Abstract
Human papillomaviruses (HPVs) are the most common sexually transmitted infections. HPVs are transmitted through anogenital sex or oral sex. Anogenital transmission/infection is associated with anogenital cancers and genital warts while oral transmission/infection is associated with head and neck cancers (HNCs) including recurrent respiratory papillomatosis. Current HPV vaccines protect against HPV types associated with ~90% of cervical cancers and are expected to protect against a percentage of HNCs. However, only a few studies have assessed the efficacy of current vaccines against oral HPV infections. We had previously developed a mixed MS2-L2 candidate HPV vaccine based on bacteriophage MS2 virus-like particles (VLPs). The mixed MS2-L2 VLPs consisted of a mixture of two MS2-L2 VLPs displaying: i) a concatemer of L2 peptide (epitope 20–31) from HPV31 & L2 peptide (epitope 17–31) from HPV16 and ii) a consensus L2 peptide representing epitope 69–86. The mixed MS2-L2 VLPs neutralized/protected mice against six HPV types associated with ~87% of cervical cancer. Here, we show that the mixed MS2-L2 VLPs can protect mice against additional HPV types; at the genital region, the VLPs protect against HPV53, 56, 11 and at the oral region, the VLPs protect against HPV16, 35, 39, 52, and 58. Thus, mixed MS2-L2 VLPs protect against eleven oncogenic HPV types associated with ~95% of cervical cancer. The VLPs also have the potential to protect, orally, against the same oncogenic HPVs, associated with ~99% of HNCs, including HPV11, which is associated with up to 32% of recurrent respiratory papillomatosis. Moreover, mixed MS2-L2 VLPs are thermostable at room temperature for up to 60 days after spray-freeze drying and they are protective against oral HPV infection.
Keywords: HPV vaccine, bacteriophage MS2-L2 VLPs, buccal immunization, Gardasil-9, mucosal adjuvants, thermostable vaccine
1. Introduction
Human papillomaviruses (HPVs) are non-enveloped DNA viruses with more than 220 different types identified to date [1–3]. Approximately, 40 of these HPV types are transmitted sexually through anogenital-to-anogenital sex or anogenital-to-oral sex [4, 5], and 19 HPV types can cause cancers. Anogenital HPV infections are associated with cervical cancer, vaginal cancer, penile cancer, anal cancer, and genital warts [6–10] whereas oral HPV infections are associated with head and neck cancers (HNCs; oral, oropharyngeal, laryngeal cancers, etc.) including recurrent respiratory papillomatosis (RRP) [11–15]. Twenty-five percent of HNCs are caused by HPVs [16]; HPV type 16 is associated with more than 70% of HPV-associated HNCs (HPV+HNCs). HPV18 is associated with 14–17% of HPV+HNCs while the remaining HPV+HNC cases are caused by other oncogenic HPV types (31, 33, 35, 45, etc.) [11, 17–19]. Two prophylactic vaccines (Gardasil-9 and Cervarix; Gardail-4 has been discontinued) are currently being used to protect against HPV infections [6, 20, 21]. Amongst these vaccines, Gardasil-9 offers the broadest level of protection against HPV types (HPV16, 18, 31, 33, 45, 52, 58) that cause ~90% of cervical cancers worldwide and HPV types (HPV6 and 11) that cause 90% of genital warts/RRP. Despite the broadness of protection offered by Gardasil-9 vaccine, vaccinated individuals are still advised to continue cervical cancer screening to make sure they are not infected with cancer-causing HPV types not included in the vaccine. The HPV types (e.g. HPV35, 39, 53, 56, etc.) associated with ~10% of cervical cancer not protected by current vaccines are significantly important especially if this involves patients infected with human immunodeficiency virus (HIV) or are suffering from acquired immunodeficiency syndrome (AIDS). Additionally, there is no data on the efficacy of Gardasil-9 vaccine against oral HPV infections.
To enhance the spectrum of protection and to circumvent these problems, we developed, in a previous study [22], two bacteriophage MS2 VLPs: i) MS-31L2/16L2 VLP displaying a concatemer of HPV31 L2 (amino acid 20–31) & HPV16 L2 (amino acid 17–31) on its surface; ii) MS2-consL2(69–86) VLP displaying a consensus sequence (amino acid 69–86) derived from the alignment of 19 cancer-causing HPV types and 4 warts-causing HPV types. L2 (the minor capsid protein) is one of the two capsid proteins of HPV; L2, especially the N-terminus, is highly conserved among HPV types and is a target for next-generation HPV vaccines [23–26]. In Zhai et al. 2017, we showed that mice immunized, intramuscularly, with a mixture of the VLPs, MS2-31L2/16L2 and MS2-consL2(69–86), elicited high-titer (>104) IgG antibodies and protected the mice from vaginal challenge with HPV pseudoviruses representing HPV16, 18, 31, 45, and HPV33 at levels similar to Gardasil-9; suboptimal protection was observed against HPV58 [22]. To build on that study, we assessed whether oral immunization with the mixed MS2-L2 VLPs can protect mice from oral and/or vaginal infection with additional HPV pseudovirus types. We also assessed whether the mixed VLPs can be developed into a heat-stable dry powder formulation with mucosal adjuvants; we assessed the thermostability/immunogenicity of the dry powder VLPs stored at room temperature for 60 days.
2. Materials and Methods
2.1. Production of MS2-L2 VLPs
Plasmids encoding recombinant MS2-L2 proteins -- MS2 coat protein with an insertion of a concatemer of HPV L2 epitope containing amino acids (aa) 20–31 from HPV31 L2 and aa 17–31 from HPV16 L2 (MS2-31L2/16L2) or MS2 coat protein with an insertion of a consensus HPV L2 epitope [MS2-consL2(69–86)] - were previously described [22]. The plasmids were used to transform C41 Escherichia coli (E. coli) cells. Protein expression and VLPs purification were conducted as previously described [22]. Briefly, transformed bacterial C41 culture was induced with 0.5 mM Isopropyl β-D-1-thiogalactopyranoside (IPTG) for 3 hours and the bacteria were lysed with 0.2% lysozyme solution [for MS2-consL2(69–86) VLPs] or 10 mM Borax (for MS2-31L2/16L2 VLPs). Soluble VLPs were precipitated with 50% ammonium sulfate and purified using sepharose CL-4B columns.
2.2. Spray-freeze drying of VLPs into dry powder formulation
Equal concentrations of MS2-31L2/16L2 VLPs and MS2-consL2(69–86) VLPs were mixed together to obtain mixed MS2-L2 VLPs. Half of the mixed MS2-L2 VLPs (100 μg) was further mixed with 2 μg each of the mucosal adjuvants, cholera toxin (CT) and monophosphoryl lipid A (MPLA); control MS2 VLPs were mixed with the same concentration of adjuvants. The mixed MS2-L2 VLPs and MS2 VLPs, with or without mucosal adjuvants, were added to a 3% w/v MTDL excipient solution (containing Mannitol (M, 75% w/w), Trehalose (T, 7.5% w/w), Dextran (D, 2.5% w/w), and L-Leucine (L, 15% w/w). The VLPs were added at a concentration of 8% w/w to the MTDL excipients and the VLPs-excipients were then spray-freeze dried (SFD) in two steps. In the first step, the VLPs-excipients suspensions were sprayed into a stainless-steel container filled partially with liquid nitrogen. This was achieved by using a two-fluid nozzle (0.7 mm) which is part of a Büchi B-290 mini spray-dryer; the spraying operating conditions were as following: nitrogen flow (Q) between 10 and 15mm, and a VLPs-excipients feed rate of ~ 4 mL/min. Subsequently, the liquid nitrogen in the steel container was allowed to evaporate and the frozen droplets were transferred into a lyophilizer (FreeZone® Triad™ Freeze Dry system, Model 74000 Series) [27] for freeze-drying (the second step). Freeze-drying was conducted under the following conditions: pre-freeze for 3 h at −80 °C, primary drying at −10 °C for 24 h with a ramp of 0.25 °C/min, followed by secondary drying at 15 °C for 4 8 h with a ramp of 0.25 °C/min, and vacuum pressure of 1.51 mBar. All SFD products were collected in glass scintillation vials and were stored under refrigeration until further use.
2.3. Assessing the thermostability of SFD VLPs
Spray-freeze dried VLP powders were loaded into capsules, transferred to amber-colored Wheaton glass bottles and sealed with 20 mm butyl septa caps. The bottles were then purged with nitrogen gas and crimped with tear-away aluminum seals using a vial seal crimper [28]. The sealed bottles were then stored at 4 °C or room temperature for 60 days. The powders were then reconstituted in 1X phosphate buffered saline (PBS) and the integrity of VLPs was assessed, in comparison to non-spray-freeze dried VLPs, by transmission electron microscopy (TEM; 30,000X magnification). The immunogenicity of the reconstituted VLPs was assessed by immunizing mice as described below.
2.4. Immunization of mice and assessing antibody responses
All animal work was conducted following Michigan Technological University Institutional Animal Care and Use Committee (IACUC) guidelines. Groups of female balb/c mice (4–6 mice per group) were immunized thrice orally, by placing VLPs underneath the tongue or by injecting into the buccal region, at two-week intervals with freshly prepared (non-spray-freeze dried) mixed MS2-L2 VLPs [50 μg each of MS2-31L2/16L2 VLPs and MS2-consL2(69–86)] mixed with either 2 μg of CT, 2 μg of MPLA, a combination of the two adjuvants (same concentrations), or without adjuvants. Other groups of mice were immunized with control MS2 VLPs and same adjuvant combinations for comparison. In all immunizations, 5 μg of Gardasil-9 was used as controls administered intramuscularly because it does not contain mucosal adjuvants.
To assess the immunogenicity of the spray-freeze dried VLPs, 100 μg of the reconstituted SFD mixed MS2-L2 or SFD control MS2 VLPs (all SFD with CT and MPLA adjuvants) were used to immunize mice as described above (same route and schedule). Other groups of mice were immunized with the same concentration of reconstituted SFD VLPs but with additional 2 μg each of fresh liquid CT and MPLA adjuvants; the latter will assess whether the activity of the adjuvants were inactivated during the SFD process. The VLPs that were SFD without the adjuvants were immunized (at same concentration) with 2 μg each of fresh liquid CT and MPLA adjuvants.
In all immunizations, sera and saliva were collected two weeks after the last immunizations. IgG antibody titers in sera were conducted by peptide-ELISA as previously described [29]. Briefly, 96-well plates were coated, overnight, with 500 ng HPV16 L2 peptide conjugated to streptavidin. The plates were blocked for two hours with 0.5 % non-fat milk in 1X PBS. The plates were incubated with 4-fold serial dilutions of serum for two hours. Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (1:5000 dilution) antibodies were added to the plates for one hour. The wells were washed and developed with 3, 3’, 5, 5’-Tetramethylbenzidine (TMB) and stopped with 1M hydrogen chloride solution. Antibody titers were determined (at optical density 450) as the reciprocal of highest sera dilutions at which reactivity of experimental sera was at least twice that of control sera. IgA antibody titers in saliva were determined also by peptide-ELISA with 100 μL saliva samples as primary antibodies. HRP-conjugated goat anti-mouse IgA (1:500) antibodies were added for one hour and the plates were developed as described above except without the stop solution. IgA antibodies were determined by comparing the optical density (405 nm) values of experimental group to those of control group.
2.5. Oral and vaginal infection with HPV pseudoviruses (PsVs)
HPVs pseudoviruses representing PsVs 11, 16, 35, 39, 52, 53, 56 and 58 were expressed and purified by cesium chloride gradient ultracentrifugation as previously described [26]. The PsVs encode a reporter plasmid that expresses green fluorescence protein and luciferase. For vaginal infection, immunized mice were treated with Depoprovera and PsVs (11, 16, 53, and 56) infection was conducted as previously described [22]. For oral infection, immunized mice were injected at the buccal region with PsVs (16, 35, 39, 52 or 58); 1 – 7 × 106 infectious unit (IU) of PsVs were used in all infections. Forty-eight hours post-infection, mice that were infected orally were injected in the buccal region with 1 mg of luciferin, while those that were infected vaginally were instilled vaginally with 0.4 mg of luciferin. In both cases, the mice were imaged using IVIS Spectrum at one-minute exposure. Average radiance (p/s/cm2/sr) was determined by drawing equally sized regions of interests surrounding the site of PsV instillation.
2.6. Statistical analysis
Statistical analyses for ELISAs and of HPV PsV challenge studies were done using unpaired two-tailed t-test and unpaired one-tailed t-test, respectively.
3. Results
3.1. Buccal immunization with mixed MS2-L2 VLPs plus mucosal adjuvants elicits protective immune responses at the vaginal and oral regions
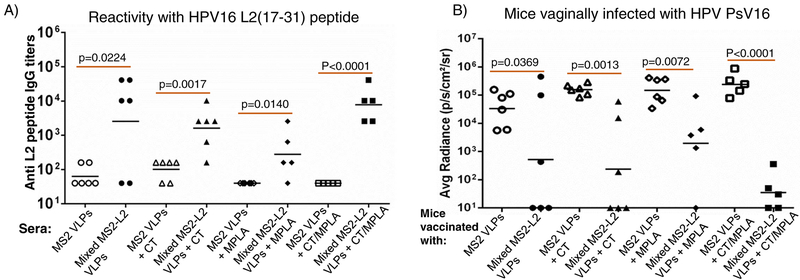
To assess which delivery method of oral immunization will elicit immune responses, mice were immunized by placing freshly prepared (non-spray-freeze dried) mixed MS2-L2 VLPs with/without cholera toxin and MPLA in the floor of the mouth or by injecting the buccal area of the mouth with the VLPs. We then assessed IgG and IgA antibody levels in sera and saliva, respectively. Mice immunized by injecting the floor of the mouth with the VLPs elicited high-titer IgG antibodies in the sera (Fig. 1A) but no detectable IgA in saliva (data not shown). Mice immunized with the mixed MS2-L2 VLPs in the presence of the two adjuvant combinations (mixed MS2-L2 VLPs + CT/MPLA) elicited a higher IgG titers (p<0.0001) compared to mice immunized with the same VLPs but with only one of the adjuvants (especially mixed MS2-L2 VLPs + MPLA; p=0.0140). Mice immunized by placing the VLPs in the floor of the mouth did not elicit immune response in the sera or in the saliva (data not shown). Thus, all subsequent immunization studies were conducted by injecting the buccal region of the mouth and with the two-adjuvant combinations (CT/MPLA).
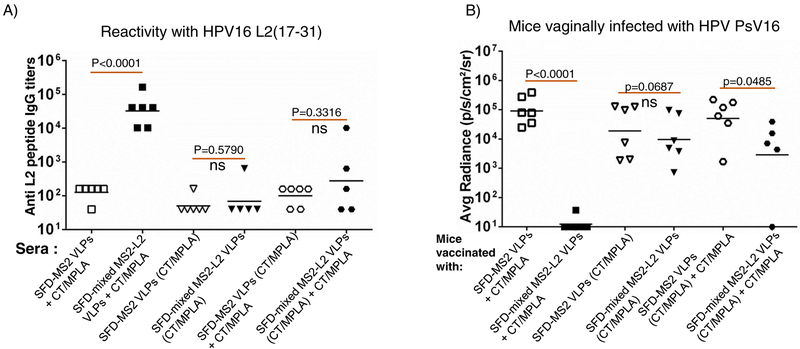
Fig. 1.
Immunogenicity of mixed MS2-L2 VLPs with/without mucosal adjuvants (CT or MPLA) and protection from PsV16, 53, 56 and 11. Mice were immunized (buccal injection) thrice with 100 mg of mixed MS2-L2 VLPs or control MS2 VLPs with/without CT, MPLA or with/out the two-adjuvant combinations at two-week intervals. Sera were collected two weeks after the last immunization. A) Anti-L2 peptide IgG titers in sera were determined by end-point dilution ELISA using 16L2 (17–31) peptide as the target peptide. Each datum represents antibody titer in each mouse and the black horizontal lines represent geometric mean for each group. B) The mice in (A) were vaginally challenged with ~3 × 106 IU of HPV PsV16. C) Additional groups of mice were immunized by buccal injection with 100 μg of mixed MS2-L2 VLPs, control MS2 VLPs, and 5 μg of Gardasil-9 vaccine (immunized intramuscularly). The mice were vaginally challenged with 1.6 – 4.4 × 106 IU of PsVs 53, 56, and 11. The average radiance (p/s/cm2/sr) of luciferase expression was determined using Living Image 4.5.5 software. Each datum represents the average radiance of an individual mouse and the lines represent the geometric mean for each group. The P-values for ELISAs and infection assays were determined by unpaired two-tailed t-test and unpaired one-tailed t-test, respectively.
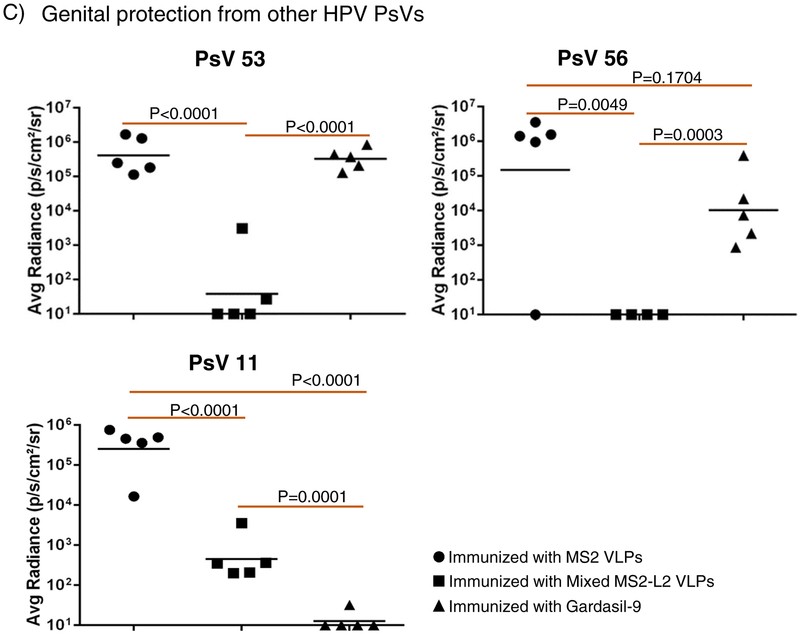
To assess if the immune responses elicited by buccal immunization were protective, we challenged the mice vaginally, using a well-characterized method for HPV infection [29, 30]. As shown in Fig. 1B, mice immunized with mixed MS2-L2 VLPs with CT/MPLA showed better levels of protection (p<0.0001) from HPV PsV16 infection compared to mice immunized with only one of the two adjuvants (especially those immunized with MPLA; p=0.0072). To test if buccal immunization with mixed MS2-L2 VLPs offered cross-protection against other HPV PsVs, vaccinated mice were vaginally infected with high-risk HPV PsV53 and PsV56, which are not included in Gardasil-9 vaccine, and with low-risk HPV PsV11. Mice immunized with mixed MS2-L2 VLPs offered complete protection against PsV53 and PsV56 while Gargasil-9 offered partial protection against PsV56 but no protection against PsV53 (Fig. 1C); both mixed MS2-L2 VLPs and Gardasil-9 elicited protective responses against PsV11 (Fig. 1C).
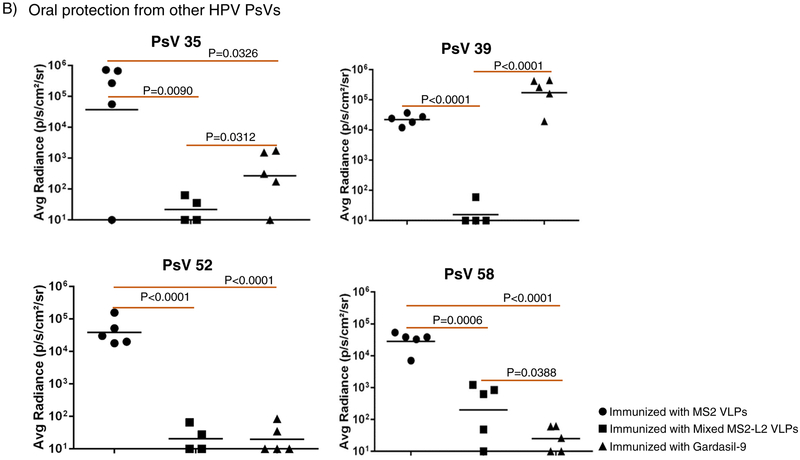
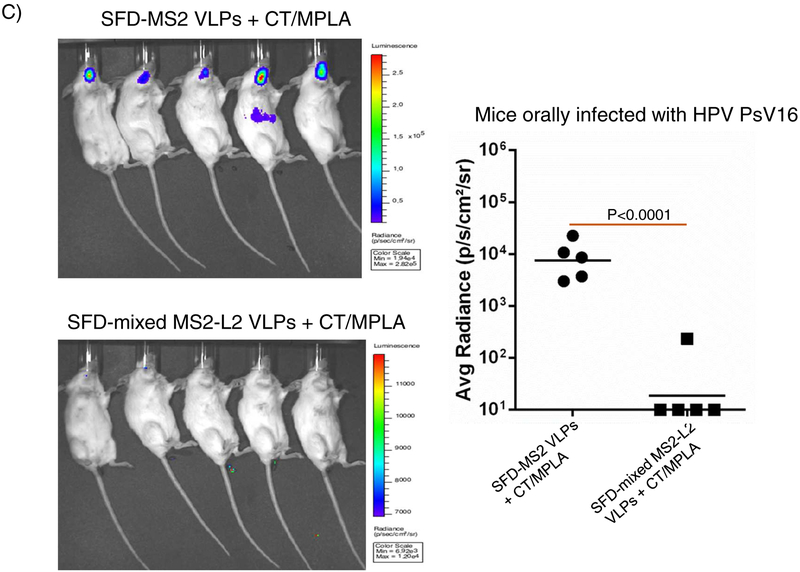
Having demonstrated that buccal immunization protected mice from vaginal infection by HPV PsVs 11, 16, 53, and 56, we decided to assess if buccal immunization can protect mice from oral infection with PsV16 and other PsVs (35, 39, 52 and 58). In Fig. 2, buccal immunization with mixed MS2-L2 VLPs + CT/MPLA protected mice from oral infection with PsVs 16, 35, 39, 52, and 58. While mixed MS2-L2 VLPs and Gardasil-9 (immunized intramuscularly) offered similar levels of protection against PsV16 and PsV52, Gardasil-9 offered better protection against PsV58 and mixed MS2-L2 VLPs offered best protection against PsV35 and PsV39 (Fig. 2B).
Fig. 2.
Buccal immunization with mixed MS2-L2 VLPs and oral protection from HPV PsVs 16, 35, 39, 52 and 58. Mice immunized (buccal injection) thrice (at two-week intervals) with 100 μg of mixed MS2-L2 VLPs, control MS2 VLPs, and 5 μg of Gardasil-9 vaccine (immunized intramuscularly) were orally challenged with: A) 7 × 106 IU of PsV16 and B) 1 – 7 × 106 IU of PsVs 35, 39, 52 and 58. Each datum represents the average radiance of an individual mouse and the lines represent the geometric mean for each group. The P-values were determined by unpaired one-tailed t-test.
3.2. Mixed MS2-L2 VLPs can be SFD without a mixture of cholera toxin/MPLA adjuvants
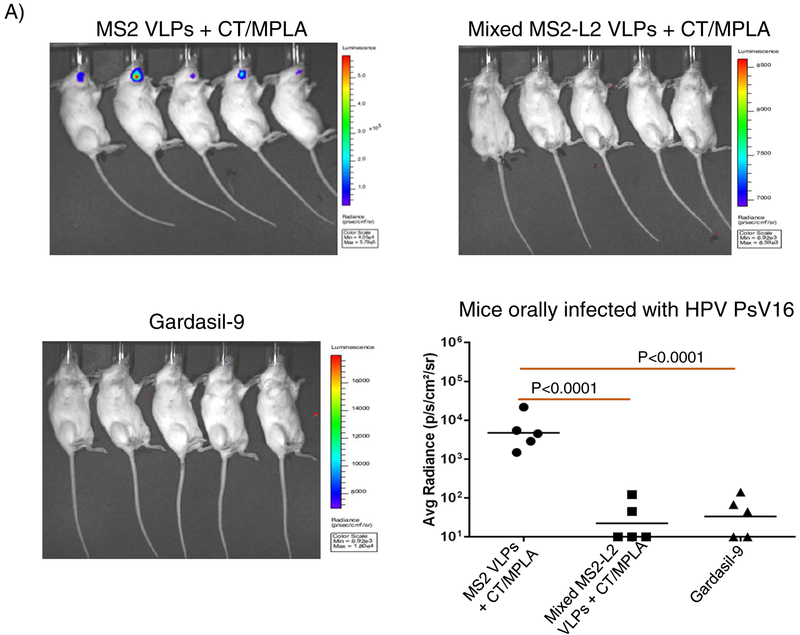
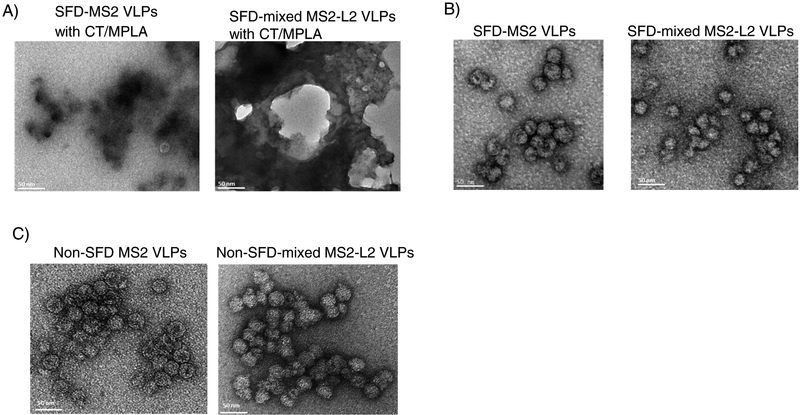
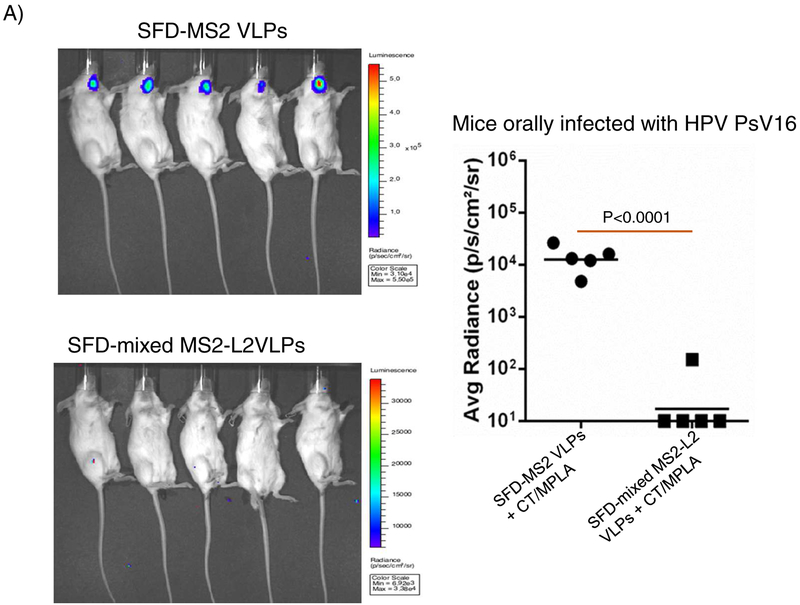
To assess if mixed MS2-L2 VLPs can be SFD into a product that is thermostable at room temperature, the VLPs with or without CT/MPLA were SFD with stabilizing excipients and the integrity of the VLPs was assessed by TEM. As a control, MS2 VLPs with same adjuvants and excipients were also SFD. While mixed MS2-L2 VLPs or MS2 VLPs in the absence of adjuvants were successfully SFD (based on the integrity of the VLPs after reconstitution of the powder in PBS buffer in comparison to non-SFD VLPs), the VLPs could not be SFD with the adjuvants (Fig. 3). SFD with adjuvants caused the VLPs to agglomerate (Fig. 3A). To assess whether the SFD VLPs (those that did not agglomerate and those that agglomerated) were still immunogenic, we immunized mice (in buccal region) with the VLPs with/without additional CT/MPLA adjuvants. Mixed MS2-L2 VLPs SFD without adjuvant, but adjuvant combinations were added (+ CT/MPLA) just prior to immunization were highly immunogenic compared to the VLPs [mixed MS2-L2 VLPs (CT/MPLA)] that were SFD together with the adjuvant combinations (Fig. 4A). The addition of more adjuvant (+ CT/MPLA) to the latter [mixed MS2-L2 VLPs (CT/MPLA)] slightly enhanced the immunogenicity of the VLPs but this was not significant. The mice immunized with mixed MS2-L2 VLPs + CT/MPLA were significantly protected from vaginal infection with HPV PsV16 while mice immunized with mixed MS2-L2 VLPs (CT/MPLA) + CT/MPLA elicited suboptimal protection against PsV16 infection (Fig. 4B). Also, the group of mice (SFD mixed MS2-L2 VLPs + CT/MPLA-immunized; Fig. 4B) that showed complete protection from vaginal infection with PsV16 was completely protected from oral infection with the same PsV type (Fig. 4C).
Fig. 3.
Transmission electron microscopy (TEM) images of spray-freeze dried (SFD) VLPs and non-spray-freeze dried VLPs. Mixed MS2-L2 VLPs and MS2 control VLPs were SFD with/without mucosal adjuvants (CT and MPLA) and the dry powders were reconstituted in PBS and analyzed (in comparison with fresh liquid VLPs) using a TEM. A) TEM of spray-freeze dried VLPs with CT/MPLA, B) TEM of spray-freeze dried VLPs without CT/MPLA, and C) TEM of fresh liquid VLPs. Images were taken under 30,000X magnification.
Fig. 4.
Buccal immunization with reconstituted SFD mixed MS2-L2 VLPs, vaginal and oral protection from HPV PsV16. Spray-freeze dried VLPs were reconstituted in PBS and mice were immunized thrice with SFD VLPs with/out addition of fresh CT/MPLA adjuvants. A) Sera were collected two weeks after the last immunization and anti-L2 peptide IgG titers in sera were determined by end-point dilution ELISA using 16L2 (17–31) peptide. Each datum represents antibody titer in each mouse and the black horizontal lines represent geometric mean for each group. B) The mice in (A) were vaginally challenged with ~3 × 106 IU of HPV PsV16. C) Mice immunized (buccal injection) with reconstituted SFD mixed MS2-L2 VLPs or control MS2 VLPs (in the presence of fresh CT/MPLA adjuvants) were orally challenged with ~7 × 106 IU of PsV16. Each datum represents average radiance of an individual mouse and the lines represent the geometric mean for each group. The P-values for ELISAs and infection assays were determined by unpaired two-tailed t-test and unpaired one-tailed t-test, respectively.
3.3. SFD mixed VLPs are thermostable at room temperature for up to 60 days and elicit protective responses
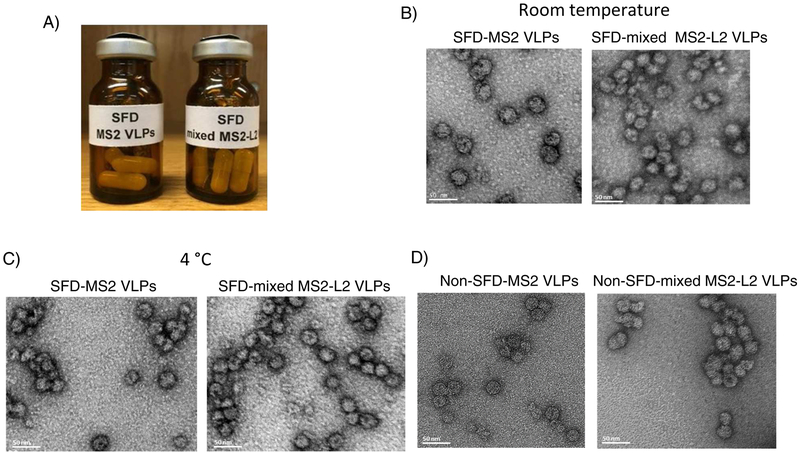
To evaluate if spray-freeze dried VLPs are thermostable, the VLPs (SFD without adjuvants; Fig. 5A) were stored at room temperature and 4°C for 60 days and their integrities were assessed using TEM. As shown in Figs. 5B and 5C, SFD VLPs stored at either temperature did not disintegrate during storage; their integrities look similar to freshly prepared non-SFD VLPs (Fig. 5D). Moreover, buccal immunization with the SFD mixed MS2-L2 VLPs (stored at room temperature for 60 days) protected mice from oral infection with HPV PsV16 (Fig. 6).
Fig. 5.
TEM images of SFD VLPs after two months storage. Mixed MS2-L2 VLPs and MS2 control VLPs spray-freeze dried without adjuvants were loaded into capsules, sealed in amber-colored Wheaton glass bottles, and purged with nitrogen gas (A). The vials with the VLPs were stored at room temperature or 4 °C for 60 days. After 60 days, the spray-freeze dried VLPs were removed from the capsules, reconstituted in PBS buffer. TEM was done and images of the VLPs stored at: B) room temperature and C) 4 °C taken. The VLPs were compared to non-SFD VLPs (D ). Images were taken at 30,000X magnification.
Fig. 6.
Buccal immunization with reconstituted SFD mixed MS2-L2 VLPs after 60-day storage and oral protection from HPV PsV16. Spray-freeze dried VLPs were reconstituted in PBS after 60-day storage at room temperature. The reconstituted VLPs in the presence of fresh CT/MPLA adjuvants were used to immunize mice thrice. Immunized mice were orally challenged with 7 × 106 IU of HPV PsV16. Each datum represents the average radiance of an individual mouse and the lines represent the geometric mean for each group. The P-values were determined by unpaired one-tailed t-test.
4. Discussion
HPV-associated HNCs are on the rise especially oropharyngeal squamous cell carcinomas (OPSCC), a type of HPV+HNC. The number of HPV-associated OPSSCs has increased by more than 225% within the last half of the century due to an increase in the number of people engaged in oral sex (a route of transmission) [11, 13, 31, 32]. It has been suggested that if the trend continues, the number of HPV+OPSCCs will surpass those of cervical cancer cases in the United States [13]. While there are validated methods (Pap smears) to screen for cervical cancer, there are no validated methods to screen for HNCs, thus making early preventive interventions for HNCs very challenging. Preventive measures therefore have to rely on preventing HPV infection of the head and neck region during oral sex.
Two prophylactic vaccines (Gardasil-9 and Cervarix; Gardasil-4 is discontinued) are currently approved to protect against HPV infection. Gardasil-4, which protected against HPV6, HPV11, HPV16 and HPV18 has been shown to elicit oral IgG antibodies [33, 34], oral antibodies neutralize/protect against HPV6, HPV16, HPV18 [35, 36], and reduce oral prevalence of vaccine types (HPV types included in the vaccine) in vaccinees [37–39]. However, a protective effect of the vaccine on HPV types not included in the vaccine was not observed. The second-generation HPV vaccine, Gardasil-9, has VLPs from seven oncogenic HPV types (HPV16, 18, 31, 33, 45, 52, 58) and two non-oncogenic HPV types (HPV6, 11) included in the vaccine. Although no studies have assessed the efficacy of the vaccine against oral HPV infection, like Gardasil-4, it is anticipated that the vaccine will offer oral protection against HPV types included in the vaccine; Gardasil-9 is therefore expected to protect against ~93% HPV+HNCs and >90% recurrent respiratory papillomatosis, respectively, based on the implication of each HPV type in HNCs [6, 16, 18]. As an alternative to Gardasil-9, we developed a bacteriophage-based MS2-L2 VLP candidate vaccine against HPVs [22]. Mixed MS2-L2 VLPs [a mixture of MS2-31L2/16L2 and MS2-consL2(69–86)] neutralized and offered vaginal protection against six oncogenic HPV pseudovirus types (HPV16, 18, 31, 33, 45 and HPV58) following intramuscular immunization. It has been reported that mucosal vaccines compared to injectable vaccines provide additional secretory antibody protection against pathogens at mucosal site [40]. Here, we assessed the immunogenicity of mixed MS2-L2 VLPs following oral immunization in the presence of two mucosal adjuvant combinations, cholera toxin and MPLA, that target different immune response pathways. Cholera toxin is a mucosal adjuvant that binds to ganglioside receptors (on all cells including epithelial cells, dendritic cells, macrophages, B- and T-lymphocytes) [41–43] and activates the mitogen activated protein kinase (MAPK) signal transduction pathway thus activating the immune system. MPLA, an approved vaccine adjuvant, binds to toll-like receptors (TLR4 on B cells, dendritic cells, and macrophages) and activates the MyD88 signaling pathway, which also activates the immune system [41, 42]. Our results show that buccal immunization of mixed MS2-L2 VLPs without adjuvant offers only suboptimal protection from vaginal infection (Fig. 1B). However, in the presence of one of the two mucosal adjuvants (cholera toxin and MPLA), the protection level was enhanced; moreover, robust protection was observed when the two-adjuvant combinations were used in immunization (Figs. 1B & 1C). These results suggest that targeting different immune signaling pathways, simultaneously, is a better strategy to enhance mucosal immune responses at the genital and the oral regions. This was further demonstrated by the broadness of protection observed at the oral region. Mixed MS2-L2 VLPs protected against five HPV types (HPV16, 35, 39, 52, 58) associated with ~80% of HNCs (Figs. 2A and 2B). The protection levels against HPV PsVs 35 & 39 were superior to that offered by Gardasil-9 vaccine (which does not include HPV35 & 39 virus-like particles). To the best of our knowledge, this is the first study that has assessed oral protection using diverse HPV pseudovirus types and has assessed, simultaneously, protection at the oral & the genital regions in the same mice. For example, the groups of mice infected orally with PsV35 (Fig. 2B) were simultaneously infected vaginally with PsV56 (Fig. 1C).
In a previous study, we observed that an MS2 VLP, MS2-16L2, with an HPV L2 insertion (amino acid 17–31 from HPV16) disintegrated when stored at room temperature for 1 month [44]; this made the MS2-16L2 VLPs less suitable for developing countries with poorly developed refrigeration & temperature-monitoring infrastructures for transportation and storage of vaccines. To this end, we assessed the potential of formulating mixed MS2-L2 VLPs together with cholera toxin and MPLA into a dry powder, with the ultimate goal of enhancing its thermostability of the candidate vaccine. We SFD the VLPs together with the adjuvants. However, the VLPs agglomerated when SFD with the adjuvants and were not immunogenic even with the addition of more adjuvant prior to immunization (Fig. 4A). Given this setback, we tried another technique, spray drying, to formulate the VLPs into dry powder; however, similar results (agglomeration) were obtained (data not shown). These results are consistent with other studies, which showed that spray drying of vaccines with adjuvant (alum) lead to vaccine agglomeration. Taken together, these results suggest that certain antigens may not be amenable to SFD or spray drying with adjuvants. Irrespective of this, the mixed MS2-L2 VLPs were successfully SFD into dry powder without the adjuvants and could be stored at room temperature, without the VLPs disintegrating, for up to 60 days (Figs. 3 and 5). Moreover, the SFD VLPs offered robust protection from oral HPV infection.
In summary, mixed MS2-L2 VLPs [MS2-31L2/16L2 VLPs and MS2-consL2(69–86)] is a candidate HPV vaccine with the potential to offer protection against multiple HPV types at the oral and genital regions. In a previous study [22], we showed that the candidate vaccine offers protection from genital infection against six oncogenic HPV types (HPV16 18, 31, 33, 45, and 58). In the current study, we show that the candidate vaccine offers protection from genital with HPV PsVs 11, 53, 56 and protection from oral infection with HPV PsVs 16, 35, 39, 52, 58. Although we did not assess, in the current study, protection from oral infection with HPV PsVs 18, 31, 33, 45, 53, 56 and PsV11, we expect immunized mice to offer oral protection against these viruses given the level of protection that was observed at the vaginal region with these PsVs in our previous [22]. This view is supported by the fact that mice immunized with the mixed MS2-L2 VLPs, in our previous study [22], protected the mice from vaginal infection with HPV PsV16 (at levels similar to Gardasil-9) and similar levels of protection against PsV16 were observed at the oral region in the current study. Overall, mixed MS2-L2 VLPs has the potential to orally protect against eleven oncogenic HPV types (16, 18, 31, 33, 35, 39, 45, 52, 53, 56, and 58) associated with ~99% of HNCs (estimates are based on the contribution of each HPV type to HNCs [16–19]). The candidate vaccine also has the potential to protect, genitally, against the same eleven oncogenic HPV types associated with ~95% of cervical cancer (estimates are based on the contribution of each HPV type to cervical cancer [6, 45]). The candidate vaccine also shows protection against HPV11 associated with 19–32% of recurrent respiratory papillomatosis [46, 47] and 36% of genital warts [48, 49]. Thus, mixed MS2-L2 VLPs is a next generation HPV vaccine that should be evaluated further, especially for patients infected with HIV or are suffering from AIDS. HPV type distribution in HIV patients seems to be different from the normal population [6]; in addition to the other HPV types (16, 18, 31, 33, 45, 52, 58) that are common in the normal population, HIV patients are infected or co-infected mostly with HPV35, 39, 53, 56, which are all protected by mixed MS2-L2 VLPs.
Acknowledgements
This work was supported by grant number 1R15 DE025812-01A1 from the US National Institute of Dental and Craniofacial Research. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
We would like to thank Dr. Sara Zimmer (University of Minnesota, Duluth) for providing access to IVIS Spectrum equipment. We would also like to thank Owen Mills and Dr. Pinaki Mukherjee at Applied Chemical and Morphological Analysis Laboratory at Michigan Tech for help with TEM imaging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial interest and conflict of interest disclosures
Ebenezer Tumban is a co-inventor of L2 bacteriophage virus-like particles-related patent applications licensed to Agilvax Biotech by the University of New Mexico. Interactions with Agilvax Biotech are managed by the University of New Mexico in accordance with its conflict of interest policies.
References
- 1.Burk RD, Harari A, and Chen Z, Human papillomavirus genome variants. Virology, 2013. 445(1–2): p. 232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Z, et al. , Classification and evolution of human papillomavirus genome variants: Alpha-5 (HPV26, 51, 69, 82), Alpha-6 (HPV30, 53, 56, 66), Alpha-11 (HPV34, 73), Alpha-13 (HPV54) and Alpha-3 (HPV61). Virology, 2018. 516: p. 86–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Institutet, K., INTERNATIONAL HUMAN PAPILLOMAVIRUS (HPV) Reference Center. 2018. [Google Scholar]
- 4.Garland SM, et al. , Human Papillomavirus Genotypes From Vaginal and Vulvar Intraepithelial Neoplasia in Females 15–26 Years of Age. Obstet Gynecol, 2018. 132(2): p. 261–270. [DOI] [PubMed] [Google Scholar]
- 5.Shah A, et al. , Oral sex and human papilloma virus-related head and neck squamous cell cancer: a review of the literature. Postgrad Med J, 2017. 93(1105): p. 704–709. [DOI] [PubMed] [Google Scholar]
- 6.Zhai L and Tumban E, Gardasil-9: A global survey of projected efficacy. Antiviral Res, 2016. 130: p. 101–9. [DOI] [PubMed] [Google Scholar]
- 7.Crow JM, HPV: The global burden. Nature, 2012. 488(7413): p. S2–3. [DOI] [PubMed] [Google Scholar]
- 8.Boscolo-Rizzo P, et al. , New insights into human papillomavirus-associated head and neck squamous cell carcinoma. Acta Otorhinolaryngol Ital, 2013. 33(2): p. 77–87. [PMC free article] [PubMed] [Google Scholar]
- 9.Leslie SW and Kumar S, Genital Warts, in StatPearls. 2018: Treasure Island (FL). [Google Scholar]
- 10.Berman TA and Schiller JT, Human papillomavirus in cervical cancer and oropharyngeal cancer: One cause, two diseases. Cancer, 2017. 123(12): p. 2219–2229. [DOI] [PubMed] [Google Scholar]
- 11.D’Souza G, et al. , Differences in the Prevalence of Human Papillomavirus (HPV) in Head and Neck Squamous Cell Cancers by Sex, Race, Anatomic Tumor Site, and HPV Detection Method. JAMA Oncol, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelwan E, et al. , Nonuniform Distribution of High-risk Human Papillomavirus in Squamous Cell Carcinomas of the Oropharynx: Rethinking the Anatomic Boundaries of Oral and Oropharyngeal Carcinoma From an Oncologic HPV Perspective. Am J Surg Pathol, 2017. 41(12): p. 1722–1728. [DOI] [PubMed] [Google Scholar]
- 13.Chaturvedi AK, et al. , Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol, 2011. 29(32): p. 4294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortes HR, et al. , Recurrent respiratory papillomatosis: A state-of-the-art review. Respir Med, 2017. 126: p. 116–121. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez GI, et al. , Human papillomavirus genotype detection in recurrent respiratory papillomatosis (RRP) in Colombia. Head Neck, 2013. 35(2): p. 229–34. [DOI] [PubMed] [Google Scholar]
- 16.Kreimer AR, et al. , Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev, 2005. 14(2): p. 467–75. [DOI] [PubMed] [Google Scholar]
- 17.Ndiaye C, et al. , HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol, 2014. 15(12): p. 1319–31. [DOI] [PubMed] [Google Scholar]
- 18.Castellsague X, et al. , HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J Natl Cancer Inst, 2016. 108(6): p. djv403. [DOI] [PubMed] [Google Scholar]
- 19.de Martel C, et al. , Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer, 2017. 141(4): p. 664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Administration, U.S.F.a.D., Gardasil 9. 2018. [Google Scholar]
- 21.Administration, U.S.F.a.D., Cervarix. 2018. [Google Scholar]
- 22.Zhai L, et al. , A novel candidate HPV vaccine: MS2 phage VLP displaying a tandem HPV L2 peptide offers similar protection in mice to Gardasil-9. Antiviral Res, 2017. 147: p. 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gambhira R, et al. , A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J Virol, 2007. 81(24): p. 13927–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondo K, et al. , Neutralization of HPV16, 18, 31, and 58 pseudovirions with antisera induced by immunizing rabbits with synthetic peptides representing segments of the HPV16 minor capsid protein L2 surface region. Virology, 2007. 358(2): p. 266–72. [DOI] [PubMed] [Google Scholar]
- 25.Schellenbacher C, Roden RBS, and Kirnbauer R, Developments in L2-based human papillomavirus (HPV) vaccines. Virus Res, 2017. 231: p. 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tumban E, et al. , VLPs displaying a single L2 epitope induce broadly cross-neutralizing antibodies against human papillomavirus. PLoS One, 2012. 7(11): p. e49751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tonnis WF, et al. , Improved storage stability and immunogenicity of hepatitis B vaccine after spray-freeze drying in presence of sugars. Eur J Pharm Sci, 2014. 55: p. 36–45. [DOI] [PubMed] [Google Scholar]
- 28.Kunda NK, et al. , A stable live bacterial vaccine. Eur J Pharm Biopharm, 2016. 103: p. 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tumban E, et al. , A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2. PLoS One, 2011. 6(8): p. e23310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts JN, et al. , Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med, 2007. 13(7): p. 857–61. [DOI] [PubMed] [Google Scholar]
- 31.Chaturvedi AK, et al. , Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol, 2013. 31(36): p. 4550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Souza G, et al. , Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis, 2009. 199(9): p. 1263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinto LA, et al. , Quadrivalent Human Papillomavirus (HPV) Vaccine Induces HPV-Specific Antibodies in the Oral Cavity: Results From the Mid-Adult Male Vaccine Trial. J Infect Dis, 2016. 214(8): p. 1276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker KH, et al. , Evaluation of HPV-16 and HPV-18 specific antibody measurements in saliva collected in oral rinses and merocel(R) sponges. Vaccine, 2018. 36(19): p. 2705–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Handisurya A, et al. , Human papillomavirus vaccination induces neutralising antibodies in oral mucosal fluids. Br J Cancer, 2016. 114(4): p. 409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahn J, et al. , Prophylactic immunization with human papillomavirus vaccines induces oral immunity in mice. Laryngoscope, 2018. 128(1): p. E16–E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirth JM, et al. , Prevalence of oral human papillomavirus by vaccination status among young adults (18–30years old). Vaccine, 2017. 35(27): p. 3446–3451. [DOI] [PubMed] [Google Scholar]
- 38.Chaturvedi AK, et al. , Effect of Prophylactic Human Papillomavirus (HPV) Vaccination on Oral HPV Infections Among Young Adults in the United States. J Clin Oncol, 2018. 36(3): p. 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.HPV Vaccine Slashes Rates of Oral Infection. Cancer Discov, 2017. 7(7): p. OF6. [DOI] [PubMed] [Google Scholar]
- 40.Rhee JH, Lee SE, and Kim SY, Mucosal vaccine adjuvants update. Clin Exp Vaccine Res, 2012. 1(1): p. 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freytag LC and Clements JD, Mucosal adjuvants. Vaccine, 2005. 23(15): p. 1804–13. [DOI] [PubMed] [Google Scholar]
- 42.Cox E, et al. , Adjuvants modulating mucosal immune responses or directing systemic responses towards the mucosa. Vet Res, 2006. 37(3): p. 511–39. [DOI] [PubMed] [Google Scholar]
- 43.Bharati K and Ganguly NK, Cholera toxin: a paradigm of a multifunctional protein. Indian J Med Res, 2011. 133: p. 179–87. [PMC free article] [PubMed] [Google Scholar]
- 44.Tumban E, et al. , Preclinical refinements of a broadly protective VLP-based HPV vaccine targeting the minor capsid protein, L2. Vaccine, 2015. 33(29): p. 3346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Sanjose S, et al. , Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol, 2010. 11(11): p. 1048–56. [DOI] [PubMed] [Google Scholar]
- 46.Omland T, et al. , Recurrent respiratory papillomatosis: HPV genotypes and risk of high-grade laryngeal neoplasia. PLoS One, 2014. 9(6): p. e99114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kocjan BJ, et al. , Identical human papillomavirus (HPV) genomic variants persist in recurrent respiratory papillomatosis for up to 22 years. J Infect Dis, 2013. 207(4): p. 583–7. [DOI] [PubMed] [Google Scholar]
- 48.Sturegard E, et al. , Human papillomavirus typing in reporting of condyloma. Sex Transm Dis, 2013. 40(2): p. 123–9. [DOI] [PubMed] [Google Scholar]
- 49.Chang L, et al. , Distribution of genital wart human papillomavirus genotypes in China: a multi-center study. J Med Virol, 2013. 85(10): p. 1765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]