Abstract
PURPOSE OF REVIEW:
Early-onset Alzheimer disease (AD) is defined as having an age of onset younger than 65 years. While early-onset AD is often overshadowed by the more common late-onset AD, recognition of the differences between early- and late-onset AD is important for clinicians.
RECENT FINDINGS:
Early-onset AD comprises about 5% to 6% of cases of AD and includes a substantial percentage of phenotypic variants that differ from the usual amnestic presentation of typical AD. Characteristics of early-onset AD in comparison to late-onset AD include a larger genetic predisposition (familial mutations and summed polygenic risk), more aggressive course, more frequent delay in diagnosis, higher prevalence of traumatic brain injury, less memory impairment and greater involvement of other cognitive domains on presentation, and greater psychosocial difficulties. Neuroimaging features of early-onset AD in comparison to late-onset AD include greater frequency of hippocampal sparing and posterior neocortical atrophy, increased tau burden, and greater connectomic changes affecting frontoparietal networks rather than the default mode network.
SUMMARY:
Early-onset AD differs substantially from late-onset AD, with different phenotypic presentations, greater genetic predisposition, and differences in neuropathologic burden and topography. Early-onset AD more often presents with nonamnestic phenotypic variants that spare the hippocampi and with greater tau burden in posterior neocortices. The early-onset AD phenotypic variants involve different neural networks than typical AD. The management of early-onset AD is similar to that of late-onset AD but with special emphasis on targeting specific cognitive areas and more age-appropriate psychosocial support and education.
INTRODUCTION
Historically, Alzheimer disease (AD) was initially characterized as a neurodegenerative disorder presenting in early life or midlife, with onset at younger than 65 years of age (what is now called early-onset AD). Alois Alzheimer originally described the extracellular amyloid-positive neuritic plaques and intracellular tau-positive neurofibrillary tangles of AD in the brain of a 51-year-old woman with memory and language impairments and behavioral changes.1 In the late 1960s and early 1970s, investigators stressed the presence of similar neuritic plaques and neurofibrillary tangles in elderly individuals with dementia, thus shifting the Alzheimer focus to the far-larger numbers of patients with late-onset AD. Despite being overshadowed by late-onset AD, patients with early-onset AD (about 5% to 6% of all those with AD2) are significantly different in their clinical and neurobiological features and require different management strategies. Some investigators even suggest that these differences are sufficient to constitute a distinct form of the disorder.3
Early-onset AD is defined as AD with clinical onset younger than 65 years of age; it is the most common cause of early-onset neurodegenerative dementia.4 Early-onset AD accounts for at least one-third of patients with young-onset dementia, with the rest having vascular cognitive impairment, frontotemporal dementia, drug-related conditions, Lewy body disease, or autoimmune or infectious causes. In those 45 to 64 years of age, early-onset AD has an incidence rate of about 6.3 per 100,000 per year5 and a prevalence rate of about 24.2 per 100,000,6 with 2006 estimates of between 220,000 and 640,000 people with early-onset AD in the United States.7 Even within this early-onset group, AD risk still increases with age, with the number of cases of early-onset AD rising exponentially as the age of onset approaches 65.8
CLINICAL FEATURES
Many differences exist between early-onset and late-onset AD (TABLE 2-19). Early-onset AD is not just AD at a younger age. First, early-onset AD has a significant genetic predisposition involving a direct autosomal dominant transmission in a subgroup and a higher polygenic susceptibility in general. Patients with an autosomal dominant familial form of early-onset AD not only have an increased risk for the development of AD among relatives, but may also have atypical clinical features, including headaches, myoclonus, seizures, gait abnormalities, pseudobulbar palsy, or hyperreflexia.10,11 Second, overall deterioration is faster in early-onset AD as compared to late-onset AD (not related to APOE).12 Several studies indicate that patients with early-onset AD have a potentially more aggressive clinical course.13–15 After controlling for the direct effects of aging on mortality, patients with early-onset AD are at a greater risk for mortality compared to those with late-onset AD,16 and early-onset AD accounts for a large number of premature deaths among those 40 to 64 years of age.17 Third, patients with early-onset AD have a longer duration of the disease before diagnosis (about 1.6 years),12,18 probably reflecting missed or delayed diagnosis or a greater extent of evaluation for diagnosis.19 Fourth, patients with early-onset AD are more likely to have a history of traumatic brain injury as a risk factor for dementia.20 However, patients with early-onset AD have decreased cerebrovascular risk factors, circulatory problems, diabetes mellitus, and obesity than those with late-onset AD.10,21,22
TABLE 2-1.
Characteristics of Early-onset Alzheimer Disease Compared to Late-onset Alzheimer Diseasea
|
Data from Mendez MF, Neurol Clin.9
Perhaps most important, the overall clinical profile of early-onset AD differs from that of late-onset AD. A high proportion of patients with early-onset AD present with language, visuospatial, or dysexecutive phenotypes rather than the usual amnestic disorder seen in late-onset AD.23 In the aggregate, patients with early-onset AD, compared to similarly impaired patients with late-onset AD, have better memory recognition scores and semantic memory but worse attention, language, executive functions, ideomotor praxis, and visuospatial skills.23–26
NEUROIMAGING AND CEREBROSPINAL FLUID BIOMARKERS
MRI of patients with early-onset AD demonstrates more widespread cortical atrophy, particularly in the parietal cortex, compared to patients with late-onset AD, in which the atrophy is more limited to temporal regions.25 Compared to late-onset AD, early-onset AD shows larger sulcal widths in the temporoparietal cortex and less volume loss in hippocampi.27–29 In early-onset AD, prominent atrophy of the posterior cingulate cortex, compared to the prominent atrophy of the medial temporal lobe in late-onset AD, is consistent with early involvement of distinct intrinsic connectivity networks (FIGURE 2-1).26 Resting-state functional MRI (fMRI) findings support this conclusion, with smaller functional changes of the cortical regions connected to the hippocampus in early-onset AD compared to late-onset AD.30
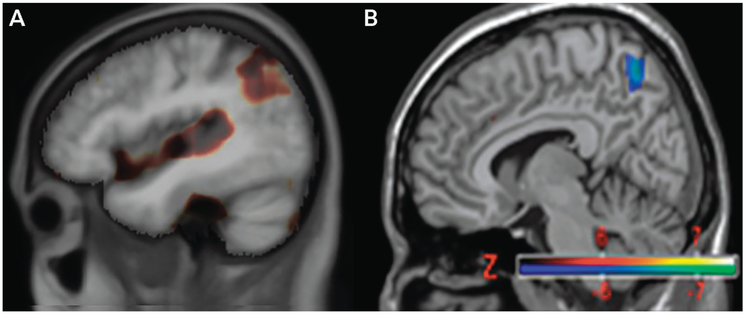
FIGURE 2-1.
Tensor-based morphometry studies of MRI (A) and fludeoxyglucose positron emission tomography (FDG-PET) overlaid on normalized T1-weighted MRI (B) showing subtraction of late-onset Alzheimer disease (AD) changes from those for early-onset AD. Both neuroimaging techniques reveal greater involvement of parietal regions in early-onset AD (brown on MRI, blue on FDG-PET).31 Greater precuneus/parietal atrophy (A) and hypometabolism (B) are seen in early-onset AD compared to with late-onset AD, indicating differences in the distribution of neuropathology.
Consistent with the MRI findings, fludeoxyglucose positron emission tomography (FDG-PET) shows greater parietal hypometabolism in early-onset AD compared to greater bilateral temporal hypometabolism in late-onset AD.31,32 The parietal hypometabolism in early-onset AD involves the temporoparietal junctions and the posterior cingulate cortex.25 FDG-PET hypometabolism is more concentrated in paralimbic regions in amnestic early-onset AD.33 FDG-PET further suggests dysfunction in the salience network among patients with early-onset AD with behavioral disturbances.34 Those with language and dysexecutive presentations have hypometabolism in the corresponding areas.
Recent developments in amyloid and tau PET imaging may revolutionize the evaluation of patients with early-onset AD. Since amyloid positivity increases with age,35 amyloid PET is particularly useful in the differential diagnosis of early-onset AD, in which it usually correlates with low CSF amyloid-β.36 Amyloid PET imaging with flutemetamol demonstrated positive scans in most patients with a pre-PET diagnosis of early-onset AD. Using amyloid PET in this population changed diagnosis in about one-fifth of patients, altered management in more than one-third of patients, and led to a nearly 20% mean increase in diagnostic confidence.37 Similar to neuropathologic studies, the relationship between the location of amyloid burden on PET does not tightly correspond to clinical symptoms, although amyloid imaging with Pittsburgh compound B (PiB) may show greater retention in the bilateral thalamus and some parts of the globus pallidus in early-onset AD compared to late-onset AD.38 However, tau PET signal better correlates with clinical symptoms.39–41 Using [18F]flortaucipir tau imaging, studies show significantly greater uptake in early-onset AD compared to late-onset AD, not only throughout the neocortex but also in prefrontal and premotor cortices as well as in the inferior parietal cortex, compared to higher [18F]flortaucipir retention in temporal lobe regions in late-onset AD.42 Tau PET regional uptake patterns also correspond to phenotypic variants and cortical atrophy patterns, with greater tau binding in the left anterior superior temporal gyrus with language dysfunction and in the parietooccipital cortex and right lingual gyrus with visuospatial dysfunction.43,44
Similar to typical late-onset AD, CSF analysis in early-onset AD shows the typical low amyloid-β1-42 and high total tau and phosphorylated tau levels that characterize AD but with some variations.45 There appears to be a better correspondence of decreased amyloid-β1-42 levels versus increased tau levels with gray matter atrophy in early-onset AD compared to late-onset AD.45 Some studies suggest lower tau levels among the phenotypic variants, particularly posterior cortical atrophy.46 Additionally, a disproportionate decrease in amyloid-β1-43, rather than the usual amyloid-β1-42, levels may be seen,47 suggesting differential deposition of amyloid-β peptides in early-onset AD compared to late-onset AD.
GENETICS
When considering early-onset AD, clinicians are often concerned with a familial form of the disease. Indeed, some, but not all, investigators have considered a PSEN1 mutation in Auguste Deter, Alzheimer’s original patient.48 In actuality, the vast majority of patients with early-onset AD have a nonfamilial, or sporadic, form.49 Only approximately 11% of those with early-onset AD (about 0.6% of the total of all patients with AD, including the late-onset form) have familial AD associated with one of the three known autosomal dominant mutations: amyloid precursor protein (APP), presenilin 1 (PSEN1), or presenilin 2 (PSEN2).50 Studies screening for these genes in patients with early-onset AD report a prevalence of 0.8% for APP, 1.1% for PSEN1, and as high as 13% for potential pathogenic variants of PSEN2, which can present even after age 65.51–53 These forms of familial AD are usually inherited from a parent with a similar age of onset of early-onset AD, generally in the forties or fifties (having a positive family history of late-onset AD has no effect).51 These three pathogenic mutations, which lead to aberrant cleavage or aggregation of the amyloid precursor protein, result in the more typical amnestic AD but can have diagnostic features such as spastic paraparesis, early myoclonus, seizures, dysarthria, pseudobulbar affect, more extensive amyloid angiopathy, and atypical amyloid plaque morphology and distribution.54 Some PSEN1 mutations (such as A79V) can be variable and sometimes mild, with ages of onset ranging from 53 to 84.55
In addition to the familial AD genes, a polygenic risk for AD exists from a number of susceptibility genes. Compared to patients with late-onset AD, those with early-onset AD have a higher frequency of two apolipoprotein E (APOE) ε4 alleles12; APOE is the main AD susceptibility gene, and the presence of an APOE ε4 allele reportedly accounts for about 9.12% of the heritability of early-onset AD.56 The APOE gene regulates the lipoprotein metabolism that binds soluble amyloid-β and influences its clearance and aggregation. The presence of an APOE ε4 allele is associated with extensive white matter disruption; the absence of any ε4 allele is associated with more focally posterior gray matter changes in the neocortex.57 For typical amnestic AD, the presence of an ε4 allele decreases the age of onset,58 whereas ε3 alleles tend to be found in variant phenotypes (language, visuospatial) of early-onset AD. Beyond APOE, recent whole-genome and whole-exome studies indicate more than 20 loci for genes involved in different metabolic pathways, each of which adds a small contributing risk for early-onset AD.56,59 These include sortilin related receptor 1 (SORL1); the anti-inflammatory microglial triggering receptor expressed on myeloid cells 2 (TREM2)50; ATP binding cassette subfamily A member 7 (ABCA7); and others involved in endocytosis or endolysosomal transport, immunologic reactivity, and lipid metabolism (eg, PLD3, PSD2, TCIRG1, RIN3, and RUFY1).49 An important development is the combination of these genes into a polygenic risk score, which may predict the occurrence of early-onset AD with accuracies of 72.9% to 75.5%.60
NEUROPATHOLOGY
Both early-onset AD and late-onset AD have neuritic plaques and neurofibrillary tangles, but patients with early-onset AD have greater tau burdens in parietal and precuneus regions and, to a lesser extent, the frontal cortex than patients with late-onset AD.23,61 Early-onset AD has faster atrophy rates in the bilateral precuneus and parietal and superior temporal lobes independent of white matter changes or APOE status.62 Patients with early-onset AD are more likely to have hippocampal sparing, particularly among the nonamnestic variants, and they have more involvement of the corresponding neocortex.61,63 It is possible that the neuropathologic cascade in early-onset AD variants is initiated through deposition of different strains of soluble oligomeric amyloid-β. Nevertheless, tau and neurofibrillary tangles, more than amyloid-β1-42 and neuritic plaques, correspond with the features of early-onset AD, with a relatively greater tau burden in early-onset AD than in late-onset AD. Whereas the distribution of amyloid is diffuse, a significant focal correlation exists between the location and density of neurofibrillary tangles and cognitive symptoms, cerebral blood flow, and atrophy or functional changes in early-onset AD.64 Early-onset AD variants also develop greater white matter involvement and selective vulnerability of long projection neurons that involve posterior cingulate and parietal regions and mediate frontoparietal functions rather than the mesial temporal involvement of late-onset AD.65
PHENOTYPIC VARIANTS OF EARLY-ONSET ALZHEIMER DISEASE
Nonamnestic variant presentations are particularly characteristic of early-onset AD. No universally accepted classification of the phenotypic variants has been established. Commonly accepted variants are those that present with language impairment (known as logopenic variant primary progressive aphasia [PPA]); those that present with visuospatial or visuoperceptual impairments (known as posterior cortical atrophy); frontal or behavioral/executive variants; and a number of parietal syndromes, such as the acalculia variant of early-onset AD.66–71 In addition, patients who meet criteria for corticobasal syndrome, with progressive limb apraxia and asymmetric motor changes, have AD in up to 25% at autopsies,72 indicating another phenotypic variant of early-onset AD. These variants, which account for one-fifth to two-thirds of those with sporadic early-onset AD,73 overlap with one another but, as a group, are distinct from the earliest typical amnestic forms of AD (FIGURE 2-274). Compared to the more typical amnestic form of early-onset AD, the phenotypic variants are more likely to be hippocampal sparing and develop prominent early posterior neocortical neurofibrillary tangles.61 The phenotypic variants appear to have early involvement of frontoparietal networks rather than the decreased posterior default mode network and medial temporal lobe-hippocampal connectivity of typical amnestic AD.75,76 Also, unlike amnestic early-onset AD, the phenotypic variants tend to be less associated with APOE ε4.74,77
FIGURE 2-2.
Phenotypic variants of early-onset Alzheimer disease compared to typical amnestic Alzheimer disease across the age spectrum. The different colored lines in the graph are meant to represent the probable distributions of different phenotypic variants of early-onset Alzheimer disease, such as posterior cortical atrophy, logopenic variant primary progressive aphasia, the behavioral/dysexecutive variant, and the acalculic variant.
Modified with permission from van der Flier WM, et al, Lancet Neurol.74 © 2010 Elsevier Ltd.
Logopenic Variant Primary Progressive Aphasia
The most common phenotypic variant of early-onset AD presents with a progressive decline in language with relatively spared memory and cognition due to focal Alzheimer neuropathology in temporoparietal language areas in the left hemisphere, especially the superior/midtemporal gyrus, angular gyrus, and midfrontal cortex (TABLE 2-2).66,78 This progressive aphasia has “logopenic” aspects, including word-finding difficulty with hesitations or false starts and repetition difficulty for both long sentences and forward digit span (CASE 2-1).79 In logopenic variant PPA, left temporoparietal degeneration disturbs the phonologic loop of verbal working or echoic memory,80 resulting in an impaired phonologic buffer for holding words or digits online, hence the characteristic inability to repeat long sentences. In addition, a history of dyslexia is common among patients with logopenic variant PPA,65 suggesting a preexisting vulnerability in language networks. Other forms of PPA, such as the nonfluent/agrammatic and semantic variants, are non-Alzheimer syndromes due to frontotemporal lobar degeneration. The presence of some degree of difficulty in episodic memory and visuospatial skills helps distinguish logopenic variant PPA from these other PPAs.
TABLE 2-2.
Characteristics of Logopenic Variant Primary Progressive Aphasia
|
CASE 2-1.
A 53-year-old woman presented with a 2-year history of a progressive decline in her ability to find words and pronounce them correctly. She also could not repeat or understand sentences when they were too long. She underwent an evaluation before presenting to clinic, which included an unremarkable MRI of the brain, normal routine laboratory results, and neuropsychological testing that showed normal intellectual abilities except for a decline in verbal fluency, with more minor changes in auditory attention and visual memory.
On examination, she had a Mini-Mental State Examination (MMSE) score of 17/30. She could not do the serial reversals and could not come up with the word watch. Memory examination was intact on delayed recall; however, her language examination was quite abnormal. She showed word-finding pauses, hesitations, and frequent phonemic paraphasic errors but no agrammatism or difficulty comprehending single words or simple commands. However, she had prominent difficulty with repetition, quickly breaking down if a sentence was more than a few words. She was unable to give the names of more than seven animals in 1 minute or name more than seven of 20 objects. On the naming task, she made phonemic paraphasic errors, such as Campbell for camel and volcan for volcano. The rest of the neurologic examination was normal.
The workup included fludeoxyglucose positron emission tomography (FDG-PET) imaging that showed asymmetric hypometabolism in the temporoparietal regions, worse on the left. In addition to treatment for Alzheimer disease with an acetylcholinesterase inhibitor and memantine, the patient underwent an extended and targeted program of speech therapy.
comment. This case is a typical example of logopenic variant primary progressive aphasia, with initial word-finding difficulty, phonemic paraphasic errors but not agrammatism, and prominent difficulty with repetition but intact single-word and short-phrase comprehension. The vast majority of patients who present with this progressive aphasia prove to have Alzheimer disease on neuropathology.
In logopenic variant PPA, neuroimaging and CSF studies usually reveal abnormalities consistent with early-onset AD. Neuroimaging shows focal atrophy and decreased metabolism in the left temporoparietal junction.81 Word-finding difficulty and anomia in logopenic variant PPA correlate with thinning in the left superior temporal gyrus,82 which is less responsive to some characteristics of auditory speech,83 and early memory impairment may also be due to this nonhippocampal temporal involvement.84 The analysis of white matter tracts and connections in logopenic variant PPA reveals bilateral but predominantly left-sided alterations in frontoparietal pathways (FIGURE 2-3)80,85,86 less involvement of the hippocampal-associated default mode network that is associated with episodic memory impairment in typical Alzheimer disease. Amyloid PET scans are positive in about 85% of patients diagnosed with logopenic variant PPA, compared to only 13% to 27% in other forms of PPA,87,88 and prominent FDG-PET hypometabolism of the left temporoparietal region is seen in patients with amyloid-positive logopenic variant PPA.89 On tau PET imaging, patients with logopenic variant PPA have increased uptake throughout the cortex, but particularly temporoparietal, in comparison to not only healthy controls but also patients with other PPA variants.90 In logopenic variant PPA, this regional tau deposition in the left inferior parietal lobule is more closely linked to hypometabolism than to amyloid density.91 Finally, in the CSF, low amyloid-β1-42 and high tau levels are seen in about 75% of patients diagnosed with logopenic variant PPA.92
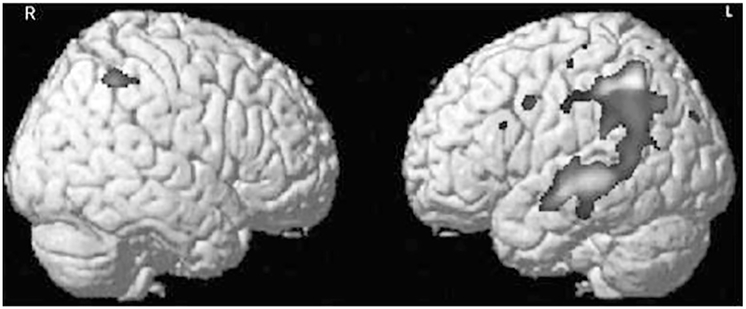
FIGURE 2-3.
Voxel-based morphometry of parietal overlap of early-onset Alzheimer disease phenotypes, showing cortical atrophy in 10 patients with logopenic variant primary progressive aphasia compared to 64 normal controls. These two composite images show the pattern of atrophy in this aphasic variant of Alzheimer disease when subtracted from the healthy controls. The dark regions show the foci of greater atrophy in logopenic variant primary progressive aphasia asymmetrically affecting the left parietal and posterior temporal region in the left hemisphere with much less involvement of the right hemisphere. The composite images particularly illustrate how this variant of Alzheimer disease results from asymmetric involvement of the left hemisphere.
Courtesy of Maria Luisa Gorno-Tempini, MD, PhD, University of California, San Francisco.
Posterior Cortical Atrophy
The second most common early-onset AD variant is posterior cortical atrophy, a syndrome characterized by progressive and disproportionate loss of visuospatial or visuoperceptual functions, usually due to Alzheimer neurodegeneration of posterior cortical regions. Patients with posterior cortical atrophy usually present with visuospatial problems in reading, manipulating or finding objects, navigating their surroundings, getting dressed, and driving. These visual symptoms frequently lead to consultations with optometrists or ophthalmologists before the suspicion is raised that the problem is from the visual brain and not the eyes. On neurologic examination, patients with posterior cortical atrophy prove to have a range of visuospatial or visuoperceptual difficulties up to and including Balint syndrome along with associated regional neurocognitive features.68,93 For more information, refer to the article “Posterior Cortical Atrophy” by Jonathan M. Schott, BSc, MD, FRCP, FEAN, SFHEA, and Sebastian J. Crutch, PhD, CPsych,94 in this issue of Continuum.
Other Variants
Beyond logopenic variant PPA and posterior cortical atrophy, the most common early-onset AD variant phenotypes, several other less recognized variants exist. Frontal variant AD, now known as behavioral/dysexecutive AD,69 presents with features suggestive of frontotemporal lobar degeneration (TABLE 2-3). Most commonly, patients with behavioral/dysexecutive AD have early apathy (or, more correctly, abulia), with decreased initiation or completion of productive behavior (CASE 2-2). Up to half, however, can have positive behaviors, notably disinhibition and impulsivity, which can contribute to a misdiagnosis of clinically possible behavioral variant frontotemporal dementia. However, when carefully evaluated, behavioral/dysexecutive AD is distinguishable from behavioral variant frontotemporal dementia because of the presence of prominent dysexecutive features accompanied by evidence of memory impairment.69 Neuroimaging in behavioral/dysexecutive AD shows significant involvement of bilateral temporoparietal regions along with milder involvement in the frontal cortex, especially noteworthy on FDG-PET.69
TABLE 2-3.
Characteristics of Behavioral/Dysexecutive Alzheimer Disease Compared to Other Dementiasa
| Clinical Features |
Typical Amnestic Alzheimer Disease |
Behavioral Early-onset Alzheimer Disease |
Dysexecutive Early-onset Alzheimer Disease |
Behavioral Variant Frontotemporal Dementia |
|---|---|---|---|---|
| Phenotype variant | Memory predominant | A behavioral predominant subtype most commonly presents with apathy | A dysexecutive predominant subtype most often presents with decreased organization and executive functioning | Behavioral |
| APOE ε4 positive | Frequent (~60%) | Frequent (59.5%) | Intermediate (40.0%) | Rare (<20%) |
| Age at onset | Late onset | Early onset | Early onset | Early onset |
| First symptom | Cognitive (memory) | Cognitive > behavioral | Cognitive (executive) | Behavioral |
| Episodic memory | Impaired | Impaired | Relatively spared | Typically spared |
| Executive function | Relatively spared | Impaired | Very impaired | Impaired |
| Behavior | Relatively spared | Impaired | Mostly spared | Impaired |
| MRI atrophy | Medial temporal, temporoparietal | Temporoparietal | Temporoparietal | Frontal and anterior temporal |
Reprinted with permission from Ossenkoppele R, et al, Brain.69 © 2015 The Authors.
CASE 2-2.
A 64-year-old man presented with several years of declining activity and failure to initiate and complete work tasks. The patient would often just sit at his desk. The patient additionally reported a significant decline in his memory. His wife described him as unable to “think properly,” reflected in wrong business decisions or judgments. She also reported he tended to eat the same foods, but he showed no inappropriate or compulsive behaviors. The patient and his wife denied that he was primarily depressed or that he was nonempathic or emotionally disengaged.
On the Mini-Mental State Examination (MMSE), he scored 28/30. In general, he had decreased spontaneous behavior and showed perseverations. Language was fluent, but he only generated four words beginning with the letter F in 1 minute, compared to 13 animal names. Memory testing was 5/10 in delayed recall. On visuospatial construction, he was able to copy intersecting pentagons but had difficulty with more complex constructions. The patient gave concrete interpretations of idioms and proverbs and had difficulty with the Luria hand sequences (repetitive fist-edge-palm motor sequences), making two mistakes in six movements. The rest of the neurologic examination was normal.
Brain MRI suggested more frontal than parietal atrophy; however, fludeoxyglucose positron emission tomography (FDG-PET) showed prominent bilateral temporoparietal hypometabolism with further involvement of the frontal lobes. After follow-up and reassessment of a progressive cognitive decline in memory and visuospatial skills, he was diagnosed with behavioral/dysexecutive early-onset Alzheimer disease (AD) and treated accordingly.
comment This case is a typical example of the behavioral/dysexecutive variant of early-onset AD, with prominent early apathy or abulia, decreased executive functioning without the entire range of abnormal behaviors characteristic of behavioral variant frontotemporal dementia, and memory and imaging evidence of early-onset AD.
Other phenotypic variants of early-onset AD have prominent parietal lobe symptoms and signs, exemplified by the acalculia variant from early Alzheimer neuropathology in the left inferior parietal lobule, particularly the intraparietal sulcus (TABLE 2-4).71 Patients with the acalculia variant of early-onset AD present with predominant and progressive difficulty with calculations out of proportion to any other cognitive impairment (CASE 2-3). Their acalculia is consistent with a primary acalculia (anarithmia) not explained by language or visuospatial impairments. Many also have anomia, ideomotor apraxia, and a complete Gerstmann syndrome, changes reflective of inferior parietal lobular dysfunction. Neuroimaging generally shows biparietal involvement, greater on the left. These patients overlap with or may eventually meet current criteria for posterior cortical atrophy or corticobasal syndrome.
table 2-4.
Characteristics of the Acalculia Variant of Early-onset Alzheimer Disease
| Acalculia |
|
| Other Elements of Gerstmann Syndrome |
|
| Alexia With Agraphia (Left) |
| Ideomotor Apraxia |
|
| Disorders of the Dorsal Visual Stream |
|
CASE 2-3.
A 62-year-old woman presented with several years of progressive difficulty with mathematics, which she used in her job. She first noticed an inability to do very simple calculations in her head as well as problems with using a tape measure and reading analog clocks or watches. The patient could not write a check or manage her checkbook and finances. She had also lost understanding of distances, such as from New York to Los Angeles. Other than some visuospatial difficulty, the patient had limited cognitive difficulty.
On examination, she had a Mini-Mental State Examination (MMSE) score of 27/30, intact language, and mildly impaired memory. However, she could not do simple written calculations, made errors in mathematical syntax in borrowing and carrying over, had transcoding difficulty (Arabic numerals to written form or vice versa), and had overall difficulty in applying numeric concepts. There were less prominent visuospatial difficulties in constructions and in interpreting overlapping or crosshatched figures. The neurologic examination was otherwise normal.
Brain MRI was nondiagnostic, but fludeoxyglucose positron emission tomography (FDG-PET) showed parietal hypometabolism, and an amyloid PET scan was positive. Treatment included donepezil (10 mg/d), memantine XR (28 mg/d), and an antidepressant for both depression and anxiety.
comment This case is a typical example of the acalculic variant of early-onset Alzheimer disease presenting with disproportionate and progressive difficulty in all aspects of numbers and calculations.
MANAGEMENT
The management of patients with early-onset AD is, in many ways, identical to the management of late-onset AD, but it differs in several respects. Acetylcholinesterase inhibitor medications, such as donepezil, galantamine, and rivastigmine, are indicated in the management of patients with early-onset AD, with the usual titration schedules. These medications, although they target memory, may also help some patients with logopenic variant PPA, posterior cortical atrophy, behavioral/dysexecutive AD, acalculia, and other variants. In these early-onset AD phenotypes, clinicians should carefully monitor response as these medications could exacerbate some behaviors. In one study, the cognitive and global responses to acetylcholinesterase inhibitor treatment and the longitudinal outcomes after 3 years did not differ between patients with early-onset AD and patients with late-onset AD.12 Another study of acetylcholinesterase inhibitors that evaluated 6-month functional responses, 3-year changes in activities of daily living, times to nursing home placement, and subsequent survival times also failed to find differences between patients with early-onset AD and patients with late-onset AD.95 Limited information is available on the use of memantine, but the aforementioned studies do not suggest a difference depending on age of onset of AD. Finally, many patients and families are eager to participate in clinical drug trials.
The management of early-onset AD may differ from late-onset AD when targeting the management of specific cognitive and behavioral deficits. For example, patients with logopenic variant PPA should have speech therapy assessment and, wherever possible, a dedicated course of therapy to improve their communication. Patients with posterior cortical atrophy benefit from techniques and services for those who are partially sighted. Patients with behavioral/dysexecutive AD may require use of psychoactive medications to manage egregious behaviors, when present. Those with the acalculia variant can benefit from the use of electronic calculation devices and those with corticobasal syndrome from referral to occupational therapists for help with ideomotor apraxia.
An important part of assessing patients with early-onset AD is determining their family history and the need for genetic testing and genetic counseling. If a family history of early-onset AD exists, genetic counseling should occur before performing genetic analysis for the PSEN1, PSEN2, and APP genes. In the presence of familial AD, implications exist for other family members, including the possibility of recognizing presymptomatic carriers of these genes, with corresponding implications for their psychological well-being and future. Currently, the polygenic risk score remains a research tool, but it may become available for increasing diagnostic certainty in diagnosing early-onset AD.
Another area that is disproportionately important in the management of early-onset AD is the provision of age-appropriate psychosocial support. Patients with early-onset AD are often in the time of life when they are most productive and in the midst of careers and families. Early-onset AD is more often associated with a sense of an unexpected loss of independence in midlife; anticipatory grief about the future; and difficulty with continued work, financial, and family responsibilities than late-onset AD.96 Moreover, patients and families need information and education on this form of AD and what it means in someone who is middle-aged or relatively young. Compared to patients with late-onset AD, patients with early-onset AD often have higher levels of disease awareness and early generalized anxiety97 with a potentially increased risk of suicide.98 Special efforts are required to provide psychological or psychiatric support and utilize local age-appropriate support groups and community resources. In many situations, support groups and other services for the elderly may not be the best fit for these patients and families. It is most helpful when support groups and services are specifically targeted to the particular phenotypic variant.
CONCLUSION
Early-onset AD differs significantly from late-onset AD, particularly in the substantial percentage of patients with early-onset AD who have phenotypic variants as well as the larger percentage of those with a familial form of the disease. Compared to typical late-onset AD, early-onset AD variants have hippocampal sparing, focal parietal and other neocortical involvement with a range of syndromes, more regional amyloid and tau accumulation, and evidence of an alternative frontoparietal network spread of the disease.61,85,99 These variants tend to differ as a group from typical amnestic AD, with a converging pattern of atrophy as the disease advances,100 as evidenced by neuroimaging studies showing overlap in posterior neocortical involvement rather than the mesial temporal cortical changes of typical amnestic AD.81,101 Recognition of early-onset AD and its variants as a specific group of dementias facilitates not only neurobiological understanding of early-onset AD but also the unique differences and issues involved in the management of these patients and helping their caregivers and families.
KEY POINTS.
Early-onset Alzheimer disease, which makes up about 5% to 6% of all cases of Alzheimer disease, is distinct from late-onset Alzheimer disease in a number of clinical, genetic, neurobiological, and management features.
Early-onset Alzheimer disease is the most common cause of early-onset neurodegenerative dementia.
Many clinical, neuropathologic, and management differences exist between early-onset and late-onset Alzheimer disease.
One major difference between early-onset and late-onset Alzheimer disease is that one-third or more of patients with early-onset Alzheimer disease present with language, visuospatial, or other phenotypes rather than the usual amnestic disorder seen in late-onset Alzheimer disease.
MRI of patients with early-onset Alzheimer disease shows more widespread cortical atrophy, particularly in the parietal cortex, compared to the more limited atrophy affecting temporal regions in patients with late-onset Alzheimer disease.
Fludeoxyglucose positron emission tomography shows greater parietal hypometabolism in early-onset Alzheimer disease compared to greater bilateral temporal hypometabolism in late-onset Alzheimer disease.
Amyloid positron emission tomography is positive in most patients with early-onset Alzheimer disease who would not be expected to have age-associated brain amyloid deposition and can be useful in diagnosis of the disorder.
Tau positron emission tomography has promise for future use in early-onset Alzheimer disease, particularly in correlating localization of changes with clinical symptoms.
CSF analysis in early-onset Alzheimer disease is similar to late-onset Alzheimer disease, showing the characteristic low amyloid-β1-42 and high total tau and phosphorylated tau levels but with some variations.
The vast majority of patients with early-onset Alzheimer disease have a nonfamilial, or sporadic, form.
Only 11% or less of those with early-onset Alzheimer disease (about 0.6% of the total of all patients with Alzheimer disease of any age) have familial Alzheimer disease associated with one of the three known autosomal dominant mutations in APP, PSEN1, or PSEN2.
An active area of genetic research is the recognition of a polygenic risk for sporadic early-onset Alzheimer disease from a number of susceptibility genes.
On neuropathology, patients with early-onset Alzheimer disease (especially with the variants) are more likely to have hippocampal sparing with increased neocortical tau pathology, particularly in the parietal cortex and, to a lesser extent, the frontal cortex, than patients with late-onset Alzheimer disease.
On neuropathology, tau and neurofibrillary tangles, more than amyloid-β1-42 and neuritic plaques, correspond with the features of early-onset Alzheimer disease, with a relatively greater tau burden in early-onset Alzheimer disease than in late-onset Alzheimer disease.
Phenotypic variants of early-onset Alzheimer disease include those that present with language impairment (known as logopenic variant primary progressive aphasia), those that present with visuospatial or visuoperceptual impairments (known as posterior cortical atrophy), frontal or behavioral/executive variants, a number of parietal syndromes (such as the acalculia variant of early-onset Alzheimer disease), and a subgroup of patients with corticobasal syndrome.
Phenotypic variants of early-onset Alzheimer disease may involve alternative frontoparietal neural networks rather than the posterior default mode network implicated in late-onset Alzheimer disease.
Logopenic variant primary progressive aphasia, the most common nonamnestic phenotypic variant of early-onset Alzheimer disease, presents with a progressive decline in language with relatively spared memory and cognition due to focal Alzheimer neuropathology in temporoparietal language areas in the left hemisphere, especially the superior/midtemporal gyrus, angular gyrus, and midfrontal cortex.
In logopenic variant primary progressive aphasia, neuroimaging and CSF studies usually reveal abnormalities consistent with early-onset Alzheimer disease, including focal atrophy and decreased metabolism in the left temporoparietal junction.
Posterior cortical atrophy, the second most common early-onset Alzheimer disease variant, presents with progressive and disproportionate loss of visuospatial or visuoperceptual functions, usually due to Alzheimer neurodegeneration of posterior visual cortical regions.
The frontal variant of Alzheimer disease, now known as behavioral/dysexecutive Alzheimer disease, presents with features suggestive of frontotemporal lobar degeneration but most commonly with apathy or abulia.
Less common phenotypic variants of early-onset Alzheimer disease may have prominent parietal lobe symptoms and signs, exemplified by the acalculia variant from early Alzheimer neuropathology in the left inferior parietal lobule, particularly the intraparietal sulcus.
Acetylcholinesterase inhibitors, such as donepezil, galantamine, and rivastigmine, are indicated in the management of patients with early-onset Alzheimer disease, with the usual precautions and titration schedules.
The management of early-onset Alzheimer disease may differ from late-onset Alzheimer disease when targeting the management of specific cognitive and behavioral deficits.
Management of patients with early-onset Alzheimer disease must also consider providing genetic counseling if patients are to be evaluated for familial Alzheimer disease when the family history is suggestive of an autosomal dominant disorder.
The provision of age-appropriate psychosocial support is important in the management of early-onset Alzheimer disease.
Acknowledgments
RELATIONSHIP DISCLOSURE:
Dr Mendez serves as a section editor for UpToDate, Inc, and as an associate editor of the Journal of Alzheimer′s Disease. Dr Mendez has received personal compensation for speaking engagements from the Medical Education Speakers Network and receives research/grant support from Biogen and the National Institutes of Health/National Institute on Aging (R01AG050967). Dr Mendez receives publishing royalties from Cambridge University Press.
Footnotes
UNLABELED USE OF PRODUCTS/INVESTIGATIONAL USE DISCLOSURE:
Dr Mendez reports no disclosure.
REFERENCES
- 1.Maurer K, Volk S, Gerbaldo H. Auguste D and Alzheimer’s disease. Lancet 1997;349(9064): 1546–1549. doi: 10.1016/S0140-6736(96)10203-8. [DOI] [PubMed] [Google Scholar]
- 2.Zhu XC, Tan L, Wang HF, et al. Rate of early onset Alzheimer’s disease: a systematic review and meta-analysis. Ann Transl Med 2015;3(3):38. doi: 10.3978/j.issn.2305-5839.2015.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendez MF. Early-onset Alzheimer’s disease: nonamnestic subtypes and type 2 AD. Arch Med Res 2012;43(8):677–685. doi:0.1016/j.arcmed.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harvey RJ, Skelton-Robinson M, Rossor MN. The prevalence and causes of dementia in people under the age of 65 years. J Neurol Neurosurg Psychiatry 2003;74(9):1206–1209. doi: 10.1136/jnnp.74.9.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bickel H, Burger K, Hampel H, et al. Presenile dementia in memory clinics-incidence rates and clinical features [in German]. Nervenarzt 2006; 77(9):1079–1085. doi: 10.1007/s00115-005-1949-y. [DOI] [PubMed] [Google Scholar]
- 6.Renvoize E, Hanson M, Dale M. Prevalence and causes of young onset dementia in an English health district. Int J Geriatr Psychiatry 2011;26(1): 106–107. doi: 10.1002/gps.2456. [DOI] [PubMed] [Google Scholar]
- 7.Alzheimer’s Association. Early-onset dementia: a national challenge, a future crisis. Published 2006. Accessed November 27, 2018 alz.org/national/documents/report_earlyonset_summary.pdf.
- 8.Lambert MA, Bickel H, Prince M, et al. Estimating the burden of early onset dementia; systematic review of disease prevalence. Eur J Neurol 2014; 21(4):563–569. doi: 10.1111/ene.12325. [DOI] [PubMed] [Google Scholar]
- 9.Mendez MF. Early-onset Alzheimer disease. Neurol Clin 2017;35(2):263–281. doi: 10.1016/j.ncl.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerritsen AA, Bakker C, Verhey FR, et al. Prevalence of comorbidity in patients with young-onset Alzheimer disease compared with late-onset: a comparative cohort study. J Am Med Dir Assoc 2016;17(4):318–323. doi: 10.1016/j.jamda.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Joshi A, Ringman JM, Lee AS, et al. Comparison of clinical characteristics between familial and non-familial early onset Alzheimer’s disease. J Neurol 2012;259(10):2182–2188. doi: 10.1007/s00415-012-6481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wattmo C, Wallin ÅK. Early- versus late-onset Alzheimer’s disease in clinical practice: cognitive and global outcomes over 3 years. Alzheimers Res Ther 2017;9(1):70. doi: 10.1186/s13195-017-0294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koedam EL, Pijnenburg YA, Deeg DJ, et al. Early-onset dementia is associated with higher mortality. Dement Geriatr Cogn Disord 2008; 26(2):147–152. doi: 10.1159/000149585. [DOI] [PubMed] [Google Scholar]
- 14.Panegyres PK, Chen HY. Differences between early and late onset Alzheimer’s disease. Am J Neurodegener Dis 2013;2(4):300–306. [PMC free article] [PubMed] [Google Scholar]
- 15.Stanley K, Walker Z. Do patients with young onset Alzheimer’s disease deteriorate faster than those with late onset Alzheimer’s disease? A review of the literature. Int Psychogeriatr 2014; 26(12):1945–1953. doi: 10.1017/S1041610214001173. [DOI] [PubMed] [Google Scholar]
- 16.Chang KJ, Hong CH, Lee KS, et al. Mortality risk after diagnosis of early-onset Alzheimer’s disease versus late-onset Alzheimer’s disease: a propensity score matching analysis. J Alzheimers Dis 2017;56(4):1341–1348. doi: 10.3233/JAD-161181. [DOI] [PubMed] [Google Scholar]
- 17.Moschetti K, Barragan N, Basurto-Dávila R, et al. Mortality and productivity losses from Alzheimer disease among US adults aged 40 to 64 years, 1999 to 2010. Alzheimer Dis Assoc Disord 2015;29(2):165–168. doi: 10.1097/WAD.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 18.van Vliet D, de Vugt ME, Bakker C, et al. Time to diagnosis in young-onset dementia as compared with late-onset dementia. Psychol Med 2013; 43(2):423–432. doi: 10.1017/S0033291712001122. [DOI] [PubMed] [Google Scholar]
- 19.Eriksson H, Fereshtehnejad SM, Falahati F, et al. Differences in routine clinical practice between early and late onset Alzheimer’s disease: data from the Swedish Dementia Registry (SveDem). J Alzheimers Dis 2014;41(2):411–419. doi: 10.3233/JAD-132273. [DOI] [PubMed] [Google Scholar]
- 20.Mendez MF, Paholpak P, Lin A, et al. Prevalence of traumatic brain injury in early versus late-onset Alzheimer’s disease. J Alzheimers Dis 2015;47(4): 985–993. doi: 10.3233/JAD-143207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Sillaire AR, Dallongeville J, et al. Low prevalence and clinical effect of vascular risk factors in early-onset Alzheimer’s disease. J Alzheimers Dis 2017;60(3):1045–1054. doi: 10.3233/JAD-170367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadohara K, Sato I, Kawakami K. Diabetes mellitus and risk of early-onset Alzheimer’s disease: a population-based case-control study. Eur J Neurol 2017;24(7):944–949. doi: 10.1111/ene.13312. [DOI] [PubMed] [Google Scholar]
- 23.Palasí A, Gutiérrez-Iglesias B, Alegret M, et al. Differentiated clinical presentation of early and late-onset Alzheimer’s disease: is 65 years of age providing a reliable threshold? J Neurol 2015; 262(5):1238–1246. doi: 10.1007/s00415-015-7698-3. [DOI] [PubMed] [Google Scholar]
- 24.Joubert S, Gour N, Guedj E, et al. Early-onset and late-onset Alzheimer’s disease are associated with distinct patterns of memory impairment. Cortex 2016;74:217–232. doi: 10.1016/j.cortex.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Aziz AL, Giusiano B, Joubert S, et al. Difference in imaging biomarkers of neurodegeneration between early and late-onset amnestic Alzheimer’s disease. Neurobiol Aging 2017;54:22–30. doi: 10.1016/j.neurobiolaging.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Dickerson BC, Brickhouse M, McGinnis S, Wolk DA. Alzheimer’s disease: the influence of age on clinical heterogeneity through the human brain connectome. Alzheimers Dement (Amst) 2016;6: 122–135. doi:110.1016/j.dadm.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho H, Jeon S, Kang SJ, et al. Longitudinal changes of cortical thickness in early- versus late-onset Alzheimer’s disease. Neurobiol Aging 2013;34(7):1921.e9–1921.e15. doi: 10.1016/j.neurobiolaging.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Migliaccio R, Agosta F, Possin KL, et al. Mapping the progression of atrophy in early- and late-onset Alzheimer’s disease. J Alzheimers Dis 2015; 46(2):351–364. doi: 10.3233/JAD-142292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamelin L, Bertoux M, Bottlaender M, et al. Sulcal morphology as a new imaging marker for the diagnosis of early onset Alzheimer’s disease. Neurobiol Aging 2015;36(11):2932–2939. doi: 10.1016/j.neurobiolaging.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 30.Park KH, Noh Y, Choi EJ, et al. Functional connectivity of the hippocampus in early- and vs. late-onset Alzheimer’s disease. J Clin Neurol 2017;13(4):387–393. doi: 10.3988/jcn.2017.13.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiaravalloti A, Koch G, Toniolo S, et al. Comparison between early-onset and late-onset Alzheimer’s disease patients with amnestic presentation: CSF and (18)F-FDG PET study. Dement Geriatr Cogn Dis Extra 2016;6(1):108–119. doi: 10.1159/000441776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaiser NC, Melrose RJ, Liu C, et al. Neuropsychological and neuroimaging markers in early versus late-onset Alzheimer’s disease. Am J Alzheimers Dis Other Demen 2012;27(7): 520–529. doi: 10.1177/1533317512459798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanhoutte M, Semah F, Rollin Sillaire A, et al. 18F-FDG PET hypometabolism patterns reflect clinical heterogeneity in sporadic forms of early-onset Alzheimer’s disease. Neurobiol Aging 2017;59:184–196. doi: 10.1016/j.neurobiolaging.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Ballarini T, Iaccarino L, Magnani G, et al. Neuropsychiatric subsyndromes and brain metabolic network dysfunctions in early onset Alzheimer’s disease. Hum Brain Mapp 2016; 37(12):4234–4247. doi: 10.1002/hbm.23305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA 2015;313(19): 1939–1949. doi: 10.1001/jama.2015.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fagan AM. What does it mean to be ‘amyloid-positive’? Brain 2015;138(pt 3):514–516. doi: 10.1093/brain/awu387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zwan MD, Bouwman FH, Konijnenberg E, et al. Diagnostic impact of [18F]flutemetamol PET in early-onset dementia. Alzheimers Res Ther 2017; 9(1):2. doi: 10.1186/s13195-016-0228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Youn YC, Jang JW, Han SH, et al. 11C-PIB PET imaging reveals that amyloid deposition in cases with early-onset Alzheimer’s disease in the absence of known mutations retains higher levels of PIB in the basal ganglia. Clin Interv Aging 2017;12:1041–1048. doi: 10.2147/CIA.S132884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bejanin A, Schonhaut DR, La Joie R, et al. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer’s disease. Brain 2017;140(12):3286–3300. doi: 10.1093/brain/awx243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nasrallah IM, Chen YJ, Hsieh MK, et al. 18F-Flortaucipir PET/MRI correlations in nonamnestic and amnestic variants of Alzheimer disease. J Nucl Med 2018;59(2):299–306. doi: 10.2967/jnumed.117.194282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xia C, Makaretz SJ, Caso C, et al. Association of in vivo [18F]AV-1451 tau PET imaging results with cortical atrophy and symptoms in typical and atypical Alzheimer disease. JAMA Neurol 2017; 74(4):427–436. doi: 10.1001/jamaneurol.2016.5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schöll M, Ossenkoppele R, Strandberg O, et al. Distinct 18F-AV-1451 tau PET retention patterns in early- and late-onset Alzheimer’s disease. Brain 2017;140(9):2286–2294. doi: 10.1093/brain/awx171. [DOI] [PubMed] [Google Scholar]
- 43.Cho H, Choi JY, Lee SH, et al. Excessive tau accumulation in the parieto-occipital cortex characterizes early-onset Alzheimer’s disease. Neurobiol Aging 2017;53:103–111. doi: 10.1016/j.neurobiolaging.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 44.Phillips JS, Das SR, McMillan CT, et al. Tau PET imaging predicts cognition in atypical variants of Alzheimer’s disease. Hum Brain Mapp 2018;39(2): 691–708. doi: 10.1002/hbm.23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ossenkoppele R, Mattsson N, Teunissen CE, et al. Cerebrospinal fluid biomarkers and cerebral atrophy in distinct clinical variants of probable Alzheimer’s disease. Neurobiol Aging 20l5;36(8):2340–2347. doi: 10.1016/j.neurobiolaging.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teng E, Yamasaki TR, Tran M, et al. Cerebrospinal fluid biomarkers in clinical subtypes of early-onset Alzheimer’s disease. Dement Geriatr Cogn Disord 20l4;37(5-6):307–3l4. doi: 10.1159/000355555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lauridsen C, Sando SB, Møller I, et al. Cerebrospinal fluid Aβ43 is reduced in early-onset compared to late-onset Alzheimer’s disease, but has similar diagnostic accuracy to Aβ42. Front Aging Neurosci 2017;9:210. doi: 10.3389/fnagi.2017.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Müller U, Winter P, Graeber MB. A presenilin 1 mutation in the first case of Alzheimer’s disease. Lancet Neurol 2013;12(2):129–130. doi: 10.1016/S1474-4422(12)70307-1. [DOI] [PubMed] [Google Scholar]
- 49.Kunkle BW, Vardarajan BN, Naj AC, et al. Early-onset Alzheimer disease and candidate risk genes involved in endolysosomal transport. JAMA Neurol 2017;74(9):1113–1122. doi: 10.1001/jamaneurol.2017.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karch CM, Goate AM. Alzheimer's disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry 2015;77(1):43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicolas G, Wallon D, Charbonnier C, et al. Screening of dementia genes by whole-exome sequencing in early-onset Alzheimer disease: input and lessons. Eur J Hum Genet 2016;24(5):710–716. doi: 10.1038/ejhg.2015.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blauwendraat C, Wilke C, Jansen IE, et al. Pilot whole-exome sequencing of a German early-onset Alzheimer’s disease cohort reveals a substantial frequency of PSEN2 variants. Neurobiol Aging 2016;37:208.e11–208.e17. doi: 10.1016/j.neurobiolaging.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 53.Barber IS, García-Cárdenas JM, Sakdapanichkul C, et al. Screening exons 16 and 17 of the amyloid precursor protein gene in sporadic early-onset Alzheimer’s disease. Neurobiol Aging 2016;39: 220.e1–220.e7. doi: 10.1016/j.neurobiolaging.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joshi A, Ringman JM, Lee AS, Juarez KO, Mendez MF. Comparison of clinical characteristics between familial and non-familial early onset Alzheimer’s disease. J Neurol Epub 2012;259(10):2182–2188. doi: 10.1007/s00415-012-6481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ringman JM. Are late-onset autosomal dominant and sporadic Alzheimer disease “separate but equal”? JAMA Neurol 2016;73(9):1060–1061. doi: 10.1001/jamaneurol.2016.1633. [DOI] [PubMed] [Google Scholar]
- 56.Bellenguez C, Charbonnier C, Grenier-Boley B, et al. Contribution to Alzheimer’s disease risk of rare variants in TREM2, SORL1, and ABCA7 in 1779 cases and 1273 controls. Neurobiol Aging 2017;59: 220.e221–220.e229. doi: 10.1016/j.neurobiolaging.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Slattery CF, Zhang J, Paterson RW, et al. ApoE influences regional white-matter axonal density loss in Alzheimer’s disease. Neurobiol Aging 2017;57:8–17. doi: 10.1016/j.neurobiolaging.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Luca V, Orfei MD, Gaudenzi S, et al. Inverse effect of the APOE epsilon4 allele in late- and early-onset Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci 2016;266(7):599–606. doi: 10.1007/s00406-015-0663-4. [DOI] [PubMed] [Google Scholar]
- 59.Carmona S, Hardy J, Guerreiro R. The genetic landscape of Alzheimer disease. Handb Clin Neurol 2018;148:395–408. doi: 10.1016/B978-0-444-64076-5.00026-0. [DOI] [PubMed] [Google Scholar]
- 60.Chaudhury S, Patel T, Barber IS, et al. Polygenic risk score in postmortem diagnosed sporadic early-onset Alzheimer’s disease. Neurobiol Aging 2018;62:244.e241–244.e248. doi: 10.1016/j.neurobiolaging.2017.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murray ME, Graff-Radford NR, Ross OA, et al. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol 2011;10(9):785–796. doi: 10.1016/S1474-4422(11)70156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fiford CM, Ridgway GR, Cash DM, et al. Patterns of progressive atrophy vary with age in Alzheimer’s disease patients. Neurobiol Aging 2018;63:22–32. doi: 10.1016/j.neurobiolaging.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phillips JS, Da Re F, Dratch L, et al. Neocortical origin and progression of gray matter atrophy in nonamnestic Alzheimer’s disease. Neurobiol Aging 2018;63:75–87. doi: 10.1016/j.neurobiolaging.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawakatsu S, Kobayashi R, Hayashi H. Typical and atypical appearance of early-onset Alzheimer’s disease: a clinical, neuroimaging and neuropathological study. Neuropathology 2017;37(2):150–173. doi: 10.1111/neup.12364. [DOI] [PubMed] [Google Scholar]
- 65.Miller ZA, Mandelli ML, Rankin KP, et al. Handedness and language learning disability differentially distribute in progressive aphasia variants. Brain 2013;136(pt 11):3461–3473. doi: 10.1093/brain/awt242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsai PH, Teng E, Liu C, Mendez MF. Posterior cortical atrophy: evidence for discrete syndromes of early-onset Alzheimer’s disease. Am J Alzheimers Dis Other Demen 2011;26(5):413–418. doi:0.1177/1533317511418955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mendez MF, Ghajarania M, Perryman KM. Posterior cortical atrophy: clinical characteristics and differences compared to Alzheimer’s disease. Dement Geriatr Cogn Disord 2002;14(1):33–40. doi: 10.1159/000058331. [DOI] [PubMed] [Google Scholar]
- 69.Ossenkoppele R, Pijnenburg YA, Perry DC, et al. The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain 2015;138(pt 9):2732–2749. doi: 10.1111/neup.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koedam EL, Lauffer V, van der Vlies AE, et al. Early-versus late-onset Alzheimer’s disease: more than age alone. J Alzheimers Dis 2010;19(4):1401–1408. doi: 10.3233/JAD-2010-1337. [DOI] [PubMed] [Google Scholar]
- 71.Mendez MF, Moheb N, Desarzant RE, Teng EH. The progressive acalculia presentation of parietal variant Alzheimer’s disease. J Alzheimers Dis 2018;63(3):941–948. doi: 10.3233/JAD-180024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee SE, Rabinovici GD, Mayo MC, et al. Clinicopathological correlations in corticobasal degeneration. Ann Neurol 2011;70(2):327–340. doi: 10.1002/ana.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mendez MF, Lee AS, Joshi A, Shapira JS. Nonamnestic presentations of early-onset Alzheimer’s disease. Am J Alzheimers Dis Other Demen 2012;27(6):413–420. doi: 10.1177/1533317512454711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Flier WM, Pijnenburg YA, Fox NC, Scheltens P. Early-onset versus late-onset Alzheimer’s disease: the case of the missing APOE ε4 allele. Lancet Neurol 2011;10(3):280–288. doi: 10.1016/S1474-4422(10)70306-9. [DOI] [PubMed] [Google Scholar]
- 75.Gour N, Felician O, Didic M, et al. Functional connectivity changes differ in early and late-onset Alzheimer’s disease. Hum Brain Mapp 2014;35(7):2978–2994. doi: 10.1002/hbm.22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lehmann M, Madison C, Ghosh PM, et al. Loss of functional connectivity is greater outside the default mode network in nonfamilial early-onset Alzheimer’s disease variants. Neurobiol Aging 2015;36(10):2678–2686. doi: 10.1016/j.neurobiolaging.2015.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smits LL, Pijnenburg YA, van der Vlies AE, et al. Early onset APOE E4-negative Alzheimer’s disease patients show faster cognitive decline on non-memory domains. Eur Neuropsychopharmacol 2015;25(7):1010–1017. doi: 10.1016/j.euroneuro.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 78.Spinelli EG, Mandelli ML, Miller ZA, et al. Typical and atypical pathology in primary progressive aphasia variants. Ann Neurol 2017;81(3):430–443. doi: 10.1002/ana.24885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giannini LAA, Irwin DJ, McMillan CT, et al. Clinical marker for Alzheimer disease pathology in logopenic primary progressive aphasia. Neurology 2017;88(24):2276–2284. doi: 10.1212/WNL.0000000000004034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whitwell JL, Jones DT, Duffy JR, et al. Working memory and language network dysfunctions in logopenic aphasia: a task-free fMRI comparison with Alzheimer’s dementia. Neurobiol Aging 2015;36(3):1245–1252. doi: 10.1016/j.neurobiolaging.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Migliaccio R, Agosta F, Rascovsky K, et al. Clinical syndromes associated with posterior atrophy: early age at onset AD spectrum. Neurology 2009;73(19):1571–1578. doi: 10.1212/WNL.0b013e3181c0d427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leyton CE, Hodges JR, Piguet O, Ballard KJ. Common and divergent neural correlates of anomia in amnestic and logopenic presentations of Alzheimer’s disease. Cortex 2017;86:45–54. doi: 10.1016/j.cortex.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 83.Hardy CJD, Agustus JL, Marshall CR, et al. Functional neuroanatomy of speech signal decoding in primary progressive aphasias. Neurobiol Aging 2017;56:190–201. doi: 10.1016/j.neurobiolaging.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Win KT, Pluta J, Yushkevich P, et al. Neural correlates of verbal episodic memory and lexical retrieval in logopenic variant primary progressive aphasia. Front Neurosci 2017;11:330. doi: 10.3389/fnins.2017.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Magnin E, Sylvestre G, Lenoir F, et al. Logopenic syndrome in posterior cortical atrophy. J Neurol 2013;260(2):528–533. doi: 10.1007/s00415-012-6671-7. [DOI] [PubMed] [Google Scholar]
- 86.Leyton CE, Piguet O, Savage S, Burrell J, Hodges JR. The neural basis of logopenic progressive aphasia. J Alzheimers Dis 2012;32(4):1051–1059. doi: 10.3233/JAD-2012-121042. [DOI] [PubMed] [Google Scholar]
- 87.Villarejo-Galende A, Llamas-Velasco S, Gómez-Grande A, et al. Amyloid pet in primary progressive aphasia: case series and systematic review of the literature. J Neurol 2017;264(1):121–130. doi: 10.1007/s00415-016-8324-8. [DOI] [PubMed] [Google Scholar]
- 88.Santos-Santos MA, Rabinovici GD, Iaccarino L, et al. Rates of amyloid imaging positivity in patients with primary progressive aphasia. JAMA Neurol 2018;75(3):342–352. doi: 10.1001/jamaneurol.2017.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krishnan K, Machulda MM, Whitwell JL, et al. Varying degrees of temporoparietal hypometabolism on FDG-PET reveal amyloid-positive logopenic primary progressive aphasia is not a homogeneous clinical entity. J Alzheimers Dis 2017;55(3):1019–1029. doi: 10.3233/JAD-160614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Josephs KA, Martin PR, Botha H, et al. [18 F]AV-1451 tau-PET and primary progressive aphasia. Ann Neurol 2018;83(3):599–611. doi: 10.1002/ana.25183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pascual B, Masdeu JC. Tau, amyloid, and hypometabolism in the logopenic variant of primary progressive aphasia. Neurology 2016;86(5):487–488. doi: 10.1212/WNL.0000000000002340. [DOI] [PubMed] [Google Scholar]
- 92.Paraskevas GP, Kasselimis D, Kourtidou E, et al. Cerebrospinal fluid biomarkers as a diagnostic tool of the underlying pathology of primary progressive aphasia. J Alzheimers Dis 2017;55(4):1453–1461. doi: 10.3233/JAD-160494. [DOI] [PubMed] [Google Scholar]
- 93.Crutch SJ, Schott JM, Rabinovici GD, et al. Shining a light on posterior cortical atrophy. Alzheimers Dement 2013;9(4):463–465. doi: 10.1016/j.jalz.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 94.Schott JM, Crutch SJ. Posterior cortical atrophy. Continuum (Minneap Minn) 2019;25(1 Dementia):52–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wattmo C, Wallin Åk. Early-versus late-onset Alzheimer disease: long-term functional outcomes, nursing home placement, and risk factors for rate of progression. Dement Geriatr Cogn Dis Extra 2017;7(1):172–187. doi: 10.1159/000455943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ducharme F, Kergoat MJ, Antoine P, et al. The unique experience of spouses in early-onset dementia. Am J Alzheimers Dis Other Demen 2013;28(6):634–641. doi: 10.1177/1533317513494443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Joshi A, Jimenez E, Mendez MF. Initial heart rate reactivity to socioemotional pictures in early-onset Alzheimer's disease. J Alzheimers Dis 2017;60(4):1325–1332. doi: 10.3233/JAD-170319. [DOI] [PubMed] [Google Scholar]
- 98.Baptista MAT, Santos RL, Kimura N, et al. Disease awareness may increase risk of suicide in young onset dementia: a case report. Dement Neuropsychol 2017;11(3):308–311. doi: 10.1590/1980-57642016dn11-030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ahmed S, de Jager CA, Haigh AM, Garrard P. Logopenic aphasia in Alzheimer's disease: clinical variant or clinical feature? J Neurol Neurosurg Psychiatry 2012;83(11):1056–1062. doi: 10.1136/jnnp-2012-302798. [DOI] [PubMed] [Google Scholar]
- 100.Ossenkoppele R, Cohn-Sheehy BI, La Joie R, et al. Atrophy patterns in early clinical stages across distinct phenotypes of Alzheimer’s disease. Hum Brain Mapp 2015;36(11):4421–4437. doi: 10.1002/hbm.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ridgway GR, Lehmann M, Barnes J, et al. Early-onset Alzheimer disease clinical variants: Multivariate analyses of cortical thickness. Neurology 2012;79(1):80–84. doi: 10.1212/WNL.0b013e31825dce28. [DOI] [PMC free article] [PubMed] [Google Scholar]