Abstract
Introduction:
In children with sickle cell disease (SCD), concomitant asthma is associated with increased morbidity and mortality when compared with children with SCD without asthma. Despite the well-established burden of asthma in children with SCD, no paradigm of care exists for the co-management of these two diseases.
Methods:
To address this gap, an integrated SCD and asthma clinic was created in a community health center that included (1) a dual respiratory therapist/asthma case manager; (2) an SCD nurse practitioner with asthma educator certification; (3) an onsite pulmonary function test laboratory; (4) a pediatric hematologist with expertise in managing SCD and asthma; and (5) application of the National Asthma Education and Prevention Program guidelines. A before (2010–2012) and after (2013–2014) study design was used to assess for improved quality of care with implementation of an integrative care model among 61 children with SCD and asthma followed from 2010 to 2014.
Results:
Asthma action plan utilization after initial diagnosis increased with the integrative care model (n=16, 56% before, 100% after, p=0.003), as did the use of spirometry in children aged ≥5 years (n=41, 65% before, 95% after, p<0.001) and correction of lower airway obstruction (n=10, 30% before, 80% after, p=0.03).
Conclusions:
Although the use of an integrative care model for SCD and asthma improved evidence-based asthma care, longer follow-up and evaluation will be needed to determine the impact on SCD-related morbidity.
Introduction
Sickle cell disease (SCD) is an inherited red blood cell disorder characterized by hemolysis and vascular occlusion, affecting an estimated 100,000 individuals in the U.S.1 Vaso-occlusive pain is the leading cause of emergency department (ED) and hospital use among children with SCD, followed by acute chest syndrome (ACS). Asthma is a common comorbidity, occurring in as many as 25% of unselected children with SCD,2 which increases the rate of vaso-occlusive pain and ACS.3 Multiple prospective studies have demonstrated that a diagnosis of asthma or history of recurrent wheezing in children with SCD is associated with premature mortality when compared with individuals with SCD without asthma or recurrent wheezing.4–6
Very few practice models exist for the management of comorbid conditions. Furthermore, children with public health insurance face barriers to specialty care access. An audit study examining scheduling behaviors among specialty clinics demonstrated disparate access to specialty care among children with public insurance versus those with private insurance, mainly attributed to specialists’ reluctance to accept public health insurance owing to decreased reimbursements.7 Specifically, 66% (179) of callers reporting Medicaid– Children’s Health Insurance Program coverage were denied an appointment for specialty care when compared with 11% (29) of callers reporting Blue Cross Blue Shield insurance.7 The Chronic Care Model is an evidence-based methodology for providing comprehensive chronic disease management and has been recommended as an effective strategy for managing individuals with multiple comorbid conditions.8 The components of the Chronic Care Model include community resources, health system organization, delivery system design, clinical information systems, self-management, and decision support. Application of the Chronic Care Model during a prospective, observational study among adults with SCD demonstrated improved adherence to care processes associated with improved health outcomes.9 Several studies have also demonstrated improved asthma-related outcomes, specifically improved guideline and medication adherence, symptom control, and quality of life, with employment of the Chronic Care Model.10–13 Given the evidence of improved outcomes with utilization of the Chronic Care Model, an integrated SCD and asthma care model was implemented to assess for improved adherence to evidence-based guidelines and outcomes when compared with a traditional model of SCD and asthma managed separately by pulmonary and hematology specialists.
Methods
This quality improvement initiative was conducted using a prospective, observational, pre–post study design. An integrated SCD and asthma care model guided by the Care Model (an expanded version of the Chronic Care Model) was created to improve asthma control and SCD morbidity in children with SCD and asthma. During the pre-intervention phase, participants were managed separately by pulmonologists and hematologists in a tertiary academic medical center. Participants were managed in an integrated SCD and asthma clinic during the intervention phase. This study was approved by the Vanderbilt University Medical Center IRB.
Setting
During the pre-intervention phase, 2010–2012, the care delivery model for SCD and asthma was composed of separate hematology and pulmonology specialty teams at a tertiary academic medical center. In the intervention years, 2013–2014, co-management of SCD and asthma was provided at a community-based federally qualified health center in Nashville, TN, which offered extended evening and weekend hours. The integration of comprehensive asthma management included
a dual respiratory therapist and asthma case manager;
an SCD nurse practitioner with asthma educator certification;
an onsite pulmonary function test laboratory;
a pediatrician with expertise in managing SCD and asthma; and
application of the National Asthma Education Prevention Program’s Expert Panel Report 3 (NAEPP) guidelines.
During the 2012 transition phase from isolated specialty clinics to the integrative model, the pediatric hematologist began seeing patients in January 2012 and the nurse practitioner in September 2012. Prior to providing medical care for children with SCD, the nurse practitioner trained 8 hours per week for 6 months with the pediatric pulmonary and asthma team.
Asthma Management
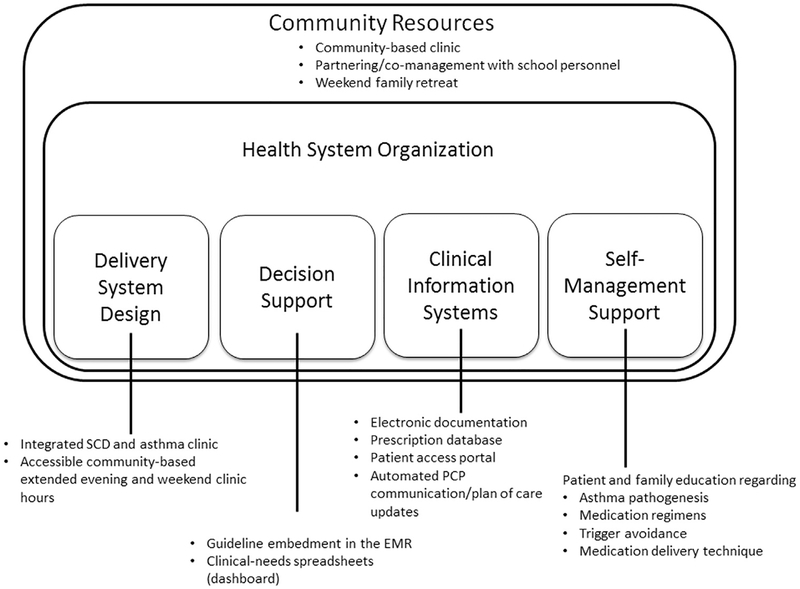
Asthma management was delivered by a multidisciplinary team following the NAEPP guidelines in accordance with the Care Model (Figure 1). The multidisciplinary team was composed of a pediatric hematologist, pediatric nurse practitioner, certified respiratory therapist, and nurse case manager. The electronic medical record (EMR) allowed for computerized documentation, electronic prescribing, communication with primary care providers, and communication with patients and families through an electronic patient portal (clinical information systems). The NAEPP guidelines include standardized assessment and educational tools and were embedded in the electronic medical record, providing a standardized, evidence-based structure for care delivery (decision support). The certified respiratory therapist reviewed asthma pathogenesis, environmental trigger recognition and avoidance, treatment regimens, and medication delivery techniques with each participant (self-management support).
Figure 1.
Working care model for integrated sickle cell disease and asthma care in a community health system.
Note: Adapted from Improving Chronic Illness. http://improvingchroniccare.org/index.php?p=The_Chronic_Care_Model&s=2. EMR, electronic medical record; PCP, primary care provider; SCD, sickle cell disease.
Asthma exacerbations are typically managed with bronchodilators and a 3- to 5-day course/burst of systemic corticorticoids.14 However, multiple reports in the literature15,16 have demonstrated a temporal risk of rebound vaso-occlusive pain in individuals with SCD who receive even a single dose of steroids, leaving some hematologists reluctant to prescribe steroids during asthma exacerbations. A onetime 2-mg/kg/day dose (max 60 mg/dose) of systemic glucocorticoids was used to treat asthma exacerbations during the intervention phase, with a 24-hour follow-up and one repeat dose of 1 mg/kg/day if needed, as the risk of untreated asthma exacerbations and resultant hypoxia outweigh the potential threat of rebound pain.
Measures
Primary outcomes related to SCD morbidity included vaso-occlusive pain and ACS. Asthma outcome measures were selected based on expert recommendations from the Asthma Outcomes Workshop, a committee of experts convened by the National Institutes of Health and Agency for Healthcare Research and Quality, and included spirometry, asthma action plan utilization, lower airway obstruction, and rates of asthma exacerbations.17 Outcome measures were reviewed by two co-authors (BM and ZI). Study participant inclusion criteria were as follows: a confirmed diagnosis of SCD based on hemoglobin analysis; consistent follow-up by the SCD team from 2010 to 2014; and a diagnosis of asthma, defined as episodic symptoms of airflow obstruction (exercise intolerance or nighttime awakenings due to coughing or respiratory symptoms), reversible airflow obstruction, and exclusion of alternative diagnoses.
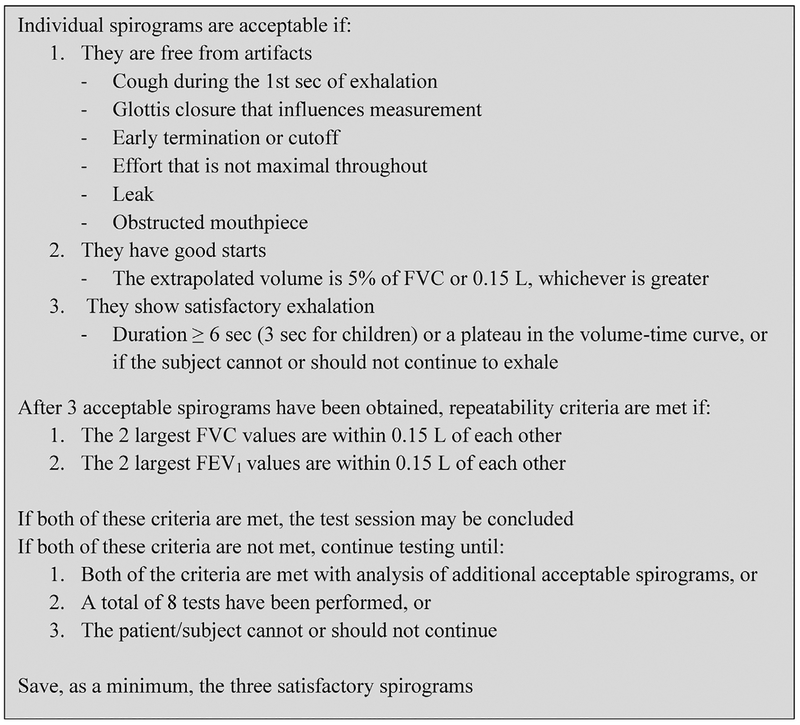
Lower airway obstruction increases the risk of ACS in children with SCD and is a clinical marker for asthma.3 Spirometry is the gold standard for detecting lower airway obstruction and is recommended annually for individuals with asthma.18,19 Spirome try was performed according to American Thoracic Society guidelines for all participants aged ≥5 years.20 The 2012 Global Lung Initiative multi-ethnic prediction equations were used as reference values.21 Spirometry was performed using the Morgan Plethysmo-graph Body Box and ComPAS PFT software. Test procedure and acceptability criteria were instituted according to American Thoracic Society guidelines (Figure 2). The spirometric variables that were measured include forced vital capacity (FVC); forced expiratory volume in 1 second (FEV1); and the ratio of FEV1 to FVC (FEV1/FVC). Lower airway obstruction was defined as FEV1/ FVC less than the fifth percentile after adjusting for age, gender, height, and race.22 Results were reviewed for quality and interpreted by pediatric pulmonary function test laboratory personnel.
Figure 2.
American Thoracic Society/European Respiratory Society spirometry acceptability criteria.
Asthma self-management is fostered through patient education and written asthma action plans, which have been shown to reduce emergent health service utilization.23 Study participants received annual written asthma action plans, as well as updated treatment plans based on steps up or down in therapy. Vaso-occlusive pain episodes were defined as hospitalizations requiring intravenous opioid administration. The total number of hospitalizations related to pain was determined through EMR extraction for discharge diagnoses indicating SCD and vasoocclusive pain and ICD-9 codes coinciding with SCD and pain.
ACS was defined as a new radiodensity on chest radiograph, accompanied by fever, increased respiratory effort, or decreased oxygen saturation.24,25 A diagnosis of pneumonia was considered an ACS episode. Hospitalizations related to ACS were determined through EMR extraction for chest radiograph findings indicating a new radiodensity, documented discharge diagnoses for SCD and ACS or pneumonia, and an ICD-9 code for ACS (517.3). Vaso-occlusive pain and ACS occurring during the same admission were counted as ACS.
Asthma exacerbations were defined as acute respiratory events resulting in worsening shortness of breath, cough, wheezing, chest tightness, or some combination of these symptoms.14 ED and hospitalizations due to asthma exacerbations were captured through EMR extraction. ICD-9 codes for asthma (493.9); plus cough (786.2); wheezing (786.07); dyspnea (786.0); chest pain (786.5); or acute exacerbation (493.82) were counted as an asthma exacerbation. Participants hospitalized for ACS with asthma symptoms were classified as ACS and acute asthma exacerbation.
Statistical Analysis
Participant demographics were examined using descriptive statistics. Pre and post utilization of spirometry, asthma actions plans, lung function, and systemic corticosteroid use were assessed using a one-tailed Fisher’s exact test. ED and hospital encounters related to asthma symptoms were compared using a two-rate person-time comparison. Rates of vaso-occlusive pain and ACS were compared using a Wilcoxon signed-rank test.
Results
The SCD practice team manages a total of 296 children with SCD, of which 31% (93/296) have a comorbid diagnosis of asthma. Of the 93 children with SCD and asthma, 61 were managed by the SCD team all 5 study years from 2010 to 2014 and were included in this study. Of the 61 participants, 73% had hemoglobin (Hb) SS and 55.7% were male. The mean age at study entry was 6.8 years. Of the 42 participants with Hb SS or Hb Sβ0 thalassemia, who were not on chronic blood transfusion therapy for primary or secondary stroke prevention, 98% (41/42) were on hydroxyurea therapy. Asthma severity was poorly documented during the pre-intervention phase, inhibiting a before and after comparison of asthma severity. Asthma severity classifications during the intervention phase were as follows: 19.6% intermittent, 27.8% mild persistent, 46% moderate persistent, and 6.5% severe persistent.
Among the 61 participants included in this study, 41 were aged ≥5 years during the study period and eligible to undergo spirometry. Spirometry was performed at either the tertiary academic medical center or community-based SCD clinic. During the pre-intervention phase, 65% (27/41) of participants received spirometry. Spiro-metry use increased to 95% (39/41) during the post-intervention phase (p<0.001).
Lower airway obstruction improved following the implementation of the integrated SCD and asthma clinic. During the pre-intervention phase, ten children with SCD had evidence of lower airway obstruction, of which 30% (3/10) had improved lower airway obstruction to above the fifth percentile with application of the separate specialty model. During the intervention phase, ten new cases of lower airway obstruction were identified, of which 80% (8/10) had a correction of lower airway obstruction to above the fifth percentile (p=0.03) with application of the NAEPP guidelines in the integrated SCD and asthma model.
Asthma action plan utilization increased after implementation of comprehensive asthma management in the community-based SCD clinic. During the pre-intervention phase, 45 participants had an established diagnosis of asthma, and 16 participants were newly diagnosed with asthma during the pre-intervention period. Of the 16 new diagnoses, nine (56%) received a written asthma action plan at the time of diagnosis. There were 16 new asthma diagnoses during the 2013–2014 intervention phase. After combining comprehensive asthma management with the community-based SCD clinic, 100% (16/16) of participants newly diagnosed with asthma received an asthma action plan at the time of diagnosis (p=0.003).
Hospital and ED encounters related to asthma exacerbation decreased with the integrative model, but did not reach statistical significance. ED and hospital encounters related to asthma symptoms before and after the integrative model occurred at a rate of 9.29 events and 6.56 events per 100 patient-years, respectively (p=0.38).
Rates of vaso-occlusive events (pain or ACS) did not decrease after implementation of comprehensive asthma management in the SCD clinic (Table 1). Hospital admissions related to vaso-occlusive pain were 54.6 per 100 patient-years during the pre-intervention phase and 48.9 per 100 patient-years during the post-invention period (p=0.54) among participants with Hb SS and Hb Sβ0 thalassemia. Incidence rates of ACS remained the same in the pre- and post-intervention phases. Hospital discharges for ACS among participants with Hb SS and Hb Sβ0 thalassemia were 21.3 and 26.6 per 100 patient-years before and after implementation of asthma care in the SCD clinic, respectively (p=0.26).
Table 1.
Overall Comparison of Standard Care Model Versus Integrative Model
| Standard asthma care model: asthma specialty clinic separate from sickle cell clinic (2010–2012) | Integrated sickle cell and asthma clinic (2013–2014) | |
|---|---|---|
| Number and location of initiation of asthma action plans for new diagnoses | ||
| Asthma clinic (n=16) | 4 (44%) | 0 |
| Sickle cell clinic (n=16) | 5 (56%) | 16 (100%)* |
| Spirometry utilization | ||
| Participants ≥5 years of age (n=41) | 27 (65%) | 39 (95%)** |
| Correction of lower airway obstruction after therapy | ||
| FEV1/FVC <5th percentile (n=10for both groups) | 3 (30%) | 8 (80%)* |
| Asthma exacerbations | ||
| Emergency department and hospital encounters per 100 patient-years | 9.29 | 6.56 |
| Systemic corticosteroids administered | 18 | 11 |
| Hospitalizations for vaso-occlusive pain per 100 patient-years | ||
| Entire cohort | 51 | 62 |
| Hemoglobin SS and sickle beta thalassemia null | 54.6 | 48.9 |
| Hemoglobin SC and sickle beta thalassemia + | 28.6 | 92.9* |
| Hospitalizations for acute chest syndrome per 100 patient-years | ||
| Entire cohort | 24 | 20 |
| Hemoglobin SS and sickle beta thalassemia null | 21.3 | 26.6 |
| Hemoglobin SC and sickle beta thalassemia + | 26.2 | 17.9 |
Note: Boldface indicates statistical significance (*p<0.05; **p<0.01).
FEV1/FVC, the ratio of forced expiratory volume in 1 second to forced vital capacity.
The use of systemic corticosteroids for asthma exacerbations did not change statistically before and after the integrative model. The intervention group (n 11) received fewer systemic corticosteroid bursts compared to the pre-intervention group (n=18); however, this decrease in systemic corticosteroid use did not reach statistical significance (p=0.10).
Discussion
Multiple studies have underlined the associated risk of increased SCD-related morbidity in children with SCD and concomitant asthma.3,5 However, no strategy has been introduced to co-manage these two conditions. Given the well-established barriers to specialty care access among children with public insurance,7 coupled with the fact that approximately 60% of children with SCD have public insurance,26 an integrative model is a promising strategy for improving access to asthma specialty care for children with SCD. The integrated SCD and asthma care model provided access to both SCD and asthma expertise in a single setting and led to improved adherence to evidence-based asthma guidelines, a critical first step for improved quality of care. As a result of improved adherence to asthma care guidelines, there was a statistically significant decrease in lower airway obstruction, a potential contributor to SCD-related morbidity.27
Only one other study has examined the impact of the Chronic Care Model in individuals with SCD. During this 10-year observational study of adults with SCD, adherence to evidence-based guidelines, hydroxyurea use, health service utilization, and hospital length of stay improved with the incorporation of standardized disease management algorithms, care coordination, multidisciplinary management, and self-management support.9 This study was different from the prior adult study in that the follow-up period was significantly shorter and was limited to children with both SCD and asthma that were followed all 5 study years. Despite these differences in implementation of the Chronic Care Model, both studies highlight the potential merits of applying the Chronic Care Model across the life span in individuals with SCD.
The integrated SCD and asthma care model did not improve incidence rates of vaso-occlusive pain, ACS, or asthma exacerbations requiring ED or hospitalization (Table 1). Given that the NAEPP guidelines have not been tested in children with SCD, it is unknown whether the current recommendations regarding preventive treatment or an increase in asthma preventive therapy beyond what is typically recommended in the general population is beneficial for children with SCD. Despite not having SCD-specific guidelines, asthma is a well-established contributor to increased SCD-related morbidity and should be detected early and managed appropriately,18 further highlighting the need for prospective clinical trials examining asthma therapies in children with SCD and a concomitant diagnosis of asthma.
Limitations
This study has several limitations. Ideally, children with SCD and asthma would have been randomly allocated to receive either standard care or the integrative care model; however, this was a before and after observational study and random allocation of participants was not feasible. Another limitation in detecting the impact of the integrative model is the relatively low baseline rates of vaso-occlusive pain and ACS in the study cohort when compared with other cohorts (0.54 and 1.0225 per 100 patient-years),28 most likely due to the high use of hydroxyurea therapy (98%) and inability to adjust for this potential confounder. Despite these findings, an integrative model improves guideline adherence, a critical determinant of treatment effectiveness.
Replication of an Integrative Care Model.
The integrated clinic was funded in part by the Junior League of Nashville Medical Home Program and Health Resources Service Administration to establish a medical home for children and adults with SCD. The funding for this novel model of care facilitated its initiation and propagation. However, based on prior and current experiences of delivering asthma care in an SCD clinic, the integrated care model can be implemented without extra funding. The effort requires cross-training, wherein mid-level and subspecialty providers are trained to manage both SCD and asthma. Further, adding extended evening and weekend hours is critical to this integrative model, as these hours of availability eliminate the barrier of missed days from school and work for many single-headed household families.
Conclusions
Comprehensive asthma management in children with SCD addresses a significant contributor to SCD-related morbidity. Innovative strategies to increase access to SCD and asthma care improve adherence to evidence-based guidelines. The Chronic Care Model provides a comprehensive framework for dual disease management of individuals with multiple comorbidities, including children with SCD and asthma. Larger multicenter studies are needed to determine the impact, if any, of integrated SCD and asthma management according to the NAEPP guidelines on rates on vaso-occlusive pain and ACS in children with SCD.
Acknowledgments
Publication of this article was supported by the Centers for Disease Control and Prevention.
This work was supported by the Junior League of Nashville, Health Resources and Services Administration grant number 5-U38-MC2222–0-04–00, the Sleep and Asthma Cohort Study, National Institute of Health grant number 5R01-HL079937–08, and the Doris Duke Charitable Foundation.
Footnotes
This article is part of the supplement issue titled Developing a Unified Approach for Sickle Cell Disease.
References
- 1.CDC. Sickle cell disease: data and statistics. www.cdc.gov/ncbddd/sicklecell/data.html. Updated July 8, 2015 Accessed July 14, 2015.
- 2.An P, Barron-Casella EA, Strunk RC, Hamilton RG, Casella JF, DeBaun MR. Elevation of IgE in children with sickle cell disease is associated with doctor diagnosis of asthma and increased morbidity. J Allergy Clin Immunol. 2011;127(6):1440–1446. 10.1016/j.jaci.2010.12.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd JH, DeBaun MR, Morgan WJ, Mao J, Strunk RC. Lower airway obstruction is associated with increased morbidity in children with sickle cell disease. Pediatr Pulmonol. 2009;44(3):290–296. 10.1002/ppul.20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knight-Madden JM, Barton-Gooden A, Weaver SR, Reid M, Green-ough A. Mortality, asthma, smoking and acute chest syndrome in young adults with sickle cell disease. Lung. 2013;191(1):95–100. 10.1007/s00408-012-9435-3. [DOI] [PubMed] [Google Scholar]
- 5.Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with increased mortality in individuals with sickle cell anemia. Haematologica. 2007;92(8):1115–1118. 10.3324/haematol.11213. [DOI] [PubMed] [Google Scholar]
- 6.Field JJ, Horst J, Strunk RC, White FV, DeBaun MR. Death due to asthma in two adolescents with sickle cell disease. Pediatr Blood Cancer. 2011;56(3):454–457. 10.1002/pbc.22891. [DOI] [PubMed] [Google Scholar]
- 7.Bisgaier J, Rhodes KV. Auditing access to specialty care for children with public insurance. N Engl J Med. 2011;364(24):2324–2333. 10.1056/NEJMsa1013285. [DOI] [PubMed] [Google Scholar]
- 8.Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1:2–4. [PubMed] [Google Scholar]
- 9.Artz N, Whelan C, Feehan S. Caring for the adult with sickle cell disease: results of a multidisciplinary pilot program. J Natl Med Assoc. 2010;102 (11):1009–1016. 10.1016/S0027-9684(15)30727-6. [DOI] [PubMed] [Google Scholar]
- 10.Fifield J, McQuillan J, Martin-Peele M, et al. Improving pediatric asthma control among minority children participating in Medicaid: providing practice redesign support to deliver a chronic care model. J Asthma. 2010;47(7):718–727. 10.3109/02770903.2010.486846. [DOI] [PubMed] [Google Scholar]
- 11.Moullec G, Gour-Provencal G, Bacon SL, Campbell TS, Lavoie KL. Efficacy of interventions to improve adherence to inhaled corticosteroids in adult asthmatics: impact of using components of the chronic care model. Respir Med. 2012;106(9):1211–1225. 10.1016/j.rmed.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Britto MT, Vockell AL, Munafo JK. Improving outcomes for underserved adolescents with asthma. Pediatrics. 2014;133(2):e418–e427. 10.1542/peds.2013-0684. [DOI] [PubMed] [Google Scholar]
- 13.Mangione-Smith R, Schonlau M, Chan KS. Measuring the effectiveness of a collaborative for quality improvement in pediatric asthma care: does implementing the chronic care model improve processes and outcomes of care? Ambul Pediatr. 2005;5(2):75–82. 10.1367/A04-106R.1. [DOI] [PubMed] [Google Scholar]
- 14.Busse WW, Boushey HA, Camargo CA, et al. National Asthma Education and Prevention Program, Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Washington, DC: National Heart Lung and Blood Institute, U.S. DHHS; 2007. [Google Scholar]
- 15.Sobota A, Graham DA, Heeney MM, Neufeld EJ. Corticosteroids for acute chest syndrome in children with sickle cell disease: variation in use and association with length of stay and readmission. Am J Hematol. 2010;85(1):24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar R, Qureshi S, Mohanty P, Rao SP, Miller ST. A short course of prednisone in the management of acute chest syndrome of sickle cell disease. J Pediatr Hematol Oncol. 2010;32(3):e91–e94. 10.1097/MPH.0b013e3181c29c52. [DOI] [PubMed] [Google Scholar]
- 17.Busse WW, Morgan WJ, Taggart V, Togias A. Asthma outcomes workshop: overview. J Allergy Clin Immunol. 2012;129(3 suppl):S1–S8. 10.1016/j.jaci.2011.12.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anim SO, Strunk RC, DeBaun MR. Asthma morbidity and treatment in children with sickle cell disease. Expert Rev Respir Med. 2011;5 (5):635–645. 10.1586/ers.11.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moeller A, Carlsen KH, Sly PD, et al. Monitoring asthma in childhood: lung function, bronchial responsiveness and inflammation. Eur Respir Rev. 2015;24(136):204–215. 10.1183/16000617.00003914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 21.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pellegrino R, Viegi G, Brusasco V, et al. Interpretive strategies for lung function tests. Eur Respir J. 2005;26:948–968. 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 23.Pinnock H, Epiphaniou E, Pearce G, et al. Implementing supported self-management for asthma: a systematic review and suggested hierarchy of evidence of implementation studies. BMC Med. 2015;13:127 10.1186/s12916-015-0361-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vance LD, Rodeghier M, Cohen RT, et al. Increased risk of severe vasoocclusive episodes after initial acute chest syndrome in children with sickle cell anemia less than 4 years old: sleep and asthma cohort. Am J Hematol. 2015;90(5):371–375. 10.1002/ajh.23959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howard J, Hart N, Roberts-Harewood M, et al. Guideline on the management of acute chest syndrome in sickle cell disease. Br J Haematol. 2015;169(4):492–505. 10.1111/bjh.13348. [DOI] [PubMed] [Google Scholar]
- 26.Rhodes KV, Bisgaier J, Lawson CC, Soglin D, Krug S, Van Haitsma M. “Patients who can’t get an appointment go to the ER”: access to specialty care for publicly insured children. Ann Emerg Med. 2013;61(4):394–403. 10.1016/j.annemergmed.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 27.Boyd JH, DeBaun MR, Morgan WJ, Mao J, Strunk RC. Lower airway obstruction is associated with increased morbidity in children with sickle cell disease. Pediatr Pulmonol. 2009;44(3):290 10.1002/ppul.20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeBaun MR, Gordon M, McKinstry RC, et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N Engl J Med. 2014;371(8):699–710. 10.1056/NEJMoa1401731. [DOI] [PMC free article] [PubMed] [Google Scholar]