Key Points
Question
Are very thin- or ultrathin-strut drug-eluting stents associated with reduced adverse cardiovascular events in all-comer patients with lesions in small coronary vessels?
Findings
In this small-vessel substudy including 1506 participants of the 3-arm randomized BIO-RESORT trial, patients experienced fewer repeated target lesion revascularizations within 3 years after treatment with ultrathin-strut sirolimus-eluting stents (2.1%) vs previous-generation thin-strut zotarolimus-eluting stents (5.3%).
Meaning
In small-vessel lesions, the implantation of the ultrathin-strut sirolimus-eluting stent that is examined in the BIO-RESORT trial may reduce the need for repeated revascularization.
Abstract
Importance
Stenting small-vessel lesions has an increased adverse cardiovascular event risk. Very thin-strut or ultrathin-strut drug-eluting stents might reduce this risk, but data are scarce.
Objective
To assess the outcome of all-comer patients with small coronary vessel lesions treated with 3 dissimilar types of drug-eluting stents.
Design
This is a prespecified substudy of the Comparison of Biodegradable Polymer and Durable Polymer Drug-eluting Stents in an All Comers Population (BIO-RESORT) trial, an investigator-initiated, randomized, patient-blinded comparative clinical drug-eluting stent trial. Patients treated with ultrathin-strut sirolimus-eluting stents, very thin-strut everolimus-eluting stents, or previous-generation thin-strut zotarolimus-eluting stents were enrolled from December 2012 to August 2015. This multicenter trial was conducted in 4 Dutch centers for cardiac intervention. Of all 3514 all-comer BIO-RESORT participants, 1506 patients with treatment in at least 1 small-vessel lesion (reference vessel <2.5 mm) were included. Data were analyzed between September 2018 and February 2019.
Main Outcomes and Measures
Target lesion failure at 3-year follow-up, a composite of cardiac death, target vessel–related myocardial infarction, or target lesion revascularization, analyzed by Kaplan-Meier methods.
Results
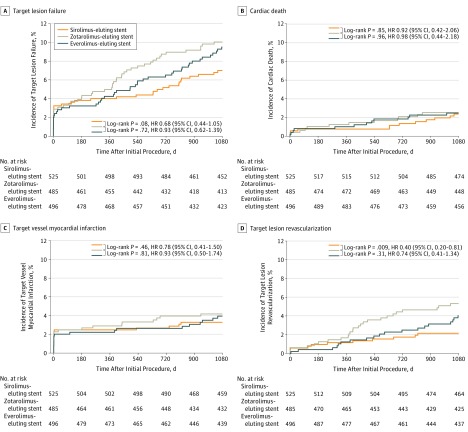
In 1452 of 1506 participants (96.4%) (1057 men [70.2%]; 449 women [29.8%]; mean [SD] age, 64.3 [10.4] years), follow-up was available. Target lesion failure occurred in 36 of 525 patients (7.0%) treated with sirolimus-eluting stents, 46 of 496 (9.5%) with everolimus-eluting stents, and 48 of 485 (10.0%) with zotarolimus-eluting stents (sirolimus-eluting vs zotarolimus-eluting hazard ratio [HR], 0.68; 95% CI, 0.44-1.05; P = .08; everolimus-eluting vs zotarolimus-eluting HR, 0.93; 95% CI, 0.62-1.39; P = .72). There was a difference in target lesion revascularizations between sirolimus-eluting and zotarolimus-eluting stents (2.1% vs 5.3%; HR, 0.40; 95% CI, 0.20-0.81; P = .009) that emerged after the first year of follow-up (1.0% vs 3.7%; P = .006); multivariate analysis showed that sirolimus-eluting stent implantation was independently associated with a lower target lesion revascularization rate at 3-year follow-up (adjusted HR, 0.42; 95% CI, 0.20-0.85; P = .02). In the everolimus-eluting stents, the revascularization rate was 4.0% (vs zotarolimus-eluting, HR, 0.74; 95% CI, 0.41-1.34; P = .31). There was no significant between-stent difference in cardiac death, target vessel myocardial infarction, or stent thrombosis.
Conclusions and Relevance
Patients stented in small coronary vessels experienced fewer repeated revascularizations if treated with ultrathin-strut sirolimus-eluting stents vs previous generation thin strut zotarolimus-eluting stents. Further research is required to evaluate the potential effect of particularly thin stent struts.
Trial Registration
ClinicalTrials.gov identifier: NCT01674803
This prespecified analysis of a randomized clinical trial assesses the outcome of all-comer patients with small coronary vessel lesions treated with 3 dissimilar types of drug-eluting stents.
Introduction
In small coronary arteries, percutaneous coronary intervention (PCI) is associated with a higher risk of adverse cardiovascular events1,2,3,4,5 including an increased need for repeated revascularization owing to in-stent restenosis.6,7,8 Depending on the definition used, small vessels have been treated in 30% to 50% of patients.2,5,9 A higher prevalence of small-vessel lesions was found in patients with diabetes and women.2,5,10 With the diabetes epidemic and the availability of stents with very small lumen diameters, the proportion of patients treated for small-vessel lesions is growing, which is reflected in all-comer stent trials reported in 2016.5,9
While first-generation drug-eluting stents (DES) used relatively thick struts, contemporary new-generation DES have substantially thinner struts. A lower strut thickness may be particularly advantageous in small target vessels because thicker struts and a smaller minimum in-stent lumen diameter are known to be independent predictors of restenosis in coronary stents.7,11,12 This can be explained by the greater relative effect of the strut size on lumen obstruction in small vessels. Therefore, the potential clinical advantage of a reduction in strut thickness may be best reflected in the outcome of patients with small target vessels. The 2-year and 3-year outcome data are of particular interest because in new-generation DES, a considerable proportion of target lesion revascularization (TLR) procedures occur beyond 1 year of follow-up.13,14
Drug-eluting stents with particularly thin struts have shown excellent clinical results in several randomized trials,15,16,17,18,19,20,21 but outcome data in all-comer patients treated in small vessels are scarce. The randomized Comparison of Biodegradable Polymer and Durable Polymer Drug-eluting Stents in an All Comers Population (BIO-RESORT) trial compares in all-comer patients 3 types of DES with thin, very thin, or ultrathin struts, showing favorable event rates at 1-year to 3-year follow-up.16,22,23 In this prespecified substudy of the BIO-RESORT trial, we assessed 3-year outcome data of all patients who were treated in small vessels (<2.5 mm in core laboratory measurements) and evaluated the hypothesis that DES with ultrathin or very thin struts might reduce the incidence of TLR as compared with a thin-strut DES.
Methods
Study Design and Patient Population
Details of the randomized, investigator-initiated, multicenter, patient-blinded BIO-RESORT (TWENTE III) trial have been reported.16 In brief, a total of 3514 all-comer patients with obstructive coronary disease were randomly assigned (1 to 1 to 1) to treatment with 1 of 3 DES: ultrathin-strut cobalt-chromium biodegradable polymer sirolimus-eluting stents (SES; Orsiro, Biotronik) or very thin-strut platinum-chromium biodegradable polymer everolimus-eluting stents (EES; Synergy, Boston Scientific) vs previous-generation thin-strut cobalt-chromium durable polymer zotarolimus-eluting stents (ZES; Resolute Integrity, Medtronic). More details are available in the eMethods in the Supplement.
The thickness of the uncoated SES strut is 60 μm in stents with a lumen diameter of 3.00 mm or less, with an asymmetrical coating distribution (abluminal/luminal coating: 7.4/3.5 μm) resulting in a thickness of 71 μm of the coated struts. In the EES, the uncoated struts measure 74 μm in stents less than 3.00 mm; the coated struts measure 78 μm (abluminal coating only: 4 μm). In ZES, the uncoated struts measure 91 μm (conformal coating: 5.6 μm), resulting in a coated strut thickness of 102 μm. All 3 types of DES were available in sizes ranging from 2.25 mm to 4.0 mm in diameter and lengths of 8 mm to 38 mm (EES and ZES) or 9 mm to 40 mm (SES). Further technical details of the stents are provided in the eFigure and eTable 1 in the Supplement.
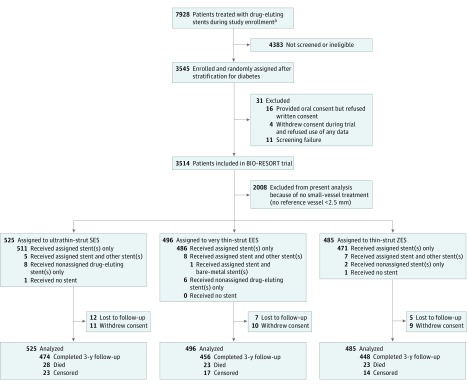
The trial complied with the Declaration of Helsinki and the 2010 CONSORT Statement and was approved by the Medical Ethics Committee Twente and the institutional review boards of all participating centers. All patients provided written informed consent. Formal trial protocols and a detailed statistical plan can be found in Supplements 2 and 3. In the overall population of the trial, noninferiority of SES vs ZES and EES vs ZES was demonstrated regarding the composite primary clinical end point at 1 year. In this prespecified substudy, we analyzed the 3-year clinical follow-up data of all patients treated with stents in small coronary vessels, defined as vessels with a reference diameter less than 2.5 mm, as measured by quantitative coronary angiography in a core laboratory analysis. This cutoff value was based on previous clinical research that suggested 2.50 mm to be a suitable threshold to identify small target vessels (Figure 1).5
Figure 1. Trial Profile.
Information on the number of patients treated with drug-eluting stents during the period of study enrollment is given irrespective of whether these patients fulfilled the inclusion and exclusion criteria because we do not have reliable data on the total number of eligible patients. EES indicates everolimus-eluting stent; SES, sirolimus-eluting stent; ZES, zotarolimus-eluting stent.
Procedures, Clinical Follow-up, and Event Adjudication
Interventional procedures were performed according to medical guidelines using standard techniques. Data monitoring, processing of clinical outcome data, and independent clinical event adjudication (clinical event committee blinded to allocated treatment) were done by an independent clinical research organization. Analysts of the clinical research organization Cardiovascular Research and Education Enschede (Enschede, the Netherlands), blinded for the used stent type, performed offline quantitative coronary angiographic analyses with QAngio XA, version 7.3 (Medis) in all patients, according to international standards.
Clinical End Points
The prespecified clinical end points of the BIO-RESORT trial were defined according to the Academic Research Consortium.24,25 The composite end point target lesion failure (TLF) is defined as a composite of cardiac death, target vessel–related myocardial infarction (MI), or clinically indicated target lesion revascularization (TLR). Target lesion failure, which is based on these 3 individual event components with different mechanisms and time courses, reflects the device and lesion-related part of the entire spectrum of adverse events that may occur during follow-up.5 Death was considered as cardiac unless an unequivocal noncardiac cause could be established. Myocardial infarction was defined by any creatine kinase concentration of more than double the upper limit of normal, with confirmatory elevated cardiac biomarkers.25 Target lesion revascularizations were considered clinically indicated if the angiographic percent diameter stenosis of the treated lesion was at least 70% or at least 50% in the presence of ischemic signs or symptoms. Stent thrombosis was classified according to the Academic Research Consortium definitions.24
Statistical Analysis
In accordance with the BIO-RESORT study protocol, 2 main hypotheses were assessed: the comparison of ultrathin-strut SES vs thin-strut ZES and very thin-strut EES vs thin-strut ZES. Data are reported as mean and standard deviation for continuous variables and as frequencies and percentages for dichotomous and categorical variables. Continuous variables were assessed with the t test, whereas categorical variables were assessed with the χ2 test or Fisher exact test as appropriate. The Kaplan-Meier method was used to assess the time to clinical end point, and the log-rank test was used for between-group comparisons. Hazard ratios (HRs), with 2-sided confidence intervals, were computed using Cox regression analysis. We performed landmark analyses of all aforementioned end points by using 1-year landmarks. In addition, we performed a sensitivity analysis of end points that had shown significant between-DES differences in the overall study population in patients with single-vessel treatment. Potential confounders were identified if in univariate analysis a P value of less than .15 was found and were entered into a multivariate Cox regression model using stepwise backward selection. A 2-sided P value less than .05 was considered significant. Statistical analyses were performed using SPSS, version 22.0 (IBM Corp).
Results
From December 2012 to August 2015, a total of 1506 patients were treated in at least 1 small vessel (42.9% of all BIO-RESORT trial participants) (Figure 1). There were 2283 target lesions, including 1819 small-vessel lesions. After 3 years, 1452 patients (96.4%) completed follow-up or had died; 24 patients (1.6%) were lost and 30 (2.0%) withdrew their consent (all were censored at moment of dropout).
Patients were aged 34 to 90 years, with a mean (SD) age of 64.3 (10.4) years. Two-thirds of the study population presented with acute coronary syndromes (n = 1005; 66.7%) and 3 of 10 patients were women (n = 449; 29.8%). Most patients presented with complex target lesions (n = 1186; 78.8%), and many were treated for at least 1 bifurcated lesion (n = 670; 44.5%). Table 1 presents the baseline characteristics of patients, lesions, and procedures, which did not show any significant difference in clinical patient characteristics between the stent groups. The small-vessel lesions of the SES group were significantly more often complex (452 of 636 [71.4%] vs 380 of 602 [63.3%]; P = .002) and less often restenotic (3 of 636 [0.5%] vs 13 of 602 [2.2%]; P = .009), and stent postdilation was more often performed in lesions treated with EES (433 of 581 [74.5%] vs 418 of 602 [69.4%]; P = .05). Further characteristics of the small-vessel lesions are presented in Table 1. Dual antiplatelet therapy duration and intensity were similar between groups (eTable 2 in the Supplement).
Table 1. Characteristics of Patients, Lesions, and Procedures at Baseline.
| Patients (n = 1506)a | No. (%) | P Value | |||
|---|---|---|---|---|---|
| SES | EES | ZES | SES vs ZES | EES vs ZES | |
| Age, mean (SD), y | 64.9 (10.2) | 64.0 (10.6) | 64.0 (10.3) | .19 | .91 |
| Female | 158 (30.1) | 142 (28.6) | 149 (30.7) | .83 | .47 |
| BMI, mean (SD) | 27.3 (4.1) | 27.5 (4.2) | 27.5 (4.1) | .38 | .91 |
| Diabetes mellitus | 101 (19.2) | 106 (21.4) | 102 (21.0) | .48 | .90 |
| Arterial hypertension | 250 (47.6) | 230 (46.4) | 249 (51.3) | .24 | .12 |
| Hypercholesterolemia | 225 (42.9) | 186 (37.5) | 194 (40.0) | .36 | .42 |
| Current smoker, No./total No. (%) | 133/514 (25.9) | 128/477 (26.8) | 129/475 (27.2) | .63 | .90 |
| Family history of CAD | 234/498 (47.0) | 215/469 (45.8) | 233/471 (49.5) | .44 | .27 |
| Previous | |||||
| Myocardial infarction | 95 (18.1) | 87 (17.5) | 109 (22.5) | .08 | .05 |
| PCI | 103 (19.6) | 93 (18.8) | 86 (17.7) | .44 | .68 |
| CABG | 33 (6.3) | 42 (8.5) | 35 (7.2) | .56 | .47 |
| Clinical syndrome | |||||
| ST-segment elevation MI | 143 (27.2) | 135 (27.2) | 113 (23.3) | .45 | .50 |
| Non–ST-segment elevation MI | 118 (22.5) | 104 (21.0) | 111 (22.9) | ||
| Unstable angina | 102 (19.4) | 86 (17.3) | 93 (19.2) | ||
| Stable angina | 162 (30.9) | 171 (34.5) | 168 (34.6) | ||
| Multivessel treatment | 154 (29.3) | 141 (28.4) | 157 (32.4) | .30 | .18 |
| Syntax scores, mean (SD)b | 13.7 (8.3) | 12.8 (7.9) | 12.9 (8.3) | .11 | .93 |
| At least 1: | |||||
| Complex lesion | 429 (81.7) | 381 (76.8) | 376 (77.5) | .10 | .79 |
| Bifurcation | 235 (44.8) | 218 (44.0) | 217 (44.7) | >.99 | .80 |
| Chronic total occlusion | 30 (5.7) | 23 (4.6) | 30 (6.2) | .75 | .28 |
| Severe calcification | 121 (23.0) | 117 (23.6) | 124 (25.6) | .35 | .47 |
| In-stent restenosis | 7 (1.3) | 12 (2.4) | 14 (2.9) | .08 | .65 |
| Lesion length >27 mm | 182 (34.7) | 169 (34.1) | 189 (39.0) | .16 | .11 |
| Total stent length per patient, mean (SD), mm | 44.6 (31.6) | 42.3 (30.6) | 45.8 (31.2) | .55 | .08 |
| Stents per patient, No., mean (SD) | 2.1 (1.2) | 2.0 (1.2) | 2.1 (1.2) | .24 | .02 |
| Implantation stent, mm | |||||
| 2.25 | 164 (31.2) | 146 (29.4) | 172 (35.5) | .15 | .04 |
| 2.50 | 250 (47.6) | 229 (46.2) | 234 (48.2) | .84 | .51 |
| 3.00 | 290 (55.2) | 274 (55.2) | 275 (56.7) | .64 | .65 |
| Implantation pressure, mean (SD), atmc | 14.9 (2.8) | 15.0 (2.7) | 14.8 (3.0) | .63 | .21 |
| IVUS use during index procedure | 4 (0.8) | 9 (1.8) | 7 (1.4) | .30 | .65 |
| OCT use during index procedure | 2 (0.4) | 1 (0.2) | 3 (0.6) | .59 | .37 |
| Small-vessel lesions (n = 1819)d | |||||
| ACC/AHA lesion type, No./total No. (%) | |||||
| A | 42/633 (6.6) | 34/581 (5.9) | 40/600 (6.7) | .01 | .20 |
| B1 | 139/633 (22.0) | 148/581 (25.5) | 180/600 (30.0) | ||
| B2 | 268/633 (42.3) | 224/581 (38.6) | 226/600 (37.7) | ||
| C | 184/633 (29.1) | 175/581 (30.1) | 154/600 (25.7) | ||
| Complex lesion (type B2 or C) | 452 (71.4) | 399 (68.7) | 380 (63.3) | .002 | .05 |
| Bifurcated lesion | 222 (34.9) | 206 (35.5) | 205 (34.1) | .75 | .61 |
| Chronic total occlusion | 24 (3.8) | 21 (3.6) | 26 (4.3) | .63 | .54 |
| Severe calcification | 113 (17.8) | 111 (19.1) | 126 (20.9) | .16 | .43 |
| In-stent restenosis | 3 (0.5) | 12 (2.1) | 13 (2.2) | .009 | .91 |
| Left main stem lesion | 5 (0.8) | 10 (1.7) | 7 (1.2) | .50 | .42 |
| Graft lesion | 4 (0.6) | 5 (0.9) | 5 (0.8) | .75 | >.99 |
| Lesion length, mean (SD), mme | 16.61 (10.07) | 16.50 (11.26) | 16.16 (11.11) | .46 | .61 |
| Reference vessel diameter, mean (SD), mm | 2.11 (0.28) | 2.12 (0.28) | 2.11 (0.28) | .83 | .61 |
| Acute lumen gain, mean (SD), mme | 1.04 (0.49) | 1.08 (0.48) | 1.02 (0.48) | .48 | .05 |
| Total stent length, mean (SD), mm | 28.2 (17.7) | 27.4 (18.0) | 28.3 (19.5) | .89 | .40 |
| Stents per lesion, No., mean (SD) | 1.4 (0.7) | 1.3 (0.7) | 1.4 (0.7) | .42 | .10 |
| Predilation performed | 516 (81.1) | 479 (82.4) | 491 (81.6) | .85 | .69 |
| Postdilation performed | 428 (67.3) | 433 (74.5) | 418 (69.4) | .42 | .05 |
| Only assigned stent used | 620 (97.5) | 574 (98.8) | 588 (97.7) | .83 | .14 |
Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association; atm, atmosphere; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CABG, coronary artery bypass graft; CAD, coronary artery disease; EES, everolimus-eluting stent; MI, myocardial infarction; PCI, percutaneous coronary intervention; SES, sirolimus-eluting stent; ZES, zotarolimus-eluting stent.
There were 525 patients assigned to SES, 496 patients assigned to EES, and 485 patients assigned to ZES.
According to the Comparison of Biodegradable Polymer and Durable Polymer Drug-eluting Stents in an All Comers Population (BIO-RESORT) study protocol, comparisons of SES vs ZES and EES vs ZES were performed. Data were available in 492 patients assigned to SES, 454 patients assigned to EES, and 450 patients assigned to ZES (not recorded for patients with previous CABG).
Data were available in 522 patients assigned to SES, 493 patients assigned to EES, and 481 patients assigned to ZES.
Of the 1819 small-vessel lesions, 636 were assigned to SES, 581 were assigned to EES, and 602 were assigned to ZES.
Data were available in 633 SES-treated lesions, 581 EES-treated lesions, and 600 ZES-treated lesions.
Table 2 and Figure 2 present the clinical outcomes until 3-year follow-up. At 3 years, the device-oriented composite clinical end point TLF occurred in 36 of 525 patients (7.0%) assigned to SES, 46 of 496 (9.5%) assigned to EES, and 48 of 485 (10.0%) assigned to ZES (SES vs ZES: HR, 0.68; 95% CI, 0.44-1.05; P = .08; EES vs ZES: HR, 0.93; 95% CI, 0.62-1.39; P = .72). At the 2-year follow-up, there was a significant difference in TLF between SES and ZES (n = 27 of 525 [5.2%] vs 42 of 485 [8.7%]; HR, 0.59; 95% CI, 0.36-0.95; P = .03). At 3 years, TLR occurred less often in SES than in ZES (11 of 525 [2.1%] vs 25 of 485 [5.3%]; HR, 0.40; 95% CI, 0.20-0.81; P = .009); after 2 years, there was also a significant difference (9 of 525 [1.7%] vs 21 of 485 [4.4%]; HR, 0.39; 95% CI, 0.18-0.85; P = .01) in TLR between SES and ZES (Table 2). In patients with EES, TLR occurred in 19 of 496 (4.0%) at 3 years (vs ZES: HR, 0.74; 95% CI, 0.41-1.34; P = .31). For SES vs ZES and EES vs ZES, there was no between-group difference in the incidence of cardiac death, target vessel MI, and stent thrombosis.
Table 2. Clinical Event Rates During 3 Years of Follow-up.
| Follow-up | No. (%) | HR (95% CI) | P Value | HR (95% CI) | P Value | ||
|---|---|---|---|---|---|---|---|
| SES (n = 525) | EES (n = 496) | ZES (n = 485) | SES vs ZES | Log Rank SES vs ZES | EES vs ZES | Log Rank EES vs ZES | |
| Until 1-y follow-up | |||||||
| Cardiac death | 4 (0.8) | 5 (1.0) | 6 (1.2) | 0.62 (0.17-2.18) | .45 | 0.81 (0.25-2.67) | .73 |
| Target vessel MI | 13 (2.5) | 11 (2.2) | 14 (2.9) | 0.86 (0.40-1.82) | .68 | 0.77 (0.35-1.69) | .51 |
| TLR | 6 (1.2) | 6 (1.2) | 8 (1.7) | 0.69 (0.24-1.99) | .49 | 0.73 (0.52-2.10) | .56 |
| TLF | 21 (4.0) | 21 (4.3) | 24 (5.0) | 0.81 (0.45-1.45) | .47 | 0.85 (0.74-1.53) | .59 |
| Definite or probable ST | 2 (0.4) | 3 (0.6) | 4 (0.8) | 0.46 (0.09-2.52) | .36 | 0.73 (0.16-3.27) | .68 |
| Definite ST | 2 (0.4) | 2 (0.4) | 2 (0.4) | 0.92 (0.13-6.55) | .94 | 0.98 (0.14-6.93) | .98 |
| Until 2-y follow-up | |||||||
| Cardiac death | 7 (1.3) | 9 (1.8) | 10 (2.1) | 0.65 (0.25-1.70) | .37 | 0.88 (0.36-2.17) | .78 |
| Target vessel MI | 14 (2.7) | 13 (2.7) | 19 (4.0) | 0.68 (0.34-1.35) | .27 | 0.67 (0.33-1.35) | .26 |
| TLR | 9 (1.7) | 12 (2.5) | 21 (4.4) | 0.39 (0.18-0.85) | .01 | 0.56 (0.27-1.13) | .10 |
| TLF | 27 (5.2) | 32 (6.5) | 42 (8.7) | 0.59 (0.36-0.95) | .03 | 0.74 (0.47-1.17) | .20 |
| Definite or probable ST | 3 (0.6) | 6 (1.2) | 7 (1.5) | 0.40 (0.10-1.53) | .16 | 0.84 (0.28-2.50) | .75 |
| Definite ST | 2 (0.4) | 3 (0.6) | 5 (1.1) | 0.37 (0.07-1.90) | .21 | 0.59 (0.14-2.46) | .46 |
| Until 3-y follow-upa | |||||||
| Cardiac death | 12 (2.4) | 12 (2.5) | 12 (2.5) | 0.92 (0.42-2.06) | .85 | 0.98 (0.44-2.18) | .96 |
| Target vessel MI | 17 (3.3) | 19 (3.9) | 20 (4.2) | 0.78 (0.41-1.50) | .46 | 0.93 (0.50-1.74) | .81 |
| TLR | 11 (2.1) | 19 (4.0) | 25 (5.3) | 0.40 (0.20-0.81) | .009 | 0.74 (0.41-1.34) | .31 |
| TLF | 36 (7.0) | 46 (9.5) | 48 (10.0) | 0.68 (0.44-1.05) | .08 | 0.93 (0.62-1.39) | .72 |
| Definite or probable ST | 3 (0.6) | 7 (1.5) | 7 (1.5) | 0.40 (0.10-1.53) | .16 | 0.98 (0.34-2.79) | .97 |
| Definite ST | 2 (0.4) | 4 (0.8) | 5 (1.1) | 0.37 (0.07-1.90) | .21 | 0.78 (0.21-2.92) | .72 |
| Landmark analysesb | SES (n = 515) | EES (n = 482) | ZES (n = 472) | ||||
| Between 1-2 y | |||||||
| Cardiac death | 3 (0.6) | 4 (0.8) | 4 (0.8) | −0.3 (−1.3 to 0.8) | .62 | 0.0 (−1.2 to 1.1) | .98 |
| Target vessel MI | 1 (0.2) | 2 (0.4) | 5 (1.1) | −0.9 (−1.9 to 0.1) | .09 | −0.7 (−1.8 to 0.5) | .25 |
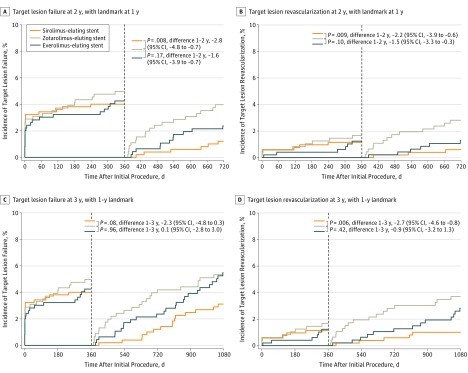
| TLR | 3 (0.6) | 6 (1.3) | 13 (2.8) | −2.2 (−3.9 to −0.6) | .009 | −1.5 (−3.3 to 0.3) | .10 |
| TLF | 6 (1.2) | 11 (2.4) | 18 (4.0) | −2.8 (−4.8 to −0.7) | .008 | −1.6 (−3.9 to 0.7) | .17 |
| Definite or probable ST | 1 (0.2) | 3 (0.6) | 3 (0.6) | −0.4 (−1.3 to 0.4) | .29 | 0.0 (−1.0 to 1.0) | .98 |
| Definite ST | 0 (0.0) | 1 (0.2) | 3 (0.6) | −0.6 (−1.4 to 0.1) | .08 | −0.4 (−1.3 to 0.4) | .31 |
| Between 1-3 y | |||||||
| Cardiac death | 8 (1.6) | 7 (1.4) | 6 (1.3) | 0.3 (−1.2 to 1.8) | .71 | 0.2 (−1.3 to 1.7) | .81 |
| Target vessel MI | 4 (0.8) | 8 (1.7) | 6 (1.3) | −0.5 (−1.8 to 0.8) | .44 | 0.4 (−1.2 to 2.0) | .62 |
| TLR | 5 (1.0) | 13 (2.7) | 17 (3.7) | −2.7 (−4.6 to −0.8) | .006 | −0.9 (−3.2 to 1.3) | .42 |
| TLF | 15 (3.0) | 25 (5.3) | 24 (5.3) | −2.3 (−4.8 to 0.3) | .08 | 0.1 (−2.8 to 3.0) | .96 |
| Definite or probable ST | 1 (0.2) | 4 (0.8) | 3 (0.6) | −0.4 (−1.3 to 0.4) | .29 | 0.2 (−0.9 to 1.3) | .73 |
| Definite ST | 0 | 2 (0.4) | 3 (0.6) | −0.6 (−1.4 to 0.1) | .08 | −0.2 (−1.1 to 0.7) | .64 |
Abbreviations: EES, everolimus-eluting stent; HR, hazard ratio; MI, myocardial infarction; SES, sirolimus-eluting stent; ST, stent thrombosis; TLF, target lesion failure; TLR, target lesion revascularization; ZES, zotarolimus-eluting stent.
According to the Comparison of Biodegradable Polymer and Durable Polymer Drug-eluting Stents in an All Comers Population (BIO-RESORT) study protocol, comparisons of SES vs ZES and EES vs ZES were performed. Three-year follow-up information was obtained from 1452 of 1506 study patients (96.4%) and analyzed using the Kaplan-Meier method; therefore, the percentages may differ slightly from straightforward nominator divided by denominator calculations.
Patients who were censored before the 1-year landmark are not included.
Figure 2. Kaplan-Meier Cumulative Event Curves for Target Lesion Failure and Its Individual Components at 3-Year Follow-up.
Target lesion failure (A), a composite of cardiac death (B), target vessel-related myocardial infarction (C), or clinically indicated target lesion revascularization (D). HR indicates hazard ratio.
Landmark analyses between 1-year and 3-year follow-up (Figure 3) showed that the difference in TLR between SES and ZES emerged during the second year of follow-up (1.0% vs 3.7%; mean difference, −2.7; 95% CI, −4.6 to −0.8; P = .006). Landmark analyses comparing EES and ZES showed no significant difference (Table 2).
Figure 3. Landmark Analyses at 1 Year for Target Lesion Failure (TLF) and Clinically Indicated Target Lesion Revascularization (TLR).
TLF until 2 years (A) and 3 years (C) of follow-up; TLR until 2 years (B) and 3 years (D) of follow-up. HR indicates hazard ratio.
Among 1054 study patients who were treated in a single small vessel, the TLF rate was 7.4% (n = 27 of 371) in SES, 7.5% in EES (n = 26 of 355), and 9.9% in patients with ZES (n = 32 of 328) (SES vs ZES, HR 0.74; 95% CI, 0.44-1.24; P = .24; EES vs ZES: HR, 0.74; 95% CI, 0.44-1.24; P = .25). In addition, TLR was performed less often in patients with SES than in patients with ZES (2.2% vs 5.0%; HR, 0.44; 95% CI, 0.19-1.02; P = .049) and in 4.1% of the patients with EES (EES vs ZES: HR, 0.80; 95% CI, 0.39-1.64; P = .55). Multivariate analysis showed that after adjustment for potential confounders (ie, in-stent restenosis) the implantation of SES was independently associated with a lower incidence of TLR (adjusted HR, 0.42; 95% CI, 0.20-0.85; P = .02) at 3-year follow-up (vs ZES).
Discussion
Main Findings
This secondary analysis of the randomized BIO-RESORT trial compared all-comers with at least 1 small-vessel lesion treated with thin-strut, very thin-strut, or ultrathin-strut DES. Drug-eluting stents with particularly thin struts were found to have the lowest adverse event rate. During 3-year follow-up, there was a significantly lower incidence of target lesion revascularization in patients treated with SES than with previous-generation ZES. This difference emerged during the second year of follow-up and was confirmed in patients who underwent single-vessel treatment only (ie, to exclude any potential confounding effect of treating additional nonsmall vessels). In addition, multivariate analysis showed that treatment with SES was independently associated with TLR. This difference in TLR contributed to a difference between SES and ZES in the composite clinical end point TLF, which reached statistical significance between 1-year and 2-year follow-up; over the entire 3 years, there was a numeric difference in TLF that did not reach statistical significance.
Previous Studies
A meta-analysis21 based on 1-year outcome data found in target vessels of various sizes no significant difference in TLR rate between patients treated with ultrathin-strut DES vs thicker-strut DES.21 As in small-vessel lesions, the strut cross-section contributes relatively more to the stent-induced luminal obstruction, the potential benefit of ultrathin-strut stents may be most pronounced in such lesions. In addition, the findings of our study suggest that the meta-analysis21 may not have been able to show a significant benefit of ultrathin strut stents because advantages may emerge after the 1-year follow-up.
A small-vessel (≤2.5 mm) subanalysis of the CENTURY-II trial9 in 525 patients compared a thin-strut SES (80 μm; Ultimaster; Terumo) vs a thin-strut EES (81 μm; Xience; Abbott Vascular). At 1-year follow-up of that study, there was no significant between-stent difference in the rates of TLR (4.0% vs 5.7%), which might be partly attributed to the similarity in strut thickness.
The overall incidence of TLR at 2 years in our study is lower than reported in previous small-vessel studies with new-generation DES that reported 2-year outcome data. In a substudy of the randomized DUTCH PEERS trial,5 comparing the previous-generation thin-strut ZES (91 μm) with a thin-strut durable polymer EES (81 μm; Promus Element; Boston Scientific), the 2-year incidence of TLR was 4.8% in all-comer patients with small vessels (n = 798). A 2019 prospective single-arm small-vessel study26 assessed 70 Japanese non–all-comer patients, who were treated with a 2.25-mm thin-strut SES (80 μm; Ultimaster) and found TLR to occur in 4.3% within 2 years. The incidence of TLR in both aforementioned studies was similar to that of patients treated with previous-generation thin-strut ZES in our study (4.4%). However, the patients in our study treated with very thin-strut EES and ultrathin-strut SES showed lower rates (2.5% and 1.7%, respectively), which supports the hypothesis that strut thickness matters for the repeated revascularization risk, in particular in small target vessels.
Yet a retrospective, observational small-vessel study assessed 1132 patients treated with 2.50-mm thin-strut EES (81 μm; Xience V or Promus) or thick-strut biolimus-eluting stents (120 μm; Nobori; Terumo). The 2-year TLR rates were similar (8.4% vs 8.3%), which might partly be explained by differences in clinical presentation between both DES groups. Overall, the TLR rates were relatively high, which might be related to the patient population.27
While most small-vessel studies reported 2-year outcome data, 5-year follow-up data were reported in a post hoc subgroup analysis in 259 patients with small target vessels (≤2.75 mm) of a previous trial that assessed the ultrathin-strut SES.28 While the TLR rates were similar for the ultrathin-strut SES and the thin-strut EES (Orsiro 8.7% vs Xience 8.9%), there was a numerically lower rate of TLF in patients treated with ultrathin strut SES (11.1%) as compared with thin strut EES (15.5%).28 The lack of a statistical significance may be attributed to the relatively small sample size as well as the choice of a more generous cutoff value for defining small vessels, which resulted in the inclusion of patients with target vessels between 2.50 mm and 2.75 mm, (ie, patients who are expected to have a lower TLR risk); these patients were not included in our analysis.
A subgroup analysis of a 2017 meta-analysis29 that assessed patients treated with various biodegradable polymer DES vs durable polymer DES in target vessels of all sizes, treated with DES that had stent struts smaller than 100 μm, found no between-group difference in the occurrence of cardiac death, MI, or stent thrombosis after a mean follow-up of 26 months. The results in patients in our study with small target vessels corroborate the aforementioned findings in patients with target vessels of all sizes, showing low stent thrombosis rates and no significant difference in cardiac death, MI, and stent thrombosis between durable and biodegradable polymer DES.
Possible Explanations for This Study’s Findings
Some aspects of our findings require further elaboration. Owing to the difference in strut thickness, the neointimal response to strut and polymer coating may have been more pronounced in ZES than in ultrathin-strut SES, most likely because in ZES there is more material to provoke a biologic response. Theoretically, in ZES, the presence of a durable polymer may have promoted and prolonged vessel wall inflammation with delayed arterial healing, and it may have accelerated the formation of neoatherosclerosis30 As a result of this, plaque burden may be larger, which may have led to more adverse events such as in-stent restenosis and TLR.
In addition, the same volume of neointimal ingrowth is much more likely to cause hemodynamically significant lumen obstructions in small vs larger target vessels. In the era of bare-metal stents, an inverse association between vessel size and angiographic restenosis or TLR was described. There was a direct association between strut thickness and late lumen loss and TLR that was most apparent in smaller vessels, suggesting an increased importance of stent strut thickness in smaller vessels.31 While DES reduced neointimal proliferation and its association with strut size, thicker DES struts required a longer time for re-endothelialization and were more thrombogenic owing to a disturbed blood flow and shear stress distribution.32 On the other hand, with increasingly thin struts, attention has to be paid to maintaining an adequate radial force. In the specific patient population of our present study, we did not notice any signal that ultrathin- or very thin-strut stents might have a clinically relevant problem with insufficient radial force.
In this study, differences in TLR (and TLF) between ultrathin-strut SES and thin-strut ZES were seen in particular beyond 1 year. A stringent cessation of DAPT after 12 months, which is common practice in the Netherlands, may have contributed to our findings by promoting some ischemic coronary events and related TLR during the second year of follow-up. In a 2017 trial,17 target vessel MI was the driver of a reduced TLF with ultrathin-strut SES as compared with thin-strut durable polymer cobalt-chromium EES both at 30 days and 1-year follow-up. Similarly, large-scale meta-analyses21,33 showed an advantage in ischemic end points for DES with particularly thin struts. This may be attributed to less flow disturbance, vascular damage, and side branch obstruction, caused by particularly thin struts.
Nevertheless, strut width and cross-sectional strut shape are also important factors that differ between contemporary devices. In this analysis, no significant difference in target vessel MI was noted, but small vessels have smaller side branches than large vessels, and obstruction of small side branches may remain more often unnoticed and may less often cause a substantial cardiac marker release. In addition, the high rate of stent postdilation might have reduced the risk of side branch compromise in all 3 stent groups, and in ZES (ie, the device with the thickest struts), the round cross-sectional strut shape may have reduced the risk of obstructing side branches.
Clinical Implications
The findings of our analysis suggest that the ultrathin-strut SES may reduce the risk of repeated revascularization in small-vessel lesions, which could have a positive effect on the patients’ comfort and morbidity as well as health care expenditures. As a consequence, operators may consider strut thickness as one of the factors when making their choice of DES for treating small-vessel lesions. Treatment of small coronary vessels has generally been associated with a higher risk of adverse clinical events, but the definition of a small target vessel has changed over time. In the eras of bare metal stents and first-generation DES, a small target vessel was usually classified by a reference vessel size of less than 3.0 mm, while later the cutoff value was lowered to 2.75 mm, and in small-vessel trials published after 2016, a cutoff value of 2.5 mm was used. Coronary lesions that previously were deemed untreatable are now considered small or very small target lesions, for which small and very small DES15,34 are available. Owing to innovations in the field and the availability of DES with very thin and ultrathin struts and increasingly smaller sizes, one may expect that PCI with DES implantation will be even more frequently the therapy of choice in patients with lesions in small and very small coronary vessels.
As an alternative to DES, drug-coated balloons can be used to treat small-vessel lesions. A 2018 meta-analysis35 of 7 studies with 1824 patients and a mean follow-up of 14.5 months showed in de novo lesions that drug-coated balloons were associated with a similar risk of TLR compared with DES. However, that analysis did not include data from studies with ultrathin-strut DES. Further research is required to evaluate whether ultrathin struts or a no-struts-at-all strategy may be best for treating small-vessel lesions.
Limitations
The potential signal of lower revascularization rates with ultrathin-strut SES should be interpreted cautiously because this hypothesis-generating finding was obtained from a prespecified subgroup analysis; therefore, no definitive conclusions can be drawn. We did not collect residual Syntax Score data (ie, after invasive treatment). In addition, during study enrollment, DES with diameters of 2.0 mm were not yet available. Nowadays, the successor (Resolute Onyx; Medtronic) of the thin-strut ZES of this study is available in 2.0-mm sizes and has struts with a thickness of 81 μm (uncoated). It has shown favorable clinical and angiographic outcomes at 1 year, including a single-arm study in 101 patients who were treated with 2.0-mm stents.15,34,36 Finally, guidance by intracoronary imaging (eg, intravascular ultrasonography or optical coherence tomography) can facilitate stent sizing in small vessels but were infrequently used (overall 1.7%); nevertheless, TLR rates were particularly low in this trial vs many previous studies.
Conclusions
Patients stented in small coronary vessels experienced fewer repeated target lesion revascularizations if they were treated with ultrathin-strut SES vs previous-generation thin-strut ZES. Study stents differed not only in strut thickness but also in stent geometry, polymer type, and eluted drug. Therefore, further research is required to definitely answer the question of whether the difference in stent strut thickness is the main driver of the observed between-stent difference.
eMethods.
eFigure. Multidimensional Representation of Study Devices
eTable 1. Technical Details of Study Devices
eTable 2. Medication at Index Procedure and at 1-Year, 2-Year and 3-Year Follow-up
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement
References
- 1.Akiyama T, Moussa I, Reimers B, et al. . Angiographic and clinical outcome following coronary stenting of small vessels: a comparison with coronary stenting of large vessels. J Am Coll Cardiol. 1998;32(6):1610-1618. doi: 10.1016/S0735-1097(98)00444-6 [DOI] [PubMed] [Google Scholar]
- 2.Schunkert H, Harrell L, Palacios IF. Implications of small reference vessel diameter in patients undergoing percutaneous coronary revascularization. J Am Coll Cardiol. 1999;34(1):40-48. doi: 10.1016/S0735-1097(99)00181-3 [DOI] [PubMed] [Google Scholar]
- 3.Wykrzykowska JJ, Serruys PW, Onuma Y, et al. . Impact of vessel size on angiographic and clinical outcomes of revascularization with biolimus-eluting stent with biodegradable polymer and sirolimus-eluting stent with durable polymer the LEADERS trial substudy. JACC Cardiovasc Interv. 2009;2(9):861-870. doi: 10.1016/j.jcin.2009.05.024 [DOI] [PubMed] [Google Scholar]
- 4.Claessen BE, Smits PC, Kereiakes DJ, et al. . Impact of lesion length and vessel size on clinical outcomes after percutaneous coronary intervention with everolimus- versus paclitaxel-eluting stents pooled analysis from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) and COMPARE (second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice) Randomized Trials. JACC Cardiovasc Interv. 2011;4(11):1209-1215. doi: 10.1016/j.jcin.2011.07.016 [DOI] [PubMed] [Google Scholar]
- 5.van der Heijden LC, Kok MM, Danse PW, et al. . Small-vessel treatment with contemporary newer-generation drug-eluting coronary stents in all-comers: insights from 2-year DUTCH PEERS (TWENTE II) randomized trial. Am Heart J. 2016;176(6):28-35. doi: 10.1016/j.ahj.2016.02.020 [DOI] [PubMed] [Google Scholar]
- 6.Elezi S, Dibra A, Mehilli J, et al. . Vessel size and outcome after coronary drug-eluting stent placement: results from a large cohort of patients treated with sirolimus- or paclitaxel-eluting stents. J Am Coll Cardiol. 2006;48(7):1304-1309. doi: 10.1016/j.jacc.2006.05.068 [DOI] [PubMed] [Google Scholar]
- 7.Kasaoka S, Tobis JM, Akiyama T, et al. . Angiographic and intravascular ultrasound predictors of in-stent restenosis. J Am Coll Cardiol. 1998;32(6):1630-1635. doi: 10.1016/S0735-1097(98)00404-5 [DOI] [PubMed] [Google Scholar]
- 8.Cassese S, Byrne RA, Tada T, et al. . Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart. 2014;100(2):153-159. doi: 10.1136/heartjnl-2013-304933 [DOI] [PubMed] [Google Scholar]
- 9.Wöhrle J, Markovic S, Rottbauer W, et al. . Bioresorbable polymer sirolimus-eluting coronary stent compared with permanent polymer everolimus-eluting coronary stent implantation for treatment of small vessel coronary artery disease: CENTURY II trial. EuroIntervention. 2016;12(2):e167-e174. doi: 10.4244/EIJV12I2A30 [DOI] [PubMed] [Google Scholar]
- 10.Dodge JT Jr, Brown BG, Bolson EL, Dodge HT. Lumen diameter of normal human coronary arteries: influence of age, sex, anatomic variation, and left ventricular hypertrophy or dilation. Circulation. 1992;86(1):232-246. doi: 10.1161/01.CIR.86.1.232 [DOI] [PubMed] [Google Scholar]
- 11.Kastrati A, Mehilli J, Dirschinger J, et al. . Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation. 2001;103(23):2816-2821. doi: 10.1161/01.CIR.103.23.2816 [DOI] [PubMed] [Google Scholar]
- 12.Briguori C, Sarais C, Pagnotta P, et al. . In-stent restenosis in small coronary arteries: impact of strut thickness. J Am Coll Cardiol. 2002;40(3):403-409. doi: 10.1016/S0735-1097(02)01989-7 [DOI] [PubMed] [Google Scholar]
- 13.von Birgelen C, van der Heijden LC, Basalus MWZ, et al. . Five-year outcome after implantation of zotarolimus- and everolimus-eluting stents in randomized trial participants and nonenrolled eligible patients: a secondary analysis of a randomized clinical trial. JAMA Cardiol. 2017;2(3):268-276. doi: 10.1001/jamacardio.2016.5190 [DOI] [PubMed] [Google Scholar]
- 14.Zocca P, Kok MM, Tandjung K, et al. . 5-year outcome following randomized treatment of all-comers with zotaroliumus-eluting resolute integrity and everolimus-eluting PROMUS Element coronary stents: final report of the DUTCH PEERS (TWENTE II) trial. JACC Cardiovasc Interv. 2018;11(5):462-469. doi: 10.1016/j.jcin.2017.11.031 [DOI] [PubMed] [Google Scholar]
- 15.von Birgelen C, Zocca P, Buiten RA, et al. . Thin composite wire strut, durable polymer-coated (Resolute Onyx) versus ultrathin cobalt-chromium strut, bioresorbable polymer-coated (Orsiro) drug-eluting stents in allcomers with coronary artery disease (BIONYX): an international, single-blind, randomised non-inferiority trial. Lancet. 2018;392(10154):1235-1245. doi: 10.1016/S0140-6736(18)32001-4 [DOI] [PubMed] [Google Scholar]
- 16.von Birgelen C, Kok MM, van der Heijden LC, et al. . Very thin strut biodegradable polymer everolimus-eluting and sirolimus-eluting stents versus durable polymer zotarolimus-eluting stents in allcomers with coronary artery disease (BIO-RESORT): a three-arm, randomised, non-inferiority trial. Lancet. 2016;388(10060):2607-2617. doi: 10.1016/S0140-6736(16)31920-1 [DOI] [PubMed] [Google Scholar]
- 17.Kandzari DE, Mauri L, Koolen JJ, et al. ; BIOFLOW V Investigators . Ultrathin, bioresorbable polymer sirolimus-eluting stents versus thin, durable polymer everolimus-eluting stents in patients undergoing coronary revascularisation (BIOFLOW V): a randomised trial. Lancet. 2017;390(10105):1843-1852. doi: 10.1016/S0140-6736(17)32249-3 [DOI] [PubMed] [Google Scholar]
- 18.Kereiakes DJ, Meredith IT, Windecker S, et al. . Efficacy and safety of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent: the EVOLVE II Randomized Trial. Circ Cardiovasc Interv. 2015;8(4):e002372. doi: 10.1161/CIRCINTERVENTIONS.114.002372 [DOI] [PubMed] [Google Scholar]
- 19.Pilgrim T, Heg D, Roffi M, et al. . Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for percutaneous coronary revascularisation (BIOSCIENCE): a randomised, single-blind, non-inferiority trial. Lancet. 2014;384(9960):2111-2122. doi: 10.1016/S0140-6736(14)61038-2 [DOI] [PubMed] [Google Scholar]
- 20.Jensen LO, Thayssen P, Maeng M, et al. . Randomized comparison of a biodegradable polymer ultrathin strut sirolimus-eluting stent with a biodegradable polymer biolimus-eluting stent in patients treated with percutaneous coronary intervention: the SORT OUT VII trial. Circ Cardiovasc Interv. 2016;9(7):e003610. doi: 10.1161/CIRCINTERVENTIONS.115.003610 [DOI] [PubMed] [Google Scholar]
- 21.Bangalore S, Toklu B, Patel N, Feit F, Stone GW. Newer generation ultra-thin strut drug-eluting stents versus older second-generation thicker strut drug-eluting stents for coronary artery disease: a meta-analysis of randomized trials. Circulation. 2018. doi: 10.1161/CIRCULATIONAHA.118.034456 [DOI] [PubMed] [Google Scholar]
- 22.Kok MM, Zocca P, Buiten RA, et al. . Two-year clinical outcome of all-comers treated with three highly dissimilar contemporary coronary drug-eluting stents in the randomised BIO-RESORT trial. EuroIntervention. 2018;14(8):915-923. doi: 10.4244/EIJ-D-18-00336 [DOI] [PubMed] [Google Scholar]
- 23.von Birgelen 3-Years BIO-RESORT: results of the 3-arm randomized study in allcomers, treated with contemporary biodegradable or durable polymer-coated drug-eluting stents. http://www.crtonline.org/Assets/73c67fcc-45ce-45ef-aac0-5e00cb570dc0/636879360117130000/4eb73a53-a46a-4cc4-92ba-13f0724e519e-pptx. Accessed March 22, 2019.
- 24.Cutlip DE, Windecker S, Mehran R, et al. ; Academic Research Consortium . Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344-2351. doi: 10.1161/CIRCULATIONAHA.106.685313 [DOI] [PubMed] [Google Scholar]
- 25.Vranckx P, Cutlip DE, Mehran R, et al. . Myocardial infarction adjudication in contemporary all-comer stent trials: balancing sensitivity and specificity: addendum to the historical MI definitions used in stent studies. EuroIntervention. 2010;5(7):871-874. doi: 10.4244/EIJV5I7A146 [DOI] [PubMed] [Google Scholar]
- 26.Saito S, Ando K, Ito Y, et al. . Two-year results after coronary stenting of small vessels in Japanese population using 2.25-mm diameter sirolimus-eluting stent with bioresorbable polymer: primary and long-term outcomes of CENTURY JSV study. Cardiovasc Interv Ther. 2019;34(1):25-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jinnouchi H, Kuramitsu S, Shinozaki T, et al. . Two-year clinical outcomes of the NOBORI biolimus-eluting stents versus XIENCE/PROMUS everolimus-eluting stents in small vessel disease. Catheter Cardiovasc Interv. 2016;88(5):E132-E138. doi: 10.1002/ccd.26360 [DOI] [PubMed] [Google Scholar]
- 28.Lefèvre T, Haude M, Neumann FJ, et al. . Comparison of a novel biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: 5-year outcomes of the randomized BIOFLOW-II trial. JACC Cardiovasc Interv. 2018;11(10):995-1002. doi: 10.1016/j.jcin.2018.04.014 [DOI] [PubMed] [Google Scholar]
- 29.El-Hayek G, Bangalore S, Casso Dominguez A, et al. . Meta-analysis of randomized clinical trials comparing biodegradable polymer drug-eluting stent to second-generation durable polymer drug-eluting stents. JACC Cardiovasc Interv. 2017;10(5):462-473. doi: 10.1016/j.jcin.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 30.Joner M, Finn AV, Farb A, et al. . Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48(1):193-202. doi: 10.1016/j.jacc.2006.03.042 [DOI] [PubMed] [Google Scholar]
- 31.Holmes DR Jr, Kereiakes DJ. The approach to small vessels in the era of drug-eluting stents. Rev Cardiovasc Med. 2005;6(suppl 1):S31-S37. [PubMed] [Google Scholar]
- 32.Kolandaivelu K, Swaminathan R, Gibson WJ, et al. . Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011;123(13):1400-1409. doi: 10.1161/CIRCULATIONAHA.110.003210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iantorno M, Lipinski MJ, Garcia-Garcia HM, et al. . Meta-analysis of the impact of strut thickness on outcomes in patients with drug-eluting stents in a coronary artery. Am J Cardiol. 2018;122(10):1652-1660. doi: 10.1016/j.amjcard.2018.07.040 [DOI] [PubMed] [Google Scholar]
- 34.Price MJ, Saito S, Shlofmitz RA, et al. . First report of the Resolute Onyx 2.0-mm zotarolimus-eluting stent for the treatment of coronary lesions with very small reference vessel diameter. JACC Cardiovasc Interv. 2017;10(14):1381-1388. doi: 10.1016/j.jcin.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 35.Megaly M, Rofael M, Saad M, et al. . Outcomes with drug-coated balloons in small-vessel coronary artery disease. Catheter Cardiovasc Interv. 2019;93(5):E277-E286. [DOI] [PubMed] [Google Scholar]
- 36.Price MJ, Shlofmitz RA, Spriggs DJ, et al. . Safety and efficacy of the next generation Resolute Onyx zotarolimus-eluting stent: primary outcome of the RESOLUTE ONYX core trial. Catheter Cardiovasc Interv. 2018;92(2):253-259. doi: 10.1002/ccd.27322 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure. Multidimensional Representation of Study Devices
eTable 1. Technical Details of Study Devices
eTable 2. Medication at Index Procedure and at 1-Year, 2-Year and 3-Year Follow-up
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement