Abstract
Background:
In humans, multiple early exposures to procedures requiring anesthesia is a significant risk factor for development of learning disabilities and disorders of attention and anxiety. In animal studies, newborns exposed to anesthetics develop long-term deficits in cognition. Previously our laboratory showed that postsynaptic density (PSD)-95, discs large homolog, and zona occludens-1 (PDZ) domains may serve as a molecular target for inhaled anesthetics. This study investigated a role for PDZ interactions in spine development, plasticity, and memory as a potential mechanism for early anesthetic exposure-produced cognitive impairment.
Methods:
Postnatal day (PND) 7 mice were exposed to 1.5% isoflurane (ISO) for 4 hrs or injected with 8 mg/kg active PSD-95 PDZ2WT peptide. Apoptosis, hippocampal dendritic spine changes, synapse density, long term potentiation (LTP), and cognition functions were evaluated (n=4-18).
Results:
Exposure of PND7 mice to ISO or PSD-95 PDZ2WT peptide causes a reduction in long thin spines (median, IQ: WT CON (0.54, 0.52 to 0.86) vs WT ISO (0.31, 0.16 to 0.38), p=0.034 and PDZ2MUT (0.86, 0.67 to 1.0) vs PDZ2WT (0.55, 0.53 to 0.59), p=0.028), impairment in LTP (median, IQ: WT CON (123, 119 to 147) and WT ISO (101, 96 to 118), p=0.0.049 and PDZ2MUT (125, 119 to 131) and PDZ2WT (104, 97 to 107), p=0.029), and deficits in acute object recognition (median, IQ: WT CON (79, 72 to 88) vs WT ISO (63, 55 to 72), p = 0.044 and PDZ2MUT (81, 69 to 84) vs PDZ2WT (67, 57 to 77), p=0.039) at PND21 without inducing detectable differences in apoptosis or changes in synaptic density. Impairments in recognition memory and LTP were preventable by introduction of a nitric oxide (NO) donor.
Conclusion:
Early disruption of PDZ domain-mediated protein-protein interactions alters spine morphology, synaptic function, and memory. These results support a role for PDZ interactions in early anesthetic exposure-produced cognitive impairment. Prevention of recognition memory and LTP deficits with a NO donor supports a role for the N-methyl-D aspartate (NMDA) receptor/PSD-95 PDZ2/neuronal nitric oxide synthase (nNOS) pathway in mediating these aspects of ISO induced cognitive impairment.
Summary Statement:
Neonatal PSD-95 PDZ2WT peptide exposure mimics isoflurane (~1 MAC) by altering spine morphology, neural plasticity, and object recognition memory without inducing detectable increases in apoptosis or changes in synaptic density.
Introduction
While pediatric anesthesia is considered safe in terms of mortality and gross morbidity, there is accumulating evidence that early exposure to anesthetic agents may interfere with brain development, cause neuronal death, and ultimately lead to permanent cognitive deficits1-6. Recently, the United States Food and Drug Administration has identified pediatric anesthetic neurotoxicity (PAN) as a potentially important public health problem 7. Evidence from epidemiologic studies suggest humans are susceptible to long-term cognitive effects after anesthesia 5, 6, 8-10. Multiple exposures before age 3 yr are associated with increased frequencies of learning disabilities and attention deficit/hyperactivity disorder 11. Recent primate studies revealed persistent abnormality in visual recognition memory 12 and emotional reactivity 13 after early repeated anesthesia exposure. Animal models have confirmed that early postnatal exposure to anesthetics results in long-lasting impairments in learning and memory 3, 14-19.
While a number of ion channels and receptors at synapses have been highlighted as potential targets for anesthetics the molecular mechanisms that underlie PAN are still poorly understood 20-24. Many of these ion channels and receptors are linked to their downstream signaling pathways through PDZ domain-mediated protein-protein interactions. Our laboratory previously showed that anesthetics can disrupt PDZ domain-mediated protein-protein interactions in vitro and in vivo 25, 26. Using clinically relevant concentrations of inhalational anesthetics we dose-dependently and specifically inhibited PDZ domain-mediated protein interactions between PSD-95 or PSD-93 and the NMDA receptor or nNOS (fig. 1) 25. These inhibitory effects are immediate, potent, and reversible and occur at a hydrophobic peptide-binding groove on the surface of the second PDZ domain of PSD-95. These findings reveal PSD-93 and PSD-95 proteins and specifically their PDZ domains as molecular targets for inhalational anesthetics. We are able to mimic this action of anesthesia with PSD-95 PDZ2WT peptide which disrupts PSD-PDZ2-mediated protein interactions by binding to interaction partners (fig.1). Specifically, we demonstrated disruption of protein-protein interactions between NMDA receptor NR2 subunits and PSD-95 27. This disruption significantly reduced MAC and righting reflex EC50 for halothane indicating that this domain and protein are important for anesthetic action.
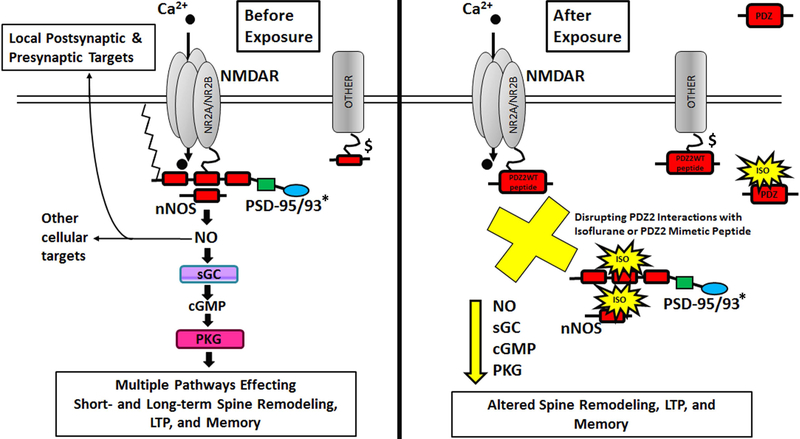
Fig. 1.
Diagram illustrating dissociation of NMDAR-PSD95/93*-nNOS interaction by ISO or PDZ2WT (active) peptide. *For simplicity, not all PSD-95 family members are represented. Before Exposure, The NMDA receptor is linked to downstream molecules such as nNOS through PSD-95: through its first and second PDZ domain, PSD-95 forms a ternary complex by binding to both the tSXV motif of NMDAR NR2 subunit and to the PDZ domain in nNOS 63. Disrupting NMDAR-PSD-95/93-nNOS complexes can reduce the efficiency by which calcium ions activate the signaling molecule nNOS. After Exposure, This disruption is achieved by exposure to inhalational anesthetics25, 26 or the intracellular introduction of PDZ2WT peptide 64; this is expected to bind to NMDAR NR2 65. $ Inhalational anesthetics (and presumably PDZ2WT peptide) can also inhibit interactions between PSD-95 PDZ2 domain and Shaker-type potassium channel Kv1.4 as well as other excitatory receptor channels related to anesthesia and other proteins not shown here for simplicity 26.
Given that 1) the PDZ domain is a molecular target for inhalational anesthetics 25, 26, 2) disruption of PSD-PDZ2 mediated protein interactions increases anesthetic sensitivity 27, 3) PSD-95 PDZ2 interacts with NMDA receptor and promotes synaptogenesis 28, 29, and 3) multi-innervated spine formation is prevented by deletion of the PSD-95 PDZ2 domain 28, we hypothesize that alteration of PDZ domain mediated protein-protein interactions contribute to the molecular mechanisms of PAN by uncoupling ion channels and receptors from their downstream signaling pathways. Here we investigate a role for PSD-95 PDZ2 domain-mediated protein-protein interactions in hippocampal development and plasticity as a potential mechanism for early anesthetic exposure-produced cognitive impairment. We examine, in vivo, the outcome of disrupting PSD-95 PDZ2 domain-mediated protein-protein interactions early in development on apoptosis, spinogenesis, synaptogenesis, long term potentiation, and object recognition memory. To specifically determine the involvement of the NMDAR NR2-PSD-95 PDZ2-nNOS pathway we introduced NO donor at the time of ISO anesthesia or PSD-95 PDZ2 exposure to test if impairments are preventable.
Materials and Methods
This study was carried out with approval from the Animal Care and Use Committee at Johns Hopkins University and was consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. No surgery was performed, and all efforts were made to minimize animal suffering and reduce the number of animals used. C57BL6 wild type (WT) and PSD-93 null mutant male and female mice were used in our study. For all experiments, mice were assessed in their sexually immature state (≤PND21). On PND7, animals from each litter were randomly assigned to control and treatment groups. Mice were maintained under standard lab housing with 12 h light/dark cycle. Water and food were available ad libitum until mice were transported to the laboratory approximately 1 h before the experiments.
Anesthesia, Peptide, and Moldsidomine Injections
PND7 control and experimental mice were placed in a clear plastic cone and body temperature maintained by a heating blanket set to 35C. Vital signs and physiological monitoring were assessed using Physiosuite and blood gasses collected. Our data suggested that the mice were adequately oxygenated and they were not overly acidotic (data not shown). Naïve animals were left with the dams. Anesthesia was initiated with 2.4% ISO in oxygen for 2 min and tapered down to 1.5% within 15 min. Exposure to 1.5% ISO was continued for 3 hours and 45 min (total 4 hours ISO). Control ‘CON’ animals were exposed to oxygen only. At the end of the exposure animals were maintained in oxygen on the heating blanket for 10 min then returned to dams. The purified fusion peptides, active Tat-PSD-95 PDZ2WT (referred to as PDZ2WT in manuscript) or inactive Tat-PSD-95 PDZ2MUT (referred to as PDZ2MUT in manuscript), at 8 mg/kg were injected into mice intraperitoneally (ip) in 150 μl of PBS and 10% glycerol, as previously described 27. Purification of fusion peptides was performed by Creative BioMart (Shirley, NY) and verified by Coomassie blue staining and Western blot analysis and then stored in 10% glycerol/phosphate-buffered saline at −80C until use. The Tat-PSD-95 PDZ2WT and MUT plasmids used to generate proteins containing an amino-terminal, in-frame, 11-amino-acid, minimal transduction domain (residues 47-57 of human immunodeficiency virus Tat protein) termed Tat. Inactive control plasmid, mutated Tat-PSD-95 PDZ2, has three sites critical for interactions between NMDARs and PSD-95 mutated (K165T, L170R and H182L)25. The NO donor Molsidomine [(N-[ethoxycarbonyl]-3- [4-morpholinosydnomine] (Sigma, St. Louis, MO) was injected at 4 mg/kg into mice ip in 100 μl sterile saline as previously described. Control animals were injected ip with the vehicle (saline).
Western Blotting
C57Bl6 WT mice were sacrificed by cervical dislocation and the brains were harvested. Hippocampi were grossly dissected from the mouse brain under a dissecting microscope. Total proteins from these tissues were extracted. The tissues were homogenized in homogenization buffer (10 mM Tris-HCl, 5 mM MgCl2, 2 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 1 μM leupeptin, 2 μM pepstatin A, and 320 mM sucrose [pH 7.4]). The crude homogenates were centrifuged at 700g for 15 min at 4 °C. Then the supernatants were combined and diluted in resuspension buffer (10 mM Tris-HCl, 5 mM MgCl2, 2mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 1 μM leupeptin, 2 μM pepstatin A, and 250 mM sucrose [pH 7.4]). Next, the protein extracts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransferred to nitrocellulose membranes. The membranes were blocked in 0.1% Tween-20 in Tris-HCl–buffered saline (TBST) containing 5% nonfat milk for 1 h at room temperature and then immunoblotted with primary antibodies (anti-caspase 3: 1:1000 and poly-(adenosine diphosphate-ribose) polymerase (PARP) 1: 1000 were from Cell Signaling Technology, Beverly, MA; anti-β-actin: 1:100,000, Sigma-Aldrich, St. Louis, MO) in TBST buffer containing 5% nonfat milk overnight at 4 °C. After being washed extensively in TBST, the membranes were incubated for 1 h with horseradish peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin (Bio-Rad Laboratories, Hercules, CA) at a dilution of 1:5000. Proteins were detected by enhanced chemiluminescence (Amersham, Piscataway, NJ). β-Actin served as a loading control.
Golgi Staining, Microscopy, and Spine Reconstruction
PND21 mice were deeply anesthetized and perfused transcardially with a brief flush of 0.01 M phosphate-buffered saline (pH 7.4) followed by 50 mL of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). After the perfusion, the brains were removed and hippocampi were grossly dissected and stained using FD Rapid GolgiStain™ Kit, (FD NeuroTechnologies, Inc., Columbia, MD) as per vendors instructions. Briefly, tissue was immersed in AB impregnation solution at room temperature in the dark for 2 weeks. Impregnation solution was replaced after the first overnight on the next day. Tissue was transferred to solution C for 72 hours. Hippocampi were embedded in tissue freezing medium (TFM) and stored at −80C. 60 μm sections were cut on a cryostat at −20C, mounted onto gelatin coated slides, and air dried overnight. Slides were rinsed in Milli-Q water, developed in the working solution DE for 10 min, rinsed, dehydrated in EtOH, cleared in xylene, and mounted with Permount®. Only samples that had optimal impregnation (contiguous staining across dendrites and spines) were taken forward for imaging (n=2 excluded animals).
Two different imaging fields per mouse each containing at least three unique dendritic segments (6 segments total per mouse), which contain dorsal hippocampus were imaged. The 6 segments were averaged per mouse to contribute one datapoint per mouse. Dentate granule cells were identified by their location within the dentate gyrus and their distinct morphology. Spines along secondary and tertiary dendrites of these neurons were selected for analysis (see fig. 3). Z-stacks of Golgi stained dendrites (optical section thickness = 0.3 μm, i.e. 50-100 images per stack) were taken at 630x magnification on a Leica SPE confocal microscope. Spine analysis was performed as described in Risher et al (2014)30 using the freely available RECONSTRUCT software31.
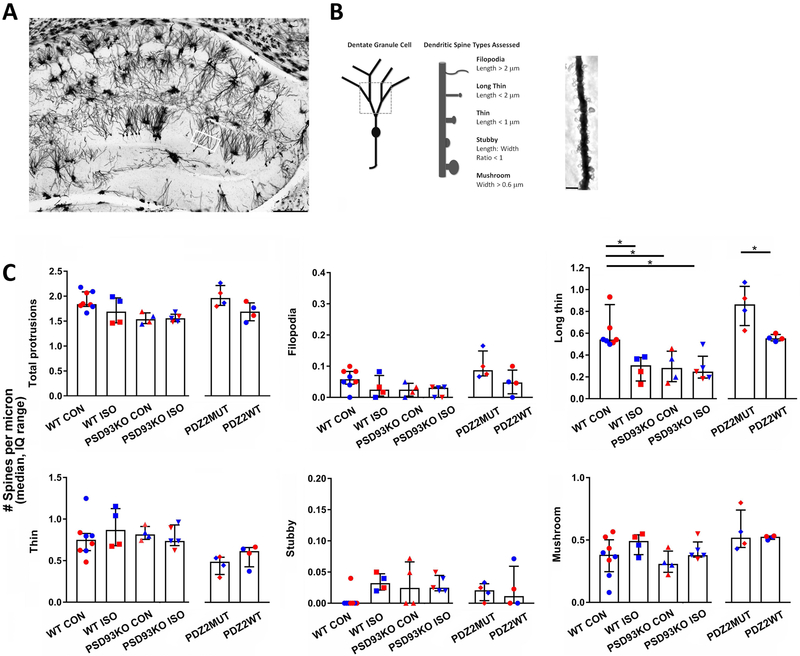
Fig. 3.
Neonatal exposure to ISO or PDZ2WT peptide alters hippocampal dendritic spine morphology and development. A, dorsal hippocampal region of a Golgi preparation illustrating dendrites from the superior blade of the dentate gyrus (DG) subregion of interest (white box); scale bar represents 250 μm. B, schematics showing dendrite branches and spine types sampled and a representative dendritic segment with spines; scale bar represents 2.5 μm. Spines were assessed on dendritic segments distal to the first and second branch points. C, Distribution of dendritic spines according to morphological type in DG among exposed groups assessed at PND21. WT and PSD-93 KO PND7 mice were exposed to O2 (control, 100% O2) or ISO (1.5% ISO in O2); WT PND7 mice were also injected with PDZ2MUT (8 mg/kg inactive peptide) or PDZ2WT (8 mg/kg active peptide). (WT CON = 8, WT ISO = 4, PSD93KO CON = 4, PSD93KO ISO = 5, PDZ2MUT = 4, PDZ2WT = 4). Data from individual animals are plotted and color coded by gender (red=female and blue=male). Data are plotted as median number of spines per micron with interquartile range). Data were analyzed with Kruskal-Wallis followed by Dunn’s multiple comparison correction and Mann-Whitney tests. A *p < 0.05 was considered significant.
Electron Microscopy
PND21 mice were deeply anesthetized and perfused transcardially with a brief flush of 35C Mammalian Ringer Solution (EMS 11763-10) with 5U/ml of Heparin (Sagent Pharmaceuticals NDC 25021-400-10) followed by 50 mL of 2.5% paraformaldehyde (freshly prepared from EM grade prill), 2% glutaraldehyde, 0.1M sodium cacodylate, 3mM MgCl2, (pH 7.2) at 1314 mOsmols at a rate of 1-ml/min. After the perfusion, the mouse heads were stored at 4C for 2 hours. After incubation in the cold, brains were removed and hippocampi were grossly dissected using a brain block and dissecting microscope. Tissue was immersed in fixative overnight and further dissected in the cold room the next morning to isolate the hippocampus (2mm × 2mm) and add notches for orientation. The following steps were kept cold (4 C) until the 70% ethanol step, then run at room temp. Samples were rinsed in 100 mM cacodylate 3.5% sucrose 3mM MgCl2, pH 7.2 at 324 mOsmols for 45 min. Following buffer rinses, samples were microwave fixed twice in 2% osmium tetroxide reduced with 1.6% potassium ferrocyanide, in the same buffer without sucrose. Sample temperatures did not exceed 9 C. Following microwave processing samples were rocked in osmium on ice for 2 hours in the dark. Tissue was then rinsed in 100 mM maleate buffer with pH 6.2, then en-bloc stained for 1 hour with filtered 2% uranyl acetate in maleate buffer, pH 6.2. Following en-bloc staining samples were dehydrated through a graded series of ethanol to 100%, transferred through propylene oxide, embedded in Eponate 12 (Pella) and cured at 60 C for two days. Sections were cut on a Riechert Ultracut E microtome with a Diatome Diamond knife (45 degree). 60nm sections were picked up on formvar coated 1 × 2 mm copper slot grids and stained with methanolic uranyl acetate. Grids were viewed on a Phillips CM 120 TEM operating at 80 kV and digital images captured with an XR80- 8 megapixel CCD by AMT. Ten images (each image 6250 nm × 7500 nm) were averaged per animal. Each animal contributed one data point to obtain median number of PSD’s for each group.
Electrophysiology
Slice preparation.
Two weeks after exposure (PND21-35) mice were euthanized and coronal brain slices containing central part of hippocampus (300μm thick) were made from Leica VT 1200S vibrotome in ice-cold ACSF containing (in mM): 128 NaCl, 3 KCl, 26 NaHCO3, 1 NaH2PO4, 1 MgSO4, 10 glucose and 2 CaCl2 and saturated with 95% O2 and 5% CO2. The slices were incubated for at least 1hr at room temperature (22-24°C) in the interface-type holding chamber filled with ACSF. Then a slice was transferred to the recording chamber, where ACSF was perfused at a rate of 1.5-2.0mL/min at room temperature. Extracellular field-potential recordings. Synaptic responses were recorded using a MultiClamp 700B amplifier, and the signal was digitized with Digidita 1440A, analyzed with pClamp10 and stored on a personal computer. Extracellular recordings of field excitatory postsynaptic potentials (fEPSPs) were made from the stratum radiatum of the hippocampal CA1 area. Evoked responses were elicited with 0.1msec constant-current pulses through a concentric electrode in the Schaffer collateral pathway every 30sec at an intensity sufficient to elicit 40-50% maximal EPSPs. After establishing a stable baseline for 20min, LTP was induced by applying three trains of 100Hz X 1sec high frequency stimulus (HFS) 20sec apart at the baseline stimulus intensity. Measurements of the fEPSP slopes were made during the rising phase (5-50% of the peak) and the values normalized to the mean values recorded in 20min baseline. The median of normalized fEPSP slopes 55-60min after HFS was used for comparison between groups. Each animal contributed one data point to obtain the median for each group.
Novel Object Recognition
The novel object recognition (NOR) procedure was based upon the original Ennaceur et al (1988) procedure and was used to assess nonspatial hippocampal memory 32, 33,34. It consisted of a training ‘familiarization’ phase followed by a testing phase. During training, mice were allowed to freely explore within an opaque box (40 cm W × 40 cm L × 34 cm H) containing two identical objects for 10 min. Data were recorded with video camera and time spent with each object was recorded using ANYmaze software (Stoelting). Object investigation time was determined by the amount of time the mouse spent in the zone immediately surrounding the object. Only mice that investigated the objects for at least 10 sec (criterion) were taken forward to the testing phase (n=6 animals were not taken forward as they did not meet criterion). After 2 h, object recognition was tested, using the same procedure as in training except that a novel object was substituted for one of the familiar training objects and mice were allowed to explore for 5 min. Mice inherently prefer to explore novel objects; thus, a preference for the novel object indicates intact memory for the familiar object.
Statistical Analysis
Statistical analysis was carried out by unpaired Student's t-test, two-tailed, Mann-Whitney, and Kruskal-Wallis followed by post-hoc Dunn’s tests with Graphpad Prism version 7.0 software (Graphpad Inc., La Jolla, CA). Datasets that failed D'Agostino & Pearson normality test were analyzed using non-parametric statistics. Student's t-test, two-tailed was used to compare novel and known object investigation times in the NOR assay. Mann-Whitney, two-tailed test was used to compare WT CON versus WT ISO and PDZ2MUT versus PDZ2WT groups (PSD quantification, NOR + NO donor, and LTP + NO donor), Mann-Whitney, two-tailed test was used to compare WT CON versus WT ISO groups (PARP WB) and PDZ2MUT versus PDZ2WT groups (spine analysis and LTP). Kruskal-Wallis test was used in analysis of spines and LTP (comparing WT CON, WT ISO, PSD93KO CON, PSD93KO ISO which included 1 family, 4 treatments, and 6 comparisons) and NOR (comparing WT NAÏVE, WT CON, WT ISO, PSD93KO CON, PSD93KO ISO which included 1 family, 5 treatments and 10 comparisons) and (PDZ2MUT, PDZ2WT, PDZ2WT+ISO that include 1 family, 3 treatments, and 3 comparisons). Data are expressed as mean± standard deviation (SD) or median, interquartile range respectively, and statistical significance was set at P < 0.05. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. Sample sizes were chosen based on previous experience and/or published literature. A sample size of N=6 was chosen for PARP WB because an N=3 was sufficient to detect significant differences following forebrain injury in rats35. A sample size of N=3 to 6 was chosen for ultrastructure analysis because this number of animals was sufficient to detect significant differences in PSDs in the DG after cerebral ischemia36. A sample size of N=4 to 8 was chosen for spine analysis because an N=4 was sufficient to show differences in hippocampal proportional densities of different types of spines following excitoxicity37. A sample size of N=4 to 7 was chosen for LTP measurements because an N=3 was sufficient to show differences in LTP amplitude induced by NMDA antagonist 38. A sample size of N=6 to 18 was chosen for NOR because sample sizes of 10 were sufficient to show age specific differences in rodents34, 39. In all experiments each animal contributed one data point to obtain the median for each group.
Results
Effect of a single dose of ISO or PDZ2WT peptide on apoptosis
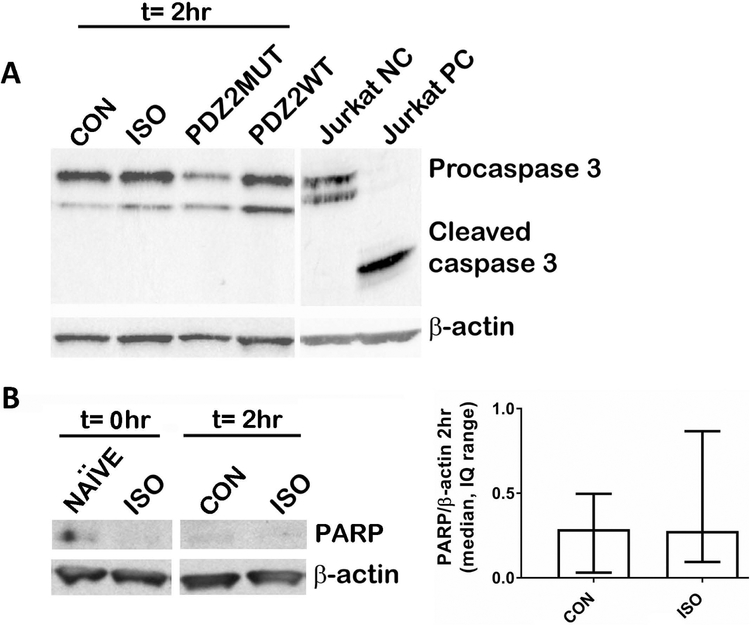
Jurkat cell control protein obtained from cell signaling technology was run to determine parameters for running Caspase-3 Western blot. The negative control was generated by lysing in Chaps cell extract buffer and positive control was treated with cytochrome C. Representative image shows expected uncleaved (procaspase 3) and cleaved (cleaved caspase 3) bands (fig. 2A). 4 h treatment in mice including ISO or PDZ2WT peptide exposure did not yield any detectable cleaved caspase at 2 h following cessation of exposure (fig. 2A). Neither were we able to detect cleaved caspase 3 at 0 h or 20 h following cessation of exposure (data not shown). Representative image shows expected cleaved PARP band (fig. 2B left). 4 h ISO treatment in mice did not yield significantly different levels of PARP compared to CON at 2 h following cessation of exposure (fig. 2B right, median, interquartile range: CON (0.29, 0.31 to 0.50) vs ISO (0.28, 0.09 to 0.87), p=0.818). Data were analyzed using Mann-Whitney test.
Fig. 2.
Western blot assays did not show any significant differences in apoptosis in our exposure paradigm. PND7 mice were exposed for 4-hours and harvested 2-hours after cessation of exposure. A, Western blot analysis for total caspase-3 revealed the presence of procaspase 3 but did not reveal any detectable cleaved caspase-3 in mice exposed to CON (O2), ISO (1.5% ISO in O2), PDZ2MUT (8 mg/kg inactive peptide), PDZ2WT (8 mg/kg active peptide). Negative and positive Jurkat control proteins were run to demonstrate sensitivity of the caspase-3 antibody. B, Western blot analysis for PARP did not reveal significant differences between CON (O2) and ISO (1.5% ISO in O2). Left, representative blot. Right, Densitometry data are plotted as median, interquartile range. CON (n=6) vs ISO (n=6), p=0.818. Data were analyzed using Mann Whitney test. Gender was not determined.
ISO, PSD93 deficiency, and PDZ2WT peptide alter hippocampal dendritic spine morphology
To determine whether inhaled anesthetics interfere with spinogenesis by disrupting synaptic PDZ interactions in the developing hippocampus, we investigated the impact of ISO, PSD-93 deficiency, and disrupting PSD-95 PDZ2 domain-mediated protein-protein interactions on dendritic spine morphology in PND21 mice two weeks following an exposure at PND7. Of note, the PSD-95 family (PSD-95, PSD-93, SAP102, SAP97) of membrane associated guanylate kinases (MAGUKs) share high sequence similarity as well as similar domain structure: three PDZ domains followed by an SH3 and a GK domain 40, 41. PDZ domains of PSD93 are remarkably similar to the PDZ domains of PSD95 in sequence and structure 42. Both PSD-93 and PSD-95 PDZ domain-mediated interactions with NMDA NR2 or nNOS can be disrupted with anesthetics (fig. 1) 25. Since PSD-93 KO mice show impaired LTP 43 similar to anesthesia exposed WT rodents 3 we used them here as a representative global knockout for the PSD-95 family of MAGUKs in assessments on spine morphology, LTP induction, and memory. Neonatal WT and PSD93KO mouse pups (PND7) were exposed to ISO or O2 for 4 hrs. A separate cohort of WT animals were also exposed to PDZ2MUT or PDZ2WT peptides. Animals were harvested two weeks later (PND21) for rapid golgi staining to visualize hippocampal dendritic spines (fig. 3). An example tiled image of a golgi stained region of the hippocampus is shown in fig. 3A. The white box indicates the subregion of interest within the superior blade of the DG. The total number of protrusions and different spine types were assessed based on dendritic segments distal to the first and second branch points (fig. 3B). Data were analyzed using Kruskal-Wallis and Mann Whitney tests.
A Kruskal-Wallis test indicated a main effect on the number of total dendritic protrusions across the following four groups (fig. 3C top left, median, IQ: WT CON (1.8, 1.8 to 2.1), WT ISO (1.7, 1.5 to 2.0), PSD93KO CON (1.5, 1.5 to 1.7), PSD93KO ISO (1.6, 1.5 to 1.6), p=0.029. However, no difference was detected following Dunn’s multiple comparison tests (WT CON vs WT ISO, p=0.766; WT CON vs PSD93KO CON, p=0.329; WT CON vs PSK93KO ISO, p=0.094; WT ISO vs PSD93KO CON, p>0.999; WT ISO vs PSD93KO ISO, p>0.999; PSD93KO CON vs PSD93KO ISO, p>0.999). No significant effect on total protrusions was observed between PDZ2MUT (2.0, 1.8 to 2.2) vs PDZ2WT (1.7, 1.5 to 1.9), p=0.114. Thus, exposure to ISO, PSD93 deficiency, or PDZ2WT peptide did not have a significant effect on total number of dendritic protrusions assessed at PND21.
ISO, PSD93 deficiency, or PDZ2WT peptide did not have significant effects on the number of filopodial type protrusions (length > 2 μm) (fig. 3C top middle, WT CON (0.06, 0.04 to 0.08), WT ISO (0.02, 0.00 to 0.07), PSD93KO CON (0.02, 0.00 to 0.05),PSD93KO ISO (0.03, 0.00 to 0.03), p=0.062. PDZ2MUT (0.09, 0.07 to 0.15) vs PDZ2WT (0.05, 0.01 to 0.09), p=0.200).
A main effect of treatment was observed across the following groups on the number of long thin spines (< 2 μm length and < 0.6 μm width) (fig. 3C top right, WT CON (0.54, 0.52 to 0.86), WT ISO (0.31, 0.16 to 0.38), PSD93KO CON (0.28, 0.16 to 0.44), PSD93KO ISO (0.25, 0.19 to 0.39), p=0.003. ISO caused a reduction in long thin spines in WT mice as did PSD93 deficiency (WT CON vs WT ISO, p=0.034; WT CON vs PSD93KO CON, p=0.042; WT CON vs PSD93KO ISO, p=0.015; WT ISO vs PSD93KO CON, p>0.999; WT ISO vs PSD93KO ISO, p>0.999). ISO did not further reduce the number of long thin spines in PSD93 KO mice (PSD93KO CON vs PSD93KO ISO, p>0.999). PDZ2WT peptide caused a reduction in long thin spines (PDZ2MUT (0.86, 0.67 to 1.0) vs PDZ2WT (0.55, 0.53 to 0.59), p=0.028.
ISO, PSD93 deficiency, or PDZ2WT peptide did not have a significant effect on number of thin spines (length< 1 μm) (fig. 3C bottom left, WT CON (0.75, 0.62 to 0.83), WT ISO (0.87, 0.68 to 1.13), PSD93KO CON (0.82, 0.76 to 0.91), PSD93KO ISO (0.74, 0.68 to 0.93), p=0.705; PDZ2MUT (0.49, 0.34 to 0.54) vs PDZ2WT (0.62, 0.43 to 0.66), p=0.200).
ISO, PSD93 deficiency, or PDZ2WT peptide did not have a significant effect on stubby spines (length:width ratio<1) (fig. 3C bottom middle, WT CON (0.00, 0.00 to 0.00), WT ISO (0.03, 0.02 to 0.05), PSD93KO CON (0.02, 0.00 to 0.07), PSD93KO ISO (0.02, 0.02 to 0.04), p=0.051; PDZ2MUT (0.02, 0.00 to 0.03) vs PDZ2WT (0.01, 0.00 to 0.06), p=0.914).
ISO, PSD93 deficiency, or PDZ2WT peptide did not have a significant effect on number of mushroom type protrusions (fig. 3C bottom right, WT CON (0.38, 0.25 to 0.50), WT ISO (0.49, 0.38 to 0.54),PSD93KO CON (0.31, 0.24 to 0.41), PSD93KO ISO (0.38, 0.36 to 0.48), p=0.283; PDZ2MUT (0.52, 0.44 to 0.74) vs PDZ2WT (0.53, 0.51 to 0.53), p>0.999).
ISO and PDZ2WT peptide do not have an effect on the number of postsynaptic densities in the hippocampus at PND21
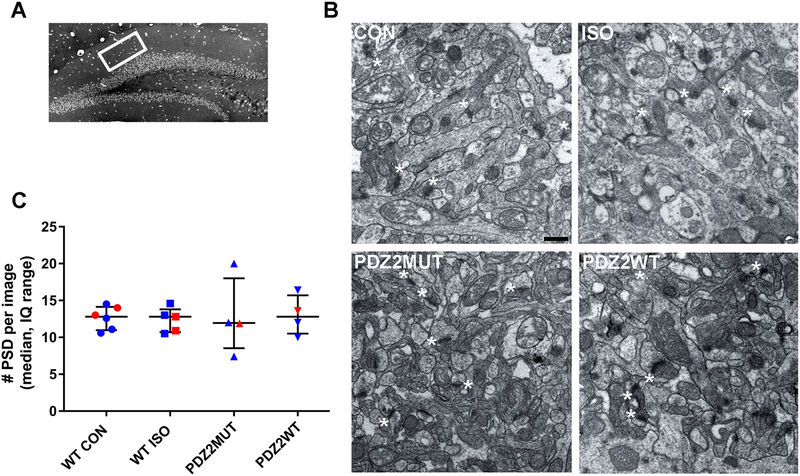
To investigate the impact of neonatal exposure to ISO or disruption of PSD-95 PDZ2 domain-mediated protein-protein interactions on synaptogenesis, we assessed the number of PSDs in the hippocampus in PND21 mice (two weeks after exposure)(fig. 4). Neonatal exposure to ISO or PDZ2WT peptide did not have a significant effect on number of PSDs as compared to controls (fig. 4C, median, IQ: WT CON (12.8, 10.9 to 14.1) vs WT ISO (12.9, 10.7 to 13.8), p=0.829; PDZ2MUT (12.0, 8.5 to 18) vs PDZ2WT (12.8, 10.5 to 15.7), p=0.743). Data were analyzed using Mann-Whitney test.
Fig. 4.
Neonatal exposure to ISO or PDZ2WT peptide did not have an acute impact on the number of hippocampal postsynaptic densities. A, dorsal hippocampal region of a semi-thin section illustrating DG subregion of interest (white box). B, representative ultrastructure images from PND21 mice exposed at PND7 to O2 (WT CON, 100% O2), WT ISO (1.5% ISO in O2), PDZ2MUT peptide (8 mg/kg inactive peptide), or PDZ2WT peptide (8 mg/kg active peptide); scale bar represents 500 nm. Asterisks showing some PSDs (not all are labeled). C, plots showing the median with interquartile range number of PSD’s. WT CON (n=6) vs WT ISO (n=5), p=0.829; PDZ2MUT (n=4) vs PDZ2WT (n=4), p=0.742. Data from individual animals are plotted and color coded by gender (red=female and blue=male). Data were analyzed with Mann-Whitney.
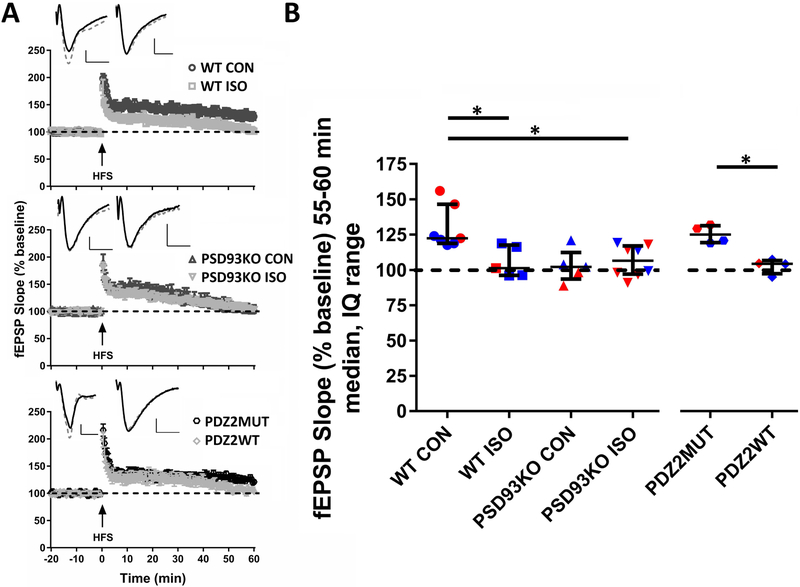
ISO, PSD93 deficiency, and PDZ2WT peptide impair LTP induction in hippocampal CA1 at PND21
To assess long-term electrophysiological effects of early ISO exposure, PSD-93 deficiency, and disruption of PSD-95 PDZ2 domain-mediated protein-protein interactions, we examined synaptic function and LTP in hippocampal slices prepared at PND21 from mice treated at PND7. Two weeks after exposure, robust LTP can be induced in WT mice that received oxygen CON exposure (fig. 5A top, 5B; median, IQ: 123, 119 to 147) or inactive PDZ2MUT peptide (fig. 5A bottom, 5B; 125, 119 to 131) 55-60 min after HFS. Expression of LTP was impaired in ISO (fig. 5A top, 5B; WT ISO, 101, 96 to 118; PSD93KO ISO, 107, 97 to 117), PDZ2WT peptide (fig. 5A bottom, 5B; 104, 97 to 107) and PSD93KO CON (fig. 5A middle, 5B; 102, 94 to 112) groups. Kruskal-Wallis test indicated a significant effect of treatment (WT CON, WT ISO, PSD93KO CON, PSD93 ISO, p=0.009). Post-hoc Dunn’s analysis showed significant differences between WT CON vs WT ISO (fig. 5B; p=0.049) and WT CON vs PSD93KO ISO (fig. 5B; p=0.026). No differences were observevd in the following comparisons (WT CON vs PSD93KO CON, p=0.056; WT ISO vs PSD93KO CON, p>0.999; WT ISO vs PSD93KO ISO, p>0.999; PSD93KO CON vs PSD93KO ISO, p>0.999). PDZ2MUT vs PDZ2WT (fig. 5B; p=0.029) groups differed during the last 5 min of recording after HFS.
Fig. 5.
Neonatal exposure to ISO or PDZ2WT peptide impairs long-term potentiation (LTP) in hippocampal CA1 at PND21. A, high frequency stimulation (HFS) induced robust LTP in WT CON (top; WT CON, 100% O2) and inactive PDZ2MUT (bottom) treated groups in hippocampal Schafer collateral to CA1 pathway. ISO (top) and PDZ2WT (bottom) exposure as well as PSD93 deficiency (middle) impaired the expression of LTP. Example traces are shown in upper left quadrants of fEPSP plots. WT CON and WT ISO (top), PSD93KO CON and PSD93KO ISO (middle), and PDZ2MUT and PDZ2WT (bottom) treated groups at baseline before HFS (solid line trace) and the average of 55-60 min after HFS (dashed line trace). B, the median of normalized fEPSP 55-60min after HFS showed significant differences between WT CON (n=7) vs WT ISO (n=5), p=0.049 and PDZ2MUT (n=4) vs PDZ2WT (n=4), p=0.028 treated groups. Significant differences were not observed between WT CON vs PSD93KO CON (n=5), p=0.056 but were observed between WT CON vs PSD93KO ISO (n=8), p=0.025 groups. Data from individual animals are plotted and color coded by gender (red=female and blue=male). Data were analyzed with Kruskal-Wallis followed by post-hoc Dunn’s test and Mann Whitney tests. Values were considered significant at *p < 0.05 or less. Data were plotted as median and interquartile range. Scale bar: 10ms, 0.25mV.
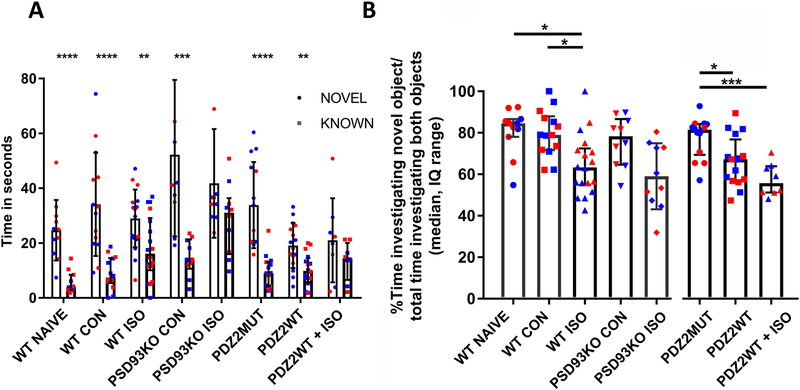
ISO and PDZ2WT peptide cause subtle but significant decreases in recognition memory in the novel object recognition test.
To determine whether the disruption of synaptic PDZ interactions contributes to cognitive impairment after early anesthetic exposure, we investigated the impact of ISO, PSD93 deficiency, and disrupting PSD-95 PDZ2 domain-mediated protein-protein interactions on memory by assessing hippocampal dependent object recognition in PND21 mice two weeks after exposure at PND7. In the majority of conditions, mice were able to discriminate at some level between novel and known objects, revealed by increased investigation times of the novel object (fig. 6A; mean, ±SD: WT Naïve (25±11 vs 6±4), p<0.0001; WTCON (34±19 vs 9±6), p<0.0001; WT ISO (29±11 vs 18±12), p=0.005; PSD93KOCON (52±30 vs 15±7), p=0.001; PDZ2MUT (34±16 vs 10±6), p<0.0001; PDZ2WT (19±8 vs 10±6), p=0.001; two-tailed t-test novel vs. known). The double hit animals were unable to significantly discriminate between novel and known objects (PSD93KO ISO (42±20 vs 29±13), p=0.098; PDZ2WT+ISO (21±15 vs 14±7), p=0.227). All groups spent more than 50% of their object interaction time with the novel object as can be seen in the recognition index (% time investigating novel object over time investigating novel object plus familiar object x 100) plot (fig. 6B). Kruskal-Wallis test indicates a significant effect of exposure treatment (fig. 6B; median, IQ: WT NAÏVE (85, 78 to 87), WT CON (79, 72 to 88), WT ISO (63, 55 to 72), PSD93KO CON (78, 65 to 87), PSD93KO ISO (57, 47 to 73), p=0.0006). Post-hoc Dunn’s comparisons across groups shows that ISO exposed WT animals have a subtle but significant decrement in recognition memory as compared to controls (WT NAÏVE vs WT ISO, p=0.023 and WT CON vs WT ISO, p= 0.0.044). PSD93 deficiency did not have a significant effect on recognition memory (WT NAÏVE vs PSD93KOCON, p>0.999 and WT CON vs PSD93KOCON, p>0.999 ). ISO exposure did not cause detectable impairment in PSD93 deficient animals (PSD93KO CON vs PSD93KO ISO, p=0.177). Kruskal-Wallis test indicates a significant effect of peptide treatment on memory (PDZ2MUT (81, 69 to 84), PDZ2WT (67, 57 to 77), PDZ2WT+ISO (56, 51 to 64), p=0.001). PDZ2WT exposed animals have a subtle but significant decrement in recognition memory as compared to PDZ2MUT controls (PDZ2MUT vs PDZ2WT, p=0.0.039 and PDZ2MUT vs PDZ2WT+ISO, p=0.001). ISO did not cause further significant decrement in recognition memory in PDZ2WT exposed animals (PDZ2WT vs PDZ2WT+ISO, p=0.386).
Fig. 6.
Neonatal exposure to ISO or PDZ2WT peptide causes a subtle but significant decrease in acute recognition memory. A, plots showing percent of time animals spent investigating novel or known objects among experimental groups(WT Naïve, n=11, p<0.0001; WTCON, n=14, p<0.0001; WT ISO, n=18, p=0.005; PSD93KO CON, n=10, p=0.001; PDZ2MUT, n=14, p<0.0001; PDZ2WT, n=16, p=0.001). The double hit animals were unable to significantly discriminate between novel and known objects (PSD93KO ISO, n=10, p=0.098; PDZ2WT+ISO, n=8, p=0.227). Data were plotted as mean and SD. Data were analyzed with two-tailed t-tests known vs. novel. B, plots showing discrimination index as percent of time animals spent investigating novel object over the total time investigating novel and known objects multiplied by 100. ISO exposed WT animals have a subtle but significant decrement in recognition memory as compared to controls (WT NAÏVE vs WT ISO, p = 0.022; WT CON vs WT ISO, p = 0.043; WT NAÏVE vs WTCON, p >0.999). PSD93 deficiency did not have a significant effect on recognition memory (WT NAÏVE vs PSD93KO CON, p>0.999; WT CON vs PSD93KOCON, p>0.999). ISO exposed PSD93 KO animals were not significantly different from PSD93 KO controls (PSD93KO CON vs PSD93KO ISO, p=0.176). ISO exposed PSD93KO animals differed from WT controls (WT NAÏVE vs PSD93KO ISO, p=0.011; WT CON vs PSD93KO ISO, p=0.022). Active peptide exposed animals have a subtle but significant decrement in recognition memory as compared to inactive peptide controls (PDZ2MUT vs PDZ2WT, p=0.038; PDZ2MUT vs PDZ2WT + ISO, p<0.001) ISO exposure did not further significantly impair peptide exposed animals (PDZ2WT vs PDZ2WT + ISO, p=0.385). Data from individual animals are plotted and color coded by gender (red=female and blue=male). Data were analyzed with Kruskal-Wallis followed by post-hoc Dunn’s test. Data were plotted as median and interquartile range. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
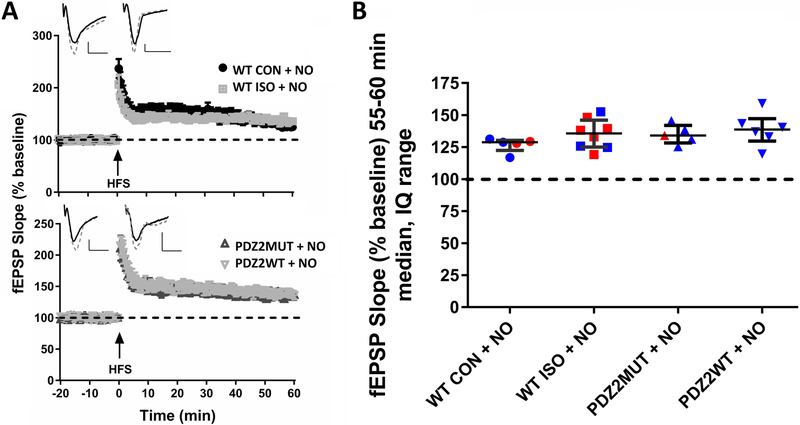
Treatment with NO donor prevents the negative effects of ISO and PDZ2WT peptide on hippocampal LTP
Treatment with NO donor prevents the impairment in LTP caused by ISO or PDZ2WT peptide as indicated by the renewed expression of LTP (fig. 7A, 7B; median, IQ: WT CON + NO (129, 123 to 130), ISO + NO (136, 125 to 146), PDZ2MUT + NO (134, 128 to 142), PDZ2WT + NO (139, 130 to 147). There is no longer a significant difference between WT CON and WT ISO when NO donor is added (fig. 7B; WT CON + NO vs WT ISO + NO, p=0.284) or between PDZ2MUT and PDZ2WT (fig. 7B; PDZ2MUT + NO vs PDZ2WT + NO, p=0.662). Data were analyzed with Mann-Whitney test. .
Fig.7.
Treatment with NO donor prevents the negative effect of ISO and PDZ2WT peptide on hippocampal LTP. A, robust LTP was induced by HFS in all groups. In upper left quadrants example traces of WT CON+NO and WT ISO+NO (top), PDZ2MUT+NO and PDZ2WT+NO (bottom) treated groups before HFS (solid line trace) and 55-60min after HFS (dashed line trace) are shown. B, the median of normalized fEPSP 55-60min after HFS no longer shows a significant difference between WT CON and WT ISO when NO donor is added (WT CON + NO (n=5) vs ISO + NO (n=8), p=0.284) or between PDZ2MUT and PDZ2WT (PDZ2MUT + NO (n=5) vs PDZ2WT + NO (n=6), p=0.662). Data were analyzed with Mann-Whitney tests. Data from individual animals are plotted and color coded by gender (red=female and blue=male). Data were plotted as median and interquartile range. Values were considered significant at *p < 0.05 or less. Scale bar: 10ms, 0.25mV.
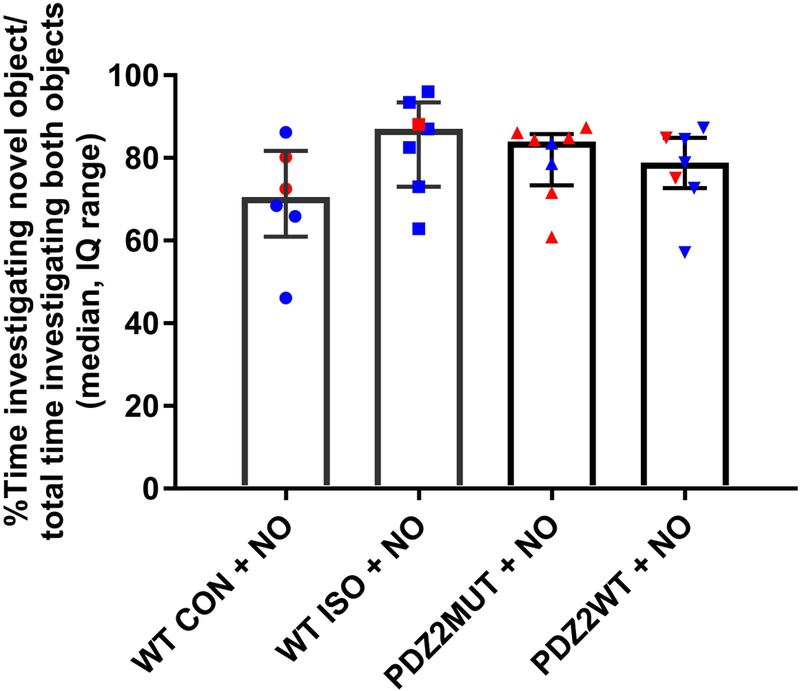
Treatment with NO donor prevents ISO or PDZ2WT induced impairment in acute recognition memory
Treatment with NO donor prevents the impairment in NOR caused by ISO or PDZ2WT peptide as indicated by the increased discrimination in NOR (fig. 8; median, IQ: WT CON + NO (71, 61 to 82), WT ISO + NO (87, 73 to 93), PDZ2MUT + NO (84, 73 to 86), PDZ22WT + NO (79, 73 to 85). There is no longer a significant difference between WT CON and WT ISO when NO donor is added (WT CON + NO vs ISO + NO, p=0.073) or between PDZ2MUT and PDZ2WT (PDZ2MUT + NO vs PDZ2WT + NO, p=0.779). Data were analyzed with Mann-Whitney test.
Fig. 8.
Treatment with NO donor prevents ISO or PDZ2WT peptide induced impairment in acute recognition memory. Plots showing discrimination index as percent of time animals spent investigating novel object over the total time investigating novel and known objects multiplied by 100. There is no longer a significant difference between WT CON and WT ISO when NO donor is added (WT CON + NO (n=6) vs ISO + NO (n=7), p=0.073) or between PDZ2MUT and PDZ2WT (PDZ2MUT + NO (n=8) vs PDZ2WT + NO (n=7), p=0.778). Data from individual animals are plotted and color coded by gender (red=female and blue=male). Data were plotted as median and interquartile range. Data were analyzed with Mann-Whitney tests. Values were considered significant at *p < 0.05 or less.
Discussion
To better understand the mechanisms mediating ISO induced cognitive impairment we focused on one specific molecular target of anesthesia. Previously, we demonstrated that inhalational anesthetics can disrupt PDZ domain-mediated protein-protein interactions in vitro and in vivo 25, 26 and specifically inhibit the PDZ domain-mediated protein interaction between PSD-95 or PSD-93 and the NMDA receptor NR2 subunits or nNOS (fig. 1) 25, 27. Here, we determined the effects of this disruption in vivo in the context of ISO exposure by specifically mimicking this isolated action of anesthesia with the PDZ2WT peptide which disrupts PSD-PDZ2-mediated protein interactions by binding to interaction partners such as NMDA receptor NR2. In contrast to earlier work 3, we found that ISO exposure did not induce a detectable increase in the level of apoptosis nor did PDZ2WT peptide (fig. 2). However, our findings are consistent to recent findings of others that showed brief exposures to anesthesia did not induce apoptosis 44-46. Further investigation into the sublethal effects of ISO, PSD-93 deficiency, and disruption of PSD-95 PDZ2 domain-mediated protein-protein interactions revealed changes in spine morphology, impairments in LTP induction, and impairments in memory. Introduction of NO donor at the time of exposure prevents impairment in LTP and recognition memory further implicating the involvement of the NMDAR NR2-PSD-95 PDZ2-nNOS signaling pathway in early anesthetic exposure-produced cognitive impairment.
Alterations in spine number and shape are associated with cognitive and developmental dysfunction in various neurological disorders 47. Anesthesia exposure, nNOS activity, and modulation of MAGUK levels have all been associated with spine morphological and density changes 28, 48, 49. The most striking result in our spine analysis is the significant loss of long thin spines (length <2 microns and width <0.6 microns) in ISO exposed, PSD-93 null mutants, and PDZ2WT peptide exposed animals compared to controls that persists 14 days after exposure. Others have also shown selective loss of spines from anesthesia such as the persistent decrease (up to 90 days) in spine density of spines with head diameter between 0.3-0.4 microns 50. Length-shortening of dendritic protrusions following sevoflurane anesthesia 51 and reduction of long protrusions following ISO anesthesia 52, 53 in culture has been reported. Platholi specifically showed that ISO reduces F-actin concentration in spines suggesting a role in spine shrinkage and loss 54. Thus the loss of long thin spines we observe in our study could be the result of length-shortening following disruption of PDZ-domain mediated interactions that lead to F-actin depolymerization. Experiments are underway to determine if the specific PDZ-domain mediated interaction involves NMDAR NR2-PSD-95 PDZ2-nNOS or other pathway. The functional significance of this loss of long thin spines remains to be determined but might involve a role in circuitry development and permanently alter neural connectivity.
Anesthesia induced changes in dendritic spine density have been shown to be accompanied by parallel changes in spine synapse number 50, 52. We did not observe any change in density of synapses following ISO or PDZ2WT peptide exposure. Our results demonstrate that exposure of immature mice to a sublethal dose of anesthesia or PDZ2WT peptide at the peak of synaptogenesis did not cause significant differences in hippocampal ultrastructural synaptic density two-weeks later at PND21. Our findings are consistent with the lack of synaptic density change in the single 2-h anesthesia exposure observed in the Amrock study 55 and lack of synaptic density loss in PSD-95 null mutants 56. Follow up studies are underway to determine whether synaptic density changes occur following our exposure paradigm in mice allowed to recover for longer periods (i.e. >PND21).
To further explore whether the disruption of synaptic PDZ interactions could contribute to learning and memory deficits through altered synaptic function after early anesthetic exposure, we investigated LTP, a widely considered major cellular mechanism underlying learning and memory 57. Previous work demonstrated early exposure to a combination anesthetic induced a profound suppression of LTP in the hippocampus of adolescent rats 3. We found suppression of LTP in ISO exposed WT animals. Similar to Carlisle et al (2008) we found LTP to be impaired in PSD93 mutant mice. Like ISO exposed animals PDZ2WT peptide exposed mice showed impaired LTP as compared to controls. Thus disrupting PDZ domain-mediated protein interactions mimicked to the effect of ISO on LTP. These results suggest synaptic PDZ interactions may contribute to the mechanism underlying anesthesia induced impairment in synaptic function in mice and therefore could contribute to learning and memory deficits.
Over a decade ago Jevtovic-Todorovic et al (2003) first reported persistent impairments in learning and memory following early exposure to anesthetics in rats 3. Subsequent studies have linked early exposure to anesthesia to impairments on hippocampal dependent recognition memory tests in rodents 44, 58-60 and humans 61. Thus, we hypothesized that if disruption of synaptic PDZ interactions contribute to impairments in learning and memory after early anesthetic exposure we should see deficits in recognition memory performance. We found WT mice exposed to ISO exhibited reduced recognition memory performance as compared to controls as we expected. Injection of PDZ2WT peptide mimicked the effect of ISO reducing recognition memory performance as compared to inactive peptide. These results indicate that intact PSD-PDZ2-mediated protein interactions are important for hippocampal dependent recognition memory performance in weanling mice.
Several PDZ domain-mediated protein-protein interactions linking ion channels and receptors to their downstream signaling pathways have been shown to be disrupted by inhalational anesthetics. We have demonstrated that PDZ domain-mediated interactions between PSD-95 or PSD-93 and NMDA receptors or nNOS 25, PSD-95 and Shaker-type potassium channel Kv1.4 26, and between AMPAR subunit GluA2 and its interacting proteins-glutamate receptor interacting protein or protein interacting with c kinase 1 26 are disrupted by clinically relevant concentrations of anesthetics. We hypothesized that introduction of certain downstream signaling components during the time of ISO or PDZ2WT peptide exposure may prevent deficits in LTP and memory. Indeed, treatment with the NO donor, Molsidomine, prevents impairment in LTP and recognition memory caused by ISO or PDZ2WT peptide suggesting the effect of NO is downstream of the disrupted PDZ interactions. These results support the involvement of the NMDAR NR2-PSD-95 PDZ2-nNOS signaling pathway in ISO mediated impairment in LTP and recognition memory. A better understanding of how NO signaling affects changes in LTP and recognition memory in this developmental context is an important area to pursue.
This study was limited in several important respects. We did not phenotype animals on noncognitive behaviors so other aspects of neurodevelopment is undefined here. In vivo evidence suggests that hyperoxia may be harmful to developing neurons 62 and there is also the potential for enhanced toxicity by combining an inhalational anesthetic with 100% oxygen so our use of 100% oxygen as a carrier gas may be questioned. This is unlikely to explain our results, however, because control mice received 100% oxygen for the same period of time and peptide injected mice were not exposed to the oxygen carrier gas or to anesthetic and showed parallel results. The number of animals in our spine and synapse analyses are small. To reduce the effect of a small N we took multiple measurements per animal in an effort to lower variation (each animal provided one data point; 6 dendritic fields were averaged per animal in the spine analysis and 10 images were averaged per animal in the EM analysis). Our ultrastructural resolution was not high enough to permit length and width assessments on PSDs.
In conclusion, our findings indicate that a single 4-hr exposure of infant mice to ISO (~1 MAC) or targeted disruption of PSD-PDZ2-mediated protein interactions (i.e. a specific molecular target of ISO) with PDZ2WT peptide results in spine morphological changes, impairments in LTP induction, and impairments in memory in mice without inducing apoptosis or changes in synaptic density. The observed impairments in LTP and object recognition memory can be prevented by introduction of an NO donor suggesting the involvement of NMDAR NR2-PSD-95 PDZ2-nNOS signaling pathway in these processes.
What We Already Know about This Topic
Certain general anesthetics have been shown to have adverse effects on neuronal development including spinogenesis and synaptogenesis that affect neural function and cognitive behavior.
Clinically relevant concentrations of inhalational anesthetics inhibit the PDZ domain-mediated protein-protein interaction between PSD-95 or PSD-93 and NMDA receptors or nNOS.
What This Article Tells Us That Is New
Neonatal PSD-95 PDZ2WT peptide exposure mimics ISO (~1 MAC) by altering dendritic spine morphology, neural plasticity (i.e. LTP), and object recognition memory without inducing detectable increases in apoptosis or changes in synaptic density.
The results indicate a sublethal effect of a single dose of ISO (~1MAC) or PSD-95 PDZ2WT peptide on the developing brain that alters dendritic spine architecture and function important to cognition and that this impairment can be prevented by NO donor, Molsidomine.
Acknowledgements:
This work was supported by a grant (R01GM110674) to RAJ from the National Institutes of Health. Department of Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Funding Statement: This work was supported by a grant (R01GM110674) to RAJ from the National Institutes of Health, Bethesda, MD.
Footnotes
Clinical trial number and registry URL: Not applicable.
Prior Presentations: Title: Neonatal Disruption of PSD-95 PDZ Domain-mediated Protein-protein Interactions Alters Dendritic Spine Morphology, Long-term Potentiation, and Causes Deficits in Learning and Memory in Mice.
Presentation 1: Association of University Anesthesiologists (AUA) Annual Meeting: Moderated Poster Discussion Session I. Thursday, 2:00-3:00 pm, April 26, 2018. Chicago.
Presentation 2: Society of Critical Care Anesthesiologists (SOCCA) Annual Meeting: Moderated Poster Discussion Session. Friday, 3:00-3:45, April 27, 2018. Chicago.
Presentation 3: International Anesthesia Research Society (IARS) Annual Meeting: Moderated Poster Discussion Session-05. Sunday, 7:30-9:00 am, April 29, 2018. Chicago.
Presentation 4: IARS Annual Meeting: Kosaka Best of Meeting Abstract Awards Session Sunday, 9:30-11:00 am, April 29, 2018. Chicago.
Conflicts of Interest: The authors declare no competing interests.
Reference List
- [1].Jackson WM, Gray CD, Jiang D, Schaefer ML, Connor C, Mintz CD: Molecular Mechanisms of Anesthetic Neurotoxicity: A Review of the Current Literature. J Neurosurg Anesthesiol 2016, 28:361–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gentry KR, Steele LM, Sedensky MM, Morgan PG: Early developmental exposure to volatile anesthetics causes behavioral defects in Caenorhabditis elegans. Anesth Analg 2013, 116:185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF: Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 2003, 23:876–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW: Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology 2010, 112:834–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR, Warner DO: Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics 2011, 128:e1053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO: Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 2009, 110:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kuehn BM: FDA considers data on potential risks of anesthesia use in infants, children. JAMA 2011, 305:1749–50, 53. [DOI] [PubMed] [Google Scholar]
- [8].DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G: A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol 2009, 21:286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].DiMaggio C, Sun LS, Li G: Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg 2011, 113:1143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ing C, DiMaggio C, Whitehouse A, Hegarty MK, Brady J, von Ungern-Sternberg BS, Davidson A, Wood AJ, Li G, Sun LS: Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics 2012, 130:e476–85. [DOI] [PubMed] [Google Scholar]
- [11].Hu D, Flick RP, Zaccariello MJ, Colligan RC, Katusic SK, Schroeder DR, Hanson AC, Buenvenida SL, Gleich SJ, Wilder RT, Sprung J, Warner DO: Association between Exposure of Young Children to Procedures Requiring General Anesthesia and Learning and Behavioral Outcomes in a Population-based Birth Cohort. Anesthesiology 2017, 127:227–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Alvarado MC, Murphy KL, Baxter MG: Visual recognition memory is impaired in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br J Anaesth 2017, 119:517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Raper J, De Biasio JC, Murphy KL, Alvarado MC, Baxter MG: Persistent alteration in behavioural reactivity to a mild social stressor in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br J Anaesth 2018, 120:761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW: Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 1999, 283:70–4. [DOI] [PubMed] [Google Scholar]
- [15].Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V: Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience 2005, 135:815–27. [DOI] [PubMed] [Google Scholar]
- [16].Ma D, Williamson P, Januszewski A, Nogaro MC, Hossain M, Ong LP, Shu Y, Franks NP, Maze M: Xenon mitigates isoflurane-induced neuronal apoptosis in the developing rodent brain. Anesthesiology 2007, 106:746–53. [DOI] [PubMed] [Google Scholar]
- [17].Slikker W Jr., Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC, Patterson TA, Hanig JP, Paule MG, Wang C: Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci 2007, 98:145–58. [DOI] [PubMed] [Google Scholar]
- [18].Kahraman S, Zup SL, McCarthy MM, Fiskum G: GABAergic mechanism of propofol toxicity in immature neurons. J Neurosurg Anesthesiol 2008, 20:233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fredriksson A, Ponten E, Gordh T, Eriksson P: Neonatal exposure to a combination of N-methyl-D-aspartate and gamma-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology 2007, 107:427–36. [DOI] [PubMed] [Google Scholar]
- [20].Franks NP: General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 2008, 9:370–86. [DOI] [PubMed] [Google Scholar]
- [21].Solt K, Forman SA: Correlating the clinical actions and molecular mechanisms of general anesthetics. Curr Opin Anaesthesiol 2007, 20:300–6. [DOI] [PubMed] [Google Scholar]
- [22].Campagna JA, Miller KW, Forman SA: Mechanisms of actions of inhaled anesthetics. N Engl J Med 2003, 348:2110–24. [DOI] [PubMed] [Google Scholar]
- [23].Rudolph U, Antkowiak B: Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci 2004, 5:709–20. [DOI] [PubMed] [Google Scholar]
- [24].Hemmings HC Jr., Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL: Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci 2005, 26:503–10. [DOI] [PubMed] [Google Scholar]
- [25].Fang M, Tao YX, He F, Zhang M, Levine CF, Mao P, Tao F, Chou CL, Sadegh-Nasseri S, Johns RA: Synaptic PDZ domain-mediated protein interactions are disrupted by inhalational anesthetics. J Biol Chem 2003, 278:36669–75. [DOI] [PubMed] [Google Scholar]
- [26].Tao F, Chen Q, Sato Y, Skinner J, Tang P, Johns RA: Inhalational anesthetics disrupt postsynaptic density protein-95, Drosophila disc large tumor suppressor, and zonula occludens-1 domain protein interactions critical to action of several excitatory receptor channels related to anesthesia. Anesthesiology 2015, 122:776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tao F, Johns RA: Effect of disrupting N-methyl-d-aspartate receptor-postsynaptic density protein-95 interactions on the threshold for halothane anesthesia in mice. Anesthesiology 2008, 108:882–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nikonenko I, Boda B, Steen S, Knott G, Welker E, Muller D: PSD-95 promotes synaptogenesis and multiinnervated spine formation through nitric oxide signaling. J Cell Biol 2008, 183:1115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kornau HC, Schenker LT, Kennedy MB, Seeburg PH: Domain Interaction between Nmda Receptor Subunits and the Postsynaptic Density Protein Psd-95. Science 1995, 269:1737–40. [DOI] [PubMed] [Google Scholar]
- [30].Risher WC, Ustunkaya T, Singh Alvarado J, Eroglu C: Rapid Golgi analysis method for efficient and unbiased classification of dendritic spines. PLoS One 2014, 9:e107591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fiala JC: Reconstruct: a free editor for serial section microscopy. J Microsc 2005, 218:52–61. [DOI] [PubMed] [Google Scholar]
- [32].Clark RE, Zola SM, Squire LR: Impaired recognition memory in rats after damage to the hippocampus. J Neurosci 2000, 20:8853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Baker KB, Kim JJ: Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learn Mem 2002, 9:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Reger ML, Hovda DA, Giza CC: Ontogeny of Rat Recognition Memory measured by the novel object recognition task. Dev Psychobiol 2009, 51:672–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Taylor DL, Joashi UC, Sarraf C, Edwards AD, Mehmet H: Consequential apoptosis in the cerebellum following injury to the developing rat forebrain. Brain Pathol 2006, 16:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Martone ME, Jones YZ, Young SJ, Ellisman MH, Zivin JA, Hu BR: Modification of postsynaptic densities after transient cerebral ischemia: a quantitative and three-dimensional ultrastructural study. J Neurosci 1999, 19:1988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gonzalez-Burgos I, Velazquez-Zamora DA, Beas-Zarate C: Damage and plasticity in adult rat hippocampal trisynaptic circuit neurons after neonatal exposure to glutamate excitotoxicity. Int J Dev Neurosci 2009, 27:741–5. [DOI] [PubMed] [Google Scholar]
- [38].Pigott BM, Garthwaite J: Nitric Oxide Is Required for L-Type Ca(2+) Channel-Dependent Long-Term Potentiation in the Hippocampus. Front Synaptic Neurosci 2016, 8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J: Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience 2006, 137:413–23. [DOI] [PubMed] [Google Scholar]
- [40].Funke L, Dakoji S, Bredt DS: Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu Rev Biochem 2005, 74:219–45. [DOI] [PubMed] [Google Scholar]
- [41].Sheng M, Hoogenraad CC: The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem 2007, 76:823–47. [DOI] [PubMed] [Google Scholar]
- [42].Fiorentini M, Nielsen AK, Kristensen O, Kastrup JS, Gajhede M: Structure of the first PDZ domain of human PSD-93. Acta Crystallogr Sect F Struct Biol Cryst Commun 2009, 65:1254–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Carlisle HJ, Fink AE, Grant SG, O'Dell TJ: Opposing effects of PSD-93 and PSD-95 on long-term potentiation and spike timing-dependent plasticity. J Physiol 2008, 586:5885–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhu C, Gao J, Karlsson N, Li Q, Zhang Y, Huang Z, Li H, Kuhn HG, Blomgren K: Isoflurane anesthesia induced persistent, progressive memory impairment, caused a loss of neural stem cells, and reduced neurogenesis in young, but not adult, rodents. J Cereb Blood Flow Metab 2010, 30:1017–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kodama M, Satoh Y, Otsubo Y, Araki Y, Yonamine R, Masui K, Kazama T: Neonatal desflurane exposure induces more robust neuroapoptosis than do isoflurane and sevoflurane and impairs working memory. Anesthesiology 2011, 115:979–91. [DOI] [PubMed] [Google Scholar]
- [46].Briner A, De Roo M, Dayer A, Muller D, Habre W, Vutskits L: Volatile anesthetics rapidly increase dendritic spine density in the rat medial prefrontal cortex during synaptogenesis. Anesthesiology 2010, 112:546–56. [DOI] [PubMed] [Google Scholar]
- [47].Blanpied TA, Ehlers MD: Microanatomy of dendritic spines: emerging principles of synaptic pathology in psychiatric and neurological disease. Biol Psychiatry 2004, 55:1121–7. [DOI] [PubMed] [Google Scholar]
- [48].Vickers CA, Stephens B, Bowen J, Arbuthnott GW, Grant SG, Ingham CA: Neurone specific regulation of dendritic spines in vivo by post synaptic density 95 protein (PSD-95). Brain Res 2006, 1090:89–98. [DOI] [PubMed] [Google Scholar]
- [49].Nikonenko I, Jourdain P, Alberi S, Toni N, Muller D: Activity-induced changes of spine morphology. Hippocampus 2002, 12:585–91. [DOI] [PubMed] [Google Scholar]
- [50].Briner A, Nikonenko I, De Roo M, Dayer A, Muller D, Vutskits L: Developmental Stage-dependent persistent impact of propofol anesthesia on dendritic spines in the rat medial prefrontal cortex. Anesthesiology 2011, 115:282–93. [DOI] [PubMed] [Google Scholar]
- [51].Zimering JH, Dong Y, Fang F, Huang L, Zhang Y, Xie Z: Anesthetic Sevoflurane Causes Rho-Dependent Filopodial Shortening in Mouse Neurons. PLoS One 2016, 11:e0159637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Head BP, Patel HH, Niesman IR, Drummond JC, Roth DM, Patel PM: Inhibition of p75 neurotrophin receptor attenuates isoflurane-mediated neuronal apoptosis in the neonatal central nervous system. Anesthesiology 2009, 110:813–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lemkuil BP, Head BP, Pearn ML, Patel HH, Drummond JC, Patel PM: Isoflurane neurotoxicity is mediated by p75NTR-RhoA activation and actin depolymerization. Anesthesiology 2011, 114:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Platholi J, Herold KF, Hemmings HC Jr., Halpain S: Isoflurane reversibly destabilizes hippocampal dendritic spines by an actin-dependent mechanism. PLoS One 2014, 9:e102978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Amrock LG, Starner ML, Murphy KL, Baxter MG: Long-term effects of single or multiple neonatal sevoflurane exposures on rat hippocampal ultrastructure. Anesthesiology 2015, 122:87–95. [DOI] [PubMed] [Google Scholar]
- [56].Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, O'Dell TJ, Grant SG: Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature 1998, 396:433–9. [DOI] [PubMed] [Google Scholar]
- [57].Cooke SF, Bliss TV: Plasticity in the human central nervous system. Brain 2006, 129:1659–73. [DOI] [PubMed] [Google Scholar]
- [58].Shih J, May LD, Gonzalez HE, Lee EW, Alvi RS, Sall JW, Rau V, Bickler PE, Lalchandani GR, Yusupova M, Woodward E, Kang H, Wilk AJ, Carlston CM, Mendoza MV, Guggenheim JN, Schaefer M, Rowe AM, Stratmann G: Delayed environmental enrichment reverses sevoflurane-induced memory impairment in rats. Anesthesiology 2012, 116:586–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Stratmann G, Sall JW, Eger EI 2nd, Laster MJ, Bell JS, May LD, Eilers H, Krause M, Heusen F, Gonzalez HE: Increasing the duration of isoflurane anesthesia decreases the minimum alveolar anesthetic concentration in 7-day-old but not in 60-day-old rats. Anesth Analg 2009, 109:801–6. [DOI] [PubMed] [Google Scholar]
- [60].Stratmann G, May LD, Sall JW, Alvi RS, Bell JS, Ormerod BK, Rau V, Hilton JF, Dai R, Lee MT, Visrodia KH, Ku B, Zusmer EJ, Guggenheim J, Firouzian A: Effect of hypercarbia and isoflurane on brain cell death and neurocognitive dysfunction in 7-day-old rats. Anesthesiology 2009, 110:849–61. [DOI] [PubMed] [Google Scholar]
- [61].Stratmann G, Lee J, Sall JW, Lee BH, Alvi RS, Shih J, Rowe AM, Ramage TM, Chang FL, Alexander TG, Lempert DK, Lin N, Siu KH, Elphick SA, Wong A, Schnair CI, Vu AF, Chan JT, Zai H, Wong MK, Anthony AM, Barbour KC, Ben-Tzur D, Kazarian NE, Lee JY, Shen JR, Liu E, Behniwal GS, Lammers CR, Quinones Z, Aggarwal A, Cedars E, Yonelinas AP, Ghetti S: Effect of general anesthesia in infancy on long-term recognition memory in humans and rats. Neuropsychopharmacology 2014, 39:2275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Felderhoff-Mueser U, Bittigau P, Sifringer M, Jarosz B, Korobowicz E, Mahler L, Piening T, Moysich A, Grune T, Thor F, Heumann R, Buhrer C, Ikonomidou C: Oxygen causes cell death in the developing brain. Neurobiol Dis 2004, 17:273–82. [DOI] [PubMed] [Google Scholar]
- [63].Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M: Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science 1999, 284:1845–8. [DOI] [PubMed] [Google Scholar]
- [64].Tao F, Su Q, Johns RA: Cell-permeable peptide Tat-PSD-95 PDZ2 inhibits chronic inflammatory pain behaviors in mice. Mol Ther 2008, 16:1776–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M: Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science 2002, 298:846–50. [DOI] [PubMed] [Google Scholar]