It is important to distinguish vaccines designed to prevent cancer from those designed to treat cancer patients. The mode of action of the HPV vaccine developed for the prevention of cervical and other HPV-associated malignancies is similar to that for vaccines developed for the prevention of infectious disease, i.e., the induction of antibodies directed against essential components of the microbe. While there have been stunning successes in the area of preventive vaccines, the history of therapeutic cancer vaccines, which principally involve the development of cell-mediated immunity, i.e., T cells, directed against tumor antigens, has been far more challenging. The renaissance of cancer immunotherapy, however, has now rendered therapeutic cancer vaccines a potential integral component of the therapies for human cancers.
The successes seen in cancer immunotherapy have, in general, shown cancers to be considered in two main groups: so-called “hot” tumors, which contain abundant anti-tumor T cells, and many of which respond to immunotherapy, and “cold” tumors, which are generally devoid of endogenous T cells (either T-cell deserts or T-cell excluded). However, these “cold” tumors constitute the majority of human solid tumors, which do not respond to checkpoint inhibitor monoclonal antibody (CIMA) therapy. Melanoma is the prototype “hot” tumor. The abundance of somatic mutations in melanoma cells leads to the expression of “neoantigens,” which the patient’s immune system recognizes as foreign, leading to the influx of T cells directed against those neoantigens. This is why, historically, subsets of patients with melanoma responded to IL-2 therapy with its ability to activate T cells. Although a very small percentage of melanoma patients developed spontaneous remissions, it remained a paradox that the majority of patients with melanoma did not respond to IL-2, given the abundance of endogenous T cells in their tumors.
The renaissance in immuno-oncology came with the use of CIMAs. Preclinical studies revealed that the T cells present in most tumors were inactive, i.e., “anergized,” and thus not able to lyse tumor cells; it was revealed that tumor cells were able to put up a defense mechanism by expressing “checkpoint molecules” such as PD-L1 on their surface to anergize T cells, an adaptive defense mechanism against the development of T-cell‒mediated autoimmunity. The use of checkpoint inhibitor MAbs, such as anti-PD-1 and anti-PD-L1, has enabled an interference with this checkpoint mechanism, allowing otherwise anergized T cells to lyse tumor cells expressing cognate antigens. However, one of the adverse effects in the use of checkpoint inhibitor MAbs is the induction of autoimmune syndromes observed in approximately 10–15% of treated patients. The use of checkpoint inhibitor MAbs as monotherapy or in combinations has led to clinical responses in approximately 10–60% of patients with melanoma,1 but in about only 10–20% of patients with solid tumors such as prostate, lung, breast and colorectal carcinomas, among others.2
These developments provide important insights into the use of therapeutic cancer vaccines. The vast majority of non-melanoma solid tumors can be characterized as “cold,” i.e., they contain few if any endogenous T cells, and do not respond to checkpoint inhibitor therapy. Thus one potential therapeutic strategy would be to generate de novo T cells directed against tumor antigens ‒ this is precisely what cancer vaccines were designed to do ‒ and to be used in combination with checkpoint inhibitor MAbs.
Historically, several Phase I and II clinical studies employing cancer vaccines as monotherapy have shown promise, but with two exceptions ‒ sipuleucel-T3 for the therapy of metastatic prostate cancer and TVEC4 for metastatic melanoma ‒ Phase III trials did not meet their primary endpoint. To further put this into historical perspective, there are two important points: (1) the Phase III trials of cancer vaccines as monotherapy in cold tumors such as prostate and breast were initiated prior to the era of checkpoint inhibitor MAbs; and (2) because more than 95% of agents entering oncology clinical testing do not get approved,5 the less than half-dozen cancer vaccine monotherapy Phase III trials that did not meet their primary endpoint does not render vaccines as a “failed” modality.
Evidence is now emerging in numerous preclinical studies and early clinical trials demonstrating synergy in the use of cancer vaccines plus checkpoint inhibitor MAbs. The use of checkpoint inhibitor MAbs with cancer vaccine therapy, however, is just the proverbial “tip of the iceberg.” Advances in basic immunology and translational immunotherapy are rapidly unravelling the complexity of the immune system and, consequently, agents and strategies are being developed that can be and are being employed to increase the efficacy of therapeutic cancer vaccines. As such, the use of vaccines could be considered a necessary, albeit not sufficient, component of an effective anti-cancer therapeutic regimen in patients with T-cell poor tumors.
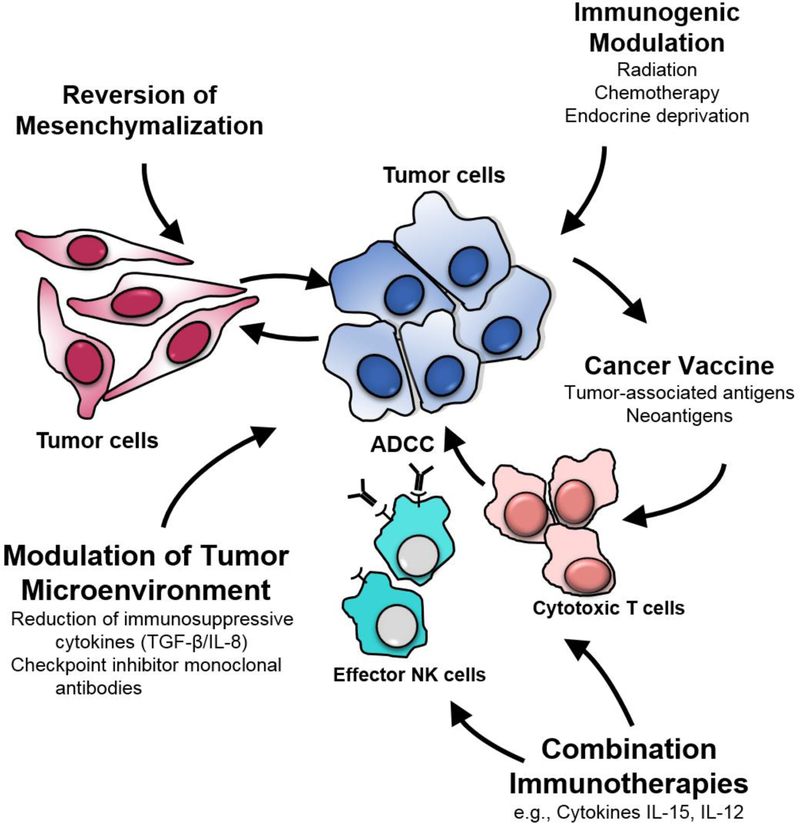
Moreover, preclinical studies are revealing that the hallmark of an effective immuno-oncology strategy for “cold” tumors is the use of multiple immuno-oncology agents, each targeting different components of the immune system (Figure 1). These include the (a) induction of an immune response, principally involving cytotoxic T cells, to tumor-associated antigens (TAA) or tumor-specific neoantigens (TSNA), caused by immunogenic mutations, via vaccine administration; (b) potentiation of that immune response by Type 1 cytokines such as IL-15 or IL-12 immunocytokine; (c) reduction of immunosuppressive entities in the tumor microenvironment (TME) with the use of checkpoint inhibitor MAbs, and agents to target immunosuppressive cytokines such as TGF-β and IL-8; and (d) use of agents to modify tumor cell phenotype to render otherwise resistant tumor cells more susceptible to T-cell‒mediated lysis. Preclinical and early clinical studies have shown that non-lethal doses of radiation, and certain chemotherapeutic and small molecule targeted agents, have this ability.
Figure 1.
Vaccines as an integral component of a multi-faceted approach to cancer immunotherapy.
TSNA are generally more immunogenic than TAA; however, algorithms for selecting which mutations are most immunogenic are imperfect and generating a patient-specific vaccine is time-consuming. In contrast, an off-the-shelf approach targeting TAA can generate effector cells that if properly facilitated can kill tumor cells and this immune-mediated killing can lead to a broadening of the immune response, or antigen spreading, that could include TSNA.
There is also a spectrum of cancer vaccine platforms, each with its unique properties. These include: (a) recombinant vectors; (b) peptides and proteins in adjuvants; and (c) autologous dendritic cells either pulsed with peptide or transfected with tumor-derived nucleic acid. Preclinical studies have shown that each platform has the ability to present different epitopes of a given TAA or TSNA to the immune system, and to activate different components of the host immune system. Preclinical and early clinical studies are providing evidence that the sequential use of two diverse vaccine platforms is more effective in inducing anti-tumor immunity than the continued use of a single platform.
Subsets of human carcinoma tumor cells have been shown to exhibit “stem-like” characteristics and are resistant to standard-of-care therapies. This process to a stem-like phenotype has been shown to be due to an epithelial to mesenchymal transition (EMT), or mesenchymalization, principally driven by transcription factors such as twist, snail and brachyury. Vaccines directed against molecules driving this and other important tumor-promoting biological processes are now in clinical trials, including vaccines targeting brachyury,6 Her2,7 and oncogenes such as MUC1-C.8 Agents targeting TGF-β and IL-8 to reverse tumor mesenchymalization are also being employed with vaccines to render tumor cells more susceptible to T-cell‒mediated lysis, one example is a bifunctional anti-PD-L1/TGFβR2 agent.9 Several checkpoint inhibitor MAbs have now been designed with the ability to also mediate antibody-dependent cell-mediated cytotoxicity (ADCC), thus engaging the patient’s innate immune system via effector NK cells to further enhancing vaccine-induced adaptive immunity (Figure 1).10 Precisely how and when cancer vaccines will be employed in regimens of immuno-oncology involving multiple agents will be the subject of ongoing and future preclinical and clinical investigations. Due to the relatively low level of toxicity being observed in the use of most immuno-oncology agents, adaptive design clinical trials are being initiated in which additional immuno-oncology agents are sequentially added to an ongoing immuno-oncology regimen when a safety signal is obtained.
In conclusion, the plethora of immune-mediating agents now available for clinical studies is designed to potentiate a vaccine-induced anti-tumor immune response, resulting in T cells directed against TAAs in the TME. Cancer vaccine therapy is now situated to be an essential component for a successful anti-tumor response for so-called “cold” tumors that currently are not responsive to the use of single or combination checkpoint inhibitor MAb therapy. The future thus appears quite promising for the integration of cancer vaccines into a range of immunotherapeutic modalities.
Footnotes
Conflict of Interest Disclosures:
Both authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Dr. Gulley did not report any disclosures. Dr. Schlom reported that the research conducted at the Laboratory of Tumor Immunology and Biology (LTIB), Center for Cancer Research, National Cancer Institute (NCI), was supported in part by the NCI’s Collaborative Research and Development Agreements (CRADAs) with EMD Serono and NantBioScience. Additionally, the NCI has financial relationships via CRADAs with six other entities relevant to the research conducted at the LTIB. This information was also reported on the submitted ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Jeffrey Schlom, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland..
James L. Gulley, Medical Oncology Service, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland..
References
- 1.Marconcini R, Spagnolo F, Stucci LS, et al. Current status and perspectives in immunotherapy for metastatic melanoma. Oncotarget 2018; 9:12452–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwai Y, Hamanishi J, Chamoto K, Honjo T. Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci 2017;24(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 2006;24:3089–3094. [DOI] [PubMed] [Google Scholar]
- 4.Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 2015;33(25):2780–2788. [DOI] [PubMed] [Google Scholar]
- 5.Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics 2018;January 31. doi: 10.1093/biostatistics/kxx069 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 6.Heery CR, Singh BH, Rauckhorst M, et al. Phase I trial of a yeast-based therapeutic cancer vaccine (GI-6301) targeting the transcription factor brachyury. Cancer Immunol Res 2015;3(11):1248–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pathangey LB, McCurry DB, Gendler SJ, et al. Surrogate in vitro activation of innate immunity synergizes with interleukin-7 to unleash rapid antigen-driven outgrowth of CD4+ and CD8+ human peripheral blood T-cells naturally recognizing MUC1, HER2/neu and other tumor-associated antigens. Oncotarget 2017;8(7):10785–10808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajabi H, Kufe D. MUC1-C oncoprotein integrates a program of EMT, epigenetic reprogramming and immune evasion in human carcinomas. Biochim Biophys Acta 2017;1868(1):117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strauss J, Heery CR, Schlom J, et al. Phase 1 trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGF-β, in advanced solid tumors. Clin Cancer Res 2018;24(6):1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyerinas B, Jochems C, Fantini M, et al. Antibody dependent cellular cytotoxicity (ADCC) activity of a novel anti-PD-L1 antibody, avelumab (MSB0010718C), on human tumor cells. Cancer Immunol Res 2015;3(10):1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]