Key Points
Question
What is the association between reductions in regional air pollutant concentration and incidence of childhood asthma?
Findings
In this longitudinal study that included 4140 children, each 4.3–parts-per-billion decrease in nitrogen dioxide was associated with a reduction of 0.83 cases per 100 person-years in asthma incidence; each 8.1-μg/m3 decrease in particulate matter less than 2.5 μm was associated with a reduction of 1.53 cases per 100 person-years in asthma incidence. There were no statistically significant associations with change in ozone and particulate matter less than 10 μm.
Meaning
Declines in nitrogen dioxide and particulate matter less than 2.5 μm may be associated with decreased childhood asthma incidence.
Abstract
Importance
Exposure to air pollutants is a well-established cause of asthma exacerbation in children; whether air pollutants play a role in the development of childhood asthma, however, remains uncertain.
Objective
To examine whether decreasing regional air pollutants were associated with reduced incidence of childhood asthma.
Design, Setting, and Participants
A multilevel longitudinal cohort drawn from 3 waves of the Southern California Children’s Health Study over a period of air pollution decline. Each cohort was followed up from 4th to 12th grade (8 years): 1993-2001, 1996-2004, and 2006-2014. Final follow-up for these data was June 2014. Population-based recruitment was from public elementary schools. A total of 4140 children with no history of asthma and residing in 1 of 9 Children’s Health Study communities at baseline were included.
Exposures
Annual mean community-level ozone, nitrogen dioxide, and particulate matter less than 10 μm (PM10) and less than 2.5 μm (PM2.5) in the baseline year for each of 3 cohorts.
Main Outcomes and Measures
Prospectively identified incident asthma, collected via questionnaires during follow-up.
Results
Among the 4140 children included in this study (mean [SD] age at baseline, 9.5 [0.6] years; 52.6% female [n = 2 179]; 58.6% white [n = 2273]; and 42.2% Hispanic [n = 1686]), 525 incident asthma cases were identified. For nitrogen dioxide, the incidence rate ratio (IRR) for asthma was 0.80 (95% CI, 0.71-0.90) for a median reduction of 4.3 parts per billion, with an absolute incidence rate decrease of 0.83 cases per 100 person-years. For PM2.5, the IRR was 0.81 (95% CI, 0.67-0.98) for a median reduction of 8.1 μg/m3, with an absolute incidence rate decrease of 1.53 cases per 100 person-years. For ozone, the IRR for asthma was 0.85 (95% CI, 0.71-1.02) for a median reduction of 8.9 parts per billion, with an absolute incidence rate decrease of 0.78 cases per 100 person-years. For PM10, the IRR was 0.93 (95% CI, 0.82-1.07) for a median reduction of 4.0 μg/m3, with an absolute incidence rate decrease of 0.46 cases per 100 person-years.
Conclusions and Relevance
Among children in Southern California, decreases in ambient nitrogen dioxide and PM2.5 between 1993 and 2014 were significantly associated with lower asthma incidence. There were no statistically significant associations for ozone or PM10.
This study uses Southern California Children’s Health Study data to examine associations between changes in regional air pollution (ozone, nitrogen dioxide, and particulate matter <10 μm and <2.5 μm) and changes in childhood asthma incidence across 3 child cohorts.
Introduction
Asthma is the most common pediatric chronic disease, estimated to have affected 14% of children globally in 2002-2003.1 There has been much interest in the effect of outdoor air pollution on asthma risk given its ubiquity and high levels in urban areas, which, compared with rural areas, have higher rates of asthma.2 Globally, outdoor air pollution has been recognized as a major public health concern and was estimated to have contributed 6.8% of the annual disability-adjusted life-years lost in 2016.3 Although ambient air pollution exposure has been causally linked to asthma exacerbations in children,4 evidence has been limited for a role in asthma development.2,4 There has been support for a link with close proximity to busy roads,4,5,6 but studies of regional pollutants, such as ozone, nitrogen dioxide, and particulate matter (PM), have provided less robust evidence.
This study was designed to take advantage of secular trends in air pollution to examine asthma incidence among children recruited and followed up longitudinally within the same set of communities over a period of air pollution decline. Since the early 1990s, air pollutant concentrations have decreased in Southern California.7 During this time, several successive cohorts of schoolchildren were enrolled from the same set of communities as part of the Southern California Children’s Health Study (CHS), a long-term study of cardiopulmonary pediatric health outcomes.8 Leveraging this unique data resource, the study examined whether observed reductions in regional air pollutants, specifically ozone, nitrogen dioxide, and PM less than 10 μm (PM10) or less than 2.5 μm (PM2.5), were associated with asthma incidence rates within these CHS communities.
Methods
Study Design and Participants
All parents or guardians of participating children provided written informed consent. The study protocol was approved by the institutional review board of the University of Southern California. The study population was drawn from 3 successive cohorts of the CHS. The CHS has been described in detail elsewhere.8,9 Briefly, 12 communities in Southern California were selected in 1993 based on historical air pollutant levels.8 Children were recruited from participating communities through public schools and followed up prospectively until 12th grade. Fourth graders aged 9 to 10 years were recruited in 1993 (n = 1798) and in 1996 (n = 2061) from the 12 communities. In 2003, kindergarteners and first graders (n = 5736) were recruited from 13 communities, resulting in a total of 16 communities contributing data to the 3 cohorts.9 These 3 cohorts will hereafter be referred to as the 1993-2001, 1996-2004, and 2006-2014 cohorts. Nine communities participated in all 3 cohorts (Alpine, Lake Elsinore, Lake Gregory, Long Beach, Mira Loma, Riverside, San Dimas, Santa Maria, and Upland; n = 6858).
Baseline questionnaires regarding the children’s health and exposures, as well as demographic information, were completed by the parents or guardians. Annual follow-up questionnaires assessing changes in the children’s health, among other factors, were completed initially by the parents or guardians and later by the participating children starting at approximately age 11 years. To better facilitate the comparison of these 3 cohorts in this analysis, follow-up for the 2006-2014 cohort was realigned to begin in the fourth year (2006) of that cohort’s original timeline, when most 2006-2014 cohort participants (>46%) were in the fourth grade. Final follow-up for these data was June 2014 and the data reported here are the most recent available at the time of this study from these cohorts.
Exposure Assessment
Ambient air pollutant monitoring stations were established in each of the study communities and have been continuously measuring regional air pollution since the inception of the CHS. Data on concentrations of ozone, nitrogen dioxide, PM10, and PM2.5 were routinely collected, as previously described.8,10 Community-specific annual mean concentrations in the baseline year for each cohort (ie, 1993, 1996, and 2006 for the 1993-2001, 1996-2004, and 2006-2014 cohorts, respectively) were calculated based on 24-hour means for nitrogen dioxide, PM10, and PM2.5, and on the 10 am to 6 pm mean for ozone due to its marked diurnal variation.
Covariate Assessment
Data were obtained from the baseline questionnaires on children’s date of birth, sex, race and ethnicity, history of asthma, participation in team sports, presence of a gas stove in the home, exposure to smoking in utero, exposure to secondhand smoke, parental education, parental history of asthma, and residential address. Determination of children’s race/ethnicity was made by the parents or guardians who completed the baseline questionnaire based on a question with fixed categories. Race/ethnicity was included as a covariate due to its role as a potential confounder of the air pollution–asthma relationship. Exposure to secondhand smoke was classified based on a positive response to either of the following questions: “Does anyone living in this child’s home currently smoke cigarettes, cigars or pipes on a daily basis inside the home?” or “In the past, has anyone living in this child’s home ever smoked cigarettes on a daily basis inside the home while the child was living there?” Use of a Spanish-language questionnaire by the parent or guardian at baseline was also recorded. Genetic ancestry, including Native American ancestry, was estimated using 233 ancestral informative markers and the STRUCTURE program.11,12,13
Baseline residential address was used to estimate exposure to local near-roadway pollution, based on a line source dispersion model as previously described.6,9 Inputs to this model included distance to roadways, vehicle counts, vehicle nitrogen oxide emission rates, wind speed and direction, and height of the mixing layer in each community. Mean temperature data were also collected during follow-up from measurements at monitoring stations. For each cohort baseline year, community-specific mean temperature was computed from monthly means in that year. Temperature data were not available for 1993, consequently 1994 levels were used instead for all 1993-2001 cohort participants. Additionally, 2006 temperature data were not available for 2 communities (Lake Gregory and San Dimas); therefore, data from 2001, the closest previous year with complete data, were used for 2006-2014 cohort participants in these 2 communities. Community-level annual mean temperatures did not vary greatly from year to year; the mean coefficient of variation from 1994 to 2001 and 2006, for those communities with data, was 4% (range, 2%-6%).
Outcome Assessment
Incident asthma was defined as a newly reported physician-diagnosed case of asthma on an annual questionnaire during follow-up (ie, first time answered “yes” to the question “Has a doctor ever diagnosed this child as having asthma?” when the parent or guardian was asked or “Has a doctor ever said you have asthma?” when the child was asked). Because incident asthma cases were defined with these annual questionnaires, specific dates of diagnoses were unknown. We imputed the date of diagnosis using the midpoint of the interval between the date of the questionnaire on which asthma diagnosis was first reported and the date of the questionnaire prior to reporting asthma status. This date was used for calculating follow-up time for all statistical analyses. Children with missing questionnaires during follow-up continued to contribute person-time until they reported an asthma diagnosis or were lost to follow-up.
Statistical Analysis
To assess association between changes in regional air quality and asthma incidence in children within community over the course of follow-up for these 3 cohorts, we fitted multilevel Poisson regression models to estimate asthma incidence rate ratios (IRRs) and 95% CIs associated with exposure to regional air pollution.10,14,15 Models included an offset term for person-time (natural log-transformed) and a fixed effect for community. Additionally, to account for clustering effects of children by cohort and community, a random effect for cohort nested within community with an unstructured covariance matrix was included in the model. Follow-up time was calculated as the number of days between joining the cohort (ie, baseline questionnaire date) and either imputed date of asthma diagnosis or date of last completed questionnaire (either 12th grade or earlier if lost to follow-up), whichever came first.
Regional air pollution exposures were defined as the community-level annual mean concentrations in the baseline year for each cohort (ie, 1993, 1996, and 2006). Data were not available for 1993 on PM10 in 4 communities (Alpine, Lake Gregory, Riverside, and Upland) and PM2.5 in any community, therefore, 1994 concentrations were used. Point estimates were scaled to the median change in community-level annual mean concentration among the 9 communities from 1993 to 2006. These models were designed to make inferences regarding within-community changes in regional air pollution and asthma incidence rates. Incidence rate differences were calculated to provide context on absolute change; these models used sampling weights for communities to make results interpretable for the entire sample. Additional details on modeling approach are reported in eMethods in the Supplement.
Potential confounders were identified a priori based on a directed acyclic graph.16 These were baseline age (continuous), sex (female, male), ethnicity (Hispanic, non-Hispanic), race (Asian/Pacific Islander, black, Native American/other, white, mixed), presence of gas stove in home (yes, no), physical activity defined here as team sports participation (yes, no), temperature defined here as community-level mean temperature for cohort baseline year (continuous), and exposure to local near-roadway pollution (continuous). To avoid loss of sample size, missing indicators were included as needed for any categorical adjustment variable. Three sets of models were fitted for each pollutant: adjusted only for community (fixed effect), additionally adjusted for all potential confounders except local near-roadway pollution, and additionally adjusted for local near-roadway pollution. Adjustment for local pollutants was conducted separately in the third model because 198 children whose residential addresses could not be geocoded were missing these data, decreasing the sample size.
We assessed heterogeneity of the regional air pollution associations by comparing nested models using a partial likelihood ratio test with and without interaction terms for the following potential effect modifiers: sex, ethnicity, race, exposure to smoking in utero, secondhand smoke exposure, parental education, parental history of asthma, Native American ancestry (only among Hispanic children), and designation of high vs low air pollution community—based on whether a community was above or below corresponding median annual mean concentration in 1993. For evaluation of effect modification, Native American ancestry among Hispanic participants was categorized into 2 groups based on having less than or greater than 50% Native American ancestry.
Robustness of the main study findings were tested with the following sensitivity analyses: (1) excluding 1 community at a time, (2) excluding participants who reported wheeze and/or 3 or more months of cough in the prior 12 months at baseline, (3) excluding data from the first year of follow-up, (4) excluding 2006-2014 cohort participants whose baseline asthma status was defined based on the year 3 rather than year 4 questionnaire, (5) reincluding participants with missing baseline asthma status, (6) imputing asthma diagnosis date to 6 months after completion date of prior questionnaire, (7) restricting to participants with longer follow-up (followed to year 5 or later, or to year 7 or later), (8) including additional potential covariates, (9) omitting the random effect for cohort nested within community and instead bootstrapping17 at the community level to assess modeling assumptions, and (10) including a fixed effect for cohort to adjust for potential temporal confounding.
Two pollutant models were fitted whenever the correlations between covariates were found to be sufficiently low to avoid multicollinearity. In addition, a set of sensitivity analyses were conducted using Cox proportional hazards regression, using the same modeling approach as the main fully adjusted model but with no random effect. These models were used to evaluate (1) the inclusion of time-varying calendar year to adjust for potential temporal confounding and (2) the use of a time-varying air pollution exposure variable. No apparent violation of the underlying assumption of proportional hazards was detected based on inclusion of a time-dependent covariate for air pollution. Due to missing air pollution data in earlier years (as noted here) and no air pollution data after 2011 as well as missing PM2.5 data for 1 community in 2005, air pollution for these years was imputed by extending the closest years’ air pollution data (ie, 1994 for 1993, 2006 for 2005, and 2011 for 2012 and later years).
All hypotheses were tested assuming a .05 significance level and a 2-sided alternative hypothesis. P values were not adjusted for multiple comparisons because the tests were hypothesis driven. All analyses were conducted using SAS software version 9.4 (SAS Institute).
Results
The characteristics of the 4140 children included in this study are described overall and by cohort in Table 1. Because the outcome was asthma incidence during follow-up, we excluded participants (in a hierarchical manner) who had no follow-up questionnaire (n = 1503), had physician-diagnosed asthma at baseline (n = 804), or were missing baseline asthma status (n = 143). For 2006-2014 cohort participants, data from the fourth year, according to the original 2006-2014 cohort timeline, were used to define baseline asthma status. If no questionnaire was completed that year, data from the prior year were used (n = 467). If data from neither the fourth-year nor third-year questionnaire were available, those participants were considered as having missing baseline asthma status and excluded from the analysis (n = 268). The final study population comprised 4140 children, including 1093, 1170, and 1877 from the 1993-2001, 1996-2004, and 2006-2014 cohorts, respectively. Descriptive statistics on participants excluded from the analysis are given in eTable 1 in the Supplement. Mean person-years observed per child were similar across the cohorts: 5.7, 5.8, and 6.0 for the 1993-2001, 1996-2004, and 2006-2014 cohorts, respectively. The crude incidence rate for asthma was the highest for the 1996-2004 cohort (2.69 cases per 100 person-years) and lowest for the 2006-2014 cohort (1.80 cases per 100 person-years).
Table 1. Distribution of Selected Participant Characteristics From the Children’s Health Study, 1993-2014.
| Characteristic | All Participants, No./Total No. (%) | Cohort Follow-up Period, No./Total No. (%) | ||
|---|---|---|---|---|
| 1993-2001 | 1996-2004 | 2006-2014 | ||
| Participants | 4140 | 1093 | 1170 | 1877 |
| Person-years of follow-up | 24 254 | 6201 | 6842 | 11 211 |
| Follow-up questionnaires per participant, mean (SD) | 5.5 (2.2) | 5.9 (2.3) | 6.7 (2.5) | 4.4 (1.2) |
| Incident asthma cases | 525 | 139 | 184 | 202 |
| Age at baseline, mean (SD), y | 9.5 (0.6) | 9.9 (0.5) | 9.5 (0.4) | 9.3 (0.7) |
| Sex | ||||
| Male | 1961/4140 (47.4) | 524/1093 (47.9) | 564/1170 (48.2) | 873/1877 (46.5) |
| Female | 2179/4140 (52.6) | 569/1093 (52.1) | 606/1170 (51.8) | 1004/1877 (53.5) |
| Ethnicity | ||||
| Hispanic | 1686/3996 (42.2) | 307/1083 (28.3) | 413/1163 (35.5) | 966/1750 (55.2) |
| Non-Hispanic | 2310/3996 (57.8) | 776/1083 (71.7) | 750/1163 (64.5) | 784/1750 (44.8) |
| Missing | 144/4140 (3.5) | 10/1093 (0.9) | 7/1170 (0.6) | 127/1877 (6.8) |
| Race | ||||
| Asian/Pacific Islander | 178/3878 (4.6) | 60/1072 (5.6) | 56/1157 (4.8) | 62/1649 (3.8) |
| Black | 145/3878 (3.7) | 50/1072 (4.7) | 54/1157 (4.7) | 41/1649 (2.5) |
| Native American/other | 890/3878 (22.9) | 182/1072 (17) | 249/1157 (21.5) | 459/1649 (27.8) |
| White | 2273/3878 (58.6) | 704/1072 (65.7) | 692/1157 (59.8) | 877/1649 (53.2) |
| Mixed | 392/3878 (10.1) | 76/1072 (7.1) | 106/1157 (9.2) | 210/1649 (12.7) |
| Missing | 262/4140 (6.3) | 21/1093 (1.9) | 13/1170 (1.1) | 228/1877 (12.2) |
| Parental education | ||||
| High school graduate or below | 1424/3900 (36.5) | 379/1068 (35.5) | 385/1113 (34.6) | 660/1719 (38.4) |
| Some college or above | 2476/3900 (63.5) | 689/1068 (64.5) | 728/1113 (65.4) | 1059/1719 (61.6) |
| Missing | 240/4140 (5.8) | 25/1093 (2.3) | 57/1170 (4.9) | 158/1877 (8.4) |
| Gas stove in home | ||||
| Yes | 3153/3937 (80.1) | 824/1067 (77.2) | 860/1147 (75) | 1469/1723 (85.3) |
| Play team sport | ||||
| Yes | 2104/4042 (52.1) | 542/1074 (50.5) | 597/1136 (52.6) | 965/1832 (52.7) |
| In utero exposure to smoking | ||||
| Yes | 484/3939 (12.3) | 187/1063 (17.6) | 177/1143 (15.5) | 120/1733 (6.9) |
| Secondhand smoke exposurea | ||||
| Yes | 874/3880 (22.5) | 302/1059 (28.5) | 308/1116 (27.6) | 264/1705 (15.5) |
| Parental history of asthma | ||||
| Yes | 687/3922 (17.5) | 175/1027 (17) | 172/1084 (15.9) | 340/1811 (18.8) |
| Spanish questionnaire | ||||
| Yes | 654/4140 (15.8) | 79/1093 (7.2) | 158/1170 (13.5) | 417/1877 (22.2) |
| Residential traffic-related pollution, mean (SD), parts per billion | 19.6 (22.1) | 27.5 (27.8) | 20.5 (23.3) | 14.9 (15.8) |
Exposure to secondhand smoke was classified based on a positive response to either of the following questions: “Does anyone living in this child’s home currently smoke cigarettes, cigars or pipes on a daily basis inside the home?” or “In the past, has anyone living in this child’s home ever smoked cigarettes on a daily basis inside the home while the child was living there?”
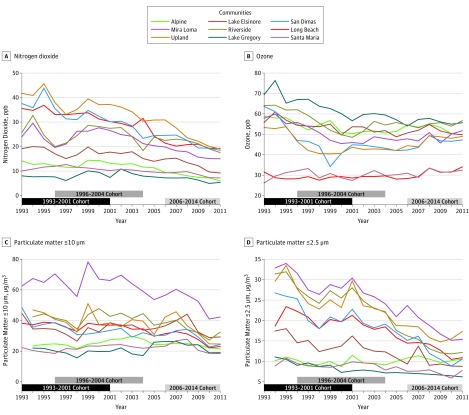
Regional air pollution concentrations generally decreased among the 9 communities over the course of the study period (Figure 1). The median changes in community-level annual mean concentration among the 9 communities from 1993 to 2006 were −8.9 parts per billion (ppb) (range, −21.4 to 4.8) for ozone, −4.3 ppb (range, −14.1 to −0.8) for nitrogen dioxide, −4.0 μg/m3 (range, −10.9 to 4.3) for PM10, and −8.1 μg/m3 (range, −15.2 to 0.7) for PM2.5. Reductions in air pollution were larger in communities with higher 1993 concentrations.
Figure 1. Annual Mean Air Pollutant Concentration During the Follow-up Period in 9 Communities of the Southern California Children’s Health Study, 1993-2011.
Black, dark gray, and light gray horizontal bars represent follow-up periods for the 1993-2001, 1996-2004, and 2006-2014 cohorts, respectively. Follow-up for the 2006-2014 cohort is truncated on the graph at 2011, the last year with air pollution data. ppb indicates parts per billion.
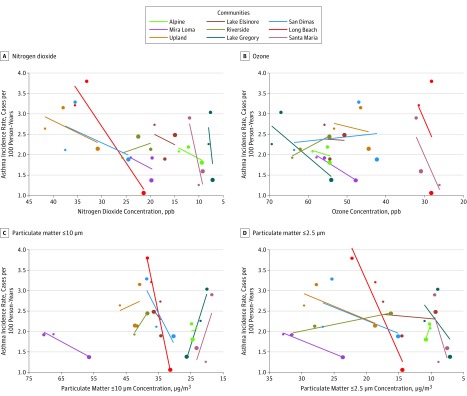
Plots of the unadjusted community-level data, along with community-specific regression lines, comparing asthma incidence rates with regional air pollution concentrations across the 3 cohorts are shown in Figure 2. Greater reductions in asthma incidence rates were observed in communities with larger decreases in either nitrogen dioxide or PM2.5 concentrations. Results were less consistent for ozone and PM10.
Figure 2. Asthma Incidence Rates and Air Pollutant Concentrations in 9 Communities During the 1993-2001, 1996-2004, and 2006-2014 Cohorts of the Southern California Children’s Health Study, 1993-2014.
Symbol colors indicate community and the size—small, medium, and large—indicates the data are from the 1993-2001, 1996-2004, and 2006-2014 cohorts, respectively. Simple linear regression models based on asthma incidence and air pollution concentration used to generate regression lines separately for each community. ppb indicates parts per billion.
Reductions in regional nitrogen dioxide and PM2.5, but not ozone or PM10, levels were statistically significantly associated with reductions in asthma incidence rate among children (Table 2). For community-level nitrogen dioxide, the IRR for asthma was 0.83 (95% CI, 0.74-0.92) when only adjusted for community (for a reduction of 4.3 ppb). When adjusted for additional potential confounders, including near-roadway pollution, the IRR was 0.81 (95% CI, 0.72-0.91). For community-level PM2.5, the IRR was 0.82 (95% CI, 0.69-0.98) (for a reduction of 8.1 μg/m3). In the adjusted model with near-roadway pollution, the IRR was 0.82 (95% CI, 0.67-0.99). Reduced risks associated with decreasing ozone and PM10 did not reach statistical significance. Results for incidence rate differences showed absolute decreases of 0.83, 1.53, 0.78, and 0.46 cases per 100 person-years for nitrogen dioxide, PM2.5, ozone, and PM10, respectively.
Table 2. Incidence Rate Ratios (IRRs) and Incidence Rate Differences (IRDs) per 100 Person-Years of Asthma Incidence Associated With Reduction in Regional Air Pollution, 1993-2014a.
| Pollutant | Community-Only Adjusted Model (n = 4140)b | Fully Adjusted Model (n = 4140)c | Fully Adjusted Model With Near-Roadway Pollution (n = 3942)d | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRR (95% CI) | P Value | IRD (95% CI)e | P Value | IRR (95% CI) | P Value | IRD (95% CI)e | P Value | IRR (95% CI) | P Value | IRD (95% CI)e | P Value | |
| Ozone | 0.86 (0.72 to 1.02) | .08 | −0.77 (−0.86 to −0.68) | <.001 | 0.85 (0.71 to 1.02) | .08 | −0.78 (−1.44 to −0.12) | .02 | 0.86 (0.71 to 1.04) | .11 | −0.76 (−1.41 to −0.11) | .02 |
| Nitrogen dioxide | 0.83 (0.74 to 0.92) | .001 | −0.88 (−0.95 to −0.80) | <.001 | 0.80 (0.71 to 0.90) | <.001 | −0.83 (−1.54 to −0.13) | .02 | 0.81 (0.72 to 0.91) | <.001 | −0.82 (−1.52 to −0.12) | .02 |
| PM10 | 0.92 (0.81 to 1.05) | .22 | −0.47 (−0.67 to −0.28) | <.001 | 0.93 (0.82 to 1.07) | .32 | −0.46 (−0.96 to 0.04) | .08 | 0.92 (0.81 to 1.04) | .17 | −0.48 (−0.90 to −0.06) | .03 |
| PM2.5 | 0.82 (0.69 to 0.98) | .03 | −1.47 (−2.11 to −0.83) | <.001 | 0.81 (0.67 to 0.98) | .03 | −1.53 (−2.95 to −0.11) | .04 | 0.82 (0.67 to 0.99) | .04 | −1.48 (−2.88 to −0.07) | .04 |
Abbreviation: PM, particulate matter.
IRR and IRD are per −8.9 ppb for ozone, −4.3 ppb for nitrogen dioxide, −4.0 μg/m3 for PM10, and −8.1 μg/m3 for PM2.5 (median changes in air pollution concentrations observed among the 9 communities between 1993 and 2006).
Community-only adjusted model adjusted for community as a fixed effect.
Fully adjusted model additionally adjusted for age at baseline, sex, ethnicity, race, gas stove in home, participation in sports, and community-level mean temperature for baseline year.
Fully adjusted model with traffic additionally adjusted for local near-roadway pollution.
Models for IRD incorporated weights for communities, based on sample size contribution, to make results interpretable for the entire sample.
Associations for nitrogen dioxide and PM2.5 did not substantially vary by sex, ethnicity, race, exposure to smoking in utero, exposure to secondhand smoke, parental education, parental history of asthma, Native American ancestry (among Hispanic children), or high or low 1993 air pollution level (eTable 2 in the Supplement).
Sensitivity analyses demonstrated results for nitrogen dioxide were robust to a variety of analytical decisions as reported in eTables 3 and 4 in the Supplement. Results for analyses excluding participants who reported wheeze and/or 3 or more months of cough in the prior 12 months at baseline (IRR, 0.81 [95% CI, 0.71-0.92]), targeting both cough- and wheeze-variant potential asthmatic cases, as well as in analyses excluding the first year of follow-up (IRR, 0.77 [95% CI, 0.68-0.88]), targeting prevalent cases, remained similar to those of the main analysis. Two pollutant models were fitted for both nitrogen dioxide and PM2.5 with PM10 (Pearson correlation coefficients were 0.50 and 0.55, respectively) and for nitrogen dioxide with ozone (correlation = 0.54). Results for nitrogen dioxide remained robust. Results for PM2.5 were not statistically significant. Two pollutant models were not fitted for nitrogen dioxide with PM2.5 (correlation = 0.60), nor PM2.5 with ozone (correlation = 0.62). Results for nitrogen dioxide and PM2.5 based on models with time-varying exposure remained statistically significant and point estimates were similar, although attenuated, compared with the results for the main baseline exposure models (eTable 4 in the Supplement). Findings for nitrogen dioxide based on models adjusted for calendar time remained robust, while those for PM2.5 did not and results for this model were null (eTable 4 in the Supplement). Overall in sensitivity analyses, associations for nitrogen dioxide remained statistically significant and generally similar in magnitude to those in the primary analysis. These analyses revealed, however, that the findings for PM2.5 were not consistently robust.
Discussion
Reductions in levels of regional nitrogen dioxide from 1993 to 2014 were statistically significantly associated with improvements in asthma incidence rates in Southern Californian children. These results were independent of changes in exposure to near-roadway pollution. Findings from this study also suggested a potential association with regional PM2.5; these results, however, were less robust to sensitivity analyses. Nitrogen dioxide results remained robust in sensitivity analyses. Associations did not appear to be substantially influenced by a single community. The inclusion of prevalent asthma cases in the study population could have resulted in exacerbation in addition to incidence being captured in the definition of the outcome. Sensitivity analyses excluding potential prevalent cases, including cough- and wheeze-variant potential asthmatic cases, did not show marked differences compared with main results, suggesting this source of bias was not of major concern. Findings for PM2.5 should be interpreted with caution because these appeared more sensitive to analytical choices, as demonstrated in sensitivity analyses. Point estimates for PM2.5 from sensitivity analyses were generally similar to those of the main models, but 95% CIs were wider, and several included the null.
This study provides evidence of a robust association between children’s exposure to community-level nitrogen dioxide and development of asthma in childhood. Previous review of the development of childhood asthma and environmental exposures concluded that although several ambient pollutants were associated with increased asthma, none were consistently identified.18 Several meta-analysis studies, however, have concluded that nitrogen dioxide exposure was associated with asthma incidence among children.5,19,20,21,22 A 2017 systematic review and meta-analysis reported an overall risk estimate of 1.05 (95% CI, 1.02-1.07) per 4 μg/m3 (2.1 ppb) nitrogen dioxide. The IRR from the current study scaled to a 2.1-ppb change in nitrogen dioxide was 1.11 (95% CI, 1.05-1.18; fully adjusted model including near-roadway pollution).
It is unclear whether nitrogen dioxide is the causal agent or rather is serving as a marker for the traffic-related air pollution mixture. One CHS study, using data from the 2006-2014 cohort only, reported an association between exposure to nitrogen dioxide and asthma incidence.6,23 Ambient nitrogen dioxide measured at community central sites was associated with increased asthma risk (hazard ratio, 2.18 [95% CI, 1.18-4.01]), although this association was attenuated after accounting for traffic-related pollutions (hazard ratio, 1.37 [95% CI, 0.69-2.71]). Results for nitrogen dioxide in the present study did not change when adjusted for near-roadway pollution. While positive associations were observed in both studies, the current study benefitted from a within-community, across-time design, which provided increased statistical power and allowed for the control of community-level unmeasured confounders. These results suggest that nitrogen dioxide is capturing effects of air pollution exposure beyond local near-roadway pollution. PM2.5 has also been found to be associated with asthma incidence,5,20,24 and while PM2.5 was positively associated with incident asthma, the results were not consistently robust to sensitivity analyses. PM2.5 mass in the current study comprises traffic-related PM2.5 and that from other sources, such as dust and ocean spray. Incorporating data on PM2.5 mass sources may help specify the relation between PM2.5 and incident asthma. From 1990 to 2012, a period corresponding to the period of study in the present analyses, levels of diesel PM have decreased by 68% in California25 and this downward trend was observed in Los Angeles.26 Furthermore, measures of diesel PM, such as elemental carbon and black carbon, are correlated with nitrogen dioxide particularly near major roadways.27,28 Studies have reported positive associations between measures of diesel PM and incident childhood asthma,5 but a lack of elemental carbon or black carbon data across all 3 cohorts precluded the evaluation of this relation in the present study.
There is evidence for the plausibility of a biological mechanism specifically for nitrogen dioxide. Studies indicate that at concentrations typical of high-income countries, exposure to nitrogen dioxide induces airway inflammation,29,30 airway hyperresponsiveness,31 and oxidative stress.32 In healthy adult humans, controlled exposure to nitrogen dioxide produced enhanced pulmonary neutrophilic inflammation and the promotion of a Th2 phenotype.33 The UK Committee on the Medical Effects of Air Pollutants identified 4 mechanisms for how air pollution might contribute to the pathogenesis of asthma: (1) oxidative stress and damage, including the depletion of antioxidants; (2) airway wall remodeling, leading to structural changes in the airways; (3) inflammatory pathways and immunological effects, including effects on the expression of inflammatory mediators; and (4) enhancement of respiratory sensitization to allergens.34
A benefit of the modeling framework used here was that communities were compared with themselves at 3 points in time, thus reducing the potential for confounding by spatial factors, under the assumption that contextual variables in the community did not change. A concern remains, however, for temporal confounding. Controlling for factors with trends across the 3 study cohorts (eg, health insurance, tobacco exposure, ethnicity) did not change results. Adjusting for cohort or time-varying calendar year showed the nitrogen dioxide findings to be robust while the PM2.5 findings were not. Furthermore, secular trends in asthma rates have been increasing over the study period,35 which would bias the results toward the null. In addition, reductions in asthma incidence rates were larger in communities with greater reductions in nitrogen dioxide concentrations, further indicating results were not likely to be simply an artifact of secular trends in asthma diagnosis or other potential temporal confounders.
Limitations
The study has several limitations. First, baseline, rather than time-varying, community-level annual average pollutant concentration was used as the exposure in the main models. Although a model with time-varying exposure was implemented using Cox proportional hazards regression in sensitivity analyses, this was not used as the main model because it was not possible to obtain estimates with the multilevel modeling approach (eg, when a fixed effect for community and a random effect of cohort nested within community was included). The sensitivity analyses using a Cox model with no random effect and time-varying air pollutant exposure generated results that were similar, although attenuated, compared with the Poisson models using baseline air pollutant exposure. These time-varying exposure data had more missing exposure data that necessitated imputation and, as such, may have resulted in greater exposure misclassification compared with the use of baseline exposure data. Additionally, these models may not capture the best time window of exposure because only a 1-year lag could be used given that no exposure data were available prior to the start of the study.
Second, the modeling framework, which controls for spatial confounding based on a fixed effect for community, remained susceptible to temporal confounding. This source of confounding was evaluated in sensitivity analyses, in which the findings for nitrogen dioxide were robust. Third, the definition of asthma incidence depended on a questionnaire-based assessment of physician-diagnosed asthma, rather than a clinical evaluation of asthma (eg, methacholine challenge test). Studies examining the validity of questionnaire-based asthma diagnosis in children, using questions similar to those used in the current study, have reported a specificity of 96% compared with health claims as the reference standard36 and a specificity of 87% compared with a clinical assessment as the standard.37 Fourth, a lack of data on measures of diesel PM (eg, elemental carbon or black carbon) and PM2.5 mass sources precluded the investigation of temporal trends in concentrations of these air pollutants and the incidence of childhood asthma.
Conclusions
Among children in Southern California, decreases in ambient nitrogen dioxide and PM2.5 between 1993 and 2014 were significantly associated with lower asthma incidence. There were no statistically significant associations for ozone or PM10.
eMethods. Data Analysis
eTable 1. Distribution of Selected Characteristics Among Participants Included and Excluded From the Current Study of the Children's Health Study, 1993-2014
eTable 2. P Values for Evidence of Effect Modification of the Asthma Incidence and Air Pollution Association Based on Partial Likelihood Ratio Tests for Interaction Terms With Pollutant and Potential Effect Modifier in the Children's Health Study, 1993-2014
eTable 3. Results of Sensitivity Analyses for Incidence Rate Ratios (IRR) Associated With Reduction in Nitrogen Dioxide (NO2) and Particulate Matter <2.5 μm (PM2.5) in the Children's Health Study, 1993-2014
eTable 4. Results of Sensitivity Analyses Using Cox Proportional Hazards Models for Ozone (O3), Nitrogen Dioxide (NO2), Particulate Matter <10 μm (PM10) and <2.5 μm (PM2.5) in the Children's Health Study, 1993-2014
eReferences
References
- 1.Pearce N, Aït-Khaled N, Beasley R, et al. ; ISAAC Phase Three Study Group . Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. 2007;62(9):758-766. doi: 10.1136/thx.2006.070169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383(9928):1581-1592. doi: 10.1016/S0140-6736(14)60617-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaborators GBDRF; GBD 2016 Risk Factors Collaborators . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1345-1422. doi: 10.1016/S0140-6736(17)32366-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.HEI Panel on the Health Effects of Traffic-Related Air Pollution Traffic-Related Air Pollution: A Critical Review of the Literature on Emissions, Exposure, and Health Effects. Boston, MA: Health Effects Institute; 2010. [Google Scholar]
- 5.Khreis H, Kelly C, Tate J, Parslow R, Lucas K, Nieuwenhuijsen M. Exposure to traffic-related air pollution and risk of development of childhood asthma: a systematic review and meta-analysis. Environ Int. 2017;100:1-31. doi: 10.1016/j.envint.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 6.McConnell R, Islam T, Shankardass K, et al. Childhood incident asthma and traffic-related air pollution at home and school. Environ Health Perspect. 2010;118(7):1021-1026. doi: 10.1289/ehp.0901232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.South Coast Air Quality Management District Final 2016: air quality management plan. http://www.aqmd.gov/docs/default-source/clean-air-plans/air-quality-management-plans/2016-air-quality-management-plan/final-2016-aqmp/final2016aqmp.pdf. Published March 2017. Accessed January 31, 2018.
- 8.Peters JM, Avol E, Navidi W, et al. A study of twelve Southern California communities with differing levels and types of air pollution, I: prevalence of respiratory morbidity. Am J Respir Crit Care Med. 1999;159(3):760-767. doi: 10.1164/ajrccm.159.3.9804143 [DOI] [PubMed] [Google Scholar]
- 9.McConnell R, Berhane K, Yao L, et al. Traffic, susceptibility, and childhood asthma. Environ Health Perspect. 2006;114(5):766-772. doi: 10.1289/ehp.8594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauderman WJ, Urman R, Avol E, et al. Association of improved air quality with lung development in children. N Engl J Med. 2015;372(10):905-913. doi: 10.1056/NEJMoa1414123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weaver GM, Gauderman WJ. Traffic-related pollutants: exposure and health effects among Hispanic children. Am J Epidemiol. 2018;187(1):45-52. doi: 10.1093/aje/kwx223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salam MT, Avoundjian T, Knight WM, Gilliland FD. Genetic ancestry and asthma and rhinitis occurrence in Hispanic children: findings from the Southern California Children’s Health Study. PLoS One. 2015;10(8):e0135384. doi: 10.1371/journal.pone.0135384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berhane K, Gauderman WJ, Stram DO, Thomas DC. Statistical issues in studies of the long-term effects of air pollution: the Southern California Children’s Health Study. Stat Sci. 2004;19(3):414-434. doi: 10.1214/088342304000000413 [DOI] [Google Scholar]
- 15.Berhane K, Chang CC, McConnell R, et al. Association of changes in air quality with bronchitic symptoms in children in California, 1993-2012. JAMA. 2016;315(14):1491-1501. doi: 10.1001/jama.2016.3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37-48. doi: 10.1097/00001648-199901000-00008 [DOI] [PubMed] [Google Scholar]
- 17.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Boca Raton, FL: CRC Press; 1993. doi: 10.1007/978-1-4899-4541-9 [DOI] [Google Scholar]
- 18.Dick S, Friend A, Dynes K, et al. A systematic review of associations between environmental exposures and development of asthma in children aged up to 9 years. BMJ Open. 2014;4(11):e006554. doi: 10.1136/bmjopen-2014-006554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gasana J, Dillikar D, Mendy A, Forno E, Ramos Vieira E. Motor vehicle air pollution and asthma in children: a meta-analysis. Environ Res. 2012;117:36-45. doi: 10.1016/j.envres.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 20.Anderson HR, Favarato G, Atkinson RW. Long-term exposure to air pollution and the incidence of asthma: meta-analysis of cohort studies. Air Qual Atmos Health. 2013;6(1):47-56. doi: 10.1007/s11869-011-0144-5 [DOI] [Google Scholar]
- 21.Takenoue Y, Kaneko T, Miyamae T, Mori M, Yokota S. Influence of outdoor NO2 exposure on asthma in childhood: meta-analysis. Pediatr Int. 2012;54(6):762-769. doi: 10.1111/j.1442-200X.2012.03674.x [DOI] [PubMed] [Google Scholar]
- 22.Gehring U, Wijga AH, Hoek G, et al. Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: a population-based birth cohort study. Lancet Respir Med. 2015;3(12):933-942. doi: 10.1016/S2213-2600(15)00426-9 [DOI] [PubMed] [Google Scholar]
- 23.Jerrett M, Shankardass K, Berhane K, et al. Traffic-related air pollution and asthma onset in children: a prospective cohort study with individual exposure measurement. Environ Health Perspect. 2008;116(10):1433-1438. doi: 10.1289/ehp.10968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowatte G, Lodge C, Lowe AJ, et al. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy. 2015;70(3):245-256. doi: 10.1111/all.12561 [DOI] [PubMed] [Google Scholar]
- 25.California Air Resources Board Overview: diesel exhaust & health. https://ww2.arb.ca.gov/resources/overview-diesel-exhaust-and-health. Accessed February 28, 2019.
- 26.McDonald BC, Goldstein AH, Harley RA. Long-term trends in California mobile source emissions and ambient concentrations of black carbon and organic aerosol. Environ Sci Technol. 2015;49(8):5178-5188. doi: 10.1021/es505912b [DOI] [PubMed] [Google Scholar]
- 27.Beckerman B, Jerrett M, Brook JR, Verma DK, Arain MA, Finkelstein MM. Correlation of nitrogen dioxide with other traffic pollutants near a major expressway. Atmos Environ. 2008;42(2):275-290. doi: 10.1016/j.atmosenv.2007.09.042 [DOI] [Google Scholar]
- 28.Polidori A, Fine PM. Ambient Concentrations of Criteria and Air Toxic Pollutants in Close Proximity to a Freeway With Heavy-Duty Diesel Traffic. Diamond Bar, CA: South Coast Air Quality Management District; 2012. [Google Scholar]
- 29.Solomon C, Christian DL, Chen LL, et al. Effect of serial-day exposure to nitrogen dioxide on airway and blood leukocytes and lymphocyte subsets. Eur Respir J. 2000;15(5):922-928. doi: 10.1034/j.1399-3003.2000.15e19.x [DOI] [PubMed] [Google Scholar]
- 30.Frampton MW, Boscia J, Roberts NJ Jr, et al. Nitrogen dioxide exposure: effects on airway and blood cells. Am J Physiol Lung Cell Mol Physiol. 2002;282(1):L155-L165. doi: 10.1152/ajplung.2002.282.1.L155 [DOI] [PubMed] [Google Scholar]
- 31.Poynter ME, Persinger RL, Irvin CG, et al. Nitrogen dioxide enhances allergic airway inflammation and hyperresponsiveness in the mouse. Am J Physiol Lung Cell Mol Physiol. 2006;290(1):L144-L152. doi: 10.1152/ajplung.00131.2005 [DOI] [PubMed] [Google Scholar]
- 32.Patel MM, Chillrud SN, Deepti KC, Ross JM, Kinney PL. Traffic-related air pollutants and exhaled markers of airway inflammation and oxidative stress in New York City adolescents. Environ Res. 2013;121:71-78. doi: 10.1016/j.envres.2012.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pathmanathan S, Krishna MT, Blomberg A, et al. Repeated daily exposure to 2 ppm nitrogen dioxide upregulates the expression of IL-5, IL-10, IL-13, and ICAM-1 in the bronchial epithelium of healthy human airways. Occup Environ Med. 2003;60(11):892-896. doi: 10.1136/oem.60.11.892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gowers AM, Cullinan P, Ayres JG, et al. Does outdoor air pollution induce new cases of asthma? biological plausibility and evidence; a review. Respirology. 2012;17(6):887-898. doi: 10.1111/j.1440-1843.2012.02195.x [DOI] [PubMed] [Google Scholar]
- 35.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980-2007. Pediatrics. 2009;123(suppl 3):S131-S145. doi: 10.1542/peds.2008-2233C [DOI] [PubMed] [Google Scholar]
- 36.Yang CL, To T, Foty RG, Stieb DM, Dell SD. Verifying a questionnaire diagnosis of asthma in children using health claims data. BMC Pulm Med. 2011;11:52. doi: 10.1186/1471-2466-11-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen TE, Evjenth B, Holt J. Validation of a questionnaire against clinical assessment in the diagnosis of asthma in school children. J Asthma. 2015;52(3):262-267. doi: 10.3109/02770903.2014.966914 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Data Analysis
eTable 1. Distribution of Selected Characteristics Among Participants Included and Excluded From the Current Study of the Children's Health Study, 1993-2014
eTable 2. P Values for Evidence of Effect Modification of the Asthma Incidence and Air Pollution Association Based on Partial Likelihood Ratio Tests for Interaction Terms With Pollutant and Potential Effect Modifier in the Children's Health Study, 1993-2014
eTable 3. Results of Sensitivity Analyses for Incidence Rate Ratios (IRR) Associated With Reduction in Nitrogen Dioxide (NO2) and Particulate Matter <2.5 μm (PM2.5) in the Children's Health Study, 1993-2014
eTable 4. Results of Sensitivity Analyses Using Cox Proportional Hazards Models for Ozone (O3), Nitrogen Dioxide (NO2), Particulate Matter <10 μm (PM10) and <2.5 μm (PM2.5) in the Children's Health Study, 1993-2014
eReferences