Abstract
Objective
Trauma, particularly when experienced early in life, can alter neurophysiologic and behavioral development, thereby increasing risk for substance use disorders and related psychopathology. However, few studies have empirically examined trauma using well-characterized developmental samples that are followed longitudinally.
Method
The association of assaultive, non-assaultive, and sexual assaultive experiences before 10 years of age with developmental trajectories of brain function during response inhibition was examined by measuring electrophysiologic theta and delta oscillations during no-go and go conditions in an equal probability go/no-go task. Data were drawn from the Collaborative Study of the Genetics of Alcoholism (COGA) prospective cohort, composed of offspring who were aged 12 through 22 years at enrollment from high-risk and comparison families, with follow-ups at 2-year intervals since 2004. In addition, other important predictors of neurophysiologic functioning (eg, substance use, impulsivity, and parental alcohol use disorders) were investigated. Moreover, associations of neurophysiologic functioning with alcohol and cannabis use disorder symptom counts and externalizing and internalizing psychopathology were examined.
Results
Individuals exposed to sexual assaultive trauma before 10 years of age had slower rates of change in developmental trajectories of no-go frontal theta during response inhibition. Importantly, effects remained significant after accounting for exposure to other traumatic exposures, such as parental history of alcohol use disorder and participants’ substance use, but not measures of impulsivity. Further, slower rates of change in no-go frontal theta adolescent and young adult development were associated with increased risk for alcohol use disorder symptoms and internalizing psychopathology, but not for cannabis use disorder symptoms or externalizing psychopathology.
Conclusion
Childhood sexual assault is associated with atypical frontal neurophysiologic development during response inhibition. This could reflect alterations in frontal lobe development, synaptic pruning, and/or cortical maturation involving neural circuits for inhibitory control. These same areas could be associated with increased risk for young adult alcohol use disorder symptoms and internalizing psychopathology. These findings support the hypothesis that changes in neurocognitive development related to early sexual trauma exposure could increase the risk for mental health and substance use problems in young adulthood.
Keywords: sexual abuse, inhibition, event-related oscillations, alcohol dependence, internalizing
Approximately 1 in 4 adolescents in the United States is exposed to a traumatic event before 16 years of age.1 Those who experience early life trauma have greater lifetime risk for substance use disorders and related mental health problems (ie, depression and anxiety2–4). Researchers have suggested that trauma, particularly when experienced early in life, might alter neurobiological and behavioral development, thereby increasing the risk for later onset of psychopathology,5 including substance use disorders.6,7 Several cross-sectional studies have reported associations between childhood trauma exposure and neurobiological and cognitive alterations.8–10 Further, many of these same neurocognitive alterations are correlates of mental health and substance use disorders.6,7 Although it has been suggested that links between early trauma exposure and later mental health and substance use problems are related to such neurocognitive alterations, few studies have empirically examined this possibility. Therefore, the longitudinal effects of early trauma exposure on neurocognitive development and the impact such effects can have on risk for later mental health and substance use disorders remain largely unknown.
Advances in understanding typical brain development have begun to elucidate why early traumatic experiences can have such a profound influence on neurobiological and behavioral development.11,12 The brain undergoes its greatest growth and development in the first years of life, with a second phase beginning in adolescence characterized by synaptic pruning, leading to anatomic and functional maturation.13–16 This second phase of development is most profound in frontal lobe regions of the brain involved in higher-order cognitive functions, including top-down control functions, such as inhibition and other aspects of executive function. This phase also is accompanied by broader developmental changes, including pubertal development, which has been shown to influence cortical maturation and synaptic pruning throughout this period.17,18 Therefore, it is important to understand whether early trauma exposure predicts differential patterns of brain development during this second maturational phase, a period of great susceptibility to environmental influences, and whether such effects are associated with increased susceptibility to mental health and substance use problems.
Studies examining the effects of early life stress on brain development have mainly implicated neural stress reactivity and emotional processing/regulation pathways,5,19–23 indicating that those exposed to early life stress exhibit deficits in cognitive and behavioral control, selective attention, and reward processing.21,24–27 Cognitive tasks such as the go/no-go (GNG) task, which requires selective attention and behavioral inhibition, could be particularly relevant to the assessment of neural functioning in individuals exposed to early trauma.28,29 The GNG task requires activation of several brain networks including the executive network,29 which facilitates the detection, monitoring, and resolution of conflict between 2 competing response tendencies—execution (go) and refraining from execution (no-go) of a motor response—thereby reflecting behavioral execution and inhibition.30–33 Behavioral inhibition is an essential regulatory executive control that undergoes substantial development during adolescence and persists through young adulthood.34 This is one developmental process that could be altered in those exposed to early life trauma.20,35 To date, 2 functional magnetic resonance imagining studies have investigated response inhibition using the GNG task in adolescents exposed to different types of early trauma or adversity (eg, abuse, neglect, or witnessing parental violence36; neglect, maltreatment, or multiple foster placements before adoption37). In these studies, decreased behavioral inhibition and activation differences in the prefrontal cortex were observed in trauma-exposed subjects.
Studies that have examined the influence of child maltreatment using electroencephalography (EEG) have the advantage of temporal resolution on the order of milliseconds, a scale at which many relevant sensory, motor, and cognitive phenomena take place at the neural level.38–41 Brain oscillations of different frequency bands are related to various cognitive functions,42–44 and task-related event-related oscillations (EROs) provide time and frequency information for a specific sensory, motor, or cognitive event. Howells et al38 reported altered cortical arousal during GNG task performance in adults who retrospectively reported different types of childhood trauma exposures. Findings were dependent on the form of childhood trauma experienced; for example, child emotional abuse was correlated with increased theta activity during the GNG task. Other electrophysiologic studies conducted in children exposed to psychosocial deprivation39 or other severe forms of neglect also found increased resting-state theta activity and decreased resting-state alpha and beta activity.40,45 In one of the few longitudinal studies conducted in this area, McLaughlin et al.40,41 reported lagged developmental trajectories of frontal resting-state EEG from 9 months to 8 years in children reared in Romanian institutions, many of whom were exposed to severe neglect. Importantly, this study also demonstrated that these changes predicted hyperactivity, impulsivity, and internalizing symptoms at approximately 4.5 years. Collectively, these findings have been interpreted as representing a maturational delay in cortical development associated with severe early life stress.39–41,46–52
Previous research has suggested that exposure to early childhood trauma is associated with a developmental lag in cortical arousal and relatedly behavioral inhibition, and that these neural responses might increase the risk for later onset of psychopathology, including mood, anxiety disorders, and behavioral disorders.39–41,46–52 However, to our knowledge, no study has explicitly examined this prospectively through emerging adulthood, the period of highest risk for the onset of many of these disorders. The studies that have examined similar questions regarding the legacy of early trauma on neurodevelopment21,40,41,49,50,53 have primarily relied on data from the Bucharest Early Intervention Study, which focuses on early development (9 months through 8 years), but not thereafter. Whether early life stress influences adolescent and young adulthood neurodevelopment and/or increases risk for young adult mental health and substance use problems remains unknown. Further, this literature has been limited by several methodologic factors, including relatively small study sizes (N < 200), cross-sectional and/or retrospective nature of most of these data, and the robustness of these associations to other confounding factors, including participants’ psychopathology, substance use, family history, and several key sociodemographic characteristics. In addition, no study to our knowledge has incorporated information on parents’ psychopathology, which often co-occurs with adverse childhood experiences and has been shown to influence neurodevelopment and risk for mental health problems.54,55 These factors pose serious challenges when attempting to disentangle which neurobiological effects are due specifically to early traumatic experiences, and whether those particular neurobiological changes influence risk for mental health and substance use problems.
The present study investigated the associations of non-assaultive, assaultive, and sexual assaultive trauma exposure before 10 years of age with developmental trajectories of frontal theta oscillations and posterior delta oscillations during no-go (response inhibition) and go conditions. Data are from a longitudinal, developmental sample of adolescents and young adults from the Collaborative Study of the Genetics of Alcoholism (COGA) prospective cohort. A second aim was to examine the role of parental history of alcohol use disorders (AUDs) and participants’ substance use, impulsivity, gender, and race/ethnicity in these associations. A third aim was to assess whether trauma-associated neurophysiologic trajectories influence risk for AUDs and cannabis use disorders (CUDs) and/or related internalizing (INT) and externalizing (EXT) psychopathology.
METHOD
Sample
The COGA prospective study began data collection in 2004 and is ongoing. Details on data collection and procedures have been published previously.56 Briefly, offspring from families densely affected by alcohol use problems and comparison community families who were 12 to 22 years old at intake and who had at least 1 parent interviewed in an earlier phase of the COGA study were enrolled, with new subjects added as they reached 12 years of age. Subjects were interviewed every 2 years with a comprehensive battery that included the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA57), covering substance use problems and other psychiatric disorders and related behavior, personality questionnaires, family history of alcohol use problems, and a neurophysiologic battery. An age-appropriate SSAGA (cSSAGA58) was used for subjects younger than 18 years. At the time of analysis, this study presents data on 2,625 offspring from 2,413 nuclear families who had at least 1 follow-up interview; 1,931 participants had a third assessment, 1,324 a fourth assessment, 842 had a fifth assessment, 428 had a sixth assessment, and thus far 8 participants have had a seventh assessment (data collection is ongoing). For the 2,625 offspring analyzed, the mean age at baseline was 17.1 years (standard deviation 3.6, range 12–26), 50.7% were female subjects, and self-reported race/ethnicity was 29.2% African American, 62.0% Caucasian, and 9.0% Asian, Pacific Islander, or “other.” Analytic sample details are presented in Table 1.
Table 1.
Analytic Sample Descriptive Statistics
| All Participants (N = 2,625) | Male Participants (n = 1,286) | Female Participants (n = 1,339) | |
|---|---|---|---|
| Mean Age At: | |||
| Baseline interview | 17.1 (12.0–26.2) | 17.2 (12.0–29.2) | 17.2 (12.0–28.7) |
| Follow-up 1 (n = 1,931) | 19.5 (13.3–32.2) | 19.2 (13.3–32.2) | 19.7 (13.8–31.6) |
| Follow-up 2 (n = 1,324) | 21.7 (15.3–32.2) | 21.7 (15.3–31.8) | 21.8 (15.8–32.2) |
| Follow-up 3 (n = 842) | 23.4 (17.4–32.3) | 23.5 (17.4–32.3) | 23.3 (17.7–31.7) |
| Follow-up 4 (n = 428) | 25.1 (19.6–32.2) | 25.0 (19.6–32.2) | 25.2 (19.8–31.9) |
| Follow-up 5 (n = 8) | 27.9 (22.1–31.9) | 27.4 (22.3–31.8) | 28.4 (22.1–31.9) |
| Most recent interview | 22.4 (12–32.0) | 22.1 (12.0–32.0) | 22.7 (12.0–32.0) |
| Self-reported race/ethnicity (%) | |||
| White/Caucasian | 62.0 | 61.9 | 62.0 |
| Black/African American | 29.2 | 29.6 | 28.7 |
| Other | 9.0 | 8.6 | 9.3 |
| Non-assaultive < 10 y, % | 16.6 | 18.0 | 15.3 |
| Assaultive < 10 y, % | 4.6 | 5.6 | 3.5 |
| Sexual assaultive < 10 y, % | 6.6 | 4.0 | 8.9 |
| Parental history of AUD, % | 41.1 | 41.6 | 40.7 |
| Alcohol ever use, % | 94.7 | 95.8 | 93.7 |
| Cannabis ever use, % | 77.4 | 82.4 | 72.5 |
| DSM-5 Symptoms | |||
| AUD (any), % | 64.6 | 65.4 | 64.0 |
| Mean (SD) | 3.0 (3.6) | 2.1 (2.4) | 1.6 (2.3) |
| CUD (any), % | 72.2 | 53.0 | 75.2 |
| Mean (SD) | 1.5 (2.7) | 2.5 (3.0) | 1.3 (2.4) |
| INT (any), % | 38.7 | 32.5 | 44.6 |
| Mean (SD) | 0.4 (0.5) | 0.3 (0.5) | 0.5 (0.5) |
| EXT (any), % | 16.8 | 19.4 | 12.0 |
| Mean (SD) | 0.2 (0.4) | 0.2 (0.4) | 0.1 (0.3) |
Note: Assaultive traumas (ie, stabbed, shot, mugged, threatened with a weapon, robbed, kidnapped, and held captive), non-assaultive traumas (ie, life-threatening accident, disaster, witnessing someone seriously injured or killed, and unexpectedly finding a dead body), and sexual assaultive traumas (ie, rape or molestation) before 10 years of age. Internalizing (INT) psychopathology count scores included DSM-IV lifetime symptoms for major depressive disorder, panic disorder, social phobia, and an additional item—suicidal ideation. Externalizing (EXT) psychopathology count scores included conduct disorder and oppositional defiant disorder symptoms. Data from each individual’s most recent interview were used. AUD = alcohol use disorder; CUD = cannabis use disorder; SD = standard deviation.
Experimental protocols were approved by each site’s institutional review board, and informed consent was obtained from all participants. Participants were excluded from neurophysiologic assessment if they had positive breath-analyzer test and/or urine screen results; hepatic encephalopathy/cirrhosis of the liver; history of head injury, seizures, or neurosurgery; uncorrected sensory deficits; history/symptoms of psychoses; self-reported positive test result for human immunodeficiency virus; other acute/chronic medical illnesses that affect brain function; or psychotropic medications that affect electrophysiologic measurement.
Measures
Traumatic Exposures
Traumatic exposures were collected using the SSAGA57 and have been described previously.56,59 The SSAGA included 21 potentially traumatic events. Several events were excluded from the present study because they did not occur before 10 years of age (eg, combat-related trauma). All events used in the present analysis are presented in Table S1, available online. Based on evidence that interpersonal assaultive events have a stronger and more enduring effect on mental health/substance use than non-assaultive events,60–62 that traumatic events cluster together,63 and to remain consistent with prior studies,59 3 composite variables were examined, representing the report of at least 1 lifetime assaultive trauma (ie, stabbed, shot, mugged, threatened with a weapon, robbed, kidnapped, and held captive), non-assaultive trauma (ie, life-threatening accident, disaster, witnessing someone seriously injured or killed, and unexpectedly finding a dead body), or sexual assaultive trauma (ie, rape or molestation by relative or non-relative). Importantly, age at occurrence of each event was recorded, and this information was used in the present study. We focused on traumatic events occurring before 10 years of age, given the suggestion that trauma exposure at early stages of development might be more influential than later exposures for neurobiological development and onset of later psychopathology23,64,65 and our desire to measure events that preceded measurement of neurophysiologic and behavioral outcomes. Any trauma experienced after 10 years was combined into a binary measure that was used as a covariate in all models, because trauma exposure is known to re-occur throughout the lifecourse.66
Parental AUD Status
Parental AUD was a lifetime measure based on available parent SSAGA interviews (60.8% of fathers and 89.8% of mothers) as described previously.56,59 For parents who were not interviewed, reports about the parent’s alcohol problems obtained in earlier COGA waves from other relatives or, less commonly, from their offspring during the prospective study assessment were used to code as affected parents with at least 2 positive family history reports based on the Family History Assessment Module.67 Maternal and paternal variables were combined to represent lifetime AUDs in either or both parents.
Substance Use and Psychopathology
Data from all offspring SSAGA and cSSAGA interviews were used to obtain lifetime reports of alcohol and cannabis use as previously detailed.56,59 Participants’ AUD symptom count scores and CUD symptom count scores were based on DSM-5 lifetime symptom counts. INT psychopathology count scores included DSM-IV lifetime diagnoses for major depressive disorder, panic disorder, social phobia, and an additional item—suicidal ideation. EXT psychopathology count scores included conduct disorder and oppositional defiant disorder diagnoses. Data from each individual’s most recent interview were used.
Barratt Impulsiveness Scale
The Barratt Impulsiveness Scale (BIS; version 11) is a 30-item scale that measures 3 aspects of impulsivity: attentional impulsiveness, motor impulsiveness, and non-planning.68 All items are answered 1 (never), 2 (occasionally), 3 (often), or 4 (always). Separate scales were developed for adolescents and adults. Total scores were computed by summing subscale items. Data from each individual’s baseline interview were used.
Sensation Seeking Scale
The Sensation Seeking Scale (SSS) measures individual differences in stimulation and arousal69 and assesses boredom susceptibility, thrill and adventure seeking, experience seeking, and disinhibition. Total scores are computed by summing all 30 items. Data from each individual’s baseline interview were used.
Theta ERO Power (GNG)
Using the protocol described by Pandey et al.,70,71 each participant was presented with 4 types of visual stimuli consisting of white isosceles triangles pointing in the up, down, right, or left direction. The stimuli were presented for 100 ms at the center of a computer screen (17 inches diagonally, 75-Hz refresh rate, 1,024 × 768 resolution) against a dark background that subtended a visual angle of approximately 1°. In the practice session, participants were instructed to press a key whenever a white triangle pointed up or down (go stimulus) and refrain from pressing the key whenever the triangle pointed toward the right or left (no-go stimulus). A dollar sign ($) appeared on the screen for 200 ms at 1,200 ms after stimulus onset when participants responded correctly, whereas a cross sign (X) appeared on the screen for 200 ms at 1,200 ms after stimulus onset when participants responded incorrectly. Participants were instructed that speed and accuracy were equally important for making a correct response. In the next, experimental, phase, EEG was recorded. Participants were informed that each correct response would earn a reward. However, each subject received a predetermined fixed amount at the end of the experiment without deductions for errors, although they were not informed of this while performing the task. The probabilities of occurrence of go and no-go stimuli were equal (50/50), and the order of stimulus presentation was randomized. The intertrial interval was 2,400 ms. Go and no-go accuracy and go reaction time at each assessment also were recorded and used in statistical analysis.
Participants were comfortably seated in front of a computer monitor screen placed 1 m away in a dimly lit, sound-attenuated, radiofrequency-shielded room (IAC Acoustics, Bronx, NY). The EEG was recorded on a Neuroscan System (versions 4.1, 4.2, 4.3, 4.4 and 4.5; Neurosoft, Inc., El Paso, TX) using a 61-channel electrode cap (Electro-cap International, Inc., Eaton, OH) that had electrode placements based on the extended 10–20 International System (Electrode Position Nomenclature; American Clinical Neurophysiology Society, 1991) with the notch filter off. The electrodes were referenced to the tip of the nose, and participants were grounded using an electrode placed on the forehead (frontal midline, 2 cm above nasion). Eye movements were recorded using a supraorbital vertical lead and a horizontal lead on the external canthus of the left eye. Electrode impedance was maintained below 5 kΩ throughout the recording. The continuous EEG signals were recorded and marked with all stimulus, response, and feedback event codes at sampling rates of 512 Hz (16-bit A/D) or 500 Hz (32-bit A/D) depending on the amplifier version, with a bandpass filter set at 0.02 to 100 Hz, and were amplified 10,000 times using a set of amplifiers (SynAmps2, Neurosoft, Inc.).
Because of prior evidence indicating the importance of frontal theta oscillations during the no-go condition and posterior delta oscillations during the go condition of the GNG task38,70,71 and a preliminary analysis to determine time-frequency regions of interest, the present study used S-transformed frontal theta total power (4–7.5 Hz, 200–400 ms, Fz) during the no-go (response inhibition) and go conditions and, for comparison, posterior delta total power (1–3.5 Hz, 200–500 ms, Pz) during the go and no-go conditions at baseline and follow-up assessments 1 through 4. Further details about the ERO signal processing using S-transformed method can be found in a previous publication (that study was conducted in a different analytic sample).72
Statistical Methods
First, we estimated an unconditional growth model that predicted log-transformed ERO measures from baseline through the most recent assessment by age by incorporating individual participant’s age at each follow-up (Mplus option: time scores; Muthén and Muthén; https://www.statmodel.com/company.shtml). This model specifies latent variables for the random intercept, the random slope for time (rate of change in ERO value by age), and a constant or individual deviation from these mean values. This approach allowed us to simultaneously estimate the variance in ERO within and between individuals across time. The slope and residual variances were fixed to be equal across all available time points. Separate models were run for delta and theta EROs (total power) during the go and no-go conditions.
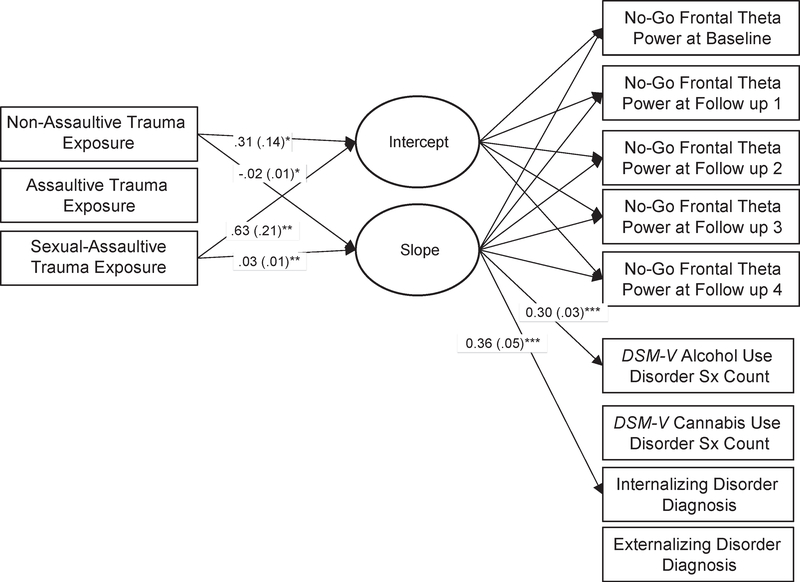
Second, we examined time-invariant predictors of ERO trajectories. We simultaneously examined the association of 3 binary measures of trauma exposure before 10 years (nonassaultive, non-sexual assaultive, and sexual assaultive traumatic exposures) with ERO intercepts and slopes (linear change from baseline through follow-up 4). This is depicted in Figure 1. Initial results indicated no evidence of nonlinear (ie, quadratic) effects. Modeling was conducted in Mplus 7.4 (Muthén and Muthén, 1998–2015) using full maximum likelihood estimation with robust standard errors. Age, gender (0 = male, 1 = female), and self-reported race/ethnicity (0 = non-Hispanic white, 1 = non-Hispanic black/African American, 2 = other) were used as covariates in all analyses. In addition, we accounted for genetic relatedness among siblings. Subsequent models included participants’ alcohol and cannabis use (0 = never used, 1 = ever used) at each interview, parental history of AUD, and participants’ impulsivity as measured by baseline BIS and SSS scores. Third, we evaluated whether residualized change in ERO from baseline to most recent follow-up was related to AUD, CUD, and INT and EXT psychopathology at each participant’s most recent interview.
FIGURE 1.
Effects of Early Trauma Exposure on No-Go Frontal Theta Power From Baseline Through Follow-Up 4 and Associations With Substance Use Disorder and Psychopathology
Note: Parameter estimates (and standard errors) are displayed only for statistically significant pathways. Not pictured, but also included in this model, are the following covariates: gender, race/ethnicity, age, alcohol use and cannabis use, and parental alcohol use disorder. Internalizing psychopathology count scores included DSM-IV lifetime symptoms for major depressive disorder, panic disorder, social phobia, and an additional item—suicidal ideation. Externalizing psychopathology count scores included conduct disorder and oppositional defiant disorder symptoms. Data from each individual’s most recent interview were used. Sx = symptom.
*p < .05; **p < .01; ***p < .001.
RESULTS
Rates of traumatic exposure in the COGA prospective sample have been described previously.56,59 When considering trauma experienced before 10 years (Table 1), 26.6% reported experiencing at least 1 type of trauma; 16.6% reported experiencing non-assaultive trauma, 4.5% reported experiencing assaultive trauma, and 6.6% reported experiencing sexual assault. Non-sexual assaultive trauma was more common for male subjects (p < .05), whereas sexual assaultive trauma was more common for female subjects (p < .05). Non-assaultive trauma exposure was higher for African-American than for white participants (p < .05).
Individuals exposed to early trauma differed with respect to measures of impulsivity as measured by the BIS and SSS, substance use behavior, and psychiatric symptoms (Table 2; associations were adjusted for gender, age at assessment, self-reported race, and parental history of AUD). Several associations withstood a Bonferroni multiple-test correction; sexual trauma before 10 years was associated with cognitive impulsivity (BIS), AUD symptom count, CUD symptom count, and INT and EXT psychopathology. Assaultive trauma exposure was associated with INT symptoms, and non-assaultive trauma was associated with EXT symptoms (Table 2). Correlations among all variables are presented in Table S2, available online. Go and no-go accuracy and go reaction time on the GNG task did not differ significantly among participants (Table S3, available online).
Table 2.
Associations of Early Trauma Exposure With Impulsivity, Substance Use, and Psychiatric Disorder Symptoms
| Non-Assaultive Trauma < 10 y (n = 418) |
Assaultive Trauma < 10 y (n = 111) |
Sexual Assaultive Trauma < 10 y (n = 121) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| B | SE | p | B | SE | p | B | SE | p | |
| BIS total score | 0.69 | 0.95 | .47 | 1.04 | 1.67 | .53 | 3.03 | 1.40 | .03 |
| Non-planning | −0.25 | 0.41 | .54 | 0.73 | 0.73 | .32 | 1.12 | 0.56 | .05 |
| Motor impulsive | 0.32 | 0.35 | .35 | 0.31 | 0.62 | .66 | 0.82 | 0.48 | .09 |
| Cognitive impulsivea | 0.82 | 0.32 | .01 | 1.31 | 0.57 | .02 | 1.21 | 0.45 | <.001a |
| Zuckerman score | 0.19 | 0.64 | .77 | 0.79 | 1.15 | .49 | 1.71 | 0.82 | .04 |
| Disinhibition | −0.07 | 0.24 | .79 | −0.57 | 0.44 | .19 | 0.44 | 0.31 | .15 |
| Boredom susceptibility | −0.16 | 0.19 | .41 | −0.08 | 0.34 | .81 | 0.43 | 0.24 | .07 |
| Thrill seeking | 0.04 | 0.29 | .19 | 0.98 | 0.52 | .06 | 0.26 | 0.37 | .48 |
| Experience seeking | 0.02 | 0.21 | .92 | 0.46 | 0.37 | .22 | 0.57 | 0.27 | .03 |
| Ever drinking | −0.01 | 0.01 | .39 | 0.01 | 0.03 | .73 | 0.05 | 0.02 | .04 |
| Ever used cannabis | −0.04 | 0.03 | .15 | 0.03 | 0.05 | .63 | 0.07 | 0.05 | .15 |
| DSM-5 AUD sxa | 0.13 | 0.15 | .40 | 0.55 | 0.29 | .05 | 0.91 | 0.25 | <.001a |
| DSM-5 CUD sxa | 0.24 | 0.16 | .14 | 0.75 | 0.31 | .02 | 1.00 | 0.27 | <.001a |
| DSM-5 INT sxa | 0.06 | 0.02 | .01 | 0.33 | 0.05 | <.001a | 0.20 | 0.06 | <.001a |
| DSM-5 EXT sxa | 0.09 | 0.03 | <.001a | 0.13 | 0.06 | .02 | 0.19 | 0.05 | <.001a |
Note: All associations are adjusted for gender, self-reported race/ethnicity, age at assessment, and parental history of alcohol dependence. Comparison groups are participants who were not exposed to any trauma type before 10 years of age. Boldface type denotes significance (p < .05). AUD = alcohol use disorder; BIS = Barratt Impulsiveness Scale; CUD = cannabis use disorder; EXT = externalizing; INT = internalizing; SE = standard error; sx = symptom.
Associations that withstood Bonferroni multiple test correction (0.05/45 tests conducted for p < .001).
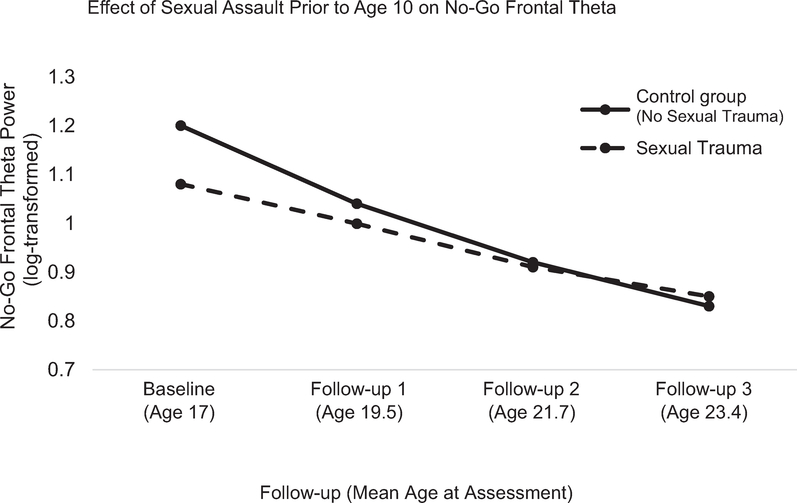
Results from ERO models, including parameter estimates and fit statistics, are presented in Tables 3 and S4, available online. The only statistically significant effect observed involved sexual assaultive trauma before 10 years and no-go frontal theta oscillation. That is, when all 3 trauma exposures were examined simultaneously (Table 3), no statistically significant effects were observed for non-assaultive trauma, non-sexual assaultive trauma, or oscillations in the go condition. In models including gender, race/ethnicity, non-assaultive trauma, and non-sexual assaultive trauma as covariates, sexual assaultive trauma before 10 years was associated with decreased no-go frontal theta oscillation at baseline (intercept, p < .01; Table 3) and a decreased rate of change in no-go frontal theta oscillation from baseline to follow-up 4 (slope, p < .001; Table 3). This is displayed in Figures 2 and 3. No significant effects were observed in the go condition. Further, no statistically significant effects were observed for posterior delta ERO in the go or no-go condition (Table S4, available online).
Table 3.
Effects of Early Trauma Exposure on the Developmental Trajectory of No-Go Frontal Theta Power From Baseline Through Follow-Up 4
| Trauma Exposure < 10 y | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Non-assaultive | ||||
| Intercept | −0.29 (1.50) | −0.29 (1.50) | 1.22 (1.98) | 0.59 (2.37) |
| Slope | −0.01 (0.07) | −0.01 (0.07) | −0.08 (0.09) | −0.01 (0.09) |
| Assaultive | ||||
| Intercept | −2.58 (1.56) | −2.58 (1.56) | 2.36 (1.99) | 3.05 (2.09) |
| Slope | 0.09 (0.07) | 0.09 (0.07) | −0.08 (0.08) | −0.09 (0.09) |
| Sexually assaultive | ||||
| Intercept | −4.41 (1.59)** | −4.41 (1.59)** | −3.11 (1.99) | 0.13 (1.89) |
| Slope | 0.22 (0.07)*** | 0.22 (0.07)*** | 0.17 (0.08)* | 0.03 (0.07) |
Note: All models Include age modeled by time scores. Model 1 (free parameters = 20; Akaike Information criterion [AIC] = 39,270.74; Bayesian Information criterion [BIC] = 39,387.91) includes gender, race/ethnicity, and age. Model 2 (free parameters = 24; AIC = 21,607.93; BIC = 21,713.31) adds to covariates in model 1 alcohol use and cannabis use. Model 3 (free parameters = 26; AIC = 21,601.10; BIC = 21,725.64) adds to covariates in model 2 parental alcohol use disorder. Model 4 (free parameters = 28; AIC = 12,627.05; BIC = 12,748.28) adds to covariates in model 3 participants’ impulsivity as measured using the Barratt Impulsiveness Scale and Sensation Seeking Scale.
p < .05
p < .01
p < .001.
FIGURE 2.
Adjusted Mean Trajectories of No-Go Frontal Theta by Sexual Assaultive Trauma Exposure
Note: Models are adjusted for gender, self-reported race/ethnicity, age at assessment, and parental history of alcohol dependence. The comparison group includes participants who were not exposed to sexual trauma before 10 years of age (93.4% of analytic sample).
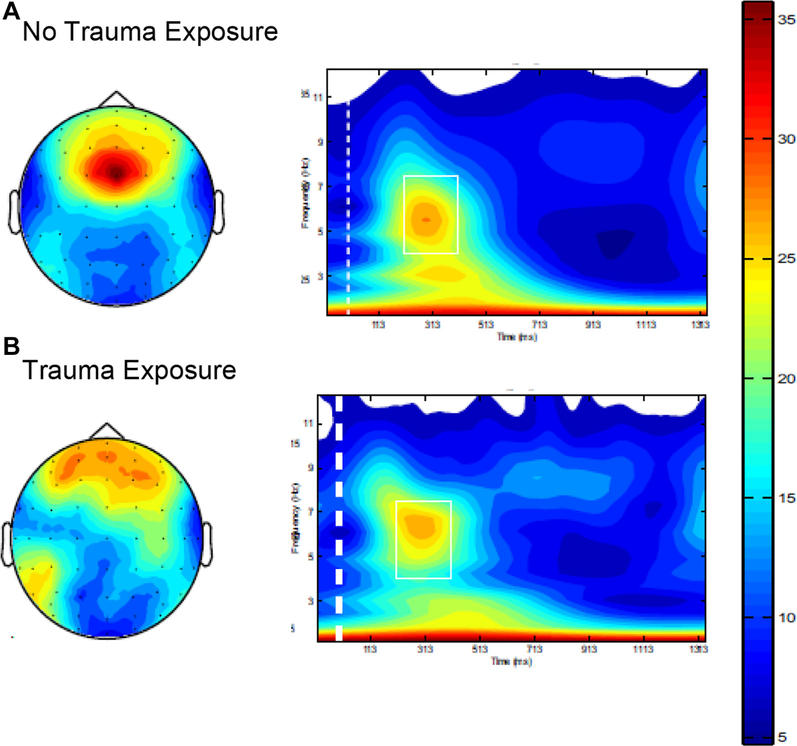
FIGURE 3.
No-Go Frontal Theta by Early Sexual Assaultive Trauma Exposure
Note: This figure depicts differences in frontal theta no-go power values at baseline observed in participants who (A) were not exposed and (B) were exposed to sexual trauma before 10 years of age. Note the more focused frontal topography and more efficient neural synchronization (ie, higher theta event-related oscillation power values) during response inhibition (no-go condition of go/no-go task) in participants who were not exposed to trauma. In contrast, the frontal topography indicates a less efficient neural synchronization (ie, lower event-related oscillation power values) during response inhibition (no-go condition of go/no-go task) in participants who were exposed to trauma. Please note color figures are available online.
Associations remained statistically significant when participants’ alcohol and cannabis use were included in the model (intercept, p < .01; slope, p < .001; Table 3). However, when parental AUD was included in the model, only a decreased rate of change in no-go frontal theta oscillation from baseline to follow-up 4 was observed (intercept, p > .05; slope, p < .05; Table 3). When cognitive impulsivity (BIS subscale, baseline assessment) was included in the model, associations were no longer statistically significant (intercept, p > .05; slope, p > .05). When additional pathways from the slope and intercept factors to INT pathology, EXT pathology, AUD symptoms, and CUD symptoms (≥18 years old) were included in the model, the rate of change in no-go frontal theta oscillation was positively associated with INT pathology and AUD symptoms at participants’ most recent follow-ups (p < .001; Figure 2). In addition, models including intercepts as covariates were examined and results remained largely unchanged (results available upon request).
DISCUSSION
Although previous studies have reported associations between childhood trauma exposure and neurobiological alterations,9,10,21,41,73–75 it remains unclear to what extent childhood trauma influences adolescent and young adult neurodevelopment, and whether these effects influence risk for onset of psychopathology in young adulthood. Findings from the present study suggest that individuals exposed to sexual assaultive trauma before 10 years of age have atypical developmental trajectories of neurophysiologic functioning during response inhibition (no-go); the typical decrease in frontal theta oscillatory activity during response inhibition (no-go) observed throughout adolescence and young adulthood occurs at a slower rate in those who have been exposed to early sexual assault. Importantly, these effects remained significant after accounting for parental history of AUD and participants’ substance use (intercept differences were no longer significant when parental AUD was included in the model; see Table 3, Model 3). However, effects were no longer significant when aspects of impulsivity were included in the model, suggesting that impulsivity could have an important role in the relation of early sexual trauma and frontal theta development during response inhibition. In addition, change in frontal no-go theta trajectories was associated with AUD symptom count and INT psychopathology (depression, anxiety, and suicidal ideation) in young adulthood.
Associations of Trauma and No-Go Theta ERO
Gradual decreases in frontal theta oscillations during response inhibition across adolescence and young adulthood were observed in all study subjects. Previous developmental ERO studies76–78 have observed similar decreases in oscillatory power globally, likely reflecting synaptic pruning (ie, fewer, but more efficient, connections) that occurs rapidly during adolescence and continues through young adult- hood.79–82 This also could correspond with gray matter development and progressive maturing of the prefrontal cortex as it assumes greater control over neural processing throughout adolescence and young adulthood.83–85
Findings from the present study suggest that individuals exposed to sexual assaultive trauma before 10 years have atypical developmental trajectories of frontal theta oscillations during response inhibition; the decrease in frontal theta power throughout adolescent and young adult development occurs at a slightly slower rate. This perhaps suggests that children exposed to early sexual assault might have atypical frontal cortical development that might be characterized by altered rates of synaptic pruning and gray matter production, which in turn could affect the development of top-down control over neural processing throughout adolescence and young adulthood. Research conducted in rodent models found that the enduring effects of early isolation and maternal separation on brain development could be a consequence of an arrested phase of synaptic overproduction.80 This is in agreement with previous studies in humans, which found maturational delay in cortical development associated with severe early life stress.39–41,46,48,50,52,75,86–90
Further support comes from studies showing an association among childhood sexual abuse, cognitive deficits, and increased behavioral disinhibition.20,35,91 In the present study, individuals exposed to early trauma also displayed higher rates of impulsivity as measured by the BIS and Zuckerman’s SSS. Interestingly, statistically significant differences in impulsivity and sensation seeking were most pronounced in those who had experienced sexual assault. When these measures of impulsivity were considered in the association of early sexual trauma and trajectories of frontal no-go theta power, effects of early sexual trauma were no longer statistically significant. There are at least 2 possible explanations for this. Impulsivity could mediate the relation of early sexual trauma and frontal no-go theta development. Alternatively, impulsivity could be a shared risk factor for early trauma exposure and atypical neurodevelopment. Thus, participants in this study who had experienced sexual assault before 10 years showed atypical trajectories of frontal no-go theta power (possibly delayed frontal cortical maturation and synaptic pruning in neural circuits involved in response inhibition) and heightened levels of impulsivity and sensation seeking (ie, behavioral disinhibition). There also is the possibility that frontal no-go theta activity might mediate the relation between early sexual abuse and impulsivity; the timing of the assessment of trauma exposure, impulsivity (BIS and SSS), and no-go frontal theta oscillation preclude the testing of this hypothesized mediation model in the present study. Future studies are needed to disentangle the influence of behavioral aspects of impulsivity with frontal theta oscillatory activity during response inhibition in the context of trauma exposure.
Results from the present study also indicated that sexual trauma-related change in frontal no-go theta trajectories influenced risk for young adult AUD symptom count and INT psychopathology, but not CUD symptom count or EXT psychopathology. Taken together, these findings support the hypothesis that early sexual trauma exposure might influence the risk for psychopathology (ie, depression, anxiety, suicidal ideation, or AUDs), in part through neurodevelopmental mechanisms. However, future longitudinal studies are needed to further characterize the potential moderating and/or mediating effects of neurodevelopmental trajectories in the associations of early trauma and later psychopathology. More research is needed to examine other aspects of neural functioning during response inhibition and other aspects of stress-reactivity, including executive control and reward processing.
Interestingly, non-sexual assaultive trauma and non-assaultive trauma exposure before 10 years were not associated with developmental trajectories of theta ERO. This could indicate that although exposure to these traumas clearly has adverse mental and physical health consequences, exposure to early sexual abuse might be a particularly potent risk factor for neurocognitive development, behavioral disinhibition, and subsequent INT and alcohol use pathology. This is in agreement with prior evidence that interpersonal assaultive events have a stronger and more enduring effect on substance use and psychopathology than non-assaultive events.60,62 In addition, GNG behavioral data (ie, go and no-go accuracy and go reaction time on the GNG task) did not differ among participants exposed to trauma (Table S4, available online). This is in agreement with previous work71,92,93 that reported that differences in neural oscillations during task performance (eg, no-go frontal ERO) can be observed even when behavioral differences are not (eg, no performance errors), suggesting that one major strength of ERO data is detection of extremely subtle effects occurring at the neural level, which have important implications for neurocognitive functioning and risk for psychopathology. However, it should be noted that no-go frontal ERO and performance on the GNG task are significantly correlated—suggesting that frontal theta ERO is relevant to task performance, although this is not reflected in a statistically significant behavioral difference among the trauma exposure groups. In the context of the present study, the atypical frontal ERO during the no-go task observed in individuals who were exposed to trauma could be a subtle index of risk for psychopathology and suggests less efficient neural processing during response inhibition, necessitating the use of alternate neural strategies to effectively inhibit their responses in the GNG task. Also of note is the effect of parental history of AUD on the associations of trauma exposure and no-go frontal theta ERO. Given previous evidence that decreased no-go frontal theta ERO is observed in individuals with a family history of AUD, this suggests that the association of sexual assaultive trauma exposure before 10 years with a slower rate of change in developmental trajectories of frontal oscillations during response inhibition (no-go frontal theta power) across adolescence and young adulthood remains after accounting for mean level differences in no-go frontal theta power due to familial risk for AUD. Future studies should investigate the extent of these findings in individuals with a family history of AUD and in community control families.
These findings should be considered in light of several caveats. First, the sample consists of offspring primarily from high-risk, densely AUD-affected families, and as such findings might not be generalizable to other populations. Second, although data across multiple waves of assessment were included in the analyses, some individuals who might have eventually developed AUD, CUD, or INT or EXT problems are treated in this study as unaffected. Third, effects of maternal AUD present in 45.3% of the analytic sample could reflect in part in utero exposure to alcohol (which is unknown for most offspring), which can affect neurodevelopment. Fourth, the present study did not have information on the frequency or duration of specific traumatic exposures. Fifth, given the relatively small number of participants meeting criteria for posttraumatic stress disorder in this sample, posttraumatic stress disorder was not incorporated into the present study. Sixth, attrition of the sample owing to participants who did not return for follow-up assessments could have affected the present study’s findings. A nonresponse analysis indicated that individuals who did not return for follow-up were younger (p < .001) and were more likely to have had a diagnosis of alcohol dependence (p < .001), and that fewer non-responders were exposed to assaultive (p < .001) and non-assaultive (p < .001) trauma and had a diagnosis of cannabis dependence (p < .001); no differences regarding gender, race/ethnicity, impulsivity, sexual trauma exposure, or ERO values were observed. In light of the absence of attrition effects for the primary findings for sexual trauma exposure and ERO power, we believe that inferences made in this report are likely to be sound. Relatedly, decreased sample sizes available in follow-ups 4 and 5 might limit the statistical power of some complex models examined in this study, leading to the possibility of type I and II errors. Despite these limitations, this is the first study to our knowledge to examine associations of early trauma exposure, neurophysiologic developmental trajectories in adolescence and young adulthood, and risk for later psychopathology. This is particularly important because this is the peak age range for the onset of substance use and mental health-related problems and has been previously understudied. Further, information provided on clinical, behavioral, and familial influences enables characterization of neurobehavioral functioning in a relatively large and racially/ethnically diverse sample.
In conclusion, findings from the present study suggest sexual assaultive trauma exposure before 10 years of age is associated with a slower rate of change in developmental trajectories of frontal oscillations during response inhibition (no-go frontal theta power) across adolescence and young adulthood and increased levels of behavioral disinhibition. In addition, this atypical neurophysiologic development, which might reflect delays in frontal cortical maturation and synaptic pruning, was associated with young adult INT and alcohol use problems. Taken together, these findings support the hypothesis that changes in neural development related to early sexual trauma exposure could increase later risk for mental health problems. These findings highlight the importance of developing effective prevention strategies to decrease exposure to childhood sexual assault and to increase treatment after trauma exposure, because this early experience significantly increases the risk for a cascade of mental and physical health problems throughout the individual’s life course. Researchers, clinicians, and policy makers should build on ongoing work aimed at identifying interventions and therapeutic strategies to mitigate the risk associated with early sexual assaultive trauma exposure.
Supplementary Material
Acknowledgments
This research was funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA; U10AA008401 to Dr. Porjesz and K01AA024152 to Dr. Salvatore) and the National Institute on Drug Abuse (NIDA; K01DA027914 to Dr. Meyers).
Drs. Meyers, McCutcheon, Almasy, and Bucholz served as the statistical experts for this research.
The Collaborative Study on the Genetics of Alcoholism (COGA; principal investigators B. Porjesz, V. Hesselbrock, H. Edenberg, and L. Bierut) includes 11 different centers: University of Connecticut (V. Hesselbrock); Indiana University (H.J. Edenberg, J. Nurnberger Jr., T. Foroud); University of Iowa (S. Kuperman, J. Kramer); SUNY Downstate (B. Porjesz); Washington University in St. Louis (L. Bierut, J. Rice, K. Bucholz, A. Agrawal); University of California at San Diego (M. Schuckit); Rutgers University (J. Tischfield, A. Brooks); Department of Biomedical and Health Informatics, The Children’s Hospital of Philadelphia and Department of Genetics, Perelman School of Medicine, University of Pennsylvania, Philadelphia (L. Almasy), Virginia Commonwealth University (D. Dick), Icahn School of Medicine at Mount Sinai (A. Goate); and Howard University (R. Taylor). Other COGA collaborators include L. Bauer (University of Connecticut); J. McClintick, L. Wetherill, X. Xuei, Y. Liu, D. Lai, S. O’Connor, M. Plawecki, and S. Lourens (Indiana University); G. Chan (University of Iowa; University of Connecticut); J. Meyers, D. Chorlian, C. Kamarajan, A. Pandey, and J. Zhang (SUNY Downstate); J.-C. Wang, M. Kapoor, and S. Bertelsen (Icahn School of Medicine at Mount Sinai); A. Anokhin, V. McCutcheon, and S. Saccone (Washington University); J. Salvatore, F. Aliev, and B. Cho (Virginia Commonwealth University); Mark Kos (University of Texas Rio Grande Valley); and A. Parsian, the NIAAA Staff Collaborator.
The authors continue to be inspired bytheir memories of Henri Begleiter, PhD, and Theodore Reich, MD, founding principal investigator and co-principal investigator of the COGA, and owe a debt of gratitude to other past organizers of COGA, including Ting-Kai Li, MD, P. Michael Conneally, PhD, Raymond Crowe, PhD, and Wendy Reich, PhD, for their critical contributions. This national collaborative study is supported by NIH grant U10AA008401 from the NIAAA and the NIDA.
Footnotes
Disclosure: Dr. Kuperman has served as a researcher of a double-blind placebo-controlled trial for neurocrine. The medication is being tested as an alternative choice for adolescents with Tourette’s syndrome. His involvement consisted of providing medical monitoring for 2 adolescent boys and the study is completed. There is no overlap with this study. Dr. Bucholz reports that her spouse has a consulting relation with a medical device company and holds several patents, but these are not related to the present work. Drs. Meyers, McCutcheon, Pandey, Kamarajan, Salvatore, Pandey, Almasy, Anokhin, Bauer, Bender, Dick, Edenberg, Hesselbrock, Kramer, Agrawal, and Porjesz, Ms. Subbie, and Mr. Chorlian report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Jacquelyn Meyers, State University of New York Downstate Medical Center, Brooklyn, NY..
Vivia V. McCutcheon, Washington University School of Medicine, St. Louis, MO..
Ashwini K. Pandey, State University of New York Downstate Medical Center, Brooklyn, NY..
Chella Kamarajan, State University of New York Downstate Medical Center, Brooklyn, NY..
Stacey Subbie, State University of New York Downstate Medical Center, Brooklyn, NY..
David Chorlian, State University of New York Downstate Medical Center, Brooklyn, NY..
Jessica Salvatore, Virginia Commonwealth University, Richmond.; Virginia Institute of Psychiatric and Behavioral Genetics,Virginia Commonwealth University.
Gayathri Pandey, State University of New York Downstate Medical Center, Brooklyn, NY..
Laura Almasy, University of Pennsylvania, Philadelphia..
Andrey Anokhin, Washington University School of Medicine, St. Louis, MO..
Lance Bauer, University of Connecticut School of Medicine, Farmington..
Annah Bender, University of Missouri, St. Louis..
Danielle M. Dick, Virginia Commonwealth University, Richmond..
Howard J. Edenberg, Indiana University School of Medicine, Indianapolis..
Victor Hesselbrock, University of Connecticut School of Medicine, Farmington..
John Kramer, University of Iowa, Iowa City..
Samuel Kuperman, University of Iowa, Iowa City..
Arpana Agrawal, Washington University School of Medicine, St. Louis, MO..
Kathleen Bucholz, Washington University School of Medicine, St. Louis, MO..
Bernice Porjesz, State University of New York Downstate Medical Center, Brooklyn, NY..
REFERENCES
- 1.Costello EJ, Erkanli A, Fairbank JA, Angold A. The prevalence of potentially traumatic events in childhood and adolescence. J Trauma Stress. 2002;15:99–112. [DOI] [PubMed] [Google Scholar]
- 2.Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the Adverse Childhood Experiences Study. Pediatrics. 2003;111:564–572. [DOI] [PubMed] [Google Scholar]
- 3.Afifi TO, Henriksen CA, Asmundson GJG, Sareen J. Childhood maltreatment and substance use disorders among men and women in a nationally representative sample. Can J Psychiatry. 2012;57:677–686. [DOI] [PubMed] [Google Scholar]
- 4.Werner KB, Grant JD, McCutcheon VV, et al. Differences in childhood physical abuse reporting and the association between CPA and alcohol use disorder in European American and African American women. Psychol Addict Behav. 2016;30: 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enoch M-A. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl). 2011;214:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbins TW, Ersche KD, Everitt BJ. Drug Addiction and the Memory Systems of the Brain. Ann N Y Acad Sci. 2008;1141:1–21. [DOI] [PubMed] [Google Scholar]
- 7.Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. [DOI] [PubMed] [Google Scholar]
- 8.Hanson JL, Chung MK, Avants BB, et al. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J Neurosci. 2010;30:7466–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dannlowski U, Stuhrmann A, Beutelmann V, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71:286–293. [DOI] [PubMed] [Google Scholar]
- 10.Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci U S A. 2012;109:E563–E572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Bellis MD, Zisk A. The biological effects of childhood trauma. Child Adolesc Psychiatr Clin North Am. 2014;23:185–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J Int Neuropsychol Soc. 2001;7:312–322. [DOI] [PubMed] [Google Scholar]
- 14.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. [DOI] [PubMed] [Google Scholar]
- 15.Kelly AMC, Di Martino A, Uddin LQ, et al. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19: 640–657. [DOI] [PubMed] [Google Scholar]
- 16.Stevens MC. The developmental cognitive neuroscience of functional connectivity. Brain Cogn. 2009;70:1–12. [DOI] [PubMed] [Google Scholar]
- 17.Herting MM, Gautam P, Spielberg JM, Dahl RE, Sowell ER. A longitudinal study: changes in cortical thickness and surface area during pubertal maturation. PLoS One. 2015;10:e0119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chechik G, Meilijson I, Ruppin E. Neuronal regulation: a mechanism for synaptic pruning during brain maturation. Neural Comput. 1999;11:2061–2080. [DOI] [PubMed] [Google Scholar]
- 19.McCrory E, De Brito SA, Viding E. The impact of childhood maltreatment: a review of neurobiological and genetic factors. Front Psychiatry. 2011;2:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hart H, Rubia K. Neuroimaging of child abuse: a critical review. Front Hum Neurosci. 2012;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLaughlin KA, Sheridan MA, Alves S, Mendes WB. Child maltreatment and autonomic nervous system reactivity. Psychosom Med. 2014;76:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lobo I, Portugal LC, Figueira I, et al. EEG correlates of the severity of posttraumatic stress symptoms: a systematic review of the dimensional PTSD literature. J Affect Disord. 2015;183:210–220. [DOI] [PubMed] [Google Scholar]
- 23.Thomason ME, Marusak HA. Toward understanding the impact of trauma on the early developing human brain. Neuroscience. 2017;342:55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mueller SC, Maheu FS, Dozier M, et al. Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia. 2010;48: 3037–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl). 2011;214:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim L, Hart H, Mehta MA, Simmons A, Mirza K, Rubia K. Neural correlates of error processing in young people with a history of severe childhood abuse: an fMRI study. Am J Psychiatry. 2015;172:892–900. [DOI] [PubMed] [Google Scholar]
- 27.Marusak HA, Martin KR, Etkin A, Thomason ME. Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology. 2015;40: 1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bokura H, Yamaguchi S, Kobayashi S. Electrophysiological correlates for response inhibition in a go/no-go task. Clin Neurophysiol. 2001;112:2224–2232. [DOI] [PubMed] [Google Scholar]
- 29.Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annu Rev Psychol. 2007;58:1–23. [DOI] [PubMed] [Google Scholar]
- 30.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. [DOI] [PubMed] [Google Scholar]
- 31.Posner MI, Sheese BE, Odludaş Y, Tang Y. Analyzing and shaping human attentional networks. Neural Networks. 2006;19:1422–1429. [DOI] [PubMed] [Google Scholar]
- 32.Coull JT. Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol. 1998;55:343–361. [DOI] [PubMed] [Google Scholar]
- 33.Coull JT, Frith CD, Frackowiak RS, Grasby PM. A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia. 1996;34:1085–1095. [DOI] [PubMed] [Google Scholar]
- 34.Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Dev Psychol. 1999;35:205–213. [DOI] [PubMed] [Google Scholar]
- 35.Anda RF, Felitti VJ, Bremner JD, et al. The enduring effects of abuse and related adverse experiences in childhood. Eur Arch Psychiatry Clin Neurosci. 2006;256:174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carrion VG, Garrett A, Menon V, Weems CF, Reiss AL. Posttraumatic stress symptoms and brain function during a response-inhibition task: an fMRI study in youth. Depress Anxiety. 2008;25:514–526. [DOI] [PubMed] [Google Scholar]
- 37.Mueller SC, Maheu FS, Dozier M, et al. Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia. 2010;48: 3037–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howells FM, Stein DJ, Russell VA. Childhood trauma is associated with altered cortical arousal: insights from an EEG study. Front Integr Neurosci. 2012;6:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otero GA, Pliego-Rivero FB, Fernández T, Ricardo J. EEG development in children with sociocultural disadvantages: a follow-up study. Clin Neurophysiol. 2003;114: 1918–1925. [DOI] [PubMed] [Google Scholar]
- 40.McLaughlin KA, Fox NA, Zeanah CH, Sheridan MA, Marshall P, Nelson CA. Delayed maturation in brain electrical activity partially explains the association between early environmental deprivation and symptoms of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;68:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLaughlin KA, Fox NA, Zeanah CH, Nelson CA. Adverse rearing environments and neural development in children: the development of frontal electroencephalogram asymmetry. Biol Psychiatry. 2011;70:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Başar E, Başar-Eroğlu C, Karakas S, Schürmann M. Brain oscillations in perception and memory. Int J Psychophysiol. 2000;35:95–124. [DOI] [PubMed] [Google Scholar]
- 43.Basar E, Basar-Eroglu C, Karakas S, Schürmann M. Oscillatory brain theory: a new trend in neuroscience. IEEE Eng Med Biol Mag. 1999;18:56–66. [DOI] [PubMed] [Google Scholar]
- 44.Başar E, Femir B, Emek-Savaş DD, Güntekin B, Yener GG. Increased long distance event-related gamma band connectivity in Alzheimer’s disease. NeuroImage Clin. 2017; 14:580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson CA, McCleery JP. Use of event-related potentials in the study of typical and atypical development. J Am Acad Child Adolesc Psychiatry. 2008;47:1252–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raine A, Venables PH, Dalais C, Mellingen K, Reynolds C, Mednick SA. Early educational and health enrichment at age 3–5 years is associated with increased autonomic and central nervous system arousal and orienting at age 11 years: evidence from the Mauritius Child Health Project. Psychophysiology. 2001;38:254–266. [PubMed] [Google Scholar]
- 47.Barry RJ, Clarke AR, McCarthy R, Selikowitz M, Johnstone SJ, Rushby JA. Age and gender effects in EEG coherence: I. Developmental trends in normal children. Clin Neurophysiol. 2004;115:2252–2258. [DOI] [PubMed] [Google Scholar]
- 48.Marshall PJ, Fox NA. Bucharest Early Intervention Project Core Group. A comparison of the electroencephalogram between institutionalized and community children in Romania. J Cogn Neurosci. 2004;16:1327–1338. [DOI] [PubMed] [Google Scholar]
- 49.Slopen N, McLaughlin KA, Fox NA, Zeanah CH, Nelson CA. Alterations in neural processing and psychopathology in children raised in institutions. Arch Gen Psychiatry. 2012;69:1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stamoulis C, Vanderwert RE, Zeanah CH, Fox NA, Nelson CA. Early psychosocial neglect adversely impacts developmental trajectories of brain oscillations and their interactions. J Cogn Neurosci. 2015;27:2512–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vanderwert RE, Marshall PJ, Nelson CA, Zeanah CH, Fox NA. Timing of intervention affects brain electrical activity in children exposed to severe psychosocial neglect. PLoS One. 2010;5:e11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vanderwert RE, Zeanah CH, Fox NA, Nelson CA. Normalization of EEG activity among previously institutionalized children placed into foster care: a 12-year follow-up of the Bucharest Early Intervention Project. Dev Cogn Neurosci. 2016;17:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stamoulis C, Vanderwert RE, Zeanah CH, Fox NA, Nelson CA. Neuronal networks in the developing brain are adversely modulated by early psychosocial neglect. J Neurophysiol. 2017;118:2275–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pandey AK, Kamarajan C, Manz N, Chorlian DB, Stimus A, Porjesz B. Delta, theta, and alpha event-related oscillations in alcoholics during go/no-go task: Neurocognitive deficits in execution, inhibition, and attention processing. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pandey AK, Kamarajan C, Tang Y, et al. Neurocognitive deficits in male alcoholics: an ERP/sLORETA analysis of the N2 component in an equal probability go/no-go task. Biol Psychol. 2012;89:170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bucholz KK, McCutcheon VV, Agrawal A, et al. Comparison of parent, peer, psychiatric, and cannabis use influences across stages of offspring alcohol involvement: evidence from the COGA prospective study. Alcohol Clin Exp Res. 2017;41:359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bucholz KK, Cadoret R, Cloninger CR, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. [DOI] [PubMed] [Google Scholar]
- 58.Kuperman S, Chan G, Kramer JR, et al. A model to determine the likely age of an adolescent’s first drink of alcohol. Pediatrics. 2013;131:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCutcheon VV, Agrawal A, Kuo SI-C, et al. Associations of parental alcohol use disorders and parental separation with offspring initiation of alcohol, cigarette and cannabis use and sexual debut in high-risk families. Addiction. 2018;113:336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Werner KB, McCutcheon VV, Agrawal A, et al. The association of specific traumatic experiences with cannabis initiation and transition to problem use: differences between African-American and European-American women. Drug Alcohol Depend. 2016;162: 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sartor CE, Lynskey MT, Heath AC, Jacob T, True W. The role of childhood risk factors in initiation of alcohol use and progression to alcohol dependence. Addiction. 2007;102: 216–225. [DOI] [PubMed] [Google Scholar]
- 62.Breslau N, Peterson EL. Assaultive violence and the risk of posttraumatic stress disorder following a subsequent trauma. Behav Res Ther. 2010;48:1063–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kristjansson S, McCutcheon VV, Agrawal A, et al. The variance shared across forms of childhood trauma is strongly associated with liability for psychiatric and substance use disorders. Brain Behav. 2016;6:e00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heim C, Binder EB. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol. 2012;233:102–111. [DOI] [PubMed] [Google Scholar]
- 65.McLaughlin KA, Peverill M, Gold AL, Alves S, Sheridan MA. Child maltreatment and neural systems underlying emotion regulation. J Am Acad Child Adolesc Psychiatry. 2015;54:753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kessler RC, Aguilar-Gaxiola S, Alonso J, et al. The associations of earlier trauma exposures and history of mental disorders with PTSD after subsequent traumas. Mol Psychiatry. 2018;23:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rice JP, Reich T, Bucholz KK, et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. [DOI] [PubMed] [Google Scholar]
- 68.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–774. [DOI] [PubMed] [Google Scholar]
- 69.Zuckerman M, Neeb M. Sensation seeking and psychopathology. Psychiatry Res. 1979; 1:255–264. [DOI] [PubMed] [Google Scholar]
- 70.Pandey AK, Kamarajan C, Tang Y, et al. Neurocognitive deficits in male alcoholics: an ERP/sLORETA analysis of the N2 component in an equal probability go/no-go task. Biol Psychol. 2012;89:170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pandey AK, Kamarajan C, Manz N, Chorlian DB, Stimus A, Porjesz B. Delta, theta, and alpha event-related oscillations in alcoholics during go/no-go task: neurocognitive deficits in execution, inhibition, and attention processing. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones KA, Porjesz B, Chorlian D, et al. S-transform time-frequency analysis of P300 reveals deficits in individuals diagnosed with alcoholism. Clin Neurophysiol. 2006;117: 2128–2143. [DOI] [PubMed] [Google Scholar]
- 73.Hanson JL, Chung MK, Avants BB, et al. Early Stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J Neurosci. 2010;30:7466–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McLaughlin KA, Fox NA, Zeanah CH, Sheridan MA, Marshall P, Nelson CA. Delayed maturation in brain electrical activity partially explains the association between early environmental deprivation and symptoms of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;68:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Slopen N, McLaughlin KA, Fox NA, Zeanah CH, Nelson CA. Alterations in neural processing and psychopathology in children raised in institutions. Arch Gen Psychiatry. 2012;69:1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yordanova J, Kolev V. Developmental changes in the event-related EEG theta response and P300. Electroencephalogr Clin Neurophysiol. 1997;104:418–430. [DOI] [PubMed] [Google Scholar]
- 77.Yordanova J, Kolev V, Heinrich H, Woerner W, Banaschewski T, Rothenberger A. Developmental event-related gamma oscillations: effects of auditory attention. Eur J Neurosci. 2002;16:2214–2224. [DOI] [PubMed] [Google Scholar]
- 78.Yordanova J, Kolev V. Event-related brain oscillations. J Psychophysiol. 2009;23: 174–182. [Google Scholar]
- 79.Petanjek Z, Judaš M, Šimic G, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108:13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–1993. [DOI] [PubMed] [Google Scholar]
- 81.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP. Developmental neurobiology of childhood stress and trauma. Psychiatr Clin North Am. 2002;25: 397–426. vii-viii. [DOI] [PubMed] [Google Scholar]
- 82.Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci USA. 2012;109:E563–E572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gogtay N, Sporn A, Clasen LS, et al. Comparison of progressive cortical gray matter loss in childhood-onset schizophrenia with that in childhood-onset atypical psychoses. Arch Gen Psychiatry. 2004;61:17. [DOI] [PubMed] [Google Scholar]
- 84.Gogtay N, Thompson PM. Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain Cogn. 2010; 72:6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Segalowitz SJ, Santesso DL, Jetha MK. Electrophysiological changes during adolescence: a review. Brain Cogn. 2010;72:86–100. [DOI] [PubMed] [Google Scholar]
- 86.Barry RJ, Rushby JA, Johnstone SJ, Clarke AR, Croft RJ, Lawrence CA. Event-related potentials in the auditory oddball as a function of EEG alpha phase at stimulus onset. Clin Neurophysiol. 2004;115:2593–2601. [DOI] [PubMed] [Google Scholar]
- 87.Nelson CA, Bos K, Gunnar MR, Sonuga-Barke EJSV. The neurobiological toll of early human deprivation. Monogr Soc Res Child Dev. 2011;76:127–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McDermott JM, Troller-Renfree S, Vanderwert R, Nelson CA, Zeanah CH, Fox NA. Psychosocial deprivation, executive functions, and the emergence of socio-emotional behavior problems. Front Hum Neurosci. 2013;7:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vanderwert RE, Westerlund A, Montoya L, McCormick SA, Miguel HO, Nelson CA . Looking to the eyes influences the processing of emotion on facesensitive event-related potentials in 7-month-old infants. Dev Neurobiol. 2015; 75:1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. [DOI] [PubMed] [Google Scholar]
- 91.Trickett PK, Noll JG, Putnam FW. The impact of sexual abuse on female development: lessons from a multigenerational, longitudinal research study. Dev Psychopathol. 2011; 23:453–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcohol Clin Exp Res. 1999;23:1125–1135. [DOI] [PubMed] [Google Scholar]
- 93.Kamarajan C, Porjesz B, Jones K, et al. Event-related oscillations in offspring of alcoholics: neurocognitive disinhibition as a risk for alcoholism. Biol Psychiatry. 2006;59:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.