Abstract
Introduction
Community-acquired pneumonia (CAP) is a common indication for antibiotic treatment in young children. Data are limited regarding the ideal dose and duration of amoxicillin, leading to practice variation which may impact on treatment failure and antimicrobial resistance (AMR). Community-Acquired Pneumonia: a randomIsed controlled Trial (CAP-IT) aims to determine the optimal amoxicillin treatment strategies for CAP in young children in relation to efficacy and AMR.
Methods and analysis
The CAP-IT trial is a multicentre, randomised, double-blind, placebo-controlled 2×2 factorial non-inferiority trial of amoxicillin dose and duration. Children are enrolled in paediatric emergency and inpatient environments, and randomised to receive amoxicillin 70–90 or 35–50 mg/kg/day for 3 or 7 days following hospital discharge. The primary outcome is systemic antibacterial treatment for respiratory tract infection (including CAP) other than trial medication up to 4 weeks after randomisation. Secondary outcomes include adverse events, severity and duration of parent-reported CAP symptoms, adherence and antibiotic resistance. The primary analysis will be by intention to treat. Assuming a 15% primary outcome event rate, 8% non-inferiority margin assessed against an upper one-sided 95% CI, 90% power and 15% loss to follow-up, 800 children will be enrolled to demonstrate non-inferiority for the primary outcome for each of duration and dose.
Ethics and dissemination
The CAP-IT trial and relevant materials were approved by the National Research Ethics Service (reference: 16/LO/0831; 30 June 2016). The CAP-IT trial results will be published in peer-reviewed journals, and in a report published by the National Institute for Health Research Health Technology Assessment programme. Oral and poster presentations will be given to national and international conferences, and participating families will be notified of the results if they so wish. Key messages will be constructed in partnership with families, and social media will be used in their dissemination.
Trial registration number
ISRCTN76888927, EudraCT2016-000809-36.
Keywords: community-acquired pneumonia, antimicrobial resistance
Strengths and limitations of this study.
This well-powered multicentre trial will provide new data on efficacy of amoxicillin treatment strategies for uncomplicated community-acquired pneumonia in young children in developed settings in the postpneumococcal vaccine era.
The Community-Acquired Pneumonia: a randomIsed controlled Trial (CAP-IT) is one of few randomised controlled trials to measure the impact of differing antibiotic treatment regimens on antimicrobial resistance.
The pragmatic approach to eligibility employed by the CAP-IT trial is aligned with clinical practice and so will facilitate rapid knowledge translation.
Although determining the optimal endpoint for pragmatic trials is challenging, use of an Endpoint Review Committee in the CAP-IT trial will strengthen the confidence in the results.
The generalisability of the CAP-IT trial findings will be maximised by the diversity of the enrolled population, in both severity and setting.
Introduction
Acute respiratory infections (including community-acquired pneumonia; CAP) are the most common indication for childhood antibiotic use in primary care and emergency departments (ED).1–3 Up to 40% of preschool children attend primary care for acute respiratory symptoms, with antibiotics prescribed in 30%.3 4 One-third of childhood ED visits are for acute respiratory symptoms or fever; up to 15% are diagnosed with CAP, with highest antibiotic prescription rates in those aged <5 years.5–7 ED attendances and hospital admissions for respiratory illness in young children increase annually, and two-thirds of antibiotics for hospitalised children aged 1–5 years are for CAP.5 8 9 The healthcare cost of childhood CAP in England is estimated at £8 million/year, though total annual societal costs are up to £17 million.10 11
CAP is a differential diagnosis in any child presenting with fever, respiratory symptoms and focal chest signs. No gold standard laboratory, microbiological or radiological tests distinguish bacterial from viral infection; diagnosis and treatment decisions are based on clinical criteria.12–14 Streptococcus pneumoniae is the most commonly implicated bacterial pathogen, even in settings with routine pneumococcal vaccination (PCV).15–17 Children have high rates of bacterial colonisation and carriage of resistant organisms which may be transmitted, with particular risks for vulnerable individuals.18–21 Interventions to maintain low antimicrobial resistance (AMR) levels in children may therefore have wider benefits.
Amoxicillin is the recommended antibiotic for childhood CAP in several international guidelines12 22–24; however, there are insufficient data to inform dose, duration and impact on AMR. The British National Formulary for Children recommends amoxicillin thrice daily at 40–80 mg/kg/day. Twice daily dosing is widely recommended due to improved adherence and non-inferiority, including by the WHO.22–26 Shorter treatment courses are effective in trials performed in low/middle-income country settings, though diagnostic and eligibility criteria limit generalisability of findings.27 28 Trials from developed settings predate PCV or include populations with high penicillin resistance rates. There are therefore no robust data to inform treatment duration, leading to variation between 3 and 7 days.23 24 27–30 Assessment of treatment efficacy is challenging, as only half of patients recover after 10 days, and 90% take up to 4 weeks to recover fully.4 31 32 While ongoing ‘minimal’ symptoms may trigger retreatment (deemed treatment failure) relatively frequently, the most widely used efficacy measure is re-exposure to antibiotics for up to 4 weeks, with recent reported rates of 15%.29 31 33
The impact of antibiotics on colonisation with resistant bacteria is complex and dynamic.34–37 Insufficiently high dosing could promote selection of resistant pathogens, and while the greatest effect on bacterial load is achieved early, resistant isolates emerge after 4–5 days.38 39 Combined effectiveness and resistance data related to antibiotic dose and duration would inform antimicrobial stewardship and childhood CAP treatment strategies.
The Community-Acquired Pneumonia: a randomIsed controlled Trial (CAP-IT) will evaluate efficacy, safety and effect on AMR of different durations and doses of oral amoxicillin treatment for young children with uncomplicated CAP. The specific primary objectives are to determine whether (1) 3 days is non-inferior to 7 days, and (2) 35–50 mg/kg/day is non-inferior to 70–90 mg/kg/day.
Methods
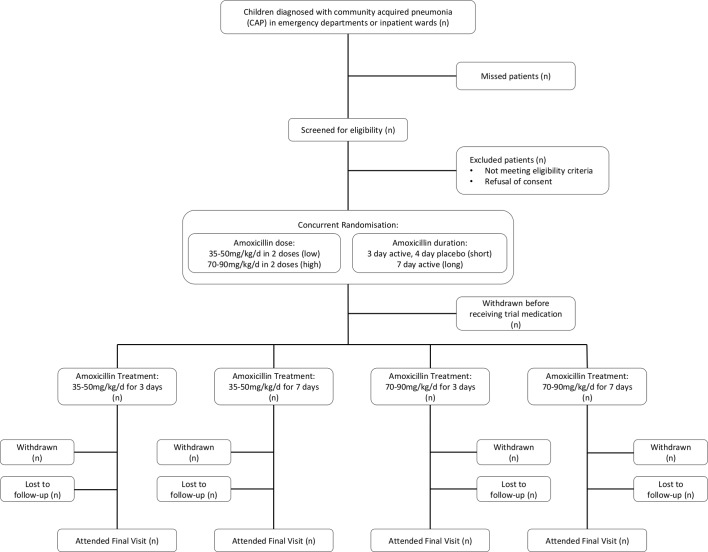
This multicentre, randomised, double-blind, placebo-controlled 2×2 factorial non-inferiority trial aims to enrol 800 patients over 2 years. Amoxicillin dose and duration are assigned simultaneously, resulting in four treatment arms (figure 1). This protocol has been written in accordance with the Standard Protocol Items: Recommendations for Interventional Trials checklist.40
Figure 1.
Possible treatment arms in the CAP-IT trial. CAP-IT, Community-Acquired Pneumonia: a randomIsed controlled Trial.
Patient and public involvement
Parents of young children have been involved throughout the development and delivery of the CAP-IT trial. In developing the research question, they advised that shorter antibiotic courses would be welcomed if equally effective, due to difficulties in giving medicine (due to palatability, or challenges with day care and daytime doses). For the same reasons, parents supported twice daily dosing. A key need identified during trial development was support for families, including clear symptomatic safety netting, close contact and availability of a contact number for the local research and clinical teams. Parents reviewed and provided input on all information materials to ensure they were clear and easy to understand. The CAP-IT Trial Steering Committee (TSC) has a parent member, who contributes to discussions as the trial progresses, and will advise on best methods of disseminating results to families as we approach the analysis phase.
Trial setting
Sites throughout the UK and Ireland are participating from Paediatric Emergency Research in the UK and Ireland (PERUKI; www.peruki.org)41 and General and Adolescent Paediatric Research in the UK and Ireland (GAPRUKI; www.gapruki.org.uk). Participating sites are tertiary or secondary hospitals with EDs and inpatient areas where potentially eligible children are managed; participating centres are listed on the trial website (www.capitstudy.org.uk). Centres are selected based on research infrastructure, likely number of recruits, participation in feasibility work and proposed training strategies. Site principal investigators (PI) are qualified by education, training and experience to assume responsibility for proper conduct of the trial.
Trial population
Participants may be enrolled from EDs, observation units, paediatric assessment units (PAU) or inpatient wards, provided they fulfil eligibility criteria.
Inclusion criteria
Children are eligible if they are aged ≥6 months, weigh 6–24 kg, have a clinical diagnosis of CAP (box 1) and will be treated with amoxicillin as the sole antibacterial agent on hospital discharge. Children must have received none or less than 48 hours of treatment with only beta-lactam antibiotics at enrolment.
Box 1. Definition of clinical diagnosis of CAP.
Clinical diagnosis of community-acquired pneumonia (CAP) is defined as all of the following:
Cough (reported by parents/guardians within 96 hours before presentation).
Temperature ≥38°C measured by any method or reported fever within 48 hours before presentation.
Signs of laboured/difficult breathing or focal chest signs (one or more of the following):
Nasal flaring.
Chest retractions.
Abdominal breathing.
Focal dullness to percussion.
Focal reduced breath sounds.
Crackles with asymmetry.
Exclusion criteria
Children are ineligible if they have (1) severe underlying chronic disease (including sickle cell anaemia, immunodeficiency, chronic lung disease, cystic fibrosis), (2) penicillin allergy or other contraindication to amoxicillin, (3) complicated pneumonia (shock, hypotension, altered mental state, ventilatory support, empyema, pneumothorax, pulmonary abscess), or (4) bilateral wheezing without focal chest signs.
Outcome measures
The primary outcome is clinically indicated treatment with systemic antibiotic other than trial medication for respiratory tract infection (including CAP) up to 4 weeks after randomisation. Reason and clinical indication are adjudicated by an Endpoint Review Committee (ERC), comprising independent and non-independent members. Independent members, including the ERC chair, are paediatricians independent of the trial and not involved in the clinical care of participants. Non-independent members include the chief investigator, project lead and trial physician. ERC members review clinical narrative summaries of retreatment primary endpoint events, supportive data from patient trial diaries, unscheduled visit forms and serious adverse event (SAE) forms; they adjudicate whether additional antibiotics were prescribed due to CAP (early failure, relapse or recurrence), new bacterial infection (respiratory tract infection or other), intolerance of trial medication or another reason. Final adjudication is based on consensus of the independent ERC members.
Secondary outcomes include (1) severity and duration of parent-reported CAP symptoms, (2) specified amoxicillin-related adverse events (AE; thrush, skin rashes and diarrhoea), (3) phenotypic resistance to penicillin at 4 weeks in nasopharyngeal (NP) S. pneumoniae isolates, and (4) adherence to trial medication.
Screening, randomisation, recruitment and consent
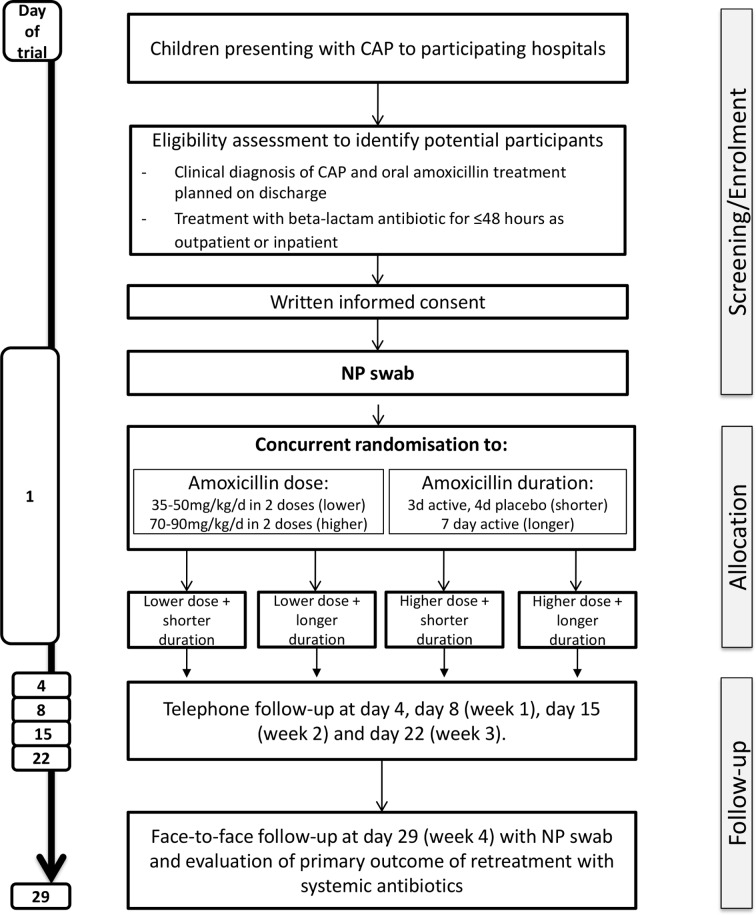
The trial flow chart is provided in figure 2. Potential participants may require hospital admission for initial CAP management or may be discharged immediately from an ED or PAU. As antibiotic treatment duration is an eligibility criterion, additional eligibility assessment procedures are undertaken for those receiving antibiotics while in hospital; these differences are outlined in the following sections, and in figures 3 and 4.
Figure 2.
CAP-IT trial flow chart. CAP-IT, Community-Acquired Pneumonia: a randomIsed controlled Trial.
Figure 3.
CAP-IT trial schema. CAP-IT, Community-Acquired Pneumonia: a randomIsed controlled Trial; NP, nasopharyngeal.
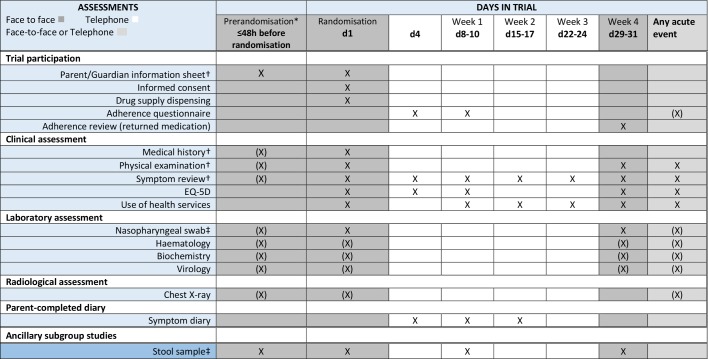
Figure 4.
CAP-IT trial assessment schedule (X) indicates tests that may be done if the child’s condition requires it or allows it, but are not mandatory. *Assessments in this column only undertaken for potential participants receiving inpatient antibiotic treatment. †May be done any time before enrolment discussion. ‡Taken before starting antibiotics where possible. CAP-IT, Community-Acquired Pneumonia: a randomIsed controlled Trial.
Screening
Potential participants are identified and screened during initial clinical assessment. Participants discharged directly from an ED or PAU undergo all trial procedures prior to discharge. Children given antibiotics while in hospital have a secondary screening episode. Participating centres keep anonymised logs of screened children, including those who were not approached and those who declined participation, which are used to identify any issues for the trial, or at a given site.
Consent
Extensive information is available for recruiting sites, including printed and video materials (accessible at www.capitstudy.org.uk); all materials have NHS Research Ethics Committee (REC) approval. Written informed consent is obtained by clinical or research staff from a parent/guardian after explanation of the aims, methods, benefits and potential hazards of the trial. Families may decline participation in all or any aspects of the trial, at any time and for any reason, without incurring any penalty or affecting treatment. Signed consent forms are kept by the site and a copy given to the family. A letter is sent to the general practitioner (GP) informing them of the trial and the child’s involvement.
Enrolment process
At enrolment, recruiting staff review trial materials with parents/guardians; these include a symptom diary, participant information sheet (PIS), investigational medicinal product (IMP) administration instructions and contact details for the trial team. The symptom diary collects information on clinical and non-clinical elements including cough, breathing, temperature, AEs, IMP administration, additional antibiotics, any other medication, time off work/childcare and other health service use. Relevant elements are incorporated into baseline data collected by recruiting staff on enrolment clinical report forms. This is done with parents/guardians to facilitate their understanding of diary content; other baseline data include demographics, symptoms, underlying disease, antibiotic exposure in the preceding 3 months, chest findings and quality of life measures. Participants are registered on the online trial database; database access is controlled through authorised usernames and passwords and all patient identifiable details are confidential.
An NP swab is collected at enrolment. S. pneumoniae is identified using culture-based techniques, AMR is detected using minimal inhibitory concentration-based techniques and respiratory viruses are detected using molecular techniques. Stool samples are collected in sites participating in ancillary studies, provided specific consent is given. There are no other mandatory radiological or laboratory tests; if done as part of routine clinical care, results of haematological, biochemical and chest radiograph investigations are recorded.
Randomisation
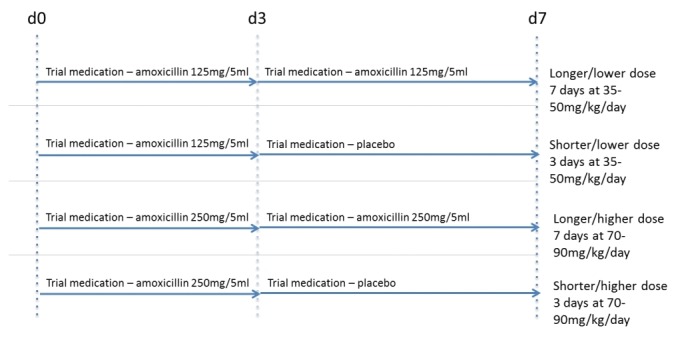
Participants are allocated 1:1 to each of the two factorial randomisations, stratified according to whether or not they receive any non-trial antibiotics in hospital before being enrolled. A randomisation list is computer generated based on random permuted blocks by the trial statistician. Based on this list, trial medication (including placebo) is labelled and packaged in a blinded manner by an independent supplier and delivered to trial sites; treatments are randomly assigned by taking the next sequentially numbered treatment from the relevant supply. As it is difficult to taste-match suspensions of amoxicillin and placebo, one brand of amoxicillin is used for all participants for the first 3 days, followed by a second bottle for days 4–7 containing either a second brand of amoxicillin or placebo; both form a yellow-coloured similar tasting suspension, and parents are instructed to expect some change in taste after the first 3 days.
Trial treatments
Trial treatment starts on the day of enrolment, with the first dose given before discharge where possible. Children receive 3 or 7 days of amoxicillin, and suspension is given twice daily with dosing by weight band; doses are 35–50 or 70–90 mg/kg/day. To ensure blinding, amoxicillin suspensions of different strengths (125 or 250 mg/5 mL) are used so that dose volume is the same regardless of treatment arm.
IMP is stored separately from routine medicines, and is dispensed in a box containing one bottle of amoxicillin and two bottles of either amoxicillin or placebo (blinded to contents). Colour labels indicate which should be used on days 1–3 and days 4–7. The importance of adherence is reinforced when dispensing IMP and during follow-up contacts. Adherence is assessed using the symptom diary, at telephone follow-up contacts, and at final follow-up when families return unused IMP.
IMP may be stopped early for reasons including toxicity, clinical change requiring treatment modification, or withdrawal of consent. All concomitant medications are allowed; if an essential medication interacts with amoxicillin, the IMP is stopped. Although participants are not required to give a reason for discontinuing IMP, a reasonable effort is made to establish this reason.
Situations necessitating unblinding are likely to be rare. Severe allergic reactions occur early during amoxicillin exposure; immediate discontinuation and trigger avoidance is recommended. Delayed reactions are generally mild and resolve with discontinuation. Where retreatment is necessary, unblinding is unlikely to impact on antibiotic choice and is therefore unnecessary. If necessary, unblinding is done using an online emergency unblinding form.
Follow-up
The trial assessment and follow-up schedule is shown in figures 3 and 4. Telephone contact occurs on days 4, 8–10, 15–17 and 22–24; a face-to-face visit occurs within 2 days of day 29. At each contact clinical signs and symptoms are reviewed, as are adverse treatment effects, acute illnesses requiring medical assessment, new antibiotics and IMP adherence. At each face-to-face visit an NP swab is collected; if there are any CAP symptoms, examination findings and physiological parameters are recorded. If a face-to-face visit is not possible for final follow-up, it is attempted by telephone. If it is not possible to contact families despite reasonable efforts, relevant data are sought from the GP if consent has been given to do so.
Additional healthcare contacts may be necessary. Parents/guardians liaise with the site trial team if they are considering acute clinical assessment during the follow-up period, though they are advised to seek immediate emergency assessment if they feel this is required. Clinician judgement determines whether investigation, treatment or hospitalisation is required. Following any unscheduled assessment, symptoms, health service utilisation and IMP adherence are reviewed by telephone, and face-to-face visits arranged if necessary.
Parents/guardians who discontinue IMP are encouraged to adhere to the follow-up schedule, and the Sponsor is informed. If follow-up is stopped early, data already collected are kept for analysis, and the children are not replaced in the trial. Samples already obtained are processed unless parents/guardians request otherwise.
Sample size and power
The sample size is based on demonstrating non-inferiority for the primary endpoint for each duration and dose. Although inflation factors have been advocated for factorial trials to account for interaction between the interventions or a reduction in the number of events, this is not necessary if either randomised intervention has a null effect (the underlying hypothesis with a non-inferiority design), as marginal analyses can then be conducted. Assuming a 15% primary outcome event rate, 8% non-inferiority margin assessed against an upper one-sided 95% CI, 90% power and 15% loss to follow-up, 800 children will be randomised. This is considered a minimum sample size; if resources permit, recruitment may continue above this number to increase statistical power and precision, with the approval of the TSC.
Analysis plan
The primary analysis will be by modified intention to treat (ITT), including all participants who take at least one IMP dose. The primary endpoint will be analysed using time-to-event methods, controlling for previous antibiotic exposure. Potential interaction effects between dose, duration and previous antibiotic exposure will be examined. For some secondary outcomes, including AEs and resistance, on-treatment analyses will be performed as well as ITT analyses.
The primary analysis of the primary endpoint will include only clinically indicated additional systemic antibiotic for respiratory tract infection (including CAP) as adjudicated by the ERC. Sensitivity analyses will be performed to include (1) all systemic antibacterial treatments other than IMP regardless of reason and indication (to guard against bias), and (2) only ERC-adjudicated clinically indicated systemic antibacterial treatment prescribed specifically for CAP (to provide clinical reassurance).
A subgroup analysis will consider CAP severity at presentation, and repeat the main efficacy analysis limited to participants with more severe disease. This will provide reassurance that an overall null effect (if observed) is not due to a dilution effect arising from inclusion of children with mild disease of viral aetiology, an important consideration as CAP is diagnosed clinically.
Lower dose and shorter duration will be considered ‘non-inferior’ to higher dose and longer duration, respectively, if the upper limit of the one-sided 95% CI for the difference in the proportion of children with the primary endpoint at day 29 is less than the non-inferiority margin of 8%. However, inference will be based primarily on point estimates and CIs rather than binary classification of a ‘non-inferior’ or ‘not non-inferior’ outcome.42
Ancillary studies
Impact on gastrointestinal microflora
Stool samples will be collected at enrolment and final follow-up from 200 children to evaluate the impact of amoxicillin exposure on different microbial communities, including AMR in gastrointestinal commensal flora. The baseline sample is collected prior to or as soon as possible after starting amoxicillin.
Symptom diary completion methodology
While widespread access to the internet and mobile devices suggest web-based questionnaires may be acceptable and reliable, data supporting this are lacking. As it is unclear which is best in terms of completion rates we will quasirandomise participants to electronic or paper questionnaires based on gender and site. We will compare overall data completeness, and completeness of individual items between paper and electronic diaries. Data supporting use of one or other approach will be important for many trials eliciting primary or key secondary parent-reported outcomes through the use of symptom diaries.
Safety reporting
Definitions for AEs and adverse reactions (AR) of the European Union (EU) Directive 2001/20/EC Article 2 based on the principles of Good Clinical Practice (GCP) apply to this trial. The symptom diary prompts for known amoxicillin ARs including gastrointestinal symptoms and rash; AEs that lead to cessation of trial treatment are also reported. When an AE or AR occurs, the PI assesses whether this is an SAE using supplied definitions, and the level of severity using provided toxicity grading. Where an SAE has occurred, an SAE form is completed and the Sponsor notified within 24 hours of the PI becoming aware. Children are followed up after an SAE until complete clinical recovery or until the event has stabilised. Staff follow their institution’s procedure for local notification requirements. PIs assess causality of SAEs in relation to IMP; assessment of expectedness is completed based on common toxicities listed in the Summary of Product Characteristics; if a serious AR (SAR) is unexpected, the event is classified as a suspected unexpected SAR (SUSAR) and reported to the Medicines and Healthcare products Regulatory Agency (MHRA), REC and site PIs. Medically qualified staff at the Sponsor and the CI (or medically qualified delegate) review all SAE reports. In case of disagreement with regard to causality, both opinions are provided in any reports. All SAEs are reported to the MHRA and REC in the annual Development Safety Update Report.
Trial monitoring
Quality assurance and quality control are based on a formal risk assessment, to ensure all elements are performed in line with applicable regulatory requirements. Site initiation visits are done to deliver trial-specific training to key clinical and research personnel, which is cascaded to all relevant staff; training and delegation logs are monitored for completeness. Screening, randomisation and consent rates are monitored by the trial team at the Medical Research Council Clinical Trials Unit (MRC CTU) at University College London (UCL), and data are checked for consistency, missing data points or possible errors, with suspect data returned as queries to ensure reliability and validity. PIs allow trial-related monitoring including audits, ethics committee review and regulatory inspections, by providing direct access to source data and documents as required. The principles of the UK Data Protection Act (DPA) are followed.
The Trial Management Group (TMG) comprises the CI, clinical and non-clinical investigators, and the CAP-IT MRC CTU at UCL team. The TMG is responsible for the day-to-day running and management of the trial. The TSC has membership from the TMG plus independent members, including an independent chair, and provides overall guidance and advice. The Independent Data Monitoring Committee (IDMC) is the only group which sees confidential accumulating data for the trial by randomised group. Formal stopping rules are not used although the IDMC Charter specifies guidelines for when to alert the TSC to consider modifying the trial design. These guidelines are conservative to guard against premature changes to trial design from early inspection of the data.
Regulatory compliance
This trial is conducted in compliance with GCP principles as laid down by the Commission Directive 2005/28/EC, Statutory Instrument 2004 No 1031: Medicines for Human Use (Clinical Trials) Regulations 2004 as amended, the UK DPA (DPA number: Z5886415) and the NHS Research Governance Framework for Health and Social Care. Sites inform the Sponsor as soon as they are aware of any possible serious breach of compliance, so that the Sponsor can report this as per regulatory requirements. This is a Clinical Trial of an IMP as defined by EU Directive 2001/20/EC. The CTA number is 17141803 and the EudraCT number is 2016-000809-36.
Ethics and dissemination
The protocol, PIS, consent form and all relevant documentation were approved centrally by the National Research Ethics Service, West London and GTAC REC (reference: 16/LO/0831; 30 June 2016). The results of the CAP-IT trial will be published in peer-reviewed journals read by health professionals managing childhood CAP in the UK and internationally, and in a report published by the National Institute for Health Research Health Technology Assessment programme. To maximise impact internationally, oral and poster presentations will be given to relevant national and international conferences. Once the trial is completed, all participating families will be notified of the results if they wish. The social media presence of the organisations involved, including PERUKI (@PERUKItweep), will be used to disseminate key messages.
Discussion and trial status
The first participant was enrolled in February 2017; as of 31 January 2019, a total of 690 patients have been enrolled from 30 sites. Recruitment is scheduled to finish in April 2019, and analysis to be completed by August 2019. The trial will end after the last follow-up visit of the last randomised participant.
Three significant changes to the CAP-IT analysis plan have been made during the course of the trial (Protocol Version 4.0, 4 December 2018) based on blinded emerging trial data. First, although patients were originally intended to be stratified based on whether they received inpatient antibacterial treatment or were discharged directly from hospital, all patients will now be jointly analysed due to significant phenotypic overlap. Subgroup efficacy analyses will be performed to consider severity of CAP at presentation. Second, the primary endpoint definition has been made more specific from ‘all antibiotic prescriptions given during follow-up’ to ‘clinically indicated antibiotic prescriptions for respiratory tract infections (including CAP) as adjudicated by an ERC’. Finally, the non-inferiority margin was adjusted as the primary endpoint event rate had been substantially underestimated.
The CAP-IT trial will provide important and robust evidence for amoxicillin treatment strategies for childhood CAP in developed settings after PCV introduction. Its factorial nature will inform amoxicillin dose and duration, and the optimal regimen for minimal AMR selection. Combined with the results of ongoing trials (including SAFER43 and SCOUT-CAP [accessible at https://clinicaltrials.gov/ct2/show/NCT02891915]) in similar healthcare systems, findings from the CAP-IT trial will improve and streamline cost-effective care on a global scale.24
The CAP-IT trial provides an important opportunity for learning and infrastructure development in acute paediatric trials in the UK and Ireland. Recruiting patients to a placebo-controlled randomised trial in EDs, PAUs, observation units and inpatient wards has required close working across clinical (emergency medicine and general paediatric) and research teams, and research networks. To date, successful recruitment has been led through close communication between the CTU and participating sites, with leadership from PERUKI, providing frequent opportunities to share best practice in overcoming any obstacles to trial delivery. This includes regular contact with sites, and a metaplanning exercise prior to the second winter of recruitment. Key obstacles identified included research training (GCP and study specific), out-of-hours recruitment, availability of site research staff and delays to discharge caused by study processes. To overcome these, a number of new initiatives were developed, including streamlining of enrolment processes, to enable families to have the opportunity to participate in this important trial.
Supplementary Material
Acknowledgments
We thank all members of the trial committees, all families and children who have taken part, and all participating sites for their invaluable contributions to the CAP-IT trial. Trial Steering Committee (TSC): Elizabeth Molyneux (chair), Chris Butler, Alan Smyth, Catherine Pritchard, Diana Gibb and Mike Sharland. Independent Data Monitoring Committee (IDMC): Tim Peto (chair), Simon Cousens and Stuart Logan. Independent Endpoint Review Committee (ERC) members: Alasdair Bamford (chair), Anna Turkova and Felicity Fitzgerald. Trial Management Group (TMG): Mike Sharland (chair), Julia Bielicki, Mark Lyttle, Colin Powell, Paul Little, Saul Faust, Adam Finn, Julie Robotham, Mandy Wan, David Dunn, Wolfgang Stöhr, Lynda Harper, Sam Barratt, Nigel Klein, Louise Rogers, Elia Vitale and Diana Gibb. MRC Clinical Trials Unit, UK: Sam Barratt, Kate Sturgeon, Anna Griffiths, Jenna Grabey, Nishdha Naufal, Lynda Harper, Wolfgang Stöhr, David Dunn, Vicky Yorke-Edwards, Jennifer Petrie and Diana Gibb.
Footnotes
Twitter: CAPIT trial: @capitstudyPERUKI: @PERUKItweepMark Lyttle: @mdlyttleDamian Roland: @Damian_Roland
Contributors: MS is the chief investigator and devised the trial concept. MS, DMG, JAB, MDL, CVEP, AF, LH, DD, MW, PL, SNF, JR and ADH secured the trial grant. MS, DMG, JAB, MDL, AF, LH and MW designed the clinical trial. CVEP, SNF, ADH, PL and JR contributed to the trial design and materials. PJ and KS contributed to the trial design and materials, and provided nursing leadership to participating sites. MDL and DR were the PERUKI leads for the trial, and CVEP was the GAPRUKI lead. DD and WS provided statistical input and developed the statistical analysis plan. SB and KS provided trial management. MDL wrote the first draft of the manuscript with input from MS and DMG. All authors contributed to subsequent drafts and agreed to the final manuscript.
Funding: The CAP-IT trial is funded by the NIHR HTA Programme, Antimicrobial Resistance Themed Call, via grant number 13/88/11. University College London (UCL) is the trial sponsor.
Disclaimer: The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. The trial funders and sponsor played no role in the trial design, writing the manuscript, or the decision to submit for publication; the responsibility for these actions lies with the Trial Management Group (TMG).
Competing interests: None declared.
Ethics approval: National Research Ethics Service, West London and GTAC Research Ethics Committee (reference: 16/LO/0831; 30 June 2016).
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Collaborators: Investigator Sites and collaborators: Alder Hey Children’s Hospital NHS Foundation Trust, Liverpool: Daniel Hawcutt (PI), Matthew Rotheram, ElizabethD Lee and Rachel Greenwood-Bibby. Birmingham Children’s Hospital: Stuart Hartshorn (PI), Deepthi Jyothish, Louise Rogers and Juliet Hopkins. Bristol Royal Hospital for Children: Mark Lyttle (PI), Stefania Vergnano, Pauline Jackson, Beth Pittaway, Alice Smith, Michelle Ross, Lucie Aplin and Sarah Sheedy. Chelsea & Westminster NHS Foundation Trust, London: James Ross (PI), Poonam Patel, Hannah Fletcher, Kribashnie Nundlall and Patricia Correia Da Costa. City Hospitals Sunderland NHS Foundation Trust: Niall Mullen (PI). Countess of Chester Hospital NHS Foundation Trust: Susie Holt (PI), John Gibbs, Caroline Burchett, Caroline Lonsdale and Sarah De-Beger. County Durham and Darlington NHS Foundation Trust: Godfrey Nyamugunuduru (PI), John Furness (former PI) and Dawn Eggington. Derbyshire Children’s Hospital, Derby: Gisela Robinson (PI), Lizzie Starkey and Mel Hayman. Evelina London Children’s Hospital: Anastasia Alcock (PI), Dani Hall (former PI), Ronny Cheung, Alyce B Sheedy and Sarah Giwa. James Cook University Hospital, Middlesbrough: Arshid Murad (PI), Katherine Jerman and Joanna Green. John Radcliffe Hospital, Oxford: Tanya Baron (PI), Chris Bird (former PI), Maria Jose Garcia Garcia, Dom Georgiou, Kirsten Beadon and Sally Beer. King’s College Hospital, London: Fleur Cantle (PI), Hannah Eastman, Paul Riozzi and Hannah Cotton. Leeds Children’s Hospital: Sean O’Riordan (PI), Alice Downes. Leicester Hospitals: Damian Roland (PI), Srini Bandi, Rekha Patel, Rachel Dunhill and Felix Hay. Nottingham University Hospitals NHS Trust: Chris Gough (PI), Megan McAulay and Louise Conner. Our Lady’s Children’s Hospital Crumlin, Dublin: Michael Barrett (PI), Jean Donnelly, Ellen Barry, Rachel Gallagher and Madeleine Niermeyer. Royal Alexandra Children’s Hospital, Brighton: Emily Walton (PI) and Vivien Richmond. Royal Hospital for Children, Glasgow: Steven Foster (PI), Ruth Bland, Ashleigh Neil, Barry Milligan and Helen Bannister. Royal London Hospital: Ben Bloom (PI), Ami Parikh, Olivia Boulton and Imogen Skene. Royal Manchester Children’s Hospital: Katherine Potier (PI), Fiona Borland, Jill L Wilson and Zainab Suleman. Sheffield Children’s Hospital NHS Foundation Trust: Judith Gilchrist (PI), Noreen West, Jayne Evans and Julie Morecombe. Southport and Ormskirk NHS Trust: Sharryn Gardner (PI), Zena Haslam and Moira Morrison. St George’s Hospital, London: Paul Heath (PI), Yasser Iqbal, Malte Kohns Vasconcelos, Elia Vitale and Claire Womack. St Mary’s Hospital, London: Ian Maconochie (PI), Suzanne Laing, Rikke Jorgensen and Ladan Ali. University Hospitals Lewisham: Maggie Nyirenda (PI), Sophie Keers, Samia Pilgrim and Emma Gardiner. University Hospital of Wales, Cardiff: Jeff Morgan (PI), Colin VE Powell (former PI) and Jennifer Muller. Southampton Children’s Hospital: Katrina Cathie (PI), Jane Bayreuther, Ruth Ensom and Emily Cornish.
Patient consent for publication: Not required.
References
- 1. Vaz LE, Kleinman KP, Raebel MA, et al. . Recent trends in outpatient antibiotic use in children. Pediatrics 2014;133:375–85. 10.1542/peds.2013-2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA 2009;302:758 10.1001/jama.2009.1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hersh AL, Shapiro DJ, Pavia AT, et al. . Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics 2011;128:1053–61. 10.1542/peds.2011-1337 [DOI] [PubMed] [Google Scholar]
- 4. Hay AD, Wilson A, Fahey T, et al. . The duration of acute cough in pre-school children presenting to primary care: a prospective cohort study. Fam Pract 2003;20:696–705. 10.1093/fampra/cmg613 [DOI] [PubMed] [Google Scholar]
- 5. Sands R, Shanmugavadivel D, Stephenson T, et al. . Medical problems presenting to paediatric emergency departments: 10 years on. Emerg Med J 2012;29:379–82. 10.1136/emj.2010.106229 [DOI] [PubMed] [Google Scholar]
- 6. Oostenbrink R, Thompson M, Lakhanpaul M, et al. . Children with fever and cough at emergency care: diagnostic accuracy of a clinical model to identify children at low risk of pneumonia. Eur J Emerg Med 2013;20:273–80. 10.1097/MEJ.0b013e32835771fd [DOI] [PubMed] [Google Scholar]
- 7. Donnelly JP, Baddley JW, Wang HE. Antibiotic utilization for acute respiratory tract infections in U.S. emergency departments. Antimicrob Agents Chemother 2014;58:1451–7. 10.1128/AAC.02039-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gill PJ, Goldacre MJ, Mant D, et al. . Increase in emergency admissions to hospital for children aged under 15 in England, 1999-2010: national database analysis. Arch Dis Child 2013;98:328–34. 10.1136/archdischild-2012-302383 [DOI] [PubMed] [Google Scholar]
- 9. Brousseau DC, Nimmer MR, Yunk NL, et al. . Nonurgent emergency-department care: analysis of parent and primary physician perspectives. Pediatrics 2011;127:e375–81. 10.1542/peds.2010-1723 [DOI] [PubMed] [Google Scholar]
- 10. Farha T, Thomson AH. The burden of pneumonia in children in the developed world. Paediatr Respir Rev 2005;6:76–82. 10.1016/j.prrv.2005.03.001 [DOI] [PubMed] [Google Scholar]
- 11. Lorgelly PK, Atkinson M, Lakhanpaul M, et al. . Oral versus i.v. antibiotics for community-acquired pneumonia in children: a cost-minimisation analysis. Eur Respir J 2010;35:858–64. 10.1183/09031936.00087209 [DOI] [PubMed] [Google Scholar]
- 12. Harris M, Clark J, Coote N, et al. . British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax 2011;66(Suppl 2):ii1–23. 10.1136/thoraxjnl-2011-200598 [DOI] [PubMed] [Google Scholar]
- 13. Courtoy I, Lande AE, Turner RB. Accuracy of radiographic differentiation of bacterial from nonbacterial pneumonia. Clin Pediatr 1989;28:261–4. 10.1177/000992288902800604 [DOI] [PubMed] [Google Scholar]
- 14. Swingler GH. Radiologic differentiation between bacterial and viral lower respiratory infection in children: a systematic literature review. Clin Pediatr 2000;39:627–33. 10.1177/000992280003901101 [DOI] [PubMed] [Google Scholar]
- 15. Cevey-Macherel M, Galetto-Lacour A, Gervaix A, et al. . Etiology of community-acquired pneumonia in hospitalized children based on WHO clinical guidelines. Eur J Pediatr 2009;168:1429–36. 10.1007/s00431-009-0943-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Honkinen M, Lahti E, Österback R, et al. . Viruses and bacteria in sputum samples of children with community-acquired pneumonia. Clin Microbiol Infect 2012;18:300–7. 10.1111/j.1469-0691.2011.03603.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McEllistrem MC, Adams JM, Patel K, et al. . Acute otitis media due to penicillin-nonsusceptible Streptococcus pneumoniae before and after the introduction of the pneumococcal conjugate vaccine. Clin Infect Dis 2005;40:1738–44. 10.1086/429908 [DOI] [PubMed] [Google Scholar]
- 18. Samore MH, Magill MK, Alder SC, et al. . High rates of multiple antibiotic resistance in Streptococcus pneumoniae from healthy children living in isolated rural communities: association with cephalosporin use and intrafamilial transmission. Pediatrics 2001;108:856–65. 10.1542/peds.108.4.856 [DOI] [PubMed] [Google Scholar]
- 19. Regev-Yochay G, Raz M, Dagan R, et al. . Nasopharyngeal carriage of Streptococcus pneumoniae by adults and children in community and family settings. Clin Infect Dis 2004;38:632–9. 10.1086/381547 [DOI] [PubMed] [Google Scholar]
- 20. Dunais B, Pradier C, Carsenti H, et al. . Influence of child care on nasopharyngeal carriage of Streptococcus pneumoniae and Haemophilus influenzae. Pediatr Infect Dis J 2003;22:589–93. 10.1097/01.inf.0000073203.88387.eb [DOI] [PubMed] [Google Scholar]
- 21. Lietzau S, Raum E, von Baum H, von BH, et al. . Household contacts were key factor for children’s colonization with resistant Escherichia coli in community setting. J Clin Epidemiol 2007;60:1149–55. 10.1016/j.jclinepi.2007.01.016 [DOI] [PubMed] [Google Scholar]
- 22. Bradley JS, Byington CL, Shah SS, et al. . The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 2011;53:e25–76. 10.1093/cid/cir531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. WHO. Pocket book of hospital care for children: second Edition. Guidelines for the management of common conditions: WHO, 2013. [PubMed] [Google Scholar]
- 24. Mathur S, Fuchs A, Bielicki J, et al. . Antibiotic use for community-acquired pneumonia in neonates and children: WHO evidence review. Paediatr Int Child Health 2018;38:S66–S75. 10.1080/20469047.2017.1409455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Appelbaum PC. Microbiological and pharmacodynamic considerations in the treatment of infection due to antimicrobial-resistant Streptococcus pneumoniae. Clin Infect Dis 2000;31 Suppl 2:S29–S34. 10.1086/314057 [DOI] [PubMed] [Google Scholar]
- 26. Calbo E, Garau J. Application of pharmacokinetics and pharmacodynamics to antimicrobial therapy of community-acquired respiratory tract infections. Respiration 2005;72:561–71. 10.1159/000089567 [DOI] [PubMed] [Google Scholar]
- 27. Lassi ZS, Das JK, Haider SW, et al. . Systematic review on antibiotic therapy for pneumonia in children between 2 and 59 months of age. Arch Dis Child 2014;99:687–93. 10.1136/archdischild-2013-304023 [DOI] [PubMed] [Google Scholar]
- 28. Chorazy ML, Lebeck MG, McCarthy TA, et al. . Polymicrobial acute respiratory infections in a hospital-based pediatric population. Pediatr Infect Dis J 2013;32:460–6. 10.1097/INF.0b013e31828683ce [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greenberg D, Givon-Lavi N, Sadaka Y, et al. . Short-course antibiotic treatment for community-acquired alveolar pneumonia in ambulatory children: a double-blind, randomized, placebo-controlled trial. Pediatr Infect Dis J 2014;33:136–42. 10.1097/INF.0000000000000023 [DOI] [PubMed] [Google Scholar]
- 30. Esposito S, Cohen R, Domingo JD, et al. . Do We Know When, What and For How Long to Treat? Antibiotic Therapy for Pediatric Community-acquired Pneumonia. Pediatr Infect Dis J 2012;31. [DOI] [PubMed] [Google Scholar]
- 31. Atkinson M, Lakhanpaul M, Smyth A, et al. . Comparison of oral amoxicillin and intravenous benzyl penicillin for community acquired pneumonia in children (PIVOT trial): a multicentre pragmatic randomised controlled equivalence trial. Thorax 2007;62:1102–6. 10.1136/thx.2006.074906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thompson M, Vodicka TA, Blair PS, et al. . Duration of symptoms of respiratory tract infections in children: systematic review. BMJ 2013;347:f7027 10.1136/bmj.f7027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Currie CJ, Berni E, Jenkins-Jones S, et al. . Antibiotic treatment failure in four common infections in UK primary care 1991-2012: longitudinal analysis. BMJ 2014;349:g5493 10.1136/bmj.g5493 [DOI] [PubMed] [Google Scholar]
- 34. Costelloe C, Metcalfe C, Lovering A, et al. . Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010;340:c2096 10.1136/bmj.c2096 [DOI] [PubMed] [Google Scholar]
- 35. Raum E, Lietzau S, von Baum H, et al. . Changes in Escherichia coli resistance patterns during and after antibiotic therapy: a longitudinal study among outpatients in Germany. Clin Microbiol Infect 2008;14:41–8. 10.1111/j.1469-0691.2007.01841.x [DOI] [PubMed] [Google Scholar]
- 36. Guillemot D, Carbon C, Balkau B, et al. . Low dosage and long treatment duration of beta-lactam: risk factors for carriage of penicillin-resistant Streptococcus pneumoniae. JAMA 1998;279:365. [DOI] [PubMed] [Google Scholar]
- 37. Schrag SJ, Peña C, Fernández J, et al. . Effect of short-course, high-dose amoxicillin therapy on resistant pneumococcal carriage: a randomized trial. JAMA 2001;286:49 10.1001/jama.286.1.49 [DOI] [PubMed] [Google Scholar]
- 38. Tam VH, Louie A, Fritsche TR, et al. . Impact of drug-exposure intensity and duration of therapy on the emergence of Staphylococcus aureus resistance to a quinolone antimicrobial. J Infect Dis 2007;195:1818–27. 10.1086/518003 [DOI] [PubMed] [Google Scholar]
- 39. Opatowski L, Mandel J, Varon E, et al. . Antibiotic dose impact on resistance selection in the community: a mathematical model of beta-lactams and Streptococcus pneumoniae dynamics. Antimicrob Agents Chemother 2010;54:2330–7. 10.1128/AAC.00331-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chan AW, Tetzlaff JM, Altman DG, et al. . SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lyttle MD, O’Sullivan R, Hartshorn S, et al. . Pediatric Emergency Research in the UK and Ireland (PERUKI): developing a collaborative for multicentre research. Arch Dis Child 2014;99:602–3. 10.1136/archdischild-2013-304998 [DOI] [PubMed] [Google Scholar]
- 42. Dunn DT, Copas AJ, Brocklehurst P. Superiority and non-inferiority: two sides of the same coin? Trials 2018;19:499 10.1186/s13063-018-2885-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pernica J, Harman S, Kam A, et al. . Short-course antimicrobial therapy for paediatric respiratory infections (SAFER): study protocol for a randomized controlled trial. Trials 2018;19:83 10.1186/s13063-018-2457-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.