Abstract
Sustainable agriculture is an important global issue. The use of organic fertilizers can enhance crop yield and soil properties while restraining pests and diseases. The objective of this study was to assess the effects of long-term use of chemical and organic fertilizers on tea and rhizosphere soil properties in tea orchards. Inductively coupled plasma mass spectrometry (ICP-MS) and high-throughput sequencing technology analyses were used to investigate heavy metals content and bacterial composition in rhizosphere soils. Our results indicated that organic fertilizer treatment significantly decreased Cu, Pb and Cd contents in rhizosphere soil sample. The results also showed that treatment with organic fertilizer significantly decreased the contents of Cd, Pb and As in tea leaves. Furthermore, organic fertilizer significantly increased the amino acids content of tea and the pH of the soil. The use of organic fertilizer significantly increased in the relative abundance of Burkholderiales, Myxococcales, Streptomycetales, Nitrospirales, Ktedonobacterales, Acidobacteriales, Gemmatimonadales, and Solibacterales, and decreased the abundance of Pseudonocardiales, Frankiales, Rhizobiales, and Xanthomonadales. In conclusion, organic fertilizer can help to shape the microbial composition and recruit beneficial bacteria into the rhizosphere of tea, leading to improved tea quality and reduced heavy metals content in rhizosphere soil and tea leaves.
Introduction
Camellia sinensis, commonly known as tea plant, is a mountainous crop and an important agricultural product for many farmers in China. Due to its richness in beneficial antioxidants, vitamins, and amino acids, the popularity of tea has been steadily growing. And the amino acids, tea polyphenols, and caffeine are key elements in determining both taste and quality of tea. In 2016, China produced 2.41 million tons of tea with a monetary value of 170.2 billion yuan. However, soil degradation and substantial quality and yield decrease have been observed in the long-term monoculture of tea bushes, and have become key problems in the sustainable development of tea orchards [1]. With growing demand and limited land availability, farmers have been increasingly using nitrogen fertilizers in order to increase crop yield. However, nitrogen fertilizers can have undesirable effects, including a decline in tea quality, soil acidification, heavy metals pollution, soil compaction, and changes in soil microbiome [2–3]. We have previously shown that long-term tea cultivation with nitrogen fertilizers altered the bacterial composition of soil and significantly decreased soil pH and microbial metabolic activity, resulting in a reduction of beneficial bacteria [1, 4–5]. Therefore, it is essential to understand the impact of nitrogen fertilizers beyond their effects on crop yield in order to achieve a balance between benefits and harms in modern agricultural practices.
The sustainability of agricultural systems is an important global issue. This has resulted in the potential benefits of organic fertilizers application have being highlighted. Organic fertilizers are derived from natural sources (e.g., livestock and poultry excreta, plant residues, biogas residue, and agricultural by-products), and their usage can have a positive impact on pollution. The potential benefits of organic fertilizers have been documented in a number of studies in which investigators observed a raise in soil microbial activities, which in turn improved crop growth and restrained pests and diseases [6–7]. Soil contains a large number of microbial species as well as other organisms that together form a highly complex ecosystem. Microorganisms are essential for nutrient recycling, healthy plant development, and decomposition of organic matter [8]. However, environmental conditions and cultivation practices are likely to influence the microbiome, resulting in alterations in soil characteristics or ecosystem [9]. Researchers have found that tea cultivated with bio-organic fertilizers has superior color and taste compared to tea treated with chemical fertilizers [10–11]. Studies have also suggested that the use of organic fertilizers resulted in higher seedling biomass and significantly improved the soil fungal to bacterial ratio as well as soil enzyme activity [12–13]. In addition, while long-term application of chemical fertilizers could lead to serious soil acidification, nutritional imbalance, and deterioration of the rhizosphere micro-ecological environment, further increased the activity of heavy metal ions in soil. The use of organic fertilizer could alleviate soil acidification, resulting in increased plant yields [14]. However, little information is available concerning the contents changes of microbial community and heavy metal ions using long-term chemical fertilizers and organic fertilizer.
Recent studies have raised concerns regarding the long-term effects of fertilization practices on biological properties of soils, but most studies focused mainly on tea plant yield and changes in soil nutrients [15–17]. Moreover, the effects of long-term use of organic fertilizer on rhizosphere bacterial composition and heavy metals in tea gardens have not been carefully investigated. In this study, we have used ICP-MS and high-throughput sequencing technology to determine the effects of organic and chemical fertilizers on bacterial taxa and heavy metals content in the rhizosphere of tea orchards. Herein we evaluated potential mechanisms of organic fertilizers application as a means to improve the quality of tea. These results could provide practical guidance to the design of sustainable tea garden ecology.
Materials and methods
Field experiment and soil sampling
The experimental station is located in the Zudun township of Nanping in the Fujian Province of China (27°24ʹN, 118°33ʹE). This is one of the main tea producing region in Fujian and is under sub-tropical monsoon climate with an average annual temperature of 18.1°C and an average annual rainfall of 1557–1743 mm. Zudun township of Fujian Province is the most important producing area of white tea in China. The two different planting and management patterns were the long-term organic fertilizer (mainly contain rape cake and sheep dung) ecological tea garden and the conventional nitrogen fertilizer management tea garden. These tea orchards had similar environmental characteristics such as altitute, slope position and slope aspect, and similar agronomic management. The tea orchards were typical red acidity soils in southern China. These experimental fields were established in 1990 and have since been used for tea planting. The tea orchards have cultivated the Fuding white tea and the tea stand ages were more than 30 years old, were selected in this study, and each had three landscape-level replications. The organic tea orchards have been certified under the US National Organic program 7 CFR Part 205 by the certification of environmental standards Gmbh (CERES) in 2015.
Soil samples were collected from the rhizosphere of fields treated with organic fertilizer (OrgS) or chemical fertilizer (NorS) fon June 4, 2018. At the same time, non-rhizosphere soils were also collected from the organic (CKOrgS) and chemical (CKNorS) fertilizer treated tea orchards. The rhizosphere and non-rhizosphere soils of tea trees were taken from each experimental plot by a 5-point sampling method. For each sample, three replicates were performed. Tea leaves from the OrgS and NorS treatment groups were sampled in the fields. After sieving (2 mm mesh) to remove stones and plant residues, soil samples were stored at −80°C.
Analysis of tea biochemical properties
Tea leaves were roasted, dried and ground into a fine powder testing. The amino acid content was measured using the ninhydrin colorimetric methods. Tea polyphenols and caffeine contents were determined using a Waters HPLC system (C18 column: Inertsil ODS-SP, 4.6 × 250 mm, 5 μm). The chromatographic conditions were as follows for tea polyphenols: mobile phase A: mixture solution (water: acetic acid: acetonitrile = 90:0.1:10, v/v/v); mobile phase B: acetonitrile; elution gradient: mobile phase B 0% (0 min)→0% (10 min)→10% (20 min)→0% (25 min)→0% (30 min); oven temperature: 40°C; detection wavelength: 280 nm; velocity: 1 mL/min. For caffeine: mobile phase A: water; mobile phase B: methanol; elution gradient: mobile phase B 65% (0 min)→65% (35 min); detection wavelength: 275 nm; velocity: 1 mL/min.
Analysis of soil chemical properties
Soil chemical properties analysis included pH, total nitrogen (TN), total phosphorus (TP), total potassium (TK), available nitrogen (AN), available phosphorus (AP), and available potassium (AK). Soil pH was determined using a glass electrode pH meter (1:2.5 soil to water suspensions). TN, TP, and TK were determined using Kjeldahl digestion, sodium carbonate fusion, and NaOH melts flamer methods, respectively [18]. AN was determined using the alkaline hydrolyzable method. AP was extracted with hydrochloric acid and ammonium fluoride, and contents were measured using the molybdenum blue method. AK was extracted with ammonium acetate, and measured by flame photometry [19].
Analysis of heavy soil metal
The microwave digestion system (Milestone ETHOS UP, Italy) was used to extract cuprum (Cu), plumbum (Pb), cadmium (Cd) and arsenic (As) from soil samples. The contents of these metals were determined by Inductively coupled plasma mass spectrometry (ICP-MS, PerkinElmer NexION 300X, U.S.A), using parameters listed in Table 1.
Table 1. The parameters for the ICP-MS.
| As | Cd | Pb | Cu | Parameters |
|---|---|---|---|---|
| Radio-frequency power | 1300 w | |||
| Plasma velocity | 13.00 L/min | |||
| Auxiliary airflow velocity | 1.40 L/min | |||
| Flow rate of the carrier gas | 0.95 L/min | |||
| Mass-to-charge ratio (m/z) | 63 | 208 | 111 | 75 |
| Atomization device | MCN | |||
| Atomizer chamber | Peltier-cooler (2°C) |
Genomic DNA purification and PCR amplification
Total soil DNA was extracted using the BioFast soil Genomic DNA Extraction kit (BioFlux, Hangzhou, China), following the manufacturer’s instructions. For each soil sample, three independent DNA extractions were performed. DNA was diluted to a concentration of 1 ng/μL in sterile water. The variable regions 3 to 4 (V3–V4) were amplified with the specific primers 338F/806R (338F, 5’-ACTCCTACGGGAGGCAGCA-3’; 806R, 5’-GGACTACHVGGGTWTCTA AT-3’). The PCR reactions were conducted in a 50 μL mixture system, using TransStart Fastpfu DNA Polymerase (TransGen Biotechnology, Beijing, U.S.A). The PCR condition was initiated denaturation with 5 min at 95°C, followed by 35 cycles of 40 s at 95°C, 40 s at 58°C, 60 s at 72°C and final elongation with 5 min at 72°C. PCR products were purified using the Qiagen Gel Extraction Kit (Qiagen, Germany), and subjected to sequencing on the Illumina HiSeq 2500 platform (Allwegene Technologies Co., Ltd., Beijing, China).
Statistical analysis
The FLASH method [20] was used to merge paired-end reads. Following quality filtering and chimera removal [21], the effective tags were used to perform OTU clustering and species annotation. Species annotation was performed using the Silva database (http://www.arb-silva.de) [22]. For each remaining sequences, the RDP classifier (Version 2.2) algorithm [23] was used to annotate taxonomic information via the GreenGen database [24]. Mothur version 1.31.2 [25] was used to analyze the alpha diversities. Beta diversities were then calculated to analyze the complexity of species diversity. The Statistical Package for the GraphPad Prism version 5.1 and the Data Processing System (DPS) version 7.05 were used for statistical analysis. Differences among the treatments were calculated and statistically analyzed using the analysis of variance (ANOVA) and the LSD multiple range tests (p < 0.05).
Results
Tea and soil chemical characteristics
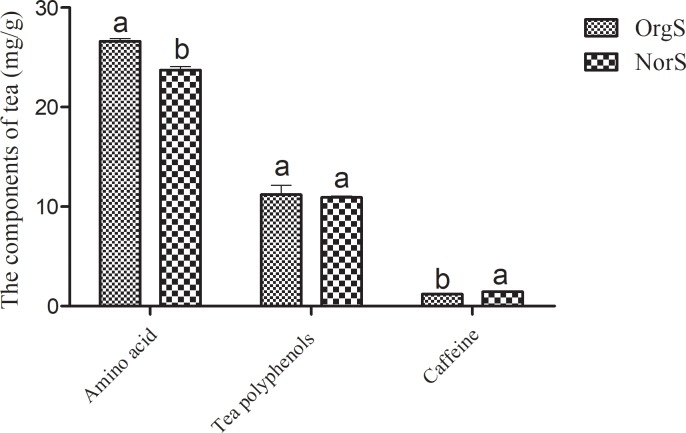
In this study, we found that the use of organic fertilizer significantly increased the amino acids content of tea (Fig 1). The contents of polyphenol did not reach statistical significance in tea samples from fields treated with organic fertilizer compared to those treated with chemical fertilizer. In contrast, tea under long-term treatment with chemical fertilizer showed significantly higher contents of caffeine compared to tea with organic fertilizer.
Fig 1. Amino acids, polyphenols, and caffeine contents of tea under treatment with organic (OrgS) or chemical (NorS) fertilizer.
Columns with different letters are statistically different (LSD test, P < 0.05).
Table 2 summarized the chemical properties of soil from tea orchards treated with either organic or chemical fertilizers. Contents of total nitrogen, total potassium, available nitrogen, available phosphorus, and available potassium were similar between the two treatment groups (P > 0.05). However, soil pH level was significantly higher in the organic fertilizer treatment group compared to the chemical fertilizer treatment group (Table 2).
Table 2. Chemical properties of soils from tea orchards with different treatments.
| Soil chemical properties | CKOrgS | CKNorS | OrgS | NorS |
|---|---|---|---|---|
| Total nitrogen (TN) (g/kg) | 1.06b | 1.38ab | 1.59ab | 1.82a |
| Total phosphorus (TP) (g/kg) | 0.27b | 0.22b | 0.36b | 1.60a |
| Total potassium (TK) (g/kg) | 6.16a | 4.73a | 6.42a | 5.47a |
| Available nitrogen (AN) (g/kg) | 0.08b | 0.13ab | 0.16ab | 0.18a |
| Available phosphorus (AP) (g/kg) | 0.01b | 0.01b | 0.03ab | 0.08a |
| Available potassium (AK) (g/kg) | 0.11a | 0.10a | 0.13a | 0.13a |
| pH | 4.24a | 4.10ab | 4.19a | 4.00b |
OrgS and NorS refer to rhizosphere soils of organic fertilizer and chemical fertilizer treatments, respectively. CKOrgS and CKNorS refer to non-rhizosphere soils. Columns with different letters are statistically different (LSD test, P < 0.05).
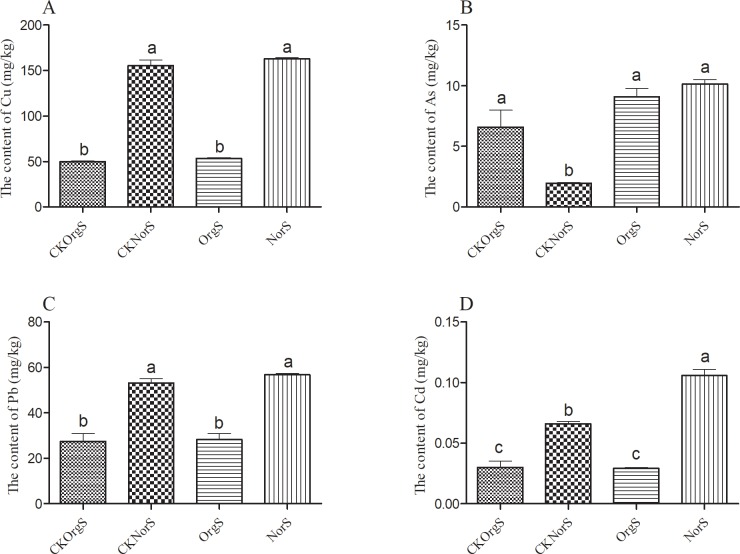
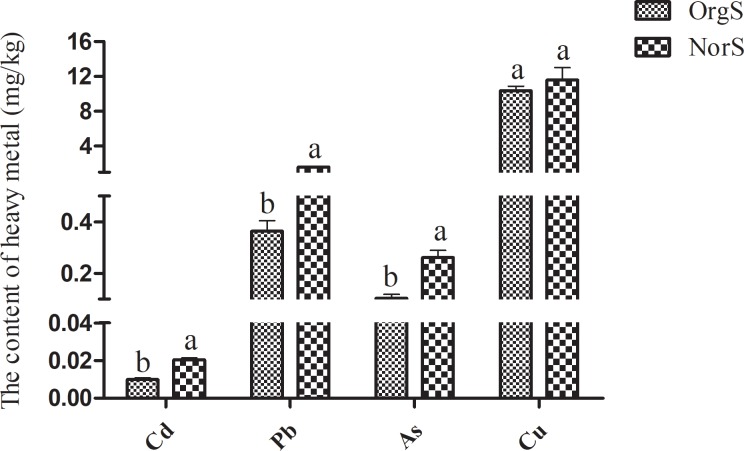
Tea orchards with long-term organic or chemical fertilizer treatment showed significant differences in soil chemical properties (Fig 2). Treatment with organic fertilizer resulted in significantly lower contents (P < 0.05) of cuprum (Cu), plumbum (Pb) and cadmium (Cd) in rhizosphere soils compared to the chemical fertilizer treatment group. A small decrease in arsenic (As) level was also detected in the organic fertilizer treatment group, but the difference was not statistically significant. Similar trends were observed in non-rhizosphere soil samples. Our results also showed that treatment with organic fertilizer significantly decreased contents of Cd, Pb and As in tea leaves (Fig 3).
Fig 2. Heavy metals content in non-rhizosphere and rhizosphere soil samples from tea orchards under organic or chemical fertilizer treatment.
Columns with different letters are statistically different (LSD test, P < 0.05).
Fig 3. Heavy metals content in tea leaf samples from tea orchards under organic or chemical fertilizer treatment.
Columns with different letters are statistically different (LSD test, P < 0.05).
Alpha diversity indices of microbial community
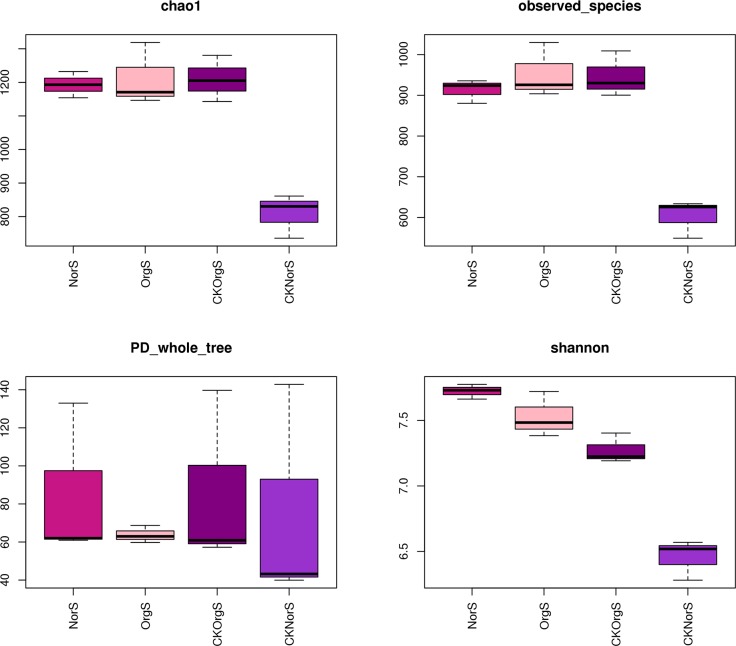
A total of 544,096 effective clean tags with bacterial species annotation were obtained from 12 soil samples. Alpha diversity was calculated to determine the complexity of species diversity. We observed a significantly higher bacterial composition and Chao1 indices with samples from the organic fertilizer treatment group compared to samples from the chemical fertilizer treatment group. Long-term organic fertilizer treatment also had a positive effect on non-rhizosphere soil. Our results showed that chemical fertilizer significantly increased Shannon’s diversity indices in rhizosphere soil in comparison to all treatments (Fig 4).
Fig 4. Observed richness, OTUs and diversity of soil samples from organic and chemical fertilizer treatment groups.
Beta diversity indices of microbial composition
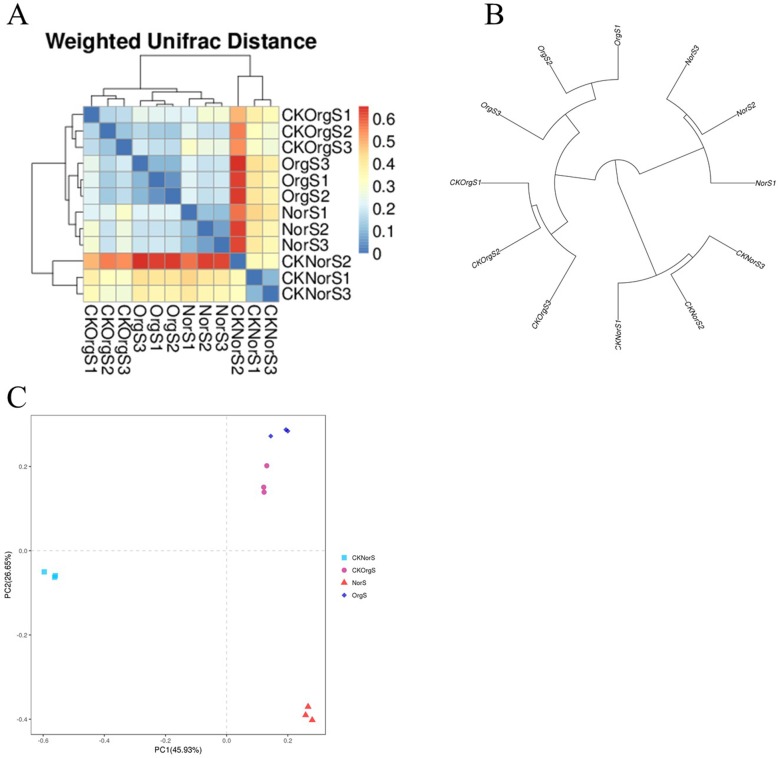
We used weighted unifrac heatmap, hierarchical clustering, and principal component analysis to identify differences in bacterial composition structure between the treatment groups (Fig 5). In comparison to CKNorS, higher distances were observed among the OrgS, CKOrgS, and NorS samples. The PC1 and PC2 components of PCoA accounted for 45.93% and 26.65% of the total bacterial composition variations, respectively. We found that the bacterial composition of OrgS and CKOrgS soil samples belonged to the same group based on the principal component analysis. In contrast, the bacterial composition of NorS and CKNorS samples fell into two separate groups that were distinct from OrgS and CKOrgS samples.
Fig 5. Beta diversity analysis of microbial composition.
(A) Weighted unifrac heatmap; (B) Hierarchical clustering analysis; (C) Principal Component Analysis.
Shifts in soil bacterial composition structure
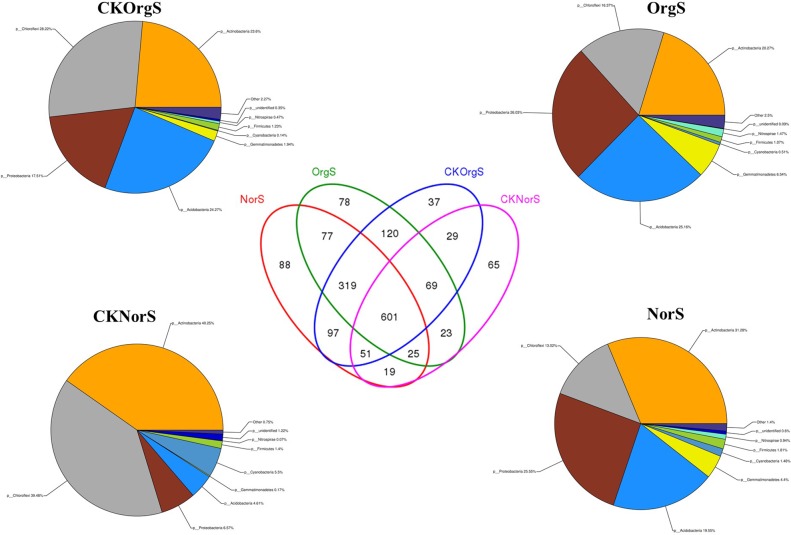
In this study, the classified sequences were affiliated with 24 bacterial phyla among the treatment groups. The majority of the phyla were assigned to Actinobacteria, Chloroflexi, Proteobacteria, Acidobacteria, Gemmatimonadetes, and Cyanobacteria (S1 Fig). Meanwhile, clear trends in variation at the phylum level were observed between the organic fertilizer and chemical fertilizer treatment groups. The number of OTUs exclusively found in OrgS and NorS samples were 78 (4.59%) and 88 (5.18%), respectively. The shared number of exclusive OTUs between OrgS and NorS were 1022 (60.19%). The shared number between OrgS and CKOrgS were 1109 (65.31%), and they dropped to 696 (41.10%) between NorS and CKNorS (Fig 6).
Fig 6. Venn diagram and relative percentages of bacterial phyla in the four different soil samples.
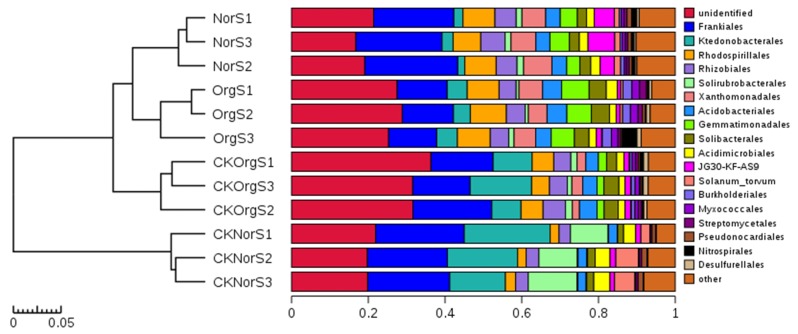
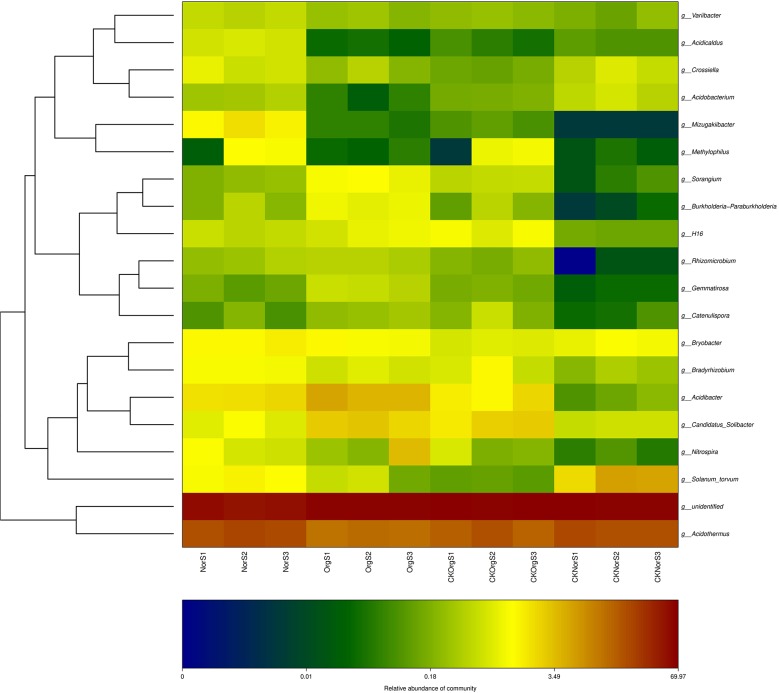
The relative abundance of these bacterial orders varied among the different soil samples. A comparison between OrgS and NorS showed that organic fertilizer treatment resulted in a significant increase in the relative abundance of Burkholderiales, Myxococcales, Streptomycetales, Nitrospirales, Ktedonobacterales, Acidobacteriales, Gemmatimonadales, and Solibacterales, and a decrease in Pseudonocardiales, Frankiales, Rhizobiales, and Xanthomonadales (S1 Table and Fig 7). Heat map analysis of the top 20 most abundant genera within the hierarchical cluster showed clear variations in bacterial composition structure across the four groups of soil samples, and these differences were statistically significant. Treatment with chemical fertilizer resulted in increased abundance of Acidothermus, Acidicaldus, and Acidobacterium, and decreased abundance of the potentially beneficial Nitrospira and Burkholderia in comparison with the organic fertilizer treatment group. No significant differences were detected in comparisons between OrgS and CKOrgS groups, as well as between NorS and CKNorS groups (Fig 8).
Fig 7. Relative abundance of the top 20 bacterial orders in the four different soil samples.
Fig 8. Heat map analysis of the top 20 bacterial orders in the four different soil samples.
Effects of soil chemical properties on dominant genera
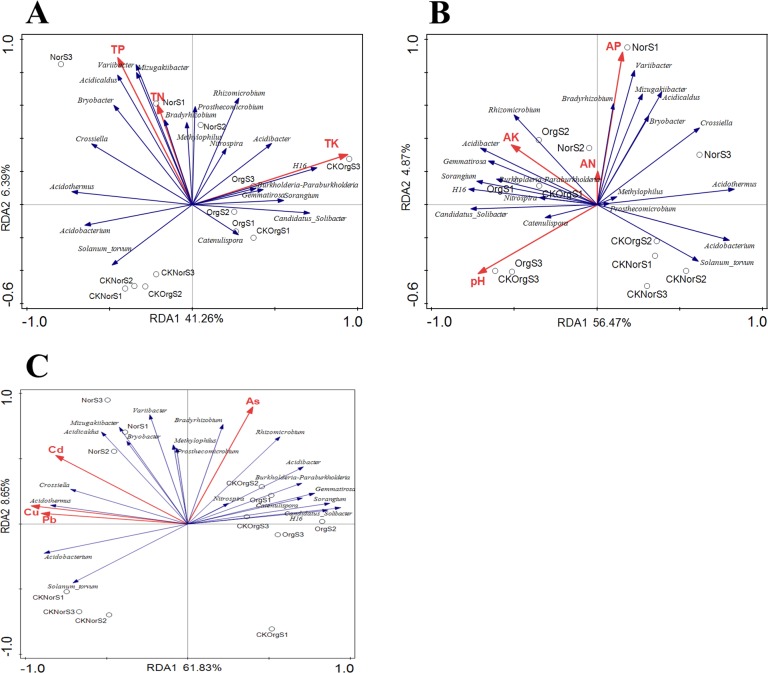
Redundancy analysis (RDA) was performed to study the relationship between soil chemical properties and abundance of dominant genera. The first two RDA components (RDA1 and RDA2) separated the organic fertilizer treated soils from the chemical fertilizer treated soils (Fig 9). The chemical fertilizer treated samples (NorS) were positively related to the cadmium (Cd), Cuprum (Cu) and plumbum (Pb).
Fig 9. Redundancy analysis (RDA) of the correlation between the most abundant genera of bacteria and soil physiochemical properties.
TP: total phosphorus, TN: total nitrogen, TK: total potassium, AN: available nitrogen, AK: available potassium, AP: available phosphorus.
The organic fertilizer treated samples (OrgS and CKOrgS) were positively related to a higher relative abundance of Catenulispora, Candidatus_Solibacter, Burkholderia-Paraburkholderia, Gemmatirosa, Nitrospira, Rhizomicrobium and negatively related to Acidobacterium, Acidothermus and Acidicaldus. Strong associations were found among total nitrogen (TN), total phosphorus (TP), available phosphorus (AP), available nitrogen (AN), cadmium (Cd), Cuprum (Cu) and plumbum (Pb) with the abundance of Acidothermus, Acidobacterium and Acidicaldus. The abundance of Acidibacter, Catenulispora, Burkholderia-Paraburkholderia, Gemmatirosa, Nitrospira, Candidatus_Solibacter, Rhizomicrobium and Sorangium were found to be highly associated with soil pH.
Discussion
For centuries, the use of organic fertilizer is a common practice to maintain soil fertility and crop yield in China [26–27]. With increased availability of chemical fertilizers since the late 1970s and rise of labor costs since the 1980s, the utilization of organic fertilizer has dramatically declined [26, 28]. In order to prevent food shortage worldwide and maximize crop yield, large amounts of chemical fertilizers have been applied to arable fields over the past few decades [29–30]. However, excessive use of chemical fertilizers has led to several issues such as serious soil degradation, nitrogen leaching, soil compaction, reduction in soil organic matter, and loss of soil carbon. In addition, the efficacy of chemical fertilizers on crop yield has been decreasing over time [29, 31–32]. Because of these concerns, there is a growing demand for development of organic agriculture. In this study, we found that N, P and K contents were not significantly different between organic and chemical fertilizer treatments. These results indicated that organic fertilizers provide similar nutrient elements as chemical fertilizer. In recent years, soil acidification has become a serious problem for modern agriculture in China [33]. The major cause of rising soil acidity is increased use of acidifying nitrogen fertilizers or incomplete cycling of nitrogen species in the soil [33–34]. Our previous studies have found tea orchards soil pH, tea amino acids, and phenolic compounds significantly decreased with increasing years of monoculturing [1, 5]. Furthermore, our data showed that organic fertilizer treatment improved soil pH, and increased the contents of tea polyphenols and amino acids. It is well known that acidification can increase the mobility of heavy metals into the soil where they could be taken up by plants [35–36]. The RDA analysis showed the some acidic microbes were highly associated with heavy metals. Previous studies have verified the heavy metal polluted soil would affect the microbial community structure, microbial biomass and microbial residues [37–39]. Some specific microorganism have the ability to adsorb heavy metal [40–41]. We found that the contents of heavy metals (Cu, Pb, Cd, As) were lower in soil and plant samples under organic fertilizer treatment compared to those treated with chemical fertilizer. The future research should focus the potential mechanism of intrinsic linkages between microbial community and heavy metal under the organic fertilizer treatments.
Healthy and asymptomatic plants in nature are colonized by a rich diversity of microbes, with the complex plant-associated microbial community referred to as a second genome of the plant because of its effect on plant growth and productivity [42–43]. In this study, we found that the long-term use of organic fertilizer significantly increased the bacterial diversity in terms of species richness. Our data are in agreement with the findings of Sun et al. [29], who reported that the NPK chemical fertilizers caused a significant decrease in bacterial diversity. Significant differences in soil bacterial composition were also observed in tea orchards under long-term treatment with chemical or organic fertilizers. The most abundantly identified bacteria phyla were assigned to the Actinobacteria, Chloroflexi, Proteobacteria, and Acidobacteria phyla. Similar results were observed with long-term continuous cropping tea orchard systems [5, 44]. Further analysis showed that Acidothermus, Acidobacterium and Acidicaldus, increased significantly in soil samples treated with chemical fertilizer, which are acidophilic and capable of proliferating in an acidic environment. In our RDA analysis, Acidibacter were found to be highly associated with soil pH. It has been well documented that soil pH has a marked influence on the composition of the microbial community [45]. Our previous study have shown the plant–microbe interactions contribute to the increased acidity and create a new environment to mediate changes in the microbial community structure in the R. pseudostellariae rhizosphere under continuous monoculture regimes [46]. We speculate that long-term application of chemical fertilizers decreased soil pH, promoted the proliferation of some specific microbes and activated the heavy metal ions in soil, further deteriorated the physicochemical properties and quality of tea. However, the long-term application of organic fertilizer was able to alleviate some of this negative effect.
Positive plant-soil feedback depends on beneficial interactions between plant roots and microorganisms for growth promotion, nutrient acquisition and disease suppression [4]. Previous studies reported that increasing years of consecutive monoculturing resulted in a significant increase in abundance of pathogens and a decrease in beneficial microorganisms in the rhizosphere of plants [47–48]. The significant decrease in beneficial plant bacteria was also observed in the rhizosphere soil of continuously monocultured tea [5]. In this study, the relative abundance of Burkholderia, Nitrospira, and Streptomycetales was significantly higher with organic fertilizer treatment. Previous studies have found that Burkholderia was able to inhibit the growth of pathogens and in effect acted as a Bio-organic fertilizer to improve plant growth [49–50]. Nitrospira is a ubiquitous bacterium that has a role in the nitrogen cycle by performing nitrite oxidation in the second step of nitrification [51–52]. Furthermore, Streptomycetales can act as PGPR (rhizosphere growth-promoting bacteria) to reduce plant disease and are associated with plant disease suppression in many soils [53–54]. These results suggested that organic fertilizer could improve the soil environment to create a new condition for the growth of potentially beneficial microbes.
In conclusion, long-term application of organic fertilizer treatment will improve the rhizosphere environment in tea orchards. And the organic fertilizer improved tea quality and decreased the level of heavy metals in rhizosphere soil. Furthermore, soil pH and shift in microbiomes were related to fertilizers treatments. Our findings suggest that organic fertilizer can shape microbial composition and recruit beneficial bacteria into the rhizosphere of tea. These results provide a promising strategy to tea orchards by treatment with organic fertilizers.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
This work was supported by by the National Natural Science Foundation of China (No. 31701329, 81573530), Fujian-Taiwan Joint Innovative Center for Germplasm Resources and Cultivation of Crop (Fujian 2011 Program, No.2015-75, China), the Natural Science Foundation of Fujian Province (No. 2016J01100), and the Project Funded by China Postdoral Science Foundation (No. 2015M580560).
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by: National Natural Science Foundation of China (No. 31701329, 81573530); Fujian-Taiwan Joint Innovative Center for Germplasm Resources and Cultivation of Crop (Fujian 2011 Program, No.2015-75, China); the Natural Science Foundation of Fujian Province (No. 2016J01100); and the Project Funded by China Postdoral Science Foundation (No. 2015M580560).
References
- 1.Li Y, Li Z, Arafat Y, Lin W, Jiang Y, Weng B, et al. Characterizing rhizosphere microbial communities in long-term monoculture tea orchards by fatty acid profiles and substrate utilization. Eur J Soil Biol. 2017; 81:48–54. [Google Scholar]
- 2.Gao G. Experiment studies on the hygienics of As、Ba、Cd、Pb in tea. Chin J Food Hygiene. 2001; 13:12–14. [Google Scholar]
- 3.Lan HX, Xia JG. Absorption and Accumulation of Lead and Cadmium in Mengshan Tea Plant. J Agro-Environ Sci. 2008; 27:1077–1083. [Google Scholar]
- 4.Arafat Y, Wei X, Jiang Y, Chen T, Saqib HAS, Lin S, et al. Spatial distribution patterns of root-associated bacterial communities mediated by root exudates in different aged ratooning tea monoculture systems. Int J Mol Sci. 2017; 18:1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li YC, Li Z, Li ZW, Jiang YH, Weng BQ, Lin WX. Variations of rhizosphere bacterial communities in tea (Camellia sinensis L.) continuous cropping soil by high-throughput pyrosequencing approach. J Appl Microbiol. 2016; 121:787–799. 10.1111/jam.13225 [DOI] [PubMed] [Google Scholar]
- 6.Zhang QC, Shamsi IH, Xu DT, Wang GH, Lin XY, Jilani G, et al. Chemical fertilizer and organic manure inputs in soil exhibit a vice versa pattern of microbial community structure. Appl Soil Ecol. 2012; 57:1–8. [Google Scholar]
- 7.Chang KH, Wu RY, Chuang KC, Hsieh TF, Chung RS. Effects of chemical and organic fertilizers on the growth, flower quality and nutrient uptake of Anthurium andreanum, cultivated for cut flower production. Sci Hortic-Amsterdam. 2010; 125:434–441. [Google Scholar]
- 8.Ahmad R, Jilani G, Arshad M, Zahir ZA, Khalid A. Bio-conversion of organic wastes for their recycling in agriculture: an overview of perspectives and prospects. Ann Microbiol. 2007; 57:471–479. [Google Scholar]
- 9.Wang W, Niu J, Zhou X, Wang Y. Long-term change in land management from subtropical wetland to paddy field shifts soil microbial community structure as determined by PLFA and T-RFLP. Pol J Ecol. 2011; 59:37–44. [Google Scholar]
- 10.Lin B, Luo GH, Xu QX, Wang QS, Guan XF. Effects of biogas residue on yield and quality of tea. Fujian J Agric Sci. 2010; 25:90–95. [Google Scholar]
- 11.Zhang Q, Wei CX. Effects of different organic fertilizers on chief qualities of tea. Guizhou Agric Sci. 2012; 40:65–67. [Google Scholar]
- 12.Sun QR, Xu Y, Xiang L, Wang GS, Shen X, Chen XS, et al. Effects of a mixture of bacterial manureand biochar on soil environment and physiological characteristics of Mals huupehens seedlings. Chin Agric Sci Bull. 2017; 33:52–59. [Google Scholar]
- 13.Xu HQ, Xiao RL, Xiang ZX, Huang Y, Luo W, Qin Z, et al. Effects of different ecological manage-ment on the soil microbial biomass and microbial population of tea plantation in hilly red soil region. Chin J Soil Sci. 2010; 41:1355–1359. [Google Scholar]
- 14.Li YC, Li ZW, Lin WW, Jiang YH, Weng BQ, Lin WX. Effects of biochar and sheep manure on rhizospheric soil microbial community in continuous ratooning tea orchards. Chin J Appl Ecol. 2018; 29:1273–1282. [DOI] [PubMed] [Google Scholar]
- 15.Owuor PO, Othieno CO, Kamau DM, Wanyoko JK. Effects of long-term fertilizer use on a high-yielding tea clone AHPS15/10: soil pH, mature leaf nitrogen, mature leaf and soil phosphorus and potassium. Int J Tea Sci. 2011; 8:15–51. [Google Scholar]
- 16.Yang J, Yang Z, Zou J. Effects of rainfall and fertilizer types on nitrogen and phosphorus concentrations in surface runoff from subtropical tea fields in Zhejiang, China. Nutr Cycl Agroecosys. 2012; 93:297–307. [Google Scholar]
- 17.Cheng Y, Wang J, Zhang JB, Müller C, Wang SQ. Mechanistic insights into the effects of N fertilizer application on N2O-emission pathways in acidic soil of a tea plantation. Plant Soil. 2015; 389:45–57. [Google Scholar]
- 18.Bao SD. Soil and Agricultural Chemistry Analysis, second ed Beijing: China Agriculture Press; 2000; 263–270. [Google Scholar]
- 19.Pansu M, Gautheyrou J. Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods. Berlin: Springer; 2006; 3–13 [Google Scholar]
- 20.Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011; 27:2957–2963. 10.1093/bioinformatics/btr507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011; 27:2194–2200. 10.1093/bioinformatics/btr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013; 41:590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007; 73:5261–5267. 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006; 72:5069–5072. 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: Open-Source, Platform-Independent, Community- Supported Software for Describing and Comparing Microbial Communities. Appl Environ Microbiol. 2009; 75:7537–7541. 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan X, Gong W. The role of chemical and organic fertilizers on yield, yield variability and carbon sequestration results of a 19-year experiment. Plant Soil. 2010; 331:471–480. [Google Scholar]
- 27.Dormaar JF, Lindwall CW, Kozub GC. Effectiveness of manure and commercial fertilizer in restoring productivity of an artificially eroded dark brown chernozemic soil under dryland conditions. Can J Soil Sci. 1988; 68:669–679. [Google Scholar]
- 28.Zhu ZL, Chen DL. Nitrogen fertilizer use in China contributions to food production, impacts on the environment and best management strategies. Nutr Cycl Agroecosys. 2002; 63:117–127. [Google Scholar]
- 29.Sun R, Zhang XX, Guo X, Wang D, Chu H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol Biochem. 2015;88:9–18. [Google Scholar]
- 30.Savci S. An agricultural pollutant: chemical fertilizer. Int J Environ Sci Te. 2012; 3:77–80. [Google Scholar]
- 31.Nkoa R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: a review. Agron Sustain Dev. 2014; 34:473–492. [Google Scholar]
- 32.Horrigan L, Lawrence RS, Walker P. How sustainable agriculture can address the environmental and human health harms of industrial agriculture. Environ Health Persp. 2002; 110: 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo JH, Liu XJ, Zhang Y, Shen JL, Han WX, Zhang WF, et al. Significant acidification in major Chinese croplands. Science. 2010; 327:1008–1010. 10.1126/science.1182570 [DOI] [PubMed] [Google Scholar]
- 34.Bolan NS, Hedley MJ, White RE. Processes of soil acidification during nitrogen cycling with emphasis on legume based pastures. Plant Soil. 1991; 134:53–63. [Google Scholar]
- 35.Blake L, Goulding KWT. Effects of atmospheric deposition, soil pH and acidification on heavy metal contents in soils and vegetation of semi-natural ecosystems at Rothamsted Experimental Station, UK. Plant Soil. 2002; 240: 235–251. [Google Scholar]
- 36.Yang SX, Liao B, Li JT, Guo T, Shu WS. Acidification, heavy metal mobility and nutrient accumulation in the soil–plant system of a revegetated acid mine wasteland. Chemosphere. 2010; 80:852–859. 10.1016/j.chemosphere.2010.05.055 [DOI] [PubMed] [Google Scholar]
- 37.Kandeler E, Tscherko D, Bruce KD, Stemmer M, Hobbs PJ, Bardgett RD, et al. Structure and function of the soil microbial community in microhabitats of a heavy metal polluted soil. Biol Fert Soils. 2000; 32:390–400. [Google Scholar]
- 38.Hemme CL, Deng Y, Gentry TJ, Fields MW, Wu L, Barua S, et al. Metagenomic insights into evolution of a heavy metal-contaminated groundwater microbial community. ISME J. 2010; 4:660–672. 10.1038/ismej.2009.154 [DOI] [PubMed] [Google Scholar]
- 39.Gremion F, Chatzinotas A, Kaufmann K, Von Sigler W, Harms H. Impacts of heavy metal contamination and phytoremediation on a microbial community during a twelve-month microcosm experiment. FEMS Microbiol Ecol. 2004; 48:273–283. 10.1016/j.femsec.2004.02.004 [DOI] [PubMed] [Google Scholar]
- 40.Goyal N, Jain SC, Banerjee UC. Comparative studies on the microbial adsorption of heavy metals. Ad Environ Res. 2003; 7:311–319. [Google Scholar]
- 41.Carpio IEM, Mangadlao JD, Nguyen HN, Advincula RC, Rodrigues DF. Graphene oxide functionalized with ethylenediamine triacetic acid for heavy metal adsorption and anti-microbial applications. Carbon. 2014; 77:289–301. [Google Scholar]
- 42.Hacquard S. Disentangling the factors shaping microbiota composition across the plant holobiont. New Phytol. 2016; 209:454–457. 10.1111/nph.13760 [DOI] [PubMed] [Google Scholar]
- 43.Berendsen RL, Pieterse CMJ, Bakker P. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012; 17:478–486. 10.1016/j.tplants.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 44.Zhao J, Wu X, Nie C, Wu T, Dai W, Liu H, et al. Analysis of unculturable bacterial communities in tea orchard soils based on nested PCR-DGGE. World J Microbiol Biot. 2012; 28:1967–1979. [DOI] [PubMed] [Google Scholar]
- 45.Rousk J, Brookes PC, Bååth E. The microbial PLFA composition as affected by pH in an arable soil. Soil Biol Biochem. 2010; 42:516–520. [Google Scholar]
- 46.Wu H, Qin X, Wang J, Wu L, Chen J, Fan J, et al. Rhizosphere responses to environmental conditions in Radix pseudostellariae under continuous monoculture regimes. Agr Ecosyst Environ. 2019; 270:19–31. [Google Scholar]
- 47.Wu L, Wang J, Huang W, Wu H, Chen J, Yang Y, et al. Plant-microbe rhizosphere interactions mediated by Rehmannia glutinosa root exudates under consecutive monoculture. Sci Rep. 2015; 5:15871 10.1038/srep15871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu H, Wu L, Wang J, Zhu Q, Lin S, Xu J, et al. Mixed Phenolic Acids Mediated Proliferation of Pathogens Talaromyces helicus and Kosakonia sacchari in Continuously Monocultured Radix pseudostellariae Rhizosphere Soil. Front Microbiol. 2016; 7:335 10.3389/fmicb.2016.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van VT, Berge O, NgôKê S, Balandreau J, Heulin T. Repeated beneficial effects of rice inoculation with a strain of Burkholderia vietnamiensison early and late yield components in low fertility sulphate acid soils of Vietnam. Plant Soil. 2000; 218:273–284. [Google Scholar]
- 50.Wu L, Chen J, Wu H, Qin X, Wang J, Wu Y, et al. Insights into the Regulation of Rhizosphere Bacterial Communities by Application of Bio-organic Fertilizer in Pseudostellaria heterophylla Monoculture Regime. Front Microbiol. 2016; 7:1788 10.3389/fmicb.2016.01788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koch H, Lücker S, Albertsen M, Kitzinger K, Herbold C, Spieck E, et al. Expanded metabolic versatility of ubiquitous nitrite-oxidizing bacteria from the genus Nitrospira. Proc Natl Acad USA. 2015; 112:11371–11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lücker S, Wagner M, Maixner F, Pelletier E, Koch H, Vacherie B, et al. A Nitrospira metagenome illuminates the physiology and evolution of globally important nitrite-oxidizing bacteria. Proc Natl Acad USA. 2010; 107:13479–13484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu L, Kloepper JW, Tuzun S. Induction of systemic resistance in cucumber by plant growth-promoting rhizobacteria: duration of protection and effect of host resistance on protection and root colonization. Phytopathology. 1995; 85:1064–1068. [Google Scholar]
- 54.Kinkel LL, Schlatter DC, Bakker MG, Arenz BE. Streptomyces competition and co-evolution in relation to plant disease suppression. Res Microbiol. 2012; 163:490–499. 10.1016/j.resmic.2012.07.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.