10 years younger; the score was 8.3 points (95% CI, 0.9-15.6 points) lower for females than males; and the score was 3.5 points (95% CI, 0.8-6.2) lower for the group with a higher dose of 20 U. For male patients aged 53 years, if the baseline score 33 months after the first injection increased by 30.6 points, the mean postinjection score after a total dose of 20 U increased 22.7 points (95% CI, 15.1-30.3). The oro-ocular synkinesis outcome was significantly associated with baseline severity (P < .001). For male patients aged 53 years, if the baseline score 33 months after the first injection increased by 42 points, the mean postinjection score after an eye dose of 5 U and a mouth dose of 5 U increased 30.6 points (95% CI, 23.3-37.9). The oculo-oral synkinesis outcome was significantly associated with baseline severity (P < .001) and perioral dose (P = .02), and perioral dose significantly modified baseline severity (P = .01). For male patients aged 53 years, if the baseline score 33 months after the first injection increased by 41.7 points, the mean postinjection score after an eye dose of 5 U and a mouth dose of 5 U increased 31.3 points (95% CI, 24.0-38.5). For every 3.8 U increase in perioral dose for patients with baseline score of 58.3, the estimated mean difference in the postinjection score was 1.04 (95% CI, −0.27 to 2.34), and this effect depended on the baseline score (for every 3.8 U increase for a baseline score of 20, the mean difference in the postinjection score was 3.66 [95% CI, 1.08-6.24], P = .01 for interaction).
Table 4. Multivariable Regression Results Using the Derived Total Score.
| Variable | Mean difference (95% CI)a | P Value | P Value for Interaction |
|---|---|---|---|
| Total synkinesis | |||
| Baseline SAQ | <.001 | ||
| Every 31-point increase for males aged 53, total dose 20 U, 33 mo after injection | 22.73 (15.14 to 30.32) | ||
| Age | .03 | .58 | |
| Every 10-y increase for a baseline score of 50 | 2.21 (0.18 to 4.23) | ||
| Sex | .03 | .42 | |
| Male vs female with the same baseline score of 50 | 8.27 (0.94 to 15.60) | ||
| Time | .55 | .71 | |
| Every 48-mo increase for a baseline score of 50 | −1.69 (−4.72 to 1.35) | ||
| Total dose | .01 | .37 | |
| Every 20-U increase for a baseline score of 50 | 3.54 (0.82 to 6.25) | ||
| Oro-ocular synkinesis (Q1-Q3) | |||
| Baseline SAQ | <.001 | ||
| Every 42-point increase for males aged 53, eye dose 5 U, mouth dose 5 U, 33 mo after injection | 30.58 (23.26 to 37.90) | ||
| Age | .66 | .76 | |
| Every 10-y increase for a baseline score of 58.3 | 1.16 (−1.43 to 3.76) | ||
| Sex | .14 | .09 | |
| Male vs female with the same baseline score of 58.3 | 7.77 (−1.12 to 16.66) | ||
| Time | .41 | .48 | |
| Every 48-mo increase for baseline score of 58.3 | −1.72 (−5.57 to 2.14) | ||
| Periocular dose | .25 | .10 | |
| Every 7.5 U increase for a baseline score of 58.3 | 1.10 (−2.82 to 5.01) | ||
| Perioral dose | .41 | .89 | |
| Every 3.75-U increase for a baseline score of 58.3 | 1.23 (−1.22 to 3.67) | ||
| Oculo-oral synkinesis (Q5-Q7) | |||
| Baseline SAQ | <.001 | ||
| Every 42-point increase for males aged 53 y, eye dose of 5 U, mouth dose of 5 U, 33 mo after injection | 31.29 (24.05 to 38.52) | ||
| Age | .26 | .77 | |
| Every 10-y increase with a baseline score of 58.3 | 1.76 (−1.20 to 4.72) | ||
| Sex | .13 | .10 | |
| Male vs female with the same baseline score of 58.3 | 9.02 (−0.07 to 18.11) | ||
| Time | .90 | .65 | |
| Every 48-mo increase for a baseline score of 58.3 | 0.33 (−3.65 to 4.31) | ||
| Periocular dose | .09 | .47 | |
| Every 7.5-U increase for a baseline score of 58.3 | 2.98 (0.31 to 5.65) | ||
| Perioral Dose | .02 | .01 | |
| Every 3.75-U increase for a baseline score of 58.3 | 1.04 (−0.27 to 2.34) | ||
| Every 3.75-U increase for a baseline score of 20.0 | 3.66 (1.08 to 6.24) |
Abbreviations: Q, question; SAQ, Synkinesis Assessment Questionnaire.
Mean difference in postinjection derived score.
Discussion
Onabotulinum toxin type A is widely used in the treatment of facial synkinesis and has been shown to improve outcomes.26,28,29 In the absence of treatment guidelines, the pattern of use and dosing is variable. This may be due to the dynamic and evolving nature of synkinesis, multitude of facial muscles and their complex interplay, patient-reported variance of effected muscle involvement and severity, and physician preferences.
At our center, BT dosing increased with time and there was purposeful targeting of prolific effector muscles. A core group of 6 muscles, the corrugator, orbicularis oculi superioris and orbicularis oculi inferioris, mentalis, risorius, and platysma, were consistently treated, and these muscles, which provide a large degree of facial muscle mass, were targeted more consistently and at increased doses over time. This is contrary to the results of Risoud and colleagues,30 who initially increased dosing after the first injection, with decreased dosing on subsequent treatments. We not only increased individual muscle dose and frequency but also found that higher doses resulted in improved SAQ outcomes; therefore, the doses were increased as tolerated. Each muscle has a unique tolerance of BT, and at later phases of treatment, doses as high as 35, 37.5, 12.5, and 32.5 U were tolerated in the upper face, midface, lower face, and neck, respectively. Overall, mean dosing increased over time for the upper face, midface, and neck while remaining stable for the lower face.
For BT-naive patients, initial treatment on the whole and for each muscle group began at a lower dose and then increased over subsequent treatment sessions. Although BT doses were progressively increased, patients did reach a “steady state” (minimal deviance from prior treatment regimen) after an initial exploratory period, similar to titrating blood pressure medication to effect after initiating therapy. This steady state was achieved after 3 treatment sessions, and most patients did not deviate from this result. Six patients never reached steady state, all of whom discontinued treatment, which constituted 40% of the 15 total patients who withdrew from therapy.
Although the risorius is a common target, dosing and consistency decreased over time for this muscle. This decrease was likely due to the recent implementation of buccinator muscle treatment, which has been shown to improve midface tightness, oral commissure retraction, cheek biting, and control of oral competence.28,29,31 During the last 2 years, the mean buccinator dose increased from 4.0 to 5.5 U and is now used in 39% of treatments, rather than 10% of earlier treatments.
There are concerns regarding complications and adverse effects of high-dose BT, and some have advocated for lower botulinum dosing.10,20,32,33 In the present study, there were no major complications, and higher doses provided statistically significant benefits when performed by skilled facial plastic surgeons with a thorough understanding of anatomy. Moreover, although not all centers implement EMG guidance for administration of BT, we find it a helpful adjunct that allows accurate administration while reducing risks.
With initial therapy starting at low doses, the core facial muscles were injected with a mean of 2 to 3 U each, and the platysma with 9 to 10 U, with an emphasis to improve periocular and perioral symptoms while minimizing risk. Lower doses initially help minimize the risk of ptosis and lagophthalmos with periocular injections, lip droop when injecting the nasalis and zygomaticus muscles, oral incompetence with overtreatment of the risorius muscles, lip biting with the depressor anguli oris, and speech changes relative to excessive buccinator treatment. The treatment of additional and contralateral muscles was implemented at the health care professional’s discretion to customize treatment for each patient.
When delivering very low doses to select muscle groups that are not immediately subdermal (eg, nasalis, zygomaticus, depressor anguli oris, or buccinator), confirmation of exact placement in the target muscle by EMG reduces intertreatment variability. The EMG can also provide immediate feedback on which mimetic muscles exhibit purely synkinetic patterns when the patient performs isolated facial movements of unrelated muscle groups and also to identify subclinical hyperkinetic or synkinetic movements.25,34 Moreover, we used EMG to assess the degree of hyperkinesis for a select muscle at rest in comparison with the contralateral, unaffected muscle to guide treatment dosing and the overall regimen.
The laterality of treatment has been debated, with some advocating for focused treatment of the synkinetic side only and others suggesting benefit from contralateral treatment. Eleven percent of our injections were bilateral, and the contralateral muscles most commonly treated included the frontalis (mean, 4.6 U), corrugator (mean, 3.6 U), depressor anguli oris (mean, 0.75 U), zygomaticus (mean, 1.4 U), and mentalis (mean, 3.9 U) muscles. The relatively larger frontalis, corrugator, and mentalis muscles received higher doses on the contralateral side, whereas the depressor anguli oris and zygomaticus muscles received smaller doses. We found this approach to improve facial symmetry and ameliorate noticeable discrepancies in mimetic expression.
At our center, the SAQ survey helped guide treatments to assess how previous injections benefited a patient and how to tailor subsequent injections. Similar to overall SAQ outcomes, we found similar positive responses for each survey component. Notably, however, in approximately 10% of treatments, a patient did not respond positively on their SAQ. Many times this was followed by subsequent improvements; therefore, these fluctuations should not deter additional treatment attempts. In general, patients greatly valued BT treatment with a median follow-up of 27 months and a median interval between injections of 91 days, and 85% of the patients at our center continue routine treatment sessions.
Compared with oro-ocular synkinesis, oculo-oral synkinesis is more common, is associated with hyperkinetic movements that are more obvious to the human eye, and may be more amenable to BT treatment.35 The findings of the present study support these statements, as shown by the greater absolute improvements in SAQ scores in oculo-oral (20.8) compared with oro-ocular (16.7) synkinesis. In addition, multivariable regression analyses showed significantly improved outcomes in oculo-oral synkinesis with worse pretreatment facial synkinesis and higher perioral BT doses. The perioral mouth dose was also an effect modifier, and patients receiving higher perioral BT dosing in oculo-oral synkinesis may have responded to an even greater extent. The reason for these findings is unclear although they may be associated with the small and spread out muscle mass of the orbicularis oculi; the disproportionate impact on patient quality of life that accompanies constant eye closure, visual field obstruction, and facial disfigurement; and the capacity for increased BT doses of the midface and lower face, whereas the contributory ocular muscles have a limited dose capacity given the risk of lagophthalmos.
It has been previously postulated that older patients and males develop worse synkinesis, and sex and age contributions were observed in the present study when controlling for baseline SAQ-reported severity.36 Younger patients and females had significantly improved outcomes when compared with their older male counterparts. Because females typically possess less facial muscle bulk than males, similar doses of BT would theoretically result in relatively greater tissue concentrations and therefore greater effects. One would then expect older patients to improve more substantially than younger patients for similar reasons; however, this expectation was not supported by data in the present study.
We chose the SAQ to longitudinally measure patient-reported outcomes because patient perception is crucial for successful treatment. Reduced social interaction and poor self-perception may contribute to reduced quality of life more so than the functional consequences of facial synkinesis. In fact, 85% of the patients at our center have continued regular BT treatments since we began collecting injection data, and we have a 90% retention rate since we began collecting SAQ data. If patient retention can be used as a surrogate measure of value, then there was a clear trend of continuing long-term BT treatments for reasons beyond what may be captured by the SAQ. Moreover, the SAQ is obtained prior to and 2 weeks after BT treatments, which captures the most severe symptomology compared with the near maximal associated treatment response. Nonetheless, the SAQ is not inclusive and lacks the ability to evaluate hyperkinesia and other facial dyskinesia and also relies on patient compliance and 2-week SAQ responses.
Similar to prior reports, the median age of the present cohort was 54 years, with a predominance of females (80%) and Bell palsy as the most common cause (41%).22,26,29,32,37 Vestibular schwannomas comprised a relatively high proportion (36%), likely owing to our neurotology referral pattern. Regardless, patients are enrolled in facial retraining by a dedicated facial rehabilitative therapist prior to initiating BT therapy. This is an integral part of the treatment process and helps identify those patients who are refractory to physical therapy alone.
Limitations
The majority of patients were referred after the onset of synkinesis, and we were not able to assess the development, incidence, or potential for prevention of the disorder, which has been previously reported.4,38,39 We collected patient-reported synkinetic outcomes without objective, physician-guided assessments, such as the Sunnybrook Facial Grading System. This is in part due to our emphasis on patient perceived severity rather than on physician interpretations, and further study is required to correlate objective and subjective assessments. Future directions of inquiry may also include determining optimal BT dosing, EMG data to elucidate best practice management for muscles demonstrating hyperkinesis vs pure synkinesis, BT treatment value and cost analysis, pathophysiology of outcome discrepancies of female sex and younger patients, and the mechanism of botulinum resistance.
Conclusions
In facial synkinesis, botulinum dosing and muscle targeting increased after the first treatment session until steady state was achieved. A group of core facial muscles was treated most commonly, with customization depending on specific patient symptoms. Patients with facial synkinesis responded significantly to therapy, especially younger patients, females, and those with greater disease severity. Higher doses were significantly associated with improved outcomes, and oculo-oral synkinesis may be more responsive to treatment than oro-ocular synkinesis.
eAppendix. Muscle-Specific Considerations
eFigure 1. Study Design
eFigure 2. Treatment Patterns and Dose and Frequency of Treatment
eTable 1. Wilcoxon Signed Rank Test Results for Individual Survey Responses
eTable 2. Linear Regression of Synkinesis Assessment Questionnaire Outcomes
References
- 1.Guerrissi JO. Selective myectomy for postparetic facial synkinesis. Plast Reconstr Surg. 1991;87(3):459-466. doi: 10.1097/00006534-199103000-00010 [DOI] [PubMed] [Google Scholar]
- 2.Salles AG, da Costa EF, Ferreira MC, Remigio AF, Moraes LB, Gemperli R. Epidemiologic overview of synkinesis in 353 patients with longstanding facial paralysis under treatment with botulinum toxin for 11 years. Plast Reconstr Surg. 2015;136(6):1289-1298. doi: 10.1097/PRS.0000000000001802 [DOI] [PubMed] [Google Scholar]
- 3.Monini S, De Carlo A, Biagini M, et al. Combined protocol for treatment of secondary effects from facial nerve palsy. Acta Otolaryngol. 2011;131(8):882-886. doi: 10.3109/00016489.2011.577447 [DOI] [PubMed] [Google Scholar]
- 4.Dalla Toffola E, Bossi D, Buonocore M, Montomoli C, Petrucci L, Alfonsi E. Usefulness of BFB/EMG in facial palsy rehabilitation. Disabil Rehabil. 2005;27(14):809-815. doi: 10.1080/09638280400018650 [DOI] [PubMed] [Google Scholar]
- 5.Choi KH, Rho SH, Lee JM, Jeon JH, Park SY, Kim J. Botulinum toxin injection of both sides of the face to treat post-paralytic facial synkinesis. J Plast Reconstr Aesthet Surg. 2013;66(8):1058-1063. doi: 10.1016/j.bjps.2013.04.012 [DOI] [PubMed] [Google Scholar]
- 6.Filipo R, Spahiu I, Covelli E, Nicastri M, Bertoli GA. Botulinum toxin in the treatment of facial synkinesis and hyperkinesis. Laryngoscope. 2012;122(2):266-270. doi: 10.1002/lary.22404 [DOI] [PubMed] [Google Scholar]
- 7.Fu L, Bundy C, Sadiq SA. Psychological distress in people with disfigurement from facial palsy. Eye (Lond). 2011;25(10):1322-1326. doi: 10.1038/eye.2011.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers CR, Schmidt KL, VanSwearingen JM, et al. Automated facial image analysis: detecting improvement in abnormal facial movement after treatment with botulinum toxin A. Ann Plast Surg. 2007;58(1):39-47. doi: 10.1097/01.sap.0000250761.26824.4f [DOI] [PubMed] [Google Scholar]
- 9.Cabin JA, Massry GG, Azizzadeh B. Botulinum toxin in the management of facial paralysis. Curr Opin Otolaryngol Head Neck Surg. 2015;23(4):272-280. doi: 10.1097/MOO.0000000000000176 [DOI] [PubMed] [Google Scholar]
- 10.Borodic G, Bartley M, Slattery W, et al. Botulinum toxin for aberrant facial nerve regeneration: double-blind, placebo-controlled trial using subjective endpoints. Plast Reconstr Surg. 2005;116(1):36-43. doi: 10.1097/01.PRS.0000169689.27829.C4 [DOI] [PubMed] [Google Scholar]
- 11.Husseman J, Mehta RP. Management of synkinesis. Facial Plast Surg. 2008;24(2):242-249. doi: 10.1055/s-2008-1075840 [DOI] [PubMed] [Google Scholar]
- 12.Mehdizadeh OB, Diels J, White WM. Botulinum toxin in the treatment of facial paralysis. Facial Plast Surg Clin North Am. 2016;24(1):11-20. doi: 10.1016/j.fsc.2015.09.008 [DOI] [PubMed] [Google Scholar]
- 13.Novak CB. Rehabilitation strategies for facial nerve injuries. Semin Plast Surg. 2004;18(1):47-52. doi: 10.1055/s-2004-823123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jowett N, Hadlock TA. An evidence-based approach to facial reanimation. Facial Plast Surg Clin North Am. 2015;23(3):313-334. doi: 10.1016/j.fsc.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 15.Terzis JK, Karypidis D. Therapeutic strategies in post-facial paralysis synkinesis in adult patients. Plast Reconstr Surg. 2012;129(6):925e-939e. doi: 10.1097/PRS.0b013e318230e758 [DOI] [PubMed] [Google Scholar]
- 16.McElhinny ER, Reich I, Burt B, et al. Treatment of pseudoptosis secondary to aberrant regeneration of the facial nerve with botulinum toxin type A. Ophthalmic Plast Reconstr Surg. 2013;29(3):175-178. doi: 10.1097/IOP.0b013e3182873d7d [DOI] [PubMed] [Google Scholar]
- 17.Mandrini S, Comelli M, Dall’angelo A, et al. Long-term facial improvement after repeated BoNT-A injections and mirror biofeedback exercises for chronic facial synkinesis: a case-series study. Eur J Phys Rehabil Med. 2016;52(6):810-818. [PubMed] [Google Scholar]
- 18.Azuma T, Nakamura K, Takahashi M, et al. Mirror biofeedback rehabilitation after administration of single-dose botulinum toxin for treatment of facial synkinesis. Otolaryngol Head Neck Surg. 2012;146(1):40-45. doi: 10.1177/0194599811424125 [DOI] [PubMed] [Google Scholar]
- 19.Couch SM, Chundury RV, Holds JB. Subjective and objective outcome measures in the treatment of facial nerve synkinesis with onabotulinumtoxinA (Botox). Ophthalmic Plast Reconstr Surg. 2014;30(3):246-250. doi: 10.1097/IOP.0000000000000086 [DOI] [PubMed] [Google Scholar]
- 20.Chua CN, Quhill F, Jones E, Voon LW, Ahad M, Rowson N. Treatment of aberrant facial nerve regeneration with botulinum toxin A. Orbit. 2004;23(4):213-218. doi: 10.1080/01676830490512233 [DOI] [PubMed] [Google Scholar]
- 21.Borodic GE, Pearce LB, Cheney M, et al. Botulinum A toxin for treatment of aberrant facial nerve regeneration. Plast Reconstr Surg. 1993;91(6):1042-1045. doi: 10.1097/00006534-199305000-00011 [DOI] [PubMed] [Google Scholar]
- 22.Toffola ED, Furini F, Redaelli C, Prestifilippo E, Bejor M. Evaluation and treatment of synkinesis with botulinum toxin following facial nerve palsy. Disabil Rehabil. 2010;32(17):1414-1418. doi: 10.3109/09638280903514697 [DOI] [PubMed] [Google Scholar]
- 23.Maria CM, Kim J. Individualized management of facial synkinesis based on facial function. Acta Otolaryngol. 2017;137(9):1010-1015. doi: 10.1080/00016489.2017.1316871 [DOI] [PubMed] [Google Scholar]
- 24.Lee JM, Choi KH, Lim BW, Kim MW, Kim J. Half-mirror biofeedback exercise in combination with three botulinum toxin A injections for long-lasting treatment of facial sequelae after facial paralysis. J Plast Reconstr Aesthet Surg. 2015;68(1):71-78. doi: 10.1016/j.bjps.2014.08.067 [DOI] [PubMed] [Google Scholar]
- 25.Cronin GW, Steenerson RL. The effectiveness of neuromuscular facial retraining combined with electromyography in facial paralysis rehabilitation. Otolaryngol Head Neck Surg. 2003;128(4):534-538. doi: 10.1016/S0194-5998(03)00005-6 [DOI] [PubMed] [Google Scholar]
- 26.Mehta RP, WernickRobinson M, Hadlock TA. Validation of the Synkinesis Assessment Questionnaire. Laryngoscope. 2007;117(5):923-926. doi: 10.1097/MLG.0b013e3180412460 [DOI] [PubMed] [Google Scholar]
- 27.Kleiss IJ, Beurskens CH, Stalmeier PF, Ingels KJ, Marres HA. Synkinesis assessment in facial palsy: validation of the Dutch Synkinesis Assessment Questionnaire. Acta Neurol Belg. 2016;116(2):171-178. doi: 10.1007/s13760-015-0528-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel PN, Owen SR, Norton CP, et al. Outcomes of buccinator treatment with botulinum toxin in facial synkinesis. JAMA Facial Plast Surg. 2018;20(3):196-201. doi: 10.1001/jamafacial.2017.1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei LA, Diels J, Lucarelli MJ. Treating buccinator with botulinum toxin in patients with facial synkinesis: a previously overlooked target. Ophthalmic Plast Reconstr Surg. 2016;32(2):138-141. doi: 10.1097/IOP.0000000000000449 [DOI] [PubMed] [Google Scholar]
- 30.Risoud M, Aljudaibi N, Duquennoy-Martinot V, Guerreschi P. Long-term sequelae treatment of peripheral facial paralysis with botulinum toxin type A: repartition and kinetics of doses used. Ann Chir Plast Esthet. 2016;61(1):10-15. doi: 10.1016/j.anplas.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 31.Chundury RV, Perry JD. Re: “Treating Buccinator with Botulinum Toxin in Patients with Facial Synkinesis: A Previously Overlooked Target”. Ophthalmic Plast Reconstr Surg. 2016;32(1):70. doi: 10.1097/IOP.0000000000000608 [DOI] [PubMed] [Google Scholar]
- 32.Armstrong MW, Mountain RE, Murray JA. Treatment of facial synkinesis and facial asymmetry with botulinum toxin type A following facial nerve palsy. Clin Otolaryngol Allied Sci. 1996;21(1):15-20. doi: 10.1111/j.1365-2273.1996.tb01018.x [DOI] [PubMed] [Google Scholar]
- 33.Ito H, Ito H, Nakano S, Kusaka H. Low-dose subcutaneous injection of botulinum toxin type A for facial synkinesis and hyperlacrimation. Acta Neurol Scand. 2007;115(4):271-274. doi: 10.1111/j.1600-0404.2006.00746.x [DOI] [PubMed] [Google Scholar]
- 34.On AY, Yaltirik HP, Kirazli Y. Agreement between clinical and electromyographic assessments during the course of peripheric facial paralysis. Clin Rehabil. 2007;21(4):344-350. doi: 10.1177/0269215507073177 [DOI] [PubMed] [Google Scholar]
- 35.Moran CJ, Neely JG. Patterns of facial nerve synkinesis. Laryngoscope. 1996;106(12, pt 1):1491-1496. doi: 10.1097/00005537-199612000-00009 [DOI] [PubMed] [Google Scholar]
- 36.Beurskens CH, Oosterhof J, Nijhuis-van der Sanden MW. Frequency and location of synkineses in patients with peripheral facial nerve paresis. Otol Neurotol. 2010;31(4):671-675. [DOI] [PubMed] [Google Scholar]
- 37.Dall’Angelo A, Mandrini S, Sala V, et al. Platysma synkinesis in facial palsy and botulinum toxin type A. Laryngoscope. 2014;124(11):2513-2517. doi: 10.1002/lary.24732 [DOI] [PubMed] [Google Scholar]
- 38.Cai ZG, Shi XJ, Lu XG, Yang ZH, Yu GY. Efficacy of functional training of the facial muscles for treatment of incomplete peripheral facial nerve injury. Chin J Dent Res. 2010;13(1):37-43. [PubMed] [Google Scholar]
- 39.Nakamura K, Toda N, Sakamaki K, Kashima K, Takeda N. Biofeedback rehabilitation for prevention of synkinesis after facial palsy. Otolaryngol Head Neck Surg. 2003;128(4):539-543. doi: 10.1016/S0194-5998(02)23254-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Muscle-Specific Considerations
eFigure 1. Study Design
eFigure 2. Treatment Patterns and Dose and Frequency of Treatment
eTable 1. Wilcoxon Signed Rank Test Results for Individual Survey Responses
eTable 2. Linear Regression of Synkinesis Assessment Questionnaire Outcomes