Summary
Heart failure has remained the leading cause of death globally for the last 15 years—and its prevalence will continue to rise. Fifty years ago, heart failure management was enriched by the possibility of a heart transplant. Despite impressive improvements in medical treatment for heart failure, a heart transplant remains the most effective long-lasting treatment for advanced heart failure in terms of mortality and quality of life. However, donor and recipient characteristics have changed dramatically in recent years, leading to more complex decision-making regarding organ acceptance and to more demanding operations and postoperative management. With improving pathophysiological understanding in the last decades, today’s scientific interest still focuses on basic knowledge. How to retrieve and conserve organs to minimize ischaemic injury; how best to allocate them, considering the likelihood of success (developing a heart-allocation scoring system similar to that for lung allocation); how to match donor/recipient characteristics (ABO blood-group antigen compatibility versus incompatibility); and how to avoid graft failure, rejection and secondary morbidities such as malignomas and cardiac allograft vasculopathy after the heart transplant—all these factors remain fundamental challenges in today’s transplant medicine. The use of ex vivo perfusion (e.g. via the Organ Care System®, TransMedics, Andover, MA, USA) may play an important role in this change. Remarkably, there are huge regional divergences in current transplant practices: Whereas the number of transplants continues to rise in most Eurotransplant countries and other major transplant networks, there are some countries in which transplant numbers are static or even dropping (as in Germany). This difference results in wide variations across different countries as to how advanced heart failure is treated using mechanical circulatory-assist devices.
Keywords: Heart failure, Heart transplant, Mechanical circulatory support, Long-term survival
INTRODUCTION
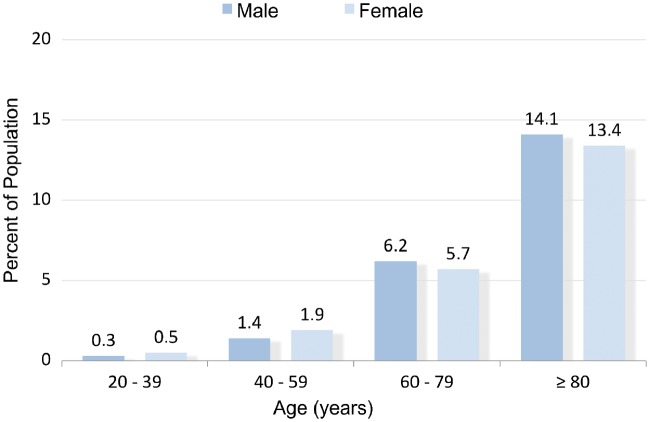
The first epidemiological analysis by the Framingham Heart Study revealed that heart failure is a fundamental factor of cardiovascular health [1]. Heart failure is usually diagnosed in adolescents and adults: The cumulative influence of risk factors over a period of years means that the incidence of heart failure increases with age and is higher in men than in women (Fig. 1) [2]. Heart failure affects 1–2% of the whole population and has a lifetime risk at the age of 55 of 33% in men and 28% in women [3]. Arterial hypertension and coronary heart disease remain the 2 most common conditions predating its onset [4, 5]. Heart failure, especially due to ischaemic heart disease, has remained the leading cause of death worldwide for the last 15 years [6]. In light of rising life expectancy across the globe and the persistence of risk factors like arterial hypertension and coronary heart disease, the prevalence of heart failure is predicted to increase by 46% from 2012 to 2030 and will therefore remain a leading cause of death [7].
Figure 1:
Prevalence of heart failure by sex and age (National Health and Nutrition Examination Survey, 2011–2014)—adopted and modified from Ref. [2].
Surgical treatment of end-stage heart failure such as a heart transplant or implantation of mechanical circulatory support (MCS) is mainly performed in patients with severely impaired systolic ventricular function due to end-stage cardiomyopathies who remain symptomatic despite optimal guideline-recommended treatment [8]. International guidelines recommend a heart transplant in end-stage heart failure and in the absence of contraindications (Table 1) [9, 10]. A consensus statement of the International Society of Heart and Lung Transplantation regulates listing and management policies for potential cardiac transplant candidates and was last updated in 2016 [11].
Table 1:
Indications and contraindications for a heart transplanta
| Indications for a heart transplant |
|
| Absolute and relative contraindications for a heart transplant |
|
Adapted from Ref. [9].
BMI: body mass index; LVAD: left ventricular assist device; NYHA: New York Heart Association.
Total implantation numbers of MCS prior to a heart transplant constantly increased in the past decade [12]. Based on the scarcity of allografts and the recently published improved long-term performance of current left ventricular assist devices (LVADs) [13], people might believe that mechanical devices can replace conventional heart transplants to a great extent.
Our goal is to outline the general management of heart failure, the therapeutic goal of which is to reduce morbidity and mortality. Furthermore, we clearly demonstrate why a heart transplant remains the gold standard in the treatment of end-stage heart failure despite widespread and emerging MCS achieved with the LVAD.
IMPROVEMENT IN STANDARD TREATMENT AMELIORATED PROGNOSIS
Morbidity and mortality rates associated with heart failure improved over the last decades [14]. However, the 5-year mortality rate for advanced heart failure remains stuck at approximately 50%. Even in 2018, the prognosis is still as limited as that for some of the common malignant cancers in both men and women [15].
CURRENT EVIDENCE-BASED TREATMENT RECOMMENDATIONS
According to the American and European Guidelines, the latest treatment recommendations for heart failure remain multimodal.
Pharmacological treatment
The CONSENSUS trial investigated ‘enalapril’ more than 30 years ago; it was the first systematic evaluation of lowering the number of heart failure-associated deaths via pharmacological treatment [16]. Since then, various substances have proven effective. Postulating an annual mortality rate of 20% and a mean survival time of 4.1 years at baseline, adding an angiotensin-converting enzyme inhibitor, beta-blocker, aldosterone antagonist and an implantable cardioverter-defibrillator (ICD) decreases annual mortality by 70% and lengthens the mean survival time by 5.6 years [17]. Results from the recently published PARADIGM-HF [Prospective Comparison of ARNI (Angiotensin Receptor–Neprilysin Inhibitor) with ACEI (Angiotensin-Converting–Enzyme Inhibitor) to Determine Impact on Global Mortality and Morbidity in Heart Failure] trial demonstrated an improvement in the mortality rate in response to applying new pharmacological treatment options. Sacubitril-valsartan proved superior in reducing the risks of death and hospitalization for heart failure compared to standard medical treatment by interacting with the neurohumoral system [18].
Risk factors and surgical treatment
The second treatment principle is to minimize the common risk factors for heart failure, such as alleviating hypertension [4]. Coronary revascularization [coronary artery bypass grafting (CABG)] for ischaemic heart disease preserves cardiac function [5] and improves outcome in combination with guideline-recommended medical therapy with excellent long-term overall mortality rates (all-cause mortality at 10 years with CABG versus optimal medical treatment: 58.9% vs 66.1%, hazard ratio 0.84, 95% confidence interval 0.73–0.97; P = 0.02; NNT = 14), as was recently demonstrated from STICH (Surgical Treatment for Ischaemic Heart Failure) and STICHES (Surgical Treatment for Ischaemic Heart Failure Extension Study) investigators [19]. The latest coronary revascularization guideline favours CABG as the preferable choice of revascularization in multivessel disease and reduced ventricular function, whereas comparable data for percutaneous coronary intervention are missing [20].
Beneficial reverse remodelling effects induced by pharmacological heart failure treatment in ischaemic heart failure led to the development of a surgical procedure to reduce left ventricular volume and wall tension, with the expectation of a similar mortality benefit [21]. Post hoc analyses from the STICH trial added further insight to this idea: CABG with additional surgical ventricular reconstruction (SVR) in cases with postinfarction dilation proved effective. So, these data revealed that SVR continues to be important in the treatment of ischaemic cardiomyopathy, with convincing results and survival benefits whenever SVR was performed in a way that reduced the ventricular geometric parameters to an almost normal size (postoperative left ventricular systolic volume index of 70 ml/m2 or less) [22].
Several interventional treatments are being investigated to address coexisting lesions, especially the treatment option using interventional edge-to-edge repair for functional mitral regurgitation associated with heart failure. The latest evidence on interventional edge-to-edge repair in this patient cohort indicates that in patients whose condition is stable and in high volume centres, this therapy can lead to survival benefits and symptomatic relief from dyspnoea [23]. However, in a more open all-comers trial on functional regurgitation, including severely impaired patients who can also be considered for a heart transplant or MCS, interventional edge-to-edge repair failed to provide a clinical benefit [24].
Arrhythmia therapy, electroresynchronization
An ICD implant to detect and alleviate life-threatening arrhythmias in patients with ischaemic and non-ischaemic cardiomyopathologies [25, 26] and cardiac resynchronization therapy [27] both play a fundamental role in the treatment of heart failure—and thus represent a pivotal recommendation in current heart failure guidelines. Remarkably, publication of the DANISH (Danish Study to Assess the Efficacy of ICDs in Patients with Non-ischaemic Systolic Heart Failure on Mortality) trial subsequent to the last European Society of Cardiology guideline recommendation on the treatment of heart failure raised uncertainty about prophylactic ICD implants: device treatment in patients with symptomatic systolic heart failure not caused by coronary heart disease was not associated with a significantly lower long-term rate of death from any cause than was usual clinical care [28]. Basically, the latest guideline recommendations are based mainly on the MADIT-II (Multicentre Automatic Defibrillator Implantation Trial II) [29] and the SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) [30] trial, which were published more than a decade ago. But pharmacological treatment and coronary revascularization in coronary heart disease have changed fundamentally since these early trials with an impact on mortality and a significant reduction in sudden cardiac deaths [31]. Hence, current recommendations should be critically reappraised and supported by further randomized controlled trials.
Mechanical circulatory support
Ventricular assist devices (VADs) evolved from research involving cardiopulmonary bypass and the total artificial heart in the 1950s and 1960s [32]. With publication of the REMATCH (Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure) trial in 2001, the VAD breakthrough began following demonstration of the longer survival of heart failure candidates with VAD support in comparison to those treated with optimal medical treatment alone [33]. Increasing numbers of VAD implants are currently designated as destination therapy, although some of them were primarily implanted with a bridge-to-transplant intention. In a patient with a stabilized cardiac condition, this VAD support might frequently make further high-urgency listings for transplants superfluous, or patients do not fulfil strict heart transplant high-urgency criteria or simply no longer want a transplant [34]. Independently of the excellent long-term data for heart transplants, patients who are denied a transplant (due to older age or relevant comorbidities) or who will not survive the long high-urgency waiting time might benefit most from a permanent LVAD and attain outpatient status with acceptable quality of life (QoL) for a certain period. One current trial is examining the optimal point to implant a VAD in patients who have been given transplantable (T−) status and who are listed for a heart transplant with an increased risk of death while on the waiting list. The study was designed to compare the superiority of an early VAD implant to the current therapeutic strategy of medical heart failure treatment and assist device implantation only after serious deterioration of the patient’s condition. Final data collection for primary outcome measures is expected in August 2022 (ClinicalTrials.gov Identifier: NCT02387112).
LEFT VENTRICULAR ASSIST DEVICE AS A CHANCE IN PROHIBITIVE TRANSPLANT CONDITIONS
LVAD support offers an excellent option for those patients with high pulmonary vascular resistance who are rejected for a heart transplant, provided they have adequate right ventricular function. VAD implantation frequently enables vascular resistance to decline via adequate unloading of the left ventricle, meaning these patients may become transplant-eligible [35]. However, the complication rate remains high [36], as outlined in the following section.
LIMITATIONS OF PERMANENT DEVICE SUPPORT
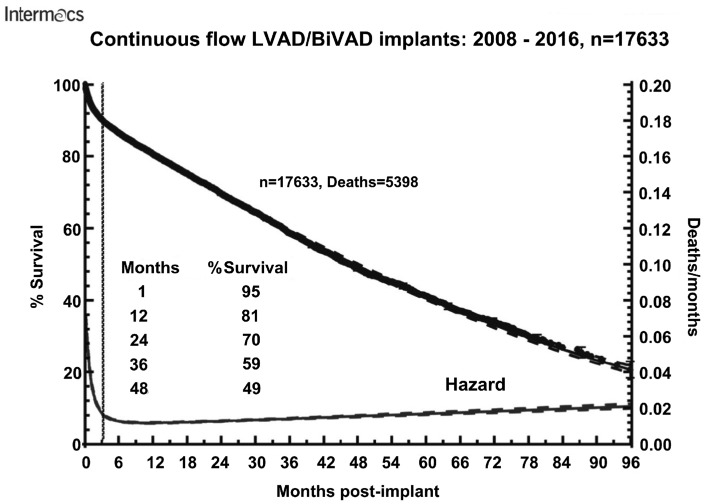
Independent of MCS flow-type, size and durability have improved over the years. Published data continue to reveal clear mid- and long-term survival benefits and superiority over optimal medical treatment in patients with end-stage heart failure [37]. However, the efficacy of VADs is limited in the long-term due to device-typical complications, resulting in a gradual drop in the survival rate (Fig. 2). A postapproval HeartMate II lifetime therapy report revealed a high probability of device-related adverse events in patients at the 2-year follow-up: driveline infections (19%), sepsis (19%), strokes (11.7%), thrombus formation (3.6%), bleeding (54%), mechanical failures requiring replacement (4%) and right heart failure (18%) [36]. Recent data from the Momentum-3 (Multicentre Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3) trial shows improvement of outcome parameters with the use of the HeartMate III device [13]. However, this trend needs to be supported by large-scale trials or registry data.
Figure 2:
Parametric survival curve and associated hazard function with 70% confidence limit for survival after implantation of a continuous-flow LVAD or BiVAD. Reprinted with permission from Ref. [12]. BiVAD: biventricular assist device; LVAD: left ventricular assist device.
The QoL of patients with MCS is far from optimal: The VAD can alleviate some heart failure symptoms, improve impaired end-organ function and raise the patient’s QoL somewhat. Nevertheless, several functional limitations persist: patients still lack independence, and MCS-related complications also significantly affect QoL. Psychological distress remains a serious problem after LVAD implantation [38]. In contrast, patients who have had a heart transplant had the best outcome compared with the LVAD group in terms of their mental health [39].
It remains unclear whether mechanical support affects the prognosis after a heart transplant: Some evidence shows that the post-heart transplant prognosis is impaired due to donor-specific antibodies triggering rejection and contributing to morbidity and mortality after the operation [8]. Moreover, recent evidence indicates that pretransplant MCS is a risk factor for primary graft failure [40]. Regarding the rising number of MCS recipients over the last few years, this fact will certainly affect transplant medicine in the future: In 2000, the International Society for Heart and Lung Transplantation reported that 19.1% of transplant recipients had been on mechanical support previously, a percentage that rose to 43.9% in the current transplant collective from 2009 to 2017 [8, 41].
HEART TRANSPLANTS: A RETROSPECTIVE
The first successful heart transplant by Christiaan Barnard in Cape Town, South Africa in 1967 can be regarded as the birth of the modern treatment of end-stage heart failure. This surgical heart failure treatment milestone led to global euphoria and unbelievable hope that heart failure could be healed, even though the first patient to receive a transplant survived only a few days. Managing immunosuppression proved to be problematic, with only a few substances available [42]. It was the introduction of the calcineurin inhibitor (CNI) cyclosporine A in 1982 in particular that helped raise the 3-year survival rate from about 40% to 70% [43]. Later developments in standardized pharmacological protocols and new immunosuppressive drugs for the induction and maintenance of permanent immunosuppression provided further insights and beneficial long-term effects. Thus, inhibition of the ‘mammalian target of rapamycin (mTOR)’ in combination with a CNI demonstrated favourable effects with less coronary allograft vasculopathy compared to standard treatment [44, 45]. Furthermore, CNI-free immunosuppression protocols demonstrated improved renal function in patients with a heart transplant and chronic renal failure compared to CNI-based protocols. This result might affect prognosis after the transplant, because CNI-related renal failure is a common problem after a cardiac transplant and a major cause of long-term morbidity [46, 47]. Also, graft preservation techniques and ex vivo perfusion (as discussed below) might contribute to the constantly improving long-term results.
HEART TRANSPLANTS: WHERE ARE WE NOW?
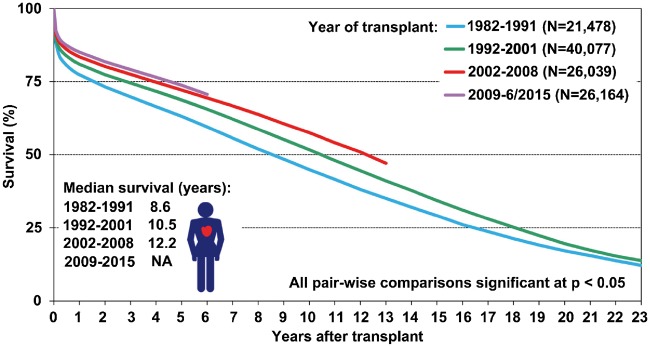
Despite recent improvements in mechanical circulatory-assist devices, it is hard to compete with the excellent median overall survival of 10.7 years for all patients who received transplants from 1982 to 2015 [8]. In particular, the mortality rate remains consistently low if the patient survives the critical first 12 months after the transplant: Long-term deaths are due mainly to infectious diseases in immunocompromised patients (e.g. multiorgan sepsis), immunosuppression-associated malignancies (such as post-transplant lymphoproliferative disease as a result of immunosuppression) and progressive cardiac allograft vasculopathy (Fig. 3) [8]. Future research should focus on these factors.
Figure 3:
Kaplan–Meier survival in adult heart transplant recipients by era (transplants: January 1982–June 2015). Reprinted with permission from Ref. [8].
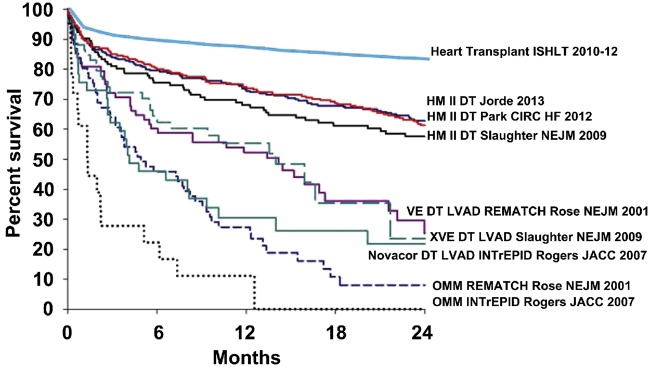
Drakos [48] outlined the survival advantage of a heart transplant over a VAD in an editorial comment by matching International Society for Heart and Lung Transplantation survival data of adult patients receiving transplants in 2010–2012 with survival curves from the study ‘Results of the Destination Therapy Post-Food and Drug Administration Approval Study With a Continuous Flow Left Ventricular Assist Device’ (Fig. 4). The included VAD data (mainly derived from early generation VAD trials) are followed by better data from the next-generation LVAD—currently seen in the MOMENTUM-3 trial [13]. These new VAD data comprising more favourable adverse event-free survival and overall survival rates are comparable with survival rates in the first 2 years after a heart transplant. But these results still neglect the constant threats associated with an MCS (e.g. bleeding, thrombosis and infection) especially in the following years in which the adverse event rate after the transplant is extremely beneficial. Thus, younger patients with missing contraindications definitely profit more from a heart transplant as destination therapy, whereas permanent MCS should be considered in older cohorts or in patients with limited prognoses [49].
Figure 4:
Survival rates in trials and registry reports of heart transplantation and chronic mechanical circulatory support as DT. Reprinted with permission from Ref. [48]. DT: lifetime therapy; HM: HeartMate; LVAD: left ventricular assist device; OMM: optimal medical management; VE/XVE: early generation ventricular assist devices (HeartMate VE/XVE, Thoratec Corp., Pleasanton, CA).
Increased demand for terminal heart failure therapy, but divergence in heart transplants
Surgical support in end-stage heart failure, either MCS or a heart transplant, will remain an ongoing topic in cardiology [7]. However, current management is not often guided by the best medical option but rather by feasibility because human donors are ‘limited’ and grafts are scarce. Additionally, a heart transplant requires international coordination, established transplant centres with considerable experience and close collaboration with practicing cardiologists. Because of the scarcity of organs in some areas, there has been a recent structural shift in the indication towards MCS in the management of end-stage heart failure.
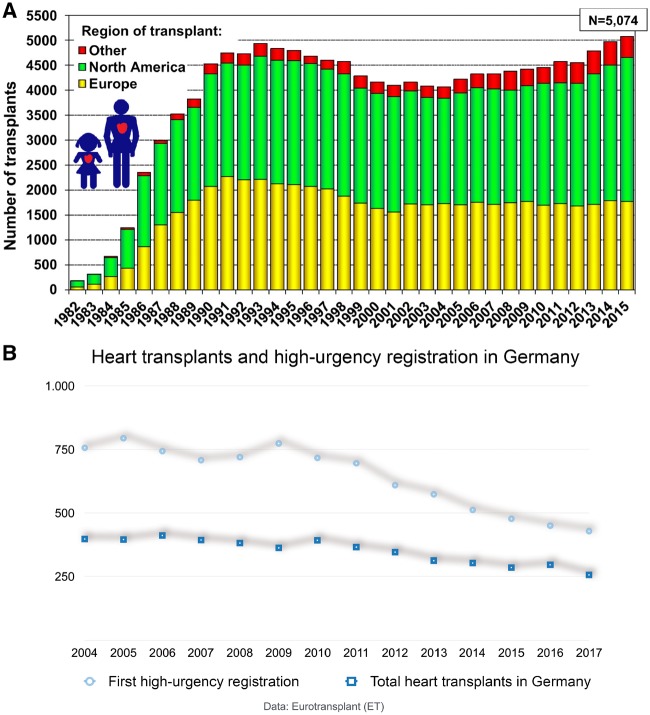
After a period of stagnation, the number of global heart transplants has risen, but the situation varies across regions (Fig. 5A). Hence, MCS predominates in those regions with low heart transplant rates—offering tolerable survival rates compared to optimal medical treatment but neglecting the survival advantage of the heart transplant over time (Fig. 4).
Figure 5:
(A) Number of adult and paediatric heart transplants by year (transplants: 1982–2015) and geographic region. Reprinted with permission from Ref. [8]. (B) Overview of new high-urgency registrations and total heart transplants 2004–2017 in Germany (data: Eurotransplant).
Germany is a country where the transplant situation is alarming in contrast to the positive global trend. Heart transplant numbers have consistently dropped since reaching a maximum at the end of the 1990s, with a historically low number carried out in 2017. Therefore, it is almost exclusively those candidates with a high-urgency status who are offered an organ. The mortality rate of patients on the highly urgent list who are waiting for a suitable organ is high and is exacerbated by a dynamic rise caused by more and more high-urgency candidates and longer waiting times (Fig. 5B) [50].
The German Organ Transplantation Foundation registered a drop in total heart transplant numbers and a decrease in new registrations for a transplant (Fig. 5B) [51]. Both factors are remarkable. The reasons are multifactorial: The organ allocation scandal in 2011–2012 in Germany seems not to be the driving force for the current organ shortage, because organ donations had been dropping before then [52]. The general attitude in the German population does not seem to have changed regarding organ donation: The favourable opinion towards organ donations is currently 84%, which is higher than ever before. Also, the numbers of card-carrying organ donors (individuals with cards indicating that they are willing to be an organ donor) continue to rise from 22% in 2012 to 36% in 2018 [53]. The most likely explanation for the drop in post-mortem organ donations in Germany is not the general attitude but rather an organizational deficit caused by the plethora of regulations and large quantities of paperwork required of explantation hospitals, the dearth of expert knowledge in small hospitals and the lack of cost-effective reimbursements, all of which result in only a small number of possible donors who approach the official board. Based on this retrospective analysis of possible organ donors, depending on the documented cause of death, the transplant rate in Germany could be increased significantly by a more effective identification system [54]. In our opinion, it is the general regulations of the donation system that have to be revised rather than the attitude of the population. Political initiatives such as improvements in the laws related to organ donations as well as further discussion about a change in the general organ donation regulation towards an active refusal of someone’s individual will might help as well.
TRANSPLANT MEDICINE FACES NEW CHALLENGES
The profiles of donors and recipients have changed fundamentally in recent years:
The increasing numbers of patients with MCS prior to a heart transplant put extreme demands on the surgical site in terms of preparation and postoperative management.
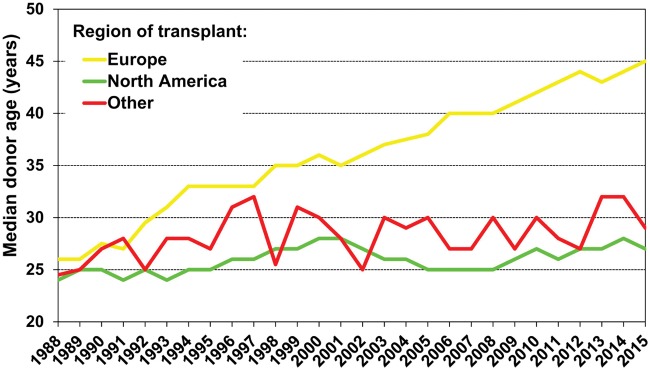
Changes in the donor’s cause of death reflect a shift from traumas to ischaemic brain injuries due to safety improvements, triggering a rise in donor age at the time of donation [55].
The rising donor age in recent decades—especially in Europe—is associated with more comorbidities and less-than-ideal graft quality (Fig. 6) [8]
Figure 6:
Adult and paediatric heart transplants according to median donor age by location and year. Reprinted with permission from Ref. [8].
Current developments in transplants: cardiac allocation score
Creating a more just system for and clarifying the risks of a patient’s prospects for success in heart allocation (with the lung-allocation score being a good example of a suitable system [56]) has long been discussed. Sufficient long-term experience with MCS is still lacking, and satisfactory data from databases are only just beginning to be available. There are no substantiated scoring systems available that are capable of estimating device-related complications. Because of this gap in knowledge, it is impossible to balance the risk-benefit ratio of patients with MCS awaiting a heart transplant against those without MCS. So, use of a VAD might currently be deemed to be responsible for a lower status on the waiting list and therefore worse organ allocation. These problems need to be solved by creating a suitable scoring system for the allocation of hearts.
Current developments in transplants: expanding the donor pool by early donor management
Expansion of the existing donor pool is crucial and includes early donor identification and comprehensive care and diagnostics prior to selecting hearts acceptable for transplant. Early donor management by scouting teams can contribute to higher organ utilization rates with comparable transplant results. In existing programmes, allocated implant centres send specialized medical staff to the possible donor and guide further diagnosis and treatment until a final decision is made about the suitability of the organ donation [57].
Current developments in transplants: ex vivo graft perfusion
Facing increasing donor ages, all means must be undertaken to assess donated hearts as thoroughly as possible. New preservation technology [Organ Care System® (OCS), TransMedics, Andover, MA, USA] has been introduced to reduce ischaemic damage to the graft and enable further examination. This commercially available device preserves the heart in an ex vivo beating, perfused, normothermic and oxygenated state (Video 1). Although longer ischaemia times worsen prognosis [8], this device can contribute to consistent outcomes or to an expanded donor pool. The PROCEED-II trial (Randomized Study of Organ Care System Cardiac for Preservation of Donated Hearts for Eventual Transplantation), a randomized study, demonstrated non-inferiority in the OCS intention-to-treat group compared to standard cold-ischaemic preservation [58]. After receiving clinical approval due to the PROCEED-II trial’s findings, other investigators confirmed a favourable outcome in conjunction with OCS use in unfavourable donor–recipient constellations [59, 60]. Ex vivo preservation is also a significant chance in marginal organ selection to enable a post-mortem examination (e.g. using coronary angiography) and to evaluate metabolism, oxygen saturation, aortic pressure and coronary blood flow as surrogate parameters for good graft status. Furthermore, the application delivers valuable time for the transplant team to expand transport distances or use time for difficult situs preparation (e.g. explantation or preparation of MCS after several previous operations in adult patients with congenital heart defects). In addition, an economic benefit became apparent with a shorter period of ischaemia [61].
Video 1:
During a training session, a porcine heart after harvesting and preparation is connected via the aorta to the ex-vivo perfusion device (Organ Care System®, TransMedics, Andover, MA, USA) and perfused with autologous blood. After 20 seconds of reperfusion, the heart starts beating and will be secured for clinical and biochemistry assessment.
Current developments in transplants: donation after circulatory death
Since the first human heart transplant [42], declaring death prior to organ procurement had historically been based on the cessation of circulation. However, following the subsequent increase in the discussions about death criteria, later heart transplants were not carried out until the death of the donor’s brain had been verified using a previously accepted definition of brain death [62]. The current shortage of organs forced the revitalization of the method of donation after circulatory death (DCD) because some dying patients will never meet formal brain death criteria. The Maastricht agreement defined 5 categories based on the circumstances of the cardiac arrest, of which only selected cohorts can lead to DCD donations [63]. Once cardiac function has ceased and death has been declared, the heart must be retrieved as soon as possible. Unlike procurement after brain death, there is perforce a period of cardiac ischaemia and ventricular distention that can compromise the vitality of the organ after removal and that was the source of initial concern regarding the vitality of DCD hearts after retrieval [64]. Ex vivo perfusion and/or normothermic regional perfusion may guarantee graft metabolism and function and ensure post-transplant function [65, 66]. Early outcome of a DCD heart transplant is apparently comparable with outcomes with a transplant from a donation after brain death [67], and thus DCD programmes have contributed to greater transplant activity in some regions [68, 69]. Whether the DCD programme can be successfully implemented worldwide remains to be seen: although some countries successfully perform transplants in compliance with the DCD, the German Medical Association declared in 1998 that organ procurement and transplants in Germany may not follow the DCD criteria because they do not fulfil the strict German ethical end-of-life guidelines.
Current developments in organ transplants: transplants incompatible with the ABO blood group
The ABO blood group-antigen system is based on carbohydrate epitopes present on different core saccharide chains that are bound to lipids (glycolipids) or to proteins (glycoproteins) and that form in early childhood. Because the ABO-antigen system is always present on the cell surface, it plays a central role in solid organ transplants due to its capacity to induce rejection [70]. Compatibility between the donor’s and the recipient’s ABO alloantigens has long been required, and an ABO-incompatible heart transplant was absolutely contradicted in adults. However, acceptable ABO-incompatible abdominal transplant outcomes—especially renal transplants [71]—in adult patients and advances in ABO-incompatible heart transplants in paediatric patients [72, 73] have led to discussions about the potential for adult ABO-incompatible heart transplants to expand the donor pool. In a recent registry study, Bergenfeldt et al. [74] demonstrated no difference in the incidence of deaths or retransplants between ABO-compatible and ABO-incompatible heart transplants in a transplant collective after 2005. However, ABO-incompatible transplants remain exceptional and are severely restricted to individual decisions due to non-standardized immunosuppression protocols, a high-risk rejection constellation and the unknown long-term prognosis.
Current developments in transplants: xenotransplants
Due to the scarcity of cardiac donors and the long waiting lists for heart transplants in many countries, the alternative of using xenografts has been explored for several years. The obviously most difficult limitation of using xenografts is the accelerated rate of rejection. Few, but very active, research groups focus on strategies to overcome the cross-species-derived rejection. Thus, genetically modified pig hearts were successfully transplanted into baboons using a modified preservation and immunosuppression protocol with an excellent 90-day survival rate with no signs of rejection [75]. This promising approach with xenotransplants must acquire further basic knowledge and long-term data before it can be tested further via large clinical examinations and human trials [76]. Comparing the major treatment options for end-stage heart failure from a future perspective, we believe that in the coming 10 years MCS will further improve and precise risk prediction can discriminate better candidates for heart transplants or MCS, hopefully. It is likely that treatment with an allograft heart transplant will be reserved for those patients who would profit the most from the donor heart in terms of the likelihood for long-term survival, such as young and otherwise healthy recipients. It is possible that a xenotransplant will be a clinical option in 10 years and that the indications for heart transplant and MCS might be discussed differently at that point.
A HEART TRANSPLANT IS THE PAST AND THE PROMISING FUTURE OF END-STAGE HEART FAILURE TREATMENT
All the aforementioned factors make a heart transplant currently the best possible therapy for patients with end-stage heart failure and offer promising future developments. In view of the improvements in MCS and the scarcity of donor organs, candidate selection and the choice of a potentially ‘optimal’ method provide further challenges. MCS can play a key role in patient selection in emergency-stabilized, haemodynamically unstable patients, as a bridge-to-bridge or bridge-to-transplant solution, to prove candidacy in some candidates with high pulmonary vascular resistance or in candidates with contraindications for a heart transplant even as destination therapy (Table 2).
Table 2:
Comparing a HTx to a VAD: benefits and limitations
| Advantages | Disadvantages | Problems to solve | |
|---|---|---|---|
| HTx |
|
|
|
| VAD |
|
|
|
ABO: ABO blood group system; HLA: human leucocyte antigen; HTx: heart transplant; OCS: Organ Care System; VAD: ventricular assist device.
CONCLUSION
In conclusion, we offer a positive answer to the question raised in the title. Based on the continuous improvement in heart transplant programmes with new therapeutic options, we believe that a heart transplant with an allograft will remain an important factor in the treatment of end-stage heart failure.
Continuous research is urgently needed to further boost the heart transplant and strengthen its position in the treatment of advanced heart failure as long as there is no effective causal therapy. Central topics remain, namely:
Increasing organ donations by improving the identification of local heart donors, optimizing the reimbursement for explanting hospitals and, finally, enhancing donor willingness.
Expanding the donor pool (establishing early donor management programmes; assessing marginal donors in ex vivo perfusion systems; DCD donations; consideration of ABO-incompatible transplants in exceptional cases).
Improving organ allocation by considering post-transplant prognosis and the judicious allocation of highly urgent patients in view of the growing numbers of patients with mechanical assistance on the waiting list.
Improving organ retrieval and preservation procedures to minimize ischaemic injury and post-transplant outcomes.
Optimizing future immunosuppression procedures to prevent rejection and adverse long-term outcomes (e.g. cardiac allograft vasculopathy, malignancies). Because cardiac allograft vasculopathy is the biggest problem in regard to long-term mortality, all strategies designed to modify the immunosuppression regimen with the therapeutic options need to focus on that. In addition, long-term morbidity with renal function impairment may be the second most important factor that needs to be improved.
Increasing research for causal healing of cardiomyopathy and total cardiac replacement (e.g. durable total artificial hearts, xenotransplants).
Funding
This paper was published as part of a supplement supported by an educational grant from Medtronic.
Conflict of interest: none declared.
REFERENCES
- 1. Ho KK, Pinsky JL, Kannel WB, Levy D.. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol 1993;22:6A–13A. [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R. et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 20177;135:e146–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bleumink GS, Knetsch AM, Sturkenboom M, Straus S, Hofman A, Deckers JW. et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure: the Rotterdam Study. Eur Heart J 2004;25:1614–19. [DOI] [PubMed] [Google Scholar]
- 4. Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK.. The progression from hypertension to congestive heart failure. JAMA 1996;275:1557–62. [PubMed] [Google Scholar]
- 5. Fox KF, Cowie MR, Wood DA, Coats AJ, Gibbs JS, Underwood SR. et al. Coronary artery disease as the cause of incident heart failure in the population. Eur Heart J 2001;22:228–36. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. World Health Statistics 2016: Monitoring Health for the SDGs Annex B: Tables of Health Statistics by Country, WHO Region and Globally [Internet]. 2016. http://www.who.int/gho/publications/world_health_statistics/2016/EN_WHS2016_AnnexB.pdf (2 April 2019, date last accessed).
- 7. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC. et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lund LH, Khush KK, Cherikh WS, Goldfarb S, Kucheryavaya AY, Levvey BJ. et al. The registry of the International Society for Heart and Lung Transplantation: thirty-fourth adult heart transplantation report—2017; focus theme: allograft ischemic time. J Heart Lung Transplant 2017;36:1037–46. [DOI] [PubMed] [Google Scholar]
- 9. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- 10. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH. et al. 2013 ACCF/AHA Guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 11. Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA. et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant 2016;35:1–23. [DOI] [PubMed] [Google Scholar]
- 12. Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL. et al. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant 2017;36:1080–6. [DOI] [PubMed] [Google Scholar]
- 13. Mehra MR, Goldstein DJ, Uriel N, Cleveland JC, Yuzefpolskaya M, Salerno C. et al. Two-year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med 2018;378:1386–95. [DOI] [PubMed] [Google Scholar]
- 14. Roger VL, Weston SA, Redfield MM, Hellermann HJ, Killian J, Yawn BP. et al. Trends in heart failure incidence and survival in a community-based population. JAMA 2004;292:344–50. [DOI] [PubMed] [Google Scholar]
- 15. Mamas MA, Sperrin M, Watson MC, Coutts A, Wilde K, Burton C. et al. Do patients have worse outcomes in heart failure than in cancer? A primary care-based cohort study with 10-year follow-up in Scotland. Eur J Heart Fail 2017;19:1095–104. [DOI] [PubMed] [Google Scholar]
- 16.CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987;316:1429–35. [DOI] [PubMed] [Google Scholar]
- 17. Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB. et al. The Seattle heart failure model: prediction of survival in heart failure. Circulation 2006;113:1424–33. [DOI] [PubMed] [Google Scholar]
- 18. McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR. et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 19. Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA. et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med 2016;374:1511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sousa-Uva M, Neumann F-J, Ahlsson A, Alfonso F, Banning AP, Benedetto U. et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg 2019;55:4–90. [DOI] [PubMed] [Google Scholar]
- 21. Athanasuleas CL, Buckberg GD, Stanley AWH, Siler W, Dor V, Di Donato M. et al. Surgical ventricular restoration in the treatment of congestive heart failure due to post-infarction ventricular dilation. J Am Coll Cardiol 2004;44:1439–45. [DOI] [PubMed] [Google Scholar]
- 22. Michler RE, Rouleau JL, Al-Khalidi HR, Bonow RO, Pellikka PA, Pohost GM. et al. Insights from the STICH trial: change in left ventricular size after coronary artery bypass grafting with and without surgical ventricular reconstruction. J Thorac Cardiovasc Surg 2013;146:1139–45.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM. et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med 2018;379:2307–18. [DOI] [PubMed] [Google Scholar]
- 24. Obadia J-F, Messika-Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N. et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 2018;379:2297–306. [DOI] [PubMed] [Google Scholar]
- 25. Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H. et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 1996;335:1933–40. [DOI] [PubMed] [Google Scholar]
- 26. Siddiqui WJ, Aggarwal S, Rafique M, Singh S, Kutalek S, Eisen HJ.. Prophylactic use of the implantable cardioverter-defibrillator and its effect on the long-term survival, cardiovascular and sudden cardiac death in nonischemic cardiomyopathy patients—a systematic review and meta-analysis. Heart Fail Rev 2018;23:181–90. [DOI] [PubMed] [Google Scholar]
- 27. Cleland JGF, Daubert J-C, Erdmann E, Freemantle N, Gras D, Kappenberger L. et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–49. [DOI] [PubMed] [Google Scholar]
- 28. Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E. et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016;375:1221–30. [DOI] [PubMed] [Google Scholar]
- 29. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS. et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–83. [DOI] [PubMed] [Google Scholar]
- 30. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R. et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–37. [DOI] [PubMed] [Google Scholar]
- 31. Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JGF. et al. Declining risk of sudden death in heart failure. N Engl J Med 2017;377:41–51. [DOI] [PubMed] [Google Scholar]
- 32. Stewart GC, Mehra MR.. A history of devices as an alternative to heart transplantation. Heart Fail Clin 2014;10:S1–12. [DOI] [PubMed] [Google Scholar]
- 33. Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W. et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435–43. [DOI] [PubMed] [Google Scholar]
- 34. Maybaum S, Kamalakannan G, Murthy S.. Cardiac recovery during mechanical assist device support. Semin Thorac Cardiovasc Surg 2008;20:234–46. [DOI] [PubMed] [Google Scholar]
- 35. Zimpfer D, Zrunek P, Sandner S, Schima H, Grimm M, Zuckermann A. et al. Post-transplant survival after lowering fixed pulmonary hypertension using left ventricular assist devices. Eur J Cardiothorac Surg 2007;31:698–702. [DOI] [PubMed] [Google Scholar]
- 36. Jorde UP, Kushwaha SS, Tatooles AJ, Naka Y, Bhat G, Long JW. et al. Results of the destination therapy post-Food and Drug Administration approval study with a continuous flow left ventricular assist device: a prospective study using the INTERMACS Registry (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol 2014;63:1751–7. [DOI] [PubMed] [Google Scholar]
- 37. Mancini D, Colombo PC.. Left ventricular assist devices: a rapidly evolving alternative to transplant. J Am Coll Cardiol 2015;65:2542–55. [DOI] [PubMed] [Google Scholar]
- 38. Adams EE, Wrightson ML.. Quality of life with an LVAD: a misunderstood concept. Heart Lung 20181;47:177–83. [DOI] [PubMed] [Google Scholar]
- 39. Heilmann C, Kaps J, Hartmann A, Zeh W, Anjarwalla AL, Beyersdorf F. et al. Mental health status of patients with mechanical aortic valves, with ventricular assist devices and after heart transplantation. Interact CardioVasc Thorac Surg 2016;23:321–5. [DOI] [PubMed] [Google Scholar]
- 40. Prieto D, Correia PM, Batista M, Antunes MJ, Primary graft failure after cardiac transplantation: prevalence, prognosis and risk factors. Interact CardioVasc Thorac Surg 2018;27:765–72. [DOI] [PubMed] [Google Scholar]
- 41. Khush KK, Cherikh WS, Chambers DC, Goldfarb S, Hayes D, Kucheryavaya AY. et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-fifth adult heart transplantation report—2018; focus theme: multiorgan transplantation. J Heart Lung Transplant 2018;37:1155–68. [DOI] [PubMed] [Google Scholar]
- 42. Cooper D. Christiaan Barnard and his contributions to heart transplantation. J Heart Lung Transplant 2001;20:599–610. [DOI] [PubMed] [Google Scholar]
- 43. Lindenfeld J, Miller GG, Shakar SF, Zolty R, Lowes BD, Wolfel EE. et al. Drug therapy in the heart transplant recipient: part II: immunosuppressive drugs. Circulation 2004;110:3858–65. [DOI] [PubMed] [Google Scholar]
- 44. Eisen HJ, Tuzcu EM, Dorent R, Kobashigawa J, Mancini D, Valantine-von Kaeppler HA. et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med 2003;349:847–58. [DOI] [PubMed] [Google Scholar]
- 45. Keogh A, Richardson M, Ruygrok P, Spratt P, Galbraith A, O’Driscoll G. et al. Sirolimus in de novo heart transplant recipients reduces acute rejection and prevents coronary artery disease at 2 years: a randomized clinical trial. Circulation 2004;110:2694–700. [DOI] [PubMed] [Google Scholar]
- 46. Groetzner J, Kaczmarek I, Schulz U, Stegemann E, Kaiser K, Wittwer T. et al. Mycophenolate and sirolimus as calcineurin inhibitor-free immunosuppression improves renal function better than calcineurin inhibitor-reduction in late cardiac transplant recipients with chronic renal failure. Transplantation 2009;87:726–33. [DOI] [PubMed] [Google Scholar]
- 47. Barten MJ, Hirt SW, Garbade J, Bara C, Doesch A, Knosalla C. et al. MANDELA study results at 18 months after heart transplantation: superior renal function with CNI-free everolimus over standard CNI-based regimen—a randomized, multi-center trial in de novo heart transplant recipients. Transplantation 2018;102:S362. [Google Scholar]
- 48. Drakos SG. The Odyssey of chronic cardiac mechanical support. J Am Coll Cardiol 2014;63:1758–60. [DOI] [PubMed] [Google Scholar]
- 49. Ammirati E, Oliva F, Cannata A, Contri R, Colombo T, Martinelli L. et al. Current indications for heart transplantation and left ventricular assist device: a practical point of view. Eur J Intern Med 2014;25:422–9. [DOI] [PubMed] [Google Scholar]
- 50.Eurotransplant International Foundation. Eurotransplant Statistical Report 2017 [Internet]. 2017. https://www.eurotransplant.org/cms/mediaobject.php?file=803150+020288+Statistical+Report+2017+%28online%2913.pdf (2 April 2019, date last accessed).
- 51.German Organ Transplantation Foundation. Annual Report: Organ Donation and Transplantation in Germany 2017 [Internet]. Frankfurt/Main: German Organ Transplantation Foundation; 2018. https://www.dso.de/uploads/tx_dsodl/JB_2017_web_01.pdf (2 April 2019, date last accessed). [Google Scholar]
- 52.German Organ Transplantation Foundation. German Organ Transplantation Foundation Annual Report: Organ Donation and Transplantation in Germany 2013 [Internet]. Frankfurt/Main. https://www.dso.de/uploads/tx_dsodl/2013_Jahresbericht.pdf (2 April 2019, date last accessed). [Google Scholar]
- 53.Bundeszentrale für gesundheitliche Aufklärung (BZgA). Studien zur Organ- & Gewebespende BZgA-Repräsentativbefragung [Internet]. Köln: Bundeszentrale für gesundheitliche Aufklärung (BZgA); 2018. https://www.organspende-info.de//infothek/studien (2 April 2019, date last accessed). [Google Scholar]
- 54. Schulte K, Borzikowsky C, Rahmel A, Kolibay F, Polze N, Fränkel P. et al. Decline in organ donation in Germany—a nationwide secondary analysis of all inpatient cases. Dtsch Arztebl Int 2018;115:463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Organización Nacional de Trasplantes Madrid, Espana. Actividad de Trasplante Cardíaco 2016. http://www.ont.es/infesp/Memorias/Memoria%20Coraz%C3%B3n.pdf (2 April 2019, date last accessed). [Google Scholar]
- 56. Gottlieb J. Lung allocation. J Thorac Dis 2017;9:2670–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Barbero C, Ravaglioli A, Page A, Parizkova B, Berman M, Sudarshan C. et al. Retrieval team initiated early donor management (scouting) increases donor heart acceptance rate for transplantation. J Heart Lung Transplant 2016;35:S220. [Google Scholar]
- 58. Ardehali A, Esmailian F, Deng M, Soltesz E, Hsich E, Naka Y. et al. Ex vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomised non-inferiority trial. Lancet 2015;385:2577–84. [DOI] [PubMed] [Google Scholar]
- 59. García Sáez D, Zych B, Sabashnikov A, Bowles CT, De Robertis F, Mohite PN. et al. Evaluation of the organ care system in heart transplantation with an adverse donor/recipient profile. Ann Thorac Surg 2014;98:2099–106. [DOI] [PubMed] [Google Scholar]
- 60. Ius F, Berchtold-Herz M, Rojas SV, Kaufeld T, Scheumann J, Avsar M. et al. Heart preservation with the organ care system in Germany—revival in high risk recipients. J Heart Lung Transplant 2018;37(Suppl 4):S410. [Google Scholar]
- 61. Rylski B, Berchtold-Herz M, Olschewski M, Zeh W, Schlensak C, Siepe M. et al. Reducing the ischemic time of donor hearts will decrease morbidity and costs of cardiac transplantations. Interact CardioVasc Thorac Surg 2010;10:945–7. [DOI] [PubMed] [Google Scholar]
- 62.Ad Hoc Committee of the Harvard Medical School. A definition of irreversible coma: report of the ad hoc committee of the Harvard Medical School to examine the definition of brain death. JAMA 1968;205:337–40. [PubMed] [Google Scholar]
- 63. Kootstra G, Daemen JH, Oomen AP.. Categories of non-heart-beating donors. Transplant Proc 1995;27:2893–4. [PubMed] [Google Scholar]
- 64. Manara AR, Murphy PG, O’Callaghan G.. Donation after circulatory death. Br J Anaesth 2012;108:i108–21. [DOI] [PubMed] [Google Scholar]
- 65. Suehiro K, Mohri M, Yamaguchi H, Takagaki M, Hisamochi K, Morimoto T. et al. Posttransplant function of a nonbeating heart is predictable by an ex vivo perfusion method. Ann Thorac Surg 2001;71:278–83. [DOI] [PubMed] [Google Scholar]
- 66. Messer S, Page A, Colah S, Axell R, Parizkova B, Tsui S. et al. Human heart transplantation from donation after circulatory-determined death donors using normothermic regional perfusion and cold storage. J Heart Lung Transplant 2018;37:865–9. [DOI] [PubMed] [Google Scholar]
- 67. Messer S, Page A, Axell R, Colah S, Hernandez-Sanchez J, Parizkova B. et al. Excellent early outcomes following heart transplantation from circulatory dead donors. J Heart Lung Transplant 2017;36:S15–16. [Google Scholar]
- 68. Noterdaeme T, Detry O, Hans M-F, Nellessen E, Ledoux D, Joris J. et al. What is the potential increase in the heart graft pool by cardiac donation after circulatory death? Transpl Int 2013;26:61–6. [DOI] [PubMed] [Google Scholar]
- 69. Messer S, Lannon J, Wong E, Hopkinson C, Fielding S, Axell R. et al. The potential of transplanting hearts from donation after circulatory determined death (DCD) donors within the United Kingdom. J Heart Lung Transplant 2015;34:S275. [Google Scholar]
- 70. Rydberg L. ABO-incompatibility in solid organ transplantation. Transfus Med 2001;11:325–42. [DOI] [PubMed] [Google Scholar]
- 71. Opelz G, Morath C, Süsal C, Tran TH, Zeier M, Döhler B.. Three-year outcomes following 1420 ABO-incompatible living-donor kidney transplants performed after ABO antibody reduction: results from 101 centers. Transplantation 2015;99:400–4. [DOI] [PubMed] [Google Scholar]
- 72. Henderson HT, Canter CE, Mahle WT, Dipchand AI, LaPorte K, Schechtman KB. et al. ABO-incompatible heart transplantation: analysis of the Pediatric Heart Transplant Study (PHTS) database. J Heart Lung Transplant 2012;31:173–9. [DOI] [PubMed] [Google Scholar]
- 73. Urschel S, Larsen IM, Kirk R, Flett J, Burch M, Shaw N. et al. ABO-incompatible heart transplantation in early childhood: an international multicenter study of clinical experiences and limits. J Heart Lung Transplant 2013;32:285–92. [DOI] [PubMed] [Google Scholar]
- 74. Bergenfeldt H, Andersson B, Bućin D, Stehlik J, Edwards L, Rådegran G. et al. Outcomes after ABO-incompatible heart transplantation in adults: a registry study. J Heart Lung Transplant 2015;34:892–8. [DOI] [PubMed] [Google Scholar]
- 75. Längin M, Mayr T, Reichart B, Michel S, Buchholz S, Guethoff S. et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature 2018;564:430–3. [DOI] [PubMed] [Google Scholar]
- 76. Knosalla C. Success for pig-to-baboon heart transplants. Nature 2018;564:352.. [DOI] [PubMed] [Google Scholar]