Abstract
Objectives
To investigate the prevalence of depressive symptoms in rheumatoid arthritis (RA) patients using two previously validated questionnaires in a large patient sample, and to evaluate depressive symptoms in the context of clinical characteristics (e.g. remission of disease) and patient-reported impact of disease.
Methods
In this cross-sectional study, the previously validated Patient Health Questionnaire (PHQ-9) and Beck-Depression Inventory II (BDI-II) were used to assess the extent of depressive symptoms in RA patients. Demographic background, RA disease activity score (DAS28), RA impact of disease (RAID) score, comorbidities, anti-rheumatic therapy and antidepressive treatment, were recorded. Cut-off values for depressive symptomatology were PHQ-9 ≥5 or BDI-II ≥14 for mild depressive symptoms or worse and PHQ-9 ≥ 10 or BDI-II ≥ 20 for moderate depressive symptoms or worse. Prevalence of depressive symptomatology was derived by frequency analysis while factors independently associated with depressive symptomatology were investigated by using multiple logistic regression analyses. Ethics committee approval was obtained, and all patients provided written informed consent before participation.
Results
In 1004 RA-patients (75.1% female, mean±SD age: 61.0±12.9 years, mean disease duration: 12.2±9.9 years, DAS28 (ESR): 2.5±1.2), the prevalence of depressive symptoms was 55.4% (mild or worse) and 22.8% (moderate or worse). Characteristics independently associated with depressive symptomatology were: age <60 years (OR = 1.78), RAID score >2 (OR = 10.54) and presence of chronic pain (OR = 3.25). Of patients classified as having depressive symptoms, only 11.7% were receiving anti-depressive therapy.
Conclusions
Mild and moderate depressive symptoms were common in RA patients according to validated tools. In routine clinical practice, screening for depression with corresponding follow-up procedures is as relevant as incorporating these results with patient-reported outcomes (e.g. symptom state), because the mere assessment of clinical disease activity does not sufficiently reflect the prevalence of depressive symptoms.
Clinical trial registration number
This study is registered in the Deutsches Register Klinischer Studien (DRKS00003231) and ClinicalTrials.gov (NCT02485483).
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease of the musculoskeletal system that leads to pain, swelling and progressive joint destruction via increased activity of pro-inflammatory cytokines [1]. Although a variety of conventional and biological disease modifying anti-rheumatic drugs are available to control disease activity in RA, the disease is still challenging and usually requires life-long treatment. Furthermore, it is associated with a substantial reduction of overall physical and emotional well-being not only due to articular symptoms including pain and loss of physical function but also as a result of fatigue and impaired quality of sleep [1, 2]. This disease burden can affect the patient’s quality of life and socioeconomic outcomes including workplace absenteeism and presenteeism (low workplace productivity) [3–5]. For patients with RA, a greater loss of workplace productivity has been associated not only with functional disability but also with depressive symptoms [6]. Moreover, depression has also been associated with high costs of absenteeism and presenteeism in a wide range of countries across a variety of socioeconomic statuses [7]. Against this background, the challenge of depression as a comorbidity of RA becomes evident: in combination, these disorders pose an even more considerable burden for patients and are likely to result in more frequent and longer periods of absence. In clinical practice, depressive symptoms and affective disorders are common in RA [8–10] with a recent meta-analysis reporting 16.8% of RA patients as having a major depressive disorder [11]. Furthermore, Dougados et al. found depression to be the most frequent comorbidity in RA (15%) [12]. Thus, detecting and addressing depression in patients with RA needs to be a part of patient care. This means, applying standardized procedures to screen for depression and in the case of positive screening results, a follow-up with a full diagnostic assessment [13].
A recent meta-analysis emphasized the need for screening tools to be validated against a clinical interview as gold standard in order to assess depressive symptoms [11]. Following these recommendations, we previously evaluated the psychometric properties of the Patient Health Questionnaire 9 (PHQ-9) [14], the Beck Depression Inventory II (BDI-II) [15] and the World Health Organization 5-Item Well-Being Index (WHO-5) [16] against a structured patient interview using the Montgomery-Ǻsberg Depression Rating Scale (MADRS) in RA patients [17]. Within this validation study (VADERA I), we were able to show that the PHQ-9 and BDI-II are valid and reliable for depression screening in patients with RA [17]. The aim of the present study–VADERA II–was to use the PHQ-9 and BDI-II to determine the prevalence of depressive symptoms in RA patients in clinical practice and to subsequently evaluate the role of covariates such as disease impact measured using the Rheumatoid Arthritis Impact of Disease (RAID) questionnaire. Although a number of instruments have been introduced to assess depressive symptoms in rheumatology [11, 18], to the best of our knowledge, VADERA II is the first study within its field to have explicit validation of self-reporting tools prior to determining prevalence of depressive symptoms.
Methods
Description of the patient sample
VADERA II was a cross-sectional study. Patients were enrolled from October 2014 to September 2015 at centres in Germany. Inclusion criteria included a documented diagnosis of RA according to the ACR/EULAR 2010 criteria, age of ≥18 years, the ability to complete the questionnaires and scheduling of a RA consultation at one of the participating sites. To avoid sampling bias and to assure a representative study population, investigators were informed of the importance of enrolling a practice-oriented and representative sample of RA patients. There were no exclusions of patients due to RA disease activity, medical treatment or comorbidities. All sites were requested to document the reasons for non-participation of patients. Institutional review board approval was obtained from the ethics committee of the Medical Faculty of the Friedrich-Alexander University Erlangen-Nuremberg (4431), and all patients completed an informed consent form before participation. This study is registered in the Deutsches Register Klinischer Studien (DRKS00003231) and ClinicalTrials.gov (NCT02485483).
The study centres were all members of the working group for the evaluation of systemic effects of RA (SYRA). The VADERA II study was conducted in 10 centres representing a spectrum of clinical settings including large academic medical centres, community hospitals and private rheumatology practices across Germany. Study participation consisted of a single clinic visit, which included self-completion of the depression questionnaires.
Sample size considerations for this study were based on a review of the medical literature for previously reported depression prevalence rates in RA patients. The reported prevalence of depression in RA patients ranged from 6.4% as reported by Covic et al. in 2012 [19] to 41.5% as reported by Isik et al. in 2007 [20]. Thus, it was assumed that with the enrolment of 1000 patients, between 64 and 415 patients in the study would have depressive symptomatology, which would be adequate for the planned analyses.
Study-related procedures and questionnaire cut-points for depressive symptoms
Data collection in VADERA II was cross-sectional. All data collected in connection with the VADERA II study were entered in an electronic case report form (eCRF) including the paper-based BDI-II and PHQ-9 questionnaires [21] and the RAID questionnaire (consisting of seven items: pain, functional disability, fatigue, sleep, physical well-being, emotional well-being, coping) [2, 22], all of which were completed during the patient’s study visit. In addition, demographic data (age, gender, height, weight, family status, educational status and employment status), RA disease activity according to the Disease Activity Score based on 28 joints and erythrocyte sedimentation rate (DAS28 (ESR)), laboratory and serologic parameters (CRP, haemoglobin, ESR, rheumatoid factor and anti-citrullinated protein antibody (ACPA)), and comorbidities were recorded. Furthermore, information on treatment with anti-rheumatic therapy in categories (conventional and biological Disease-Modifying Anti-rheumatic Drugs (cDMARDs/bDMARDs), glucocorticoids, nonsteroidal anti-inflammatory drugs (NSAIDs), opioids, other medication groups) were recorded as well as anti-depressive treatment in categories (tricyclic antidepressants (TCAs), serotonin or norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors (MAOIs), psychotherapy, other). Comorbidities at the time of study participation and potential adverse events were also documented. Standardized cut-offs of the PHQ-9 and the BDI-II as defined by the questionnaire developers [15, 23] were applied.
Cut-off values for positive depressive symptomatology were PHQ-9 ≥5 or BDI-II ≥14 which corresponds to the presence of at least mild depressive symptoms. Numbers for the frequency of depressive symptomatology were derived by frequency analysis. Point prevalence rates (patients with positive depressive symptoms / total study population) and their 95% confidence intervals (CI) were calculated using the Clopper-Pearson (exact) method.
Covariates of depressive symptoms
The risk of depressive symptomatology was further evaluated by taking into account the effects of other covariates, which were included in univariate and multiple logistic regression analysis. The potential effects of covariates on the risk of depressive symptomatology were evaluated by the odds ratio (OR) and 95% CIs. All potentially important covariates were included in a full model, and a step-down procedure was used to evaluate the impact of the covariates in the model. The corresponding variables (and arbitrary cut-offs) of interest were as follows: Age (<60 years, ≥60 years), sex (0 = female, 1 = male), family status (0 = single, 1 = family), BMI kg/m2 (0 = <25, 1 = ≥25), education (ten years of school education or more = 1, less than ten years of school education = 0), employment (other = 0, 1 = employed), duration of RA (continuous variable), DAS28 (ESR) remission (<2.6, ≥2.6), RAID patient acceptable symptom state (PASS) (i.e. RAID ≤2 vs. RAID >2) [24], presence of chronic pain (0 = no, 1 = yes), presence of a comorbidity other than mental illness or chronic pain (0 = no, 1 = yes), and current medication–cDMARD (0 = no, 1 = yes), bDMARD (0 = no, 1 = yes), glucocorticoids (0 = no, 1 = yes), NSAIDs (0 = no, 1 = yes), opiates (0 = no, 1 = yes). Due to indications of multicollinearity in the multiple logistic regression when referring to RAID single numerical rating scales, we used the PASS criterion for the total score as the outcome of clinical relevance.
The potential effects of RAID single items on the risk of depressive symptomatology were measured by the OR and 95% CIs addressing distinct symptoms in the univariate analyses.
Sensitivity analyses were conducted using cut-off points for moderate depressive symptoms as previously defined by the developers of the PHQ-9 and BDI-II questionnaires. In order to evaluate the impact of the cut-off values of the PHQ-9 and the BDI-II on depression prevalence and the essential covariates, the cut-off values for moderate depressive symptomatology were set to a summary score ≥10 for the PHQ-9 questionnaire and a summary score of ≥20 for the BDI-II questionnaire, respectively.
All adverse events (AE), patients experienced between informed consent and completion of questionnaires were recorded, independent of AE severity or relationship to treatment. Continuous descriptive results are presented as mean (standard deviation; SD) if not stated otherwise. Descriptive analysis of nominal characteristics was conducted by referring to frequency analysis. Statistical analyses were computed using SAS Version 9.4 (SAS; Cary, NC, USA). The study results are reported according to the STROBE guidelines for observational studies [25].
Results
Patient sample
A total of 1015 patients with RA had given informed consent to the VADERA II study and 1004 patients (99%) completed at least one of the depression questionnaires and were included in the analysis. During this cross-sectional study, no serious AEs or adverse drug reactions occurred. Complete questionnaires (all questions answered) were available for evaluation of the PHQ-9 in 975 patients (97.1%) and the BDI II in 971 patients (96.7%). For the 36 of 1051 screened RA patients (3%) who did not complete the two depression questionnaires, the most common reasons were: ‘not enough time’ and ‘questions too personal’.
Patient characteristics
Mean patient age was 61.0 (SD 12.9) years; 75.1% were female. The most common family status was living with a partner (58.4%), followed by single/living alone (23.5%), or living with partner and children (16.0%). Amongst others, 52.1% of the patients reported being retired, 39.4% were employed at the time of study participation.
Mean duration of RA in the study population was 12.2 (SD 9.9) years. Serology results indicated that 52.7% of the patients were rheumatoid factor (RF) positive and 61.2% ACPA positive.
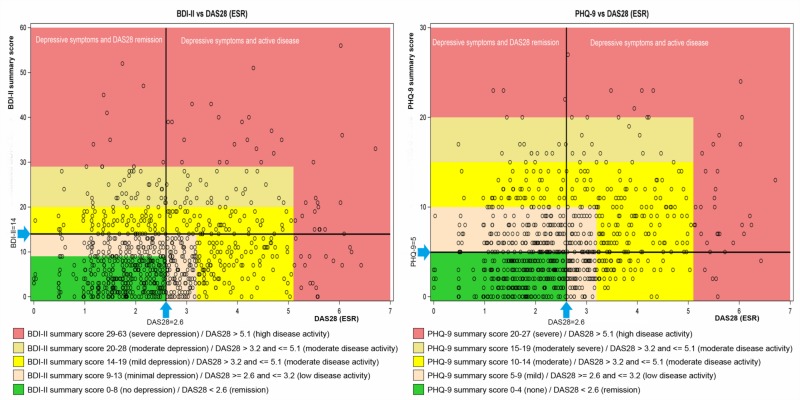
Mean DAS28 (ESR) was 2.5 (SD 1.2) while the median number of both swollen joints and tender joints was 0. The majority of patients (59.1%) were in DAS28 remission (DAS28 <2.6). ACR/EULAR Boolean remission [22] was reported for 25.6% of patients. DAS28 remission was found in 50.8% of patients having at least mild depressive symptoms in the PHQ-9 or the BDI-II (see Fig 1A and 1B). On average, the mean RAID score was 3.6 (SD 2.2) with 269 patients (27.0%) fulfilling RAID PASS with a total score ≤2 and 728 patients (73.0%) with a RAID score >2.
Fig 1. A/1B BDI-II and PHQ-9 scores by DAS28 (ESR).
Legend: (Fig 1A) DAS28 (ESR) scores by BDI-II score with black lines showing cut-point for mild depression and low disease activity. (Fig 1B) DAS28 (ESR) scores by PHQ-9 score with black lines showing cut-point for mild depressive symptoms and low disease activity.
Presence of a comorbidity at time of participation was reported by 60.9% of patients (58.6% of patients excluding mental illness). The most common comorbidities were cardiovascular disease (42.7%) including hypertension (38.1%), diabetes mellitus (9.6%), chronic pain due to conditions other than RA (9.4%) and chronic respiratory diseases (8.0%). Of the total study population, 7.5% were receiving anti-depressive therapy.
Details on sample characteristics are shown in Table 1.
Table 1. Study population characteristics.
| PHQ-9 or BDI-II indicating depressive symptoms | PHQ-9 or BDI-II not indicating depressive symptoms | Total | |
|---|---|---|---|
| Characteristics | (N = 556) | (N = 448) | (N = 1004) |
| Years of age [Mean (SD)] | 60.2 (12.2) | 62.0 (13.5) | 61.0 (12.9) |
| Gender [n (%)] | |||
| Female Male |
429 (77.2%) 127 (22.8%) |
325 (72.5%) 123 (27.5%) |
754 (75.1%) 250 (24.9%) |
| Duration of RA in years [Mean (SD)] | 12.3 (10.0) | 12.1 (9.6) | 12.2 (9.9) |
| Disease Activity Scores | |||
| DAS28 (ESR) Score [Mean (SD)] | 2.8 (1.3) | 2.2 (1.0) | 2.5 (1.2) |
| Boolean Remission [n (%)] | 88 (16.3%) | 162 (37.2%) | 250 (25.6%) |
| RAID score [Mean (SD)] | 4.6 (1.9) | 2.3 (1.7) | 3.6 (2.2) |
| Current RA therapy [n (%)] | |||
| cDMARDs | 441 (80.6%) | 364 (82.2%) | 805 (81.3%) |
| Glucocorticoids | 303 (55.4%) | 198 (44.7%) | 501 (50.6%) |
| bDMARDs | 269 (49.0%) | 197 (44.5%) | 466 (47.0%) |
| NSAIDs | 233 (42.5%) | 131 (29.6%) | 364 (36.7%) |
| Opioids | 36 (6.5%) | 10 (2.3%) | 46 (4.6%) |
| Presence of a comorbidity [n (%)] | 360 (64.7%) | 251 (56.0%) | 611 (60.9%) |
| Cardiovascular diseases | 239 (43.0%) | 190 (42.4%) | 429 (42.7%) |
| Diabetes mellitus | 54 (9.7%) | 42 (9.4%) | 96 (9.6%) |
| Other chronic pain | 73 (13.1%) | 21 (4.7%) | 94 (9.4%) |
| Chronic respiratory diseases | 47 (8.5%) | 33 (7.4%) | 80 (8.0%) |
| Mental illness (including depression) |
54 (9.7%) | 10 (2.2%) | 64 (6.4%) |
| Depression | 42 (7.6%) | 8 (1.8%) | 50 (5.0%) |
| Malignancies | 20 (3.6%) | 17 (3.8%) | 37 (3.7%) |
| Comorbidity without mental illness | 342 (61.5%) | 246 (54.9%) | 588 (58.6%) |
| Anti-depressive therapy [n (%)] | 65 (11.7%) | 10 (2.2%) | 75 (7.5%) |
a Number (%) of patients with non-missing data unless stated otherwise. Abbreviations: SD = standard deviation, RA = rheumatoid arthritis, DAS = Disease Activity Score, ESR = erythrocyte sedimentation rate, RAID = Rheumatoid Arthritis Impact of Disease questionnaire, cDMARDs = conventional Disease-Modifying Anti-rheumatic Drugs, bDMARDs = biological Disease-Modifying Anti-rheumatic Drugs, NSAIDs = Nonsteroidal Anti-inflammatory Drugs
Prevalence of depressive symptoms in RA
Overall, the median PHQ-9 score was 5.0 (range 0.0–27.0). Based on the categorized results from the PHQ-9, 55.1% (95% CI: 51.89–58.23) (537 / 975 patients) of patients reported at least mild depressive symptoms (Table 2) with the following distribution across the predefined categories: 44.9% (none), 34.2% (mild), 15.5% (moderate), 3.6% (moderately severe), and 1.8% (severe). The median BDI-II score was 8.0 (range 0.0–56.0). Based on the categorized results from the BDI-II, 27.7% (95% CI: 24.91–30.63) (269 / 971 patients) of the RA patients had at least mild depressive symptoms (Table 2) while 20.4% had minimal depressive symptoms, which was below the predefined cutpoint of interest in this study. Point prevalence rates for depressive symptoms based on the BDI-II were as follows: 51.9%, (none), 20.4% (minimal), 15.7% (mild), 7.8% (moderate), and 4.2% (severe).
Table 2. Point prevalence of depressive symptoms by depression questionnaire(s).
| Point Prevalence % (n / total) |
95% CI | |
|---|---|---|
| ≥ Mild depressive symptoms | ||
| PHQ-9 Positive (PHQ-9 ≥5) | 55.1% (537 / 975) | 51.89–58.23 |
| BDI-II Positive (BDI-II ≥14) | 27.7% (269 / 971) | 24.91–30.63 |
| BDI-II ≥14 or PHQ-9 ≥5 | 55.4% (556 / 1004) | 52.24–58.48 |
| ≥ Moderate depressive symptoms | ||
| PHQ-9 Positive (PHQ-9 ≥10) | 20.9% (204 / 975) | 18.41–23.61 |
| BDI-II Positive (BDI-II ≥20) | 12.0% (117 / 971) | 10.07–14.26 |
| BDI-II ≥20 or PHQ-9 ≥10 | 22.8% (229 / 1004) | 20.25–25.53 |
a Number (%) of patients with non-missing data
Considering all patients with a BDI-II ≥14 or a PHQ-9 ≥5, the point prevalence of at least mild depressive symptoms based on one or the other questionnaire was 55.4% (95% CI: 52.24–58.48) (556 / 1004 patients; see Table 2). Using more restrictive cut-points indicating at least moderate depressive symptoms, i.e. BDI-II ≥20 or PHQ-9 ≥10, the point prevalence was 22.8% (95% CI 20.25–25.53) (229 / 1004 patients) (Table 2).
Patients with at least mild depressive symptoms were less likely to have ACR/EULAR Boolean remission than patients with no depressive symptoms (16.3% vs 37.2%), and other chronic pain was more frequently reported by these patients than patients with no depressive symptoms (13.1% vs 4.7%).
Characteristics related to depressive symptoms in RA
The unadjusted ORs and the 95% CIs for the risk of depressive symptoms stratified by covariates suggested that age (<60 years), BMI (≥25), not being in DAS28 remission (DAS28 (ESR) ≥2.6), a RAID total score >2, steroid use, NSAID use, opiate use and presence of chronic pain lead to a higher risk of having depressive symptoms. Gender, RA disease duration, family status, education, employment, cDMARDs, bDMARDs and the presence of comorbidity excluding mental illness or chronic pain were not related to depressive symptoms in the univariate analyses. ORs obtained by multiple logistic regression which adjusted for all other covariates in the model, confirmed that age (<60 years), a RAID total score >2 and presence of chronic pain lead to a statistically significant higher risk of having depressive symptoms. In this context, not having RAID PASS (i.e. RAID total score >2) seemed to be the most important risk factor (OR: 10.54, 95% CI: 6.87–16.16) followed by presence of chronic pain (OR: 3.25, 95% CI: 1.65–6.39) and age < 60 years (OR: 1.78, 95% CI: 1.13–2.80). This pattern of results was found for the ORs given for both at least mild depressive symptoms (BDI-II ≥14 or PHQ-9 ≥5) and at least moderate depressive symptoms (BDI-II ≥20 or PHQ-9 ≥10), whereas the results for RAID PASS showed considerably increased ORs in both the univariate and multiple analyses (Table 3). Furthermore, the univariate RAID single item analysis addressing distinct symptoms indicated that all items were related to the occurrence of depressive symptoms (Table 4). The step-down multiple regression procedure verified significance of RAID score, chronic pain and age, and showed that NSAID medication was marginally significant for at least moderately depressive symptoms (OR: 1.47 95% CI: 1.02–2.13).
Table 3. Odds ratios of at least mild or moderate depressive symptoms by patient and RA-related characteristics.
| ≥ Mild Odds Ratio (95% CI) |
≥ Moderate Odds Ratio (95% CI) |
|||
|---|---|---|---|---|
| Influence Variable | Unadjusted | Adjusted | Unadjusted | Adjusted |
| Age (1: <60, 0: ≥60) |
1.50 (1.16–1.93) |
1.78 (1.13–2.80) |
1.76 (1.31–2.37) |
2.16 (1.31–3.56) |
| Sex (0 = female, 1 = male) |
0.78 (0.59 - 1.04) |
0.93 (0.63–1.36) |
0.88 (0.62–1.25) |
0.96 (0.62–1.50) |
| Family status (family = 1, alone = 0) |
1.00 (0.74–1.34) |
1.07 (0.72–1.59) |
0.99 (0.70–1.42) |
1.20 (0.75–1.93) |
| BMI (kg/m2) (≥25 = 1, <25 = 0) |
1.49 (1.15–1.93) |
1.09 (0.77–1.55) |
1.27 (0.93–1.74) |
1.02 (0.68–1.53) |
| Education (>10 years = 1, ≤10 years = 0) |
0.97 (0.75–1.27) |
0.98 (0.70–1.38) |
0.80 (0.59–1.10) |
0.75 (0.51–1.10) |
| Employment (employed = 1, other = 0) |
1.09 (0.84–1.41) |
1.14 (0.73–1.79) |
1.22 (0.90–1.64) |
1.11 (0.68–1.82) |
| RA Duration (years) |
1.00 (0.99–1.02) |
1.00 (0.98–1.01) |
0.99 (0.97–1.00) |
0.99 (0.97–1.01) |
| DAS28 (ESR) (<2.6, ≥2.6) |
2.13 (1.61–2.80) |
1.27 (0.90–1.80) |
1.89 (1.37–2.61) |
1.34 (0.91–1.97) |
| RAID Score (≤2, >2) |
11.34 (7.94–16.21) |
10.54 (6.87–16.16) |
19.23 (8.43–43.86) |
13.61 (5.80–31.92) |
| cDMARDs (yes = 1, no = 0) |
0.90 (0.65–1.25) |
0.88 (0.57–1.37) |
0.75 (0.52–1.08) |
0.62 (0.39–1.00) |
| bDMARDs (yes = 1, no = 0) |
1.20 (0.93–1.54) |
0.99 (0.69–1.42) |
1.07 (0.79–1.44) |
0.83 (0.55–1.26) |
| Glucocorticoids medication (yes = 1, no = 0) |
1.54 (1.19–1.98) |
1.03 (0.74–1.44) |
1.50 (1.11–2.03) |
1.02 (0.70–1.49) |
| NSAID medication (yes = 1, no = 0) |
1.76 (1.35–2.30) |
1.24 (0.87–1.75) |
1.74 (1.29–2.35) |
1.42 (0.97–2.08) |
| Opiates medication (yes = 1, no = 0) |
3.03 (1.49–6.18) |
1.50 (0.64–3.48) |
2.77 (1.51–5.06) |
1.67 (0.79–3.55) |
| Comorbidity without mental illness or chronic pain (0 = no, 1 = yes) |
0.79 (0.62–1.02) |
1.03 (0.71–1.49) |
0.81 (0.60–1.09) |
1.18 (0.77–1.81) |
| Chronic pain (0 = no, 1 = yes) |
3.07 (1.86–5.08) |
3.25 (1.65–6.39) |
2.55 (1.64–3.97) |
2.90 (1.55–5.43) |
aReference category for ORs: age ≥60, female sex, DAS28 <2.6, RAID ≤2, no for each type of RA medication, and no for presence of a comorbidity without mental illness or chronic pain, no for chronic pain. Adjusted odds ratios adjusted in multiple logistic regression for other covariates in table. Abbreviations: RA = rheumatoid arthritis, DAS = Disease Activity Score, ESR = erythrocyte sedimentation rate, RAID = Rheumatoid Arthritis Impact of Disease questionnaire
Table 4. Risk of depressive symptoms (≥ Mild, ≥ Moderate) for RAID single item scores.
| RAID single item scoresa | Unadjusted Odds Ratio | 95% CI | Unadjusted Odds Ratio | 95% CI |
|---|---|---|---|---|
| ≥ Mild | ≥ Moderate | |||
| Pain | 1.40 | 1.31–1.49 | 1.37 | 1.28–1.46 |
| Functional disability assessment | 1.45 | 1.36–1.55 | 1.42 | 1.32–1.52 |
| Fatigue | 1.74 | 1.62–1.87 | 1.64 | 1.52–1.77 |
| Sleep | 1.50 | 1.42–1.59 | 1.46 | 1.38–1.56 |
| Physical well-being | 1.63 | 1.52–1.75 | 1.57 | 1.45–1.69 |
| Emotional well-being | 2.31 | 2.08–2.56 | 1.95 | 1.78–2.14 |
| Coping | 1.81 | 1.67–1.96 | 1.67 | 1.55–1.81 |
a Score range: 0–10. “Unadjusted” refers to the univariate relation of the independent variable to the dependent variable in logistic regression.
Discussion
To the best of our knowledge, this is the first time the RAID questionnaire and its PASS criterion have been applied to a study addressing depressive symptoms in RA. Furthermore, this is the first time depression questionnaires that were previously validated specifically in RA patients have been used in a prevalence study.
In summary, our findings show that depressive symptoms measured with previously validated questionnaires are common in RA, with frequencies ranging from 12.0% (at least moderate, BDI-II) to 55.1% (at least mild, PHQ-9), depending on the respective category threshold and the questionnaire’s level of detail. In both severity categories of interest, the BDI-II as tool with a higher level of detail, returned lower frequencies of depressive symptoms compared to the PHQ-9. Moreover, the frequency of depressive symptoms on the PHQ-9 in the present study (20.9% at least moderate symptom severity) was lower than in a corresponding meta-analysis (38.8%) [11]. In our sample, patients lacking PASS according to the RAID questionnaire, patients less than 60 years of age, or patients with chronic pain were prone to depressive symptoms. The RAID PASS result clearly supports previous findings that highlight the relationship between pain, fatigue, quality of sleep, coping (all measured in the RAID), and depressive symptomology [26–33].
Applying the BDI-II or the PHQ-9 along with the RAID in clinical routine is likely to benefit patients and rheumatologists not only due to an improved detection of depressiveness but also by shared decision making with the help of structured patient-reported outcomes incorporated into the clinical evaluation. A point that still is frequently neglected in clinical routine. Outcomes such as the RAID or the PHQ-9 may explain why patients with RA, despite clinical remission, still might show impaired emotional or physical well-being.
Chronic pain, another explanation for considerably reduced well-being, also increased the risk of depressive symptoms in our sample. Previous research has shown that inflammation (with pain as a prominent component) might be triggering depression. Nevertheless, further proof is needed to determine whether chronic pain is a predictor of depression in RA, vice versa, or whether the relationship is bidirectional [34–38]. In our sample, moderate depressive symptoms occurred twice as frequently in patients below 60 years of age compared to patients aged 60 years and above. A previous meta-analysis found similar results [11]. Accordingly, depression seems to be more common in phases of personal life frequently characterized by founding a family, raising children, career development, establishing a work-life balance, and maintaining health while still working–all of that while facing the everyday burden of RA. Other commonly discussed covariates such as sex, drug class of anti-rheumatic medication, comorbidities other than chronic pain and mental illness, or DAS28 remission of RA could not be confirmed to be related to depressive symptoms.
For follow-up on borderline scores, the authors of the PHQ-9 suggest that not all patients showing mild depressive symptoms require taking action [14]. However, only 20.7% of our study participants with positive test results for moderate depressive symptoms in both questionnaires were previously diagnosed with depression or another mental disorder. Moreover, merely 11.7% of the patients classified as having depressive symptoms were receiving anti-depressive treatment (including psychotherapy) at the time of questioning. This is a clear call for proactive depression screening. Rheumatologists regularly see patients for routine check-ups and follow them up, and depression or emotional disturbances can impair RA treatment adherence [4, 5] [34, 39]. Thus, the rheumatologist’s role in screening for depressive disorders is crucial for both physical and mental well-being. Nevertheless, screening for depression only makes sense when coupled with follow-up routines for diagnostic workup and initiation of anti-depressive therapy [40, 41]. Thus, before the initiation of screening routines, rheumatologists should collaborate with mental-health professionals and/or therapy coordination services to establish efficient transfer of patients with depressive symptomology. The screening workflow should fit the clinical routine and address results indicating depressiveness at the very same patient visit. There should also be routines planned in case of a patient reporting suicidal ideations. This is especially pertinent for resident rheumatologists without instant access to the hospital environments.
Although we were able to recruit a patient sample from a wide variety of rheumatologic facilities in Germany, the reproducibility of our results and their practical application may be limited to the situation in Western Europe. Worldwide generalization of our results may not be possible due to differences in culture, health care system and limited availability of anti-rheumatic treatment. The low disease activity in our sample (e.g. median ESR: 14mm/h, median CRP: 0 mg/dl, median TJC: 0, median SJC: 0)–likely the result of adequate clinical care and sufficient access to effective bDMARDs–may accurately reflect treatment at German centres, but may also limit the generalizability of these results to large populations of patients with severe RA and patients with early or persistent RA. Furthermore, to ensure comparability with other studies, analysis of these data used standard PHQ-9 ≥5 and BDI-II ≥14 cut-offs to determine mild depressive symptomology. Validation of these instruments specifically in RA patients found mild depressive symptomology cut-offs to be PHQ-9 ≥6 and BDI-II ≥12 [17].
Overall, we think that these data are robust and therefore translate to other countries with good access to anti-rheumatic therapy. Especially as these data show that despite good control of inflammatory symptoms of RA, comorbid depression constitutes a significant burden for patients. Coupling depression screening routines with consultation by a mental health professional at the same visit may already suffice to lower unrecognized episodes of depression and improve clinical outcomes.
Supporting information
(XLSX)
Acknowledgments
The authors thank the patients and their relatives for their participation in and support of the study.
Data Availability
All relevant data are within the manuscript and its supporting information files.
Funding Statement
This work was supported by Roche Pharma AG and Chugai Pharma Europe Ltd. Drs. Englbrecht, Alten, Aringer, Baerwald, Burkhardt, Fliedner, Kleinert, Kneitz, Krueger, Schett, Schmalzing, Tausche, Tony, and Wendler received funding. Data analysis was done by AMS – Advanced Medical Services GmbH (Mannheim, Germany) and was funded by Roche Pharma AG and Chugai Pharma Europe Ltd.
References
- 1.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–19. Epub 2011/12/14. 10.1056/NEJMra1004965 . [DOI] [PubMed] [Google Scholar]
- 2.Gossec L, Dougados M, Rincheval N, Balanescu A, Boumpas DT, Canadelo S, et al. Elaboration of the preliminary Rheumatoid Arthritis Impact of Disease (RAID) score: a EULAR initiative. Ann Rheum Dis. 2009;68(11):1680–5. Epub 2008/12/05. 10.1136/ard.2008.100271 . [DOI] [PubMed] [Google Scholar]
- 3.Gunnarsson C, Chen J, Rizzo JA, Ladapo JA, Naim A, Lofland JH. The Employee Absenteeism Costs of Rheumatoid Arthritis: Evidence From US National Survey Data. J Occup Environ Med. 2015;57(6):635–42. 10.1097/JOM.0000000000000461 . [DOI] [PubMed] [Google Scholar]
- 4.Mattila K, Buttgereit F, Tuominen R. Influence of rheumatoid arthritis-related morning stiffness on productivity at work: results from a survey in 11 European countries. Rheumatol Int. 2015;35(11):1791–7. 10.1007/s00296-015-3275-4 . [DOI] [PubMed] [Google Scholar]
- 5.Verstappen SM. Rheumatoid arthritis and work: The impact of rheumatoid arthritis on absenteeism and presenteeism. Best Pract Res Clin Rheumatol. 2015;29(3):495–511. 10.1016/j.berh.2015.06.001 . [DOI] [PubMed] [Google Scholar]
- 6.Sruamsiri R, Mahlich J, Tanaka E, Yamanaka H. Productivity loss of Japanese patients with rheumatoid arthritis—A cross-sectional survey. Mod Rheumatol. 2018;28(3):482–9. 10.1080/14397595.2017.1361893 . [DOI] [PubMed] [Google Scholar]
- 7.Evans-Lacko S, Knapp M. Global patterns of workplace productivity for people with depression: absenteeism and presenteeism costs across eight diverse countries. Soc Psychiatry Psychiatr Epidemiol. 2016;51(11):1525–37. 10.1007/s00127-016-1278-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moll LT, Gormsen L, Pfeiffer-Jensen M. [Higher prevalence of depression in patients with rheumatoid arthritis—a systematic review]. Ugeskr Laeger. 2011;173(41):2564–8. Epub 2011/10/12. . [PubMed] [Google Scholar]
- 9.Rathbun AM, Reed GW, Harrold LR. The temporal relationship between depression and rheumatoid arthritis disease activity, treatment persistence and response: a systematic review. Rheumatology (Oxford). 2013;52(10):1785–94. Epub 2012/12/14. 10.1093/rheumatology/kes356 . [DOI] [PubMed] [Google Scholar]
- 10.Dickens C, McGowan L, Clark-Carter D, Creed F. Depression in rheumatoid arthritis: a systematic review of the literature with meta-analysis. Psychosom Med. 2002;64(1):52–60. Epub 2002/01/31. . [DOI] [PubMed] [Google Scholar]
- 11.Matcham F, Rayner L, Steer S, Hotopf M. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology (Oxford). 2013;52(12):2136–48. Epub 2013/09/05. 10.1093/rheumatology/ket169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dougados M, Soubrier M, Antunez A, Balint P, Balsa A, Buch MH, et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis. 2014;73(1):62–8. Epub 2013/10/08. 10.1136/annrheumdis-2013-204223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baillet A, Gossec L, Carmona L, Wit M, van Eijk-Hustings Y, Bertheussen H, et al. Points to consider for reporting, screening for and preventing selected comorbidities in chronic inflammatory rheumatic diseases in daily practice: a EULAR initiative. Ann Rheum Dis. 2016;75(6):965–73. Epub 2016/03/18. 10.1136/annrheumdis-2016-209233 . [DOI] [PubMed] [Google Scholar]
- 14.Kroenke K, Spitzer RL. The PHQ-9: A New Depression Diagnostic and Severity Measure. Psychiatr Ann. 2002;32(9):509–15. 10.3928/0048-5713-20020901-06 [DOI] [Google Scholar]
- 15.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 16.World Health Organization info package: mastering depression in primary care. Frederiksborg: Regional Office for Europe, Psychiatric Research Unit. [Google Scholar]
- 17.Englbrecht M, Alten R, Aringer M, Baerwald CG, Burkhardt H, Eby N, et al. Validation of Standardized Questionnaires Evaluating Symptoms of Depression in Rheumatoid Arthritis Patients: Approaches to Screening for a Frequent Yet Underrated Challenge. Arthritis Care Res (Hoboken). 2017;69(1):58–66. Epub 2016/08/03. 10.1002/acr.23002 . [DOI] [PubMed] [Google Scholar]
- 18.Smarr KL, Keefer AL. Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S454–66. Epub 2012/05/25. 10.1002/acr.20556 . [DOI] [PubMed] [Google Scholar]
- 19.Covic T, Cumming SR, Pallant JF, Manolios N, Emery P, Conaghan PG, et al. Depression and anxiety in patients with rheumatoid arthritis: prevalence rates based on a comparison of the Depression, Anxiety and Stress Scale (DASS) and the hospital, Anxiety and Depression Scale (HADS). BMC Psychiatry. 2012;12:6 Epub 2012/01/25. 10.1186/1471-244X-12-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isik A, Koca SS, Ozturk A, Mermi O. Anxiety and depression in patients with rheumatoid arthritis. Clin Rheumatol. 2007;26(6):872–8. Epub 2006/08/31. 10.1007/s10067-006-0407-y . [DOI] [PubMed] [Google Scholar]
- 21.Kohlmann T, Raspe H. [Hannover Functional Questionnaire in ambulatory diagnosis of functional disability caused by backache]. Rehabilitation (Stuttg). 1996;35(1):I–VIII. Epub 1996/02/01. . [PubMed] [Google Scholar]
- 22.Gossec L, Paternotte S, Aanerud GJ, Balanescu A, Boumpas DT, Carmona L, et al. Finalisation and validation of the rheumatoid arthritis impact of disease score, a patient-derived composite measure of impact of rheumatoid arthritis: a EULAR initiative. Ann Rheum Dis. 2011;70(6):935–42. Epub 2011/05/05. 10.1136/ard.2010.142901 . [DOI] [PubMed] [Google Scholar]
- 23.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. Epub 2001/09/15. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dougados M, Brault Y, Logeart I, van der Heijde D, Gossec L, Kvien T. Defining cut-off values for disease activity states and improvement scores for patient-reported outcomes: the example of the Rheumatoid Arthritis Impact of Disease (RAID). Arthritis Res Ther. 2012;14(3):R129 Epub 2012/06/01. 10.1186/ar3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9. Epub 2008/03/04. 10.1016/j.jclinepi.2007.11.008 . [DOI] [PubMed] [Google Scholar]
- 26.Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Fitzgerald JD, Ranganath VK, et al. Sleep loss exacerbates fatigue, depression, and pain in rheumatoid arthritis. Sleep. 2012;35(4):537–43. Epub 2012/04/03. 10.5665/sleep.1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharpe L, Sensky T, Allard S. The course of depression in recent onset rheumatoid arthritis: the predictive role of disability, illness perceptions, pain and coping. J Psychosom Res. 2001;51(6):713–9. Epub 2001/12/26. . [DOI] [PubMed] [Google Scholar]
- 28.Wolfe F, Michaud K. Predicting depression in rheumatoid arthritis: the signal importance of pain extent and fatigue, and comorbidity. Arthritis Rheum. 2009;61(5):667–73. Epub 2009/05/01. 10.1002/art.24428 . [DOI] [PubMed] [Google Scholar]
- 29.Ziarko M, Mojs E, Piasecki B, Samborski W. The mediating role of dysfunctional coping in the relationship between beliefs about the disease and the level of depression in patients with rheumatoid arthritis. ScientificWorldJournal. 2014;2014:585063 Epub 2014/02/28. 10.1155/2014/585063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Covic T, Adamson B, Spencer D, Howe G. A biopsychosocial model of pain and depression in rheumatoid arthritis: a 12-month longitudinal study. Rheumatology (Oxford). 2003;42(11):1287–94. Epub 2003/06/18. 10.1093/rheumatology/keg369 . [DOI] [PubMed] [Google Scholar]
- 31.Pollard LC, Choy EH, Gonzalez J, Khoshaba B, Scott DL. Fatigue in rheumatoid arthritis reflects pain, not disease activity. Rheumatology (Oxford). 2006;45(7):885–9. Epub 2006/02/02. 10.1093/rheumatology/kel021 . [DOI] [PubMed] [Google Scholar]
- 32.Englbrecht M, Gossec L, DeLongis A, Scholte-Voshaar M, Sokka T, Kvien TK, et al. The impact of coping strategies on mental and physical well-being in patients with rheumatoid arthritis. Semin Arthritis Rheum. 2012;41(4):545–55. Epub 2012/02/22. 10.1016/j.semarthrit.2011.07.009 . [DOI] [PubMed] [Google Scholar]
- 33.Conner TS, Tennen H, Zautra AJ, Affleck G, Armeli S, Fifield J. Coping with rheumatoid arthritis pain in daily life: within-person analyses reveal hidden vulnerability for the formerly depressed. Pain. 2006;126(1–3):198–209. Epub 2006/08/15. 10.1016/j.pain.2006.06.033 . [DOI] [PubMed] [Google Scholar]
- 34.Sturgeon JA, Finan PH, Zautra AJ. Affective disturbance in rheumatoid arthritis: psychological and disease-related pathways. Nat Rev Rheumatol. 2016;12(9):532–42. Epub 2016/07/15. 10.1038/nrrheum.2016.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sergeeva M, Rech J, Schett G, Hess A. Response to peripheral immune stimulation within the brain: magnetic resonance imaging perspective of treatment success. Arthritis Res Ther. 2015;17:268 Epub 2015/10/20. 10.1186/s13075-015-0783-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krishnadas R, Nicol A, Sassarini J, Puri N, Burden AD, Leman J, et al. Circulating tumour necrosis factor is highly correlated with brainstem serotonin transporter availability in humans. Brain Behav Immun. 2016;51:29–38. Epub 2015/08/11. 10.1016/j.bbi.2015.08.005 . [DOI] [PubMed] [Google Scholar]
- 37.Brown GK. A causal analysis of chronic pain and depression. J Abnorm Psychol. 1990;99(2):127–37. Epub 1990/05/01. . [DOI] [PubMed] [Google Scholar]
- 38.Husted JA, Tom BD, Farewell VT, Gladman DD. Longitudinal study of the bidirectional association between pain and depressive symptoms in patients with psoriatic arthritis. Arthritis Care Res (Hoboken). 2012;64(5):758–65. Epub 2012/01/11. 10.1002/acr.21602 . [DOI] [PubMed] [Google Scholar]
- 39.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–7. Epub 2000/07/25. . [DOI] [PubMed] [Google Scholar]
- 40.O'Connor EA, Whitlock EP, Beil TL, Gaynes BN. Screening for depression in adult patients in primary care settings: a systematic evidence review. Ann Intern Med. 2009;151(11):793–803. Epub 2009/12/02. 10.7326/0003-4819-151-11-200912010-00007 . [DOI] [PubMed] [Google Scholar]
- 41.Pignone MP, Gaynes BN, Rushton JL, Burchell CM, Orleans CT, Mulrow CD, et al. Screening for depression in adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136(10):765–76. Epub 2002/05/22. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its supporting information files.