Abstract
Harnessing an antitumor immune response has been a fundamental strategy in cancer immunotherapy. For over a century, efforts have primarily focused on amplifying immune activation mechanisms that are employed by humans to eliminate invaders such as viruses and bacteria. This “immune enhancement” strategy often results in rare objective responses and frequent immune-related adverse events (irAEs). However, in the last decade, cancer immunotherapies targeting the B7-H1/PD-1 pathway (anti-PD therapy), have achieved higher objective response rates in patients with much fewer irAEs. This more beneficial tumor response-to-toxicity profile stems from distinct mechanisms of action that restore tumor-induced immune deficiency selectively in the tumor microenvironment, here termed “immune normalization,” which has led to its FDA approval in more than 10 cancer indications and facilitated its combination with different therapies. In this article, we wish to highlight the principles of immune normalization and learn from it, with the ultimate goal to guide better designs for future cancer immunotherapies.
Introduction
Successful generation of a T cell-mediated immunity to eliminate antigen includes but it is not limited to the following steps: (1) tumor-antigen uptake and processing by antigen-presenting cells (APCs), (2) migration of APCs to lymphoid organs, (3) tumor-antigen presentation fine-tuned by co-stimulation and co-inhibitory signals that regulate the activation of tumor-specific naive T cells to become effector T cells in lymphoid organs, (4) the regression of tumor-specific effector T cells from lymphoid organs into peripheral blood and trafficking to tumor tissues, (5) tumor-antigen recognition and tumor lysis, and (6) death of effector T cells and the generation of tumor-specific memory T cells. Based on the understanding of these cellular and molecular mechanisms, various types of immunotherapies were developed to “push” immune activation through the modulation of general regulatory and/or activatory mechanisms governing these steps to improve antitumor immune responses in quantity and/or quality. This general approach aims to activate and increase the immune response, and we have termed this as “enhancement immunotherapies.”
However, cancer does not simply grow to race with the development of immune responses, but rather actively employs various tactics to delay, alter, or even stop antitumor immunity. These tactics, collectively termed “immune evasion mechanisms,” often defeat intrinsically developed antitumor immunity, leading to a failure in the control of tumor growth. These mechanisms develop continuously during the progression of cancer and become more diverse and complex in late-stage cancers. New approaches to improve the immune response against cancer consist of blocking these immune evasion mechanisms. One of the first and most characterized immune evasion mechanisms is the programmed cell death (PD) pathway. This pathway inhibits an effector T cell antitumor immune response when it is upregulated in the tumor microenvironment, and therapies blocking this pathway have proven effective at improving an antitumor immune response against multiple tumor types. This approach is conceptually different from the previous enhancement approach. While enhancement approaches are designed based on the knowledge of the general activation process, anti-PD therapy exploits new knowledge based on immune evasion mechanisms. Furthermore, while in the enhancement approach, we assume that the general mechanisms of immune system activation are always the same, anti-PD therapy first requires a careful study of the tumor microenvironment (TME) to identify that the PD pathway is upregulated, making this therapy more “personalized.” We believe that this approach represents the first of an emerging group of strategies in the future of cancer immunology research and will expand as we understand better mechanisms of immune escape. Because this new approach aims to restore a lost antitumor immunity, we have termed it “normalization cancer immunotherapy.”
The Beginning: Enhancement Cancer Immunotherapy
Starting from its inception, mainstream cancer immunotherapy has involved enhancing the processes believed to be necessary for a successful and powerful immune response. These enhancement strategies have generally been grouped in two: the first approach is to use effector cells/molecules of the immune system to directly attack tumor cells, which is called “passive” immunotherapy. This category includes antibody-targeted therapy and its derivatives (e.g., antibody-drug conjugates), as well as adoptive immune cell therapies and, more recently, genetically engineered T cells (chimeric antigen receptor [CAR]-T, T cell receptor [TCR]-T, etc.). Passive immunotherapy employs the power of modern technology and brings the immune system to much higher, sometimes extraordinary levels. The most well-known examples are anti-Her2/neu monoclonal antibody (mAb) for breast cancer, anti-EGFR mAb for colorectal or head and neck cancer, and anti-CD20 mAb for B lymphoma, among others. The second approach is to enhance immune system activation through the modulation of endogenous regulatory and/or activatory immune mechanisms, which is also called “active” immunotherapy. In accordance with the immune response step that these strategies enhance, we can (1) enhance antigen uptake, processing, and presentation to T cells by APCs, such as antigen/adjuvant vaccines and dendritic cell vaccines—this could also extend to cytokines or agents that promote APC activity such as type I interferons (IFNs), Toll-like receptor (TLR) agonists, and stimulator of interferon genes (STINGs) agonists; (2) enhance the activation and expansion of naive T cells: examples are dendritic cell vaccines and anti-cytotoxic T lymphocyte antigen-4 (CTLA-4) mAb; and (3) intensify the effector phase of the immune response: examples are adoptive cell therapy using ex vivo stimulated and expanded tumor infiltrate T cells to infuse back into cancer patients. In this article, we will focus our discussion and analysis mainly on active immunotherapies, as well as CAR-T cell therapy. For antibody-targeted therapy, we will refer to several excellent review articles (Scott et al., 2012; Weiner, 2015; Weiner et al., 2010), and it will not be discussed in depth here.
It is important to understand that the enhancement strategies are not designed to correct or overcome an existing or known deficiency during the development of the antitumor immune response. For instance, interleukin-2 (IL-2) therapy was not chosen based on a defective expression on IL-2 and its receptors in cancer patients. It is also unknown whether or not CTLA-4 overexpression or altered B7–1/B7–2 expression constitutes a tumor-induced immune-deficient mechanism in patients treated with anti-CTLA-4 mAbs. Likewise, cancer vaccines or adoptive therapy are also given to patients without knowledge of any defects in presentation/priming in patients. This suggests that perhaps, in some patients, these enhancement strategies are indeed providing a needed supply to the immune activation process. Therefore, these patients may benefit greatly from this treatment. However, in most of these cases, these strategies may simply be general activators of the immune system that are increasing the immune response against tumors but that are also pushing the immune system to supraphysiological levels with a subsequent risk of increasing immune-related adverse events (irAEs). This may explain the low tumor response-to-toxicity profile during enhancement immunotherapy.
Despite these conceptual limitations, these early strategies resulted in objective tumor responses and subsequent FDA approvals for the treatment of a few tumors (Grupp et al., 2013; Kantoff et al., 2010; Parkinson et al., 1990; Porter et al., 2011; Rosenberg et al., 1987), as summarized in Figure 1. However, it became evident that successful treatment was the exception (only a small portion of patients showed objective responses), and often, these strategies failed to demonstrate a significant clinical benefit in multiple tumor types. In most of the cases, these therapies failed to extend their approval beyond classically immunogenic tumors due to an unfavorable response/toxicity ratio (i.e., IL-2 and anti-CTLA-4 mAbs). We focus this discussion on why we consider these therapies as “enhancement” and the reasons for their less favorable response-to-toxicity ratio. Because it is well beyond this Perspective to review all possible immunotherapies that failed during past and present years, we have chosen to focus our analysis here only on FDA-approved cancer immunotherapies that have demonstrated a significant antitumor activity in at least one tumor type.
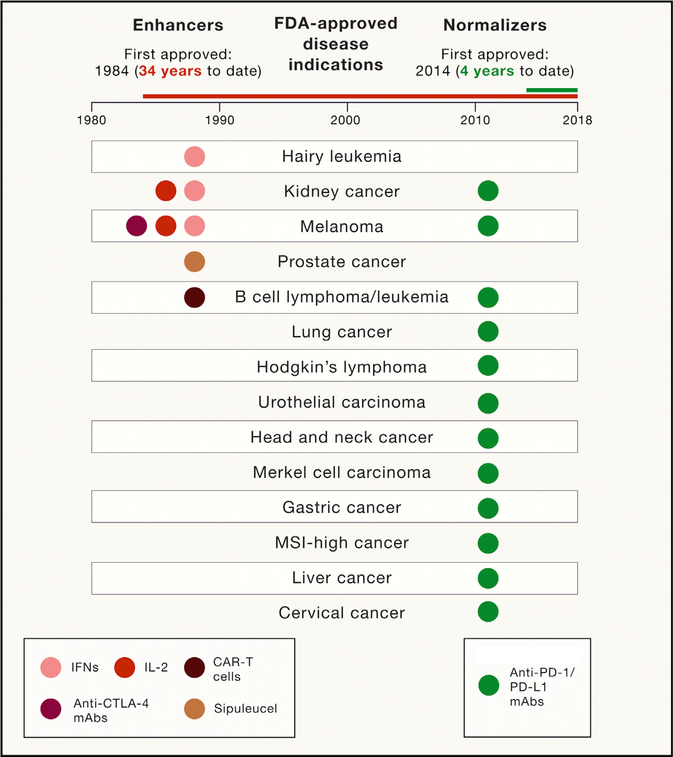
Figure 1. FDA-Approved Cancer Immunotherapies and Their Indications under the Categories of the Immune Enhancement versus Immune Normalization.
All FDA-approved cancer immunotherapies are illustrated. These cancer immunotherapies are divided into two categories. The immune enhancement category (IFNs, IL-2, anti-CTLA-4, cancer vaccine, and CAR-T cells) is listed in the left panel with the first approval of type I IFN for the treatment of hairy cell leukemia in 1984. The immune normalization category (anti-PD-1/PD-L1 mAbs) is listed in the right panel with the first approval of anti-PD-1 mAb nivolumab in 2014. Approved disease indications are listed in the middle panel.
Cancer Vaccines
Following the idea of vaccination in infectious diseases, the most popular cancer immunotherapy practice has been to immunize patients against tumor antigens through many different approaches, including cell-based, DNA-based, and protein/ peptide-based preparations. Unfortunately, after thousands of cancer vaccine trials have been conducted and tested in multiple tumor types, only one cancer vaccine has been approved with moderate effect (sipuleucel in prostate cancer) (Kantoff et al., 2010). Ironically, many of these vaccination strategies were able to induce a peripheral tumor-specific T cell response while failing to show objective antitumor activity (Melero et al., 2014; Rosenberg et al., 2004). These findings suggest that tumor-specific T cell priming may not be the major hurdle in these cancer patients. Furthermore, the appearance of vitiligo, a sign of autoimmunity, is often associated with objective tumor response after cancer vaccination in some melanoma patients, indicating that tumor response and autoimmunity are frequently associated (Overwijk and Restifo, 2000). With the advent of neoantigen tumor vaccines, this strategy has been reinvigorated proposing the need for new antigens without central tolerance and higher T cell affinity (Ott et al., 2017; Sahin et al., 2017).
Cytokines
One of the most illustrative examples of the enhancement of cancer immunotherapy is the use of IL-2. IL-2 is a growth factor for antigen-specific T cells and natural killer (NK) cells. IL-2 was first identified in 1976 (Morgan et al., 1976), and isolation of the cDNA clone was described in 1983 (Taniguchi et al., 1983). Subsequently, recombinant IL-2 was shown to have antitumor activity in a number of murine tumor models (Rosenberg et al., 1985). Based on animal model data, IL-2 was tested in cancer patients and received FDA approval to treat renal cell carcinoma (1992) and melanoma (1998) with a 5%–15% objective response rate (Rosenberg, 2014). While IL-2 induces an effective antitumor immune response in a few cases, in most patients, IL-2 induces a significant toxicity in multiple organs and tissues, mostly related with general capillary leak syndrome (Atkins et al., 1999). Thus, the antitumor effect seems to be the exception, while broad stimulation of the immune system seems to be the rule. With the arrival of new versions of IL-2 receptor agonists, efficacy and toxicity are expected to be improved, based first on the design of engineered IL-2 that preferentially binds CD8 and NK IL-2 receptor over Treg IL-2 receptor (Charych et al., 2016) and second on bispecific constructs targeting tumor antigens and refocusing most of the effect of IL-2 into the tumor microenvironment (Klein et al., 2017).
Anti-CTLA-4 mAbs
CTLA-4 is a cell-surface receptor induced in conventional T cells after TCR engagement that acts as a regulator of naive and effector antigen-specific T cell activation (Leach et al., 1996; Walunas et al., 1994). Additionally, CTLA-4 is highly expressed in Tregs and has proven to be critical for the development and function of induced Tregs (Takahashi et al., 2000). Mice lacking CTLA-4 develop an early-onset general T cell activation, resulting in inflammatory infiltration and death around 3 weeks of age, highlighting the importance of CTLA-4 to control self-reactive T cell responses (Waterhouse et al., 1995). In the clinic, anti-CTLA-4 mAbs induce frequent autoimmune reactions that confirm the importance of this pathway in controlling self-reactive T cells. Whether these autoimmune reactions occur due to the loss of CTLA-4 function in conventional T cells, regulatory T cells, or both is under debate. A recent study using a humanized CTLA-4 mouse model casts doubt on the blockade of the B7s/CTLA-4 interaction using clinical-grade anti-CTLA-4 mAbs (ipilimumab) (Du et al., 2018), and numerous works during recent years suggest that the main effect of anti-CTLA-4 mAbs may be mediated by Treg depletion (Bulliard et al., 2013; Selby et al., 2013; Simpson et al., 2013, Arce Vargas et al., 2018). These results thus call for a reassessment of the “immune checkpoint blockade” concept for anti-CTLA-4 therapy. On the other hand, while a small group of melanoma patients developed an objective tumor response (15%–20%), severe toxicities (grades 3–5) were more common (30%), suggesting that this agent more effectively activates self-reactive rather than tumor-specific T cells. Judging from the mechanisms of action, the CTLA-4 blocking strategy may be another type of enhancement cancer immunotherapy, because there is no evidence that this pathway is induced by tumors as an immune evasion mechanism, and furthermore, we do not have evidence that anti-CTLA-4 mAbs preferentially activate tumor-specific T cells over self-reactive T cells in patients. In contrast, clinical data illustrate that irAEs are more frequent than tumor response (ORR) as it happens with the use of unspecific T cell growth factor IL-2 (Table 1). This unfavorable response/toxicity ratio most likely is the reason why ipilimumab has been approved to treat metastatic melanoma (Hodi et al., 2010) but failed to show clinical benefit in other tumor types where it was tested as a single-agent therapy (Bilusic et al., 2017; Lynch et al., 2012).
Table 1.
ORR and Severe Treatment-Related AEs in Metastatic Melanoma Patients Treated with FDA-Approved Immunotherapies
| Drug | Phase | Drug and Schedule | Number of Patientsa | Objective Response Rate (%) | Treatment-Related Toxicitiesb | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Any Grade (%) | Grades 3–4 (%) | |||||||
| IL-2 | ||||||||
| II | high dose IL-2 | 46 | 10 (24) | >32 (>68) | >17 (>35) | Parkinson et al. (1990) | ||
| II | high dose IL-2 | 134 | 23 (17) | 134 (100) | >78 (>37) | Rosenberg et al. (1994) | ||
| IIc | high dose IL-2 | 270 | 43 (16) | >64 (>24) | >45 (>17) | Atkins et al. (1999) | ||
| II | high dose IL-2 | 26 | 5 (19) | 26 (100) | 13 (50) | Tarhini et al. (2007) | ||
| III | low dose IL-2 | 153 | 5 (3) | N/A | 59 (39) | Agarwala et al. (2002) | ||
| III | high dose IL-2 | 93 | 6 (6) | >36 (>39) | 74 (80) | Schwartzentruber et al. (2011) | ||
| Average | (14) | (>43) | ||||||
| Anti-CTLA-4 mAbs | ||||||||
| II | ipilimumab | 57 | 9 (16) | 54 (95) | 27 (47) | Weber et al. (2009) | ||
| III | ipilimumab | 137 | 15 (11) | 105 (80) | 30 (23) | Hodi et al. (2010) | ||
| III | ipilimumab | 278 | 33 (12) | 187 (73) | 51 (20) | Robert et al. (2015a) | ||
| III | ipilimumab | 315 | 60 (19) | 268 (85) | 85 (27) | Larkin et al. (2015) | ||
| II | ipilimumab | 47 | 5 (11) | 34 (74) | 9 (19) | Hodi et al. (2016) | ||
| III | ipilimumab | 727 | 100 (14) | 324 (45) | 190 (26) | Ascierto et al. (2017) | ||
| Average | (14) | (27) | ||||||
| Anti-PD-1 mAbs | ||||||||
| I | pembrolizumab | 135 | 44 (38) | 107 (79) | 17 (13) | Hamid et al. (2013) | ||
| II | nivolumab | 107 | 33 (31) | 90 (84) | 24 (22) | Topalian et al. (2014) | ||
| I | pembrolizumab | 173 | 41 (26) | 142 (82) | 20 (12) | Robert et al. (2014) | ||
| III | nivolumab | 316 | 138 (44) | 257 (82) | 51 (16) | Larkin et al. (2015) | ||
| III | pembrolizumab | 556 | 185 (33) | 423 (76) | 65 (12) | Robert et al. (2015a) | ||
| III | nivolumab | 210 | 84 (40) | 153 (74) | 24 (12) | Robert et al. (2015b) | ||
| II | pembrolizumab | 357 | 84 (23) | 252 (76) | 45 (13) | Ribas et al. (2015) | ||
| III | nivolumab | 227 | 74 (27) | 206 (77) | 37 (14) | Larkin et al. (2018) | ||
| Average | (33) | (14) | ||||||
N/A, not available.
Number of patients available for ORR or treatment-related AE assessment vary across tde studies.
Indicated as “>” when frequency per toxicity grade was not available, and it was estimated based on tde most common treatment-related adverse event reported.
Data combination of eight phase II clinical trials.
Chimeric Antigen Receptor
CAR-T cells are genetically engineered T cells with an antigen-binding domain (typically a single-chain variable fragment [scFv]) and additional intracellular costimulatory domains from receptors, such as CD28 and/or CD137. The advantage of these engineered T cells is that the recognition of antigens by CAR-T is not restricted by major histocompatibility complex (MHC) expression. This design is one of the most paradigmatic examples of using engineered effector immune cells to more effectively attack tumor cells, and the infusion of a large number of these cells is an illustrative case of the enhancement strategy. A main disadvantage is that it requires extracellular surface expression of the targets on the tumor cells, and this limits CAR-T cells’ specificity and broadness of application. Since the invention of hybridoma technology, numerous mAbs have been generated against tumor cells with the hope to discover “magic bullets,” and it is now clear that there are very few tumorspecific cell-surface antigens. CAR-T cells are extremely effective at recognizing and destroying target cells. In this regard, this type of adoptive cell therapy is equally effective to clear tumor as normal cells when the target antigen is shared by tumor and non-tumor tissues. For instance, CAR-T cells targeting CD19 proteins are very effective at recognizing and destroying B cell lymphoma and leukemia cells but also destroying all the normal CD19+ B cells in the body of the patient. While this offtarget effect is acceptable, since B cell depletion in patients can be functionally replaced by administering polyclonal human immunoglobulin Gs (IgGs), the consequences can be more dramatic when the target is expressed in normal epithelial cells. In addition to this off-target toxicity, the most important irAE related with CAR-T cells is acute cytokine release syndrome (CRS) as a consequence of producing supraphysiologic levels of cytokines via CAR-T cells upon antigen recognition (Brudno and Kochen-derfer, 2016; Namuduri and Brentjens, 2016). All of these are important limitations to the extension of these therapies to solid tumors as was shown in the preliminary results of HER2-specific CAR-T cells (Ahmed et al., 2015; Morgan et al., 2010).
In summary, the common experience for the use of enhancement cancer immunotherapy is that general activation of the immune system leads to more frequent irAEs than objective antitumor responses (with the exception of CAR-T therapy in hematological malignancies). This unfavorable response-to-toxicity ratio has limited the use for most of these therapies, and none of these agents have had a broad spectrum of indications thus far (Figure 1).
The Normalization Cancer Immunotherapy Has Come of Age
An important clinical observation in the last decade is that systemic immune activation does not necessarily result in cancer regression, especially in solid tumors. In fact, ample evidence exists that the presence of fully activated tumor-specific T cells in peripheral blood does not often correlate with the regression of tumors or a better prognosis in cancer patients (Melero et al., 2014; Rosenberg et al., 2005). This discrepancy has been better understood recently with the discovery and characterization of immune escape mechanisms developed by tumors that lead to a local, rather than a systemic, immunosuppression (Taube et al., 2012, Chen and Han, 2015). It has been well documented that human cancer can develop various mechanisms to escape specific and non-specific immune attacks (Vinay et al., 2015). These mechanisms prevent immune attack, inhibiting T cell activity in the TME. Unfortunately, the mechanisms governing immune escape in tumors discovered thus far are often similar, if not identical, to those governing self-tolerance (Phan et al., 2001), making it difficult to develop a therapeutic that generates an antitumor response but avoids irAEs. This limitation, however, has recently been challenged by encouraging basic and clinical findings using mAbs to block B7-H1 and PD-1 interactions (collectively named anti-PD therapy) (Chen and Han, 2015). Indeed, anti-PD therapies are the first FDA-approved immunotherapies to demonstrate more frequent objective tumor responses than severe treatment-related AEs in cancer patients, illustrating that it is possible to increase efficacy without increasing toxicity. We have highlighted this achievement by comparing the objective response rate and treatment-related AEs across clinical trials using FDA-approved IL-2, anti-CTLA-4, and anti-PD-1 mAbs as single agents to treat metastatic melanoma patients (Table 1). Also, a recent side-by-side comparison of nivolumab (anti-PD-1) and ipilimumab in a randomized clinical trial of advanced and localized melanoma patients confirmed that nivolumab has three times more activity and three times less toxicity than ipilimumab (Larkin et al., 2015; Robert et al., 2015a; Weber et al., 2017). Furthermore, clinical results have repeatedly demonstrated that this strategy can induce durable responses in a broad spectrum of large and disseminated late-stage human malignancies, increasing the frontiers of immunotherapy beyond the traditional set of previously categorized immunogenic tumors, such as melanoma (Callahan et al., 2016; Zou et al., 2016). Currently, anti-PD therapy has been approved by the FDA for the treatment of metastatic melanoma, lung cancer, head and neck cancer, renal cell carcinoma, urothelial carcinoma, liver cancer, gastric cancer, Hodgkin’s lymphoma, Merkel cell carcinoma, large B cell lymphoma, cervical cancer, and any MSI+ tumors (Ribas and Wolchok, 2018) (Figure 1). Moreover, anti-PD therapy is effective in >25 different types of solid tumors and several hematopoietic malignancies, which will likely lead to future FDA approval (Ribas and Wolchok, 2018). One of the factors that facilitated this unprecedented success is a more favorable response-to-toxicity profile, with a 40% objective tumor response rate and a 7%–12% grades 3–5 irAEs across multiple tumor types (Naidoo et al., 2015; Ribas and Wolchok, 2018). This new tumor response-to-toxicity profile and this antitumor activity across multiple tumor types (beyond those classically considered “immunogenic tumors”) reflect a different mechanism of action, termed here immune normalization, which we will discuss in detail in the following section. We believe that there are other potential immune normalizers, and learning from the principles of anti-PD therapies, we can choose immunotherapies that can reproduce the success of anti-PD therapy.
Principles of Normalization Cancer Immunotherapy
The concept of immune normalization emphasizes the importance of identifying the particular defects or dysfunctions of the immune response during tumor progression and to develop strategies to specifically correct these deficiencies to restore a natural antitumor immune capacity. Although the end result of the normalization strategy may lead to elevated immune responses, these responses should fluctuate transiently in limited ranges and, in theory, should not cause permanent damage to normal organs/tissues. This “controlled” elevation of immune responses during normalization immunotherapy may be due to immune responses under normal feedback regulation. While there are some cases where severe irAEs occur in patients treated with anti-PD therapy, under a normalization immunotherapy approach, these patients may be predisposed to or in a “subclinical” status of inflammatory or autoimmune disease in which anti-PD therapy will serve as a “final nail in the coffin.”
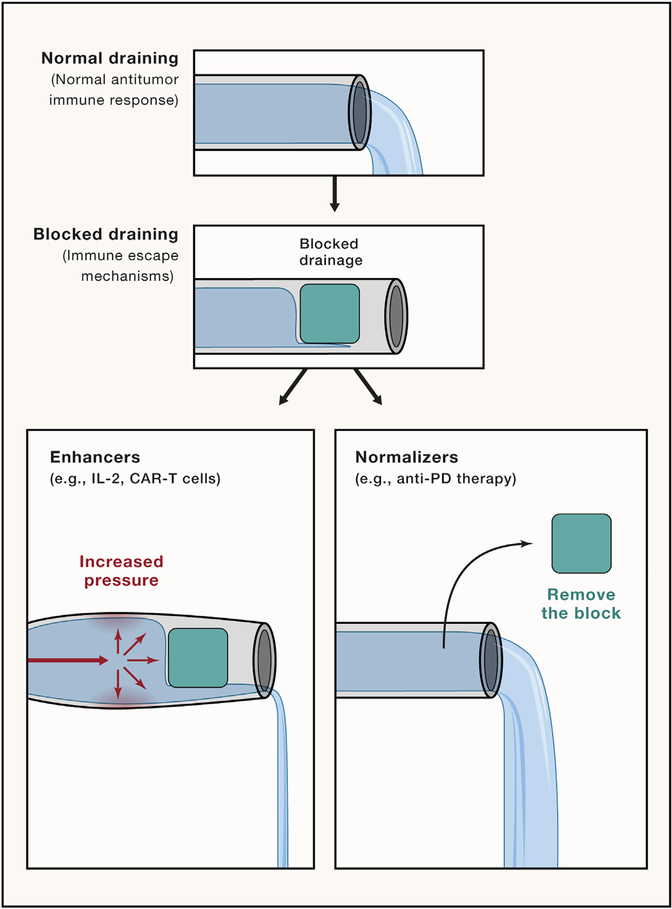
Normalization strategies aim to unblock a blocked immune response to increase the antitumor responses in contrast to enhancers that aim to generally activate the immune system to improve the antitumor responses. To illustrate this concept, we can think of the process of immune response as a big pipeline with water flow. Normal immune response, in this case, would be proper drainage. Thus, if the pipeline gets blocked, the flow would be impaired, and the pipeline would not drain sufficiently. In this situation, the “enhancer” approach can be illustrated as an increase in the pressure on the pipeline to overcome deficient drainage with the associated risk of breaking the pipeline if we increase the pressure too much. In contrast, the normalizer approach can be illustrated by strategies that aim to identify and unlock the blockade to restore the normal flow without risking the pipeline walls (Figure 2).
Figure 2. Illustration of the Immune-Normalization versus Immune-Enhancement Approaches.
Using proper flow and drainage of a pipeline as a comparison for the antitumor immune response. The flow of the pipeline can be insufficient when a blockade impairs flow, as the antitumor immune response can be insufficient when there is an immune impairment. The immune enhancement approach is illustrated as an increase in flow or pressure to return to proper function/flow with the risk of breaking the pipe (adverse effects). In contrast, the immune normalization approach would be to identify and try to unblock this specific blockage and restore the flow.
Anti-PD therapy is the clearest example of this approach thus far. Immune inhibitory B7-H1 protein is overexpressed in the TME leading to an overregulation of tumor-specific effector T cells, thus creating a locally deficient immune response that prevents tumor cell destruction. Blocking the B7-H1/PD-1 pathway results in selective repair of this defect and restores immune competence against tumors without leading to a general immune activation. Taking the B7-H1/PD-1 pathway as an example, we can illustrate three main principles that explain why the normalization approach is more suitable to reach antitumor immunity with reduced adverse events.
Targeting a Tumor-Induced Immune Escape Mechanism
Ample evidence indicates that B7-H1/PD-1 is a major counter-receptor interaction that leads to suppression of immune responses in both preclinical and clinical settings (Chen and Han, 2015; Ribas and Wolchok, 2018; Zou et al., 2016). Although either B7-H1 or PD-1 could also interact with other proteins (B7-H1/B7–1, PD-1/PD-L2), the biological significance of these interactions in humans is not yet fully understood. While T cells are a primary target for suppression, as shown in the majority of studies, the PD pathway could also impair the functions of dendritic cells (Yao et al., 2009), macrophages (Yao et al., 2009), and NK cells (Benson et al., 2010; Huang et al., 2015; Vari et al., 2018). The PD-1 mediated suppression mechanisms appear to be complex, including apoptosis, induction of suppressive cytokines, anergy, exhaustion, and Treg induction (Chen and Han, 2015; Zou and Chen, 2008; Zou et al., 2016). It is also evident that B7-H1 could also act as a receptor to transmit an anti-apoptotic signal to various types of cells, which may be responsible for the resistance of tumor cells to T cell-mediated lysis (Azuma et al., 2008; Chin et al., 2018). How these potential mechanisms contribute to human cancer progression, as well as new mechanistic insights, are under intensive investigation. The B7-H1/PD-1 pathway represents the archetypal tumor-adaptive immune escape mechanism. Upon recognition of tumor antigens, tumor-specific effector T cells upregulate PD-1 and release IFN-γ that induces B7-H1 in tumor and myeloid cells in the TME. B7-H1 inhibits T cells through PD-1 engagement, inter-rupting antitumor T cell attack. This inter-ruption of the antitumor T cell response represents a form of local immunodeficiency that allows tumors to escape and has been termed “adaptive immune resistance” (Dong et al., 2002, Taube et al., 2012). This interruption of the antitumor immune response can be restored by terminating B7-H1/PD-1 pathway signaling, described first in preclinical tumor models (Dong et al., 2002). Additionally, chronic viral infection models (e.g., hepatitis B virus [HBV] or lymphocytic choriomeningitis virus [LCMV]) demonstrated that chronic expression of B7-H1 is associated with T cell dysfunction, which can be restored when the B7-H1/PD-1 pathway is blocked (Barber et al., 2006). These findings have also been explored in human chronic viral infections (Gardiner et al., 2013; Gay et al., 2017). Although ample evidence indicates that chronic viral infection also utilizes the B7-H1/PD-1 pathway to suppress anti-viral immunity, the lessons learned from these studies are not always applicable to understanding the B7-H1/ PD-1-mediated tumor escape mechanisms. In contrast to frequent systemic immune suppression in peripheral organs and lymphoid organs during viral infection, tumor escape mechanisms occur frequently at tumor sites, while immune responses in peripheral organs, as well as lymphoid organs, are relatively normal. This may explain, in part, the dichotomy of progressive tumor growth accompanied with elevated tumor immunity in blood, indicating that the priming of T cell immune responses are not impaired, as described above. This led to the second important principle of the normalization cancer immunotherapy: selectively modulating immunity in the tumor microenvironment.
Selectively Modulating Immunity in the Tumor Microenvironment
A major difference of the B7-H1/PD-1 pathway with other immune inhibitory pathways is that B7-H1/PD-1 is minimally active in non-lymphoid tissues under normal physiological conditions. In this regard, B7-H1 protein is not expressed in steady-state normal human tissues (with the exception of placenta, tonsil, and a small portion of macrophage-like cells in lung and liver), although the mRNA of B7-H1 is broadly present in various normal tissues/cells (Chen and Flies, 2013; Dong et al., 2002; Petroff et al., 2002). In contrast, this membrane receptor can be broadly induced by type I and type II IFNs (Lee et al., 2006; Sanmamed and Chen, 2014) and displayed on the cell membrane of hematopoietic and non-hematopoietic cells within inflammatory tissues, including tumoral and virally infected tissues (Chen and Han, 2015). Because PD-1 is broadly present on effector memory T cells in peripheral blood and in tumor and non-tumor lymphoid organs (Gros et al., 2014), the TME-specific effect of anti-PD therapy is determined by selective expression of B7-H1 in the TME.
This minimal expression of B7-H1 in non-inflamed tissues ensures a TME-selective effect of anti-PD therapy by preventing damage to normal tissues and allows for a more focused and precise immune response with less systemic immune activation (Sanmamed and Chen, 2014). Such minimal systemic toxicity was demonstrated in early phase I clinical trials with anti-PD therapy when a maximum tolerated dose was not reached during dose escalation of anti-PD-1 or anti-B7-H1 mAbs (Brahmer et al., 2010, 2012; Topalian et al., 2012). A dose-dependent increase in toxicity was similarly absent, as the 10-mg/kg dose did not show a significant increase in the frequency of severe adverse events when compared with the 1-mg/kg dose (Brahmer et al., 2010, 2012; Topalian et al., 2012).
The tumor-site specificity of the pathway is, therefore, mainly determined by the localized induction of B7-H1 by IFN-γ. In this manner, B7-H1 expression is often a sign of a local ongoing but impaired antitumor immune response. Histological analyses corroborate this concept, as most human tumors show a pattern of B7-H1 expression that is focal or clustered rather than diffused and is often co-localized with T cell infiltration (Taube et al., 2012). It is still not well understood why a soluble cytokine like IFN-γ does not diffuse to a large area of tissues to have a more profound effect. One possible explanation could be the broad distribution of IFN-γ receptor on various types of cells in the TME (Bach et al., 1997), which may prevent IFN-γ from traveling further. Additional evidence supporting tumor selectivity of the immune response during anti-PD therapy is the selective expansion and functional improvement of T cells, mainly at the tumor site, and a lack of correlation between tumor regression and immune cell activation markers detected in the peripheral blood (Das et al., 2015; Herbst et al., 2014). This is in sharp contrast with enhancement immunotherapies that are often effective in activating a systemic immune response but show weak immune activation at the tumor site.
Resetting Immunity in the Tumor Microenvironment
A most-intriguing but less-understood observation in basic and clinical research in anti-PD therapy is its capacity to reset or reprogram antitumor immunity in the TME. The B7-H1/PD-1 pathway seems like a “master switch” that determines the fate of the entire TME in some cancer patients, since manipulation of a single pathway (the blockade of the B7-H1/PD-1 interaction) and, in some cases, even with a single dose of anti-PD therapy, can change from an initially highly suppressive TME to a highly active inflammatory site. During phase 1 clinical trials with anti-PD therapy, some patients showed objective tumor shrinkage with a single dose of anti-PD therapy (Gainor et al., 2016). Because immune dysfunction or deficiency in the TME are often multifaceted at the cellular and molecular levels (Zou, 2005; Zou and Chen, 2008; Zou et al., 2016), these observations are encouraging and implicate that, at least in some patients, normalizing a single master pathway is sufficient to trigger the resetting process, and it is not necessary to correct all defects in the TME.
From the currently available data, we speculate that the majority of, if not all, patients with late-stage cancer do not have systemic immune defects, and their immune systems still work to continuously provide newly generated effector T cells into the TME. This constitutes an important immunological basis for normalization immunotherapy. The TME in late-stage cancer patients could be considered a hostile work environment where various tactics of immune evasion mechanisms have already developed during tumor progression. The mechanisms of action for anti-PD therapy are likely multifaceted, including restoring functions of those already dysfunctional T cells, as well as preventing newly arrived effector T cells from becoming dysfunctional in the TME. These reinvigorated T cells, upon PD-pathway blockade, and newly arriving immune cells protected against PD-pathway inhibition may contribute to the resetting of immune responses in the TME while behaving as effector cells against tumor cells. An important but less-understood consequence of this normalization process is the generation of memory T cells, which are believed to give the durable antitumor effect of anti-PD therapy. Currently, we do not know the origin of tumor-antigen-specific memory T cells, how and why they become memory T cells, and how to modulate them. Understanding these issues will determine how and when we can use these therapies with precision.
The Normalization of Cancer Immunotherapy: The End of the Beginning
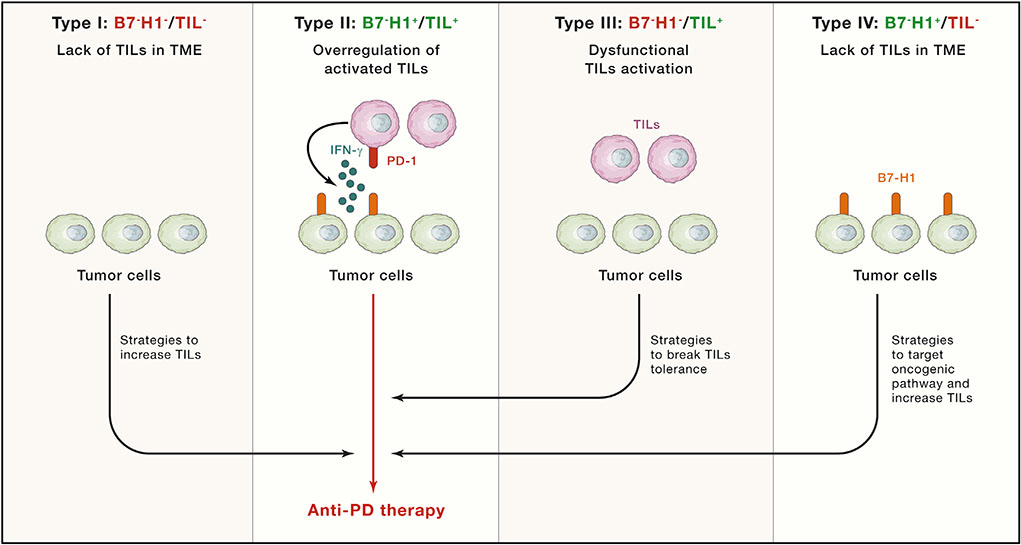
Beyond B7-H1,there are arrays of molecular pathways that cause immune defects in the antitumor immune response that can be targeted to restore the competence of this response. A new classification system of human cancer, termed tumor immunity in the microenvironment (TIME) was proposed based on the level of tumor-infiltrating leukocytes (TILs) and B7-H1 expression levels in the TME as a road map to search for potential immune defects that need to be normalized (Sznol and Chen, 2013; Taube et al., 2012; Zhang and Chen, 2016) (Figure 3). Molecular entities and mechanisms of these immune defects, once identified and characterized, could be potential targets for normalization immunotherapies and should provide an alternative for those patients that do not respond to anti-PD therapy. Among the potential different antitumor immunity defects, we can broadly classify cancer patients into three categories: (1) defects in the entry and/or proliferation of immune cells in which there is a lack of significant TILs in the TME, as indicated in TIME types I and IV, (2) overregulation of activated TILs, largely due to the effect of the B7-H1/PD-1 pathway and potentially other T cell regulatory pathways, indicated as type II, and (3) dysfunctional TILs in the TME due to suppression by molecular pathways (non-B7-H1/PD-1), indicated as type III. It is critical to identify which antitumor immune defect is predominant in each patient, since using the same strategy for all patients will be inefficient, costly, and wasteful. For instance, targeting local immune inhibitory pathways like the B7-H1/PD-1 pathway in a patient with a cancer that lacks immune infiltration may be pointless as a single therapy.
Figure 3. Tumor Immunity in the Microenvironment Classification.
Four different TME groups with potential implications for mechanism and therapy have been identified according to B7-H1 (PD-L1) expression and the presence of TILs in tumor biopsies: I, B7-H1-negative tumors without TILs, considered immunological ignorant because immune cells do not accumulate at the tumor site, II, B7-H1-positive tumors with TILs, considered a paradigm of adaptive resistance of tumors mediated by the B7-H1/PD-1 pathway, III, B7-H1-negative tumors with TILs, considered a situation of tolerance because TILs are present, but they do not induce B7-H1 expression in the tumor microenvironment through IFN-γ production, and IV, B7-H1-positive tumors without TILs, considered as a scenario of intrinsic induction of B7-H1 expression in tumor cells through oncogenic pathways that may be susceptible to be targeted. Groups I, III, and IV can be converted into group II through different strategies, synergizing with the action of anti-PD therapies that are more effective in the presence of TILs and B7-H1 expression.
Since we are just starting to understand the complexity of the TME in the regulation of immune responses, it is obvious that this TIME classification for human cancer is preliminary, and this may also raise more questions than answers. For example, in type II and type III TIME, we do not know which kinds of TIL components and other regulatory cells will determine the outcomes and responses to immunotherapy. In addition to IFNs, are there any other cytokines and molecular pathways that could regulate the expression of B7-H1? In types I and IV, what makes the TME stop the entry of TILs, and what are the mechanisms that prevent the proliferation of TILs? It is unlikely that these human cancer types do not have sufficient antigens, because our previous data have shown that among melanomas, which have the highest mutation burden of nearly all human cancers, up to 45% fall into this category (Taube et al., 2012). Extensive studies of the identification and characterization of molecular pathways and detailed molecular profiling of the TME may help address these questions and provide targets for future normalization cancer immunotherapy.
Challenges in Developing New Normalization Cancer Immunotherapies
With the success of anti-PD therapy, immune normalization as an approach for cancer immunotherapy has already shown great promise. However, there are still quite a few hurdles that hinder the development of new normalization over enhancement immunotherapies. Some of these limitations are conceptual, while others may be more technical.
Identification of the Dominant Antitumor Immune Defect
The development of normalization immunotherapy relies on identifying specific defects in the antitumor immune response. However, tumor-induced immune defects are highly heterogeneous (Schalper et al., 2017). This heterogeneity of immune defects not only occurs among different patients, but also extends to different areas in a single tumor lesion. These observations speak to the importance of identifying which of the immune defects is the dominant or master switch ultimately facilitating tumor escape. However, the definition of master switch may be patient specific and should be functionally demonstrated based on the capacity to reset the tumor microenvironment when this is modulated. Selecting the right master switch in each patient is the real challenge. Currently, the only way to demonstrate among all immune defects identified in a TME which factor is dominant is empirically. We then need better in vivo models to characterize the dominance of the different immune pathways in a patient TME and the effect of their modulation. Humanized mouse models or explant 3D culture models may provide support in this way (Jenkins et al., 2018; Sanmamed et al., 2016; Zitvogel et al., 2016).
Technological Difficulties to Study Immune Defects in the TME
Similar to the B7-H1/PD-1 pathway, many other immunosuppressive mechanisms that favor tumor progression occur in the local TME. One major challenge to characterize these mechanisms will be the capacity to evaluate immune responses at the TME level, requiring sequential tumor tissue collection and analyses. This is distinct from enhancement immunotherapies, whose systemic effects can be more readily monitored largely via blood analysis. Tumor tissue analysis represents a tremendous technical challenge, as sequential biopsies in cancer patients are not always possible, and most samples obtained are tiny and have large areas of necrosis.
The study of these immune defects requires the use of technology that can perform single-cell analyses with high phenotypical resolutions. New platforms such as single-cell sequencing, mass cytometry, and in vivo imaging techniques (e.g., PET imaging) may help uncover the complexity of the TME (Burel et al., 2016). With rapid progress in the identification and characterization of various immune defects in the TME, a future challenge will be how to identify among the multiple immune inhibitory pathways one or few that are dominant in a specific patient to decide on the best therapeutic approach.
The Principles to Combine Other Therapeutics with Anti-PD Therapy
Anti-PD therapy as a representative of an immune normalization approach has an inherent and significant advantage in the context of its broad therapeutic effect and minimal toxicity, which facilitates its use in combinatorial treatment. While combination therapy represents a popular, current strategy for the treatment of cancer, this strategy is largely driven by a mix of demands in clinical cancer care, corporate finance, and market competition with limited or modest scientific rationales. Currently, >1,500 clinical trials are ongoing that combine anti-PD therapy with nearly all available cancer therapeutics, including chemotherapy, radiotherapy, oncolytic virus, targeted therapy, and other immunotherapies (Tang et al., 2018). However, many of these clinical trials are not designed based on the basic principles of anti-PD therapy, and some of them may even impair the effects of anti-PD therapies. The future direction of combination cancer immunotherapies should mainly be guided by science. For example, a minimal requirement for a successful combination with anti-PD therapy should be chosen based on the biology of the pathway. It is important to keep in mind that our main driving force here is immune cells—specifically effector T cells and memory T cells, for which survival and activation of effector T cells in the TME and the generation of memory T cells for long-term effect need to be respected and potentiated. We will not cover all ongoing combinatorial approaches here, because it is beyond the scope of our Perspective, but we will mention some examples for consideration.
Combination with Radiotherapy and Chemotherapy
Radiotherapy may potentially destroy valuable tumor-specific T cells present in the tumor tissue, whereas it would be safer to perform local radiotherapy in those tumors without tumor-infiltrated T cells at the moment of starting radiotherapy (TIME classification types I and IV). Similarly, some chemotherapy drugs may also damage the function of effector T cells in the TME, which may lead to a less-than-additive effect of the combination therapy. In this case, a sequential treatment, rather than combination therapy, may be a better approach, since immunotherapy treatments may promote tumor-specific T cell proliferation while we are providing cytotoxic agents that target critical processes for cell division. Furthermore, currently, it is unknown whether or not radiotherapy or chemotherapy can affect the generation of memory T cells, which is the holy grail of adaptive immunity and may directly determine the durability of immunotherapy. This issue should also be addressed in the future. In the context of this concern, overall survival may be a better indicator than tumor response rate to appraise the impact of tumor-specific memory formation. It is encouraging that anti-PD therapy plus chemotherapy showed an improved overall survival over chemotherapy alone during a short period of time (1–2 years) (Gandhi et al., 2018). It is more important to compare this combination therapy with anti-PD therapy alone, as well as the evaluation of the generation of memory T cells, a major force for durable immune responses.
Combination with Local Therapies
Direct injection of various biological and chemical agents into tumors as an approach for cancer therapy has a longstanding history with William B. Coley’s efforts in the late 19th century (Aznar et al., 2017; Marabelle et al., 2017). These agents include but are not limited to TLRs, RIG-I-like receptor (RLR) agonists, STING pathway modulators (Corrales et al., 2016; Li et al., 2017), and oncolytic viruses (Lawler et al., 2017). Local therapies are believed to (1) trigger innate immunity so as to initiate adaptive immunity, (2) induce death of tumor cells, which could make more tumor antigens available to the immune system, and (3) generate a more inflammatory environment, which may support a better T cell response. These therapies can induce the regression of injected and distal tumors in experimental models. Very promising results have been reported in clinical trials with local injection of oncolytic viruses in melanoma lesions (Tang et al., 2018) and brain tumors (Desjardins et al., 2018; Lang et al., 2018). The roles of local therapies in long-term survival and the regression of distal metastases remain to be studied in the clinic. Furthermore, some fundamental questions on the immunological effect of these approaches in the patients’ TMEs remain to be fully characterized. In normal tissue, these local therapies could generate potential innate immunity, which helps initiate antigen-specific responses. However, in the TME, various tumor escaping mechanisms may impair antigen-specific responses, explaining infrequent objective response rates observed in the clinic using these therapies alone. In the context of these observations, it seems reasonable to combine local therapies with anti-PD therapy to neutralize the immunosuppressive mechanisms developed in the TME as long as the B7-H1/PD-1 pathway is one of the major immunosuppressive mechanisms. It is also possible that this combination may deviate the T cell response against viral antigens instead of tumor antigens due to possible immunological dominancy by anti-PD therapy (Martínez-Usatorre et al., 2018; Chen et al., 2018). Local treatment, on the other hand, has shown to be an effective approach to increase T cell infiltration into tumors. Recently, some promising results were published that illustrated the synergistic effect of local virotherapy as a way to increase tumor T cell infiltration and synergize with anti-PD therapy (Ribas et al., 2017).
Combination with Targeted Therapies
The combination between anti-PD therapy and targeted therapies (antibody or small molecules) has been explored extensively in animal models and currently in the clinic (Hughes et al., 2016; Vanneman and Dranoff, 2012). While these therapies are not capable of achieving durable, complete responses as single agents, in most cases, they are useful at inhibiting tumor growth and changing or even resetting the TME. It is possible that rapid lysis of tumor cells by targeted therapies generates an acute inflammation environment that may boost tumor immunity. It could not be excluded that in this context, B7-H1 may be upregulated. If this is the case, the combination of target therapies and anti-PD therapy seems reasonable, since at least an additive effect is expected. Target therapies, however, should be chosen carefully to avoid intrinsic metabolic and activation pathways that are required for TILs’s proliferation and survival. Also, pathways required for tumor-specific T cell memory formation should be considered.
Combination with Adoptive Cell Therapies
One of the reasons that adoptive cell therapy fails in some patients could be the suppression of transferred activated T cells when they enter the TME. Before adoptive transfer, T cells are activated in vitro, leading to PD-1 expression. Activated effector T cells upon tumor-antigen recognition in the TME can rapidly release IFN-γ; therefore, B7-H1 upregulation is expected, although this needs to be validated in the clinic. Therefore, as long as in vitro activated T cells can arrive in the TME, TILs and anti-PD therapy may represent an attractive direction for future cancer immunotherapy.
Combination with Other Immunotherapies
Currently, there are multiple immunotherapies under development that could be combined with anti-PD therapy. These potential combinations have been reviewed elsewhere (Melero et al., 2015; Smyth et al., 2016). To select the immunotherapy strategies most likely to succeed by combining with anti-PD therapies, some aspects need to be considered. First, as was explained in the principles section above, a favorable response-to-toxicity ratio of anti-PD therapy is attributed to the selective expression of B7-H1 in the TME, with minimal expression in normal tissues in the steady state. However, therapies that promote inflammation in normal tissues may trigger an anti-PD therapy effect out of the TME, losing this favorable response-to-toxicity ratio. An example is the combination with anti-CTLA-4 mAb. CTLA-4 mAbs, as we reviewed before, as a single therapy show more frequent irAEs than objective tumor responses. These irAEs in non-tumoral tissues can be amplified by anti-PD therapy (Larkin et al., 2015), which could explain why anti-CTLA-4 plus anti-PD therapy shows a synergistic toxic effect but an additive antitumor effect. Immunotherapies with a more selective treatment in the TME but a different mechanism of action (e.g., targeting myeloid cells or other immune escape mechanisms) may be better immunotherapy partners to be combined with anti-PD therapy. It is important to have a better understanding of how T cell memory formation is developed and collaborated to not only achieve strong effector T cell responses, but also to build strong T cell memory that ensures a long-lasting immune response.
An ideal scenario for combination therapy is synergism, which is commonly formulated as “1 + 1 > 2.” Without such a synergistic effect with two or more drugs, sequential use of these drugs may achieve the same goals. With an understanding of the mechanisms and limitations of anti-PD therapy, it is ideal that the TIME classification type I, type III, and type IV tumors be treated differently, with a goal to convert them to type II tumors. In this case, these treatments are likely to synergize with anti-PD therapy, since anti-PD therapies are most likely to be effective in the presence of TILs and B7-H1 expression. Therefore, a future effort would be to carefully analyze the defects of the antitumor immune response for each patient with a set of biomarkers and design a rationale for an effective combinatorial treatment that will achieve a potential synergistic efficacy. For instance, types I and IV will require strategies that increase the trafficking of T cells into the TME, while type III will require strategies to break TIL tolerance or reversal of TIL dysfunction (Figure 3).
Concluding Remarks
Cancer immunotherapy is undergoing an important transition from traditional immune enhancement approaches that activate systemic immune responses based on general knowledge of immune-activation processes to a more effective and less toxic treatment of immune normalization that targets the tumor microenvironment based on tumor-induced immune escape mechanisms. Anti-PD therapy has set an example that it is possible to increase the antitumor effect while minimizing irAEs. These results set a new standard in the field of cancer immunotherapy, and we believe that future cancer immunotherapy should aim not only to boost antitumor immunity, but also to understand the specific defects in tumor immunity and then normalize them to selectively modify a specific type of antitumor response in the right location rather than exacerbate a systemic immune response with the risk of increased irAEs. Our analyses reveal multiple principles that are fundamental and essential for the success of anti-PD therapy. Therefore, a simplistic description of the B7-H1/PD-1 pathway as another “immune checkpoint” may deviate our efforts to target those pathways that have low therapeutic value. The principles we have learned from the immunology of the B7-H1/PD-1 pathway and the development of anti-PD therapy will lead us to design more effective normalization cancer immunotherapies, allowing for optimal combinatorial therapies and to extend the frontiers of more successful cancer treatment.
ACKNOWLEDGMENTS
We thank Beth Cadugan for editing the manuscript. This work is partially supported by US NIH grants P50 CA196530 and P30 CA16359 and a Yale University endowed chair from the United Technologies Corporation. M.F.S. is supported by a Miguel Servet contract from Instituto de Salud Carlos III, Fondo de Investigación Sanitaria (Spain).
Footnotes
DECLARATION OF INTERESTS
L.C. is a consultant/board member of NextCure, Pfizer, Tayu, Vcanbio, and GenomiCare and currently has sponsored research funds from NextCure.
REFERENCES
- Agarwala SS, Glaspy J, O’Day SJ, Mitchell M, Gutheil J, Whitman E, Gonzalez R, Hersh E, Feun L, Belt R, et al. (2002). Results from a randomized phase III study comparing combined treatment with histamine dihydrochloride plus interleukin-2 versus interleukin-2 alone in patients with metastatic melanoma. J. Clin. Oncol 20, 125–133. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, Liu E, Dakhova O, Ashoori A, Corder A, et al. (2015). Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J. Clin. Oncol 33, 1688–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce Vargas F, Furness AJS, Litchfield K, Joshi K, Rosenthal R, Ghorani E, Solomon I, Lesko MH, Ruef N, Roddie C, et al. (2018). Fc Effector Function Contributes to the Activity of Human Anti-CTLA-4 Antibodies. Cancer Cell 33, 649–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascierto PA, Del Vecchio M, Robert C, Mackiewicz A, Chiarion-Sileni V, Arance A, Lebbé C, Bastholt L, Hamit O, Rutkowski P, et al. (2017). Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastaticmelanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 18, 611–622. [DOI] [PubMed] [Google Scholar]
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, et al. (1999). High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol 17, 2105–2116. [DOI] [PubMed] [Google Scholar]
- Aznar MA, Tinari N, Rullán AJ, Sánchez-Paulete AR, Rodriguez-Ruiz ME, and Melero I (2017). Intratumoral Delivery of Immunotherapy-Act Locally, Think Globally. J. Immunol 198, 31–39. [DOI] [PubMed] [Google Scholar]
- Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, and Chen L (2008). B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood 111, 3635–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach EA, Aguet M, and Schreiber RD (1997). The IFN γ receptor: a paradigm for cytokine receptor signaling. Annu. Rev. Immunol 15, 563–591. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, and Ahmed R (2006). Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687. [DOI] [PubMed] [Google Scholar]
- Benson DM Jr., Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, Baiocchi RA, Zhang J, Yu J, Smith MK, et al. (2010). The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 116, 2286–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilusic M, Madan RA, and Gulley JL (2017). Immunotherapy of Prostate Cancer: Facts and Hopes. Clin. Cancer Res. 23, 6764–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al. (2010). Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol 28, 3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. (2012). Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med 366, 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudno JN, and Kochenderfer JN (2016). Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 127, 3321–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulliard Y, Jolicoeur R, Windman M, Rue SM, Ettenberg S, Knee DA, Wilson NS, Dranoff G, and Brogdon JL (2013). Activating Fc g receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J. Exp. Med 210, 1685–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burel JG, Apte SH, and Doolan DL (2016). Systems Approaches towards Molecular Profiling of Human Immunity. Trends Immunol. 37, 53–67. [DOI] [PubMed] [Google Scholar]
- Callahan MK, Postow MA, and Wolchok JD (2016). Targeting T Cell Coreceptors for Cancer Therapy. Immunity 44, 1069–1078. [DOI] [PubMed] [Google Scholar]
- Charych DH, Hoch U, Langowski JL, Lee SR, Addepalli MK, Kirk PB, Sheng D, Liu X, Sims PW, Vanderveen LA, et al. (2016). NKTR-214, an Engineered Cytokine with Biased IL2 Receptor Binding (Increased Tumor Exposure, and Marked Efficacy in Mouse Tumor Models). [DOI] [PubMed]
- Chen L, and Flies DB (2013). Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol 13, 227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, and Han X (2015). Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J. Clin. Invest 125, 3384–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Azuma T, Yu W, Zheng X, Luo L, and Chen L (2018). B7-H1 maintains the polyclonal T cell response by protecting dendritic cells from cytotoxic T lymphocyte destruction. Proc. Natl. Acad. Sci. USA 115, 3126–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin YT, Wei PL, Ho Y, Nana AW, Changou CA, Chen YR, Yang YS, Hsieh MT, Hercbergs A, Davis PJ, et al. (2018). Thyroxine inhibits resveratrol-caused apoptosis by PD-L1 in ovarian cancer cells. Endocr. Relat. Cancer 25, 533–545. [DOI] [PubMed] [Google Scholar]
- Corrales L, McWhirter SM, Dubensky TW Jr., and Gajewski TF (2016). The host STING pathway at the interface of cancer and immunity. J. Clin. Invest 126, 2404–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R, Verma R, Sznol M, Boddupalli CS, Gettinger SN, Kluger H, Callahan M, Wolchok JD, Halaban R, Dhodapkar MV, and Dhodapkar KM (2015). Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J. Immunol 194, 950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins A, Gromeier M, Herndon JE 2nd, Beaubier N, Bolognesi DP, Friedman AH, Friedman HS, McSherry F, Muscat AM, Nair S, et al. (2018). Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med 379, 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. (2002). Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med 8, 793–800. [DOI] [PubMed] [Google Scholar]
- Du X, Tang F, Liu M, Su J, Zhang Y, Wu W, Devenport M, Lazarski CA, Zhang P, Wang X, et al. (2018). A reappraisal of CTLA-4 checkpoint blockade in cancer immunotherapy. Cell Res. 28, 416–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, Huynh TG, Zhao L, Fulton L, Schultz KR, et al. (2016). EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin. Cancer Res. 22, 4585–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, et al. ; KEYNOTE-189 Investigators (2018). Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med 378, 2078–2092. [DOI] [PubMed] [Google Scholar]
- Gardiner D, Lalezari J, Lawitz E, DiMicco M, Ghalib R, Reddy KR, Chang K-M, Sulkowski M, Marro SO, Anderson J, et al. (2013). A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS ONE 8, e63818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay CL, Bosch RJ, Ritz J, Hataye JM, Aga E, Tressler RL, Mason SW, Hwang CK, Grasela DM, Ray N, et al. ; AIDS Clinical Trials 5326 Study Team (2017). Clinical Trial of the Anti-PD-L1 Antibody BMS-936559 in HIV-1 Infected Participants on Suppressive Antiretroviral Therapy. J. Infect. Dis 215, 1725–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME, et al. (2014). PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J. Clin. Invest 124, 2246–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et al. (2013). Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med 368, 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid O, Robert C, Daud A, Hodi FS, Hwu W-J, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. (2013). Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med 369, 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst RS, Soria J-C, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al. (2014). Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, Linette GP, Meyer N, Giguere JK, Argarwala SS, et al. (2016). Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advancedmelanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2trial. Lancet Oncol. 17, 1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med 363, 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang BY, Zhan YP, Zong WJ, Yu CJ, Li JF, Qu YM, and Han S (2015). The PD-1/B7-H1 pathway modulates the natural killer cells versus mouse glioma stem cells. PLoS ONE 10, e0134715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes PE, Caenepeel S, and Wu LC (2016). Targeted Therapy and Checkpoint Immunotherapy Combinations for the Treatment of Cancer. Trends Immunol. 37, 462–476. [DOI] [PubMed] [Google Scholar]
- Jenkins RW, Aref AR, Lizotte PH, Ivanova E, Stinson S, Zhou CW, Bowden M, Deng J, Liu H, Miao D, et al. (2018). Ex Vivo Profiling of PD-1 Blockade Using Organotypic Tumor Spheroids. Cancer Discov. 8, 196–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. ; IMPACT Study Investigators (2010). Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med 363, 411–422. [DOI] [PubMed] [Google Scholar]
- Klein C, Waldhauer I, Nicolini VG, Freimoser-Grundschober A, Nayak T, Vugts DJ, Dunn C, Bolijn M, Benz J, Stihle M, et al. (2017). Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy: Overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines. OncoImmunology 6, e1277306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang FF, Conrad C, Gomez-Manzano C, Yung WKA, Sawaya R, Weinberg JS, Prabhu SS, Rao G, Fuller GN, Aldape KD, et al. (2018). Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol 36, 1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. (2015). Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med 373, 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Minor D, D’Angelo S, Neyns B, Smylie M, Miller WH Jr., Gutzmer R, Linette G, Chmielowski B, Lao CD, et al. (2018). Overall Survival in Patients With Advanced Melanoma Who Received Nivolumab VersusInvestigator’s Choice Chemotherapy in CheckMate 037: A Randomized, Controlled, Open-LabelPhase III Trial. J. Clin. Oncol 36, 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler SE, Speranza M-C, Cho C-F, and Chiocca EA (2017). Oncolytic Viruses in Cancer Treatment: A Review. JAMA Oncol 3, 841–849. [DOI] [PubMed] [Google Scholar]
- Leach DR, Krummel MF, and Allison JP (1996). Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736. [DOI] [PubMed] [Google Scholar]
- Lee S-J, Jang B-C, Lee S-W, Yang Y-I, Suh S-I, Park Y-M, Oh S, Shin J-G, Yao S, Chen L, and Choi IH (2006). Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274). FEBS Lett. 580, 755–762. [DOI] [PubMed] [Google Scholar]
- Li K, Qu S, Chen X, Wu Q, and Shi M (2017). Promising Targets for Cancer Immunotherapy: TLRs, RLRs, and STING-Mediated Innate Immune Pathways. Int. J. Mol. Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H, Cuillerot J-M, and Reck M (2012). Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, doubleblind, multicenter phase II study. J. Clin. Oncol 30, 2046–2054. [DOI] [PubMed] [Google Scholar]
- Marabelle A, Tselikas L, de Baere T, and Houot R (2017). Intratumoral immunotherapy: using the tumor as the remedy. Ann. Oncol 28 (suppl_12), xii33–xii43. [DOI] [PubMed] [Google Scholar]
- Martínez-Usatorre A, Donda A, Zehn D, and Romero P (2018). PD-1 Blockade Unleashes Effector Potential of Both High- and Low-Affinity Tumor-Infiltrating T Cells. J Immunol. 201, 792–803. [DOI] [PubMed] [Google Scholar]
- Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, Thatcher N, Wagstaff J, Zielinski C, Faulkner I, et al. (2014). Therapeutic vaccines for cancer: an overview of clinical trials. Nat. Rev. Clin. Oncol 11, 509–524. [DOI] [PubMed] [Google Scholar]
- Melero I, Berman DM, Aznar MA, Korman AJ, Pérez Gracia JL, and Haanen J (2015). Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat. Rev. Cancer 15, 457–472. [DOI] [PubMed] [Google Scholar]
- Morgan DA, Ruscetti FW, and Gallo R (1976). Selective in vitro growth of T lymphocytes from normal human bone marrows. Science 193, 1007–1008. [DOI] [PubMed] [Google Scholar]
- Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, and Rosenberg SA (2010). Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther 18, 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, and Wolchok JD (2015). Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol 26, 2375–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namuduri M, and Brentjens RJ (2016). Medical management of side effects related to CAR T cell therapy in hematologic malignancies. Expert Rev. Hematol 9, 511–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, et al. (2017). An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk WW, and Restifo NP (2000). Autoimmunity and the immunotherapy of cancer: targeting the “self” to destroy the “other”. Crit. Rev. Immunol 20, 433–450. [PMC free article] [PubMed] [Google Scholar]
- Parkinson DR, Abrams JS, Wiernik PH, Rayner AA, Margolin KA, Van Echo DA, Sznol M, Dutcher JP, Aronson FR, Doroshow JH, et al. (1990). Interleukin-2 therapy in patients with metastatic malignant melanoma: a phase II study. J. Clin. Oncol 8, 1650–1656. [DOI] [PubMed] [Google Scholar]
- Petroff MG, Chen L, Phillips TA, and Hunt JS (2002). B7 family molecules: novel immunomodulators at the maternal-fetal interface. Placenta 23 (Suppl A), S95–S101. [DOI] [PubMed] [Google Scholar]
- Phan GQ, Wang E, and Marincola FM (2001). T-cell-directed cancer vaccines: mechanisms of immune escape and immune tolerance. Expert Opin. Biol. Ther 1, 511–523. [DOI] [PubMed] [Google Scholar]
- Porter DL, Levine BL, Kalos M, Bagg A, and June CH (2011). Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med 365, 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A, and Wolchok JD (2018). Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, Hodi FS, Schachter J, Pavlick AC, Lewis KD, et al. (2015). Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 16, 908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon J, Fernandez E, et al. (2017). Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell 170, 1109–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu W-J, Gangadhar TC, et al. (2014). Antiprogrammed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 384, 1109–1117. [DOI] [PubMed] [Google Scholar]
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al. ; KEYNOTE-006 investigators (2015a). Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med 372, 2521–2532. [DOI] [PubMed] [Google Scholar]
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al. (2015b). Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med 372, 320–330. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA (2014). IL-2: the first effective immunotherapy for human cancer. J. Immunol 192, 5451–5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Mulé JJ, Spiess PJ, Reichert CM, and Schwarz SL (1985). Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J. Exp. Med 161, 1169–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S, Linehan WM, Robertson CN, Lee RE, Rubin JT, et al. (1987). A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N. Engl. J. Med 316, 889–897. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, and White DE (1994). Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA 271, 907–913. [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, and Restifo NP (2004). Cancer immunotherapy: moving beyond current vaccines. Nat. Med 10, 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, Royal RE, Kammula U, Restifo NP, Hughes MS, et al. (2005). Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J. Immunol 175, 6169–6176. [DOI] [PubMed] [Google Scholar]
- Sahin U, Derhovanessian E, Miller M, Kloke B-P, Simon P, Löwer M, Bukur V, Tadmor AD, Luxemburger U, Schrörs B, et al. (2017). Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226. [DOI] [PubMed] [Google Scholar]
- Sanmamed MF, and Chen L (2014). Inducible expression of B7-H1 (PD-L1) and its selective role in tumor site immune modulation. Cancer J. 20, 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmamed MF, Chester C, Melero I, and Kohrt H (2016). Defining the optimal murine models to investigate immune checkpoint blockers and their combination with other immunotherapies. Ann. Oncol. 27, 1190–1198. [DOI] [PubMed] [Google Scholar]
- Schalper KA, Carvajal-Hausdorf D, McLaughlin J, Altan M, Velcheti V, Gaule P, Sanmamed MF, Chen L, Herbst RS, and Rimm DL (2017). Differential Expression and Significance of PD-L1, IDO-1, and B7-H4 in Human Lung Cancer. Clin. Cancer Res 23, 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, et al. (2011). gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N. Engl. J. Med 364, 2119–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AM, Wolchok JD, and Old LJ (2012). Antibody therapy of cancer. Nat. Rev. Cancer 12, 278–287. [DOI] [PubMed] [Google Scholar]
- Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, and Korman AJ (2013). Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol. Res 1, 32–42. [DOI] [PubMed] [Google Scholar]
- Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, et al. (2013). Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med 210, 1695–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Ngiow SF, Ribas A, and Teng MWL (2016). Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol 13, 143–158. [DOI] [PubMed] [Google Scholar]
- Sznol M, and Chen L (2013). Antagonist antibodies to PD-1 and B7-H1 (PDL1) in the treatment of advanced human cancer–response. Clin. Cancer Res. 19, 5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, and Sakaguchi S (2000). Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med 192, 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Shalabi A, and Hubbard-Lucey VM (2018). Comprehensive analysis of the clinical immuno-oncology landscape. Ann. Oncol 29, 84–91. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Matsui H, Fujita T, Takaoka C, Kashima N, Yoshimoto R, and Hamuro J (1983). Structure and expression of a cloned cDNA for human interleukin-2. Nature 302, 305–310. [DOI] [PubMed] [Google Scholar]
- Tarhini AA, Kirkwood JM, Gooding WE, Cai C, and Agarwala SS (2007). Durable complete responses with high-dose bolus interleukin-2 in patients with metastatic melanoma who have experienced progression after biochemotherapy. J. Clin. Oncol 25, 3802–3807. [DOI] [PubMed] [Google Scholar]
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, and Chen L (2012). Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med 4, 127ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. (2012). Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med 366, 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, et al. (2014). Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol 32, 1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneman M, and Dranoff G (2012). Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 12, 237–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vari F, Arpon D, Keane C, Hertzberg MS, Talaulikar D, Jain S, Cui Q, Han E, Tobin J, Bird R, et al. (2018). Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood 131, 1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HMCS, et al. (2015). Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 35 (Suppl), S185–S198. [DOI] [PubMed] [Google Scholar]
- Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, and Bluestone JA (1994). CTLA-4 can function as a negative regulator of T cell activation. Immunity 1, 405–413. [DOI] [PubMed] [Google Scholar]
- Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, and Mak TW (1995). Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270, 985–988. [DOI] [PubMed] [Google Scholar]
- Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, Ridolfi R, Assi H, Maraveyas A, Berman D, et al. (2009). A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin. Cancer Res. 15, 5591–5598. [DOI] [PubMed] [Google Scholar]
- Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V, Marquez-Rodas I, et al. (2017). Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med 377, 1824–1835. [DOI] [PubMed] [Google Scholar]
- Weiner GJ (2015). Building better monoclonal antibody-based therapeutics. Nat. Rev. Cancer 15, 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner LM, Surana R, and Wang S (2010). Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat. Rev. Immunol 10, 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Wang S, Zhu Y, Luo L, Zhu G, Flies S, Xu H, Ruff W, Broad-water M, Choi I-H, et al. (2009). PD-1 on dendritic cells impedes innate immunity against bacterial infection. Blood 113, 5811–5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, and Chen L (2016). Classification of Advanced Human Cancers Based on Tumor Immunity in the MicroEnvironment (TIME) for Cancer Immunotherapy. JAMA Oncol. 2, 1403–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Pitt JM, Daillère R, Smyth MJ, and Kroemer G (2016). Mouse models in oncoimmunology. Nat. Rev. Cancer 16, 759–773. [DOI] [PubMed] [Google Scholar]
- Zou W (2005). Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer 5, 263–274. [DOI] [PubMed] [Google Scholar]
- Zou W, and Chen L (2008). Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 8, 467–477. [DOI] [PubMed] [Google Scholar]
- Zou W, Wolchok JD, and Chen L (2016). PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med 8, 328rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]