Abstract
Objective
The negative effect of sepsis on the myocardium affects its electric functionality. This study aims to evaluate the incidence of atrial fibrillation (AF) in patients with septic shock, and the mortality rate of patients with AF versus patients that maintained sinus rhythm (SR).
Methods
This is a one-year observational prospective pilot study. It was conducted at the Department of Anaesthesia and Intensive Care of Pisa University. Patients with septic shock were enrolled in this study. They were divided in two groups based on the occurrence of AF while in the ICU. Data were collected at admission and after 72 hours, and the data consisted of anamnesis, vital parameters, blood results and severity score.
Results
Out of 27 patients, 9 developed AF during the first 72 hours. At admission and at 72 hours, SOFA was statistically higher in the patients with AF (p=0.012 and p=0.002, respectively). In the AF group, the overall mortality was 66.7%, whereas, it was 11.1% (p=0.006) in the patients with SR. Age, rhythm and noradrenaline dosage were univariate predictors of total mortality.
Conclusion
In patients with septic shock, AF has a high incidence, and it correlated with a worse outcome. Patients with higher SOFA score are at a greater risk of developing arrhythmia.
Keywords: Arrhythmia, atrial fibrillation, mortality, sepsis, septic shock, SOFA score
Introduction
Sepsis is a clinical state that cannot be simply explained by infection. It is characterised by a profound dysregulation of the immune system, and can cause a multi-organ dysfunction (1). Among these, the cardiovascular system (both vascular tone and cardiac function) can be impaired by this condition (2, 3). The negative effects of sepsis on the heart are not limited to the contractile function and/or ventricular relaxation, but they also extend to the electric function (4). The underlying mechanism that can explain this phenomenon is unknown, but inflammation seems to play an important role. In the past, the electrical instability of cardiomyocytes in patients with sepsis was considered associated to vasoactive drugs and serum electrolytes. However, according to recent findings, atrial fibrillation (AF) could be the result of the necrosis and fibrosis induced by inflammation (5, 6). The alterations of the myocardial tissue are supposed to be able to start the arrhythmia directly thanks to a fluctuation in the myocardial cells’ membrane potential (7).
One of the first studies that retrospectively analysed the incidence and the clinical course of AF in a medical-surgical ICU found that 5.8% of patients with sepsis developed AF (8). Remarkably, they also observed that the mortality was statistically higher in patients with sepsis with new-onset AF (p=0.034). A 2015 systematic review on 11 studies showed that the mean incidence of new-onset AF was 8% in patients with sepsis and 23% in patients with septic shock. The authors of that study also observed a significant increase in ICU length of stay in this group of patients (9). Guenancia et al. (10) conducted a prospective study on patients with septic shock, and they found an incidence of new-onset AF of 44%. New-onset AF was associated with lower ejection fraction, older age, higher level of troponin-HS and NT-pro-BNP, longer QRS duration and higher risk of arrhythmias. Noteworthy, a 34% of new-onset AF would not be diagnosed without Holter ECG monitoring (silent AF). Moreover, no difference was observed in mortality at 28 or 90 days in patients with or without new-onset AF. In literature, the wide range of AF incidence during sepsis may be due to the different criteria used to define sepsis and septic shock, or the method used for the diagnosis of AF. Therefore, the first aim of this study was to determine the incidence of AF in patients with septic shock admitted to the ICU of Pisa University-Hospital using continuous ECG monitoring. The second aim was to evaluate the mortality rate of patients with AF in comparison with patients that maintained sinus rhythm (SR).
Methods
The study was design as a one-year (from May 2016 to May 2017) observational prospective pilot study, and it was conducted at the Department of Anaesthesia and Intensive Care of Pisa University-Hospital. After approval by the Research Ethics Committee of Pisa, patients were enrolled in this study (approval number 853; May 2016). Written informed consent was obtained from all the participants who were conscious or regained consciousness during the ICU stay.
Study population
We prospectively recruited adult patients admitted to the ICU for septic shock. We excluded patients aged <18 years, pregnant women, those with valvular heart diseases and with chronic AF or presence of implantable pacemaker.
Study design
At admission, septic shock was defined according to the International Guidelines for Management of Severe Sepsis and Septic Shock 2016 (11). All patients admitted with a diagnosis of septic shock were managed as follows: sedation, intubation and mechanical ventilation with low tidal volume (6 mL kg−1) and a positive-end expiratory pressure setting. Standard monitoring was used that included a continuous electrocardiogram (three or five derivations), finger pulse oximetry and end-tidal carbon dioxide monitoring. Furthermore, arterial and central venous catheters were inserted, and fluid resuscitation was started. Blood cultures, bronchoalveolar lavage and surveillance swabs were collected. This was followed by the administration of broad-spectrum antibiotics. Due to the persistence of hypotension, noradrenaline was continuously administered. Blood gas analysis was performed at least every 6 hours, and serum electrolytes (Na+, K+, Ca2+) and fluid balance were carefully corrected. In case of failure to obtain haemodynamic stability, regardless of vasoactive administration and adequate fluid administration, hydrocortisone infusion was started. Procalcitonin (PCT) was also measured at admission and for the following days.
When an arrhythmia was suspected during the ECG monitoring, a standard 12 derivation ECG was obtained for the careful evaluation of the rhythm. In patients presenting with AF, cardioversion was attempted when considered appropriate in accordance with 2015 advanced cardiac life support guidelines (12). In particular, in case of unstable patients (e.g. hypotension, acute heart failure, signs of shock), an immediate synchronised cardioversion was performed. If cardioversion was attempted, data were collected regarding its outcome. Anamnestic information was gathered (i.e. hospital admission diagnosis, history of AF, diagnosis of diabetes, hypertension, previous myocardial infarction, thyroid disorders, and pulmonary, gastrointestinal tract or the central nervous system diseases). Moreover, information was collected regarding infection source. SAPS II, APACHE II and SOFA score were calculated at admission. SOFA score was also calculated at T72 h.
Haemodynamic (i.e. mean invasive arterial pressure, heart rate, respiratory rate) and blood gas analysis parameters (i.e. pH, PaO2, PaCO2, PaO2/FiO2) were also recorded at T0 and T72h.
Blood samples were taken for laboratory tests at admission (T0) and after 72 hours from admission (T72h). Electrolytes, blood glucose, haemoglobin, serum lactate, blood count, C reactive protein (CRP), PCT, creatinine, liver enzymes, bilirubin and troponin were determined according to the standard laboratory procedures. Information regarding need for haemodynamic support with vasoactive drugs or inotropes, length of recovery and the need for mechanical ventilation or renal replacement therapy was recorded.
Follow-up data
Follow-up data were obtained from at least one of four sources: review of the patient’s hospital record, personal communication with the patient’s physician, review of the patient’s chart and a telephonic interview with the patient conducted by trained personnel and/or a staff physician. Total mortality was the primary end point.
Statistical analysis
The statistical analyses included descriptive statistics (frequency and percentage of categorical variables and mean and standard deviation of continuous variables), Kaplan-Meier survival curves and Cox proportional hazards models. Differences between survival curves were compared with the log-rank test. All analyses were performed using the Statistical Package for the Social Sciences statistical software (SPSS Inc.; Chicago, IL, USA). A p value <0.05 was considered statistically significant.
Results
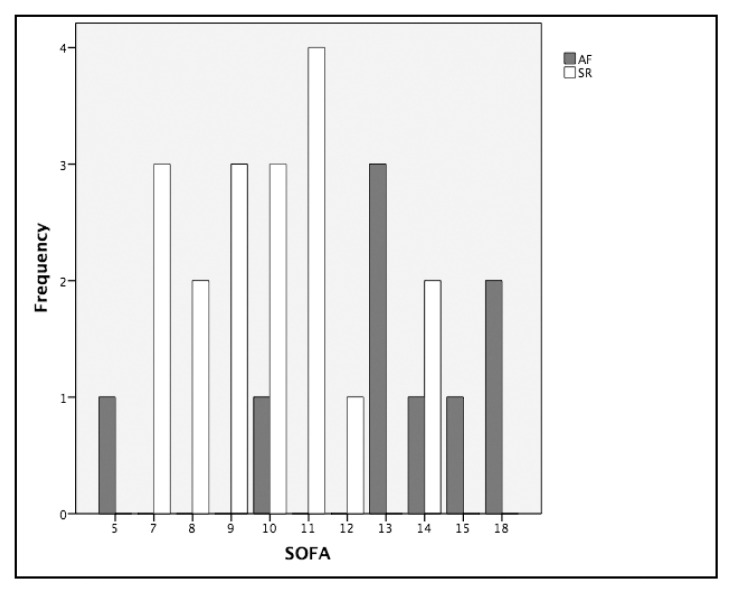
During the study period, 31 septic shock admissions were recorded. Three patients were excluded because of chronic AF, and one because of the presence of a pacemaker. The final enrolment was 27 patients. Demographic characteristics of participants are shown in Table 1. Out of 27 patients with septic shock admitted to the ICU during the one-year period, 9 developed AF during the first 72 hours. Of these nine patients, seven developed arrhythmia in the first 24 hours. Considering the two groups, no significant differences were found in terms of demographic characteristics, anamnestic information and primary source of infection when compared with the patients with SR (Table 1). The incidence of AF in patients with septic shock was 33.3%; and because only two patients had a prior history of an AF episode, the incidence of new-onset AF was 25.9%. The severity of illness was evaluated by SAPS II, APACHE II and SOFA (Table 2). The SOFA score was the only one significantly different variable between the two groups (SR vs. AF) at admission (p=0.012), and the difference was even more marked at 72 hours (p=0.002) (Table 2). The values of SOFA score calculated for patients in both groups are displayed in Figure 1. Vital parameters, blood results and blood gas obtained at admission (T0) and on the third day (T72h) were also compared. In the AF group at T72h, mean arterial blood pressure was significantly higher [AF 127.71 (17.64) mmHg vs. SR 108.22 (14.91) mmHg; p=0.025] as well as BE serum concentration (AF 0.40 (3.02) mEq L−1 vs. SR 3.46 (4.24) mEq L−1 p=0.043), whereas, HCO3− was lower [AF 24.83 (2.44) mmol L−1 vs. SR 27.73 (3.43) mmol L−1; p=0.040]. All these results are shown in Appendix Table A. Moreover, there was no significant major request for haemodynamic support with noradrenaline in patients with AF (Table 3). Length of recovery, need for mechanical ventilation, vasopressor support and renal replacement therapy were also recorded and compared between the two groups without significant differences, as shown in Table 3.
Table 1.
Demographic characteristics of participants
| Characteristics | Overall study population | AF group (n=9) | SR group (n=18) | p |
|---|---|---|---|---|
|
| ||||
| Mean (SD) | Mean (SD) | Mean (SD) | ||
|
| ||||
| Age (year) | 72.04 (11.81) | 76.78 (5.93) | 69.76 (13.37) | 0.085 |
| Height (cm) | 169.59 (6.95) | 170.89 (7.80) | 168.94 (6.63) | 0.668 |
| Weight (kg) | 74.15 (15.71) | 75.89 (19.10) | 73.28 (14.26) | 0.860 |
| BMI | 25.78 (5.38) | 26.00 (6.58) | 25.66 (4.88) | 0.940 |
| N | N (%) | N (%) | ||
| Gender (male) | 18 | 5 (27.78) | 13 (72.22) | 0.423 |
| Medical patient | 7 | 4 (57.14) | 3 (42.86) | 0.175 |
| Surgical patient | 20 | 5 (25) | 15 (75) | |
|
| ||||
| Comorbidities | ||||
|
| ||||
| N | N (%) | N (%) | ||
| Hypertension | 16 | 6 (66.7) | 10 (55.6) | 0.692 |
| Diabetes | 11 | 2 (22.2) | 9 (50.0) | 0.231 |
| Coronary artery disease | 8 | 1 (11.1) | 7 (38.9) | 0.201 |
| History of AF | 4 | 2 (22.2) | 2 (11.1) | 0.582 |
|
| ||||
| Source of infection | ||||
|
| ||||
| N | N (%) | N (%) | ||
| Primary blood stream | 1 | 0 (0) | 1(100) | 1.00 |
| Respiratory tract | 5 | 2 (40) | 3 (60) | 1.00 |
| Abdominal | 14 | 4 (29) | 10 (71) | 0.695 |
| Genito-urinary tract | 6 | 2 (33) | 4 (67) | 1.00 |
| Skin or soft tissue | 1 | 1 (100) | 0 (0) | 0.33 |
Values are expressed as mean and standard deviation (SD) or as total number (N) and percentage (%) where appropriate; p value was obtained using Mann-Whitney’s non-parametric U test for Fisher’s exact test where appropriate. BMI: body mass index; AF: atrial fibrillation; SR: sinus rhythm
Table 2.
Survival score between AF group and SR group
| AF group (n=9) | SR group (n=18) | ||
|---|---|---|---|
|
| |||
| Mean (SD) | Mean (SD) | p | |
|
| |||
| SOFA | 13.22 (3.99) | 9.89 (2.14) | 0.012 |
| SAPS II | 56.89 (11.15) | 46.17 (13.43) | 0.059 |
| APACHE | 23.00 (5.61) | 18.94 (5.59) | 0.194 |
| SOFA at T72h | 12.43 (2.64) | 7.44 (3.79) | 0.002 |
Values are expressed as mean and standard deviation (SD); p value was obtained using Mann-Whitney’s non-parametric U test. AF: atrial fibrillation; SR: sinus rhythm; SOFA: Sequential Organ Failure Assessment Score; SAPS II: Simplified Acute Physiology Score; APACHE: The Acute Physiology and Chronic Health Evaluation
Figure 1.
The SOFA score values distribution among studied patients based on rhythm
AF: atrial fibrillation; SR: sinus rhythm; frequency of SOFA score displayed on a clustered bar chart
Table 3.
Survival score between AF group and SR group
| AF group (n=9) | SR group (n=18) | ||
|---|---|---|---|
|
| |||
| Mean (SD) | Mean (SD) | p | |
|
| |||
| Length of recovery | 13.00 (11.10) | 15.44 (13.01) | 0.959 |
| Days on ventilation support | 9.11 (7.92) | 7.44 (9.88) | 0.264 |
| Days on vasopressor therapy | 7.11 (5.35) | 7.72 (8.70) | 0.958 |
| Noradrenaline-T0 (μg kg−1 min−1) | 0.33 (0.36) | 0.16 (0.14) | 0.172 |
| Noradrenaline-T72h (μg kg−1 min−1) | 0.30 (0.28) | 0.09 (0.13) | 0.098 |
| Days on renal replacement therapy | 5.11 (5.32) | 4.17 (8.46) | 0.192 |
Values are expressed as mean and standard deviation (SD); p value was obtained using Mann-Whitney’s non-parametric U test.
Synchronised electrical cardioversion
Synchronised electrical cardioversion (ECV) was attempted in five patients due to haemodynamic instability. In three patients, the procedure was not effective, whereas, in one patient, SR was restored. However, AF recurred shortly afterwards; and in one case, a stable SR was obtained. When ECV was not clinically indicated, appropriated drugs were administered (amiodarone, beta-blockers or calcium antagonist) to control the patient’s heart rate, if clinically needed. Noteworthy, out of the four patients in which ECV was ineffective or AF recurred shortly afterwards, three died during their ICU stay.
Follow-up data
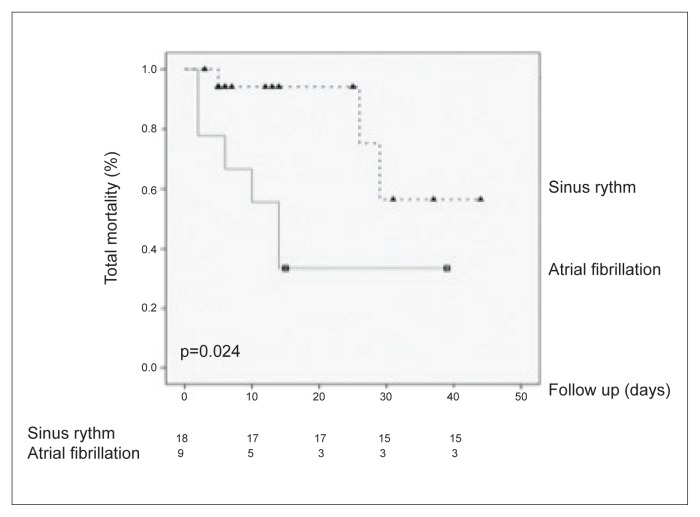
By inclusion criteria, follow-up data were obtained in all patients (mean value: 14.63 days). Total mortality was the primary end points. Hospital and physician records as well as death certificates were used to ascertain the cause of death. The total mortality was 29.6%. In the AF group, six deaths were recorded, consisting of the 66.7% of the patients. By contrast, in the SR group, only 11.1% of the patients had a negative outcome (overall mortality AF vs. SR, p=0.024; as shown in Figure 2).
Figure 2.
Kaplan-Meier survival curves (considering total mortality as an endpoint)
With an interactive procedure, we analysed the predictive characteristic of the model considering the following: anagraphic data (i.e. age and sex), physiological variables (i.e. BMI, mean blood pressure, heart rate, respiratory frequency), haematological data (as described previously), blood gas analysis parameters, noradrenaline therapy, presence of SR or AF. Univariate predictors of total mortality were reported in Table 4. Using stepwise Cox’s proportional hazard model, only age [p=0.015; HR-(95% CI); 1.187 (1.039–1.357)] and noradrenaline dosage in the first day (μ kg−1 min−1) [p=0.013; HR-(95% CI); 32.669 (2.082–512.497)] were independent predictors of death.
Table 4.
Significant univariate predictors of total mortality
| p | HR (95%CI) | |
|---|---|---|
|
| ||
| Age | 0.009 | 1.170 (1.040–1.316) |
| Male sex | 0.044 | 4.821 (1.044–22.259) |
| AF rhythm | 0.04 | 4.414 (1.070–18.208) |
| Noradrenaline 1 day | 0.007 | 27.992 (2.441–320.1) |
Noradrenaline 1 day: Mean noradrenaline dosage in the first day of sepsis diagnosis
Discussion
In our one-year prospective observational pilot study, data emerging suggest that AF is a common occurrence during septic shock, and that its presentation is usually within 24 hours from admission. A major risk factor associated with the development of the arrhythmia was an elevated SOFA score. In our study, hypertension, diabetes and coronary artery disease, commonly associated with AF in general population, did not seem to increase the occurrence of AF in patients with septic shock. This underlined that in case of AF appearance in patients with septic shock, inflammation could have an important role.
Moreover, no major difference regarding vital parameters, blood result and blood gas recorded at T0 and T72h was observed suggesting SOFA to be a more sensible factor associated with this population.
In our study, patients presenting with atrial arrhythmia or those who developed AF were more likely to have a negative outcome (Figure 2), and usually presented with a higher SOFA score, both at admission and after 72 hours.
Furthermore, we remarked that those patients that required ECV, most of the times, where not effectively cardioverted, and in three out of four patients, the failure to restore SR was associated with a negative outcome.
Physiopathological mechanisms
Sepsis is characterised by a systemic release of pro-inflammatory factors and by a profound dysregulation of the immune system. Inflammation has been implicated in the pathological processes of various cardiovascular diseases (e.g. unstable angina, myocardial infarction) as well as AF. In fact, the conventional risk factors associated with the occurrence of AF (such as diabetes mellitus, cardiovascular diseases) did not seem responsible of the increased incidence of new-onset AF in patients with sepsis (13, 14). This phenomenon could be explained by the fact that patients with standard cardiovascular AF risk factors may have already developed a chronic AF or have been treated for AF. However, other pathophysiological pathways are likely to play an important role in the development of AF during sepsis and warrant further evaluation. Inflammation, oxidative stress, apoptosis and fibrosis are all pathological mechanisms that may promote AF genesis in patients with sepsis (5).
Comparison with previous studies
Regarding the incidence of AF in patients with septic shock, our results are in accordance with previous literature. Although, small sample size studies already suggested that the occurrence of AF had a difference rate in patients with sepsis according to their severity of illness (15), a larger study conducted by Walkey et al. (14), which retrospectively analysed data from over 60,000 patients admitted for sepsis, confirmed this hypothesis showing an overall incidence of AF during sepsis of 25.5%. This number rose to 31.6% when considering only the ICU population. Moreover, these authors found that new-onset AF during sepsis was characterised by different risk factors profile in comparison to the general population of patients with AF (14). Older age, white race and severity of acute illness seemed to be the major factors influencing the development of new-onset AF. This difference in risk factors profile for AF in patients with septic shock, and in particular the association with acute illness, is coherent with our findings. Noteworthy, Launey et al. (16) observed that low-dose hydrocortisone represented a risk factor of AF. However, hydrocortisone was administered to all the patients included in our study. Consequently, it was not possible to obtain a control group to determine whether the use of hydrocortisone actually affected the occurrence of AF in our group of patients.
Our study confirms the present literature on the detrimental outcome in patients with sepsis that developed the arrhythmia in comparison with those who maintained SR (8, 15, 17, 18). Even when the probability for a negative outcome was adjusted for severity of illness, there was an increased risk for mortality related to the presence of AF. Although other two studies did not show this difference in mortality in patients with sepsis with AF, in one, the authors themselves suggested that there was a trend towards a worse outcome in patients with AF, and the negative results was due probably to the small sample size of the study (19). In the second study, Holter ECG monitoring was used to identify the arrhythmia, and since the authors stated that 34% of the patients with AF would not have been diagnosed with the arrhythmia without monitoring, we could assume that roughly in one third of this study population, the arrhythmia was not clinically significant (10). This was confirmed by another retrospective study (20). In fact, Moss at al. (20) observed that new-onset AF was subclinical or went undiagnosed in about 8% of the ICU patients. This aspect highlights the importance of the analysis of continuous electrocardiogram. Furthermore, in the aforementioned study, the authors found that new-onset AF was associated with increased hospital mortality (OR, 1.63; 95% CI, 1.01–2.63) and hospital length of stay (OR 2.25; CI, 0.58–3.92). Consequently, they concluded that new-onset AF appeared to be related with poor hospital outcomes. These results are in accordance with another retrospective study (21). Shahreyar et al. (21) observed that after correcting for possible confounders, patients with severe sepsis were at higher risk for AF, and cardiac arrest, with higher in-hospital mortality (OR, 1.41; 95% CI, 1.37–1.45), length of stay (OR, 1.50; 95% CI, 1.46–1.53) and total hospital charges (OR, 1.37; 95% CI, 1.34–1.41). Finally, in a 2018 systematic review and meta-analysis of 12 studies, the authors found a statistically significant difference in the rate of hospital mortality between patients with and without new-onset AF (OR 2.7; 95% CI 2.43–3.00) (22). Furthermore, the authors performed subgroup analysis of patients with sepsis or septic shock and they observed a significant association between new-onset AF and mortality (OR 2.32; 95% CI 1.88–2.85; I2=0%).
Study limitations
Our study has some limitations. Firstly, the small sample size of our study may have affected the possibility to detect differences between the two groups of patients regarding the measured parameters. Secondly, Holter EGG monitoring was not used for AF diagnosis, consequently, only AF episode detectable with continuous ECG monitoring were recorded, in accordance with usual clinical practice. Moreover, we decided to include the patients with history of AF episode in the study, if there was evidence of their being in SR before the occurrence of septic shock. Out of 27 patients, 4 had history of AF; 2 maintained SR, whereas, 2 again developed arrhythmia.
Conclusion
This study confirms the high incidence of AF in patients with septic shock. Those with a higher SOFA score are at greater risk of developing the arrhythmia. In this particular subset of patients, history of a prior AF episode had no influence on the occurrence of AF during septic shock as well as other risk factors commonly associated with AF.
For these reasons, AF in patients with septic shock should be considered as a sign of a greater severity of illness. Critically ill patients developing this arrhythmia should be considered being at more risk for a negative outcome. Additionally, in most of these cases, cardioversion is not effective, at least not in the first few days of admission.
In this particular subset of ICU population, the occurrence of AF may be considered as a manifestation of the severity of septic shock. Therefore, standardised ICU protocol should be directed towards the detection of any AF episode.
You can reach the questionnaire of this article at https://doi.org/10.5152/TJAR.2019.44789
Supplementary Material
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Pisa University (approval number 853; May 2016).
Informed Consent: Written informed consent was obtained from all participants who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – I.S., E.B., F.F.; Design – I.S., E.B., F.F.; Supervision – I.S., E.B., L.P., D.T., G.G., E.Bignami., F.F.; Materials – I.S., E.B., L.P., D.T., G.G., E.Bignami., F.F.; Data Collection and/or Processing – I.S., E.B., L.P., D.T., G.G., E.Bignami., F.F.; Analysis and/or Interpretation – I.S., E.B., L.P., D.T., G.G., E.Bignami., F.F.; Literature Search – I.S., E.B., L.P., D.T., G.G., E.Bignami., F.F.; Writing Manuscript – I.S., E.B., L.P., D.T., G.G., E.Bignami., F.F.; Critical Review – I.S., E.B., L.P., D.T., G.G., E.Bignami., F.F.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Deutschman CS, Tracey KJ. Sepsis: current dogma and new perspectives. Immunity. 2014;40:463–75. doi: 10.1016/j.immuni.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Court O, Kumar A, Parrillo JE, Kumar A. Clinical review: Myocardial depression in sepsis and septic shock. Crit Care. 2002;6:500–8. doi: 10.1186/cc1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gamcrlidze MM, Intskirveli NA, Vardosanidze KD, Chikhladze Kh E, Goliadze L, Ratiani LR. Vasoplegia in septic shock (review) Georgian Med News. 2015;239:56–62. [PubMed] [Google Scholar]
- 4.Seguin P, Signouret T, Laviolle B, Branger B, Malledant Y. Incidence and risk factors of atrial fibrillation in a surgical intensive care unit. Crit Care Med. 2004;32:722–6. doi: 10.1097/01.CCM.0000114579.56430.E0. [DOI] [PubMed] [Google Scholar]
- 5.Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J. 2015;79:495–502. doi: 10.1253/circj.CJ-15-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hadi HA, Alsheikh-Ali AA, Mahmeed WA, Suwaidi JMA. Inflammatory cytokines and atrial fibrillation: current and prospective views. J Inflamm Res. 2010;3:75–97. doi: 10.2147/JIR.S10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galea R, Cardillo MT, Caroli A, Marini MG, Sonnino C, Narducci ML, et al. Inflammation and C-Reactive protein in atrial fibrillation: cause or effect? Tex Heart Inst J. 2014;41:461–8. doi: 10.14503/THIJ-13-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christian SA, Schorr C, Ferchau L, Jarbrink ME, Parrillo JE, Gerber DR. Clinical characteristics and outcomes of septic patients with new-onset atrial fibrillation. J Crit Care. 2008;23:532–6. doi: 10.1016/j.jcrc.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Kuipers S, Klouwenberg PMK, Cremer OL. Incidence, risk factors and outcomes of new-onset atrial fibrillation in patients with sepsis: a systematic review. Crit Care. 2014;18:688. doi: 10.1186/s13054-014-0688-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guenancia C, Binquet C, Laurent G, Vinault S, Bruyere R, Prin S, et al. Incidence and predictors of new-onset atrial fibrillation in septic shock patients in a medical ICU: data from 7-day holter ECG monitoring. PloS One. 2015;10:e0127168. doi: 10.1371/journal.pone.0127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–77. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 12.Link MS, Berkow LC, Kudenchuk PJ, Halperin HR, Hess EP, Moitra VK, et al. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S444–64. doi: 10.1161/CIR.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 13.Walkey AJ, McManus D. When rhythm changes cause the blues: new-onset atrial fibrillation during sepsis. Am J Respir Crit Care Med. 2017;195:152–4. doi: 10.1164/rccm.201608-1617ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walkey AJ, Greiner MA, Heckbert SR, Jensen PN, Piccini JP, Sinner MF, et al. Atrial fibrillation among medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J. 2013;165:949–55.e3. doi: 10.1016/j.ahj.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salman S, Bajwa A, Gajic O, Afessa B. Paroxysmal atrial fibrillation in critically ill patients with sepsis. J Intensive Care Med. 2008;23:178–83. doi: 10.1177/0885066608315838. [DOI] [PubMed] [Google Scholar]
- 16.Launey Y, Lasocki S, Asehnoune K, Gaudriot B, Chassier C, Cinotti R, et al. Impact of low-dose hydrocortisone on the incidence of atrial fibrillation in patients with septic shock. J Intensive Care Med. 2017 doi: 10.1177/0885066617696847. 885066617696847. [DOI] [PubMed] [Google Scholar]
- 17.Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306:2248–54. doi: 10.1001/jama.2011.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells GL, Morris PE. Incidence and prognosis of atrial fibrillation in patients with sepsis. Cardiol Res. 2011;2:293–7. doi: 10.4021/cr108w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meierhenrich R, Steinhilber E, Eggermann C, Weiss M, Voglic S, Bogelein D, et al. Incidence and prognostic impact of new-onset atrial fibrillation in patients with septic shock: a prospective observational study. Crit Care. 2010;14:R108. doi: 10.1186/cc9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moss TJ, Calland JF, Enfield KB, Gomez-Manjarres DC, Ruminski C, DiMarco JP, et al. New-onset atrial fibrillation in the critically Ill. Crit Care Med. 2017;45:790–7. doi: 10.1097/CCM.0000000000002325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shahreyar M, Fahhoum R, Akinseye O, Bhandari S, Dang G, Khouzam RN. Severe sepsis and cardiac arrhythmias. Ann Transl Med. 2018;6:6. doi: 10.21037/atm.2017.12.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanjanahattakij N, Rattanawong P, Krishnamoorthy P, Horn B, Chongsathidkiet P, Garvia V, et al. New-onset atrial fibrillation is associated with increased mortality in critically ill patients: a systematic review and meta-analysis. Acta Cardiol. 2018:1–8. doi: 10.1080/00015385.2018.1477035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.