Abstract
Given prior reports of adverse effects of cannabis use on working memory, an executive function with a protracted developmental course during adolescence, we examined associations between developmental patterns of cannabis use and adult working memory (WM) processes. Seventy-five adults with longitudinal assessments of cannabis use (60 with reported use, 15 with no reported use) and prenatal drug exposure assessment completed a spatial WM task during fMRI at age 28. All subjects passed a multi-drug urine screen on the day of testing and denied recreational drug use in the past week. A fast event-related design with partial trials was used to separate the BOLD response associated with encoding, maintenance, and retrieval periods of the WM task. Behavioral results showed that subjects who began using cannabis earlier in adolescence had longer reaction times (RT) than those with later initiation. Cannabis age of onset was further associated with reduced posterior parietal cortex (PPC) encoding BOLD activation, which significantly mediated age of onset WM RT associations. However, cannabis age of onset brain-behavior associations did not differ between groups with a single reported use and those with repeated use, suggesting age of onset effects may reflect substance use risk characteristics rather than a developmentally-timed cannabis exposure effect. Within repeated cannabis users, greater levels of total cannabis use were associated with performance-related increases in dorsolateral prefrontal cortex (DLPFC) activation during maintenance. This pattern of significant results remained unchanged with inclusion of demographic and prenatal measures as covariates. Surprisingly, however, at the group level, cannabis users generally performed better than participants who reported never using cannabis (faster RT, higher accuracy). We extend previous investigations by identifying that WM associations with cannabis age of onset may be primary to PPC stimulus encoding activity, while the amount of cannabis use is associated with DLPFC maintenance processes. Poorer performance of participants who reported never using cannabis and the consistency of cannabis age of onset associations across single and repeated users limit interpretation of direct developmental effects of cannabis on WM in adulthood.
Keywords: Cannabis, Working memory, Executive function, Substance use, Functional MRI, Development
Introduction
Marijuana and other forms of cannabis are the most widely used illicit drugs in the United States (Johnston et al, 2010). The first onset of cannabis use is typically during adolescence (Johnston et al., 2010), a period of normative functional brain development and the refinement of cognitive functions (Luna et al., 2010). However, the impact of adolescent cannabis exposure on brain systems supporting adult cognitive functioning remains largely unknown.
Cannabis use has been associated with deficits in working memory (WM), a core component of executive function that continues to develop through adolescence into adulthood (Luna et al., 2004). Dose-dependent effects of acute cannabis exposure have been associated with accuracy in delayed match to sample tasks (Lane et al., 2005) and regular cannabis users have been shown to make more spatial WM errors than non-regular users in the Cambridge Neuropsychological Test Automated Battery (CANTAB; Robbins et al., 1994; Harvey et al., 2007).
While the exact mechanisms of cannabis-induced cognitive impairment are still unknown, behavioral pharmacology indicates Δ9 THC (tetrahydrocannabinol), the primary psychoactive component of cannabis, induces memory impairments (Mallet and Beninger, 1998; Varvel et al., 2001). THC is a partial CB1 receptor agonist and THC-induced memory impairments are reversed by CB1 receptor antagonists (Mallet and Beninger, 1998; Varvel et al., 2001). CB1 receptors are highly expressed in several subcortical brain regions, including the cerebellum and basal ganglia (Herkenham et al., 1990), as well as cortical association areas (Tsou et al., 1998). Importantly, subcortical regions (Giedd et al., 1996) and lateral prefrontal (Ordaz et al., 2013) and posterior parietal cortices (PPC) (Olesen et al., 2003) undergo significant maturation during adolescence. Recent work has also shown CB1 levels in primate dorsolateral prefrontal cortex (DLPFC) continue to mature through adolescence (Eggan et al., 2009).
Given changes in the endocannabinoid system in developmentally sensitive regions, including the DLPFC and PPC, adolescents may be particularly sensitive to the effects of cannabis on WM and/or other executive functions that rely on WM. To this end, initial research has provided support for an association between early cannabis initiation and later cognitive differences, with studies demonstrating both domaingeneral (e.g., IQ) and domain-specific (e.g., visual attention) effects. For example, early cannabis initiation (e.g., prior to 17-years-old) (Pope et al., 2003), as well as persistent cannabis use in adolescence (Meier et al., 2012), has been associated with lower adult IQ. However, other work suggests the effect of cannabis age of onset, as a continuous variable (Ehrenreich et al., 1999), or when comparing those who initiated prior to and following 16-years-old (Becker et al., 2010), may be specific to increased response times but not accuracy measures in executive function tasks. Increased response times may be due to primary differences in cognitive processing speed (Varma et al., 1988) or visual attention (Ehrenreich et al., 1999; Jacobsen et al., 2004) that are associated with adolescent cannabis onset.
Previous fMRI studies have shown associations between early cannabis onset and BOLD activation in key regions of executive function and WM, including DLPFC (Chang et al., 2006; Tapert et al., 2007), PPC (Becker et al., 2010; Schweinsburg et al., 2008; Tapert et al., 2007), and anterior cingulate cortex (Gruber et al., 2012). However, previous investigations have examined cannabis associations in the context of a general-process WM activation, where the BOLD signal is not specific to different periods of the task (cue, delay, response) and therefore not representative of subprocesses of WM (encoding, maintenance, retrieval). Moreover, no studies have examined brain-behavior relationships in the context of cannabis age of onset BOLD activation differences. Together, these limitations make it difficult to assess whether observed BOLD differences support domain-general or domain-specific age of onset cognitive associations and whether reported BOLD activation differences are relevant to behavior.
An additional complexity of examining cannabis age of onset effects arises from the association between age of onset and cumulative cannabis exposure, where those with early initiation accumulate greater total cannabis use (cf., Ehrenreich et al., 1999). Total cannabis use has been shown to predict cognitive performance (Solowij et al., 2002) and may thus partially explain previously reported cannabis age of onset WM associations. However, an exposure effect of early cannabis use predicts cannabis age of onset associations to be dose-dependent, where any lasting effect of early use would be larger for those adolescents who consume greater amounts of cannabis. The absence of dose-dependent age of onset effects might suggest differences attributed to early cannabis use are driven by baseline differences in neurocognitive function (cf., Jackson et al., 2016).
The primary aim of the present study was to characterize the association between adolescent cannabis use and brain systems supporting WM in adulthood. Importantly, utilizing a sample with longitudinal cannabis use assessments, we examined associations of cannabis age of onset and total cannabis usage with WM performance and investigated how these are associated with distinct patterns of cortical engagement during WM encoding, maintenance, and retrieval. Further, through comparisons with non-users we examined the extent to which observed cannabis associations supported poorer neurocognitive outcomes in adulthood. Consistent with the reviewed literature (cf., Ehrenreich et al., 1999; Becker et al., 2010), we hypothesized cannabis age of onset would be associated with WM reaction time, while total cannabis use would be associated with a more general decrease in WM performance. We also hypothesized cannabis effects would be observed in developmentally sensitive, DLPFC and PPC regions associated with WM performance. However, to fully characterize cannabis use associations and WM brain- behavior relationships, we performed full-brain analyses.
Methods and materials
Participants
Participants were drawn from the Maternal Health Practices and Child Development Project (MHPCD), a longitudinal, prospective cohort study of the effects of prenatal drug exposure on children from low-income families in Pittsburgh, PA (cf., Day et al., 1994). From this larger cohort, 86 subjects were eligible based upon exclusion criteria and consented (see below) and 75 subjects successfully completed neuroimaging protocols at age 28. Reasons for unsuccessful neuroimaging protocols were claustrophobia during acquisition (n = 3), follow-up report of metal in the body (n = 1), positive pregnancy test (n = 1), THC positive urine screen on the day of neuroimaging (n = 2), current psychiatric medication (n = 2), and non-compliance (n = 2). Study exclusion criteria included childhood (age 10) IQ scores below 80, current psychiatric disorder (assessed with the Mini International Neuropsychiatric Interview; Sheehan et al., 1998) or current psychiatric medication, past head injury with loss of consciousness, and MRI contraindications, including pregnancy, claustrophobia, and non-removable metal in the body. One subject only completed three of four fMRI runs and was consequently excluded from fMRI analysis. The Institutional Review Board at the University of Pittsburgh approved this study.
Measures
Cannabis use
The MHCPD substance use assessment procedure (detailed in Day et al., 2006; Goldschmidt et al, 2012) was designed to maximize honest and accurate substance use self-report. Cannabis use was assessed at previous assessment phases at 14-, 16-, and 22-years-old, and at the time of testing (28-years-old). At each visit, cannabis age of onset was obtained (“How old were you when you first tried marijuana?”). Quantity of cannabis use was assessed through self-report of the usual dose over the past year at each assessment phase. As in previous work from the MHCPD (Day et al., 1991; Sonon et al., 2015), all consumption was converted to a dose of marijuana joints based on THC estimates from previous literature (Gold, 1989). For example, reported hashish dose was counted as equivalent to three joints of marijuana; a blunt of was counted as four joints. Frequency of the usual quantity of use was also obtained over the past year at each assessment phase (every day, 3–4 times per week, 1–2 times per week, once per month, 6–11 times per year, 1–5 times per year, no use). As in previous work from the MHCPD, our primary dosage estimate combined frequency and quantity measures (the product of average frequency and quantity) to create an average daily dose of cannabis (joints per day) for each assessment period.
Based on age of onset and total cannabis use measures, subjects were distributed across three groups (Table 1): non-users (NU, n = 15), those who did not report cannabis use at any time point, cannabis experimenters (EXP, n = 14), those who reported an age of cannabis initiation but no other cannabis use, and repeated cannabis users (REP, n = 46), those who reported initiation and any additional cannabis use. No subjects reported use without age of initiation. If subject report of cannabis age of onset differed between assessment periods, the cannabis age of onset from the earliest assessment period was used, as it was more proximal to the onset time.
Table 1.
Participant characteristics and bivariate relationships with cannabis usage groups.
| Variable | Non-Users (NU) n = 15 |
Cannabis Experimenters (EXP) n = 14 |
Cannabis Repeated (REP) n = 46 |
p-value |
|---|---|---|---|---|
| Age (Years) | 28.16 | 28.14 | 28.22 | n.s. |
| (0.71) | (0.66) | (0.72) | ||
| Gender (n Female) | n = 13 | n = 7 | n = 27 | NU by EXP = .050 |
| NU by REP = .067 | ||||
| WISC-Full Scale IQ (Age 14) | 94.83 | 99.12 | 93.99 | n.s. |
| (12.05) | (16.57) | (13.32) | ||
| Highest Level of Education (Years) | 14.73 | 14.14 | 13.76 | n.s. |
| (2.40) | (1.79) | (1.58) | ||
| Family Income | 2185 | 2320 | 2216 | n.s. |
| (Age 16; Dollars/Month) | (1363) | (1364) | (1295) | |
| Cannabis Age of Onset (Years) | - | 17.18 | 15.14 | EXP > REP = .006 |
| - | (2.20) | (2.27) | ||
| Total Cannabis Use | - | - | 1.45 | - |
| (Total Joints Per Day) | (2.67) | |||
| Peak Cannabis Use | 1.14 | |||
| (Max joints per day 14-, 16-, 22-, 28-years-old) | (2.14) | |||
| Mean Cannabis Use | .367 | |||
| (Mean joints per day 14-, 16-, 22-, 28-years-old) | (.683) | |||
| Cannabis Use within Last Year | - | n = 0 | n = 15 | REP > EXP = .013 |
| (Any Use in Last Year) | ||||
| Total Alcohol Use | 0.58 | 2.31 | 3.30 | EXP > NU = .015 |
| (Total Drinks Per Day) | (0.70) | (2.63) | (3.95) | REP > NU = .0001 |
| Alcohol Use Within Last Year | 0.33 | 0.81 | 0.92 | EXP > NU = .037 |
| (Drinks Per Day) | (0.56) | (0.85) | (1.14) | REP > NU = .011 |
| Total Cigarette Use | 1.21 | 0.037 | 14.82 | NU > EXP = .044 |
| (Total Cigarettes Per Day) | (4.70) | (0.11) | (19.64) | REP > NU < .0001 |
| REP > EXP = .001 | ||||
| Cigarette Use Within Last Year | n = 1 | n = 0 | n = 21 | REP by NU = .001 |
| (Any Use in Last Year) | REP by EXP = .011 | |||
| Other Drug Usea | n = 0 | n = 2 | n = 12 | NU by REP = .027 |
| (Other Drug Use at Any Visit) | n = 0 | n = 0 | n = 1 | |
| (Other Drug Use in Last Year) | ||||
| Prenatal Cannabisb | 0.19 | 0.16 | 0.32 | n.s. |
| (Total Joints Per Day) | (0.64) | (0.33) | (0.75) | n.s. |
| (Any Exposure) | n = 4 | n = 6 | n = 15 | |
| Prenatal Alcoholb | 0.55 | 0.67 | 0.63 | n.s. |
| (Total Drinks Per Day) | (1.24) | (1.06) | (1.31) | n.s. |
| (Any Exposure) | n = 9 | n = 8 | n = 36 | |
| Prenatal Cigaretteb | 27.77 | 25.71 | 31.48 | n.s. |
| (Total Cigarettes Per Day) | (39.14) | (36.10) | (36.79) | n.s. |
| (Any Exposure) | n = 9 | n = 8 | n = 30 | |
Note. p-value, pairwise significant and trending differences (p < .10) from Welch’s unequal variance t-test (continuous variables), Mann-Whitney U (substance use measures), or Fisher’s exact test (categorical variables). Other drug use (at any visit) and cannabis and cigarette use within the year of testing were dichotomized into use and non-use groups, as they were relatively uncommon in the sample.
A total of 14 subjects reported drug use other than cannabis, alcohol, or cigarettes at any visit, including cocaine, LSD, and recreational use of prescription drugs. One subject reported other drug use in the last year (recreational use of opioid pain medication).
Prenatal measures were collected longitudinally across three assessment periods (1 per trimester) and summed to create total exposure measures.
In statistical analysis, cannabis age of onset was examined as a continuous variable within both cannabis groups (EXP and REP). Total cannabis use, measured as the sum of joints per day across assessments (14-, 16-, 22-, 28-years-old) and conceptualized as a general measure of cannabis use severity, was examined only in those with a reported cannabis dose (REP). Total cannabis use measures were log transformed to reduce the impact of the few high dose participants, evident in the strong positive skew of the total cannabis use distribution (Supplemental Fig. S1). On the day of testing, all subjects included in the current analysis (N = 75) denied recreational drug use of any kind during the past week and had a negative multi-drug urine screen (Uritox Medical, Toledo, OH, THC Threshold 50 ng/ml). Accordingly, we consider all subjects abstinent at testing (no recent use).
Demographics and participant characteristics
As detailed in previous work (Day et al., 1994, 2006), extensive participant demographics and characteristics were collected as part of MHCPD assessments. In the current work, we included measures associated with cannabis use risk in previous work in the MHCPD (e.g., Day et al., 2006) and other studies of adolescent cannabis use (e.g., Pope et al., 2003) (see Table 1). Measures examined as potential covariates included socioeconomic status (highest level of education, family income), IQ (WISC-III Full Scale; Wechsler, 1991), other substance use (alcohol and cigarette use), and prenatal drug exposure (cannabis, alcohol, and cigarette). In cases where measures were available from multiple assessment phases (e.g., IQ, family income), we selected the measure that was closest to the year of neuroimaging testing (age 28). For five subjects, the most recent IQ measurement (age 14) was missing but age 10 IQ scores were available for these subjects. Similarly, for seven subjects, the most recent family income data (age 16) were missing but age 10 family income data was available for these subjects. In both cases, data from these earlier assessments were included and adjusted based on regression models predicting the most recent assessment from the rest of the cohort and used in covariate models (IQ, Standford-Binet Intelligence Scale 4th Edition (Thorndike et al., 1986) age 10 predicting WISC at age 14, B = .986, t = 8.87, p < .0001; family income at age 10 predicting family income at age 16 (n = 7), B = .848, t = 8.68, p < .0001).
fMRI task design
Across four fMRI runs, participants performed 96 full trials of a Sternberg type spatial working memory task (see Fig. 1) with a 2 (load) x 2 (delay length) x 2 (cue validity) factorial design. The task included three epochs: a cue (encoding) epoch (1.5s) where subjects were shown to-be-remembered stimuli (one or three spatial locations), a delay (maintenance) epoch, where subjects maintained information (1.5 or 6s), and a target (retrieval) epoch, where subjects had to retrieve information and respond to whether a probe (four spatial locations) matched any of the cued information (<3s). An additional 48 partial trials with either the cue epoch alone (n = 24) or the cue and delay epochs but not the target epoch (n = 24) were included in order to estimate the hemodynamic response for each epoch (Ollinger et al., 2001). The task utilized a mixed block/event-related fMRI design with interleaved task blocks (102s) and fixation only periods (12s). Inter-trial intervals within task blocks were pseudo-randomized and ranged from 1.5s to 5.5s.
Fig. 1.
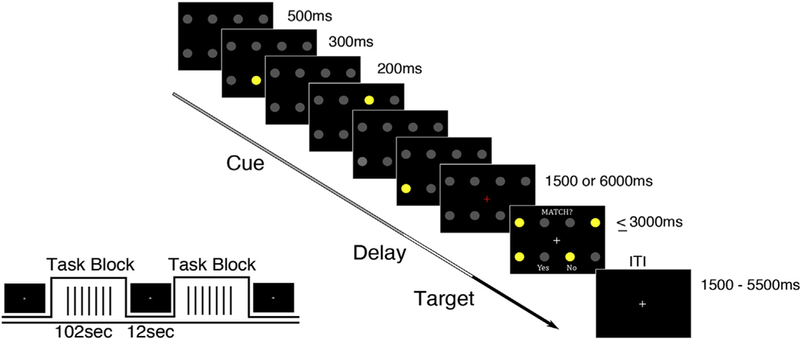
Working Memory Task. Three cues (yellow circles) were presented sequentially (300 ms presentation, 200 ms ISI) in one of eight possible locations (2 row X 4 column grid).
fMRI data acquisition
Stimuli were presented onto a screen behind the scanner using E- Prime software (Psychology Software Tools, Inc., Pittsburgh, PA) and were visible to the subject through a mirror mounted to the head coil. Reaction time in the working memory task was recorded as the time in milliseconds from the probe display (four yellow circles) to response (button press on MRI safe button box). Trials were marked correct if the button response (Pointer Finger = yes/match; Middle Finger = no/no match; right hand glove) correctly indicated whether or not one of the probes occupied a previously cued location. If no response was given after 3000 ms, the trial was “timed-out” and marked as an omission error.
Imaging data were collected using a 3.0-T Siemens Magnetom TIM Trio (Erlangen, Germany) at the Magnetic Resonance Research Center at the University of Pittsburgh. Structural images were collected using a magnetization prepared rapid acquistion gradient-echo (MP-rage) pulse sequence with a 192 slices (1 mm slice thickness; 1 mm isotropic voxels). Functional data were collected using an echo-planar imaging (EPI) sequence with the following parameters: TR = 2.0s,TE = 20ms, Flip Angle = 80°, and 128 × 120 acquisition matrix with a field of view of 220 mm. Thirty-three slices were collected in the axial plane with an anisotropic voxel size of 1.72 mm × 1.72 mm × 3 mm and a .75 mm gap between slices.
Standard techniques were used to preprocess the functional data and used the same pipeline as previous work from our group (cf., Paulsen et al., 2015). This included wavelet despiking, slice timing correction, motion correction (mcflirt; Jenkinson et al., 2002), brain extraction, non-linear registration of functional data to a standardized anatomical brain (MNI-152 template), spatial smoothing with FWHM of 4.25 mm (SUSAN; Smith and Brady, 1997), high pass filtering at 0.008 Hz, and rescaling to a 10,000 unit global median.
Statistical analysis
Selection of covariates
We tested bivariate associations between usage groups (NU, EXP, REP) and our primary variables of interest (cannabis age of onset, total cannabis use) and participant demographics and characteristics as reported in Tables 1 and 2. For associations with categorical usage groups (NU, EXP, REP), statistical testing utilized Welch’s unequal variance t-test (continuous variables), Mann-Whitney U (substance use measures), or Fisher’s exact test (categorical variables). For bivariate associations with continuous cannabis use measures (cannabis age of onset, total cannabis), statistical testing utilized Pearson correlation (continuous variables) and Welch’s unequal variance t-test (categorical variables). Any measures that had a significant or trending association (p < .10, uncorrected) to cannabis age of onset or total cannabis use and/or distinguished usage groups (Tables 1 and 2) were used as covariates in behavior and neuroimaging analysis according to the procedures outlined below.
Table 2.
Cannabis user (n = 60) characteristics and bivariate relationships with cannabis age of onset and total cannabis use.
| Variable | Cannabis Age of Onset | Total Cannabis Use (log) |
|---|---|---|
| Age (Years) | r = .101 | r = .124 |
| Sex | t = 0.05 | t = −0.87,s |
| (Male: 1 vs. Female: 0) | ||
| WISC-Full Scale IQ | r = .091 | r = −.162 |
| (Age 14) | ||
| Highest Level of Education (Years) | r = .370** | r = −.497** |
| Family Income | r = −.0002 | r = −.265+ |
| (Age 16; Dollars/Month) | ||
| Cannabis Age of Onset (Years) | - | r = −.236° |
| Cannabis Use within Last Year | t = 0.88 | t = 2.41*,s |
| (Any Use in Last Year) | ||
| Total Alcohol Use | r = −.057 | r = .323* |
| (Total Drinks Per Day) | ||
| Alcohol Use Within Last Year | r = −.175 | r = .203 |
| (Drinks-Per-Day) | ||
| Total Cigarette Use | r = −.432** | r = .319* |
| (Total Cigarettes Per Day) | ||
| Cigarette Use Within Last Year | t = −2.00+ | t = 2.37*,s |
| (Any Use in Last Year) | ||
| Other Drug Use | t = 4.17** | t = 3.87**,s |
| (Other Drug Use at Any Visit) | ||
| Prenatal Cannabis | r = −.110 | r = .162 |
| (Total Joints Per Day) | t = −0.12 | t = 2.51*,s |
| (Any Exposure) | ||
| Prenatal Alcohol | r = −.116 | r = 1.63s |
| (Total Drinks Per Day) | t = 1.04 | t=0.19s |
| (Any Exposure) | ||
| Prenatal Cigarette | r = .079 | r = −.117 |
| (Total Cigarettes Per Day) | t = 0.04 | t = −0.19s |
| (Any Exposure) | ||
Note. 0, p = .114,
p < .10,
p < .05.,
p < .01.
r - Pearson correlation. t - Welch’s unequal variance t-test.
sign flipped to represent higher values of total cannabis as positive value. Cannabis age of onset associations performed with full sample of cannabis users (EXP + REP, n = 60). Total Cannabis use (log of total joints per day) associations performed with only users with repeated use (REP, n = 46).
Based on this covariate testing procedure, for cannabis age of onset analyses, highest level of education, total cigarette use, cigarette use within the last year, other drug use, and cannabis group (EXP/REP) were used as covariates (covariate set A). For total cannabis use analysis, highest level of education, family income, cannabis age of onset (see below), cannabis use within the last year, total alcohol use, total cigarette use, cigarette use within the last year, and other drug used were used as covariates (covariate set B). One subject (cannabis age of onset = 12.33, REP) was missing highest level of education data and was therefore not included in covariate models with this measure. See Supplemental Table 1:4 for significance of covariates in primary cannabis age of onset and total cannabis use analyses. For comparison between usage groups (NU, EXP, REP), total alcohol use, alcohol use within the last year, total cigarette use, and cigarette use within the last year were used as covariates (covariate set C).
Of note, the NU group contained a small proportion of men (2 out of 15). Therefore, instead of model adjustment, secondary analyses were run including only women when comparing NU, REP, and EXP. Additionally, given the MHCPD assessment of prenatal exposure, which has been associated with IQ and WM (Day et al., 1994; Goldschmidt et al., 2008; Richardson et al., 2002), secondary analyses were also run with prenatal cannabis, alcohol, and cigarette exposure as covariates and moderators when examining both cannabis age of onset and total cannabis use (Supplemental Fig. S2). Furthermore, based on the reviewed literature (cf., Ehrenreich et al., 1999) and the differences in cannabis age of onset between EXP and REP (Table 1), we performed secondary analysis examining cannabis age of onset associations in the REP group while covarying total cannabis use. Finally, one subject (cannabis age of onset =13.75, REP) reported use of a drug other than cannabis, alcohol, or cigarettes during the year prior to testing (recreational use of opioid pain medication). The pattern of significant results was unchanged when excluding this subject.
WM behavior
Primary behavioral analysis was performed in R 3.1.2 (Team, 2014). Mixed-effects models (lme4 package; Bates et al., 2013) were used to examine main effects and interactions between task conditions and categorical (NU, EXP, REP) and continuous (age of onset, total cannabis use) cannabis measures. Accuracy data were analyzed using a generalized-linear mixed-effects model with a logit link function since trial level data were binomially distributed (correct vs. incorrect). Trials with omission errors (4.02% of all trials) were excluded from the accuracy analysis, where omissions were not counted toward the trial count (see Supplemental Tables 5 and 6 for omission error analysis). Reaction time data were log transformed to reduce heteroscedasticity of residuals and analyzed using a linear mixed-effects model including only correct trials. Both linear- and generalized-linear mixed-effects models were estimated with maximum likelihood. Random intercepts were estimated for each subject. Task conditions (load, delay length, cue validity), cannabis measures, and covariates were included as fixed effects in two phases: a baseline model, with only the task conditions and cannabis measure in question, and a full model with all potential confounding variables as covariates (see Tables 1 and 2). Significance values for fixed effects were obtained through the car package (chi-square test; Fox et al., 2016). Simple effects were obtained through the lsmeans package (Lenth and Hervé, 2015). Potential influential observations were examined using Cook’s distance and dfbetas (both cutoffs > 1) on mean performance measures. No subjects exceeded these thresholds.
WMfMRI
All imaging analysis was performed with Analysis and Visualization of Functional Neuroimages (AFNI, Bethesda, MD) software (Cox, 1996). In order to estimate the BOLD response at both the trial- and WM epoch-level (cue, delay, target), two level-1 GLM analyses were run for every subject using AFNI’s 3dDeconvolve tool.
GLM-1.
To estimate the average HRF response to the task, trial time courses for correct, incorrect, and partial trials were modeled using TENT basis functions spanning 28 s with 15 time steps. Due to the temporal properties of the HRF, conditions with different durations were modeled separately (1500 ms and 6000 ms delay trials). Six rigid-body head motion parameters and their derivatives, as well as run-wise 0 through 3rd order polynomials, were used as nuisance regressors. The current and preceding TR were censored if the Euclidean norm head motion distance surpassed 0.9 mm. This choice of censoring threshold was guided by work examining motion outliers in task-based fMRI (Siegel et al., 2014).
GLM-2.
To estimate the HRF to individual epochs of the task, a second model was run with individual regressors for cue, delay, and target epochs. Relevant partial trials were included as examples of the epoch in question to aid in epoch-specific HRF estimation. Cue epochs were modeled with a 1500 ms boxcar convolved with a gamma function (AFNI’s block 4) and scaled to have an amplitude of 1. Delay epochs were modeled with a single regressor composed of a 1500 ms (short delay trials) or 6000 ms (long delay trials) boxcar convolved with a gamma function. Target epochs were modeled using constant and parametric terms, each convolved with a gamma function. For each trial, reaction time determined the width of the constant term and the height of the parametric term (Grinband et al., 2008). The constant term was scaled to have an amplitude of 1. The same nuisance regressors and motion censoring were used as in GLM-1. Comparison of a reconstructed time series from GLM-2 and the observed time series of GLM-1 (Supplemental Fig. S3) suggested the epoch-based analysis of GLM-2 adequately captured primary amplitude components of the piecewise time series in GLM-1.
Voxelwise testing.
In order to detect potential subtle differences in the shape of the HRF as a function of cannabis measures, omnibus group effects were examined on correct trial time courses (GLM −1), entered into a voxel-wise multivariate model (3dMVM; Chen et al., 2015). TR (15 time points) and condition (1500 ms/6000 ms delay trials) were entered as within-subject effects and cannabis measures (age of onset or total use) were entered as between-subject effects. An interaction term between TR and the cannabis measure was used to identify voxels whose correct, trial-wise HRF significantly varied as a function of age of onset or total use. Importantly, this analysis was blind to the direction of the effect (F-test) and epoch amplitudes, such that differences as a function of a cannabis measure in any epoch could contribute to a trial-wise difference. Voxelwise testing was masked to only include voxels with a 50% or greater probability of being grey matter in the MNI-152 template and full EPI coverage in all subjects across all runs. Voxels were further constrained such that each had a main effect of TR (F-test), suggesting activation to the WM task significantly differed (positively or negatively) from baseline. Results were corrected for multiple comparisons using the intersection of voxelwise FDR correction (q < .05) and cluster size within the voxelwise space as defined above. Cluster size thresholds were determined through a Monte Carlo simulation using AFNI’s 3dClustSim program with mean spatial autocorrelation parameters estimated from GLM-1 residuals. This analysis specified that 16 or more contiguous (faces touching) voxels with a single voxel threshold of p = .0018 (q < .05) for cannabis age of onset and p = .0023 (q < .05) for total cannabis use were required to achieve corrected, cluster-level alphas of less than .05.
Epoch testing.
Post-hoc comparisons (using GLM-2) and brain-behavior analysis were performed on the mean activation within clusters with a significant cannabis by TR interaction (as defined above in GLM-1) for cue, delay, and target epochs. Significance values were Bonferroni corrected across epochs within each cluster. Covariates (Tables 1 and 2) were utilized in the same procedure as behavioral analysis (baseline and full models). Brain-behavior analyses were performed on mean performance measures (mean of log reaction time and mean accuracy). Reaction time models used linear regression. Accuracy models used beta regression in order to reduce the impact of mean accuracy distribution ceiling (Cribari-Neto and Zeileis, 2009). For all epoch testing, potential influential observations were removed if Cook’s distance or dfbetas exceeded 1 in either the post-hoc epoch or brain-behavior model. This never resulted in more than 2 subjects being removed.
Mediation analysis.
To examine whether differences in activation could account for behavioral differences, mediation analysis was performed on epoch amplitudes that had significant, corrected associations to 1) cannabis measures and 2) WM performance (while covarying the cannabis measure). Significance values for indirect effects were obtained using 5000 draws in a bootstrap procedure (mediation package; Tingley et al., 2014).
Results
WM behavior in cannabis users
Cannabis age of onset
In the groups that reported use (EXP, REP), cannabis age of onset was not a significant predictor of WM accuracy (baseline: z = 1.30, p = .193; full model (covariate set A): z = 0.52, p = .601) but showed a significant, negative relationship with WM RT in both baseline (, t = −2.99, p = .003) and full models ( t = −3.11, p = .002) (Fig. 2). Importantly, this relationship remained significant after adjusting for prenatal measures (full model + prenatal cannabis exposure: t = - 3.10, p = .002) and the association between cannabis age of onset and RT did not differ in exposed vs. unexposed groups ( t = −0.54, p = .589, Supplemental Fig. S2). Of the task conditions (load, delay length, cue validity), only WM load had a significant interaction with cannabis age of onset while predicting RT (full model: t = −2.19, p = .028). Post hoc testing revealed the association between cannabis age of onset and RT was significantly greater in the low load condition (t(69.27) = −3.22, p = .002) compared to the high load condition (t(70.10) = −2.54, p = .013).
Fig. 2.
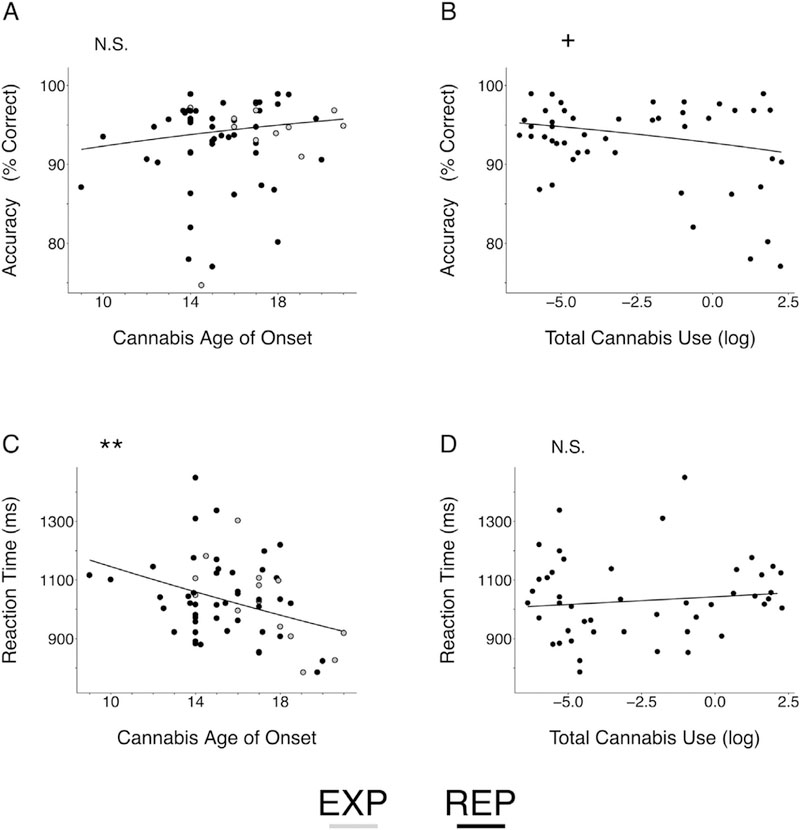
Behavioral Effects of Cannabis Age of Onset and Total Cannabis Use on Working Memory Performance. N.S. Non-significant, + p < .10, **p < .01. EXP, cannabis experimenters; REP, repeated cannabis users. Model estimates were back transformed from log (reaction time) and logit (accuracy) space for ease of interpretation. Visualized estimates are from intercept onlybaseline models. (A), Cannabis age of onsetwas not a significant predictor of WM accuracy (baseline: z = 1.30, p = .193; full model: z = 0.52, p = .601), but had a significant, negative relationship with WM RT (C; baseline: t = −2.99, p = .003; full model: t = −3.11, p = .002). In contrast, total cannabis use (log) was not a significant predictor of working memory RT (D; baseline: t = 0.88, p = .381; full model: t = 1.40, p = .162) but had a near significant, negative relationship with WM accuracy in the baseline model (B; z = −1.93, p = .054). However, this effect was no longer significant after adjusting for prenatal cannabis exposure (z = −1.41, p = .159).
An interaction between cannabis use group (EXP + REP) and cannabis age of onset in predicting WM RT did not reach significance (baseline: t = 1.75, p = .079; full model: t = 1.41, p = .160), suggesting the association between age of onset and WM RT did not differ in EXP and REP groups. However, the simple effect of age of onset predicting WM RT was significant in the EXP group (baseline: t(64.81) = −2.91, p = .005; full model: t(68.79) = −2.66, p = .010) and in the REP group in the full model (t(68.79) = −2.02, p = .048) but only a trend in the REP group in the baseline model (t(64.81) = −1.91, p = .060). Within the REP group, the association between cannabis age of onset and WM RT was relatively unchanged when additionally covarying total cannabis use (baseline model + total cannabis use (log): t = −1.72, p = .085; full model (covariate set A) + total cannabis use (log): t = −2.23, p = .025) and total cannabis use was not a significant predictor of WM RT (see below).
Total cannabis use
In the REP group, total cannabis use (log) was not significantly related to WM RT (baseline: χ2(1) = 0.77, t = 0.88, p = .381; full model (covariate set B): χ2(1) = 2.10, t = 1.45, p = .147), but showed a near-significant, negative association with WM accuracy in the baseline model (baseline: χ2(1) = 3.72, z = −1.93, p = .054). However, this effect was no longer evident after adjusting for prenatal cannabis exposure (baseline + prenatal cannabis exposure: χ2(1) = 1.98, z = −1.41, p = .159). No significant task interactions were observed for total cannabis use. See Fig. 2 for a summary of behavioral results.
interactions between cannabis age of onset and total cannabis use
Within the REP group, significant interactions were not observed between cannabis age of onset and total cannabis use for WM accuracy (baseline model: χ2(1) = 0.09, z = 0.29, p = .768; full model (covariate set B): χ2(1) = 0.10, z = 0.31, p = .753) or WM RT (baseline model: χ2(1) = 0.51, z = −0.72, p = .474; full model (covariate set B): χ2(1) = 0.29, z = −0.54, p = .592), suggesting there were no combined effects of cannabis age of onset and total cannabis use on WM behavior and age of onset associations were not dose-dependent.
Cannabis use in the last year
Cannabis use in the last year did not moderate the effects of cannabis age of onset or total cannabis use nor was it a significant predictor of WM accuracy or WM RT (Supplemental Fig. S4).
Group differences in WM behavior
To examine whether cannabis use effects generalized to potential group differences with non-user participants (NU), we first compared a categorical model of cannabis use (EXP + REP) to NU (Fig. 3). There was a trend for cannabis users to have higher accuracy than NU (baseline: χ2(1) = 3.79, z = 1.95, p = .052), which was relatively unchanged by the inclusion of distinguishing covariates (full model (covariate set C): χ2(1) = 4.78, z = 2.19, p = .029) and was significant when the analysis only included women (full model (w): χ2(1) = 5.71, z = 2.39, p = .017). A combined cannabis group also had significantly faster RT than NU in both a baseline (χ2(1) = 5.49, t = −2.34, p = .019) and full model (χ2(1) = 5.35, t = −2.31, p = .021). However, this was not significant when the analysis only included women (full model (w): χ2(1) = 1.46, t = −1.21, p = .227). In contrast, cannabis use groups (REP vs. EXP) did not differ from one another in accuracy (baseline: χ2(1) = 1.40, z = −1.19, p = .237; full model: χ2(1) = 1.02, z = −1.01, p = .312) or RT (baseline: χ2(1) = 0.13, t = 0.36 p = .719; full model: χ2(1) = 0.47, t = −0.69, p = .492).
Fig. 3.
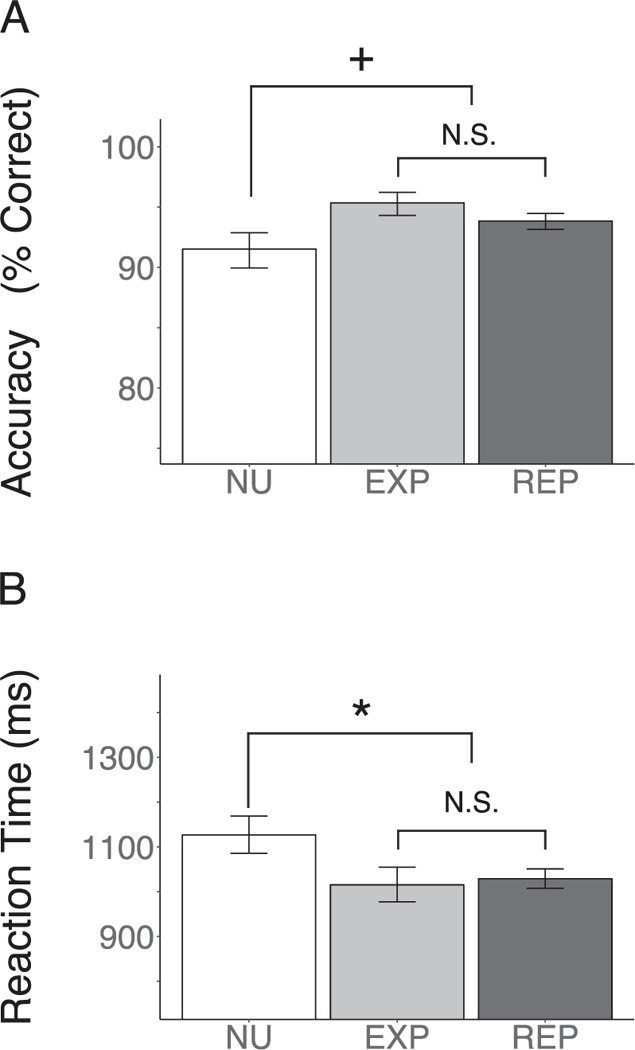
Performance Differences in Usage Groups. NU, non-user; EXP, cannabis experimenters; REP, repeated cannabis users. N.S., Non-significant, + p < .10, *p < .05. Visualized estimates are from intercept only baseline models. There was a trend (baseline: z = 1.95, p = .052) for a combined cannabis group (EXP + REP) to have higher WM accuracy than subjects that reported not using cannabis (NU). Additionally, a combined cannabis group (EXP + REP) had significantly lower RT (baseline: t = −2.34, p = .019). In contrast, usage groups (REP vs. EXP) did not significantly differ on WM accuracy (baseline: z = −1.19, p = .237) or RT (baseline: t = 0.36 p = .719).
Follow up post-hoc comparisons were made between NU and EXP and REP separately to determine if the general pattern of users performing better than NU was held across the two cannabis groups (EXP, REP). EXP had higher WM accuracy than NU in both baseline (z = 2.28, p = .023) and full models (covariate set C: z = 2.33, p = .020). Furthermore, EXP still had higher WM accuracy when the analysis included only women (full model (w): z = 3.05, p = .002). There was a trend for EXP to have faster WM RT than NU (baseline: t (78.24) = 1.96, p = .053; full model: t(84.02) = 1.89, p = .062). However, this was not significant when the analysis included women only (full model (w): t(57.60) = 1.39, p = .168). There was a trend for REP to have higher WM accuracy than NU (baseline: z = 1.61, p = .110; full model: z = 1.73, p = .083) and this was unchanged when the analysis included only women (full model (w): z = 1.68, p = .093). REP had faster WM RT than NU in the both the baseline (t (78.24) = −2.15, p = .034) and full models (t (84.11) = 2.00, p = .049). However, this was not significant when the analysis included women only (full model (w): t(57.78) = 0.75, p = .459).
Cannabis use and WM fMRI
Session-wise motion (mean Euclidean norm) was not associated with cannabis age of onset (r = .087, p = .511) or total cannabis use (log, r = - .226, p = .131) and did not differ between usage groups (p’s > .5). Likewise, the number of censored volumes was not associated with cannabis age of onset (r = .124, p = .339) or total cannabis use (log, r = .160, p = .287) and did not differ between usage groups (p’s > .5). Robust, trail-wise BOLD activation was observed in canonical working memory regions during the task (Supplemental Fig. S4). Omnibus testing of the HRF time series revealed activation differences as a function of cannabis age of onset in the posterior parietal cortex (PPC) and the posterior cingulate (Table 3). When considering total cannabis use, omnibus activation differences were observed in bilateral DLPFC and the inferior frontal gyrus (Table 3).
Table 3.
fMRI time series activation interactions for cannabis age of onset and total cannabis use.
| Region | BA | Number of Voxels |
F- value (peak) |
MNI |
||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Cannabis Age of Onset | ||||||
| Posterior Cingulate | 30,31 | 51 | 5.15 | −8 | 53 | 11 |
| R-Posterior Parietal CortexA |
4,40 | 23 | 5.43 | −35 | 32 | 53 |
| R-Posterior Parietal CortexB |
7,40 | 16 | 6.72 | −38 | 53 | 62 |
| Total Cannabis Use | ||||||
| R-Dorsolateral Prefrontal Cortex |
46,10,9 | 108 | 5.49 | −47 | −38 | 29 |
| L-Dorsolateral Prefrontal CortexA |
6,8,9 | 49 | 6.10 | 44 | −5 | 56 |
| L-Dorsolateral Prefrontal CortexB |
9,8 | 44 | 5.13 | 50 | −11 | 38 |
| R-Posterior Parietal CortexC |
40 | 26 | 5.34 | −59 | 44 | 41 |
| R-Posterior Parietal CortexD |
40 | 22 | 3.73 | −38 | 47 | 44 |
| R-Superior Frontal Gyrus | 10 | 19 | 5.15 | −35 | −62 | 8 |
| L-Inferior Frontal Gyrus | 44, 45 | 16 | 3.68 | 35 | −29 | 5 |
Note. BA, Brodmann areas; F-value, cluster peak test statistic for cannabis age of onset, F(14,714), or total cannabis use (log), F(14,616); MNI, MNI-152 coordinates at peak.
designations used to distinguish clusters within the same region.
WM epochs
Epoch specific comparisons revealed a significant (after Bonferonni correction) positive relationship between cue (encoding) epoch activation and cannabis age of onset in the PPC (cluster A), where those who began using earlier had reduced activation (standardized regression coefficient (β = .428, t(56) = 3.55, p = .002, corrected; Table 4; Fig. 4). The association between cannabis age of onset and PPC cue BOLD activation did not differ between EXP and REP groups (t(54) = 0.43, p = .672), although the association was significant in the REP group (β = .423, t(44) = 3.10, p = .003, uncorrected, p = .010, corrected) but not the EXP group (β = .262, t(10) = 0.86, p = .410). In the REP group, the association between cannabis age of onset and PPC (A) cue BOLD activation remained significant while covarying total cannabis use (β = .413, t(43) = 2.91, p = .006 uncorrected, p = .017, corrected) and total cannabis use was not a significant predictor of PPC cue BOLD activity (P = −.046, t(43) = −0.32, p = .749) nor did it moderate the effect of cannabis age of onset (age of onset by total cannabis use interaction: t(42) = −0.43, p = .673). Epoch-specific BOLD associations with cannabis age of onset in other clusters (e.g., posterior cingulate) were not significant after multiple comparison correction (Table 4).
Table 4.
Epoch amplitudes and relationships with cannabis age of onset and working memory reaction time.
| Cluster | M | SD | Cannabis Age of Onset β |
Working Memory Reaction Time β |
|---|---|---|---|---|
| Posterior Cingulate | ||||
| Cue | −5.50 | 11.18 | .070 | −.270+C,P |
| Delay | −9.73 | 14.19 | .009 | .102 |
| Target | 18.74 | 18.55 | −.079 | −.204 |
| R-Posterior Parietal Cortex (A) | ||||
| Cue | 3.24 | 9.22 | .428**C,P | −.358*C,P |
| Delay | 1.56 | 11.09 | .004 | .098 |
| Target | 5.76 | 11.33 | .138 | .090 |
| R-Posterior Parietal Cortex (B) | ||||
| Cue | 20.64 | 15.05 | .253 | −.153 |
| Delay | 11.18 | 14.08 | .232 | .105 |
| Target | 20.38 | 15.89 | .164 | −.174 |
Note.
p < .10,
p < .05.,
p < .01 (corrected);
p < .05 with full model covariates (set A);
p < .05 with full model + prenatal measures. Epoch means and standard deviations of clusters with an omnibus cannabis age of onset effect. β, standardized regression coefficients for cannabis age of onset and reaction time (mean of log reaction time). Significant and trending effects (p < .10, corrected) are bolded.
Fig. 4.
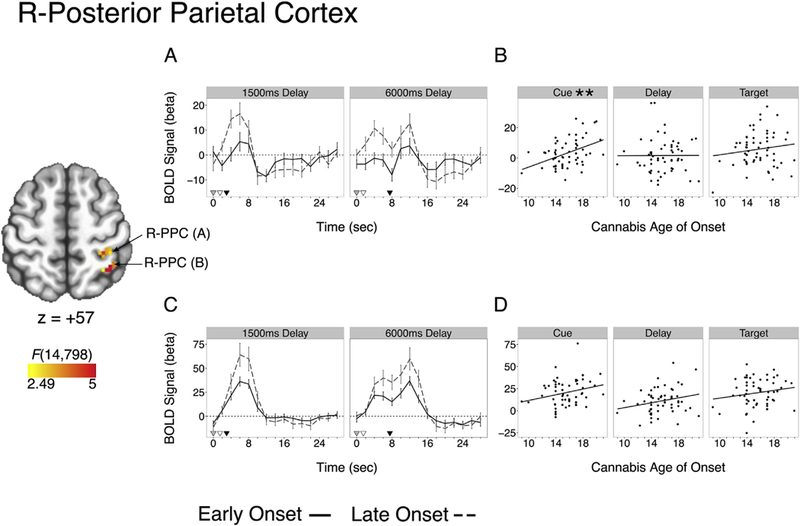
Clusters of Omnibus Activation Differences as a Function of Cannabis Age of Onset in the Right Posterior Parietal Cortex (cluster A, top; cluster b, bottom. **p < .01 (corrected); (A,C) Mean cluster time course with reference markers of cue (grey), delay (white), and target (black). Model was run with a continuous age of onset function and data are median split for visualization only. (B,D) Epoch activation differences as a function of cannabis age of onset.
Higher levels of total cannabis use in the REP group were associated with increased activation in the R-DLPFC during the delay (maintenance) (β = .432, t(44) = 3.18, p = .008, corrected) and target (retrieval) (β = .478, t(44) = 3.61, p = .002, corrected) epochs (Table 5, Fig. 5). R- DLPFC delay and target BOLD activation remained significant while covarying cannabis age of onset (delay: β = .430, t(43) = 3.04, p = .012, corrected; target: β = .539, t(43) = 4.08, p = .0006, corrected). Further, cannabis age of onset was not a significant predictor of R-DLPFC delay BOLD activation in these models (delay: β = −.011, t(43) = −0.08, p = .940; target: β = .259, t(43) = 1.96, p = .057, corrected) nor did it moderate the effect of total cannabis use (delay: t(42) = −0.53, p = .600; target: t(42) = 0.68, p = .500). Epoch-specific BOLD associations with total cannabis use in other clusters (e.g., L-DLPFC, inferior frontal gyrus) were not significant after multiple comparison correction and adjusting for covariates (Table 5).
Table 5.
Epoch amplitudes and relationships with total cannabis use and working memory Accuracy.
| Cluster | M | SD | Total Cannabis Use (log) P |
Working Memory Accuracy O.R. |
|---|---|---|---|---|
| R-Dorsolateral Prefrontal Cortex | ||||
| Cue | 1.69 | 7.05 | .293 | .997 |
| Delay | 3.80 | 10.21 | .432**C‘P | .978+C,P |
| Target | 13.04 | 10.35 | .478**C,P | .997 |
| L-Dorsolateral Prefrontal Cortex (A) | ||||
| Cue | 6.41 | 12.08 | .468** | 1.007 |
| Delay | 3.58 | 11.44 | .303 | 1.010 |
| Target | 8.55 | 12.40 | .498** | 1.018 |
| L-Dorsolateral Prefrontal Cortex (B) | ||||
| Cue | 8.22 | 11.20 | .227 | 1.005 |
| Delay | 7.07 | 11.87 | .309 | .990 |
| Target | 15.54 | 12.77 | .464** | .988 |
| L-Inferior Frontal Gyrus | ||||
| Cue | 0.69 | 8.57 | .005 | 1.015 |
| Delay | 14.19 | 12.50 | .255 | .998 |
| Target | 19.15 | 12.96 | .420* | .995 |
Note.
p < .10,
p < .05.,
p < .01 (corrected).
p < .05 with full model covariates (set B);
p < .05 with full model + prenatal measures. Epoch means and standard deviations of clusters with an omnibus total cannabis use effect and a significant (corrected) associations with epoch amplitudes. β, standardized regression coefficients for total cannabis use (log). O.R., odds ratio (based on one unit of BOLD amplitude) from beta regression model performed on mean accuracy data with logit link function. Significant and trending effects (p < .10, corrected) are bolded.
Fig. 5.
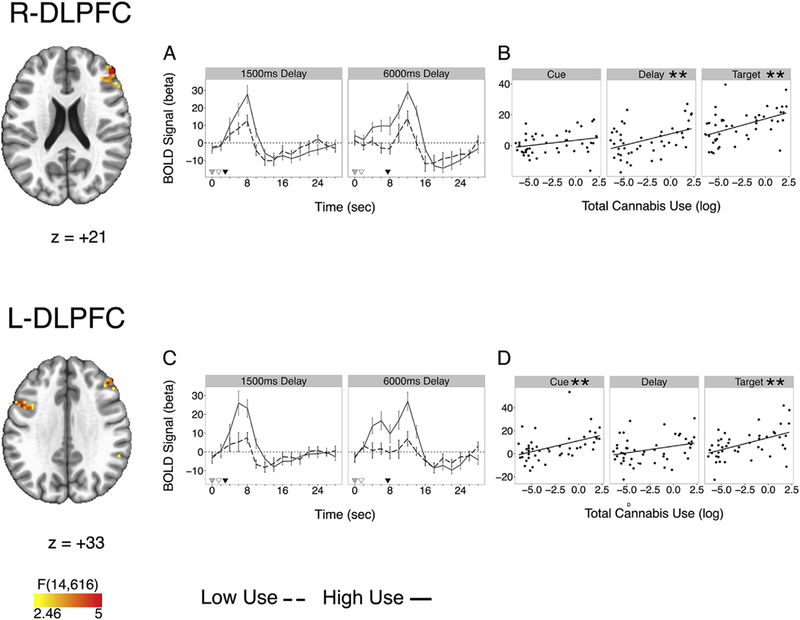
Clusters of Omnibus Activation Differences as a Function of Total Cannabis Use in Right (top) and Left (bottom) Dorsolateral Prefrontal Cortex (DLPFC). Left DLPFC cluster is represented as L-DLPFC (A) in tables. (A,C) Mean cluster time course with reference markers for cue (grey), delay (white), and target (black). Model was run with a continuous total cannabis use (log) function and data are median split for visualization only. (B,D) Epoch activation differences as a function of total cannabis use (log).
Brain-behavior relationships
Based on behavioral results, brain-behavior analyses were limited to RT for age of onset clusters, and accuracy for total use clusters. A significant (corrected) negative relationship was observed between cue epoch activation and RT (controlling for cannabis age of onset) in the PPC (cluster A), where those who had greater activation had faster RT’s (P = −.358, t (55) = −2.75, p = .024, corrected) (Fig. 6A; Table 4). Mediation analysis revealed that partialing out the variance of PPC (A) cue epoch activation significantly attenuated the relationship between cannabis age of onset and RT (baseline: average indirect pathway, β = −.153, 95% C.I., −.307: −.043, p = .003; full model (covariate set A): β = −.140,95% C.I., −.342: −.008, p = .029) (Fig. 6B). PPC cue epoch BOLD was also associated with WM RT in the REP group while covarying total cannabis use (β = −.459, t (42) = −3.01, p = .010, corrected) where mediation was also significant (average indirect pathway, P = −.189, 95% C.I., −.415: −.026, p = .017) (Fig. 6B).
Fig. 6.
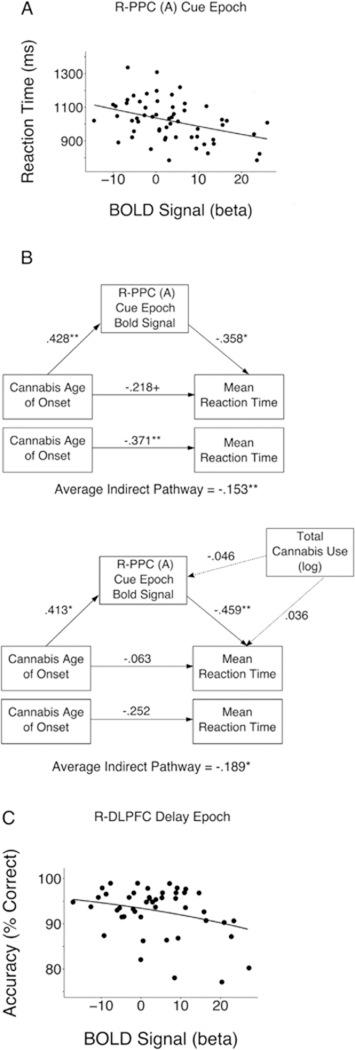
Brain Behavior Relationships for R-Posterior Parietal Cortex (A) (R-PPC(A)) and R-Dorsolateral Prefrontal Cortex (R-DLPFC). + p < .10, *p < .05, **p < .01.(A) Cue epoch activation in the R-PPC (A) was a significant predictor of WM RT (β = −.358, t (55)) = −2.75, p= .024, corrected. (B) Top panel, R-PPC (A) cue epoch activation significantly mediates the relationship between cannabis age of onset and reaction time (baseline: average indirect pathway, β= −.153, 95% C.I., −.307: −.043, p = .003; full model: β= −.140, 95% C.I., −.342: −.008, p = .029). Bottom Panel, R-PPC (A) cue epoch activation significantly mediates the relationship between cannabis age of onset and reaction time in the REP only group while covarying total cannabis use (average indirect pathway, β= −.189, 95% C.I., −.415: −.026, p = .017). (C), Delay epoch activation in R-DLPFC had a negative trend with working memory accuracy (O.R. = .978, z = −2.16, p = .093, corrected).
In contrast, delay period R-DLPFC activation, which was positively associated with increased total cannabis use (Table 5), displayed a trend towards a negative relationship with accuracy (Odds Ratio (O.R.) = .978, z = −2.16, p = .032, uncorrected, p = .093, corrected) (Fig. 6C). This relationship remained when including cannabis age of onset as a covariate (O.R. = .983, z = −2.09, p = .037, corrected, p = .110, corrected).
Group differences in fMRI
As in the behavioral analysis, we examined whether the epoch- specific brain-behavior BOLD relationships within user groups (EXP, REP) generalized to differences with non-user (NU) participants. For PPC (A) cue BOLD activation (Fig. 7A), a combined user group (EXP + REP) did not differ from NU (welch’s t(24.35) = 1.56, p = .132). Post-hoc testing examining EXP and REP separately demonstrated EXP had significantly greater PPC (A) cue BOLD activation compared to NU (t(22.08) = 2.10, p = .047), whereas EXP and REP (t(17.03) = 1.42, p = .176) and REP and NU (t(26.62) = 1.16, p = .255) did not differ.
Fig. 7.
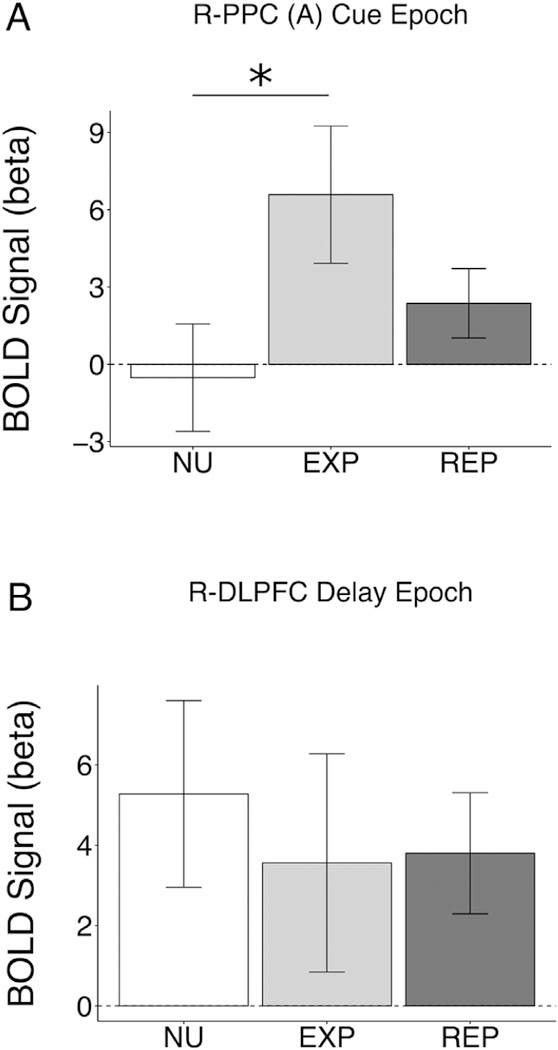
*p < .05. Usage groups (NU, non-user; EXP, cannabis experimenters; REP, repeated cannabis users) BOLD activation in performance-related fMRI regions from cannabis age of onset (A; R-PPC (A) cue epoch) and total cannabis use (B; R-DLPFC delay epoch) analyses.
No significant group differences where observed for delay period R- DLPFC (EXP + REP vs. NU: t(23.68) = −0.57, p = .572; EXP vs. NU: t(26.62) = −0.64, p = .635; EXP vs. REP: t(26.62) = −0.08, p = .930; REP vs. NU:t(26.62) = −0.53, p = .599).
Combined, the lack of consistent differences between user groups (EXP, REP) and NU suggest the observed brain-behavior relationships in PPC and DLPFC within cannabis groups were distinct from performance differences in NU.
Discussion
In this study, we aimed to identify brain processes that underlie the association between adolescent cannabis use and adult working memory (WM) performance. Results indicated that early cannabis age of onset was associated with slower reaction times and this relationship was mediated by reduced BOLD activation of right posterior parietal cortex (PPC) during stimulus encoding. Independently of age of onset, higher levels of total cannabis use were associated with greater delay period DLPFC activation and a trend towards lower WM accuracy. Nevertheless, within this sample, cannabis users either had better WM performance or did not differ from non-user participants and no additive effects of cannabis age of onset and total cannabis use were observed, providing evidence against cognitive deficits induced by adolescent cannabis use.
Cannabis age of onset
Our results indicating differential engagement of posterior parietal cortex in those with earlier age of onset cannabis during a spatial WM task are consistent with a prior study using a verbal WM task (Becker et al., 2010), supporting the hypothesis that cannabis age of onset is associated with BOLD activation in developmentally sensitive regions. In addition, our study was able to further specify that association between cannabis age of onset and parietal activation are particularly evident during encoding processes of WM and that encoding PPC activation mediated the relationship between cannabis age of onset and working memory reaction time. Therefore, for the first time, we demonstrate brain-behavior relationships between cannabis age of onset and WM performance, adding specificity to the role of posterior parietal cortex activation in the association between adolescent cannabis age of onset, executive subprocesses, and WM performance.
Interestingly, cannabis age of onset associations with WM reaction time and PPC encoding BOLD activation did not differ between users who only reported an age of onset (EXP) and those who reported repeated use (REP). Furthermore, within the REP group, cannabis age of onset behavior associations were not moderated by total cannabis use and total cannabis use was not associated with WM reaction time or PPC encoding BOLD activation. Combined, these results suggest that, in the present high-risk cohort, cannabis age of onset associations were relatively independent of the severity of cannabis use. Accordingly, our results appear inconsistent with a developmentally timed cannabis exposure effect, which would predict cannabis age of onset effects to be dose-dependent. From this perspective, the observed cannabis age of onset associations may reflect pre-onset characteristics that predict both brain-behavior development and substance use initiation.
Based on the brain-behavior associations of cannabis age of onset, cognitive processing speed, or the efficiency in which information is integrated in complex cognitive tasks, may serve as mechanism for preonset characteristics distinguishing early and late cannabis users. Supporting this idea, cannabis age of onset was a significant predictor of working memory reaction time, but not working memory accuracy. Age of onset and reaction time associations were significantly greater in low load trials, where individual differences are likely less influenced by working memory representation. Further, we observed performance- related differences in BOLD activation as a function of age of onset during the cue (encoding) epoch in the PPC. Individual differences in WM encoding may be more sensitive to how quickly the stimulus can be perceived, processed, and stored. Supporting this, previous research has demonstrated that BOLD activation in the parietal cortex (Takeuchi et al., 2012) and structural integrity of white matter tracks between parietal and frontal cortices (superior longitudinal fasciculus; Turken et al., 2008) are involved in cognitive processing speed. An additional explanation for the observed brain-behavior assocations may be that cannabis age of onset is associated with individual differences in visual attention, which has been shown to modulate WM RT (Awh and Jonides, 2001) and PPC BOLD activation (Corbetta et al., 2000).
Contrary to our hypothesis, we did not find significant activation differences as a function of cannabis age of onset in DLPFC. This is consistent with previous work examining cannabis age of onset during a WM task in adults (cf., Becker et al., 2010). Previous work has shown DLPFC activation differences may be moderated by WM accuracy differences between groups (cf., Van Snellenberg et al., 2006). Accordingly, we may have not observed activation differences in DLPFC as cannabis age of onset was not associated with WM accuracy. This idea is supported by the association between total cannabis use and DLPFC activation (see 4.2). Another possibility is that WM maintenance and manpulation processes associated with DLPFC (cf., Veltman et al., 2003; D’Esposito et al., 1999) are not associated with cannabis age of onset.
Together, our results suggest that WM performance in early-onset cannabis users is particularly associated with processing speed or visual attention subserved by the integrity of PPC function. However, prospective, longitudinal studies are needed to test possible pre-cannabis use limitations in PPC that may contribute to WM differences and predict cannabis onset.
Total cannabis use
Greater total cannabis use was associated with a trend toward lower working memory accuracy, providing some support for the hypothesis that cumulative cannabis use is associated with lower cognitive performance. Behavioral differences were accompanied by increased activation in bilateral DLPFC during multiple WM epochs. Given developmental patterns and network properties of CB1 receptors in the DLPFC (cf., Eggan et al., 2009), activation differences may reflect long-term changes in task-evoked activity. Greater delay period activation in right DLPFC was associated with higher levels of total cannabis use and a corrected trend towards lower WM accuracy. That higher levels of total cannabis use were associated with greater DLPFC BOLD is consistent with previous work suggesting compensatory-like DLPFC activation in groups with poorer executive function performance (Van Snellenberg et al., 2016; Ordaz et al., 2013). Furthermore, our results are consistent with recent work demonstrating that DLPFC activation is positively associated with cannabis use frequency and negatively associated with WM performance (Taurisano et al., 2016). We extend this previous result by demonstrating brain-behavior relationships that are specific to the WM delay period. Given the associations between total cannabis use and WM accuracy and delay period activation, our result appears more consistent with WM representational differences, as opposed to the processing speed effects of cannabis age of onset. However, total cannabis use WM accuracy differences were no longer significant while adjusting for prenatal cannabis exposure and we did not find evidence supporting a brain-behavior mediation model. Therefore, it is difficult to conclude whether these results represent neurocognitive deficits or pre-cannabis use cognitive differences and whether the identified circuits fully explain behavioral effects.
Cannabis users and non-users
Despite the specificity in brain-behavior relationships demonstrated for cannabis age of onset and total cannabis use, there was little evidence for general cannabis use differences in WM in the group comparisons. In particular, the cannabis group who only reported initiation, but no other use (EXP), had higher WM accuracy than non-users (NU), which is consistent with a recent large cross-sectional study of cannabis use and neurocognitive performance (Scott et al., 2017). Given the likelihood of very low cannabis use in the EXP group (recall a specific amount of cannabis use could not be established in the EXP group, as EXP only reported an age of initiation) its possible group differences between EXP and NU reflect alternative mechanisms that either precede or are independent of cannabis initiation. Again, however, prospective longitudinal studies are needed to better test this hypothesis.
Given the high-risk population of the study, it is possible group differences between NU and EXP reflect unique characteristics of substance use risk, rather than substance use itself. To this end, previous work has suggested executive function deficits in cannabis users compared to nonusers in more normative samples, without high-risk characteristics (Pope and Yurgelun-Todd, 1996; Meier et al., 2012; Ehrenreich et al., 1999) (see Crean et al., 2011 for a review). Within our high-risk cohort, most participants endorsed some level of cannabis use during adolescence. Accordingly, the participants who did not endorse cannabis use at any time point (NU) may have unique cognitive characteristics that both protect against cannabis use during adolescence and predict differential WM performance in adulthood. One possibility is anxiety and behavioral inhibition, which have been shown to predict poorer visuospatial WM performance (Shackman et al., 2006) and in social contexts may be protective against substance use involvement during adolescence (Myers et al., 2003). Given the exclusion criteria for the current study included meeting criteria for a mental health disorder, the associations between cannabis use, WM, and anxiety, would have to function at subclinical levels. Accordingly, future research with dimensional assessments may examine anxiety as a moderating factor in cognitive differences between cannabis users and non-users in high-risk cohorts.
Another explanation for the observed WM differences between EXP and NU could be gender, as the NU group was mostly women (13 out of 15). Although we attempted to account for the specific discrepancy of male to female ratios among our groups by performing secondary behavior analysis with only the women from each group, future work with larger sample sizes of male and female users and non-users is justified.
In sum, the reasons for worse performance in the non-user group (compared to the cannabis group) remain unclear. The present study was designed to examine age-of-onset and cannabis severity brain-behavior associations within cannabis groups and not the precipitating factors and features associated with cannabis abstinence in high-risk cohorts, limiting our ability to make stronger conclusions. Further work is needed to determine whether this result replicates to other cohorts and whether more subtle, dimensional features of high-risk cohorts might explain group differences.
Limitations
This study was designed to take advantage of the unique aspects of the population sample, including the prospective longitudinal assessment of cannabis use and prenatal cannabis exposure. We also utilized an approach to characterize specific epochs of the WM task to better identify which cognitive processes may be associated with age of onset and total cannabis use. However, several limitations should also be noted. First, this study utilized a high-risk prospective cohort sample with varying degrees of cannabis use throughout adolescence and adulthood. Accordingly, participants were not excluded from the study based on previous cannabis use, unless they had a positive urine screen on the day of testing or reported recreational drug use in the past week. In our analyses examining the REP group, in which fifteen subjects reported cannabis use in the last year, we covaried cannabis use within the last year. However, future investigations with larger samples should further investigate associations between cannabis use history, recent cannabis use, and neurocognitive function. An additional limitation of the study was the binary threshold (positive/negative) for the urine screen. Future work may utilize drug screening procedures that provide a more dimensional biological measure of recent cannabis use and examine whether this moderates cannabis age of onset and cumulative cannabis use WM associations. Finally, although cannabis use was prospectively assessed (cohort years 14-, 16-, 22-, 28-years-old), the interview only asked about the previous year. Usage in the intervening years was not measured. Accordingly, two participants who reported the same use at an assessment year (e.g., age 28) could have varied in their usage in the years between other assessment phases. Future work with prospective longitudinal assessment should incorporate more frequent assessments of cannabis use in order to better estimate total cannabis use trajectories. Despite these limitations, the current project highlights the value in examining associations between cannabis use and cognitive function in adults with longitudinal cannabis use history and prenatal substance exposure assessments.
Conclusion
Our results support evidence suggesting that reported early cannabis use in the adolescent period is associated with individual differences in the parietal cortex in adulthood. We extend this line of inquiry by providing evidence that cannabis age of onset may be specifically associated with information processing speed and stimulus encoding. However, this pattern of results was evident in both those who reported a onetime use and those with repeated use, suggesting these effects may represent pre-onset substance use risk characteristics rather than cannabis exposure effects. In contrast, our results suggested total cannabis use may be specifically associated with processes underlying the ability to maintain information in WM through the DLPFC, independent of cannabis age of onset. Together with previous reports, our data help refine distinct neurocognitive phenotypes of adolescent cannabis use. However, this model necessitates future testing within prospective studies where neuroimaging and cognitive assessment are performed prior to cannabis use onset.
Supplementary Material
Acknowledgments
This work was supported by the Pennsylvania Department of Health, Common Universal Research Enhancement (C.U.R.E), Program SAP #4100055579.
Footnotes
Financial disclosures
All authors report no financial interests or potential conflicts of interest.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.neuroimage.2017.12.041.
References
- Awh E, Jonides J, 2001. Overlapping mechanisms of attention and spatial working memory. Trends Cognit. Sci. 5 (3), 119–126. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, 2013. lme4: Linear Mixed-effects Models Using S4 Classes. Retrieved from. http://CRAN.R-project.org/package=lme4.
- Becker B, Wagner D, Gouzoulis-Mayfrank E, Spuentrup E, Daumann J, 2010. The impact of early-onset cannabis use on functional brain correlates of working memory. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 34 (6), 837–845. 10.1016/j.pnpbp.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Cloak C, Ernst T, 2006. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain 129 (5), 1096–1112. [DOI] [PubMed] [Google Scholar]
- Chen G, Saad ZS, Adleman NE, Leibenluft E, Cox RW, 2015. Detecting the subtle shape differences in hemodynamic responses at the group level. Front. Neurosci. 9 Retrieved from. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4620161/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL, 2000Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat. Neurosci. 3 (3), 292–297. [DOI] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29 (3), 162–173. [DOI] [PubMed] [Google Scholar]
- Crean RD, Crane NA, Mason BJ, 2011. An evidence based review of acute and longterm effects of cannabis use on executive cognitive functions. J. Addiction Med. 5 (1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribari-Neto F, Zeileis A, 2009. Beta Regression in R. Retrieved from. http://epub.wu.ac.at/726/. [Google Scholar]
- Day N, Sambamoorthi U, Taylor P, Richardson G, Robles N, Jhon Y, Jasperse D, 1991. Prenatal marijuana use and neonatal outcome. Neurotoxicol. Teratol. 13 (3), 329–334. [DOI] [PubMed] [Google Scholar]
- Day NL, Richardson GA, Goldschmidt L, Robles N, Taylor PM, Stoffer DS, Geva D, 1994. Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol. Teratol. 16 (2), 169–175. [DOI] [PubMed] [Google Scholar]
- Day NL, Goldschmidt L, Thomas CA, 2006. Prenatal marijuana exposure contributes to the prediction of marijuana use at age 14. Addiction 101 (9), 1313–1322. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Ballard D, Lease J, 1999. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cognit. 41 (1), 66–86. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Mizoguchi Y, Stoyak SR, Lewis DA, 2009. Development of cannabinoid 1 receptor protein and messenger RNA in monkey dorsolateral prefrontal cortex. Cerebr. Cortex bhp 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Hoehe MR, 1999. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology 142 (3), 295–301. [DOI] [PubMed] [Google Scholar]
- Fox J, Weisberg S, Adler D, Bates D, Baud-Bovy G, Ellison S, et al. , 2016. Package “car.” Companion to Applied Regression. R Package Version, 2–1. [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Rapoport JL, 1996. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebr. Cortex 6 (4), 551–560. [DOI] [PubMed] [Google Scholar]
- Gold MS, 1989. Marijuana. Plenum Publishing Corporation, New York, NY, US. [Google Scholar]
- Goldschmidt L, Richardson GA, Willford J, Day NL, 2008. Prenatal marijuana exposure and intelligence test performance at age 6. J. Am. Acad. Child Adolesc. Psychiatr. 47 (3), 254–263. [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Cornelius MD, Day NL, 2012. Prenatal cigarette smoke exposure and early initiation of multiple substance use. Nicotine Tob. Res. 14 (6), 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinband J, Wager TD, Lindquist M, Ferrera VP, Hirsch J, 2008. Detection of time- varying signals in event-related fMRI designs. Neuroimage 43 (3), 509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gönenc A, Killgore WDS, 2012. Age of onset of marijuana use impacts inhibitory processing. Neurosci. Lett. 511 (2), 89–94. 10.1016/j.neulet.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey MA, Harvey MA, Sellman JD, Harvey MA, Sellman JD, Porter RJ, et al. , 2007. The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev. 26 (3), 309–319. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, De Costa BR, Rice KC, 1990. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. Unit. States Am. 87 (5), 1932–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson NJ, Isen JD, Khoddam R, Irons D, Tuvblad C, Iacono WG, Baker LA, 2016. Impact of adolescent marijuana use on intelligence: results from two longitudinal twin studies. Proc. Natl. Acad. Sci. Unit. States Am. 201516648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Westerveld M, Pugh KR, 2004. Impact of cannabis use on brain function in adolescents. Ann. N. Y. Acad. Sci. 1021 (1), 384–390. 10.1196/annals.1308.053. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S, 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17 (2), 825–841. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE, 2010. Monitoring the Future: National Results on Adolescent Drug Use. Overview of Key Findings, 2009 NIH Publication No. 10–7583. National Institutes of Health; Retrieved from. http://eric.ed.gov/?id=ED514371. [Google Scholar]
- Lane SD, Cherek DR, Lieving LM, Tcheremissine OV, 2005. Marijuana effects on human forgetting functions. J. Exp. Anal. Behav. 83 (1), 67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth RV, Hervé M, 2015. Lsmeans: Least-squares Means, Version 2.14. [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA, 2004. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 75 (5), 1357–1372. [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K, 2010. What has fMRI told us about the development of cognitive control through adolescence? Brain Cognit. 72, 101–113. 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet PE, Beninger RJ, 1998. The cannabinoid CB1 receptor antagonist SR141716A attenuates the memory impairment produced by Δ9-tetrahydrocannabinol or anandamide. Psychopharmacology 140 (1), 11–19. 10.1007/s002130050733. [DOI] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, Moffitt TE, 2012. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc. Natl. Acad. Sci. Unit. States Am. 109 (40), E2657–E2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Aarons GA, Tomlinson K, Stein MB, 2003. Social anxiety, negative affectivity, and substance use among high school students. Psychol. Addict. Behav. 17 (4), 277. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T, 2003. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Cognit. Brain Res. 18 (1), 48–57. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M, 2001. Separating processes within a trial in event-related functional MRI: I. The method. Neuroimage 13 (1), 210–217. [DOI] [PubMed] [Google Scholar]
- Ordaz SJ, Foran W, Velanova K, Luna B, 2013. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J. Neurosci. 33 (46), 18109–18124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen DJ, Hallquist MN, Geier CF, Luna B, 2015. Effects of incentives, age, and behavior on brain activation during inhibitory control: a longitudinal fMRI study. Developmental Cognitive Neuroscience 11, 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Yurgelun-Todd D, 1996. The residual cognitive effects of heavy marijuana use in college students. Jama 275 (7), 521–527. [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D, 2003. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 69 (3), 303–310. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Ryan C, Willford J, Day NL, Goldschmidt L, 2002. Prenatal alcohol and marijuana exposure: effects on neuropsychological outcomes at 10 years. Neurotoxicol. Teratol. 24 (3), 309–320. [DOI] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P, 1994. Cambridge neuropsychological test automated battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia 5, 266–281. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF, 2008. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatr. Res. 163 (1), 40–51. 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Wolf DH, Calkins ME, Bach EC, Weidner J, Ruparel K, Gur RE, 2017. Cognitive functioning of adolescent and young adult cannabis users in the Philadelphia Neurodevelopmental Cohort. Psychol. Addict. Behav. 31 (4), 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Sarinopoulos I, Maxwell JS, Pizzagalli DA, Lavric A, Davidson RJ, 2006. Anxiety selectively disrupts visuospatial working memory. Emotion 6 (1), 40. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC, 1998. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM- IV and ICD-10. J. Clin. Psychiatr. Retrieved from http://doi.apa.org/psycinfo/1998-03251-004. [PubMed] [Google Scholar]
- Siegel JS, Power JD, Dubis JW, Vogel AC, Church JA, Schlaggar BL, Petersen SE, 2014. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Hum. Brain Mapp. 35 (5), 1981–1996. 10.1002/hbm.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Brady J, 1997. SUSAN - a new approach to low level image processing. Int. J. Comput. Vis. 23 (1), 45–78. [Google Scholar]
- Van Snellenberg JX, Torres IJ, Thornton AE, 2006. Functional neuroimaging of working memory in schizophrenia: task performance as a moderating variable. Neuropsychology 20, 497–510. [DOI] [PubMed] [Google Scholar]
- Van Snellenberg JX, Girgis RR, Horga G, van de Giessen E, Slifstein M, Ojeil N, et al. , 2016. Mechanisms of working memory impairment in schizophrenia. Biol. Psychiatr. Retrieved from http://www.sciencedirect.com/science/article/pii/S0006322316001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Vendetti J, 2002. Cognitive functioning of long-term heavy cannabis users seeking treatment. Jama 287 (9), 1123–1131. [DOI] [PubMed] [Google Scholar]
- Sonon KE, Richardson GA, Cornelius JR, Kim KH, Day NL, 2015. Prenatal marijuana exposure predicts marijuana use in young adulthood. Neurotoxicol. Teratol. 47, 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Sugiura M, Sassa Y, Sekiguchi A, Yomogida Y, Taki Y, Kawashima R, 2012. Neural correlates of the difference between working memory speed and simple sensorimotor speed: an fMRI study. PLos One 7 (1), e30579. 10.1371/journal.pone.0030579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SPA, Paulus MP, Brown SA, Yang TT, Frank LR, 2007. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology 194 (2), 173–183. 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taurisano P, Antonucci LA, Fazio L, Rampino A, Romano R, Porcelli A, et al. 2016. Prefrontal activity during working memory is modulated by the interaction of variation in CB1 and COX2 coding genes and correlates with frequency of cannabis use. Cortex 81, 231–238. [DOI] [PubMed] [Google Scholar]
- Team RC, 2014. R: A Language and Environment for Statistical Computing, 2013. R Foundation for Statistical Computing, Vienna, Austria: ISBN 3–900051-07–0. [Google Scholar]
- Thorndike RL, Hagen EP, Sattler JM, 1986. Stanford-binet Intelligence Scale. Riverside Publishing Company. [Google Scholar]
- Tingley D, Yamamoto T, Hirose K, Keele L, Imai K, 2014. Mediation: R Package for Causal Mediation Analysis. Retrieved from. http://dspace.mit.edu/handle/1721.1/91154.
- Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM, 1998. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83 (2), 393–411. 10.1016/S0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Turken AU, Whitfield-Gabrieli S, Bammer R, Baldo J, Dronkers NF, Gabrieli JDE,Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. Neuroimage 42 (2), 1032–1044. 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma VK, Malhotra AK, Dang R, Das K, Nehra R, 1988. Cannabis and cognitive functions: a prospective study. Drug Alcohol Depend. 21 (2), 147–152. [DOI] [PubMed] [Google Scholar]
- Varvel S, Hamm R, Martin B, Lichtman A, 2001. Differential effects of Δ9-THC on spatial reference and working memory in mice. Psychopharmacology 157 (2), 142–150. [DOI] [PubMed] [Google Scholar]
- Veltman DJ, Rombouts SA, Dolan RJ, 2003. Maintenance versus manipulation in verbal working memory revisited: an fMRI study. Neuroimage 18 (2), 247–256. [DOI] [PubMed] [Google Scholar]
- Wechsler D, 1991. WISC-III: Wechsler Intelligence Scale for Children: Manual. Psychological Corporation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


