ABSTRACT
Background
Understanding measurement error in sodium and potassium intake is essential for assessing population intake and studying associations with health outcomes.
Objective
The aim of this study was to compare sodium and potassium intake derived from 24-h dietary recall (24HDR) with intake derived from 24-h urinary excretion (24HUE).
Design
Data were analyzed from 776 nonpregnant, noninstitutionalized US adults aged 20–69 y who completed 1-to-2 24HUE and 24HDR measures in the 2014 NHANES. A total of 1190 urine specimens and 1414 dietary recalls were analyzed. Mean bias was estimated as mean of the differences between individual mean 24HDR and 24HUE measurements. Correlations and attenuation factors were estimated using the Kipnis joint-mixed effects model accounting for within-person day-to-day variability in sodium excretion. The attenuation factor reflects the degree to which true associations between long-term intake (estimated using 24HUEs) and a hypothetical health outcome would be approximated using a single 24HDR: values near 1 indicate close approximation and near 0 indicate bias toward null. Estimates are reported for sodium, potassium, and the sodium: potassium (Na/K) ratio. Model parameters can be used to estimate correlations/attenuation factors when multiple 24HDRs are available.
Results
Overall, mean bias for sodium was −452 mg (95% CI: −646, −259), for potassium −315 mg (CI: −450, −179), and for the Na/K ratio −0.04 (CI: −0.15, 0.07, NS). Using 1 24HDR, the attenuation factor for sodium was 0.16 (CI: 0.09, 0.21), for potassium 0.25 (CI:0.16, 0.36), and for the Na/K ratio 0.20 (CI: 0.10, 0.25). The correlation for sodium was 0.27 (CI: 0.16, 0.37), for potassium 0.35 (CI: 0.26, 0.55), and for the Na/K ratio 0.27 (CI: 0.13, 0.32).
Conclusions
Compared with 24HUE, using 24HDR underestimates mean sodium and potassium intake but is unbiased for the Na/K ratio. Additionally, using 24HDR as a measure of exposure in observational studies attenuates the true associations of sodium and potassium intake with health outcomes.
Keywords: sodium, potassium, 24-h dietary recall, urine, measurement error
Introduction
Hypertension is a major risk factor for cardiovascular disease (CVD) morbidity and mortality (1). Evidence indicates sodium intake is related positively and directly to blood pressure, with lower concentrations of sodium intake associated with lower blood pressure (1–5). However, variability in the association of sodium intake with blood pressure and other health outcomes has led some to question this relation (6). In addition, studies suggest a higher intake of potassium can lower blood pressure and may blunt the blood-pressure raising effects of excess sodium intake (4, 5). Further, potassium salts have been used as a replacement for sodium in manufactured foods to lower the sodium content (6, 7). Some studies suggest the sodium: potassium (Na/K) ratio may have a stronger association with blood pressure than either electrolyte examined alone (3, 8–10).
In research studies and population surveys, including the NHANES, the intake of individual nutrients is commonly derived from measures of self-reported dietary intake of foods, such as 24-h dietary recall (24HDR). However, 24HDR is subject to recall bias, requires up-to-date nutrient databases, may not capture sodium intake from salt added at the table or potassium from salt substitutes, and requires >1 24HDR to estimate usual long-term intake. Measurement error associated with 24HDR can be attributable to systematic bias that include: 1) intake-related bias, such as participants who consume a higher intake of sodium foods may be less likely to report these foods, or 2) additive systematic bias, for example the instrument is inaccurate, indicating that a constant error is added to each person's reported intake, or 3) person-specific bias, for instance one participant consistently overreports sodium intake and another participant consistently underreports sodium intake (11). Measurement error can also be due to random error that includes within-person bias due to day-to-day variability in food consumption (11). Evaluating measurement error in the assessment of sodium and potassium intake is essential for assessing population intake in order to monitor the impact of sodium reduction strategies, and to determine associations in observational studies with health outcomes, such as blood pressure (12,13).
The 24-hour urinary excretion (24HUE) of sodium is considered the reference biomarker for sodium intake (14, 15). When collection is complete, urinary sodium excretion captures all sources of sodium intake, represents ∼90% of sodium consumed, and does not rely on self-report (12, 14). In contrast, urinary potassium excretion captures ∼50–90% of potassium intake (16–19). Previous validity studies evaluating the ability of 24HDR to capture accurate sodium and potassium intake compared with 24HUE were conducted among convenience samples of adults (20, 21). Thus, the objective of this study was to evaluate the measurement error in sodium and potassium intake, assessed using 24HDR compared with 24HUE, in a national sample of US adults.
Methods
Data source
NHANES is a continuous nationally representative cross-sectional survey of civilian noninstitutionalized persons in the US with data publicly released in 2-y cycles, e.g. 2013–2014 (22, 23). In 2014, a random half sample of 1,103 nonpregnant adults aged 20–69 y who completed the examination component of NHANES were selected to collect ≤2 24HUE samples (Figure 1) (24, 25). Due to lower response rates among adults aged ≥70 y who were interviewed (61.4% compared with 71.0% overall in 2013–2014) and examined (59.8% compared with 68.5% overall), participants aged ≥70 years were not selected to collect 24HUE (24). Of the participants selected to collect 24HUE, 827 completed one 24HUE. Of these, about half, 436, were selected and completed a second 24HUE 3–10 d later. Details related to selection and completions are available elsewhere (24, 25). For our analysis, we excluded 48 participants with unreliable dietary data and 3 participants with missing covariates, leaving an analytical sample of 776 US adults who completed ≤2 24HUE and 24HDR, totaling 1190 urine specimens and 1414 dietary recalls. The National Center for Health Statistics Research Ethics Review Board reviewed and approved NHANES including 24-h urine collection, and participants gave written informed consent.
FIGURE 1.
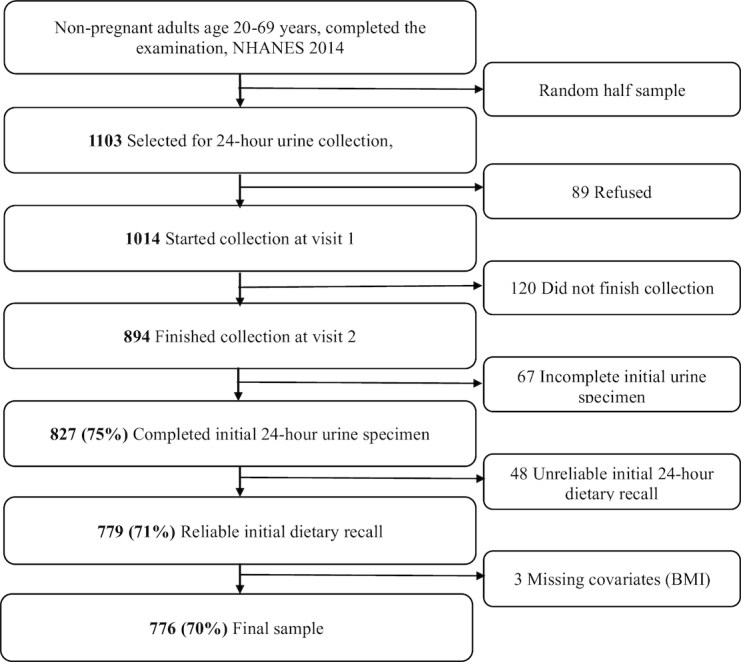
Flow diagram of analytical sample, adults aged 20–69 y, US, 2014.
Biomarkers
Complete details on urine collection procedures, specimen processing, storage, shipping, quality assurance, and control procedures have been previously described (26–31). Most collections were started and completed in person in the Urine Mobile Examination Center (MEC) and collections were completed on all days of the week (26, 27). Urine collections did not occur on the same day as dietary recall. Urine specimens were considered incomplete if: start and end times were not documented, length of collection was <22 hours, total urine volume was <400 mL, a female participant reported menstruating, or if a participant reported more than a few drops of urine were missed during the collection (26). Urine specimens were analyzed for electrolytes including sodium and potassium using the Roche Ion-Selective Electrode technique and for creatinine using the Roche enzymatic assay on the Roche Cobas 6000 Analyzer (27–29). The total amount of sodium and potassium excreted in each 24HUE was standardized to 24 h by multiplying the concentration by the adjusted 24-h urine volume based on duration of collection (27–30).
24HDR
For the 24HDR, trained interviewers asked participants to report foods and beverages consumed in the past 24 h using USDA's 5-step Automated Multiple Pass Method (AMPM) (31–33). In NHANES, ≤2 24HDR are collected, the initial 24HDR in the MEC and a second 24HDR 3–10 days later by telephone (31, 32). Furthermore, 24HDRs were collected for all days of the week (31, 32). Recalls were conducted in English or Spanish. Interpreters were also available for all other languages. Only 24HDRs coded as reliable were used in the analysis (Figure 1). The 24HDRs that were incomplete (coded as not reliable or did not meet minimum criteria) were excluded from analysis (e.g. food/beverages were missing for a reported meal or eating occasion, or the interview was terminated before details of the food/beverages were provided) (31, 32).
Statistical analysis
To help assess generalizability of results to the broader US population, weighted characteristics of participants in the analytic sample were described along with those of the larger sample of potentially eligible participants in the public release data, NHANES 2013–2014. Potentially eligible participants were defined as nonpregnant participants, aged 20–69 y in NHANES 2013–2014 who were examined in the MEC and completed ≥1 dietary recall (n = 4246). Rao-Scott chi-square tests were used to examine differences in weighted distributions of demographic characteristics and BMI of the analytical sample (n = 776) by sex. The 24-h urine sample weights were used for the analyses using the NHANES 2014 analytic sample, and the dietary day one (initial day) sample weights were used for the analyses of NHANES 2013–2014 participants to account for the complex survey sample design. P values <0.05 were considered statistically significant. A percentage point difference of >5% in the distribution of characteristics by sex was noted as significant.
The weighted mean population intake of sodium, potassium, and the Na/K ratio were estimated using 24HDR and 24HUE at the initial visit, the follow-up visit, and the mean of ≤2 d per individual. Due to the observed distributions of sodium and potassium intake, dietary recall and biomarker measures, assessed by 24HDR and 24HUE, respectively, were kept on the original scale, rather than a log scale. In determining the appropriate scale, we evaluated normality, residuals and difference in residuals, correlations between mean and SD for individuals with 2 observations, and distribution of mean differences. All analyses used survey sample weights. First, normality was assessed for each nutrient for first and second recalls and biomarkers. Normality was determined through assessment of skewness and kurtosis, as well as a visual assessment of the distributions for each nutrient value for the first and second recalls and biomarkers. Second, we evaluated and compared residuals for nutrient measurements on the original scale and log scale as follows: For both 24HDR and 24HUE measurements, linear regression models were used to model first and second day nutrient values as the outcome (in separate models) and age, BMI, race and Hispanic origin, and day of intake as predictors and to examine corresponding residuals for each model. Then, the difference in residuals between first and second day nutrient values from these models was assessed, and the normality of the differences evaluated using PROC UNIVARIATE [SAS Institute, Inc.]. Third, for participants with 2 observations, distribution was additionally assessed by evaluating correlations between mean and SD for each individual, and evaluating the skewness and kurtosis, as well as visual assessment of the distribution of mean difference between the first and second day for recalls and biomarkers.
To evaluate measurement error in the 24HDR, we estimated the population mean bias, correlation between person-level 24HUE and 24HDR, and the attenuation factor for sodium intake, potassium intake, and the Na/K ratio assessed in mg/d. The mean bias in intake, assessed using 24HDR, is estimated as the mean of the differences in each individual's mean intake calculated from ≤2 24HDR minus the mean intake from ≤2 24HUE divided by the total number of participants. For individuals with 1 measurement, a single (initial) measurement was used. SAS PROC SURVEYMEANS [SAS Institute, Inc.]was used to estimate the weighted population mean intakes and 95% CIs, using 24-h urine sample weights, and accounting for complex survey and sampling design. Estimated population means were compared using t-tests. Pair-wise t-tests were used to compare group level mean bias.
The attenuation factor reflects the degree to which true association between long-term intake (estimated by 24HUE accounting for within-person random error) and a hypothetical health outcome would be approximated using 24HDRs. Attenuation factor values near 1 indicate close approximation to the true association, with increased bias toward the null as the attenuation factor value approaches zero. The attenuation factor, when values are between 0 and 1, represents the multiplicative bias or shrinkage factor in the estimated regression coefficient for the association of interest, in this case the association between intake and a health outcome. When a health outcome is regressed on continuous intake assessed with each individual's single dietary recall, for example, rather than his or her true usual dietary intake as approximated by 24HUE accounting for within-person day-to-day variability, both random and systematic measurement error may bias or attenuate the association toward null. In relation to true usual long-term intake, model parameters can be used to estimate correlations/attenuation factors when multiple 24HDRs such as the mean of 2, 3, or infinite numbers of 24HDRs are available.
The correlation coefficient reflects the degree of linear association between methods and is used to measure loss of statistical power to detect the association with health outcomes that is attributable to measurement error. It is estimated as the correlation between nutrient intake, assessed using 24HDRs, and the biomarker value, assessed using 24HUEs, accounting for within-person measurement error (11). Values of <0.4 generally indicate weak associations whereas values >0.7 indicate strong associations (11).
Attenuation factors and correlations were estimated using the Kipnis joint-mixed effect models (11) adjusting for BMI (continuous), age (continuous), race and Hispanic origin (non-Hispanic white, non-Hispanic black, Hispanic, and non-Hispanic other), and sex (for overall estimates). The Kipnis model uses the nutrient biomarker (24HUE) as a referent measure. We assume that sodium and potassium biomarkers are unbiased for measurement of longer-term intake based on the following assumptions: Biomarker concentrations provide an unbiased estimate of short-term intake, within-person variation is independent of personal characteristics, and intake did not vary systematically with time. The Kipnis model accounts for systematic error (additive and intake-related bias) and random error (person-specific and within-person day-to-day variability) associated with 24HDR. Up to 2 24HDRs and 2 24HUEs for each individual were used. In the Kipnis model, the attenuation factor and correlations for the measurement being evaluated (i.e., intake assessed using 24HDR) were estimated related to true intake (long-term usual intake approximated using 24HUEs accounting for within-person day-to-day variability). As indicated earlier, measurement error in intake as assessed with 1 or the mean of 2, 3, 6, or an infinite number of 24HDRs was approximated in relation to “true” intake. The mean of an infinite number of 24HDRs estimates what would happen if random day-to-day error was eliminated as a cause of measurement error. In this case (infinite 24HDR), the correlations and attenuation factors represent systematic, but not random, measurement error related to the use of 24HDRs.
For the Kipnis model, estimates were calculated using a 2-step process. First, SAS PROC CALIS [SAS Institute, Inc.]was used because it was able to fit the model and estimate starting values for α coefficients, β coefficients, and variance of nutrient measurements. Next, due to improved efficiency in comparison to PROC CALIS, SAS PROC NLMIXED [SAS Institute, Inc.] was used to calculate estimates of attenuation factors and correlations using sample weights and using the starting coefficients and variance previously calculated with PROC CALIS. SEs and 95% CIs were calculated using NLMIXED with Balanced Repeat Replication weights based on 24-h urine sample weights to account for sampling and nonresponse. A relative SE/estimate > 30% was considered statistically unreliable.
Furthermore, we evaluated the influence of nutrient recovery, correcting for the amount of intake excreted in urine for sodium and potassium, on estimates of mean bias, attenuation factor, and the correlation coefficient. A correction factor of 0.90 was applied to all urinary sodium excretion measurements (24HUE/0.90). Using Turban et al. as a reference, participants who consumed a control diet (low in potassium), showed little difference in potassium excreted by race (73% for non-Hispanic white adults and 74% for non-Hispanic black) (19). Therefore, we applied a correction factor of 0.735 to all urinary potassium excretion measurements (24HUE/0.735) (19). Values for 24HUE mean intake, mean bias, attenuation, and correlation were reported with and without application of a correction factor for sodium and potassium.
Statistical analyses were completed in SAS, version 9 (SAS Institute, Inc.). To protect the confidentiality of survey respondents, all analyses involving data from 24-h urine collection in 2014 were performed on-site at the National Center for Health Statistics Research Data Center (34). For purposes of reproducing the results, all SAS code is available to other researchers upon request to the corresponding author. NHANES 2014 24-h urine study procedures are publicly available, as are NHANES 2013–2014 data and procedures (24, 26–33).
Sensitivity analyses
Sensitivity analyses were conducted in subsets of the analytic sample to explore whether results could be influenced by 1) extreme dietary intake values (outliers) or 2) potential incomplete urine collection. In addition, unweighted analyses were conducted to determine if results could be influenced by weighting the sample to the US population. Outliers for sodium and potassium intake, measured using 24HDR, were defined as outside the range of [25th percentile – 2.5IQR; 75th percentile + 2.5IQR] and with visual inspection (11). Potentially incomplete 24-h urine collection was defined by measured 24-h urinary creatinine excretion being < 70% of estimated 24-h urinary creatinine excretion based on age, sex, weight, and height. Two separate equations were used to calculate estimated 24-h urinary creatinine excretion (35, 36).
Results
The number of participants and weighted frequencies of demographic groups and BMI are shown overall and by sex in Table 1. After additional weighting for sample design and nonresponse, population estimates from the analytical sample (n = 776) were similar in their distribution of sex, age, race and Hispanic origin, and BMI to estimates from all nonpregnant adult participants aged 20–69 y examined in NHANES 2013–2014. Using the analytical sample, estimates from men and women were similar in distributions of age, and race and Hispanic origin, and BMI.
TABLE 1.
Estimated population characteristics, adults aged 20–69 y, US
| NHANES 2013–20141 N2 (%)3 | Analytical sample, NHANES 20144 N2 (%)5 | |||||
|---|---|---|---|---|---|---|
| Overall | Men | Women | Overall | Men | Women | |
| 4246 | 2056 | 2190 | 776 | 394 | 382 | |
| Age, y | ||||||
| 20–44 y | 2152 (50.6) | 1062 (52.5) | 1090 (48.8) | 406 (52.8) | 206 (55.3) | 200 (50.4) |
| 45–69 y | 2094 (49.4) | 994 (47.5) | 1100 (51.2) | 370 (47.2) | 188 (44.7) | 182 (49.6) |
| Race and Hispanic origin | ||||||
| Non-Hispanic white | 1729 (63.7) | 838 (64.0) | 891 (63.4) | 328 (64.6) | 172 (64.7) | 156 (64.5) |
| Non-Hispanic black | 879 (11.7) | 434 (11.0) | 445 (12.5) | 203 (11.3) | 102 (10.5) | 101 (12.1) |
| Hispanic | 1026 (16.1) | 490 (16.2) | 536 (16.0) | 132 (16.0) | 65 (16.9) | 67 (15.2) |
| Non-Hispanic other | 612 (8.5) | 294 (8.8) | 318 (8.2) | 113 (8.1) | 55 (7.9) | 58 (8.1) |
| BMI (kg/m2) | ||||||
| <30 | 2636 (61.7) | 1346 (64.5)6 | 1238 (59.1) | 454 (59.4) | 248 (62.2) | 206 (56.8) |
| ≥ 30 | 968 (38.3) | 700 (35.5) | 938 (40.9) | 322 (40.6) | 146 (37.8) | 176 (43.2) |
1Participants aged 20–69 y in NHANES 2013–2014, who came to the mobile examination center (MEC) and participated in the examination component and who completed ≥1 24-h dietary recall. Pregnant women were excluded.
2Unweighted number; participants in subgroups may not add up to the total sample size due to missing information on the designated characteristic.
3Weighted % based on dietary day 1 sample weights, NHANES 2013–2014.
4Participants in the 1-y 24-h urine collection in NHANES 2014 who also had information from dietary recalls.
5Weighted % based on 1-y 24-h urine sample weights, NHANES 2014.
6 P values <0.05 for difference in means between men and women for the specified subgroup.
The uncorrected 24HUE mean values for sodium, potassium, and their ratio differ from those corrected for percentage recovery of intake in urine (24HUE CF). As shown in Table 2, overall, sodium intake assessed using 24HUE CF (corrected for 90% recovery of sodium in urine; 4,017 mg/d (95% CI: 3,817, 4,217), was greater than the mean intake assessed using 24HDR (3,565 mg/d [CI: 3,365, 3,765]). Using 24HUE CF as the reference, mean bias in sodium assessed using 24HDR was −452 mg/d (CI: −646, −259; P value <0.05). Similarly, the mean bias was −487 mg/d (CI: −786, −188) for men and −420 mg/d (CI: −628, −212) for women. Thus, mean sodium intake, assessed using 24HDR, was 10 to 12% lower for US adults overall, for men, and for women, than 24HUE CF measures with 95% CIs that did not include zero.
TABLE 2.
Weighted mean and mean bias for sodium and potassium intake, mg/d, and Na/K, with and without applying a correction factor for sodium (0.90) and potassium (0.735) adults aged 20–69 y, US 20141
| Sodium | Potassium | Na/K ratio | ||
|---|---|---|---|---|
| N2 | Weighted mean (95% CI) | Weighted mean (95% CI) | Weighted mean (95% CI) | |
| Overall | 776 | |||
| Mean 24HDR | — | 3565 (3365, 3765) | 2642 (2494, 2791) | 1.46 (1.37, 1.55) |
| Mean 24HUE | — | 3616 (3435, 3796) | 2173 (2029, 2318) | 1.84 (1.68, 2.01) |
| Mean bias | — | h-51 (−235, 134) | 469 (361, 577)3 | −0.38 (−0.52, −0.24)3 |
| Mean 24HUE CF | — | 4017 (3817, 4217) | 2957 (2760, 3154) | 1.50 (1.37, 1.64) |
| Mean bias CF | −452 (−646, −259)3 | −315 (−450, −179)3 | −0.04 (−0.15, 0.07) | |
| Men | 394 | |||
| Mean 24HDR | — | 4172 (3899, 4445) | 2991 (2758, 3224) | 1.52 (1.42, 1.63) |
| Mean 24HUE | — | 4193 (3927, 4459) | 2422 (2266, 2577) | 1.92 (1.71, 2.14) |
| Mean bias | — | −21 (−304, 262) | 569 (345, 794)3 | −0.40 (−0.62, −0.18)3 |
| Mean 24HUE CF | — | 4658 (4363, 4954) | 3295 (3083, 3506) | 1.57 (1.39, 1.74) |
| Mean bias CF | −487 (−786, −188)3 | −304 (−550, −58)3 | −0.05 (−0.23, 0.14) | |
| Women | 382 | |||
| Mean 24HDR | — | 2994 (2837, 3151) | 2314 (2149, 2479) | 1.41 (1.31, 1.51) |
| Mean 24HUE | — | 3073 (2909, 3236) | 1940 (1776, 2104) | 1.77 (1.61, 1.92) |
| Mean bias | — | −79 (−275, 118) | 374 (209, 540)3 | −0.36 (−0.47, −0.25)3 |
| Mean 24HUE CF | — | 3414 (3232, 3596) | 2639 (2416, 2862) | 1.44 (1.31, 1.57) |
| Mean bias CF | −420 (−628, −212)3 | −325 (−527, −123)3 | −0.04 (−0.13, 0.06) |
1Mean of each individual's average intake from ≤2 24-h dietary recalls or 2 24-h urinary excretions. Mean bias = mean of each individual's average intake from ≤2 24-h dietary recalls minus the average intake from ≤2 24-h urinary excretions. 24HDR, 24-h dietary recalls; 24HUE, 24-h urinary excretions; CF, correction factor.
2Unweighted number of participants in the analytical sample.
3 P value <0.05 when comparing mean intake from 24-h dietary recalls with mean urinary excretion from 24-h urine collection.
Potassium intake, assessed using 24HDR, was significantly lower than that assessed using 24HUE CF (corrected for 73.5% recovery of potassium in urine) overall and by sex (P value <0.05 for each). Overall, mean 24HDR potassium was 2642 mg/d (CI: 2494, 2791), mean 24HUE CF potassium was 2957 mg/d (CI: 2760, 3154), and the mean bias was −315 mg/d (CI: −450, −179). The mean bias for 24HDR potassium was −304 mg/d (CI: −550, −58) for men and −325 mg/d (CI: −527, −123) for women. In contrast to sodium and potassium mean bias, the mean bias for the Na/K ratio, assessed using 24HDR, was unbiased, compared with 24HUE CF where the 95% CI includes zero for US adults overall and by sex.
The mean bias in intake assessed using 24HDR compared with 24HUE CF, significantly differed by age, race and Hispanic origin, and BMI status, but differences were not necessarily consistent by sex subgroup or across nutrients (sodium, potassium, or the Na/K ratio) (Table 3). Overall, for sodium, mean bias differed across BMI status groups (BMI ≥30 kg/m2: Mean bias = −927 mg/d [CI: −1205, −649]; BMI <30: Mean bias = −128 mg/d [CI: −377, 120]; P value <0.05). For potassium, mean bias differed by age group, where adults aged 45–69 y had a greater mean bias (−507 mg/d [CI: −673, −340]) than adults aged 20–44 y (−143 mg/d [CI: −347, 62]), P value <0.05. Mean bias also differed by race. The mean bias for non-Hispanic black adults was 74 mg/d (CI: −159, 306) and for Hispanic adults was −70 mg/d (CI: −326, 186) compared with non-Hispanic white adults, −440 mg/d (CI: −605, −274, P value <0.05). For the Na/K ratio, mean bias differed by race and Hispanic origin. Non-Hispanic black adults and Hispanic adults had a higher mean bias Na/K, −0.25 (CI: −0.42, −0.08) and −0.28 (CI: −0.55, 0.00) respectively, than non-Hispanic white adults (0.04 [CI: −0.04, 0.13]) (P value <0.05).
TABLE 3.
Weighted mean bias for sodium and potassium intake, mg/d, and Na/K, by population subgroups, correcting for urinary excretion for sodium (0.90) and potassium (0.735), adults aged 20–69 y, US 20141
| Mean bias (95% CI) | ||||
|---|---|---|---|---|
| N2 | Sodium | Potassium | Na/K ratio | |
| Overall | 776 | |||
| Age 20–44 y | 406 | −391(−679, −102) | −143 (−347, 62) | −0.08 (−0.23, 0.08) |
| Age 45–69 y | 370 | −521(−685, −358) | −507 (−673, −340)3 | 0.00 (−0.09, 0.08) |
| BMI <30 | 454 | −128 (−377, 120) | −203 (−402, −4) | 0.00 (−0.17, 0.16) |
| BMI ≥30 | 322 | −927 (−1205, −649)3 | −479 (−743, −214) | −0.09 (−0.19, 0.00) |
| Non-Hispanic white | 328 | −401 (−659, −143) | −440 (−605, −274) | 0.04 (−0.04, 0.13) |
| Non-Hispanic black | 203 | −566 (−900, −232) | 74 (−159, 306)3 | −0.25 (−0.42, −0.08)3 |
| Hispanic | 132 | −608 (−1157, −59) | −70 (−326, 186)3 | −0.28 (−0.55, 0.00) 3 |
| Non-Hispanic Other | 113 | −395 (−1070, 281) | −344 (−603, −84) | 0.06 (−0.30, 0.41) |
| Men | 394 | |||
| Age 20–44 y | 206 | −562 (−1020, −103) | −172 (−471, 127) | −0.12 (−0.39, 0.15) |
| Age 45–69 y | 188 | −394 (−604, −184) | −466 (−794, −138) | 0.05 (−0.08, 0.17) |
| BMI <30 | 248 | −61 (−468, 346) | −98 (−380, 184) | −0.02 (−0.26, 0.21) |
| BMI ≥30 | 146 | −1188 (−1571, −805)3 | −643 (−1036, −250)3 | −0.08 (−0.23, 0.07) |
| Non-Hispanic white | 172 | −474 (−822, −126) | −424 (−760, −88) | 0.02 (−0.14, 0.17) |
| Non-Hispanic black | 102 | −385 (−926, 155) | 218 (−86, 522)3 | −0.30 (−0.60, −0.01)3 |
| Hispanic | 65 | −802 (−1647, 44) | −163 (−478, 152) | −0.31 (−0.81, 0.19) |
| Non-Hispanic other | 55 | −57 (−895, 782) | −310 (−672, 52) | 0.33 (−0.19, 0.84) |
| Women | 382 | |||
| Age 20–44 y | 200 | −214 (−525, 97) | −112 (−344, 120) | −0.03 (−0.16, 0.10) |
| Age 45–69 y | 182 | −629 (−840, −418)3 | −541 (−764, −318)3 | −0.04 (−0.14, 0.05) |
| BMI <30 | 206 | −198 (−391, −5) | −311 (−548, −73) | 0.02 (−0.12, 0.15) |
| BMI ≥30 | 176 | −712 (−1090, −335)3 | −343 (−715, 28) | −0.10 (−0.23, 0.02) |
| Non-Hispanic white | 156 | −332 (−621, −43) | −454 (−688, −221) | 0.07 (−0.03, 0.16) |
| Non-Hispanic black | 101 | −714 (−1087, −341) | −44 (−407, 318)3 | −0.21 (−0.31, −0.10)3 |
| Hispanic | 67 | −406 (−907, 95) | 27 (−366, 420)3 | −0.24 (−0.39, −0.09)3 |
| Non-Hispanic other | 58 | −705 (−1501, 91) | −375 (−694, −55) | −0.19 (−0.59, 0.21) |
1Mean bias = Mean of each individual's average intake from ≤2 24-h dietary recalls minus the average intake from ≤2 24-h urinary excretions.
2Unweighted number of participants in the analytical sample.
3 P value <0.05 and calculated using pairwise t-test when comparing mean bias across subgroups with ref = age 20–44 y for age, ref = BMI < 30 for BMI, and ref = non-Hispanic white for race/Hispanic origin.
By sex subgroup, for sodium, mean bias differed across BMI subgroups. Adults with BMI ≥30 had a greater mean bias than adults with BMI <30 for both men (−1,188 mg/d [CI: −1,571, −805] compared with −61 mg/d [CI: −468, 346], P value <0.05) and women (−712 mg/day [CI: −1,090, −335] compared with −198 [CI: −391, −5], P value <0.05). For women, mean bias differed significantly by age group where women aged 45–69 y had a mean bias of −629 mg/d (CI: −840, −418) and women aged 20–44 y, −214 mg/d (CI: −525, 97) (P value <0.05). For potassium, mean bias differed by race and Hispanic origin for both men and women. Non-Hispanic black men had a mean bias of 218 mg/d (CI: −86, 522) compared with non-Hispanic white men −424 mg/d (CI: −760, −88; P value <0.05). Similarly, non-Hispanic black women had a mean bias of −44 (CI: −407, 318) and Hispanic women, 27 mg/d (CI: −366, 420), compared with non-Hispanic white women who had a mean bias of −454 mg/d (CI: −688, −221; P value <0.05).
For the Na/K ratio, mean bias varied by race and Hispanic origin for both men and women. Non-Hispanic black adults, compared with non-Hispanic white adults, had higher a Na/K ratio for men (−0.30 [CI: −0.60, −0.01] compared with 0.02 [CI: −0.14, −0.17], P value <0.05), and for women (−0.21 [CI: −0.31, −0.10] compared with 0.07 [CI: −0.03, 0.16], P value <0.05). Hispanic women had greater mean bias (−0.24 mg/d [CI: −0.39, −0.09]) than non-Hispanic white women (P value <0.05).
The weighted attenuation factors overall and by sex for sodium, potassium, and Na/K ratios were similar with and without correcting for percentage recovery of intake in urine (Table 4). Overall, the attenuation factors, when correcting for urinary excretion, for using a single 24HDR were low: 0.16 (CI: 0.09, 0.21) for sodium, 0.25 (CI: 0.16, 0.36) for potassium, and 0.20 (CI: 0.10, 0.25) for the Na/K ratio.
TABLE 4.
Weighted and adjusted attenuation factor for sodium, potassium, Na/K overall, men, and women with and without applying a correction factor for sodium (0.90) and potassium (0.735)1
| Attenuation factor, λ (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Sodium | ||||||
| Overall (N = 776) | Men (N = 394) | Women (N = 382) | ||||
| Without CF | CF | Without CF | CF | Without CF | CF | |
| 1 24HDR | 0.14 (0.08, 0.21) | 0.16 (0.09, 0.21) | 0.15 (0.07, 0.24) | 0.17 (0.08, 0.20) | 0.12 (0.02, 0.23)2 | 0.14 (0.02, 0.10)2 |
| 2 24HDR | 0.21 (0.11, 0.30) | 0.23 (0.13, 0.30) | 0.21 (0.09, 0.33) | 0.24 (0.11, 0.27) | 0.19 (0.03, 0.35)2 | 0.21 (0.03, 0.15)2 |
| 3 24HDR | 0.24 (0.13, 0.35) | 0.27 (0.14, 0.35) | 0.25 (0.10, 0.39) | 0.27 (0.12, 0.31) | 0.23 (0.03, 0.43)2 | 0.26 (0.03, 0.18)2 |
| 6 24HDR | 0.29 (0.15, 0.43) | 0.32 (0.17, 0.40) | 0.29 (0.11, 0.47)2 | 0.32 (0.13, 0.35)2 | 0.30 (0.03, 0.56)2 | 0.33 (0.03, 0.21)2 |
| Infinite 24HDR | 0.36 (0.16, 0.56) | 0.40 (0.18, 0.47) | 0.35 (0.10, 0.60)2 | 0.39 (0.12, 0.38)2 | 0.41 (0.00, 0.82)2 | 0.46 (0.00, 0.23)2 |
| Potassium | ||||||
| Overall (N = 776) | Men (N = 394) | Women (N = 382) | ||||
| Without CF | CF | Without CF | CF | Without CF | CF | |
| 1 24HDR | 0.19 (0.11, 0.26) | 0.25 (0.16, 0.36) | 0.14 (0.05, 0.24)2 | 0.20 (0.07, 0.19)2 | 0.24 (0.12, 0.37) | 0.33 (0.16, 0.40) |
| 2 24HDR | 0.24 (0.14, 0.34) | 0.33 (0.19, 0.44) | 0.19 (0.06, 0.32)2 | 0.26 (0.08, 0.25)2 | 0.31 (0.11, 0.51)2 | 0.42 (0.15, 0.43)2 |
| 3 24HDR | 0.27 (0.15, 0.39) | 0.37 (0.20, 0.48) | 0.21 (0.06, 0.36)2 | 0.29 (0.08, 0.27)2 | 0.34 (0.10, 0.58)2 | 0.46 (0.13, 0.43)2 |
| 6 24HDR | 0.30 (0.16, 0.45) | 0.41 (0.22, 0.52) | 0.24 (0.06, 0.42)2 | 0.33 (0.09, 0.29)2 | 0.38 (0.07, 0.68)2 | 0.51 (0.10, 0.40)2 |
| Infinite 24HDR | 0.34 (0.17, 0.52) | 0.47 (0.22, 0.56) | 0.28 (0.06, 0.50)2 | 0.38 (0.09, 0.32)2 | 0.42 (0.02, 0.82)2 | 0.58 (0.03, 0.34)2 |
| Na/K ratio | ||||||
| Overall (N = 776) | Men (N = 394) | Women (N = 382) | ||||
| Without CF | CF | Without CF | CF | Without CF | CF | |
| 1 24HDR | 0.24 (0.12, 0.36) | 0.20 (0.10, 0.25) | 0.25 (0.08, 0.42)2 | 0.20 (0.07, 0.20)2 | 0.24 (0.05, 0.42) | 0.19 (0.04, 0.16)2 |
| 2 24HDR | 0.38 (0.20, 0.55) | 0.31 (0.16, 0.39) | 0.36 (0.12, 0.60)2 | 0.30 (0.10, 0.30)2 | 0.39 (0.11, 0.66) | 0.32 (0.09, 0.30)2 |
| 3 24HDR | 0.46 (0.25, 0.67) | 0.38 (0.20, 0.49) | 0.43 (0.15, 0.71)2 | 0.35 (0.12, 0.35)2 | 0.50 (0.17, 0.83)2 | 0.40 (0.14, 0.40)2 |
| 6 24HDR | 0.60 (0.32, 0.87) | 0.49 (0.26, 0.63) | 0.52 (0.17, 0.87)2 | 0.43 (0.14, 0.43)2 | 0.68 (0.26, 1.10)2 | 0.56 (0.21, 0.59)2 |
| Infinite 24HDR | 0.84 (0.39, 1.29) | 0.69 (0.32, 0.81) | 0.67 (0.21, 1.13)2 | 0.55 (0.17, 0.53)2 | 1.10 (0.34, 1.85)2 | 0.90 (0.28, 0.86)2 |
1Weighted estimates calculated using PROC Calis and adjusting for sex (when applicable), age, race and Hispanic origin, and BMI. 24HDR, 24-h dietary recalls; 24HUE, 24-h urinary excretions; CF, correction factor.
2Relative standard error >30%.
If the mean of 2 24HDRs was used to assess sodium intake, the attenuation factor in relation to use of usual 24HUE CF was estimated as 0.23, whereas if the mean of an infinite number of 24HDRs was used, the attenuation factor was 0.40. For potassium, if the mean of 2 24HDRs was used, the attenuation factor was 0.33, whereas using an infinite number of 24HDRs, the attenuation factor was 0.47. For the Na/K ratio, the attenuation factors were estimated at 0.31 and 0.69, for the mean of 2 and the mean of an infinite number of 24HDRs, respectively. Attenuation factors for sodium, potassium, and the Na/K ratio were similar by sex, but the relative SEs were >30% among most sex subgroups.
Correlations between the 2 methods are shown in Table 5 for sodium, potassium, and the Na/K ratio. Similar to attenuation factors, correlation coefficients between sodium intake measured using a single 24HDR and usual 24HUE CF were low; 0.27 (CI: 0.16, 0.37) overall, 0.27 (CI: 0.14, 0.34) for men, and 0.30 (CI: 0.03, 0.20) for women. For potassium, the correlation coefficient was 0.35 (CI: 0.26, 0.55) overall, 0.32 (CI: 0.12, 0.34) for men, and 0.39 (CI: 0.30, 0.63) for women. The correlation coefficient for the Na/K ratio was 0.27 (CI: 0.13, 0.32) overall, 0.23 (CI: 0.02, 0.14) for men, and 0.35 (CI: 0.13, 0.36) for women.
TABLE 5.
Weighted and adjusted correlation coefficient for sodium, potassium, Na/K overall, men, and women with and without applying a correction factor for sodium (0.90) and potassium (0.735)1
| Correlation coefficient (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Sodium | ||||||
| Overall (N = 776) | Men (N = 394) | Women (N = 382) | ||||
| Without CF | CF | Without CF | CF | Without CF | CF | |
| 1 24HDR | 0.27 (0.16, 0.38) | 0.27 (0.16, 0.37) | 0.27 (0.13, 0.41) | 0.27 (0.14, 0.34) | 0.30 (0.03, 0.58)2 | 0.30 (0.03, 0.20)2 |
| 2 24HDR | 0.33 (0.19, 0.46) | 0.33 (0.20, 0.45) | 0.32 (0.16, 0.48) | 0.32 (0.16, 0.40) | 0.37 (0.02, 0.73)2 | 0.37 (0.03, 0.24)2 |
| 3 24HDR | 0.35 (0.21, 0.50) | 0.35 (0.21, 0.49) | 0.34 (0.17, 0.51) | 0.34 (0.17, 0.43) | 0.41 (0.03, 0.80)2 | 0.41 (0.03, 0.25)2 |
| 6 24HDR | 0.39 (0.22, 0.55) | 0.39 (0.23, 0.53) | 0.37 (0.18, 0.56) | 0.37 (0.18, 0.46) | 0.47 (0.02, 0.92)2 | 0.47 (0.02, 0.26)2 |
| Infinite 24HDR | 0.43 (0.24, 0.62) | 0.43 (0.25, 0.58) | 0.41 (0.19, 0.63) | 0.41 (0.20, 0.49) | 0.55 (0.00, 1.12)2 | 0.55 (−0.02, 0.25)2 |
| Potassium | ||||||
| Overall (N = 776) | Men (N = 394) | Women (N = 382) | ||||
| Without CF | CF | Without CF | CF | Without CF | CF | |
| 1 24HDR | 0.35 (0.25, 0.45) | 0.35 (0.26, 0.55) | 0.32 (0.12, 0.52)2 | 0.32 (0.12, 0.34)2 | 0.39 (0.30, 0.48) | 0.39 (0.30, 0.63) |
| 2 24HDR | 0.40 (0.29, 0.52) | 0.40 (0.29, 0.62) | 0.37 (0.13, 0.61)2 | 0.37 (0.13, 0.38)2 | 0.44 (0.32, 0.55) | 0.44 (0.32, 0.69) |
| 3 24HDR | 0.42 (0.30, 0.54) | 0.42 (0.30, 0.65) | 0.39 (0.14, 0.64)2 | 0.39 (0.14, 0.40)2 | 0.46 (0.33, 0.58) | 0.46 (0.33, 0.71) |
| 6 24HDR | 0.45 (0.31, 0.58) | 0.45 (0.32, 0.69) | 0.42 (0.14, 0.70)2 | 0.42 (0.14, 0.42)2 | 0.48 (0.33, 0.63) | 0.48 (0.33, 0.72) |
| Infinite 24HDR | 0.48 (0.33, 0.63) | 0.48 (0.33, 0.72) | 0.45 (0.14, 0.76)2 | 0.45 (0.14, 0.44)2 | 0.51 (0.32, 0.70) | 0.51 (0.32, 0.73) |
| Na/K ratio | ||||||
| Overall (N = 776) | Men (N = 394) | Women (N = 382) | ||||
| Without CF | CF | Without CF | CF | Without CF | CF | |
| 1 24HDR | 0.27 (0.12, 0.42) | 0.27 (0.13, 0.32) | 0.23 (0.01, 0.46)2 | 0.23 (0.02, 0.14)2 | 0.35 (0.12, 0.57)2 | 0.35 (0.13, 0.36)2 |
| 2 24HDR | 0.34 (0.16, 0.52) | 0.34 (0.16, 0.41) | 0.28 (0.01, 0.55)2 | 0.28 (0.02, 0.17)2 | 0.44 (0.17, 0.71)2 | 0.44 (0.18, 0.49)2 |
| 3 24HDR | 0.38 (0.18, 0.57) | 0.38 (0.18, 0.45) | 0.31 (0.02, 0.59)2 | 0.31 (0.02, 0.19)2 | 0.50 (0.21, 0.79) | 0.50 (0.21, 0.57) |
| 6 24HDR | 0.43 (0.20, 0.65) | 0.43 (0.21, 0.52) | 0.34 (0.02, 0.66)2 | 0.34 (0.03, 0.21)2 | 0.59 (0.27, 0.91) | 0.59 (0.27, 0.70) |
| Infinite 24HDR | 0.51 (0.24, 0.77) | 0.51 (0.25, 0.62) | 0.38 (0.02, 0.74)2 | 0.38 (0.03, 0.24)2 | 0.74 (0.38, 1.11) | 0.74 (0.39, 0.94) |
1Weighted estimates calculated using PROC Calis and adjusting for sex (when applicable), age, race and Hispanic origin, and BMI. 24HDR, 24-h dietary recalls; 24-h urinary excretions; CF, correction factor.
2RSE >30%.
Similarly, modeling the use of the mean of 2 24HDRs to estimate sodium intake, for sodium the correlation with usual 24HUE was estimated at 0.33, whereas using the mean of an infinite number of 24HDRs to approximate usual long-term intake, the correlation was 0.43. For potassium the correlation using 2 24HDRs was 0.40 and 0.48 when using an infinite number of 24HDRs overall. Similar results were found when stratified by sex. Correlation for the Na/K ratio was 0.34 using the mean intake of 2 24HDRs and 0.51 using an infinite number of 24HDRs overall. Relative SEs were >30% for most correlations when stratified by sex for sodium, potassium, and the Na/K ratio. Regardless of the application of a correction factor, estimates of attenuation factor and correlation coefficient remained low for 24HDR overall and by sex for sodium, potassium, and the Na/K ratio.
Weighted mean intake assessed using 24HDR and 24HUE for sodium, potassium, and the Na/K ratio stratified by race and Hispanic origin are detailed in Supplemental Table 1. Weighted mean intake assessed using 24HDR and 24HUE for sodium, potassium, and the Na/K ratio were similar after excluding outliers and after excluding participants with <70% of expected 24-h urinary creatinine excretion, see Supplemental Tables 2–4. Unweighted, adjusted attenuation factors, and correlation coefficients overall and for men and women were similar to the weighted values, see Supplemental Tables 5 and 6. Additionally, attenuation and correlation were similar for sodium despite excluding extreme outliers or participants with <70% of expected 24-h urinary creatinine, see Supplemental Tables 7–9. The measurement error structure is shown in Supplemental Table 10. The variance of within-person error for sodium, assessed using both 24HUE and 24HDR, was significantly higher than for potassium. The ratio of person-specific bias of the Na/K ratio assessed using 24HDR to variance of true intake, as estimated using the Kipnis model, was significantly lower than each nutrient alone.
Discussion
This study is novel in its application of the Kipnis model to data from a national survey in order to understand the implications of measurement error in 24HDR for determining mean sodium and potassium intake in a US population and for evaluating associations with health outcomes in observational studies (11). Our findings indicate that the 24HDR may underestimate mean sodium intake for the US population, overall and by sex, and for those with obesity, after applying correction factors that estimate percentage recovery in 24HUE. Similarly, the 24HDR may underestimate mean potassium intake, where estimates of mean intake using 24HDR are significantly lower than estimates based on 24HUE overall and by sex. Furthermore, mean potassium intake, assessed using 24HDR when compared with 24HUE, varied by race, which may reflect underestimation of potassium intake using 24HUE for some population subgroups (19). In contrast to sodium and potassium alone, assessment of the Na/K ratio using 24HDR was unbiased overall and by sex. Additionally, the low attenuation factors and correlations suggest that the true associations of health outcomes with sodium or potassium intake and their ratio can be substantively attenuated by both random and systematic error when intake is assessed using 24HDR.
In general, despite differences in participant characteristics, study methods, and analysis, results of mean bias, attenuation, and correlation for sodium and potassium in the current study were generally consistent with previous studies (20, 21, 37–40). Prior studies evaluating mean bias in population intake, attenuation, and correlation used a factor for both sodium and potassium to correct for the amount of intake excreted in urine (20, 39, 40). Although no one correction factor is universally applied, our results indicate the application of a correction factor affects estimates of bias, whereas attenuation factors and correlations are similar. Although differential correction factors for population subgroups such as various racial-ethnic groups are not yet available, when an overall correction factor is applied, the 24HDR underestimated mean sodium and potassium intake overall, for men, and for women and the 24HDR was unbiased for estimating the mean Na/K ratio. Whereas 24-h urinary sodium excretion captures all sources of sodium consumed, sodium intake based on 24HDR excludes salt added at the table and assumes the consistent addition of salt in home food preparation (41, 42). Furthermore, variability in urinary excretion of sodium and potassium intake overall and by race, as demonstrated in previous feeding trials, may influence the magnitude of estimation for sodium and potassium (16–19, 43, 44).
Similar to previous studies (21, 38), among individuals with obesity, 24HDR significantly underestimated mean population sodium intake when compared with 24HUE sodium excretion, overall, among men, and, to a lesser degree, among women. Inconsistent with a previous study (38), 24HDRs in the current study underestimated sodium intake among older women, but not men. One potential explanation is that energy and sodium intake are highly correlated (38). Individuals who are obese are more likely to underreport the amount of food they consume and thus, energy and sodium intake (38), and a greater proportion of women than men were obese in the current study. Underestimation of sodium intake with 24HDR among individuals with obesity and older adults may also be attributable in part to a greater consumption of salt added at the table or during home food preparation (42).
The relation between mean bias for potassium and race/Hispanic origin was consistent with a prior study by Turban et al. (19), where non-Hispanic white adults had greater urinary potassium excretion than non-Hispanic black adults. Turban et al. found that among participants with prehypertension and hypertension, when non-Hispanic black adults consumed the same Dietary Approaches to Stop Hypertension diets as non-Hispanic white adults, black participants had lower urinary potassium excretion. The lower urinary potassium excretion observed among non-Hispanic black adults may reflect differences in percentage recovery of ingested potassium in urine. Although studies are limited, possible causes for this observation include differences in potassium excretion from other routes (stool, sweat, and insensible losses), differences in total body potassium concentrations, and genetic differences in renal potassium handling (aldosterone secretion and plasma renin activity) and retention (19, 45–49).
Estimates of attenuation and correlation for a single 24HDR were low in the current study, however, more variation and somewhat higher values were observed across studies pooled by Freedman et al. (19). The differences in results across studies may reflect the differences in study participants. For example, previous studies were conducted in a more homogenous convenience sample of adults with respect to age, sex, and race and Hispanic origin. Additionally, low estimates of attenuation and correlation may partly be explained by greater random error including within-person variability. The Kipnis model accounts for contributions of intake-related bias, person-specific bias, and within-person variability (11). For instance, our results show that in unadjusted analyses, attenuation factors and correlation coefficients are low. However, as the number of 24HDRs used per individual is increased to an infinite number to approximate usual long-term intake adjusting for random error due to within-person day-to-day variability, attenuation factors and correlation coefficients are somewhat higher, and more consistent with the results of Freedman et al. using different data with the same model (20).
Our study findings, in addition to the findings from the Freedman study, suggest that evaluation of the association of sodium and potassium intake, assessed by 24HDR, with health outcomes would be strongly biased towards the null. For example, Jackson et al. studied the association between urinary sodium and potassium excretion and blood pressure among adults in the US using NHANES 2014 24-h urinary sodium and potassium measures adjusted for within-person error (5). The study found, for example, that on average, a 4.58 mmHg higher systolic blood pressure was associated with each gram of sodium intake as measured using estimated usual urinary sodium excretion. Using the Jackson study as our reference for the association of blood pressure with usual sodium intake and the attenuation factor estimated in the current study, the association observed using a single 24HDR to estimate sodium intake would have been 0.73 mmHg higher systolic blood pressure per gram or 16% of the true association. If we completely adjust for random error, using multiple 24HDR to calculate usual sodium intake, the estimated increase in systolic blood pressure would be 1.83 mmHg per gram or 40% of the true association. The lack of association between sodium and potassium intake and health outcomes in previous observational studies could be partly explained by this measurement error.
Similar to our study results, Freedman et al. found that Na/K ratios had the highest attenuation factors and correlations among the components investigated (20). Lower measurement error may be one explanation for higher observed associations when using the Na/K ratio rather than sodium alone to assess intake with 24HDRs in observational studies of health outcomes. For example, Yang et al. found that a higher Na/K ratio, assessed using 24HDRs, was associated with a significantly increased risk of CVD and all-cause mortality. Results from our study suggest that there is less attenuation associated with using the Na/K ratio and might explain why the Na/K ratio, assessed using 24HDR, shows a stronger association with CVD and all-cause mortality, when compared with sodium alone (50). Measurement error, e.g. due to underreporting of intake, associated with either sodium or potassium when assessed separately, may be diminished when using the ratio (20).
Results from this study are subject to limitations. First, due to the small sample size, estimates of attenuation and correlation across demographic and BMI status by sex had a relative SE >30% and may be unreliable. Second, results are only generalizable to nonpregnant adults aged 20–69 y. Third, unaccounted factors related to nonresponse may affect generalizability. Fourth, 24HUE also has limitations where the accuracy of estimates relies on collection of all urine over the 24-h period. Fifth, estimates of sodium intake based on 24HDR exclude salt added at the table. Furthermore, the percentages of ingested sodium or potassium recovered in urine were estimated from feeding studies with small convenience samples whose characteristics differ from the population included in this study. Thus, the correction factors based on percentage recovery may be less accurate for some subgroups.
The findings from this study suggest that, compared with 24HUE, the 24HDR as used in NHANES might be a biased measure for estimating mean sodium and mean potassium intake for the US population overall and by sex. However, the 24HDR was not biased for estimating the Na/K ratio. Furthermore, low attenuation factors and correlations suggest associations of health outcomes with both sodium and potassium, using 24HDR, are strongly biased towards the null. The lack of associations of health outcomes with sodium and potassium intake and higher associations when using the Na/K ratio in previous observational studies using 24HDRs could be partly explained by measurement error. Results of the measurement error structure from our study could potentially be applied to future work to better assess mean sodium and potassium intake in the US population and associations with health outcomes in observational studies.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the contribution of Victor Kipnis and Doug Midthune for planning and creating the Kipnis model and their assistance with its application to NHANES 2014.
The authors’ contributions were as follows——PV , KWD, LZ, AMT-P, CIM, ALT, SLJ, CW, CML, AJM, DGR, and MEC: designed the research; PV, LZ, MEC: analyzed data or performed statistical analysis; PV, KWD, AMT-P, MEC: wrote the manuscript; PV, KWD, MEC: had primary responsibility for final content; KWD, ALT, CW, AJM, DGR: provided essential reagents or provided essential materials; ALT, CW: conducted research; PV, KWD, LZ, AMT-P, CIM, ALT, SLJ, CW, CML, AJM, DGR, MEC: performed other-data interpretation; and all authors: read and approved the final manuscript.None of the authors report a conflict of interest related to the research presented in this article.
Notes
This study was funded by Epidemic Intelligence Service, Centers for Disease Control and Prevention.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the National Cancer Institute, the National Heart, Lung, and Blood Institute; the NIH; or the US Department of Health and Human Services.
Supplemental Tables 1–10 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CVD, cardiovascular disease; 24HDR, 24-h dietary recall; 24HUE, 24-h urinary excretions.
References
- 1. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW et al.. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;71:2199-2269:[]. [DOI] [PubMed] [Google Scholar]
- 2. Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, Lim S, Danaei G, Ezzati M, Powles J. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371(7):624–34. [DOI] [PubMed] [Google Scholar]
- 3. Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: Systematic review and meta-analyses. BMJ. 2013;346:f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Department of Health and Human Services, Department of Agriculture. Dietary guidelines for Americans 2015–2020. [Internet]. 8th ed 2015. Available from: http://health.gov/dietaryguidelines/2015/guidelines (Accessed 27 April 2018). [Google Scholar]
- 5. Jackson SL, Cogswell ME, Zhao L, Terry AL, Wang C, Wright J, Coleman King SM, Bowman B, Chen T, Merritt R et al.. Association between urinary sodium and potassium excretion and blood pressure among adults in the United States National Health and Nutrition Examination Survey 2014. Circulation. 2018;137:237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Newberry SJ, Chung M, Anderson C, Fu W, Chen C, Tang A, Zhao N, Booth M, Marks J, Hollands S et al.. Effects of dietary sodium and potassium intake on chronic disease outcomes and related risk factors. Systematic Review No. 206. [Internet]. (Prepared by the Southern California Evidence-based Practice Center under Contract No. 290-2015-00010-I). AHRQ Publication No. 18-EHC009-EF Rockville, MD: Agency for Healthcare Research and Quality; 2018. Available from: www.effectivehealthcare.ahrq.gov/reports/final.cfm. [Google Scholar]
- 7. Van Buren L, Dötsch-Klerk M, Seewi G, Newson RS. Dietary impact of adding potassium chloride to foods as a sodium reduction technique. Nutrients. 2016;8(4):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Binia A, Jaeger J, Hu Y, Singh A, Zimmermann D. Daily potassium intake and sodium-to-potassium ratio in the reduction of blood pressure: A meta-analysis of randomized controlled trials. J Hypertens. 2015;33(8):1509–152. [DOI] [PubMed] [Google Scholar]
- 9. Cook NR, Obarzanek E, Cutler JA, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK; Trials of Hypertension Prevention Collaborative Research Group. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: The trials of hypertension prevention follow-up study. Arch Intern Med. 2009;169(1):32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cook NR, Appel LJ, Whelton PK. Lower levels of sodium intake and reduced cardiovascular risk. Circulation. 2014;129(9):981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kipnis V, Subar AF, Midthune D, Freedman LS, Ballard-Barbash R, Troiano RP, Bingham S, Schoeller DA, Schatzkin A, Carrol RJ. Structure of dietary measurement error: Results of the OPEN biomarker study. Am J Epidemiol. 2003;158:14–21. [DOI] [PubMed] [Google Scholar]
- 12. Institute of Medicine. Strategies to reduce sodium intake in the United States. Washington, DC: National Academies Press; 2010. [Google Scholar]
- 13. Cogswell ME.and Frieden TR.(2016) Dietary Sodium and Cardiovascular Disease Risk. N. Engl. J. Med. 375, 2407–2408. 10.1056/NEJMc161230427974027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cogswell ME, Maalouf J, Elliott P, Loria CM, Patel S, Bowman BA. Use of urine biomarker to assess sodium intake: Challenges and opportunities. Ann Rev Nutr. 2015;35:349–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johansson G, Akesson A, Berglund M, Nermell B, Vahter M. Validation with biological markers for food intake of a dietary assessment method used by Swedish women with three different dietary preferences. Public Health Nutr. 1998;1:199–206. [DOI] [PubMed] [Google Scholar]
- 16. Mickelson O, Makdani D, Gill JL, Frank RL.. Sodium and potassium intakes and excretions of normal men consuming sodium chloride or a 1:1 mixture of sodium and potassium chlorides. Am J Clin Nutr. 1997;30:2033–40. [DOI] [PubMed] [Google Scholar]
- 17. Holbrook JT, Patterson KY, Bodner JE, Douglas LW, Veillon C, Kelsay JL, Mertz W, Smith JC Jr. Sodium and potassium intake and balance in adults consuming self-selected diets. Am J Clin Nutr. 1984;40(4):786–93. [DOI] [PubMed] [Google Scholar]
- 18. Tasevska N, Runswick SA, Bingham SA. Urinary potassium is as reliable as urinary nitrogen for use as a recovery biomarker in dietary studies of free living individuals. J Nutr. 2006;136(5):1334–40. [DOI] [PubMed] [Google Scholar]
- 19. Turban S, Thompson CB, Parekh RS, Appel LJ. Effects of sodium intake and diet on racial differences in urinary potassium excretion: Results from the DietaryApproaches to Stop Hypertension (DASH)-Sodium trial. Am J Kidney Dis. 2013;61(1):88–95. [DOI] [PubMed] [Google Scholar]
- 20. Freedman LS, Commins JM, Moler JE, Willett W, Tinker LF, Subar AF, Spiegelman D, Rhodes D, Potischman N, Neuhouser ML et al.. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for potassium and sodium intake. Am J Epidemiol. 2015;181(7):473–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mercado CI, Cogswell ME, Valderrama AL, Wang CY, Loria CM, Moshfegh AJ, Rhodes DG, Carriquiry AL. Difference between 24 h diet recall and urine excretion for assessing population sodium and potassium intake in adults aged 18–39 y. Am J Clin Nutr. 2015;101(2):376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention (CDC). National Center for Health Statistics. National Health and Nutrition Examination Survey. NHANES 2013–2014. [Internet]. Available from: https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2013 (Accessed 27 April 2018). [Google Scholar]
- 23. Centers for Disease Control and Prevention (CDC), National Center for Health Statistics. National Health and Nutrition Examination Survey. Questionnaires, Datasets, and Related Documentation. [Internet]. Response Rates Available from: https://wwwn.cdc.gov/nchs/nhanes/ResponseRates.aspx (Accessed 27 April 2018). [Google Scholar]
- 24. Cogswell ME, Loria CM, Terry AL, Zhao L, Wang CY, Chen TC, Wright JD, Pfeiffer CM, Merritt R, Moy CS et al.. Estimated 24-hour urinary sodium and potassium excretion in US adults. JAMA. 2018;319(12):1209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Terry AL, Cogswell ME, Wang CY, Chen TC, Loria CM, Wright JD, Zhang X, Lacher DA, Merritt RK, Bowman BA. Feasibility of collecting 24-h urine to monitor sodium intake in the National Health and Nutrition Examination Survey. Am J Clin Nutr. 2016;104(2):480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. National Center for Health Statistics, Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: 24-hour urine study procedures manual. [Internet]. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/manuals/24_Hour_Urine_Study_Procedures_Manual.pdf (Accessed 11 May 20). [Google Scholar]
- 27. Centers for Disease Control and Prevention. National Center for Environmental Health: Laboratory procedures manual. [Internet]. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/labmethods/URLT_H_R_MET_Electrolytes.pdf (Accessed 5 May 2017). [Google Scholar]
- 28. Centers for Disease Control and Prevention, National Health and Nutrition Examination Survey. Laboratory procedure manual. [Internet]. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/labmethod/U1KM_H_R_MET_CREATININE.pdf (Accessed 5 May 2017). [Google Scholar]
- 29. National Center for Health Statistics, Centers for Disease Control and Prevention (CDC). National health and nutrition examination survey. [Internet]. 2014-2014 Data Documentation, Codebook, and Frequencies. 24-hour Urine Collection Data Process- First Collection (UR1_H_R). March2016. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/limited_access/UR1_H_R.htm (Accessed 1 May 2017). [Google Scholar]
- 30. National Center for Health Statistics, Centers for Disease Control and Prevention (CDC). National health and nutrition examination survey. 2014-2014 Data Documentation, Codebook, and Frequencies. 24-hour Urine Collection Data Process- Second Collection (UR2_H_R). March2016. [Google Scholar]
- 31. National Center for Health Statistics, Centers for Disease Control and Prevention. National health and nutrition examination survey: MEC in-person dietary interviewers procedure manual. [Internet]. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/manuals/mec_in_person_dietary_procedures_manual_jan_2014.pdf (Accessed 11 May 2018). [Google Scholar]
- 32. National Center for Health Statistics, Centers for Disease Control and Prevention. National health and nutrition examination survey: Phone follow-up dietary interviewer procedures manual. [Internet]. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/manuals/Phone_Follow-up_Dietary_Interviewers_Manual.pdf (Accessed 11 May 2018). [Google Scholar]
- 33. Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, Ingwersen LA et al.. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88:324–32. [DOI] [PubMed] [Google Scholar]
- 34. National Center for Health Statistics, Centers for Disease Control and Prevention. National health and nutrition examination survey: 2014-2014 Data documentation, codebook, and frequencies. [Internet]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/limited_access/UR1_H_R.htm (Accessed 11 May 2017). [Google Scholar]
- 35. Joossens JV, Geboers J. Monitoring salt intake of the population: Methodological considerations. In: De Backer GG, Pedo HT, Ducimetiere P, editors. Surveillance of the dietary habits of the population with regard to cardiovascular diseases, EURONUT report 2. Wageningen (The Netherlands): Department of Human Nutrition, Agricultural University; 1984;61–73. [Google Scholar]
- 36. Mage DT, Allen RH, Kodali A. Creatinine corrections for estimating children's and adult's pesticide intake doses in equilibrium with urinary pesticide and creatinine concentrations. J Expo Sci Environ Epidemiol. 2008;18:360–8. [DOI] [PubMed] [Google Scholar]
- 37. Rhodes DG, Murayi T, Clemens JC, Baer DJ, Sebastian RS, Moshfegh AL. The USDA automated multiple-pass method accurately assess population sodium intakes. Am J Clin Nutr. 2013;97:958–64. [DOI] [PubMed] [Google Scholar]
- 38. Huang Y, Van Horn L, Tinker LF, Neuhouser ML, Carbone L, Mossavar-Rahmani Y, Thomas F, Prentice RL. Measurement error corrected sodium and potassium intake estimation using 24-hour urinary excretion. Hypertension. 2014;63(2):238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Park Y, Dodd KW, Kipnis V, Thompson FE, Potischman N, Schoeller DA, Baer DJ, Midthune D, Troiano RP, Bowles H et al.. Comparison of self-reported dietary intakes from the automated self-administered 24-h recall, 4-d food records, and food-frequency questionnaires against recovery biomarkers. Am J Clin Nutr. 2018;107(1):80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Freedman LS, Midthune D, Carroll RJ, Krebs-Smith S, Subar AF, Troiano RP, Dodd K, Schatzkin A, Ferrari P, Kipnis V. Adjustments to improve the estimation of usual dietary intake distributions in the population. J Nutr. 2004;134:1836–43. [DOI] [PubMed] [Google Scholar]
- 41. Sebastian RS, Enns CW, Steinfeldt LC, Goldman JD, Moshfegh AJ. Discontinuation of data processing step: Salt adjustment on designated foods likely to be home prepared. [Internet]. Available from: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/0910/discontinuation%20of%20data%20processig%20step-salt%20adjustment.pdf (Accessed 24 May 2018). [Google Scholar]
- 42. Harnack LJ, Cogswell ME, Shikany JM, Gardner CD, Gillespie C, Loria CM, Zhou X, Yuan K, Steffen LM. Sources of sodium in US adults from 3 geographic regions. Circulation. 2017;135:1775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lucko AM, Doktorchik C, Woodward M, Cogswell M, Neal B, Rabi D, Anderson C, He FJ, MacGregor GA, L'Abbe MTRUE Consortium, et al., TRUE Consortium . Percentage of ingested sodium excreted in 24-hour urine collections: A systematic review and meta-analysis. J Clin Hypertens (Greenwich). 2018;20(9):1220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chatterjee R, Yeh HC, Shafi T, Anderson C, Pankow JS, Miller ER, Levine D, Selvin E, Brancati FL. Serum potassium and the racial disparity in diabetes risk: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2011;93(5):1087–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Palacios C, Wigertz K, Martin BR, Braun M, Pratt JH, Peacock M, Weaver CM. Racial differences in potassium homeostasis in response to differences in dietary sodium in girls. The Am J Clin Nutr. 2010;91(3):597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wong CM, O'Connor DT, Martinez JA, Kailasam MT, Parmer RJ. Diminished renal kallikrein responses to mineralocorticoid stimulation in African Americans: Determinants of an intermediate phenotype for hypertension. Am J Hypertens. 2003;16:281–9. [DOI] [PubMed] [Google Scholar]
- 47. Price DA, Fisher ND, Osei SY, Lansang MC, Hollenberg NK. Renal perfusion and function in healthy African Americans. Kidney Int. 2001;59(3):1037–43. [DOI] [PubMed] [Google Scholar]
- 48. Cohen SL, Jhetam D, Da Silva J, Milne FJ, van der Walt A. Sodium and potassium status, plasma renin and aldosterone profiles in normotensive and hypertensive Johannesburg blacks. S Afr Med J. 1982;62:941–4. [PubMed] [Google Scholar]
- 49. Barlow RJ, Connell MA, Milne FJ. A study of 48-hour faecal and urinary electrolyte excretion in normotensive black and white South African males. J Hypertens. 1986;4(2):197–200. [DOI] [PubMed] [Google Scholar]
- 50. Yang Q, Liu T, Kuklina EV, Flanders WD, Hong Y, Gillespie C, Chang MH, Gwinn M, Dowling N, Khoury MJ et al.. Sodium and potassium intake and mortality among US adults: Prospective data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2011;171(13):1183–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


