Abstract
As small regulatory transcripts, microRNAs (miRs) act as genetic ‘fine tuners’ of posttranscriptional events, and as genetic switches to promote phenotypic switching. The miR miR26a targets the BMP signalling effector, smad1. We show that loss of miR26a leads to hemorrhage (a loss of vascular stability) in vivo, suggesting altered vascular differentiation. Reduction in miR26a levels increases smad1 mRNA and phospho-Smad1 (pSmad1) levels. We show that increasing BMP signalling by overexpression of smad1 also leads to hemorrhage. Normalization of Smad1 levels through double knockdown of miR26a and smad1 rescues hemorrhage, suggesting a direct relationship between miR26a, smad1 and vascular stability. Using an in vivo BMP genetic reporter and pSmad1 staining, we show that the effect of miR26a on smooth muscle differentiation is non-autonomous; BMP signalling is active in embryonic endothelial cells, but not in smooth muscle cells. Nonetheless, increased BMP signalling due to loss of miR26a results in an increase in acta2-expressing smooth muscle cell numbers and promotes a differentiated smooth muscle morphology. Similarly, forced expression of smad1 in endothelial cells leads to an increase in smooth muscle cell number and coverage. Furthermore, smooth muscle phenotypes caused by inhibition of the BMP pathway are rescued by loss of miR26a. Taken together, our data suggest that miR26a modulates BMP signalling in endothelial cells and indirectly promotes a differentiated smooth muscle phenotype. Our data highlights how crosstalk from BMP-responsive endothelium to smooth muscle is important for smooth muscle differentiation.
Author summary
The structural integrity of a blood vessel is critical to ensure proper vessel support and vascular tone. Vascular smooth cells (vSMCs) are a key component of the vessel wall and, in their mature state, express contractile proteins that help to constrict and relax the vessel in response to blood flow changes. vSMCs differentiate from immature vascular mural cells that lack contractile function. Here, we use a zebrafish model to identify a small microRNA that regulates vascular stabilization. We show that a small regulatory RNA, microRNA26a is enriched in the endothelial lining of the blood vessel wall and, through signalling, communicates to the smooth muscle cell to control its maturation. Providing a mechanistic insight into vSMC differentiation may help develop and produce feasible miR-based pharmaceutical to promote SMC differentiation.
Introduction
Vascular smooth muscle cells (vSMCs) provide structural integrity to the vessel wall. Guided control of signalling cascades, including Platelet derived growth factor (Pdgf), Notch, and Transforming Growth Factor-β/Bone morphogenic Protein (TGF-β/BMP) recruits and induces differentiation of perivascular mural cells (vSMCs and pericytes) to create a two-layered vessel wall with an internal endothelial cell lining and a muscle cell covering [1–3]. Once the vSMCs surround the vessel, they begin depositing extracellular matrix (ECM) proteins Laminin, Collagen IV and Fibulins to support the vessel wall [4]. vSMCs then take on a mature phenotype that stabilizes the underlying endothelial cells through induction of quiescence, expression of junctional and attachment proteins, and expression of contractile proteins to provide myogenic tone [2,5–7].
vSMCs maintain phenotypic plasticity and can undergo a phenotypic switch from a quiescent contractile state to a proliferative synthetic state in response to cellular stimuli [4,8]. Contractile vSMCs are defined by an elongated and thin ‘spindle-shaped’ morphology and low rates of proliferation. The expression of key differentiation markers such as smooth muscle (α)-actin (Acta2), smooth muscle β-myosin heavy chain (Myh11), and transgelin (Sm22α) allows vSMCs to perform their contractile function and provide vascular tone. In contrast, the immature synthetic vSMCs have reduced expression of contractile genes, produce ECM proteins, are highly proliferative, and have a rhomboid or rounded morphology [9–12].
Numerous studies have demonstrated that BMP signaling through Smad1 modulate vSMC plasticity (reviewed by [13]). Defective BMP signalling can affect both endothelial and vSMC cells [14–21]. Aberrant vSMCs phenotype switching plays a critical role in the pathogenesis of vascular diseases such as hereditary hemorrhagic telangiectasia (HHT) and pulmonary arterial hypertension (PAH). In canonical Smad-mediated BMP signaling, Smad1 is phosphorylated by the serine-threonine kinase activity of a type 1 BMP receptor (ACVRL1 (ALK1)/ BMPR1A, BMPR1B) allowing it to associate and dimerize with the co-mediator Smad4 and translocate to the nucleus to control gene transcription. Murine homozygous null mutants for BMPR-1a (Activin like kinase 3, ALK3) or the type II receptor BMPR-2 (which is mutated in human patients with PAH) [22,23] and their ligand Bmp4 or downstream co-Smad4 are embryonic lethal, and present with vascular deformities attributable to a loss of Smad1 mediated signalling [24]. Mutations in ALK1 lead to HHT2, a disease characterized by arteriovenous malformations (AVMs) [25]. Deletion of Alk1 in mice leads to cranial hemorrhages, AVM-like fusion of micro-vessel plexi, dilation of large vessels and reduced coverage of vessels by vSMCs [26]. In zebrafish, disruption of Alk1 signalling results in pathological arterial enlargement and maladaptive responses to blood flow that generate AVMs. Potential vSMCs defects in this model have not been assessed [27].
As small noncoding RNAs, microRNAs (miRs) regulate gene expression of key vSMC marker genes to control vSMC dynamics. [28–31]. A number of miRs have been identified as modulators of the vSMC phenotype in vitro and in vivo, including miR-145, miR-21, miR-221, miR-222 and miR-146a [32–40]. We previously showed that miR-145 promotes visceral smooth muscle differentiation via controlling cross-talk between epithelial cells and smooth muscle [32,41].
Here, we investigate the role of microRNA26a (miR26a) in regulating vSMC dynamics using the zebrafish model of vessel stabilization. miR26a regulates proliferation, migration and differentiation of vSMCs and has been shown to target smad1, a key intracellular mediator of BMP signalling, in cultured vSMCs in vitro [42–45].
miR26a expression is altered during abdominal aortic aneurysm (AAA) and neointimal lesion formation [43,45]. However, the role of miR26a in vivo in an intact animal in the context of developing vSMCs are largely unknown. Using a combination of genetic gain and loss of function methods to understand the role of miR26a in vivo, we show that miR26a acts within a BMP responsive pathway to fine tune vSMC maturation via targeting smad1. Interestingly, we find that active BMP signalling and changes in Smad1 activation are observed within endothelium in vivo, and not in smooth muscle cells. Together the evidence suggests that miR26a plays a role in regulating blood vessel stabilization via a non-autonomous mechanism.
Results
miR26a is expressed in developing blood vessels
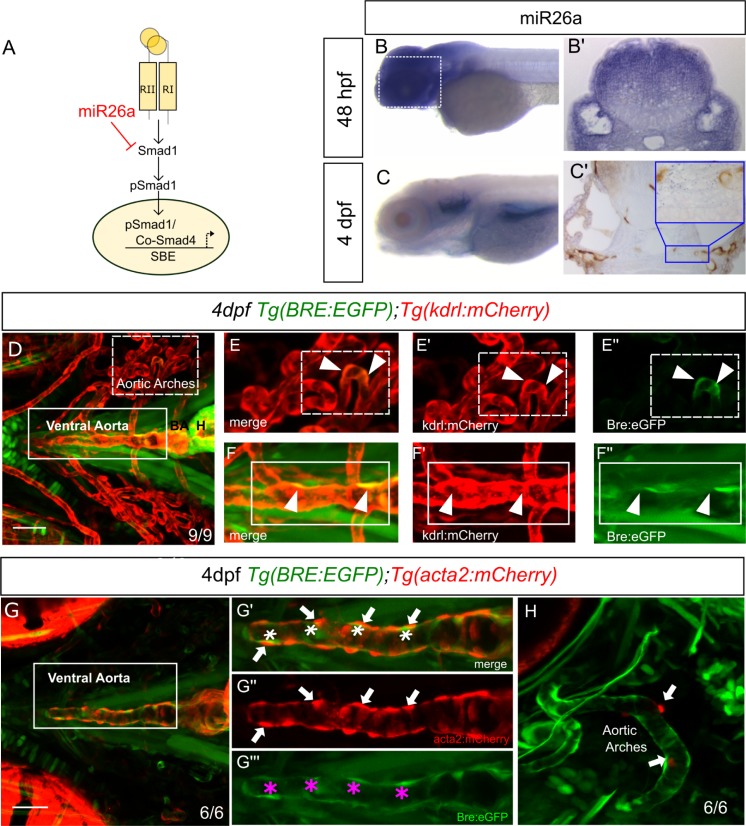
miR26a targets smad1 and thereby directly regulates BMP signalling ([36,43] Fig 1A). To observe the spatial gene expression pattern of miR26a in developing embryos we used in situ hybridization. At 48 hpf, miR26a has a ubiquitous expression pattern (Fig 1B and 1B’), however by 4 dpf expression becomes enriched in the ventral head of the embryo, with strong expression in the pharyngeal region, bulbus arteriosus and ventral aorta (Fig 1C). miR26a is expressed in and around the blood vessel endothelium where it could potentially play a role modulating BMP signalling in blood vessels (compare to kdrl: GFP stain; Fig 1C’, inset). In order to further analyze the cell specific expression of miR26a, we used fluorescent-activated cell sorting (FACS) to isolate EGFP+ve vSMCs and mCherry+ve endothelial cells from 4 dpf Tg(acta2:EGFP;kdrl:mCherry) embryos. In keeping with the in-situ hybridization data, RT-qPCR showed that miR26a is indeed expressed in both cell types, although it is not significantly enriched in endothelial cells (S1 Fig). FACS sorting efficiently separated vSMCs and endothelial cells; we find that acta2: EGFP+ve vSMCs cells have an average 37.4-fold enrichment in acta2 expression, and minimal expression of alk1 or smad1 when compared to kdrl: mCherry endothelial cells. However, smad1 is 14-fold enriched and acvrl1 is 3.5-fold enriched in mCherry+ve endothelial cells while there is nearly no expression of acta2 (S1 Fig). Thus, a miR26a target, smad1 is enriched in endothelial cells.
Fig 1. miR26a is expressed in blood vessels; endothelial cells have active BMP signalling.
A) Model of how miR26a controls BMP signaling via direct targeting of smad1. B) Lateral view of whole mount in situ expression of miR26a at 48 hpf shows ubiquitous expression pattern, with strong expression in the ventral head of the embryo. B’) Cross section of the head at 48 hpf. C) At 4 dpf miR26a is expressed in the pharyngeal arches, bulbous arteriosus and ventral aorta. C’) Cross section of the head showing miR26a expression in blood vessels (purple; punctate stain) compared with endothelial stain (brown; kdrl:GFP transgenic). Inset is an enlargement of image in C’. D) Ventral view of the pharyngeal region of a 4 dpf double transgenic Tg(BRE:EGFP);Tg(kdrl:mCherry) embryo shows BRE:EGFP (green) expression within endothelial cells in aortic arches (red, white arrowheads in E’-E”‘) and ventral aorta (red, white arrowheads F’-F”‘). G-H) Ventral and lateral views of a 4 dpf double transgenic Tg(BRE:EGFP); Tg(acta2:mCherry) zebrafish shows that acta2 positive cells are in direct contact with BMP-responsive endothelial cells but do not express BRE:EGFP. Scale bar represents 50μm.
BMP signalling is active in the aorta endothelium
We next tested the relationship between mir26a expression and activated BMP signaling using an in vivo reporter of Smad1/5 activity. Tg(BRE:EGFP] transgenic fish encode EGFP driven by an upstream Bmp Response Element (BRE) that contains multiple short Smad-binding sites from the id1 promoter, a major transcriptional target of canonical Bmp/Smad1 signaling [46,47]. We crossed Tg(BRE:EGFP] to endothelial Tg(kdrl:mCherry) or vSMC Tg(acta2:mCherry) lines to observe BMP activation in endothelial and vSMCs, respectively (Fig 1D and 1G). We use the 4 dpf time point as vSMC cells first differentiate and begin to express the mature marker acta2 between 3 and 4 dpf [7,48]. Surprisingly, although miR26a has been implicated in controlling Smad1 regulated vSMC dynamics directly, we found that transgenic BRE:EGFP signals are restricted to the endothelium of the vessel wall and have co-localized expression with kdrl:mCherry (Fig 1E, 1E’, 1F and 1F’). The acta2:mCherry-positive vSMCs lie directly adjacent to BRE:EGFP-expressing cells, with no detectable expression of BRE:EGFP in vSMCs on the ventral aorta or in pharyngeal aortic arch arteries (Fig 1G, 1G” and 1H, both ventral and lateral projections are shown). Similarly, acta2:mCherry-positive cells are closely associated with pSmad1-positive endothelial cells but do not show pSmad1 staining (S1D Fig). Together, our data suggest that in early development, miR26a and smad1 are expressed within endothelial cells where BMP signaling is also active, as visualized by two methods of detection.
Loss of miR26a leads to upregulation of smad1 mRNA
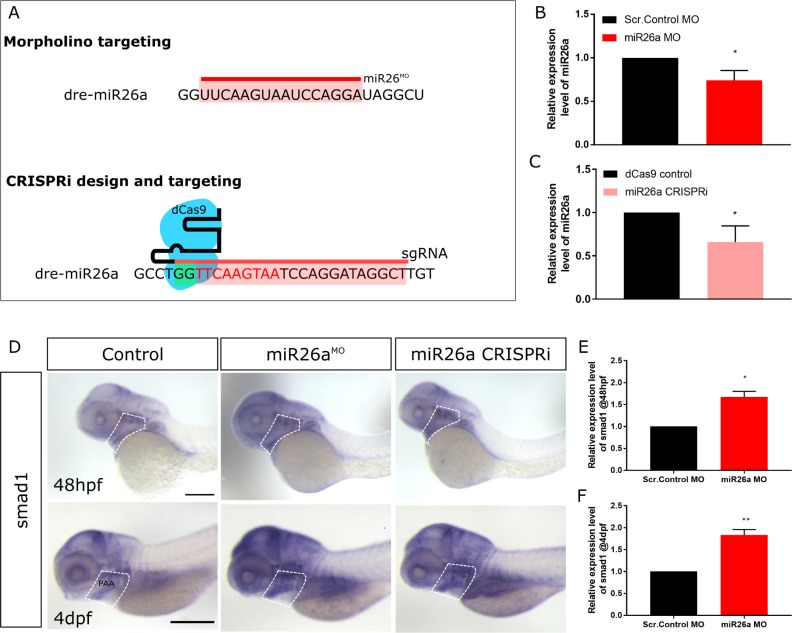
The highly conserved miR-26 family constitutes miR26a-1, miR26a-2, miR26a-3 and miR26b [44] as identified by their seed sequences and accessory sequence. In zebrafish and humans, miR26a-1, miR26a-2 and miR26a-3 have the same mature sequence, and only differ from the mature miR-26b sequence by two nucleotides [42,49]. To investigate the role of miR26a in vascular development in vivo, we knocked down miR26a using an antisense morpholino that targets the mature miRNA seed sequence of all three miR26a isoforms. A 6bp mismatch scrambled control morpholino was used as a control. 1 ng doses of morpholino were used, as suggested by current guidelines [50,51]. In parallel, we designed a second genetic knockdown approach using CRISPR interference (CRISPRi) [52] to target the pri-miR hairpin structure using the complementary sequence to the mature miRNA (Fig 2A). RT-qPCR shows a 26% (0.74±0.65) reduction in miR26a following miR26a MO knockdown and 34% (0.65±0.10) reduction of miR26a using CRISPRi (Fig 2B and 2C) confirming that both knockdown methods result in decreased miR26a expression. smad1 is a demonstrated target of miR26a in vitro [36,43]. In support of smad1 being a miR26a target in vivo, miR26a knockdown results in increased smad1 expression in 48 hpf and 4 dpf injected embryos as compared to controls by in situ hybridization (Fig 2D) and resulted in an average 1.6-fold and 1.8-fold increase by RT-qPCR, respectively (Fig 2E and 2F).To further determine whether miR26a can regulate smad1 expression in vivo, we designed a sensor assay and fused the smad1 3′UTR to EGFP (EGFP: smad1pA). This was co-injected with an internal mCherry control into single-cell zebrafish embryos in the presence or absence of a miR26a morpholino. When fluorescence levels were examined at 24 hpf, injections of EGFP: smad1pA sensor mRNA alone resulted in EGFP expression; however, this fluorescence was enhanced by over 65% by co-injection of miR26a morpholino (S2A and S2B Fig).
Fig 2. miR26a knockdown increases smad1 expression.
A) Schematic of miR26a transient knockdown methods. B and C) Relative expression level of miR26a in morpholino and CRISPRi injected embryos at 48 hpf (n = 3). D) Whole mount in situ hybridization staining for smad1 at 48 hpf and 4 dpf shows increased expression of smad1 in miR26a knockdown embryos particularly in the ventral aorta, aortic arches and pharyngeal region (dotted outline). Scale bar represents 200μm. E) Relative expression of smad1 in 48 hpf morphants is increased compared to control embryos (n = 3). F) Relative expression of smad1 in 4 dpf miR26a morphants is increased compared to control embryos (n = 4). RT-qPCR data show the mean ± SEM, Student's two-tailed t-test *p < 0.05, n = number of biological replicates.
At 4 dpf, upregulation of smad1 in miR26a morphants and CRISPRi knockdown embryos is more prominent in the ventral pharyngeal region, with staining in the ventral aorta, aortic arches and bulbous arteriosus (Fig 2D, highlighted areas), similar to where miR26a is expressed most strongly (Fig 1B and 1C). In a complementary approach, we injected a miR26a mimic to overexpress miR26a and observed an increase in miR26a expression by RT-qPCR (S2C Fig), as well as a marked reduction of smad1 expression in the ventral pharyngeal region by in situ hybridization (S2E Fig). Overexpression of miR26a results in mildly dorsalized embryos by 48 hpf with pericardial edema, dorsal axis defects and poor circulation (S2D Fig), suggesting overexpression of miR26a disrupts the BMP pathway that patterns early embryonic axes.
Loss of miR26a leads to increased phosphorylated Smad1 in endothelium
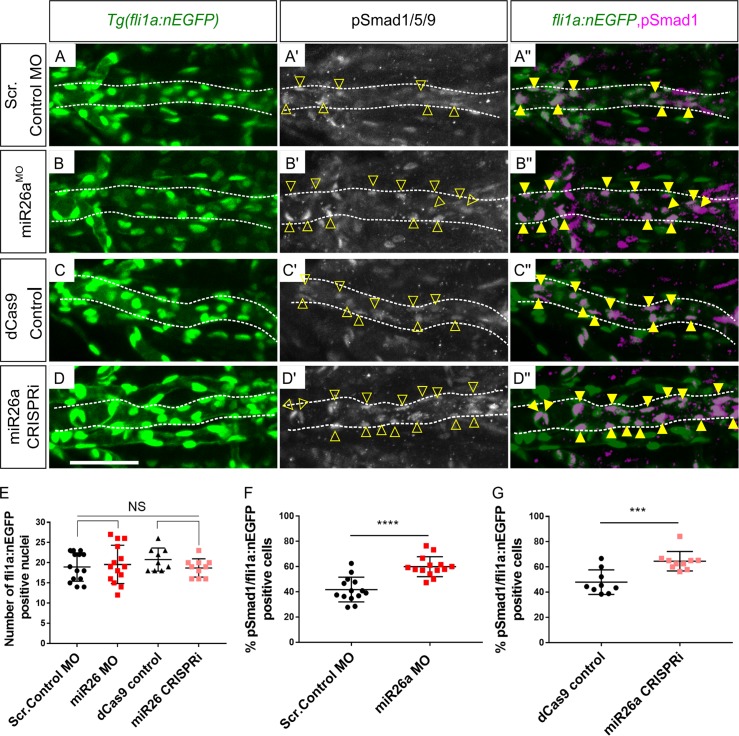
We next tested whether the increased expression of smad1 mRNA in miR26a knockdown embryos leads to enhanced Smad1 phosphorylation. Wildtype immunostaining showed pSmad1/5/9 is high in endothelium but not in vSMCs (S1D–S1D” Fig). miR26a knockdown embryos do not show any significant difference in endothelial cell number as compared to controls, using endothelial nuclear transgenic lines (Tg(fli1a:nEGFP; kdrl:mCherry); Fig 3A–3E, and S3 Fig). However, there is a significant 20% increase in pSmad1 positive/ fli1a:nEGFP nuclei in miR26a knockdown embryos as compared to controls, with an average of 60±3.1% and 64.9±3.9% in miR26a morphants (Fig 3B–3B” and 3F) and CRISPRi embryos (Fig 3D, 3D” and 3G), respectively as compared to 41±2.9 and 46±6.5% in controls.
Fig 3. miR26a knockdown embryos have increased endothelial pSmad1.
A-D) Ventral view confocal projections of the 4 dpf ventral aorta (dotted outline). Endothelial nuclei (fli1a:nEGFP; A-D, arrowheads) and pSmad1/5/9 (pSmad1,white A’- D’) and overlay (magenta, A”- D”) in 4 dpf Scr. Control (A), miR26a MO (B), dCas9 control (C) and miR26a CRISPRi (D) embryos. Yellow arrowheads indicate double positive pSmad1 + fli1a:nEGFP nuclei in the ventral aorta. E) Quantification of total number of fli1a:nEGFP nuclei in the ventral aorta. F-G) Quantification of the percentage of double pSmad1; fli1a:nEGFP positive nuclei in miR26a morphants (F) and miR26a CRISPRi (G) embryos. N = 3 experiments. Total embryos are as follows: Scr. Control MO n = 14, miR26a MO n = 14, dCas9 control n = 9 and miR26a CRISPRi n = 10. Student's two-tailed t-test, p***< 0.0001 and p ****< 0.00001 as compared to WT, error bars = SD. Scale bar: represents 50μm.
Increased levels of smad1 lead to vascular stability defects
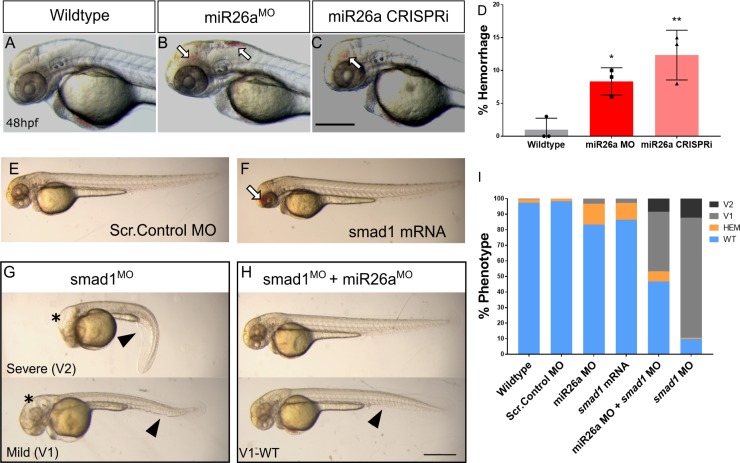
Loss of miR26a leads to compromised vessel integrity at 48 hpf. miR26a morphants have an average 13±2% hemorrhage (Fig 4B and 4D) and CRISPRi embryos have an average 15±1% hemorrhage (Fig 4C and 4D) as compared to 2–3% rate of the controls. The phenotype is dose-dependent as higher doses of morpholino lead to an increase in hemorrhage to 40% and a 1.8-fold reduction in miR26a expression (S4C and S4D Fig). As smad1 overexpression has not been previously connected to vascular stability defects, we next tested whether overexpressed smad1 could lead to hemorrhage. Injection of smad1 mRNA into single cell stage embryos resulted in significantly higher hemorrhage rate of 12±0.9% in injected embryos as compared to uninjected controls (Fig 4E, 4F and 4I).
Fig 4. Increased levels of smad1 result in defects in the vascular system and body axis.
A-C) Representative 48 hpf miR26a knockdown embryos with hemorrhage, as indicated by arrows. D) Quantification of average rates of hemorrhage. (Error bars = SD Unpaired t test, miR26a MO *p< 0.01 and mi26 CRISPRi **p< 0.001 as compared to WT, N = 3, Wildtype n = 224, miR26a MO n = 124, miR26a CRISPRi n = 180). E-F) Representative morphology after smad1 overexpression. G-I) miR26a and smad1 double knockdown experiments. G) Representative 48 hpf smad1 MO embryos with mild (V1) and severe (V2) ventralization phenotypes. H) Representative 48 hpf double miR26a and smad1 knockdown embryos with rescued hemorrhage and normal body axis showing only mild (V1-WT) ventralization phenotypes. I) Quantification of observed phenotypes double knockdown experiments (N = 4, total n wildtype = 193, Scr. Control MO = 157, smad1 MO = 175, smad1 mRNA = 95, miR26a MO = 190, and miR26a MO + smad1 MO = 190. One Way ANOVA of hemorrhage phenotype; Wildtype/Scr. Control MO vs. miR26a MO p< 0.0001 Wildtype/Scr. Control vs. SMAD1 mRNA p< 0.0001 miR26a MO vs. miR26a MO+ smad1 MO p< 0.0001 One Way ANOVA of V1 phenotype: Wildtype/Scr. Control MO; vs. smad1 MO p< 0.0001 vs. miR26a MO+ smad1 MO p< 0.0001. Error Bars = SEM. Scale bar represents 500μm.
Further, as miR26a knockdown leads to increased smad1 levels, we predicted that reduction in smad1 would rescue hemorrhage in miR26a knockdown embryos. Double knockdown by co-injection of smad1 [53] and miR26a morpholinos reduced hemorrhage rates to below 5±0.8% (Fig 4H, top embryo and Fig 4I). Of note, smad1 MO alone did not result in hemorrhage; however it did result in a range of phenotypes associated with smad1 knockdown including dorsal-ventral axis defects and hydrocephalus as previously reported [53]. smad1 knockdown led to an average a 77±8.6% of embryos with a mild (V1) ventralized defect and 12±17% with a more severe (V2) phenotype (Fig 4G–4I). Both defects were reduced in double knockdown embryos (Fig 4G and 4H, bottom). Thus, reducing miR26a or increasing smad1 in vivo leads to leads to a loss of vascular stability and hemorrhage.
Loss of miR26a leads to increased numbers of acta2-positive vSMCs and upregulation of pathways involved in endothelial-vSMC crosstalk
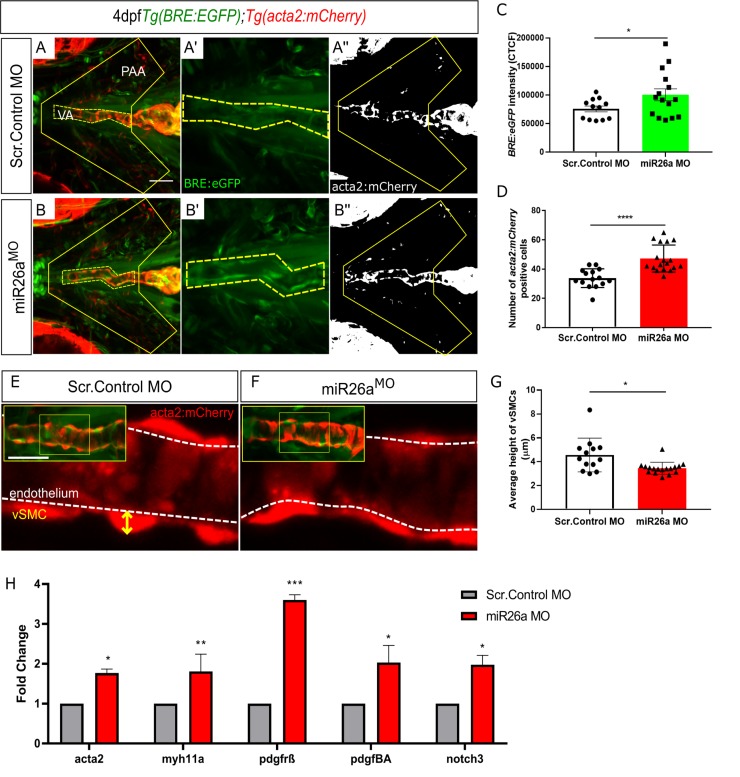
To demonstrate functional consequences of increased endothelial BMP signalling on vSMCs, we next investigated vSMC investment on the ventral aorta and pharyngeal arch arteries of Tg(BRE:EGFP;acta2:mCherry) embryos in miR26a knockdown embryos. This assay allowed us to make three key observations. Firstly, BRE:EGFP signal intensity is enhanced in miR26a morphants (Fig 5A’, 5B’ and 5C), which correlates with the increased pSmad1 staining we observed in endothelial nuclei of knockdown embryos (Fig 3). Secondly, the number of acta2:mCherry positive cells along the ventral aorta and pharyngeal arch arteries (PAA) is increased in miR26a knockdown embryos (33.8±1.6 in controls vs 47.2 ±2.2 in miR26a knockdown, Fig 5A”, 5B” and 5D). Thirdly, the increase in acta2 positive cell number is accompanied by a change in cell morphology in miR26a knockdown embryos (Fig 5E–5G). In control embryos, acta2 positive vSMCs have a rounded, punctate morphology, and ‘sit’ high on the vessel wall with an average height of 4.5±0.4 μm, above the underlying endothelium. In miR26a morphants, vSMCs are have a significantly reduced vSMC height of 3.4 ± 0.1μm, and appear flatter and more closely apposed to the endothelium when compared to control embryo vSMCs. These data suggest that loss of miR26a results in increased vSMC coverage along blood vessels and a shift to a differentiated morphology.
Fig 5. Loss of miR26a morphants leads to increased expression of vSMC genes and acta2-positive vSMCs.
A-B) Representative ventral views of 4 dpf Tg(BRE:EGFP); Tg(acta2:mCherry) embryos. Scr. Control embryos (A-A”) and miR26a morphant embryos (B-B”) showing qualitative upregulation of BRE:EGFP in the ventral aorta (VA) and pharyngeal arch arteries (PAA). C) Quantification of green fluorescent marker (BRE:EGFP) along the VA, taken from the highlighted yellow region in A’ and B’, and represented as corrected total cell fluorescence (CTCF) (N = 3, miR26a MO n = 15, Scr. Control n = 12, Unpaired t test, ****p< 0.0001 as compared to control, error bars = SEM). D) Quantification of acta2 positive cell number on VA and PAAs, within area outlined in A” and B”. Number of acta2 positive cells is significantly increased in miR26a morphants (N = 3, miR26a MO n = 18, Scr. Control n = 15, Unpaired t test, ****p< 0.0001 as compared to control, error bars = SEM). E and F) Measurement of vSMC height (yellow axis) from the endothelium (white dashed line). Representative images of ventral aorta (from insets), Scr. Control (E) and miR26a morphants (F). G) Quantification of average vessel heights along the length of the VA (N = 3, miR26a MO n = 18, Scr. Control n = 13, Student's two-tailed t-test, ****p< 0.0001 as compared to control, error bars = SEM). H) RT-qPCR quantification of vSMC differentiation genes in injected controls and miR26a morphants (n = 3). RT-qPCR data show the mean ± SEM, Student's two-tailed t-test *p < 0.05, n, number of biological replicates.
In parallel, we quantitated gene expression for vSMC differentiation genes. RT-qPCR using isolated embryonic head mRNA at 4 dpf showed a 1.7-fold increase in acta2 and 1.8-fold increase in myh11a mRNA in miR26a morphants (Fig 5H). Further, using in situ hybridization, we found that miR26a morphants have increased expression of acta2 and myh11a in the pharyngeal region (S5 Fig), similar to the location of increased smad1 staining (Fig 2D). Conversely by 4 dpf, miR26a mimic injected embryos had reduction in acta2 and sm22 expression by in situ hybridization (S5 Fig). The Bmp/ Notch3/ Pdgf signalling axis is an important regulator of vSMC proliferation and subsequent differentiation. We found that the vSMC notch receptor notch3 has a 2.0-fold increase in miR26a morphants (Fig 5H). Furthermore, the endothelial expressed ligand pdgfba and its mural cell receptor pdgfrβ, had a 3.6 and 2.0-fold increase, respectively, in miR26a morphants as compared to controls. This suggests that increased vSMC numbers could potentially arise from enhanced proliferation via activation of the Pdgfrβ pathway downstream of Smad1 activation. Increases in acta2, myh11 and notch3 may therefore reflect increased cell numbers in addition to increased vSMC differentiation.
Endothelial overexpression of smad1 promotes vSMC differentiation
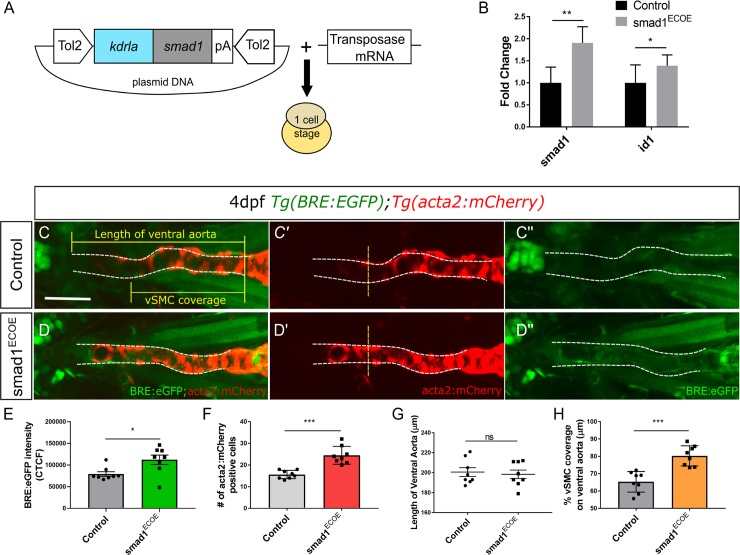
To demonstrate that smad1 expression in endothelial cells promotes vSMC differentiation, we expressed smad1 under an endothelial promoter in a transposon vector (TolCG2:kdrl:smad1, hereafter smad1ECOE; Fig 6A). The vector and transposase or transposase alone control were injected into Tg(BRE:EGFP;acta2:mCherry) embryos and scored at 48 hpf and 4 dpf. At 48 hpf, 10% of smad1ECOE embryos hemorrhage, similar to the increased hemorrhage observed in mirR26 knockdown and global smad1 mRNA overexpression (Fig 4). Higher doses of the vector result in significant cranial and pericardial edemas (S6A and S6B Fig). At 4 dpf, RT-qPCR of smad1ECOE embryos shows a 1.9-fold increase in smad1 and 1.4-fold increase in the BMP responsive gene id1 expression as compared to control (Fig 6B). Similarly, BRE:EGFP fluorescence is also increased in smad1ECOE embryos by 30% as compared to controls (Fig 6C”, 6D” and 6F). Together, the data show that activation of Smad1 was significantly increased in smad1 injected embryos. smad1ECOE embryos do not show a change in the length of the ventral aorta, however the BRE:EGFP signal extends further along the ventral aorta (Fig 6C”, 6D” and 6G). The total number of acta2:mCherry positive vSMCs along the ventral aorta in smad1ECOE embryos was significantly increased from 15±0.6 in controls to an average 24±1.4 cells (Fig 6C’, 6D’ and 6E). The percent vSMC coverage of the ventral aorta is also increased by 20% with an average of 80 ± 2.05% in smad1ECOE as opposed to 65.3±2.1% in controls (Fig 6C’, 6D’ and 6H). Our data suggests that upregulation of smad1 in endothelial cells is sufficient to increase vSMC number and coverage of the ventral aorta of 4 dpf embryos.
Fig 6. smad1 overexpression in endothelial cells results in increased vSMC coverage.
A) Vector construct for overexpression of smad1 under the endothelial cell promoter kdrla. B) RT-qPCR fold change in smad1 and id-1 expression levels in endothelial specific smad1 overexpressing embryos (smad1ECOE) embryos at 4 dpf (n = 3). RT-qPCR data show the mean ± SEM, Student's two-tailed t-test *p < 0.05, n, number of biological replicates. C–D) Representative orthogonal projections of ventral views of 4 dpf Tg(BRE:EGFP); Tg(acta2:mCherry) embryos. Control embryos (C-C”) and smad1ECOE embryos (D-D”) showing endothelial BRE:EGFP and vSMC acta2:mCherry expression in the ventral aorta (VA) and pharyngeal arch arteries (PAA). E) Quantification of green fluorescent marker (BRE:EGFP) along the VA, highlighted within the yellow region in C” and D”, as corrected total cell fluorescence (CTCF). F) Quantification of acta2 positive cell number on VA, within area outlined in C and D. Number of acta2 positive cells is significantly increased in smad1ECOE embryos. G) Quantification of length of VA, within area outlined in C and D. H) Quantification of the percent vSMC coverage of ventral aorta. For each quantification, N = 3, smad1ECOE embryos n = 8, Control n = 8, Student's two-tailed t-test, *-***p< 0.01–0.0001 as compared to control. Error bars = SEM, Scale bar represents 50μm.
BMP inhibition rescues the effect of on vSMC differentiation after miR26a knockdown
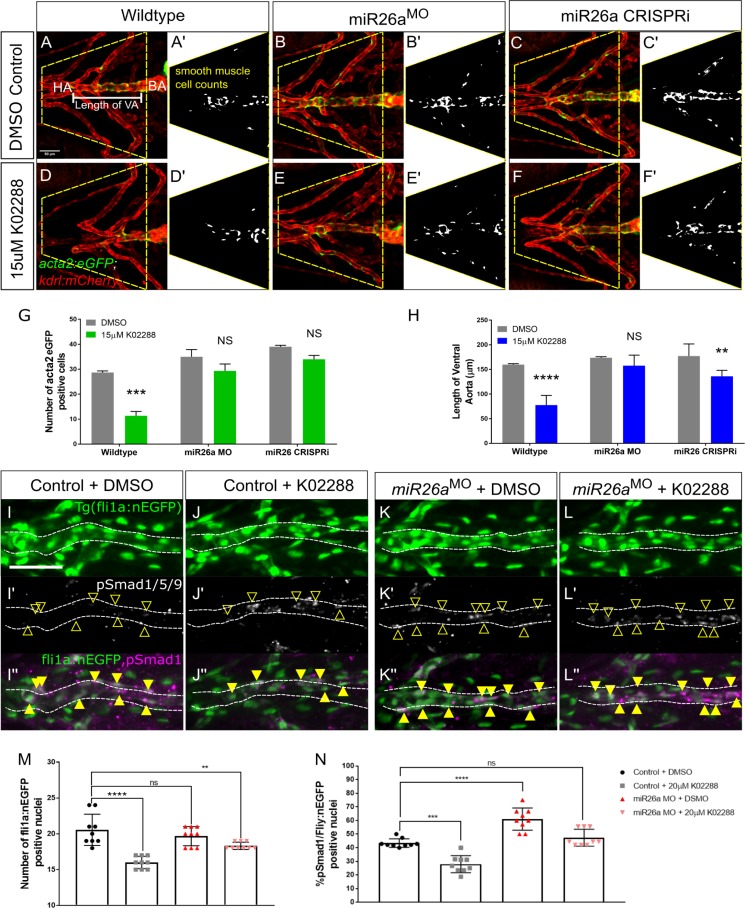
Our results showed that miR26a knockdown leads to an increased number of acta2 positive vSMCs on the ventral aorta and upregulation of Smad1 activation in the endothelium. To further investigate the interplay between BMP signalling in endothelial cells and vSMC differentiation, we tested whether the increase in vSMC number and differentiation after loss of miR26a could be reversed by blocking endothelial BMP signalling. K02288 is a selective and potent small molecule inhibitor of BMP signalling that blocks Smad1 phosphorylation by type I receptor Activin like kinase 1 (Alk1) and Alk2 [54,55]. We show that alk1 expression is enriched in endothelial cells at this developmental stage, but not vSMCs (S1C Fig). We selected a time point for drug application when the endothelium of the major blood vessels is patterned [56], but when vSMC coverage of the ventral aorta and PAA is only starting [7]. Tg(acta2:EGFP;kdrl:mCherry) embryos were treated with 15μM K02288 from 52 hpf to 4 dpf. As expected, miR26a morphant and miR26a CRISPRi treated embryos have significantly more vSMCs than wildtype embryos (Fig 7B, 7B’, 7C, 7C’ and 7G). Wildtype embryos treated with K02288 show a 62% reduction in the total average number acta2:EGFP positive cells compared to vehicle control alone (Fig 7A’, 7D’ and 7G. 29±1 to 11±3). In miR26a knockdown embryos (Fig 7B’ and 7C’), the effects of K02288 were rescued; miR26a morphants had a non-significant 17% reduction in vSMC numbers (35±5 to 29±5) and miR26a CRISPRi embryos had a non-significant reduction from 39±1 to 34±3 (Fig 7E’, 7F’ and 7G). We also found that BMP inhibition not only affects vSMC number, but also reduces ventral aorta length by 55% in K02288 treated wildtype embryos, from an average 159±2.08 μm to 77±19.5 μm (Fig 7A, 7D and 7H). However, miR26a morphants treated with K02288 are rescued and have a ventral aorta length not significantly different than wildtype. miR26a CRISPRi embryos showed a smaller rescue and had a 20% decrease in length when treated (Fig 7B, 7C, 7E and 7F, 177±8.6 to 136±5.6). Of note, there was no statistical difference in ventral aorta length between miR26a knockdown and control embryos, which supports our finding that endothelial cell number is not affected by loss of miR26a.
Fig 7. miR26a controls vSMC differentiation via smad1-mediated BMP signaling.
Ventral aorta showing endothelial (red) and smooth muscle (green) cells in miR26a morphants or CRISPRi- injected embryos treated with vehicle control (DMSO) or 15μM K02288 from 52 hpf to 4 dpf. A-C) DMSO-treated vehicle control embryos. D-F) K02288 treated control embryos. (A, D), miR26a morphant (B, E), miR26a CRISPRi knockdown (C, F). A’-F’ are threshold adjusted images of acta2-EGFP expression. G) Quantification of acta2 positive cell number on VA and PAAs, within area outlined in A and B. Number of acta2 positive cells is significantly reduced in K02288 treated embryos as compared to DMSO control. There is no significant decrease in miR26a knockdown embryos (two Way ANOVA, N = 3, miR26a MO n = 15, Wildtype n = 15, Unpaired t test, ****p< 0.0001 as compared to control, Error Bars = SEM. H) Quantification of length of VA, within area outlined in A and B. Length of VA is significantly reduced in K02288 treated embryos as compared to DMSO control. There is no significant decrease in miR26a knockdown embryos (Two Way ANOVA, N = 3, miR26a MO n = 15, Scr.Control n = 15, Unpaired t test, ****p< 0.0001 as compared to control, Error Bars = SEM. VA = ventral aorta, HA = hyoid artery, BA = bulbous arteriosus. (N = 3, 8–9 embryos per treatment group. One Way ANOVA, p< 0.001–0.0001***-****. Scale bar represents 50μm. I-L) pSmad1/59 staining in K02288 treated embryos. Endothelial nuclei (fli1a:nEGFP; I-L, arrowheads) and pSmad1/5/9 (pSmad1, white I’-L’) and overlay (magenta, I”-L”) in 4 dpf Scr. Control and miR26a morphants. Solid yellow arrowheads in I”-L” indicate pSmad1 + fli1a:nEGFP double positive nuclei in the ventral aorta. M) Quantification of total number of fli1a:nEGFP nuclei in the ventral aorta. N) Quantification of the percent pSmad1; fli1a:nEGFP double positive nuclei in miR26a morphants and miR26a CRISPRi embryos. N = 3 experiments, total embryos Scr. Control MO n = 9, miR26a MO n = 9. One Way ANOVA, p< 0.001–0.0001***-****. Scale bar represents 50μm.
We next tested whether pSmad1 levels are rescued in K02288 treated miR26a knockdown embryos as compared to controls (Fig 7I–7L). Using the endothelial nuclear marker fli1a:nEGFP, we confirmed that there was no significant difference in endothelial cell number between untreated control and miR26a morphants (Fig 7M). In control embryos, treatment with K02288 (Fig 7I, 7I’, 7J and 7J’) significantly reduced the number fli1a:nEGFP positive cells to 16± 0.2, which is 20% less than controls. Similarly, the proportion of pSmad1 positive/nEGFP nuclei also decreased from 43±1% to 28±2% (Fig 7N). Although K02288 treated miR26a morphants have a slight reduction in the total number fli1a:nEGFP positive cells (Fig 7M), there is no significant decrease in the proportion of pSmad1 positive/fli1a:nEGFP nuclei (Fig 7K, 7K’, 7L, 7L’ and 7N), and they remain similar to untreated controls. Taken together, our results further suggest that the endothelial cell is a critical site of Smad1-mediated BMP signalling and blocking its activation can significantly affect vSMC coverage. Loss of miR26a is able to rescue these defects to maintain both endothelial signalling and vSMC coverage.
Discussion
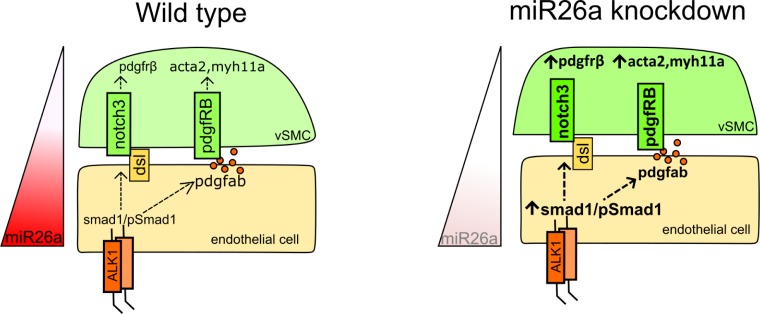
Compromised structural vascular integrity, vessel weakening and rupture (hemorrhage) can result from aberrant BMP signalling [57–59]. Hemorrhage ultimately results from weak endothelial junctions, however defects in mural cell coverage (attachment and ECM secretion) are implicated in the pathological progression of vascular diseases. We show that the endothelium of the ventral aorta in zebrafish has activated pSmad1 at 4 dpf, but that pSmad1 is not detectable in mural cells. At a stage when mature vSMC are normally present, embryos with loss of miR26a have upregulation of pSmad1, increased vSMC coverage and a change in vSMC morphology, with no observable changes in the number or morphology of the pSmad1-expressing endothelial cells. We show that inhibition of BMP signalling reduces both vSMC coverage and the length of the ventral aorta while dual miR26a knockdown and BMP receptor inhibition leads to a rescue such that animals maintain normal vSMC number, length of the ventral aorta, and vSMC coverage. We therefore suggest that miR26a modulates BMP signalling in endothelial cells to control vSMC differentiation via a paracrine mechanism potentially involving Notch and/or Pdgfrβ signalling. We propose that miR26a therefore functions in vivo to fine tune endothelial signals to the vSMCs (Fig 8).
Fig 8. Mechanistic model by which miR26a modulates BMP signaling to promote vSMC differentiation via interactions with endothelial cells.
miR26a modulates vascular stability by directly targeting smad1. At developmental stages when smooth muscle appears, the endothelium has active BMP signaling. Loss of miR26a results in increased BMP signaling in endothelial cells where Smad1 becomes phosphorylated. Increased pSmad1 in endothelial cells leads to increased differentiation (acta2 expression) and increased vSMC cell number, while blocking BMP signaling leads to a decrease of both. (Dashed arrows indicated the indirect effect on vSMC marker expression and cell number).
Studies in cultured vSMC have suggested that miR26a controls Smad1-mediated BMP signalling within vSMCs to modulate their phenotype [28]. However, these studies do not address whether the levels of pathway activation in vitro are relevant to tissues in vivo. Additionally, data collected from in vitro culture systems do not address the role of cell to cell communication (autonomous and non-autonomous signalling) that is critical in vivo [1,9]. We therefore sought to use an in vivo model of vascular development with intact tissue and cellular contexts to assess how loss of miR26a and subsequent increases can affect vSMC coverage. We show multiple lines of evidence that suggest that endothelial pSmad1 levels correlate with increased vSMC coverage of blood vessels. Our use of BMP-reporter transgenic fish reveals that during normal development, and under physiological conditions, vSMCs directly contact BRE and pSmad1 positive endothelial cells but have undetectable BRE or pSmad1 signal themselves.
In parallel to loss of miR26a resulting in a subsequent increase in smad1 and vSMC coverage, we also demonstrate that endothelial specific overexpression of smad1 (smad1ECOE) results in increased vSMC coverage. Our data therefore inversely complement the murine knockout models of HHT that have noted reduced vSMC coverage when endothelial Smad1 signalling is reduced. Of note, endothelial specific knockdown of Alk1 or Smad4 leads to a reduction of αSMA/Acta2 coverage on larger arterial vessels. Interestingly, there is a context-dependant shift in vSMC coverage in these studies, as ectopic expression of vSMCs is seen on venous and capillary vessel beds [60]. This hypervascularization was presumed to be in response to increased flow from AVM affected vessels into finer retinal vessels. Similar shifts are seen when BMP9/10 blocking antibodies are used [61]. Our study did not address changes in vSMC coverage in venous beds, but it would be interesting to see if overexpression of smad1 leads to increased vSMC coverage across both arterial and venous vessel beds.
Our data suggest that the normal function of miR26a is to reduce Smad1 protein activation within the endothelium, and indirectly inhibit vSMC differentiation in early development. Treatment with K02288, a potent ALK1/2 inhibitor, significantly reduced both acta2-positive vSMC coverage and reduced the length of the ventral aorta. These effects could be rescued by loss of miR26a. Thus, we suggest that enhanced Smad1 activation in these embryos compensates for receptor inhibition. ALK1, ALK2 and ALK3 are expressed in both endothelial and vSMCs [17–21], however in zebrafish alk1 is highly expressed only in the endothelium at 36 hpf [47]. Violet beauregarde (vbgft09e) alk1 loss of function zebrafish mutants develop striking cranial vessel abnormalities by 48 hpf due to increased endothelial cell proliferation [19]. vbgft09e are also unable to limit the diameter of arteries carrying increasing flow from the heart [27]. Based on our data involving indirect control by endothelial signalling, we would predict there is an additional defect in vSMC recruitment in alk1 mutants, although this remains to be tested.
Endothelial and mural cells signal through several paracrine pathways to stabilize vessels [62,63]. BMP signalling in endothelial cells activates an axis of BMP/ Notch3/ Pdgf signalling to promote the expression of contractile vSMCs genes such as Acta2 and Myh11a in in vitro co-culture systems [64]. Specifically, BMP9 signalling via endothelial cells induces NOTCH3 in vSMCs, which in turn induces expression of Pdgfrβ and maintains the proper response to Pdgf ligands [17,65]. There is evidence that mouse MiR26a is modulated by Pdgf-BB signalling [45]. For instance, neointimal hyperplasia results in elevated levels of Pdgfbb associated with upregulation of MiR26a and accumulation and proliferation of vSMC at sites of injury. Furthermore, treatment of primary mouse aortic vSMCs with MiR26a mimic drives cells to a synthetic vSMC state [45]. We found that notch3, pdgfrβ and contractile vSMC markers were significantly increased in miR26a knockdown embryos, suggesting that increases in endothelial Smad1 in zebrafish may be transmitted to vSMCs through a BMP/ Notch3/ Pdgf signalling axis. Pdgf ligands are primarily released by endothelial cells, and we observe an increase in pdgfba in miR26a morphants, providing a potential mechanism by which active BMP signaling in endothelium can recruit and induce vSMC differentiation via paracrine non-autonomous signalling pathways.
While we found increased differentiation of vSMCs at the later stage 4 dpf time point, at 48 hpf loss of miR26a results in hemorrhage. The 48 hpf to 4 dpf window is a common window for vascular instability phenotypes to emerge in zebrafish [3,66–68]. BMP signalling is initiated in endothelium at this time point and perturbations can affect endothelial cell junction development [63]. We have previously shown mural cells present around vessels by 48 hpf, although they are mesenchymal and immature [3]. These cells express pdgfrβ but have no expression of mature vSMC markers [69], suggesting the 48 hpf time point represents a critical window for vascular mural cell attachment to endothelium and differentiation to a mature phenotype. It is paradoxical then that we see increased maturation of vSMCs at 4 dpf when mir26a is reduced. We suggest that the altered receptor and ligand expression in miR26a morphants may promote morphological change towards maturation, but may not regulate all aspects of maturation, leading to destabilization. For instance aberrant ECM deposition would not be visible in our assays and could lead to vascular instability at the earlier time points [63].
As critical modulators of vascular cell function and with roles in cell differentiation, contraction, migration, proliferation and apoptosis, miRs are attractive targets of therapeutic treatments aimed at modulating the vSMC phenotypic switch. Specific to TGF-β/BMP signalling, the miR-145/143 family has direct involvement in SMC differentiation by repressing the Klf4 to induce a contractile morphology and reduced rates of proliferation [40]. miR-21 controls vSMC differentiation through cross-talk with miR-143/-145 [35] and by mediating TGF-β/BMP induction to promote miR-21 cleavage to its mature form and a more contractile phenotype (Fig 5). miR26a is unique in this group in that it represses smooth muscle differentiation, likely via a paracrine signalling from endothelial cells. As drug delivery to the endothelium is relatively straightforward, modulation of miR26a might be therapeutically useful for post-transcriptional control of key genes involved in vSMC phenotypic switching.
Materials and methods
Ethics statement
All animal procedures were approved by the University of Calgary Animal Care Committee (AC17-0189). Anesthesia and euthanasia used MS-222 (Tricaine) at 10–40 mg/L.
Zebrafish maintenance and husbandry
Zebrafish (Danio rerio) embryos were collected and incubated at 28.5°C in E3 embryo medium and staged in hours post-fertilization (hpf) or days post fertilization (dpf). Endogenous pigmentation was inhibited from 24 hpf by the addition of 0.003% 1-phenyl-2-thiourea (PTU, Sigma-Aldrich, St. Louis, MO) in E3 embryo medium. The fluorescent transgenic endothelial mCherry-expressing Tg(kdrl:mCherry)ci5, GFP-expressing Tg(kdrl:EGFP)la116 report endothelial expression and Tg(fli1a:nEGFP)y7 [19] reports EGFP cDNA fused to a nuclear localization sequence in endothelial nuclei. Tg(acta2:GFP)ca7 and Tg(acta2:mCherry)ca8 report smooth muscle expression [7]. BMP-reporter fish Tg(BRE-AAVmlp:EGFP)mw29 [BRE:EGFP] report active BMP signaling [46].
Morpholino knockdown, CRISPRi and mRNA overexpression
Both MO and mimic were injected into one- to four-cell stage embryos within recommended dosage guidelines [50,70]. Injected doses were 1ng/ embryo for miR26a MO, Scrambled (Scr.) control, miR26a, and smad1 MO. Morpholinos (MO) were obtained from Gene Tools LLC (Corvallis, OR, USA). mir-26a MO blocks the mature microRNA (5 AGCCTATCCTGGATTACTTGAAC-3’), miR26a Scrambled control has 6bp mismatch (5’-ACCGTATCGTGCATTACTTCAAC-3’), and smad1 MO blocks Smad1 translation (5’-AGGAAAAGAGTGAGGTGACATTCAT-3’) [53]. For rescue experiments, embryos were first injected with miR26a MO and then smad1 MO. To control for non-specific neural cell death that occurs from nonspecific activation of p53 with morpholinos, a standard p53 MO was co-injected with high dose morpholino to establish dosage curve. Hsa miR26a miRIDIAN mimic was obtained from Dharmacon (Chicago, IL) and injected in a dose of 3ng/ embryo.
For CRISPRi mediated knockdown of miR26a, sgRNA were designed using CHOPCHOP [71,72] to target the seed sequence of miR26a family members, to reduce miR26a processing. MiR26a-1, miR26a-2, miR26a-3, are independent genes located on different chromosomes. miR26b differs by one nucleotide. To generate sgRNA we followed a method established by [73]. 10 μmol of forward primer (5’ TAATACGACTCACTATAGGATCCT GGATTACTTGAACCAGTTTTAGAGCTAGAA-3′) and 50 μmol of a universal reverse primer (5′AAAAGCACCGACTCGGTGCCACTTTTTCAAGTTGATAACGGACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAAC-3′), (IDT Oligos, Coralville, Iowa were annealed and filled in [72], purified (Qiagen PCR purification kit) and in vitro transcribed (T7 mMESSAGE mMACHINE kit, Ambion, Austin, TX. Zebrafish codon optimized dCas9 plasmid [74] was linearized with XbaI and in vitro transcribed using Ambion Maxi Kit (Life Technologies Inc., Burlington, ON), and RNA purified using an RNeasy Mini Kit (Qiagen, Hilden, Germany). Zebrafish embryos at the one-cell stage were injected with 200pg of a solution containing 75 ng/ μl of sgRNA with 150 ng/ μl of Cas9 mRNA. For overexpression of smad1, mRNA was in vitro transcribed as described (McReynolds et al. 2007; gift from Todd Evans Lab) using mMessage mMachine (Life Technologies Inc., Burlington, ON). 40 pg of mRNA was injected per embryo at the 1 cell stage.
Plasmid construction
For endothelial specific overexpression, smad1 was amplified from zebrafish cDNA using primers that incorporate attb1/b2 recombination sites (5’-GGGGACAAGTTTGTACAAAAAAGCAGGCTTCACCATGAATGTCACCTCACTCTTTTCC-3’ and 3’- GGGGACCACTTTGTACAAGAAAGCTGGGTGCTAGGACACTGAAGAAATGGGGT-5’ and inserted into pDONR221 to create a pME-smad1 vector. Three way Tol2 gateway cloning [75] was used to insert smad1 downstream of the kdrla promoter to achieve a TolCG2:kdrla:smad1 vector. One-cell stage zebrafish embryos were injected with a solution consisting of 5–20 ng/ μl kdrla-smad1 plasmid and 50 ng/ μl transposase mRNA.
For the in vivo sensor test, smad1 3′UTR forward and reverse oligos (IDT) were designed incorporating BamHI and Bsrg1 sites using the prediction software TargetScan [76] for miR26 targets within the 3’UTR of zebrafish smad1 (underlined). (5’GCGTGTACACCGGATGACTAGAGGGTTAGGTTGTGTACTACTTGAAGGCAGTTTGTTAGGGTGGGGGTCATCGAATCTGGCTGAAGAGTCCTCAGTTTTCAGCCCGTGAGAATCTGGAAGATACTTGACAACTCTGTGGCCGGATCCATA-3’ and 3’- TATGGATCCGGCCACAGAGTTGTCAAGTATCTTCCAGATTCTCACGGGCTGAAAACTGAGGACTCTTCAGCCAGATTCGATGACCCCCACCCTAACAAACTGCCTTCAAGTAGTACACAACCTAACCCTCTAGTCATCCGGTGTACACGC-5’). Oligos were digested and ligated into the p3E-polyA vector. This construct was then recombined into pDestTol2pA2 by Gateway cloning to achieve a CMV-SP6 promoter upstream of EGFP: smad1 3′UTR:EGFP or a control EGFP: p3E-polyA 3′UTR. Sensor mRNA and mCherry mRNA were in vitro transcribed from the pCS2 Gateway compatible vector (39) by using the mMessage Machine SP6 kit (Ambion). One-cell zebrafish embryos were injected with 150 pg sensor mRNA and 100 pg mCherry mRNA. When applicable, miR26a MO or miRNA mimic were added. Live embryos were imaged with an identical exposure time at 24 hpf (n = 10/group). The average pixel intensity for fluorescence was measured as described (17)
Cell sorting, RNA isolation and RT-qPCR
For FACS analysis ~200 embryos were collected from 4 dpf Tg(acta2:EGFP;kdrl:mCherry) fish. Embryos were anesthetized with 0.4% Tricaine (Sigma) and heads dissected and pooled. Single cell dissociation was performed according to Rougeot et al. 2014. Briefly dissected embryo heads were washed once with calcium-free Ringers Solution and gently triturated 5–10 times before dissociation solution was added and incubated in a 28.5°C water bath with shaking and periodic trituration for 45 min. The reaction was stopped, centrifuged and resuspended in Dulbecco’s Phosphate-Buffered Saline (GE Healthcare Life Sciences, Logan, Utah, USA, centrifuged and resuspended in fresh resuspension solution. The single cell suspension was filtered with 75 μm, followed by 35 μm filters. Cells were then sorted with a BD FACSAria III (BD Bioscience, San Jose, USA) and collected.
Total RNA from 48 hpf whole embryos, 4 dpf dissected embryo heads or FACS sorted cells was isolated using the miRNeasy Mini Kit (Qiagen). For microRNA RT-qPCR, 5 ng of total RNA from each sample was reverse transcribed using the miRCURY LNA Universal RT cDNA Synthesis Kit and expression assayed using the miRCURY LNA Universal RT microRNA PCR System (Qiagen). Primers were ordered for miR26a-5p (MIMAT0000082, Target sequence: UUCAAGUAAUCCAGGAUAGGCU), and, expression levels normalized to that of miR-103a-3p (MIMAT0000425, Target sequence: CAGUGCAAUGUUAAAAGGGCAU) or miR122 (MIMAT0000421, Target sequence: AGCUACAUUGUCUGCUGGGUUUC for miRNA expression)
For gene expression, zebrafish specific Taqman assays (Thermo Fisher Scientific, Waltham, Massachusetts, USA) were used: smad1 (Cat# 4351372, Clone ID: Dr03144278_m1), acta2 (4331182, Dr03088509_mH), myh11a (444889, Dr03141711_m1), pdgfrβ (4441114, ARKA4GC), pdgfba (4441114, ARWCXGT), nothch3 (4448892, Dr03432970_m1) and normalized to β-actin (4448489, Dr03432610_m1). 500 ng of total RNA from each sample were reverse transcribed into cDNA using and assayed using according to manufacturer’s protocols in a 5ng/ 10ul final reaction using TaqMan Fast Advanced Master Mix (Thermo Fisher). Reactions were assayed using a QuantStudio6 Real-time system (Thermo Fisher).
The ΔΔCt method was used to calculate the normalized relative expression level of a target gene from triplicate measurements. Experiments were repeated independently at least three times, unless stated otherwise.
Small molecule inhibition
K02288 was used at a dose of 15μM (SML1307, Sigma). DSMO (D8418, Sigma) was used as a vehicle and control. Drug stocks were heated for 20 min at 65C and then diluted in E3 embryo medium. Drug or control was applied to the media from 52 hpf until 4 dpf. Embryos were grown at 28.5C in the dark until imaging, and drug changed once.
In situ hybridization and immunostaining
All embryos were fixed in 4% paraformaldehyde in PBS with 0.1% Tween-20 at 4°C overnight, followed by 100% methanol at −20°C. Digoxigenin (DIG)-labeled antisense RNA probes were used for in situ hybridization. Probes for smad1 (construct described by [53]) sm22a, acta2, myh11a were synthesized from PCR fragments previously described [7,48]. Probes were synthesized by using SP6 or T7 RNA polymerase (Roche, Basel, Switzerland). miR26a double-DIG-labeled LNA probe was obtained from Exiqon, (Copenhagen, Denmark. In situ hybridization was performed as described [32]using a Biolane HTI robot (Holle and Huttner AG, Tubingen, Germany). For microRNA in situ hybridization, a double-DIG-labeled Locked Nucleic Acid (LNA) probe (Exiqon) was used to detect the mature miR26a in whole-mount embryos as recommended by the manufacturer with the modification that hybridization was at 54°C.
For wholemount immunostaining an antigen retrieval protocol optimized from [77] was used. Briefly embryos are hydrated into PBST, washed twice with 15 mM Tris–HCl pH 9.5, 150 mM EDTA and then heated in 15 M Tris–HCl pH 9.5, 150 mM EDTA at 70°C for 15 min. Embryos are then washed 3 times in PBST at room temperature and incubated in 10% normal sheep serum in PBST with 1% triton block and incubated for at least 48 hours at 4°C in primary antibody. Phospho-SMAD1/5/9 (pSMAD1/5/9) was detected with Rabbit anti-Phospho-Smad1 (Ser463/465)/Smad5(Ser463/465)/Smad9(Ser426/428) (1:400; Cell Signaling Technology, Danvers, Massachusetts, USA), GFP was detected with mouse anti-GFP antibody, JL8 (1:500, Clontech, Mountain View, California, USA) and detected with Alexafluor 647 or 488 secondary antibodies for 1 hour at room temp in 5% normal sheep serum in PBST with 0.1% triton (1:500; Invitrogen Molecular Probes).
Imaging and data analysis
For imaging, embryos were immobilized in 0.004% Tricaine (Sigma) and mounted in 0.8% low melt agarose on glass bottom dishes (MatTek, Ashland, MA). Confocal images were collected on a Zeiss LSM 700 inverted microscope. Image stacks were processed in Zen Blue and are presented as maximal intensity projections and analyzed using FIJI/ImageJ [78] For cell counts images were converted to 16-bit using ImageJ and the threshold adjusted to allow counting of cells over a region of the VA from the anterior bulbous arteriosus to the most anterior PAA.
To measure intensity, total cell fluorescence (CTCF) was calculated using the formula: CTCF = Integrated Density—(Area of selected cell X Mean fluorescence of background readings). The area for measurement was gated by tracing the aorta from bulbous where the bulbous arteriosus merges with the ventral aorta to the distal tip of the ventral aorta or to the bifurcation point of the ventral aorta using the free form drawing tool, whichever was shorter [56].
For measurement of vSMC cell heights, measurements were made from the endothelial kdrla:EGFP expression to the highest point of the vSMC. 8 measurements were taken for each sample where possible. Ventral head measurements were taken from the ventral aorta and the aortic arch arteries. Measurements represent mean vessel diameter ± standard deviation in micrometers.
Statistical analysis
Distribution of data points are expressed as mean ± standard error of the mean (S.E.M.), or as relative proportion of 100% as mentioned in the appropriate legends. Depending on the number of the groups and independent factors, student's t-tests, one-way or two-way analyses of variance (ANOVA) with non-parametric tests were used as indicated in the figures. Two treatment groups were compared using Student’s t-test, using Welch’s correction. Three or more treatment groups were compared by one- or two-way ANOVA followed by post hoc analysis adjusted with a least significant-difference correction for multiple comparisons using GraphPad Prism version 7.00 (La Jolla California USA). Results were classed as significant as follows: *P < 0.05, **P < 0.01, and ***P < 0.001.
Supporting information
A) Schematic of FACS sorting strategy for acta2:EGFP+ and kdrl:mCherry+ cells from 4 dpf Tg(acta2:EGFP;kdrl:mCherry) dissected embryo heads. B) Expression level of miR26a in acta2:EGFP+ and kdrl:mCherry+ isolated by FACS (n = 2). C) Expression level of smad1, acvrl (alk1) and acta2 in acta2:EGFP+ and kdrl:mCherry+ isolated by FACS (n = 2). RT-qPCR analysis of values represent mean ± SEM, n = biological replicates. D) Wholemount immunohistochemistry of pSmad1/5/9 in Tg(acta2:EGFP)ca7; Tg(kdrl:mCherry)ci5 shows nuclear staining of pSmad1 in endothelial cells. pSmad 1/5/9 is observed in endothelium (red) but not smooth muscle (green) of the hyoid artery and afferent branchial arches. Magenta asterisk indicates pSmad1/5/9 stain; arrows indicate smooth muscle cells. Scale bar represents 50μm.
(EPS)
A) EGFP:smad1 sensor assay showing EGFP-smad1 sensor expression vs mCherry control. B) Quantification of EGFP fluorescence in sensor assay as compared to controls. Student's two-tailed t-test *p< 0.05, N = 3, total of 9 embryos per group, Error Bars = SEM, Scale bar represents 50μm. C) RT-qPCR of relative expression of miR26a in miR26a-mimic injected embryos at 48 hpf. Values are means of 3 replicates and normalized to miR122; Unpaired t test, *p< 0.01 as compared to control; Error Bars = SD. D) miR26a mimic injected embryo with mild dorsalization phenotype, heart edema (arrowhead), dorsal axis defects (arrows) and poor circulation (arrowhead at tail) at 48 hpf. E) Whole mount in situ hybridization staining for smad1 at 48 hpf in uninjected control, negative control mimic and miR26a injected embryos. There is decreased expression of smad1 in miR26a mimic injected embryos (boxes and arrow).
(EPS)
Ventral view confocal projections of 4 dpf Tg(kdrl:mCherry; fli1a:nEGFP ventral aorta (dotted outline. A-D) Endothelial cytoplasm (kdrl:mCherry) and endothelial nuclear (fli1a:nEGFP) staining. E-H) fli1a:nEGP endothelial nuclear stain. I-L) pSmad1/5/9 staining. M-P) overlay image, in 4 dpf Scr. Control (A,E,I,M), miR26a MO (B,F,J,N), dCas9 control (C,G,K,O) and miR26a CRISPRi (D,H,L,P). Q) Average number of fli1a:nEGFP nuclei. R) Average percentage pSmad1/fli1a:nEGFP double positive nuclei. Scale bar represents 50μm.
(EPS)
A) RT-qPCR of relative expression of miR26a at 48 hpf in miR26a MO (28 ng/ embryo). Values are means of 3 replicates and normalized to miR122, n = 3: UIC vs. Neg. ctl. MO (Scr.Control MO) p = 0.16. UIC vs. Pre-miR26a MO: p < .0001. UIC vs. miR-26a mature MO: p = 0.0002. RT-qPCR analysis of values represent mean ± SEM, n = 2 biological replicates. B) Whole-mount in situ hybridization staining for smad1 at 48 hpf shows increased expression of smad1 at higher dose of miR26a MO. C) Representative images of wildtype (uninjected) and high dose miR26a morpholino-injected embryos showing normal body axis, but with hemorrhage and mild hydrocephalus. D) Average rates of hemorrhage for miR26a MO at 28 ng/ embryo and 6 ng/ embryo (Student's two-tailed t-test, ****p< 0.0001 as compared to WT, Error Bars = SEM. N = 5, n total miR26a MO 28 ng = 488, 6 ng = 501 and Wildtype = 535).
(EPS)
A-D) Whole-mount in situ hybridization staining for acta2 and myh11a at 4 dpf shows increased expression in miR26a knockdown embryos in the aortic arches and pharyngeal region. E-J) Whole-mount in situ hybridization staining for acta2 and sm22α at 4 dpf shows decreased expression in miR26a mimic injected embryos.
(EPS)
A) Phenotypes observed at 48 hpf with increasing doses of smad1ECOE. B) Quantification of phenotypes, Student's two-tailed t-test **-****p< 0.005–0.00005 as compared to control. Error Bars = SEM. N = 3, n total smad1-ECOE 5 ng, n = 60, 10 ng, n = 61, 20 ng, n = 63 and control n = 72. C) Ventral view confocal projections of the 4 dpf ventral aorta of Tg(BRE:EGFP) embryos. D) Average number of acta2:mCherry cells, BRE intensity and vSMC coverage.
(EPS)
File of raw data underlying graphs.
(XLSX)
Acknowledgments
We would like to thank past and present members of the Childs lab Corey Arnold, Michela Goi, Tom Whitesell, Jasper Greysson-Wong, Nabila Bahrami, in addition to members of Dr. Peng Huang’s lab for helpful comments on the project and paper. We would like to thank the members of the Flow Cytometry core facility.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
SJC received an Natural Science and Engineering Research Council Discovery Grant RGPIN/06360-2014 (www.nserc.ca). CW received an Eyes Hish Studentship from the University of Calgary. LZ received fellowships from the Canadian Institutes for Health Research Training Program in Genetics, Child Health and Development and from the Kertland fellowship. SJC received salary support from the Canada Research Chairs program and the Alberta Innovates Health Solutions. The funders played no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Gaengel K, Genové G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29(5):630–8. 10.1161/ATVBAHA.107.161521 [DOI] [PubMed] [Google Scholar]
- 2.Lamont RE, Vu W, Carter AD, Serluca FC, MacRae CA, Childs SJ. Hedgehog signaling via angiopoietin1 is required for developmental vascular stability. Mech Dev. 2010;127(3):159–68. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Fraser SD, Faloon PW, Rollins EL, Vom Berg J, Starovic-Subota O, et al. A βPix–Pak2a signaling pathway regulates cerebral vascular stability in zebrafish. Proc Natl Acad Sci. 2007;104(35):13990–5. 10.1073/pnas.0700825104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rensen SSM, Doevendans P, Van Eys G. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Netherlands Hear J. 2007;15(3):100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.David L, Mallet C, Keramidas M, Lamande N, Gasc JM, Dupuis-Girod S, et al. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res [Internet]. 2008;102 Available from: 10.1161/CIRCRESAHA.107.165530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owens GK, Vernon SM, Madsen CS. Molecular regulation of smooth muscle cell differentiation. J Hypertens Suppl Off J Int Soc Hypertens. 1996;14(5):S55–64. [PubMed] [Google Scholar]
- 7.Whitesell TR, Kennedy RM, Carter AD, Rollins E-L, Georgijevic S, Santoro MM, et al. An?-Smooth Muscle Actin (acta2/?sma) Zebrafish Transgenic Line Marking Vascular Mural Cells and Visceral Smooth Muscle Cells. PLoS One [Internet]. 2014. March 3;9(3):e90590 Available from: 10.1371/journal.pone.0090590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendrix JA, Wamhoff BR, McDonald OG, Sinha S, Yoshida T, Owens GK. 5′ CArG degeneracy in smooth muscle α-actin is required for injury-induced gene suppression in vivo. Vol. 115, Journal of Clinical Investigation. 2005. p. 418–27. 10.1172/JCI22648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev [Internet]. 2004. July 1 [cited 2015 Mar 8];84(3):767–801. Available from: http://physrev.physiology.org/content/84/3/767 10.1152/physrev.00041.2003 [DOI] [PubMed] [Google Scholar]
- 10.Mack CP, Owens GK. Regulation of smooth muscle α-actin expression in vivo is dependent on CArG elements within the 5′ and first intron promoter regions. Circ Res. 1999;84(7):852–61. [DOI] [PubMed] [Google Scholar]
- 11.Miano JM, Cserjesi P, Ligon KL, Periasamy M, Olson EN. Smooth muscle myosin heavy chain exclusively marks the smooth muscle lineage during mouse embryogenesis. Circ Res. 1994;75(5):803–12. [DOI] [PubMed] [Google Scholar]
- 12.Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med. 2006;259(4):381–92. 10.1111/j.1365-2796.2006.01624.x [DOI] [PubMed] [Google Scholar]
- 13.Cai J, Pardali E, Sánchez-Duffhues G, ten Dijke P. BMP signaling in vascular diseases. FEBS Lett. 2012;586(14):1993–2002. 10.1016/j.febslet.2012.04.030 [DOI] [PubMed] [Google Scholar]
- 14.Li L, Miano JM, Mercer B, Olson EN. Expression of the SM22alpha promoter in transgenic mice provides evidence for distinct transcriptional regulatory programs in vascular and visceral smooth muscle cells. J Cell Biol. 1996;132(5):849–59. 10.1083/jcb.132.5.849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Bizri N, Guignabert C, Wang L, Cheng A, Stankunas K, Chang C-P, et al. SM22α-Targeted Deletion of Bone Morphogenetic Protein Receptor IA in Mice Impairs Cardiac and Vascular Development and Influences Organogenesis. Vol. 135, Development (Cambridge, England). 2008. p. 2981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torihashi S, Hattori T, Hasegawa H, Kurahashi M, Ogaeri T, Fujimoto T. The expression and crucial roles of BMP signaling in development of smooth muscle progenitor cells in the mouse embryonic gut. Differentiation [Internet]. 2009. March [cited 2016 May 10];77(3):277–89. Available from: http://www.sciencedirect.com/science/article/pii/S0301468108000261 10.1016/j.diff.2008.10.003 [DOI] [PubMed] [Google Scholar]
- 17.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125(9):1591–8. [DOI] [PubMed] [Google Scholar]
- 18.Lan Y, Liu B, Yao H, Li F, Weng T, Yang G, et al. Essential role of endothelial Smad4 in vascular remodeling and integrity. Mol Cell Biol. 2007;27(21):7683–92. 10.1128/MCB.00577-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roman BL, Pham VN, Lawson ND, Kulik M, Childs S, Lekven AC, et al. Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development [Internet]. 2002. June 15;129(12):3009–19. Available from: http://dev.biologists.org/content/129/12/3009.abstract [DOI] [PubMed] [Google Scholar]
- 20.Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, et al. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol [Internet]. 2000. May 1 [cited 2016 May 24];221(1):249–58. Available from: http://www.sciencedirect.com/science/article/pii/S0012160600996702 10.1006/dbio.2000.9670 [DOI] [PubMed] [Google Scholar]
- 21.Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995. December;9(24):3027–37. [DOI] [PubMed] [Google Scholar]
- 22.Paul BY, Beppu H, Kawai N, Li E, Bloch KD. Bone morphogenetic protein (BMP) type II receptor deletion reveals BMP ligand-specific gain of signaling in pulmonary artery smooth muscle cells. J Biol Chem. 2005;280(26):24443–50. 10.1074/jbc.M502825200 [DOI] [PubMed] [Google Scholar]
- 23.Orvis GD, Jamin SP, Kwan KM, Mishina Y, Kaartinen VM, Huang S, et al. Functional redundancy of tgf-Beta family type I receptors and receptor-smads in mediating anti-mullerian hormone-induced mullerian duct regression in the mouse. Biol Reprod [Internet]. 2008;78 Available from: 10.1095/biolreprod.107.066605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sirard C, de la Pompa JL, Elia A, Itie A, Mirtsos C, Cheung A, et al. The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes Dev. 1998. January;12(1):107–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald J, Bayrak-Toydemir P, Pyeritz RE. Hereditary hemorrhagic telangiectasia: An overview of diagnosis, management, and pathogenesis. Genet Med [Internet]. 2011. May 4;13:607 Available from: 10.1097/GIM.0b013e3182136d32 [DOI] [PubMed] [Google Scholar]
- 26.Park C, Lavine K, Mishina Y, Deng CX, Ornitz DM, Choi K. Bone morphogenetic protein receptor 1A signaling is dispensable for hematopoietic development but essential for vessel and atrioventricular endocardial cushion formation. Development [Internet]. 2006;133 Available from: 10.1242/dev.02499 [DOI] [PubMed] [Google Scholar]
- 27.Corti P, Young S, Chen C-Y, Patrick MJ, Rochon ER, Pekkan K, et al. Interaction between alk1 and blood flow in the development of arteriovenous malformations. Development [Internet]. 2011. March 22;138(8):1573–82. Available from: http://dev.biologists.org/content/138/8/1573.abstract 10.1242/dev.060467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albinsson S, Suarez Y, Skoura A, Offermanns S, Miano JM, Sessa WC. miRNAs are necessary for vascular smooth muscle growth, differentiation and function. Arter Thromb Vasc Biol. 2010;30(6):1118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007/05/05. 2007;100(11):1579–88. 10.1161/CIRCRESAHA.106.141986 [DOI] [PubMed] [Google Scholar]
- 30.Xie C, Zhang J, Chen YE. MicroRNA and vascular smooth muscle cells. Vitam Horm. 2011/12/01. 2011;87:321–39. 10.1016/B978-0-12-386015-6.00034-2 [DOI] [PubMed] [Google Scholar]
- 31.Zhang C. MicroRNAs in vascular biology and vascular disease. J Cardiovasc Transl Res. 2010;3(3):235–40. 10.1007/s12265-010-9164-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng L, Carter AD, Childs SJ. miR-145 directs intestinal maturation in zebrafish. Proc Natl Acad Sci [Internet]. 2009. October 20;106(42):17793–8. Available from: http://www.pnas.org/content/106/42/17793.abstract 10.1073/pnas.0903693106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rangrez AY, Massy ZA, Metzinger-Le Meuth V, Metzinger L. MiR-143 and miR-145 molecular keys to switch the phenotype of vascular smooth muscle cells. Circ Cardiovasc Genet. 2011;4(2):197–205. 10.1161/CIRCGENETICS.110.958702 [DOI] [PubMed] [Google Scholar]
- 34.Xu J, Wu C, Che X, Wang L, Yu D, Zhang T, et al. Circulating MicroRNAs, miR‐21, miR‐122, and miR‐223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog. 2011;50(2):136–42. 10.1002/mc.20712 [DOI] [PubMed] [Google Scholar]
- 35.Sarkar J, Gou D, Turaka P, Viktorova E, Ramchandran R, Raj JU. MicroRNA-21 plays a role in hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration. Am J Physiol Cell Mol Physiol. 2010;299(6):L861–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali SS, Kala C, Abid M, Ahmad N, Sharma US, Khan NA. Pathological microRNAs in acute cardiovascular diseases and microRNA therapeutics. J Acute Dis [Internet]. 2015. October [cited 2015 Oct 26]; Available from: http://www.sciencedirect.com/science/article/pii/S2221618915000797 [Google Scholar]
- 37.Sun S, Zheng B, Han M, Fang X, Li H, Miao S, et al. miR‐146a and Krüppel‐like factor 4 form a feedback loop to participate in vascular smooth muscle cell proliferation. EMBO Rep. 2011;12(1):56–62. 10.1038/embor.2010.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Yu M, Yu G, Bian J, Deng X, Wan X, et al. Serum miR-146a and miR-223 as potential new biomarkers for sepsis. Biochem Biophys Res Commun. 2010;394(1):184–8. 10.1016/j.bbrc.2010.02.145 [DOI] [PubMed] [Google Scholar]
- 39.Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev [Internet]. 2009. August 31; Available from: http://genesdev.cshlp.org/content/early/2009/08/31/gad.1842409.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460(7256):705–10. 10.1038/nature08195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng L, Childs SJ. The smooth muscle microRNA miR-145 regulates gut epithelial development via a paracrine mechanism. Dev Biol. 2012;367(2):178–86. 10.1016/j.ydbio.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 42.Icli B, Dorbala P, Feinberg MW. An emerging role for the miR-26 family in cardiovascular disease. Trends Cardiovasc Med. 2014;24(6):241–8. 10.1016/j.tcm.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leeper NJ, Raiesdana A, Kojima Y, Chun HJ, Azuma J, Maegdefessel L, et al. MicroRNA‐26a is a novel regulator of vascular smooth muscle cell function. J Cell Physiol. 2011;226(4):1035–43. 10.1002/jcp.22422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bai Y, Wang L, Sun L, Ye P, Hui R. Circulating microRNA-26a: potential predictors and therapeutic targets for non-hypertensive intracerebral hemorrhage. Med Hypotheses. 2011/07/19. 2011;77(4):488–90. 10.1016/j.mehy.2011.06.017 [DOI] [PubMed] [Google Scholar]
- 45.Yang X, Dong M, Wen H, Liu X, Zhang M, Ma L, et al. MiR-26a contributes to the PDGF-BB-induced phenotypic switch of vascular smooth muscle cells by suppressing Smad1. Vol. 8, Oncotarget. 2017. p. 75844–53. 10.18632/oncotarget.17998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collery RF, Link BA. Dynamic Smad-mediated BMP signaling revealed through transgenic zebrafish. Vol. 240, Developmental dynamics: an official publication of the American Association of Anatomists. 2011. p. 712–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laux DW, Febbo JA, Roman BL. Dynamic analysis of BMP-responsive smad activity in live zebrafish embryos. Dev Dyn [Internet]. 2011. March 1;240(3):682–94. Available from: 10.1002/dvdy.22558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Georgijevic S, Subramanian Y, Rollins E, Starovic‐Subota O, Tang ACY, Childs SJ. Spatiotemporal expression of smooth muscle markers in developing zebrafish gut. Dev Dyn. 2007;236(6):1623–32. 10.1002/dvdy.21165 [DOI] [PubMed] [Google Scholar]
- 49.Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36(suppl 1):D154–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bill BR, Petzold AM, Clark KJ, Schimmenti LA, Ekker SC. A primer for morpholino use in zebrafish. Zebrafish. 2009;6(1):69–77. 10.1089/zeb.2008.0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bedell VM, Westcot SE, Ekker SC. Lessons from morpholino-based screening in zebrafish. Br Funct Genomics. 2011/07/13. 2011;10(4):181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Long L, Guo H, Yao D, Xiong K, Li Y, Liu P, et al. Regulation of transcriptionally active genes via the catalytically inactive Cas9 in C. elegans and D. rerio. Cell Res. 2015;25(5):638 10.1038/cr.2015.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McReynolds LJ, Gupta S, Figueroa ME, Mullins MC, Evans T. Smad1 and Smad5 differentially regulate embryonic hematopoiesis. Vol. 110, Blood. Washington, DC; 2007. p. 3881–90. 10.1182/blood-2007-04-085753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kerr G, Sheldon H, Chaikuad A, Alfano I, von Delft F, Bullock AN, et al. A small molecule targeting ALK1 prevents Notch cooperativity and inhibits functional angiogenesis. Angiogenesis [Internet]. 2015. April;18(2):209–17. Available from: 10.1007/s10456-014-9457-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanvitale CE, Kerr G, Chaikuad A, Ramel M-C, Mohedas AH, Reichert S, et al. A New Class of Small Molecule Inhibitor of BMP Signaling. PLoS One [Internet]. 2013. April 30;8(4):e62721 Available from: 10.1371/journal.pone.0062721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Isogai S, Horiguchi M, Weinstein BM. The Vascular Anatomy of the Developing Zebrafish: An Atlas of Embryonic and Early Larval Development. Dev Biol [Internet]. 2001;230(2):278–301. Available from: http://www.sciencedirect.com/science/article/pii/S0012160600999950 10.1006/dbio.2000.9995 [DOI] [PubMed] [Google Scholar]
- 57.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8(11):1249–56. 10.1038/nm1102-1249 [DOI] [PubMed] [Google Scholar]
- 58.Milewicz DM, Kwartler CS, Papke CL, Regalado ES, Cao J, Reid AJ. Genetic variants promoting smooth muscle cell proliferation can result in diffuse and diverse vascular diseases: evidence for a hyperplastic vasculomyopathy. Genet Med. 2010;12(4):196–203. 10.1097/GIM.0b013e3181cdd687 [DOI] [PubMed] [Google Scholar]
- 59.Nebbioso A, Carafa V, Benedetti R, Altucci L. Trials with “epigenetic” drugs: an update. Mol Oncol [Internet]. 2012. December 12 [cited 2015 Jul 13];6(6):657–82. Available from: http://www.moloncol.org/article/S1574789112000968/fulltext 10.1016/j.molonc.2012.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roxana O, H. KS, Feng Z, Gael G, Raja C, Laurence P-F, et al. SMAD4 Prevents Flow Induced Arteriovenous Malformations by Inhibiting Casein Kinase 2. Circulation [Internet]. 2018. November 20;138(21):2379–94. Available from: 10.1161/CIRCULATIONAHA.118.033842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ola R, Dubrac A, Han J, Zhang F, Fang JS, Larrivée B, et al. PI3 kinase inhibition improves vascular malformations in mouse models of hereditary haemorrhagic telangiectasia. Nat Commun [Internet]. 2016. November 29;7:13650 Available from: 10.1038/ncomms13650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mack CP. Signaling Mechanisms That Regulate Smooth Muscle Cell Differentiation. Vol. 31, Arteriosclerosis, thrombosis, and vascular biology. 2011. p. 1495–505. 10.1161/ATVBAHA.110.221135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winkler EA, Bell RD, Zlokovic BV. Lack of Smad or Notch Leads to a Fatal Game of Brain Pericyte Hopscotch. Dev Cell [Internet]. 2011. March 15 [cited 2018 Aug 1];20(3):279–80. Available from: https://www.sciencedirect.com/science/article/pii/S1534580711000840 10.1016/j.devcel.2011.03.002 [DOI] [PubMed] [Google Scholar]
- 64.Tachida Y, Izumi N, Sakurai T, Kobayashi H. Mutual interaction between endothelial cells and mural cells enhances BMP9 signaling in endothelial cells. Biol Open [Internet]. 2017. March 15;6(3):370 LP– 380. Available from: http://bio.biologists.org/content/6/3/370.abstract 10.1242/bio.020503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science (80-). 1997;277(5323):242–5. [DOI] [PubMed] [Google Scholar]
- 66.Zheng X, Xu C, Di Lorenzo A, Kleaveland B, Zou Z, Seiler C, et al. CCM3 signaling through sterile 20–like kinases plays an essential role during zebrafish cardiovascular development and cerebral cavernous malformations. J Clin Invest [Internet]. 2010. August 2;120(8):2795–804. Available from: 10.1172/JCI39679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Montero-Balaguer M, Swirsding K, Orsenigo F, Cotelli F, Mione M, Dejana E. Stable Vascular Connections and Remodeling Require Full Expression of VE-Cadherin in Zebrafish Embryos. PLoS One [Internet]. 2009. June 3;4(6):e5772 Available from: 10.1371/journal.pone.0005772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.High FA, Lu MM, Pear WS, Loomes KM, Kaestner KH, Epstein JA. Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proc Natl Acad Sci [Internet]. 2008. February 12;105(6):1955 LP– 1959. Available from: http://www.pnas.org/content/105/6/1955.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ando K, Fukuhara S, Izumi N, Nakajima H, Fukui H, Kelsh RN, et al. Clarification of mural cell coverage of vascular endothelial cells by live imaging of zebrafish. Vol. 143, Development (Cambridge, England). 2016. p. 1328–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schulte-Merker S, Stainier DYR. Out with the old, in with the new: reassessing morpholino knockdowns in light of genome editing technology. Development. 2014;141(16):3103–4. 10.1242/dev.112003 [DOI] [PubMed] [Google Scholar]
- 71.Labun K, Montague TG, Gagnon JA, Thyme SB, Valen E. CHOPCHOP v2: a web tool for the next generation of CRISPR genome engineering. Nucleic Acids Res. 2016;gkw398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Montague TG, Cruz JM, Gagnon JA, Church GM, Valen E. CHOPCHOP: a CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 2014;gku410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Narayanan A, Hill-Teran G, Moro A, Ristori E, Kasper DM, A. C, et al. In vivo mutagenesis of miRNA gene families using a scalable multiplexed CRISPR/Cas9 nuclease system. Sci Rep [Internet]. 2016. August 30;6:32386 Available from: 10.1038/srep32386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rossi A, Kontarakis Z, Gerri C, Nolte H, Hölper S, Krüger M, et al. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature [Internet]. 2015. July 13;524:230 Available from: 10.1038/nature14580 [DOI] [PubMed] [Google Scholar]
- 75.Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, et al. The Tol2kit: A multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn [Internet]. 2007. November 1;236(11):3088–99. Available from: 10.1002/dvdy.21343 [DOI] [PubMed] [Google Scholar]
- 76.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. 10.1016/j.cell.2004.12.035 [DOI] [PubMed] [Google Scholar]
- 77.Lopez-Rios J. Sensing BMP Pathway Activity by Immune Detection of Phosphorylated R-Smad Proteins in Mouse Embryonic Kidney In: Michos O, editor. Kidney Development: Methods and Protocols. Totowa, NJ: Humana Press; 2012. p. 267–73. [DOI] [PubMed] [Google Scholar]
- 78.Rasband W. ImageJ [Internet]. U. S. National Institutes of Health, Bethesda, Maryland, USA; Available from: https://imagej.nih.gov/ij/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Schematic of FACS sorting strategy for acta2:EGFP+ and kdrl:mCherry+ cells from 4 dpf Tg(acta2:EGFP;kdrl:mCherry) dissected embryo heads. B) Expression level of miR26a in acta2:EGFP+ and kdrl:mCherry+ isolated by FACS (n = 2). C) Expression level of smad1, acvrl (alk1) and acta2 in acta2:EGFP+ and kdrl:mCherry+ isolated by FACS (n = 2). RT-qPCR analysis of values represent mean ± SEM, n = biological replicates. D) Wholemount immunohistochemistry of pSmad1/5/9 in Tg(acta2:EGFP)ca7; Tg(kdrl:mCherry)ci5 shows nuclear staining of pSmad1 in endothelial cells. pSmad 1/5/9 is observed in endothelium (red) but not smooth muscle (green) of the hyoid artery and afferent branchial arches. Magenta asterisk indicates pSmad1/5/9 stain; arrows indicate smooth muscle cells. Scale bar represents 50μm.
(EPS)
A) EGFP:smad1 sensor assay showing EGFP-smad1 sensor expression vs mCherry control. B) Quantification of EGFP fluorescence in sensor assay as compared to controls. Student's two-tailed t-test *p< 0.05, N = 3, total of 9 embryos per group, Error Bars = SEM, Scale bar represents 50μm. C) RT-qPCR of relative expression of miR26a in miR26a-mimic injected embryos at 48 hpf. Values are means of 3 replicates and normalized to miR122; Unpaired t test, *p< 0.01 as compared to control; Error Bars = SD. D) miR26a mimic injected embryo with mild dorsalization phenotype, heart edema (arrowhead), dorsal axis defects (arrows) and poor circulation (arrowhead at tail) at 48 hpf. E) Whole mount in situ hybridization staining for smad1 at 48 hpf in uninjected control, negative control mimic and miR26a injected embryos. There is decreased expression of smad1 in miR26a mimic injected embryos (boxes and arrow).
(EPS)
Ventral view confocal projections of 4 dpf Tg(kdrl:mCherry; fli1a:nEGFP ventral aorta (dotted outline. A-D) Endothelial cytoplasm (kdrl:mCherry) and endothelial nuclear (fli1a:nEGFP) staining. E-H) fli1a:nEGP endothelial nuclear stain. I-L) pSmad1/5/9 staining. M-P) overlay image, in 4 dpf Scr. Control (A,E,I,M), miR26a MO (B,F,J,N), dCas9 control (C,G,K,O) and miR26a CRISPRi (D,H,L,P). Q) Average number of fli1a:nEGFP nuclei. R) Average percentage pSmad1/fli1a:nEGFP double positive nuclei. Scale bar represents 50μm.
(EPS)
A) RT-qPCR of relative expression of miR26a at 48 hpf in miR26a MO (28 ng/ embryo). Values are means of 3 replicates and normalized to miR122, n = 3: UIC vs. Neg. ctl. MO (Scr.Control MO) p = 0.16. UIC vs. Pre-miR26a MO: p < .0001. UIC vs. miR-26a mature MO: p = 0.0002. RT-qPCR analysis of values represent mean ± SEM, n = 2 biological replicates. B) Whole-mount in situ hybridization staining for smad1 at 48 hpf shows increased expression of smad1 at higher dose of miR26a MO. C) Representative images of wildtype (uninjected) and high dose miR26a morpholino-injected embryos showing normal body axis, but with hemorrhage and mild hydrocephalus. D) Average rates of hemorrhage for miR26a MO at 28 ng/ embryo and 6 ng/ embryo (Student's two-tailed t-test, ****p< 0.0001 as compared to WT, Error Bars = SEM. N = 5, n total miR26a MO 28 ng = 488, 6 ng = 501 and Wildtype = 535).
(EPS)
A-D) Whole-mount in situ hybridization staining for acta2 and myh11a at 4 dpf shows increased expression in miR26a knockdown embryos in the aortic arches and pharyngeal region. E-J) Whole-mount in situ hybridization staining for acta2 and sm22α at 4 dpf shows decreased expression in miR26a mimic injected embryos.
(EPS)
A) Phenotypes observed at 48 hpf with increasing doses of smad1ECOE. B) Quantification of phenotypes, Student's two-tailed t-test **-****p< 0.005–0.00005 as compared to control. Error Bars = SEM. N = 3, n total smad1-ECOE 5 ng, n = 60, 10 ng, n = 61, 20 ng, n = 63 and control n = 72. C) Ventral view confocal projections of the 4 dpf ventral aorta of Tg(BRE:EGFP) embryos. D) Average number of acta2:mCherry cells, BRE intensity and vSMC coverage.
(EPS)
File of raw data underlying graphs.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.