Abstract
Background:
Ukraine, a country of 45.5 million people, has one of the most volatile HIV and HCV epidemics in the world. In this paper, we estimate the prevalence of HIV and HCV among PWID in five Ukrainian cities.
Methods:
A cross-sectional study was conducted in 2014-2015, based on stratified hybrid sampling with random and respondent driven sampling in 5 cities: Kyiv, Odesa, Mykolaiv, Dnipro and Lviv. Using data on HIV and HCV antibody testing from 1,613 respondents, we evaluate selection bias in the sampling methods by analyzing spatial and network patterns of sampling processes. We develop and apply inverse probability weights in order to estimate the HIV and HCV prevalence in each city, as well as in the overall sample.
Findings:
The aggregate HIV prevalence for the five cities is 35.1% (95% CI: 29.5%-38.5%) but this varied considerably by city: in Kyiv the HIV prevalence is 26.6% (95% CI: 20.3.8%-33.4%), in Odesa - 38.2% (95% CI: 29.8% and 47.1%), in Mykolaiv - 42.0% (95% CI: 34.3%-49.2%), in Dnipro - 58.8% (95% CI: 52.2%- 65.8%), and in Lviv 24.6% (95% CI: 18.8%-30.8%). The aggregate HCV prevalence estimate for the five cities is 58.6% (95% CI: 54.9%-61.7%). The highest HCV prevalence is estimated in Kyiv – 84.8% (95% CI: 78.5%-90.1%).HCV prevalence in Odesa is the lowest and estimated to be 36.5% (95% CI: 29.5%-45.1%), in Mykolaiv - 49.1% (95% CI: 41.5%- 57.0%), in Dnipro - 56.1% (95% CI: 50.3-63.4) and in Lviv 38.5% (95% CI: 31.8%- 45.0%).
Conclusions:
Monitoring behavioral and health outcomes of PWID on a regular basis is necessary for determining prevention and treatment priorities for HIV and HCV infections in Ukraine and elsewhere. The heterogeneity of the local epidemics provides insights into the best prevention and treatment strategies to be deployed in low-resource settings.
Keywords: HIV, HCV, prevalence, PWID, Ukraine, respondent-driven sampling, sample selection bias
Background
Ukraine, a country of 45.5 million people, has one of the most volatile HIV and HCV epidemics in the world. While HIV transmission and mortality have decreased globally, the HIV epidemic in Ukraine continues to grow (Booth, et al., 2016; UNAIDS, 2017). Ukraine’s harsh criminalization of drug use favors incarceration over rehabilitation, which further contributes to ongoing HIV and HCV transmission (Altice, et al., 2016). Stigma, discrimination, and fear of criminal prosecution contribute to the marginalization of people who inject drugs (PWID), many of whom refuse or underuse addiction treatment and harm reduction programs (A. Mazhnaya, Bojko, MJ, Marcus, R, Filippovych, S, Islam, Z, Dvoriak, S, Altice, FL, 2016). The hidden nature of PWID poses significant challenges to estimating the size of the population and disease prevalence among this group (Semaana, et al., 2009). Among the estimated 340,000 PWID (172,000-520,000) in Ukraine, the HIV prevalence is approximately 19.1% (16.2-22.2), while the estimated HCV prevalence is 53.9% (49.2-58.7) (Balakirieva, et al., 2014; Barska & Sazanova, 2016; L. Degenhardt, et al., 2017). In 2016, the HCV prevalence among PWID was estimated to be 56% based on a sample of 8,512 PWID who were prescribed opioid agonist therapies (OAT) with methadone or buprenorphine in a healthcare setting (Alliance for Public Health, 2016). Data from treatment facilities, however, may not be fully representative of all PWID, the majority (approximately 97%) of whom do not receive OAT.
Both HIV and HCV prevalence throughout Ukraine, however, is variable and potentially related to regional micro-epidemics that require further exploration (Zaller, et al., 2015).The HIV prevalence estimates between different geographical locations range from 3% to 50%, while the HCV prevalence ranges from 8.8% to 60.1% (Balakirieva, et al., 2014). Regional variation may, however, also reflect selection biases inherent in the method of sampling, rendering accurate comparisons across time within geographical areas highly problematic. For example, the mean of the square deviation of HIV prevalence within cities from 2009 to 2015 is as high as 40%, indicating a lack of consistent estimates across time. (Balakirieva, et al., 2014; G. Berleva, Sazonova, Y, 2017). The data for HCV estimates among PWID are even more limited than for HIV, and exhibit a similar pattern of variation within cities. City-level HCV prevalence estimates collected in 2013 through RDS are an order of magnitude higher than HCV estimates collected in the same set of cities in 2011, despite the similarity of sampling methods used (Balakirieva, et al., 2014). Given the variability of the estimates due to sampling biases, the overall objective of this study is to assess the performance of the sampling methods and to estimate HIV and HCV prevalence among PWID in five Ukrainian cities using a combination of random sampling and RDS. In the process of estimation, we aim to identify and correct for sample selection bias.
Methods
Setting
The HIV and HCV epidemics in Ukraine are concentrated in PWID, with 56% of new HIV infections in Ukraine attributable to drug injection (Ministry of Health of Ukraine, 2010; UNAIDS, 2010). In 2015, there were over 292,000 registered HIV cases, while over 1.2 million people are estimated to be infected with HCV (Ministry of Health of Ukraine, 2014, 2015); there is no HCV registration program nationally. Initially, in the post-Soviet era, the HIV prevention and treatment efforts focused on the introduction of needle and syringe exchange (∣NSP) and expanded gradually to incorporate Opiod agonist therapies (OAT) for treating opioid use disorder. OAT with buprenorphine were first introduced in Ukraine in 2004 (Bruce, et al., 2007; Lawrinson, et al., 2008), followed by methadone maintenance treatment in 2008 (Schaub, et al., 2010), and was prioritized for HIV-positive patients. Despite the reported effectiveness of OAT, scale-up in Ukraine remains limited (Bojko, et al., 2015; L. Degenhardt, et al., 2014; Mazhnaya A, 2015; A. Mazhnaya, Bojko, MJ, Marcus, R, Filippovych, S, Islam, Z, Dvoriak, S, Altice, FL, 2016), as less than 3% of PWID are currently receiving OAT treatment.
Study Design and Data
From January 2014 to March 2015, self-administered surveys with linked HIV and HCV testing was conducted in five large cities: Kyiv (Kiev), Odesa (Odessa), Mykolaiv (Nikolaev), Dnipro (Dnepropetrovsk), and Lviv (Lvov). Recruitment occurred sequentially (approximately 60–90 days per city) between 2014 and 2015. The sample was stratified by three groups of PWID: 1) currently on OAT; 2) previously on OAT; and 3) never on OAT. The overall objective of the study was to understand drug use behavior, health outcomes, risk factors, and barriers and facilitators of accessing OAT treatment among PWID in Ukraine.
The eligibility criteria and sampling methods have been previously described (Kutsa, 2016). Briefly, participants were over the age of 18 and met ICD-10 criteria for opioid dependence. Study participants who were currently or previously on OAT were randomly selected from client lists obtained from OAT sites in each city and invited by outreach workers to participate in the study. Respondent Driven Sampling (RDS) was used primarily to recruit clients who had never been on OAT, but also included some clients prescribed it. RDS seeds were selected based on criteria that included residential location, age, injection duration, and gender. After completing the online survey, all respondents underwent point-of-care HCV and HIV testing by medical personnel, with post-test counseling. Participants were paid 100 UAH (~US$4-10) for completing the survey, and earned 20 UAH (~US$1-2) for up to 3 recruited PWID peers (Salganik & Heckathorn, 2004). The study was approved at Institutional Review boards at Yale University and the Gromashevskiy Institute at the National Academy of Medical Sciences. Additional details on the study design and implementation are contained in Section A of the online Supplementary Appendix.
Study Definitions
The primary outcomes, HIV and HCV, were assessed using rapid tests (CITO TEST HIV 1/2/0, Pharmasco and CITO TEST HCV, Pharmasco) based on the presence of disease antibodies. The testing was administered by professionally-trained medical staff. Participation in OAT was confirmed based on review of clinical records in the case of the random sampling, while in the RDS, the frequency and type of OAT prescribed was gathered by self-report. The population size statistics for the OAT groups were taken directly from the administrative records of OAT programs, as well as based on medical records, while population size estimates of PWID who had no OAT experience were based on previously published research (G. Berleva, et al., 2012). Network size for each RDS participant was defined in terms of the number of PWID each person reported knowing personally in his/her city by name or nickname, who also knew the respondent by his/her name. In addition, each respondent was asked to name an intersection of streets closest to his or her residential locations, which were geocoded into latitude and longitude coordinates and used in the spatial analysis.
Statistical Methods
Overview of Analytical Strategy
Traditional probability-based sampling, including stratified random sampling, has a crucial advantage over non-probability-based methods, such as snowball sampling and RDS. This is because the former increases the likelihood of obtaining samples that are representative of the target population due to individuals’ having an equal probability of being chosen for the sample, usually by chance. If random probability-based sampling is carried out independently in each stratum, the prevalence of infectious diseases for each city can be estimated as a weighted average of the disease prevalence in each city’s strata, where the weights are equal to the share of the population in each strata, following the conventional stratified sampling approach (Cochran, 1977; Seber, 2013). In the case of non-random sampling, the estimator based on the weighted average of the prevalence in each strata is likely to be biased. Due to the hidden nature of the PWID population, random selection has proven to be challenging and RDS has emerged as an effective sampling method for PWID recruitment (Malekinejad, et al., 2008; Salganik & Heckathorn, 2004; Volz, 2008). Many researchers, however, have raised concerns regarding RDS, as a chain referral method that starts from a convenient sample and suffers from selection bias that may not disappear as the sample grows in size (Erickson, 1979; Heimer, 2005; Zelenev, et al., 2016). Given the shortcomings of RDS, we adopted a hybrid sampling approach in our study. We divided the population into strata based on OAT experience and used a combination of random sampling along with RDS to collect the survey data. Stratification based on OAT experience ensured that the individuals who participated in OAT would enter the sample, as they represent less than 5% of PWID in the population and have a high risk of being excluded by RDS. We assess the performance of the RDS sampling method by comparing RDS to the random sampling method, and we develop a sample-selection-bias correction using inverse probability weights. The novelty of the method is the effort to develop a new type of probability weight that combines random sampling, RDS, and total population size estimates. We evaluate the bias within the OAT strata, and, using the proposed correction, we estimate HIV and HCV prevalence in each city and for the entire sample.
Data Visualization
We compare spatial and temporal patterns of HIV and HCV prevalence in the RDS and random samples. First, we plot a series of cumulative sample prevalence estimates for each disease, HIV and HCV, in each city and for each method of sampling. The cumulative prevalence estimates are based on the proportion of HIV and HCV positive individuals recruited over time, and provide means to identify a lack of convergence, as well as examine the dependence among observations in the data over time (Bazazi, 2015; Gile, 2015). In addition, we create several visual diagrams to display geographical and network variability in the data and examine the patterns of clustering among observations. We used ArcGIS software to map the residential location of disease clusters in both RDS and random samples, and we use the package igraph in R to produce network plots of the disease among RDS respondents (ESRI, 2011; Gabor, 2015).
Spatial Statistical Methods
In order to identify the possible sources of selection bias due to recruitment within networks and residential locations, we compare the spatial properties between RDS and random sampling methods. We compute Ripley’s K statistic, which is related to the variance of the point process and measures the expected number of points within a specified distance of an observed event (Ripley, 1977). We assess the significance of the difference in Ripley’s K functions between random and RDS samples using a Monte Carlo procedure suggested by Diggle and Chetwynd, in which we randomly reshuffled points between the two sampling groups and plotted the boundary of the difference in K-functions (P. Diggle & Chetwynd, 1991). Significant differences between the sampling processes would indicate differences in the amount of clustering between two processes over distances, comparing them not only to each other, but also to a complete spatially random process (CSR), simulated by a Poisson process (P. Diggle, Ribiero Jr, PJ, 2007). We then computed auto-spatial correlations of the two diseases for both RDS and random samples for each city using spatial probit models (LeSage, 2009; Zelenev, et al., 2016). In the spatial probit models, we condition on several variables that include age, gender, years of injection, previous heroin use, and network size. We varied the distance in the contiguity matrix, which is a matrix that is used to specify the relative spatial position in the spatial probit model. In addition to auto-spatial correlations, we also estimate auto-correlation of disease within the recruitment network of the RDS sample in each of the five cities. The analysis was implemented in R and Matlab (R Core Team, 2017; The MathWorks Inc).
Prevalence Estimation with a Selection-Bias Correction
To assess the magnitude of bias in the RDS, we take advantage of the overlapping strata based on OAT participation, which allows us to compare the disease distribution in the RDS directly to a random sample. The problem of selection bias, including in the RDS, can be reframed as a problem of missing data, in which the mechanism for the missingness is non-ignorable (Rubin, 1976). Inverse probability weighting (IPW), a method in which each point is weighted by the probability of being in the sample, was first proposed by Horvitz and Thomson in 1952 and has been widely used to correct for sample selection bias (Horvitz, 1952; Little, 1987; Wooldridge, 2002). For example, the relatively popular Volz-Heckathorn estimator is based on the idea of inverse probability weighting, in which the weights are proportional to the size of respondent’s network (Volz, 2008). Using data on the size of the population in the OAT strata, we can estimate probabilities of non-response for the OAT sample directly and use the estimated probabilities to construct weights and adjust the estimates for sample selection bias. The appendix contains the technical details describing the procedure used to construct the inverse probability weights (see Section C in the Supplementary Appendix). The 95% confidence interval for prevalence was estimated by the non-parametric bootstrap, in which the sampling probabilities were recalculated based on resampling with replacement from the different strata (Rao, 1992). In cities with very small OAT samples within RDS, confidence intervals were not computed due to insufficient number of observations, and sampling correction was applied based on the observed data.
Results
The total sample size was 1,613. Figure 1 presents the cumulative disease prevalence for each infection over time, for each city and each sampling method. Figure 2 presents geographic spatial and network patterns of participant residential locations in the RDS and random samples. Table 1 provides HIV and HCV prevalence estimates within sampled and non-sampled OAT strata, and contains derivations of sampling weights, which are used to calculate HIV and HCV prevalences for each city. The final estimates of disease by city are contained in Tables 2A and 2B.
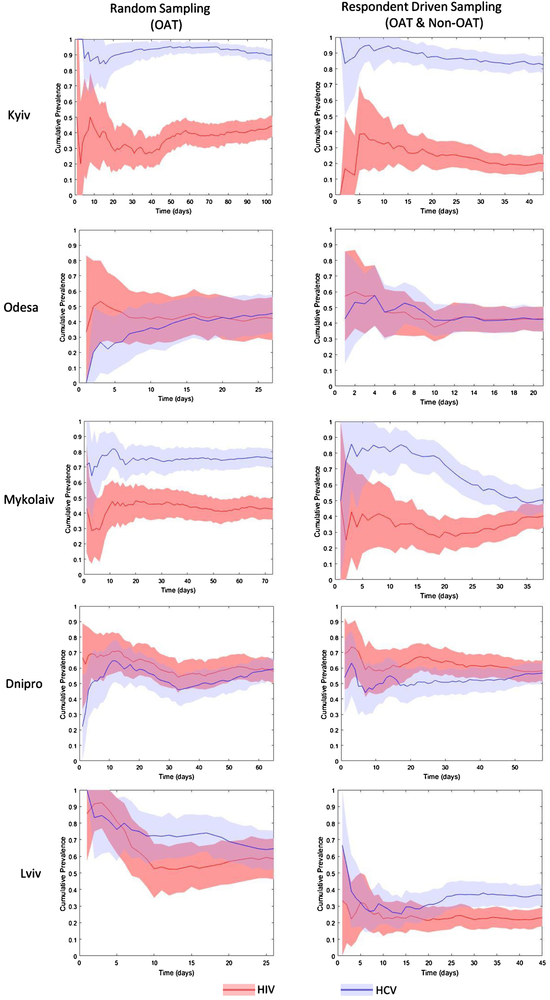
Figure 1: HIV and HCV Cumulative Prevalence by City and Sampling Method (N=1,613).
Note: Shaded regions represents 95% Confidence Interval estimated by nonparametric bootstrap. The cumulative prevalence is based on unweighted sample proportion of HIV and HCV positive individuals calculated over time leading up to each time point.
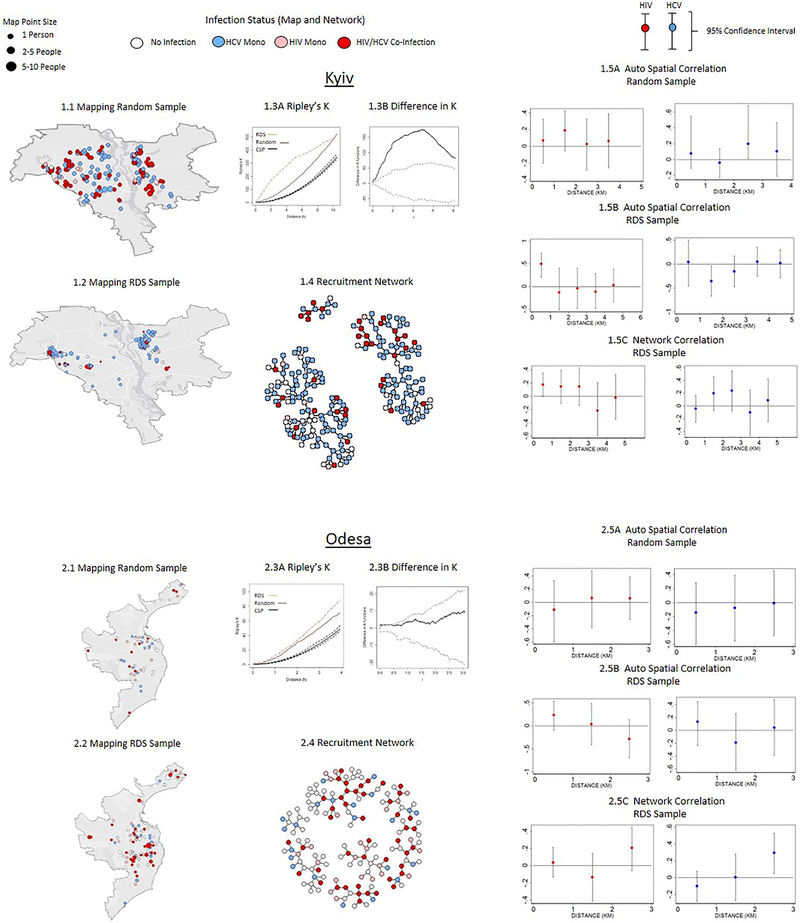
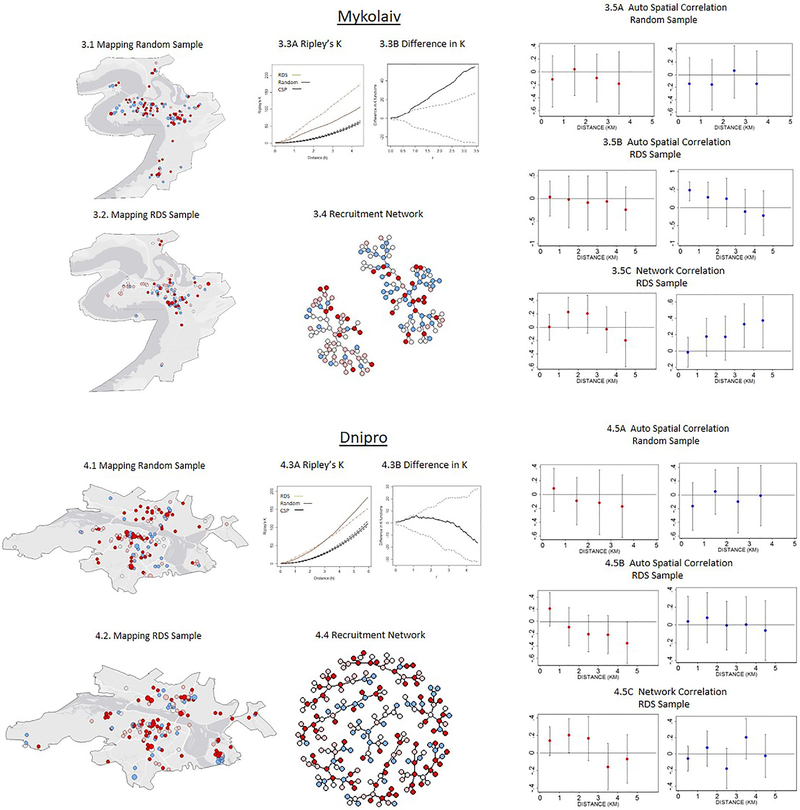
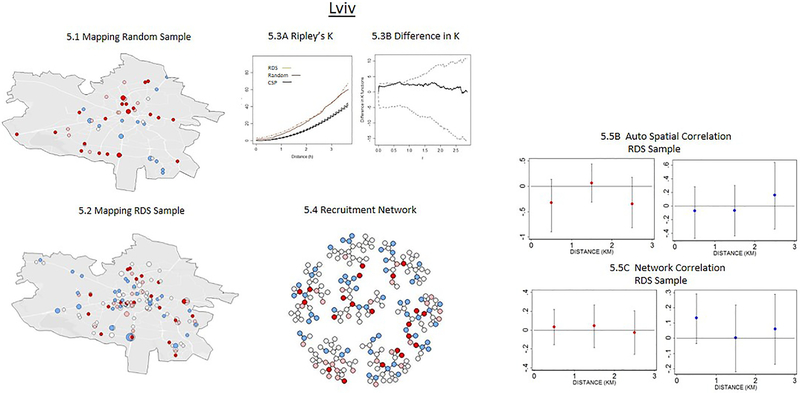
Figure 2:
Comparison of Spatial and Network Patterns in RDS and Random Sampling (N=1,613)
Table 1:
Prevalence of HIV and HCV infection in PWID within the Opioid Agonist Treatment (OAT) Group
| Location | Total | RandomSample | RDS SubSample | Not Sampled | Probability of Being Sampled Conditional on Disease |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| HIV Infection | N | N | % HIV+ | N | % HIV+ | N | % HIV+ | P(Obs ∣ HIV+) | P(Obs ∣ HIV−) | Risk Ratio |
| Kyiv | 1080 | 208 | 44.7 (38.1,51.2) |
44 | 13.6 (4.5,25.0) |
828 | 46.3 (39.1,53.2) |
0.205 | 0.256 | 0.80 |
| Odesa | 259 | 57 | 41.8 (28.1,54.8) |
9 | 66.7 (33.3,88.9) |
193 | 40.7 (27.5,54.5) |
0.275 | 0.240 | 1.15 |
| Mykolaiv | 894 | 190 | 42.8 (35.85 50.5) |
13 | 30.8 (7.7,57.7) |
691 | 43.1 (36.0,50.0) |
0.223 | 0.230 | 0.97 |
| Dnipro | 383 | 160 | 55.7 (44.4,58.6) |
5 | 54.8 NA |
218 | 56.1 (46.8,64.4) |
0.431 | 0.431 | 1.00 |
| Lviv | 157 | 65 | 55.9 (46.6,64.1) |
3 | 33.33 NA |
89 | 56.7 (44.9,68.6) |
0.425 | 0.443 | 0.96 |
| HCV Infection | N | N | % HCV+ | N | % HCV+ | N | % HCV+ | P(Obs ∣ HCV+) | P(Obs ∣ HCV−) | Risk Ratio |
| Kyiv | 1080 | 208 | 89.7 (84.2,93.2) |
44 | 81.8 (70.5,90.9) |
828 | 90.2 (85.6,94.7) |
0.230 | 0.265 | 1.15 |
| Odesa | 259 | 57 | 47.0 (34.3,60.5) |
9 | 77.7 (44.4,100) |
193 | 45.6 (32.4,58.9) |
0.278 | 0.235 | 0.85 |
| Mykolaiv | 894 | 190 | 75.8 (69.6,81.7) |
13 | 84.6 (61.5,100) |
691 | 75.6 (69.3,81.6) |
0.229 | 0.222 | 0.97 |
| Dnipro | 383 | 160 | 51.6 (43.5,59.8) |
5 | 60.0 NA |
218 | 51.4 (43.1,59. 6) |
0.433 | 0.429 | 0.99 |
| Lviv | 157 | 65 | 66.6 (55.8,77.8) |
3 | 33.3 NA |
89 | 67.7 (56.3,79.3) |
0.424 | 0.452 | 1.07 |
Note: The prevalence within OAT group is based on a weighted average of the proportion of HIV and HCV positive individuals in two strata: previously on OAT and currently on OAT. The total population estimates of OAT participation were obtained from the administrative records of OAT programs. Relative risk ratios are calculated by dividing probability of being observed conditional on testing positive for disease by probability of being observed conditional on testing negative for disease.
Table 2A :
HIV Prevalence among PWID in 5 cities: Weighted Estimates for OAT, non-OAT and Total Population. (N=1,613)
| Non-OAT | OAT | Total | |||
|---|---|---|---|---|---|
| Location | N | Prevalence (CI%) | N | Prevalence (CI%) | Prevalence (CI%) |
| Kyiv | 205 | 25.8 (18.7,33.5) |
208 | 44.7 (38.1, 51.2) |
26.6 (20.3,33.3) |
| Odesa | 158 | 38.2 (28.1, 48.1) |
57 | 41.8 (28.1, 54.8) |
38.2 (29.8, 47.1) |
| Mikolaiv | 154 | 41.9 (33.3, 50.4) |
190 | 42.8 (35.85 50.5) |
42.0 (34.3, 49.2) |
| Dnipro | 208 | 58.6 (52.5, 65.9) |
160 | 55.7 (44.4, 58.6) |
58.8 (52.2, 65.8) |
| Lviv | 208 | 23.7 (17.8 30.6) |
65 | 55.9 (46.6, 64.1) |
24.6 (18.8,30.8) |
| Total Estimate | 933 | 34.2 (28.9, 36.7) |
680 | 46.6 (42.2,51.1) |
35.1 (29.5,38.5) |
Table 2B:
HCV Prevalence among PWID in 5 cities: Weighted Estimates for OAT, non-OAT and Total Population. (N=1,613)
| Non-OAT | OAT | Total | |||
|---|---|---|---|---|---|
| Location | N | Prevalence (CI%) | N | Prevalence (CI%) | Prevalence (CI%) |
| Kyiv | 205 | 84.6 (78.1, 90.1) |
208 | 89.7 (84.2, 93.2) |
84.8 (78.5, 90.1) |
| Odesa | 158 | 36.2 (28.2, 45.1) |
57 | 47.0 (34.3, 60.5) |
36.5 (29.5, 45.1) |
| Mikolaiv | 154 | 46.7 (38.3, 55.4) |
190 | 75.8 (69.6, 81.7) |
49.1 (41.5, 57.0) |
| Dnipro | 208 | 56.4 (50.1,62.8) |
160 | 51.6 (43.5, 59.8) |
56.1 (50.3, 63.4) |
| Lviv | 208 | 38.1 (31.2, 45.3) |
65 | 66.6 (55.8, 77.8) |
38.5 (31.8, 45.0) |
| Total Estimate | 933 | 58.2 (55.3, 62.0) |
680 | 70.3 (66.2, 74.1) |
58.6 (54.9, 61.8) |
Convergence Plots: RDS vs Random Sampling
Generally, the HIV and HCV convergence plots appear to be relatively stable with some notable exceptions. The plots that indicate a probable lack of convergence include the HCV plots in the RDS sample in Mykolaiv, the HCV plots in the random sample in Dnipro, and the HCV plots in the random sample in Lviv. These plots have large fluctuations over time that do not appear to settle around a particular value (Figure 1). In Mykolaiv, the lack of convergence in the RDS data indicates that the respondents that arrived in the second half of the study had significantly lower HCV rates compared to respondents in the first half of the study. In Lviv, the large fluctuations over time in the random sample in HIV and HCV stem from the small sample size, as the random sampling proved to be more challenging to implement compared to the RDS. In other cities such as Kyiv, Odesa, and Lviv, the RDS samples have attained relative convergence in HIV and HCV prevalence rates; while in Dnipro there is not enough evidence to either corroborate or refute the convergence in HIV and HCV cumulative prevalence in the RDS sample.
Spatial and Network Patterns: RDS vs Random Sampling
The comparison of RDS and the random samples is presented in Figure 2 and suggests two distinct groups. In the first group, which consists of Odesa, Dnipro, and Lviv, the RDS and random samples are similar with respect to spatial and network patterns in three ways. First, the differences in the Ripley’s K statistic are not statistically significant between RDS and random samples, as indicated by the Diggle and Chetwynd simulated bounds within which the estimated difference line falls (Figures 2.3B, 3.3B & 5.3B). Similarities in Ripley’s K statistic suggest that RDS and random sampling processes have a similar clustering pattern over distance. Second, the HIV and HCV status of respondents are not significantly associated with the average HIV and HCV status of social peers and neighbors over distance in both the RDS and the random samples, as evident from the non-significant auto-correlation coefficient from the probit regressions (Figures 2.5A-2.5C; 3.5A-3.5C & 5.5A-5.5C). Third, RDS and the random sample penetrate similar physical areas, which can be seen from the geographical maps.
In the second group, which includes Kyiv and Mykolaiv, the RDS and random samples are significantly different with respect to the spatial and network patterns. First, in both Kyiv and Mykolaiv the RDS is much more highly concentrated spatially, as indicated by the higher values of Ripley’s K statistic. The differences in the Ripley’s K statistic are significantly different from one another, as evident from the estimated difference lying outside the Diggle and Chetwynd simulated bounds in Figures 1.3B & 4.3B. Second, in the random sample in both cities, the HIV and HCV infection statuses are not correlated between individuals over short distances, whereas in the RDS sample these are highly correlated either spatially or in the network (Figures 1.5 A & 4.5A). In Kyiv, for example, the HIV status of respondents is highly correlated with the average HIV status of friends and neighbors residing within close distances (<1km) who are recruited through the RDS, although this correlation declines and becomes non-significant as social and physical distance between respondents increases. In Mykolaiv, the HCV status of respondents is correlated with the average HCV status of neighbors residing in close distances (<1km); while the HCV status is not correlated with the average HCV status of social network peers recruited through RDS (Figures 1.5B, 1.5C, 3.5B, 3.5C). Third, RDS is confined in geographical range, penetrating fewer neighborhoods relative to the random sample. In Kyiv, more than 80% of the individuals recruited through RDS resided in 3 of the 10 neighboring districts, and some districts were excluded from the sampling. In the random sample, respondents from all 10 districts were present (Figures 2.1 and 2.2). Similar to Kyiv, the Mykolaiv RDS failed to reach several neighborhoods that the random sample was able to access, particularly in the South.
Comparison of Disease Prevalence and Sampling Rates among OAT Participants
The RDS’s performance in terms of sampling different risk groups varied among cities, and evidence for the presence of selection bias was found in several cases. The fraction of OAT participants in all five cities in the RDS sample was approximately 8% of the total RDS sample, whereas in the administrative data from the OAT programs, the estimated fraction of OAT participants is closer to 4%, and the differences are statistically significant when Fischer’s exact confidence bounds were calculated (not shown). Kyiv had the highest rate of oversampling of OAT participants, as 21% of the RDS sample indicated having experience with OAT. By contrast, the OAT participation rate gleaned from administrative data suggests 4% of the total PWID population in Kyiv participated in OAT. In other cities that include Dnipro, Mykolaiv, and Lviv, the rate of OAT participation in the RDS sample corresponded closely to the population rate, which was estimated from the administrative data, and the sample size was very small. In Odesa, the fraction of OAT participants in RDS was below the fraction of OAT participants estimated from the administrative data (1.5% vs 5.7%).
In most cities, with the exception of Kyiv, the HIV prevalence estimates based on the OAT subsample are indistinguishable from the estimates based on the random sample when uncertainty and sample size are taken into account (Table 1). In Kyiv, by contrast, the HIV prevalence among OAT participants was significantly different from the HIV prevalence among members of the random sample (13.6% vs 44.7%) at the 5% significance level. The difference appears to be due to a lower rate of sampling of HIV-positive individuals relative to HIV-negative individuals – the odds that a PWID with HIV is sampled is 20% lower than the odds that an HIV-negative person is sampled (Table 1). By contrast, in Odesa, the odds that HIV-positive individuals appear in the sample are 15% higher than the odds of HIV-negative individuals appearing, leading to an observed difference in estimated HIV prevalence rates (41.8% vs 66.7%). In other cities where the sampling rate was similar for HIV-positive and HIV-negative groups, as the relative risk proportions are close to unity, the difference in the HIV prevalence rates between random and RDS, were not statistically significant (Table 1).
In all five cities, the HCV prevalence estimates based on an RDS OAT subsample are indistinguishable from the estimates based on the random sample (Table 1). The largest differences in the sampling rates between HCV-positive and HCV-negative individuals in the RDS were observed in the Kyiv and Odesa samples, yet those differences did not affect the HCV prevalence estimates. In Kyiv, the HCV-positive individuals on OAT recruited through RDS, on average, were 15% more likely to appear in the sample than HCV-negative individuals on OAT. The HCV prevalence in the random and RDS samples among OAT participants were 81.8% and 89.7%, respectively, though not statistically significant. In Odesa, HCV-positive individuals were 15% less likely to appear in the sample than HCV-negative individuals, and the HCV prevalence in random and RDS samples were 47.0% and 77.7% respectively, though not statistically significant due to uncertainty from a small sample. In other cities, the odds ratio relating to the sampling probabilities conditional on HCV were close to unity, suggesting the conditional probabilities of being sampled were close to one another in the different disease groups.
The Estimates of HIV and HCV (with a Correction for Selection Bias)
Once we apply the bias correction, in the form of inverse probability weights based on the differential response within the OAT sample, the aggregate HIV prevalence for the five cities is estimated to be 35.1% (95% CI: 29.5%-38.5%) and varies considerably by city. The HIV prevalence estimate is highest in Dnipro - 58.8% (95% CI: 52.5%- 65.8%) and lowest in Kyiv - 26.6% (95% CI: 20.3%-33.3%) and Lviv - 24.6% (95% CI: 18.8%-30.8%). Estimates for Odesa - 38.2% (95% CI: 29.8% - 47.1%) and Mykolaiv - 42.0% (95% CI: 34.3%-49.2%) were in between these extremes.
The aggregate HCV prevalence estimate for the five cities is 58.6% (95% CI: 54.9%-61.8%). Estimated HCV prevalence is highest in Kyiv - 84.8% (95% CI: 78.5%-90.1%) and lowest in Lviv - 38.5% (95% CI: 31.8% - 45.0%). In Odesa, Mykolaiv, and Dnipro, the HCV prevalence is 36.5% (95% CI: 29.5%-45.1%), 49.1% (95% CI: 41.5%- 57.0%), and 56.1% (95% CI: 50.3%-63.4%), respectively.
Discussion
Our estimates for HIV and HCV prevalence using a different sampling frame and correction factors is 35.1% and 58.6%, respectively. In the case of HIV, the estimates here are higher than a 2014 country-wide study based on a national city-wide survey (19.1%) of PWID (Balakirieva, et al., 2014; L. Degenhardt, et al., 2017). When disaggregating the reported data from a 2014 survey, HIV prevalence was markedly different in the five cities we studied: Kyiv (14.9%), Odesa (30.2%), Mykolaiv (31.8%), Dnipro (31.2%), and Lviv (23.5%). In four cities (Kyiv, Odesa, Mykolaiv, and Dnipro), our point estimates for HIV prevalence were 10-20% higher compared to the 2014 multi-city survey. Our estimate for the HIV prevalence in Lviv, however, was identical to the estimate from the national multi-city survey. The largest differences between our estimates of HIV prevalence and the national survey are remarkable for Kyiv and Mykolaiv. According to the biennial multi-city surveys of PWID, HIV prevalence in Kyiv ranged from 15%-20% from 2013 to 2015, and 27%-31.8% in Mykolaiv, suggesting that these surveys may underestimate the “true” HIV prevalence in those two cities(Balakirieva, et al., 2014; Barska & Sazanova, 2016). Our estimates for HIV prevalence in Kyiv and Mykolaiv align more closely with estimations from previous years in the national survey
In the case of HIV, findings here have important implications for study designs that employ RDS and other convenience sampling schemes. For example, in Kyiv, we found some evidence of selection bias in the RDS sample relative to respondents randomly selected from OAT lists. The RDS respondents were more likely to participate in the OAT program compared to the overall population of PWID in Kyiv, but they were also more likely to be HIV-negative relative to the respondents that were drawn from the same HIV distribution within the OAT groups. Overall, the RDS sample in Kyiv was less representative than the sample in other cities: RDS was more likely to recruit PWID who resided in similar neighborhoods in a smaller geographic area, but also included respondents with HIV and HCV statuses that were similar to their network peers. In the case of Kyiv, the HIV convergence plot for RDS failed to indicate the presence of selection bias, suggesting the need for the development of a better diagnostic toolkit. In addition, RDS in Kyiv did not provide geographic diversity to the same extent that the random sampling method did, excluding neighborhoods from the sample that clearly had PWID residents. Kyiv is the capital of Ukraine and one of the most populous cities, with an estimated PWID population of more than 31,000, which is as high as the combined estimate of PWID population in Lviv, Dnipro, and Mykolaiv. An improvement in the RDS design would entail a strategy that accounts for the size of the population and geographical variability by selecting seeds that could reach a wider range of neighborhoods as well as selecting research sites that are accessible to residents from more neighborhoods. This finding is similar to those in Kuala Lumpur, Malaysia, where HIV prevalence in PWID differed markedly based on the neighborhood where recruitment occurred (Zelenev, et al., 2016). Compared to Kyiv, the selection bias in RDS samples was minimized more in the other cities sampled (Dolan, et al., 2016; Joint United Nations Programme on HIV/AIDS (UNAIDS), 2016). We found evidence that in Odesa, Dnipro, and Lviv, the RDS exhibited patterns that were similar to the patterns of the random sample, including similar levels of clustering, geographical coverage, and a lack of dependence of HIV or HCV status within a network and across geographical locations. Some of the similarities between our random and RDS samples may be due to the geographic compactness of smaller cities and a well-developed system of transportation that connects different parts of the cities. Although OAT samples were too small to conclude definitively, in Dnipro, Mykolaiv and Lviv, the OAT subsamples in the RDS and the random sample had similar levels of HIV and HCV prevalence. While RDS does not incorporate the sampling timeframe, using available statistics on the size of the population participating in harm reduction programs and OAT might help to understand and quantify possible selection biases. Using the hybrid sampling strategy in this analysis, we were able to develop a correction for the selection bias. When incorporating the corrective sampling weights, the HIV prevalence in Kyiv increased, while in other cities, such as Mykolaiv, Dnipro, and Lviv, where the selection bias was not as pronounced, the weights did not affect the city-level HIV and HCV estimates.
The high levels of HIV prevalence in the random and RDS samples, particularly in Odesa, Mykolaiv, and Dnipro, raise alarm about the possible convergence of HIV prevalence between OAT and non-OAT groups. This was not the case when OAT was introduced over a decade ago, when HIV-infected PWID were prioritized for OAT. The decline in the gap in HIV prevalence between OAT and non-OAT respondents underscores the importance of continued scale-up of OAT throughout Ukraine, as well as other evidence-based HIV prevention initiatives like needle and syringe programs (NSP) and antiretroviral therapy (ART). Mathematical modeling from Ukraine suggests that the combined scale-up of OAT and ART is the most cost-effective way to reduce new HIV infections and prevent transmission, with OAT scale-up alone as the most cost-effective (Alistar, et al., 2011). OAT coverage remains woefully inadequate throughout Ukraine, with fewer than 3% of PWID currently receiving treatment. In settings where HIV prevalence is highest, OAT scale-up would result in increased engagement in the HIV continuum of care as secondary prevention (Low, et al., 2016), but in cities where it remains lower, its benefit would be highest for primary prevention (Gowing, et al., 2013).
Our overall HCV prevalence estimate of 58.6% (95% CI: 54.9%-61.8%) ranges from 37%-85% in the five cities, and is comparable to that appearing in non-peer reviewed reports (mean=54.9%; range: 56%-73%) (Balakirieva, et al., 2014). HCV prevalence in this report was 77.7% in Kyiv, 63.3% in Odesa, 65.8% in Mykolaiv, 65.5% in Dnipro, and 58.1% in Lviv. In Kyiv our estimate is higher than the estimate published in the report, while in Odesa, Mykolaiv, Dnipro and Lviv, our HCV estimates are slightly lower. The sheer magnitude of the HCV epidemic among PWID in Ukraine is compounded by the lack of access to effective treatment and prevention. This is due to a number of structural barriers, including prohibitive treatment cost, restrictive protocols, stigma and discrimination in healthcare settings, amongst other issues (Wolfe, et al., 2015). HCV treatment using direct-acting antivirals (DAA) has recently been introduced but remains limited (A. Mazhnaya, et al., 2017). Effective scale-up of HCV treatment sufficient to reduce HCV transmission and incidence will likely require integrating such services into OAT services for PWID (Grebely, et al., 2015; Sylla, et al., 2007). Continued reduction in the costs of diagnostics and treatment, as well as increased availability of DAA will become crucial steps in improving the scale up of treatment and prevention efforts for HCV.
Despite the new findings presented, the study is not without limitations. First, sample size could bias our estimates and influence the bootstrap confidence intervals, resulting in a failure to capture the true variability in the HIV and HCV prevalence levels. Second, the sampling weight estimates were not found to be highly reliable in cities where the OAT strata was particularly small (such as Lviv and Dnipro). Third, HCV measures were based on antibody detection, and as such, overestimates true chronic viral infection. Fourth, inverse probability weighting is a powerful remedy against selection bias but may not be a panacea because it requires additional assumptions on the ratio of sampling rates that may render the estimator inconsistent if the assumption fails to hold. Fifth, though the inclusion criteria restricted our sample to opioid-dependent PWID and limits the generalizability to other PWID groups, this is partially mitigated by findings that nearly all PWID meet this criteria, even polysubstance users. Notwithstanding these limitations, however, the findings here introduce new methods for adjusting prevalence estimates based on the at-risk population and provide insights into prevention and treatment strategies for PWID with or at-risk for blood-borne viruses.
Conclusion
Monitoring behavioral and health outcomes of PWID on a regular basis is necessary for determining prevention and treatment priorities for HIV and HCV infections in Ukraine and elsewhere. This includes the sustained support for expanding evidence-based HIV and HCV prevention interventions for HIV, including OAT, NSP, and ART for PWID, as well as diagnostics and treatment for HCV. The heterogeneity of the local epidemics provides insights into the best prevention and treatment strategies to be deployed in low resource settings.
Supplementary Material
Acknowledgements and Source of Funding:
The authors would like to acknowledge the National Institute on Drug Abuse for funding for research (FLA: R01 DA029910, R01 DA033679) and career development (FLA: K24 DA017072, AZ: K01 DA037826), and the Global Health Equity Scholars Program funded by the Fogarty International Center and the National Institute of Allergy and Infectious Diseases (AM: Research Training Grant R25 TW009338).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All Authors declare that they do not have any conflict of interests.
References
- Alistar SS, Owens DK, & Brandeau ML (2011). Effectiveness and cost effectiveness of expanding harm reduction and antiretroviral therapy in a mixed HIV epidemic: a modeling analysis for Ukraine. Plos Medicine, 8, e1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliance for Public Health. (2016). Annual Report 2015. In. Kiev, Ukraine. [Google Scholar]
- Altice F, Azbel L, J S, Brooks-Pollock E, Smyrnov P, Dvoriak S, Taxman F, N E.-B. l., Martin N, Booth R, Stöver H, K D, & Vickerman P (2016). The perfect storm: incarceration and the high-risk environment perpetuating transmission of HIV, hepatitis C virus, and tuberculosis in Eastern Europe and Central Asia. Lancet, 388, 1228–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakirieva O, Bondar T, Loktieva I, Sazonova Y, & Sereda Y (2014). Summary of the analytical report: monitoring the behavior and HIV-infection prevalence among people who inject drugs as a component of the HIV second generation surveillance. Kiev: International HIV/AIDS Alliance in Ukraine. In: International HIV/AIDS Alliance in Ukraine. [Google Scholar]
- Barska J, & Sazanova Y (2016). Monitoring of Behavior and HIV Prevalence in People Who Inject Drugs and Their Sexual Partners in Ukraine, 2015. In. Kiev, Ukraine: Alliance for Public Health. [Google Scholar]
- Bazazi A, Crawford F, Zelenev A, Heimer R, Kamarulzaman A, Altice FL. (2015). HIV Prevalence Among People Who Inject Drugs in Greater Kuala Lumpur Recruited Using Respondent-Driven Sampling. Aids and Behavior, 19, 2347–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berleva G, Dumchev K, Kasianchuk M, Nikolko M, Saliuk T, Shvab I, & Yaremenko O (2012). Estimation of the Size of Populations Most-at-Risk for HIV Infection in Ukraine. In. Kiev, Ukraine: ICF International Alliance in Ukraine. [Google Scholar]
- Berleva G, Sazonova Y (2017). Estimation of the Size of Populations Most-at-Risk for HIV Infection in Ukraine. In. Kiev, Ukraine: IFC Alliance for Public Health in Ukraine. [Google Scholar]
- Bojko MJ, Mazhnaya A, Makarenko I, Marcus R, Dvoriak S, Islam Z, & Altice FL (2015). “Bureaucracy & Beliefs”: Assessing the barriers to accessing opioid substitution therapy by people who inject drugs in Ukraine. Drugs: education, prevention, and policy, 22, 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth R, Davis J, Dvoryak S, Brewster J, Lisovska O, Strathdee S, & Latkin C (2016). HIV incidence among people who inject drugs (PWIDs) in Ukraine: results from a clustered randomised trial. Lancet HIV, 3, 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce RD, Dvoryak S, Sylla L, & Altice FL (2007). HIV treatment access and scale-up for delivery of opiate substitution therapy with buprenorphine for IDUs in Ukraine--programme description and policy implications. International Journal of Drug Policy, 18, 326–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran W (1977). Sampling Techniques (3rd ed.). New York:: Wiley. [Google Scholar]
- Degenhardt L, Mathers BM, Wirtz AL, Wolfe D, Kamarulzaman A, Carrieri MP, Strathdee SA, Malinowska-Sempruch K, Kazatchkine M, & Beyrer C (2014). What has been achieved in HIV prevention, treatment and care for people who inject drugs, 2010-2012 ? A review of the six highest burden countries. International Journal of Drug Policy, 25, 53–60. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, Stone J, Cunningham EB, Trickey A, Dumchev K, Lynskey M, Griffiths P, Mattick R, Hickman M, & Larney S (2017). Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Global Health, 5, 1192–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle P, & Chetwynd A (1991). Second-order analysis of spatial clustering for inhomogeneous populations. Biometrics, 47, 1155–1163. [PubMed] [Google Scholar]
- Diggle P, Ribiero PJ Jr. (2007). Model-based Geostatistics. New York NY: Springer. [Google Scholar]
- Dolan K, Wirtz AL, Moazen B, Ndeffo-Mbah M, Galvani A, Kinner SA, Courtney R, McKee M, Amon JJ, Maher L, Hellard M, Beyrer C, & Altice FL (2016). Global burden of HIV, viral hepatitis, and tuberculosis in prisoners and detainees. Lancet, 388, 1089–1102. [DOI] [PubMed] [Google Scholar]
- Erickson B (1979). Some Problems of Inference from Chain Data. Sociological Methodology, 10, 276–302. [Google Scholar]
- ESRI. (2011). ArcGIS Desktop,. In (Vol. Release 10). Redlands, CA: Environmental Systems Research Institute. [Google Scholar]
- Gabor C, Nepusz T (2015). The igraph software package for complex network research. In. International Journal of Complex Systems. [Google Scholar]
- Gile K, Johnston LG, Salganik MJ. (2015). Diagnostics for Respodent Driven Sampling Journal of Royal Statistical Society A, 178, 241–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing LR, Hickman M, & Degenhardt L (2013). Mitigating the risk of HIV infection with opioid substitution treatment. Bull World Health Organ, 91, 148–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebely J, Bruggmann P, Treloar C, Byrne J, Rhodes T, & Dore GJ (2015). Expanding access to prevention, care and treatment for hepatitis C virus infection among people who inject drugs. International Journal of Drug Policy, 26, 893–898. [DOI] [PubMed] [Google Scholar]
- Heimer R (2005). Critical issues and further questions about respondent driven sampling: comment on Ramirez-Valles et al (2005). Aids and Behavior, 9, 403–408. [DOI] [PubMed] [Google Scholar]
- Horvitz D, Thompson D (1952). A Generalization of Sampling without Replacement from a Finite Population. Journal of the American Statistical Association, 47. [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS). (2016). Global AIDS Update 2016. In (pp. Accessed on May 28, 2016 at: http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016en.pdf). Geneva, Switzerland. [Google Scholar]
- Kutsa O, Marcus R, Bojko M, Zelenev A, Mazhnaya A, Dvoriak S, Filippovych S, Altice FL. (2016). Factors associated with physical and sexual violence by police among people who inject drugs in Ukraine: implications for retention on opioid agonist therapy. Journal of International Aids Society, 19, 20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrinson P, Ali R, Buavirat A, Chiamwongpaet S, Dvoryak S, Habrat B, Jie S, Mardiati R, Mokri A, Moskalewicz J, Newcombe D, Poznyak V, Subata E, Uchtenhagen A, Utami DS, Vial R, & Zhao C (2008). Key findings from the WHO collaborative study on substitution therapy for opioid dependence and HIV/AIDS. Addiction, 103, 1484–1492. [DOI] [PubMed] [Google Scholar]
- LeSage J, Pace RK (2009). Introduction to Spatial Econometrics Boca Raton, FL: CRC Press/Taylor & Francis Group, LLC. [Google Scholar]
- Little R, Rubin D (1987). Statistical Analysis with Missing Data (1st ed.). New York: John Wiley and Sons. [Google Scholar]
- Low AJ, Mburu G, Welton NJ, May MT, Davies CF, French C, Turner KM, Looker KJ, Christensen H, McLean S, Rhodes T, Platt L, Hickman M, Guise A, & Vickerman P (2016). Impact of Opioid Substitution Therapy on Antiretroviral Therapy Outcomes: A Systematic Review and Meta-Analysis. Clin Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekinejad M, Johnston LG, Kendall C, Kerr LRFS, Rifkin MR, & Rutherford GW (2008). Using Respondent-Driven Sampling Methodology for HIV Biological and Behavioral Surveillance in International Settings: A Systematic Review. Aids and Behavior, 12, 105–130. [DOI] [PubMed] [Google Scholar]
- Mazhnaya A, B. M., Marcus R, Filippovych S, Islam Z, Dvoriak S, et al. (2015). Breaking the cycle: Qualitative assessment of opioid-dependent people who inject drugs (PWIDs) in Ukraine. Drugs: Education, Prevention and Policy, In Press. [Google Scholar]
- Mazhnaya A, Bojko MJ, Marcus R, Filippovych S, Islam Z, Dvoriak S, Altice FL. (2016). In Their Own Voices: Breaking the Vicious Cycle of Addiction, Treatment and Criminal Justice Among People who Inject Drugs in Ukraine. Drugs (Abingdon, England), 23, 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazhnaya A, Meteliuk A, Barnard T, Zelenev A, Fillipovych S, & Altice FL (2017). Implementation and Scale-Up of HCV Treatment Services for People Who Inject Drugs in Ukraine. International J Drug Policy, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health of Ukraine. (2010). Ukraine: National Report on Monitoring Progress Towards the UNGASS Declaration of Commitment on HIV/AIDS. Reporting Period: January 2008–December 2009. In. Kiev. [Google Scholar]
- Ministry of Health of Ukraine. Immune Prevention of Hepatitis is the Most Efficient Way to Fight Against the Disease. Retrieved 7 March 2016. from http://moz.gov.ua/ua/portal/pre_20140728_d.html.
- Ministry of Health of Ukraine. (2015). Ukrainian Center for Socially Dangerous Diseases Control In HIV Infection In Ukraine Informational Bulletin (Vol. 43). Kiev: Gromashevsky Institute of Epidemiology and Infectious Diseases. [Google Scholar]
- R Core Team. (2017). R: A Language and Environment for Statistical Computing. In. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rao J, Wu CFJ, Yue K (1992). Some Recent Work On Resampling Methods For Complex Surveys. . Survey Methodology, 18, 209–217. [Google Scholar]
- Ripley B (1977). Modelling spatial patterns, . Journal of the Royal Statistical Society, Series B, 39, 172–192. [Google Scholar]
- Rubin D (1976). Inference and missing data. Biometrika, 63, 581–592. [Google Scholar]
- Salganik MJ, & Heckathorn DD (2004). Sampling and Estimation in Hidden Populations Using Respondent-Driven Sampling. Sociological Methodology, 34, 193–239. [Google Scholar]
- Schaub M, Chtenguelov V, Subata E, Weiler G, & Uchtenhagen A (2010). Feasibility of buprenorphine and methadone maintenance programmes among users of home made opioids in Ukraine. International Journal of Drug Policy, 21, 229–233. [DOI] [PubMed] [Google Scholar]
- Seber G, Salehi MM. (2013). Adaptive Sampling Designs Inference for Sparse and Clustered Populations. Heidelberg New York Dordrecht London: Springer. [Google Scholar]
- Semaana S, Santibanez S, Garfein R, Heckathorn D, & DesJarlais D (2009). Ethical and regulatory considerations in HIV prevention studies employing respondent-driven sampling. International Journal of Drug Policy, 20, 14–27. [DOI] [PubMed] [Google Scholar]
- Sylla L, Bruce RD, Kamarulzaman A, & Altice FL (2007). Integration and co-location of HIV/AIDS, tuberculosis and drug treatment services. Int J Drug Policy, 18, 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The MathWorks Inc. MATLAB Release 2015b,. In. Natick, Massachusetts, USA. [Google Scholar]
- UNAIDS. (2010). Global report: UNAIDS report on the global AIDS epidemic 2010. In. Geneva, Switzerland. [Google Scholar]
- UNAIDS. (2017). Ending AIDS: progress towards the 90-90-90 targets. . In. [Google Scholar]
- Volz E, Heckathorn DD. (2008). Probability based estimation theory for respondent driven sampling. . Journal of Official Statistics, 24, 79–97. [Google Scholar]
- Wolfe D, Luhmann N, Harris M, Momenghalibaf A, Albers E, Byrne J, & Swan T (2015). Human rights and access to hepatitis C treatment for people who inject drugs. International Journal of Drug Policy, 26, 1072–1080. [DOI] [PubMed] [Google Scholar]
- Wooldridge J (2002). Inverse probability weighted M-estimators for sample selection, attrition, and stratification. Portuguese Economic Journal, 1, 117–139. [Google Scholar]
- Zaller N, Mazhnaya A, Larney S, Islam Z, Shost A, Prokhorova T, Rybak N, & Flanigan T (2015). Geographic variability in HIV and injection drug use in Ukraine: implications for integration and expansion of drug treatment and HIV care. Int J Drug Policy, 26, 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenev A, Long E, Bazazi A, Kamarulzaman A, & FL A (2016). The complex interplay of social networks, geography and HIV risk among Malaysian Drug Injectors: Results from respondent-driven sampling. International Journal of Drug Policy, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.