This cohort study examines the utility of indocyanine green angiography to identify clinical factors that may be associated with neovascularization of paramedian forehead flaps in patients requiring multiple-staged nasal reconstruction surgery.
Key Points
Question
What patient or procedural factors are associated with the neovascularization of paramedian forehead flaps in staged nasal reconstruction surgical procedures as assessed by indocyanine green angiography?
Findings
In this cohort study of 71 participants, an increased number of days between the stages of the nasal reconstruction surgical procedures was independently and positively associated with the flap-to-cheek ingress (arterial inflow) ratio, whereas cartilage graft use was negatively associated with the flap-to-cheek outflow ratio.
Meaning
These findings suggest that indocyanine green angiography may help identify factors associated with flap perfusion, such as time between stages of surgery and cartilage graft use.
Abstract
Importance
Identifying factors affecting forehead flap neovascularization during nasal reconstruction surgical procedures using quantitative dynamics of fluorescence from indocyanine green angiography may be associated with reduced vascular complications.
Objectives
To identify quantifiable forehead flap perfusion measures using indocyanine green angiography during nasal reconstruction procedures and to evaluate clinical factors associated with neovascularization.
Design, Setting, and Participants
Retrospective cohort study of 71 patients at a tertiary referral center of Stanford University, Stanford, California, between January 1, 2010, and March 31, 2018, undergoing forehead flap nasal reconstruction surgery with flap perfusion assessed by indocyanine green angiography.
Exposures
Indocyanine green angiography was performed intraoperatively to record forehead flap neovascularization during the second stage of nasal reconstruction surgery after temporary clamping of the pedicle.
Main Outcomes and Measures
With use of quantifiable data of fluorescence dynamics, flap perfusion in association with a reference point in the cheek after pedicle clamping was assessed by 2 methods: (1) ingress (arterial inflow) and egress (venous outflow) flap-to-cheek ratio and (2) flap-to-cheek perfusion (fluorescence) ratio at 3 time points (midpoint of indocyanine green flap inflow, maximum fluorescence [peak], and midpoint of indocyanine green flap outflow) and their calculated mean. Association of the perfusion measures with patient and procedural factors was performed using linear regression models.
Results
Of the 71 patients included in the study, 43 (61%) were men; the mean (SD) age was 71.1 (11.0) years. The mean (SD) flap-to-cheek inflow ratio was 0.48 (0.40), peak fluorescence ratio was 0.59 (0.34), and outflow ratio was 0.88 (0.42). The calculated mean (SD) flap-to-cheek perfusion ratio of these measures was 0.65 (0.35). The mean (SD) flap-to-cheek ingress ratio was 0.54 (0.36) and egress ratio was 0.65 (0.98). With use of a multivariable regression model, the time between stages was positively associated with flap-to-cheek ingress ratio (β, 0.015; 95% CI, 0.001 to 0.030), and cartilage grafting was negatively associated with flap-to-cheek outflow ratio (β, –0.240; 95% CI, –0.472 to –0.008).
Conclusions and Relevance
The findings suggest that indocyanine green angiography is an effective method to quantify relative neovascularization perfusion of forehead flaps. Future applications may include the use of this technology to aid in early flap division and ensure adequate perfusion among high-risk patients.
Level of Evidence
NA.
Introduction
Management of structural defects of the head and neck, particularly of the nose, can frequently result in problematic impairment of both function and cosmesis. A thorough understanding of the external nasal anatomy and its blood supply is crucial to achieve optimal nasal reconstruction. Each defect is different in its size, location, and depth, requiring a unique reconstructive plan based on these factors. Likewise, each patient presents with various characteristics (ie, age or tobacco use) or comorbidities (ie, diabetes or radiotherapy exposure) that may affect the optimal reconstructive plan. Therefore, it is crucial to evaluate all of these factors when planning a nasal reconstruction surgical procedure.
Paramedian forehead flaps are commonly used to reconstruct large defects of the nose.1 Forehead flaps are axial-pattern flaps supplied by the supratrochlear blood vessels; they have a reliable pedicle that can provide abundant skin coverage of similar color, texture, and thickness to the nasal exterior of the face. The vascularity is also sufficient to simultaneously vascularize associated grafts such as cartilage or chondromucosal grafts.2 These flaps are generally used in a staged reconstruction procedure to provide sufficient time for neovascularization of the flap independent of the pedicle. Neovascularization develops from the vascular bed and/or the peripheral edges of the recipient site.3,4 The time between the flap placement and pedicle division varies among surgeons, ranging from 2 to 6 weeks.4,5 Although several patient and anatomic defect characteristics may affect neovascularization, quantitative studies assessing forehead flap perfusion have been limited.5,6,7,8
Various technologies to provide objective assessment of flap perfusion have been developed to provide a better understanding of flap hemodynamics.9 Among these technologies, indocyanine green (ICG) angiography using the SPY Elite Imaging System (LifeCell Corp Inc, Novadaq Technologies Inc) is a frequently used, minimally invasive technique to assess flap perfusion. Indocyanine green is a water-soluble dye that fluoresces maximally at 835 nm, which lies within the optical window of skin. The pharmacokinetics of ICG help for sequential monitoring of skin flap perfusion because of its short half-life of 2.5 to 4 minutes and its strict occupancy to the intravascular space.10,11 It has been safely used to assess cardiac output, liver function, choroid perfusion, skin perfusion, and recently, bone perfusion after osteotomy procedures.11,12,13,14,15,16,17 The SPY Elite system uses an infrared-sensitive camera to detect intravascular-injected fluorescent dye. The system also contains software that provides quantitative data of vascular perfusion and blood flow according to fluorescence dynamics. The SPY-Q software (LifeCell Corp Inc, Novadaq Technologies Inc) uses an algorithm to set the normal peripheral tissue perfusion at 100% and subsequently reports the perfusion percentage of the tissue in the flap under investigation. This technique has been previously used in smaller cohorts of patients to assess forehead flap perfusion.5,6
Better understanding of factors affecting the neovascularization of pedicled flaps may be associated with lower rates of vascular and functional complications. Understanding of the effect of time between the stages of the procedures may also be associated with lower morbidity and increased tolerability of staged nasal reconstructive procedures. The objective of this study was to evaluate whether clinically important patient and procedural factors hypothesized to have an influence on flap perfusion are associated with intraoperative assessment of flap perfusion using ICG angiography in a large cohort of patients undergoing paramedian forehead flap reconstruction of nasal defects.
Methods
A retrospective review of patients with nasal defects requiring paramedian forehead flap reconstruction between January 1, 2010, and March 31, 2018, was conducted after approval from the Stanford University institutional review board, Stanford, California. Participant consent was waived by the Stanford University institutional review board because no protected health information shared in the study and the study was retrospective. Additional inclusion criteria included patients older than 18 years with available ICG angiography data from the SPY Elite Imaging System for both the first- and second-stage surgical procedures. Patients younger than 18 years who were pregnant, required reconstruction of other facial defects, or had an iodide hypersensitivity were excluded. Data from ICG angiography were reviewed, and patients with incomplete data or corrupted recording files were also excluded. One of the considerations during video recording was for the patients to avoid any movement for approximately 90 seconds during the dye inflow and outflow. This restraint of movement provided consistent data for the software to automatically calculate fluorescence dynamics and avoided calculation errors. Perfusion data were collected from the recorded Digital Imaging and Communications in Medicine or Audio Video Interleaved files and were analyzed using the SPY-Q software.
During the first-stage surgical procedure, the flap was designed according to the defect size and site using aesthetic subunit principles.18 Real-time ICG angiography was performed to assess perfusion of the distal forehead flap after flap placement. At the second-stage surgical procedure, the pedicle was temporarily clamped followed by ICG angiography, which was again performed at the end of the procedure after pedicle division and flap inset. For laser-assisted ICG angiography, 2 mL of ICG (5 mg) were infused followed by a 10-mL saline bolus.
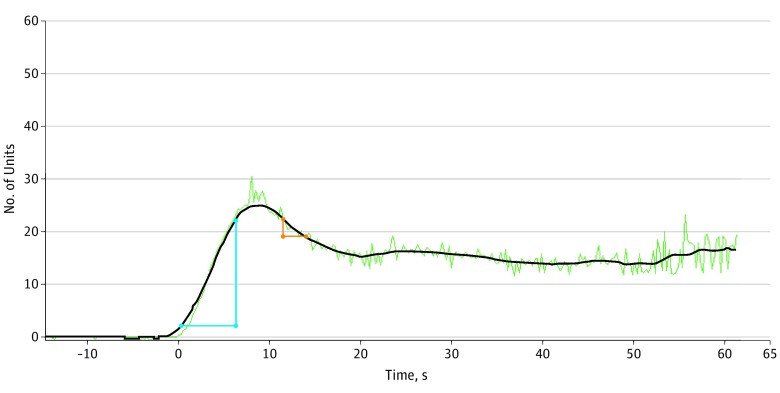
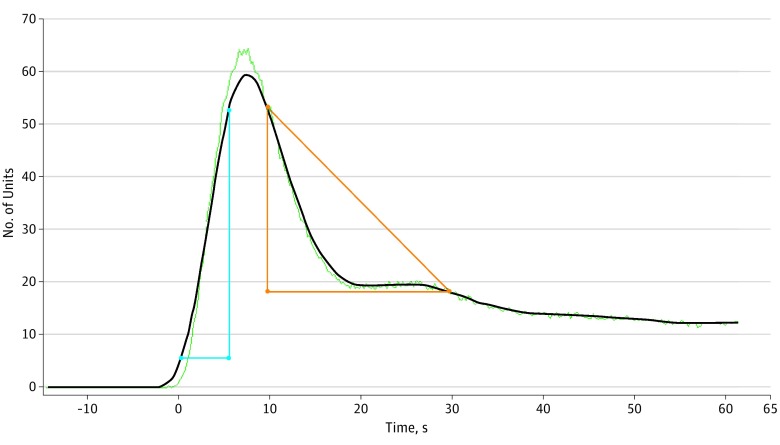
With the SPY-Q software, 2 methods were used to assess neovascularization perfusion of the forehead flap. First, the ratio of distal flap perfusion compared with a reference point on the cheek was measured at 3 different times: the midpoint of ICG inflow, at maximum (peak) fluorescence of the flap, and the midpoint of ICG outflow. The mean of these measures was also calculated. The most fluorescent region on the cheek (away from a distinct blood vessel, such as branches of the facial artery) was used as the reference. The second method assessed the ratio of the flap-to-cheek ingress (arterial inflow) and egress (venous outflow), representing a relative difference in velocity of blood flow between these 2 areas independent of a particular time, as shown in Figure 1 and Figure 2. Flap neovascularization perfusion measures were assessed after clamping the pedicle at the beginning of the second-stage procedure.
Figure 1. Ingress and Egress Rates of the Flap After Pedicle Clamping.
The blue triangle corresponds to the ingress rate, and the red triangle corresponds to the egress rate. Baseline equals 2 U. Ingress equals 25 U, with ingress rate of 3.3 U/s. Egress equals 6 U, with egress rate of 1.3 U/s.
Figure 2. Ingress and Egress Rates of Cheek.
The blue triangle corresponds to the ingress rate, and the red triangle corresponds to the egress rate. Baseline equals 1 U. Ingress equals 59 U, with ingress rate of 8.9 U/s. Egress equals 47 U, with egress rate of 1.7 U/s.
Statistical Analysis
Standard descriptive statistics were calculated for the patient population and perfusion measures using SPSS, version 25.0 (IBM Corp). Univariable linear regressions were used to assess the association of age, sex, diabetes, tobacco use, radiotherapy exposure, pathologic diagnosis, time between stages, full- vs partial-thickness defect, cartilage graft use, and defect surface area with each perfusion measure. Tests of collinearity between these variables were performed, in particular with time between the stages. Factors that were both statistically and clinically significant were used to construct the multivariable linear regression model. A P < .05 was considered to be statistically significant; β values of the linear regressions and 95% CIs were reported.
Results
Of 85 patients who met the inclusion criteria for this study, complete angiography data were available for 71 patients; 14 patients were excluded because of absent or corrupted angiography data files owing to patient movement during data collection. Baseline patient and defect characteristics are shown in Table 1. Most of the patients in this study were male (43 of 71 [61%]), with a mean (SD) age of 71.1 (11.0) years. Most patients had a diagnosis of basal cell carcinoma (54 [76%]), followed by squamous cell carcinoma (8 [11%]), melanoma (6 [8%]), and reconstruction because of other defects including infection or trauma (3 [4%]). Comorbidities assessed included tobacco use (31 [44%]), radiotherapy exposure of the head and neck (8 [11%]) with 5 exposures directly to the nose, and diabetes (12 [17%]). The mean (SD) interval between flap placement and pedicle division with flap inset was 21.3 (6.9) days. Defect depth included partial thickness defects in 55 patients (77%), full thickness defects in 16 patients (23%), mean (SD) defect surface area of 14.2 (17.3) cm2, and cartilage graft use in 43 patients (61%). The excluded 14 patients did not have characteristics significantly different from the study population after χ2 or independent t test analysis except less cartilage graft use (61% vs 28%; P = .02), and they did not encounter more complications.
Table 1. Baseline Patient and Recipient Site Characteristics.
| Characteristic | Patients (N = 71)a |
|---|---|
| Sex | |
| Male | 43 (61) |
| Female | 28 (39) |
| Age, mean (SD), y | 71.1 (11.0) |
| Pathologic diagnosis | |
| Basal cell carcinoma | 54 (76) |
| Squamous cell carcinoma | 8 (11) |
| Melanoma | 6 (8) |
| Otherb | 3 (4) |
| Tobacco use | 31 (44) |
| Head and neck radiotherapy exposurec | 8 (11) |
| Diabetes | 12 (17) |
| Time between stages, mean (SD), dd | 21.3 (6.9) |
| Defect depth | |
| Partial thickness | 55 (77) |
| Full thickness | 16 (23) |
| Defect surface area, mean (SD), cm2 | 14.2 (17.3) |
| Cartilage graft use | 43 (61) |
Data are presented as number (percentage) of patients unless otherwise indicated.
Other includes defects caused by infection or trauma.
Five of 8 radiotherapy exposures were directly to the nose.
Stage 1 defined as flap placement and stage 2 as pedicle division with flap inset.
The assessed patients did not experience flap necrosis or nasal obstruction. However, 2 patients (3%) experienced minor complications: 1 patient had distal superficial epidermal sloughing, and another patient had partial distal wound dehiscence. One patient was a nonsmoking woman in her 80s with a history of deep vein thrombosis and a 13-day interval between the 2 procedure stages. The other patient was a man in his 60s with a history of tobacco use who had a full-thickness defect and cartilage graft with a 23-day interval between procedure stages.
Perfusion data obtained by each method are shown in Table 2. The mean (SD) flap-to-cheek inflow ratio was 0.48 (0.40), the peak fluorescence ratio was 0.59 (0.34), and the outflow ratio was 0.88 (0.42). The calculated mean (SD) flap-to-cheek perfusion ratio of these measures (inflow, peak fluorescence, and outflow) was 0.65 (0.35). The mean (SD) flap-to-cheek ingress ratio was 0.54 (0.36) and egress ratio was 0.65 (0.98). All of these measures showed normal distribution except the flap-to-cheek egress ratio, which showed greater variability in values. These data revealed that there was a greater relative amount of fluorescence during blood outflow from the flap compared with during inflow, suggesting that the flap filled and drained at a slower rate than the cheek; similarly, the mean flap-to-cheek ingress ratio was lower than the egress ratio, signifying more venous congestion of the flap compared with the cheek.
Table 2. Flap-to-Cheek Perfusion Measures.
| Flap-to-Cheek Perfusion Measure | Value, Mean (SD) |
|---|---|
| Time-dependent ratio | |
| Midpoint of indocyanine green flap inflow | 0.48 (0.40) |
| Peak fluorescence | 0.59 (0.34) |
| Midpoint of indocyanine green outflow | 0.88 (0.42) |
| Calculated mean of inflow, peak, and outflow measures | 0.65 (0.35) |
| Time-independent ratio | |
| Flap ingress or arterial inflow | 0.54 (0.36) |
| Flap egress or venous outflow | 0.65 (0.98) |
Each perfusion measure was analyzed by univariable linear regression; the patient and defect characteristics are listed in the eTable in the Supplement. Only 2 perfusion measures—the flap-to-cheek outflow ratio and the flap-to-cheek ingress ratio—were statistically significantly associated with individual patient or defect characteristics, and thus only these 2 outcome variables were used to create the multivariable model. Although not statistically significant, diabetes had a consistently negative association with flap perfusion across all 6 perfusion measures (β ranging from −0.38 to −0.107), whereas tobacco use, radiotherapy exposure, and defect characteristics had more variable associations (β ranging from −0.242 to 0.256) (eTable in the Supplement).
The time between flap placement and pedicle division and flap inset had the greatest association with perfusion measures using the univariable linear model. Further assessment was performed regarding the association between this variable and other patient or defect characteristics to assess for collinearity and aid in construction of the multivariable model. Factors associated with greater time between stages by linear regression included tobacco use (β, 3.93; 95% CI, 0.76-7.10), radiotherapy exposure (β, 5.05; 95% CI, 0.01-10.10), full-thickness defect (β, 6.76; 95% CI, 3.18-10.33), defect surface area (β, 0.15; 95% CI, 0.06-0.24), and cartilage graft use (β, 5.28; 95% CI, 2.17-8.39). These factors, along with time between stages, were used in the multivariable linear regression for flap-to-cheek outflow ratio and flap-to-cheek ingress ratio to identify factors independently associated with flap perfusion.
The multivariable linear regression of flap-to-cheek outflow ratio and flap-to-cheek ingress ratio is shown in Table 3. Regarding the flap-to-cheek outflow, cartilage graft use was reported to have statistically significant negative association with flap perfusion (β, –0.240; 95% CI, –0.472 to –0.008). Other factors did not reach statistical significance on multivariable regression; however, time between stages, which was statistically significant in the univariable model, showed the next closest association with flap perfusion (β, 0.014; 95% CI, –0.0002 to 0.030). When assessing flap-to-cheek ingress ratio, time between stages had a statistically significant independent association with flap perfusion (β, 0.015; 95% CI, 0.001-0.030).
Table 3. Multivariable Analysis.
| Factors | β (95% CI) | |
|---|---|---|
| Outflow Ratio | Ingress Ratio | |
| Tobacco use | 0.075 (−0.133 to 0.283) | −0.076 (−0.261 to 0.110) |
| Radiotherapy exposure | −0.011 (−0.344 to 0.321) | 0.026 (−0.269 to 0.320) |
| Time between stages, d | 0.014 (−0.003 to 0.031) | 0.015 (0.001 to 0.030) |
| Defect depth | ||
| Partial thickness | 1 [Reference] | 1 [Reference] |
| Full thickness | 0.054 (−0.223 to 0.331) | −0.106 (−0.352 to 0.140 |
| Defect area, cm2 | 0.004 (−0.002 to 0.011) | 0.003 (−0.003 to 0.008) |
| Cartilage graft use | −0.240 (−0.472 to −0.008) | 0.039 (−0.169 to 0.247) |
Discussion
Although forehead flaps are the most commonly used type of flap to reconstruct large nasal defects, there are few studies assessing flap perfusion and hemodynamics and their clinical implications. To our knowledge, this was the largest study of patients undergoing intraoperative perfusion assessment of forehead flaps used for nasal reconstruction. This study provided quantitative relative perfusion data and clinical outcomes of the 2-stage nasal reconstruction. As shown in Table 2, the time-independent perfusion measures assessing flow (flap ingress [arterial inflow] and flap egress [venous outflow]) were both less than 1, signifying slowing perfusion of the flap compared with the cheek. This finding was also supported by the time-dependent factors assessing fluorescence of the flap compared with the cheek (flap-to-cheek inflow ratio, peak fluorescence ratio, outflow ratio, and the calculated mean of these measures). All mean results reported were less than 1, and they increased with time (0.48 [inflow ratio] to 0.59 [peak fluorescence ratio] to 0.88 [outflow ratio]), meaning that the flap became increasingly more fluorescent compared with the cheek with time, whereas the flap was both perfused with blood and drained at a slower rate than the cheek. As shown by the low rate of complications, these mean perfusion values were sufficient to provide adequate blood supply to the flap after division of the pedicle. In this study, no major complications and a 3% rate of minor complications were reported, which reflects a relatively low complications rate of this surgical procedure compared with reported complications rates varying between 6.1% and 14.1%.19,20 Use of ICG angiography resulted in no instances of complete flap necrosis, which has been reported in other studies at rates between 1.2% and 2.0%.19,21
In this study, patient factors investigated included age, sex, tobacco use, diabetes, and radiotherapy exposure. Other than diabetes, none of these factors showed consistent associations with the neovascularization perfusion measures assessed. Diabetes had a negative association with all perfusion measures; however, this finding was not statistically significant. This observation was supported by previously published studies that used color duplex–Doppler ultrasonography to assess flap perfusion and reported no association between age, sex, or tobacco use and the resistance index of blood flow in forehead flaps.7 Although tobacco use was shown to be associated with greater odds of flap necrosis in some studies,21 others showed that it did not have a significant association with wound dehiscence, scars, flap necrosis, or notching.19,22
Factors of the recipient site (defect) evaluated included pathologic diagnosis, defect surface area, defect depth (partial vs full thickness), cartilage graft use, and time between stages. As expected, longer time between the 2 stages was positively associated with the flap-to-cheek ingress ratio, suggesting that arterial inflow to the flap was faster with increased duration of time between stages. A negative association was observed between the flap-to-cheek outflow ratio and cartilage graft use, suggesting that cartilage graft use may slow venous neovascularization of the flap. One implication of this finding was that venous outflow may be more dependent on the wound bed than arterial inflow, although this was not independently examined in this study. Examination of other recipient site factors’ association with perfusion measures did not reach statistical significance. In previous studies, full-thickness defects were associated with significantly higher rates of developing a major complication, including flap necrosis, notching, and obstruction, compared with partial-thickness defects.21 Although no statistically significant association between perfusion measures and defect thickness were found in our study, 1 of the 2 patients who experienced a minor complication (small area of dehiscence) had reconstruction of a full-thickness defect.
In nasal reconstruction procedures, ICG angiography was first introduced as a qualitative assessment in a few case reports23,24,25 and as comparative quantitative assessment in a case series by Woodard and Most.6 The modality was also assessed to minimize the time between the stages of reconstruction to 2 weeks5 and evaluate the cost-effectiveness of ICG angiography in nasal reconstruction.8 Another technology used to provide comparative quantitative data of forehead flap perfusion is color duplex–Doppler ultrasonography to measure the flap resistance index.7 With use of this method, a statistically significant decrease in the early high resistance index (first day: 0.871 in the proximal flap and 0.869 in the distal flap) after a 2-week interval (0.597 in the proximal flap and 0.582 in the distal flap) was observed, and it was concluded that the resistance index decreased gradually, leading to detectable venous outflow by the end of the first postoperative week.7 This finding was supported by our results, because the flap-to-cheek ingress ratio (arterial inflow) was significantly associated with increased time between stages.
In plastic and reconstructive surgery, ICG angiography has been used to assess groin flaps, vertical rectus abdominis flaps, sural island flaps, and reversed forearm flap.11 It has also been used to detect arterial and venous anastomotic patency,26 to measure depth of burns to predict scar contracture,27 and extensively in breast reconstruction.28,29,30,31,32 In addition, ICG angiography has been used after nasal osteotomy procedures13 and renal,33 liver, and pancreatic transplant.34 The method used for perfusion assessment was variable among these studies, with some authors reporting flap fluorescence with ICG angiography as qualitative proof of vascularity24,34 and other authors providing quantitative data interpreted by the SPY-Q software.6,13,28 One quantitative method used was to report the ratio of ICG fluorescence dynamics to the flap in contrast with nonsurgically touched tissue,5,6,13 and another method reported the rate of arterial (ingress) and venous (egress) blood flow of flaps.28 Despite the variation of methods, in all the previously referenced studies, ICG angiography was shown to provide a reliable perfusion assessment.
In this study, we reported 2 distinct methods using the SPY-Q software to assess flap perfusion using a point on the cheek as a reference. One method was time dependent (comparing fluorescence between these 2 areas at the midpoint of flap inflow, peak fluorescence, flap outflow, and the calculated mean of these values), and one method was time independent (the ratio of ingress [arterial inflow] to the flap compared with the cheek and the ratio of egress [venous outflow] from the flap compared with the cheek). Further investigation is needed to assess which perfusion method is optimal. Given the low rate of vascular complications in this cohort, it was difficult to assess the association of perfusion measures with the rate of complications. Assessment of more high-risk patients or patients with shorter time between stages of reconstruction may help elucidate the optimal perfusion measures because these patients are at greater risk for vascular compromise. Another area of interest is determining whether primary neovascularization originates from the periphery (ie, coaptation of the subdermal plexus), via the deep side of the flap (ie, subcutaneously), or both equally.
Further applications of this technology include assessing a larger number of patients to capture more complications, as well as those at greater risk for vascular complications. This technology can also be used to assess the perfusion of flaps at decreased time intervals between staged reconstruction to safely perform an accelerated pedicle division and help improve patients’ quality of life.
Strengths and Limitations
A strength of this study includes the large sample size and a standardized technique for perfusion assessment. The study provided a comparative quantitative assessment of forehead flap perfusion and assessment of the association of potential patient and defect factors with neovascularization.
Limitations include the variation of comparative quantitative data obtained from patients because of variable microcirculation and hemodynamics. Thus, a ratio between the flap and patient’s own nonsurgically touched tissue was used to provide more accurate comparison between patients. In addition, this study used data from a single institution based on the work of a single surgeon and with a retrospective study design.
Conclusions
The findings suggest that indocyanine green angiography is an effective method to qualify and quantify neovascularization perfusion of forehead flaps. Among factors studied, time between stages and cartilage graft use were significantly associated with perfusion measures. Future applications may include use of this technology to aid in early flap division and ensure adequate perfusion of forehead flaps among high-risk patients.
eTable 1. Univariable Linear Regression
References
- 1.Burget GC. Aesthetic restoration of the nose. Clin Plast Surg. 1985;12(3):463-480. [PubMed] [Google Scholar]
- 2.Hessam S, Georgas D, Sand M, Bechara FG. Penetrating defect of the ala nasi: combined reconstruction with a myocutaneous hinge- and paramedian forehead flap. J Dtsch Dermatol Ges. 2014;12(2):169-171. [DOI] [PubMed] [Google Scholar]
- 3.Park SS. Reconstruction of nasal defects larger than 1.5 centimeters in diameter. Laryngoscope. 2000;110(8):1241-1250. doi: 10.1097/00005537-200008000-00001 [DOI] [PubMed] [Google Scholar]
- 4.Menick FJ. Nasal reconstruction with a forehead flap. Clin Plast Surg. 2009;36(3):443-459. doi: 10.1016/j.cps.2009.02.015 [DOI] [PubMed] [Google Scholar]
- 5.Surowitz JB, Most SP. Use of laser-assisted indocyanine green angiography for early division of the forehead flap pedicle. JAMA Facial Plast Surg. 2015;17(3):209-214. doi: 10.1001/jamafacial.2015.0171 [DOI] [PubMed] [Google Scholar]
- 6.Woodard CR, Most SP. Intraoperative angiography using laser-assisted indocyanine green imaging to map perfusion of forehead flaps. Arch Facial Plast Surg. 2012;14(4):263-269. doi: 10.1001/archfacial.2011.1540 [DOI] [PubMed] [Google Scholar]
- 7.Eskiizmir G, Tanyeri Toker G, Ozgur E, Tarhan S, Cengiz Ozyurt B. Hemodynamic changes in paramedian forehead flap. J Craniofac Surg. 2018;29(1):159-162. [DOI] [PubMed] [Google Scholar]
- 8.Calloway HE, Moubayed SP, Most SP. Cost-effectiveness of early division of the forehead flap pedicle. JAMA Facial Plast Surg. 2017;19(5):418-420. doi: 10.1001/jamafacial.2017.0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pawar SS, Kim MM. Updates in forehead flap reconstruction of facial defects. Curr Opin Otolaryngol Head Neck Surg. 2013;21(4):384-388. doi: 10.1097/MOO.0b013e328362ce42 [DOI] [PubMed] [Google Scholar]
- 10.Partington EJ, Moore LS, Kahmke R, et al. Laser-assisted indocyanine green dye angiography for postoperative fistulas after salvage laryngectomy. JAMA Otolaryngol Head Neck Surg. 2017;143(8):775-781. doi: 10.1001/jamaoto.2017.0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holm C, Mayr M, Höfter E, Becker A, Pfeiffer UJ, Mühlbauer W. Intraoperative evaluation of skin-flap viability using laser-induced fluorescence of indocyanine green. Br J Plast Surg. 2002;55(8):635-644. doi: 10.1054/bjps.2002.3969 [DOI] [PubMed] [Google Scholar]
- 12.Ishihara H, Otomo N, Suzuki A, Takamura K, Tsubo T, Matsuki A. Detection of capillary protein leakage by glucose and indocyanine green dilutions during the early post-burn period. Burns. 1998;24(6):525-531. doi: 10.1016/S0305-4179(98)80004-1 [DOI] [PubMed] [Google Scholar]
- 13.Salman S, Fattahi T, Fernandes R, Steinberg B. Dynamic analysis of maxillary perfusion during Le Fort I osteotomy using indocyanine green. Int J Oral Maxillofac Surg. 2018;47(10):1311-1315. doi: 10.1016/j.ijom.2018.01.010 [DOI] [PubMed] [Google Scholar]
- 14.Ott P. Hepatic elimination of indocyanine green with special reference to distribution kinetics and the influence of plasma protein binding. Pharmacol Toxicol. 1998;83(suppl 2):1-48. doi: 10.1111/j.1600-0773.1998.tb01945.x [DOI] [PubMed] [Google Scholar]
- 15.Flower RW, Hochheimer BF. A clinical technique and apparatus for simultaneous angiography of the separate retinal and choroidal circulations. Invest Ophthalmol. 1973;12(4):248-261. [PubMed] [Google Scholar]
- 16.Wood EH. Diagnostic applications of indicator-dilution technics in congenital heart disease. Circ Res. 1962;10:531-568. doi: 10.1161/01.RES.10.3.531 [DOI] [PubMed] [Google Scholar]
- 17.Zenn MR. Fluorescent angiography. Clin Plast Surg. 2011;38(2):293-300. doi: 10.1016/j.cps.2011.03.009 [DOI] [PubMed] [Google Scholar]
- 18.Burget GC, Menick FJ. The subunit principle in nasal reconstruction. Plast Reconstr Surg. 1985;76(2):239-247. doi: 10.1097/00006534-198508000-00010 [DOI] [PubMed] [Google Scholar]
- 19.Paddack AC, Frank RW, Spencer HJ, Key JM, Vural E. Outcomes of paramedian forehead and nasolabial interpolation flaps in nasal reconstruction. Arch Otolaryngol Head Neck Surg. 2012;138(4):367-371. doi: 10.1001/archoto.2012.69 [DOI] [PubMed] [Google Scholar]
- 20.Blázquez-Sánchez N, Fernández-Canedo I, Repiso-Jiménez JB, Rivas-Ruiz F, De Troya Martín M. Usefulness of the paramedian forehead flap in nasal reconstructive surgery: a retrospective series of 41 patients. Actas Dermosifiliogr. 2016;107(2):133-141. [DOI] [PubMed] [Google Scholar]
- 21.Little SC, Hughley BB, Park SS. Complications with forehead flaps in nasal reconstruction. Laryngoscope. 2009;119(6):1093-1099. doi: 10.1002/lary.20243 [DOI] [PubMed] [Google Scholar]
- 22.Dixon AJ, Dixon MP, Dixon JB, Del Mar CB. Prospective study of skin surgery in smokers vs. nonsmokers. Br J Dermatol. 2009;160(2):365-367. doi: 10.1111/j.1365-2133.2008.08846.x [DOI] [PubMed] [Google Scholar]
- 23.Christensen JM, Baumann DP, Myers JN, Buretta K, Sacks JM. Indocyanine green near-infrared laser angiography predicts timing for the division of a forehead flap. Eplasty. 2012;12:e41. [PMC free article] [PubMed] [Google Scholar]
- 24.Shah A, Au A. Laser-assisted indocyanine green evaluation of paramedian forehead flap perfusion prior to pedicle division. Eplasty. 2013;13:e8. [PMC free article] [PubMed] [Google Scholar]
- 25.Lee LN, Smith DF, Boahene KD, Byrne PJ. Intraoperative laser-assisted indocyanine green imaging for objective measurement of the vascular delay technique in locoregional head and neck flaps. JAMA Facial Plast Surg. 2014;16(5):343-347. doi: 10.1001/jamafacial.2014.106 [DOI] [PubMed] [Google Scholar]
- 26.Holm C, Mayr M, Höfter E, Dornseifer U, Ninkovic M. Assessment of the patency of microvascular anastomoses using microscope-integrated near-infrared angiography: a preliminary study. Microsurgery. 2009;29(7):509-514. doi: 10.1002/micr.20645 [DOI] [PubMed] [Google Scholar]
- 27.Still JM, Law EJ, Klavuhn KG, Island TC, Holtz JZ. Diagnosis of burn depth using laser-induced indocyanine green fluorescence: a preliminary clinical trial. Burns. 2001;27(4):364-371. doi: 10.1016/S0305-4179(00)00140-6 [DOI] [PubMed] [Google Scholar]
- 28.Wang CY, Wang CH, Tzeng YS, et al. Intraoperative assessment of the relationship between nipple circulation and incision site in nipple-sparing mastectomy with implant breast reconstruction using the SPY Imaging System. Ann Plast Surg. 2018;80(2S)(suppl 1):S59-S65. doi: 10.1097/SAP.0000000000001496 [DOI] [PubMed] [Google Scholar]
- 29.Phillips BT, Lanier ST, Conkling N, et al. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plast Reconstr Surg. 2012;129(5):778e-788e. doi: 10.1097/PRS.0b013e31824a2ae8 [DOI] [PubMed] [Google Scholar]
- 30.Munabi NC, Olorunnipa OB, Goltsman D, Rohde CH, Ascherman JA. The ability of intra-operative perfusion mapping with laser-assisted indocyanine green angiography to predict mastectomy flap necrosis in breast reconstruction: a prospective trial. J Plast Reconstr Aesthet Surg. 2014;67(4):449-455. doi: 10.1016/j.bjps.2013.12.040 [DOI] [PubMed] [Google Scholar]
- 31.Kanuri A, Liu AS, Guo L. Whom should we SPY? a cost analysis of laser-assisted indocyanine green angiography in prevention of mastectomy skin flap necrosis during prosthesis-based breast reconstruction. Plast Reconstr Surg. 2014;133(4):448e-454e. doi: 10.1097/PRS.0000000000000025 [DOI] [PubMed] [Google Scholar]
- 32.Duggal CS, Madni T, Losken A. An outcome analysis of intraoperative angiography for postmastectomy breast reconstruction. Aesthet Surg J. 2014;34(1):61-65. doi: 10.1177/1090820X13514995 [DOI] [PubMed] [Google Scholar]
- 33.Arichi N, Mitsui Y, Ogawa K, et al. Intraoperative fluorescence vascular imaging using indocyanine green for assessment of transplanted kidney perfusion. Transplant Proc. 2014;46(2):342-345. doi: 10.1016/j.transproceed.2013.11.129 [DOI] [PubMed] [Google Scholar]
- 34.Panaro F, Benedetti E, Pineton de Chambrun G, et al. Indocyanine green fluorescence angiography during liver and pancreas transplantation: a tool to integrate perfusion statement’s evaluation. Hepatobiliary Surg Nutr. 2018;7(3):161-166. doi: 10.21037/hbsn.2017.07.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Univariable Linear Regression