Abstract
Nitriles are found in many bioactive compounds, and are among the most versatile functional groups in organic chemistry. Despite many notable recent advances, however, there are no approaches that may be used for preparation of di- or trisubstituted alkenyl nitriles. Related approaches which are broad in scope and can deliver the desired products in high stereoisomeric purity are especially scarce. Here, we describe the development of several efficient catalytic cross-metathesis strategies, which provide direct access to a considerable range of Z- or E-disubstituted cyano-substituted alkenes or their corresponding trisubstituted variants. Depending on the reaction type, a molybdenum-based monoaryloxide pyrrolide (MAP) or chloride (MAC) complex may be the optimal choice. The utility of the approach, enhanced by an easy-to-apply protocol for utilization of substrates bearing an alcohol or a carboxylic acid moiety, is highlighted in the context of applications to synthesis of biologically active compounds.
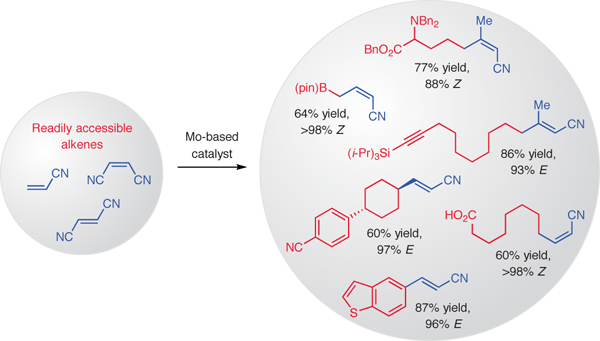
Graphical Abstract
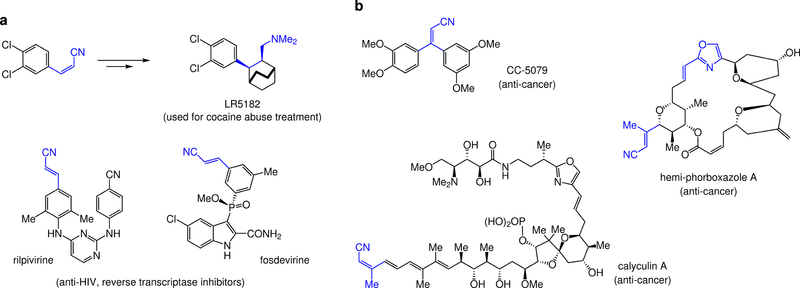
Nitrile compounds are important to chemistry, medicine1, and materials research2. Cyano-substituted alkenes are particularly attractive, as these robust and highly polarized alkenes3 may be the source of biological activity, or provide a site for irreversible and covalent inhibition4. Alkenyl nitriles may be readily modified at the olefin site (e.g., catalytic enantioselective hydrogenation5,6,7, or conjugate additions8) and/or the nitrile moiety. Z- and E-Disubstituted variants can be used in stereoselective preparation of medicinally relevant compounds, such as LR5182 (Fig. 1a)9. A nitrile unit can be the key component of a biologically active molecule, examples being anti-HIV reverse transcriptase inhibitors rilpivirine10, and fosdevirine11 (Fig. 1a). Stereochemically defined trisubstrituted alkenyl nitriles are found within anti-cancer agents CC-507912,13, phorboxazoles and their analogues14,15, where the alkenyl oxazole moiety may also be generated by modification of a cyano-substituted olefin16,17, and calyculin A18 (Fig. 1b). Sequential addition of two different nucleophiles may be induced to occur to an alkenyl nitrile at the CN bond, generating N-H amines19 without oxidation-state adjustments or protection/deprotection schemes.
Figure 1. Biologically active compounds with an alkenyl nitrile or a related moiety.
a, Stereoisomerically pure 1,2-disubstituted olefins bearing a nitrile substituent may be used to prepare medicinally relevant agents, such as LR5182, a polycyclic tertiary amine used to battle cocaine abuse. Furthermore, stereochemically defined alkenyl nitriles reside in a range of biologically active molecules. Examples are rilpivirine and fosdevirine, entities relevant to the fight against AIDS. b, Stereoisomerically pure trisubstituted alkenyl nitriles are desirable as well. These moieties are found in biologically active entities, represented by anti-cancer agents CC-5079, various phorboxazoles, and calyculin A. In the case of phorboxazoles, the oxazole ring and its adjacent olefin may be generated from an alkenyl nitrile as well.
There are catalytic protocols for synthesis of disubstituted alkenyl nitriles involving palladium-20,21, nickel-22,23, iron-24, gallium-25, copper-26,27,28, or rhodium-based29 complexes. Major shortcomings remain to be addressed however. Toxic24,25 or costly reagents28 or catalysts bearing a precious metal20,21,29 are required in several cases. Some reactions produce hydrogen cyanide21,27,29. Limitations in scope is another significant issue, as methods that furnish alkyl-substituted alkenyl nitriles are uncommon21,23,27,29. Effective control of stereochemistry can be problematic. Wittig-30,31 and Peterson-type reactions32,33 have been used to obtain Z-alkenyl nitriles, but stereoselectivities can be moderate30,32, and stoichiometric amounts of a strong base (i.e., n-butyllithium or hexamethyldisilazide) and cryogenic conditions (–78 °C)30,33 are often needed. Only two reported procedures offer access to a cyano-substituted Z olefin selectively21,27; these require a stereochemically defined Z-alkenyl bromide or iodide, stereoselective synthesis of which is non-trivial.
There are catalytic approaches for stereoselective preparation of trisubstituted alkenyl nitriles but these are confined to aryl- or polyaryl-substituted products21,24,34,35,36,37, or demand forcing conditions (≥120 °C)38,39. In some instances high loadings of precious metal salts39,40, or excess amounts (2.0 equiv.) of a strong Lewis acid (BCl3)36 are needed. To synthesize aliphatic trisubstituted alkenyl nitriles, expensive reagents must be used, slight structural variations can result in low stereoselectivity28, or substrates are valuable stereochemically defined trisubstituted alkenyl iodides27.
The large majority of the above protocols, regardless of the degree of substitution in the product olefin, require an acetylenic compound as the starting material22–23,25,29,35–40; methods that involve alkenes as starting materials would be strategically distinct and especially desirable, as olefins are more abundant and less costly.
Catalytic cross-metathesis represents an attractive strategy for preparation of stereochemically defined alkenyl nitriles. However, such methods are scarce. The first examples were disclosed more than two decades ago by Crowe and Goldberg, who showed that Mo bis-alkoxide complexes can be used to synthesize Z-1,2-disubstituted alkenyl nitriles41. Later studies with Ru-based complexes led protocols that are either similarly42,43 or less stereoselective44. Regardless of the catalyst type, Z:E ratios were variable, depended on the olefin type, and did not exceed 90:10. What is more, only reactions of unhindered n-alkyl-substituted olefins were reasonably efficient. There are only three reported instances where a trisubstituted alkenyl nitrile has been prepared by cross-metathesis (again, from n-alkyl olefins)45,46,47, and stereoselectivity was minimal in every case (e.g., 66:34 Z:E).
Results
Key challenges and their origins.
Because cyano group is small, development of a highly stereoselective cross-metathesis that generate alkenyl nitriles is especially challenging. The energy difference between the isomers of cyano-propene has been calculated by Wiberg et al.48 to be just 0.26±0.04 kcal/mol in favor of the Z isomer (61:39 Z:E). It is not surprising then that, whereas most cross-metathesis reactions generate E isomers preferentially, cyano-substituted alkenes are formed with low to moderate Z selectivity, an attribute that was recently attributed to stereoelectronic factors49.
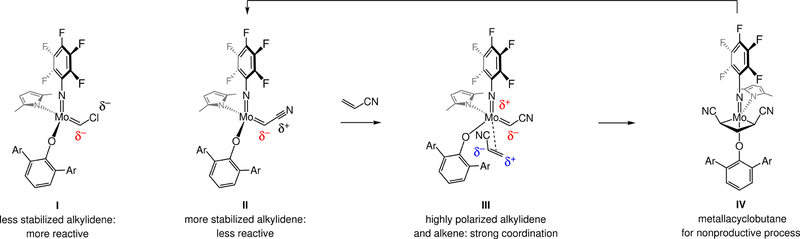
Another complication originates from the strongly electron-withdrawing nature of a nitrile unit. With an alkenyl halide50,51 the electron-withdrawing effect of a C–halogen bond is partially offset by electron–electron repulsion caused by the halide’s non-bonding electrons and the accumulated electron density at the carbon atom of a strongly polarized Mo alkylidene (see I, Fig. 2). In contrast, the presence of a cyano moiety has one overarching effect: stabilization of electron density at the alkylidene carbon (II), which translates to diminished catalyst activity. The small size of a nitrile group and the strongly polarized C=C bond in acrylonitrile further complicate matters, as these factors favor reaction via the electronically matched III (Fig. 2), which is precursor to the symmetrical metallacyclobutane IV, an intermediate for nonproductive self-metathesis.
Figure 2. Challenges in designing reactions that deliver stereodefined alkenyl nitriles.
Unlike other types of Mo alkylidenes, such as those that contain a chlorine atom (I), a nitrile-substituted variant (II) is more strongly stabilized due to electronic factors, and is therefore less reactive. The higher polarizability of Mo=C bond of a CN-substituted alkylidene and the alkene of acrylonitrile facilitates reaction via III, generating metallacyclobutane IV and causing nonproductive olefin metathesis. Ar, aryl.
Z-Disubstituted alkenyl nitriles.
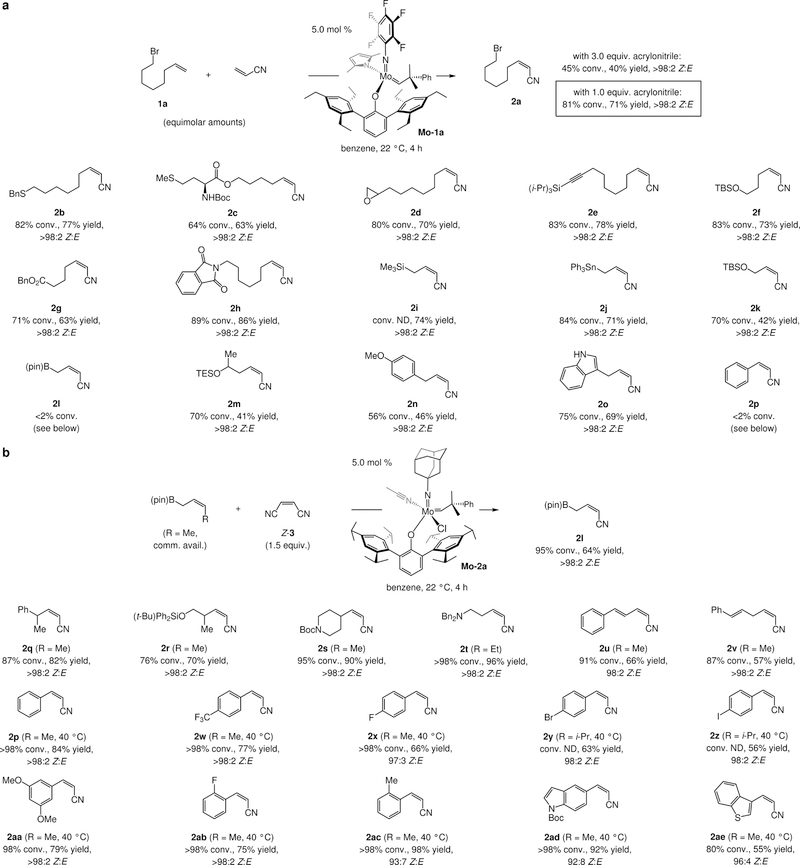
We began by examining a model transformation that could generate a Z-alkenyl nitrile, opting to use a terminal alkene (1a) and commercially available acrylonitrile (Fig. 3a). To minimize homo-metathesis of 1a, we initially used excess acrylonitrile (i.e., 3.0 equiv.). Among Mo MAP complexes, Mo-1a emerged as the most effective. Z-Alkenyl nitrile 2a was formed with complete stereochemical control (<2% E isomer), but there was only 45% consumption of 1a after four hours at ambient temperature, with no further progress after extended periods. On the basis of the hypothesis vis-à-vis the adventitious influence of nonproductive self-metathesis (via IV, Fig. 2), we probed the effect of lower acrylonitrile concentration on efficiency. With equimolar amounts of the two olefin substrates, there was 81% conversion to 2a, which was isolated in 71% yield as the pure Z isomer (Fig. 3a). It is noteworthy that, typically, excess amounts of one reaction partner is needed for high conversion, especially in kinetically Z-52,53 or E-selective51,54 cross-metathesis.
Figure 3. A broadly applicable approach to Z-disubstituted alkenyl nitriles.
a, In the presence of Mo-1a, Z-selective cross-metathesis between a terminal alkene and acrylonitrile may be performed efficiently and with high stereoselectivity. Transformations are more efficient with equimolar amounts of the alkene substrates (vs. excess acrylonitrile), probably because nonproductive metathesis is minimized. The method is applicable to an assortment of α-olefins. However, reactions with sterically demanding olefins are severely inefficient (e.g., 2l and 2p). b, The latter shortcoming may be addressed by stereoretentive processes involving easily accessible Z-disubstituted alkenes and maleonitrile (Z-3), and a monoaryloxide chloride (MAC) catalyst (Mo-2a). See the Supplementary Information Section 3 for experimental and analytical details. Bn, benzyl; pin, pinacolato; Boc, tert-butoxycarbonyl; TBS, tert-butyldimethylsilyl; TES, triethylsilyl.
Various linear alkenes were transformed to the corresponding Z-alkenyl nitriles under the conditions used to access 2a (Fig. 3a). Products bearing a sulfide (2b-c), an epoxide (2d), an alkyne (2e), a silyl ether (2f), or a Lewis basic carbonyl unit (2g-h) were isolated in 63–86% yield. Linear alkenes wherein a relatively long C–Si or C–Sn bond separates a large substituent and the alkene were similarly efficient (2i-j). A bulky and/or an electron-withdrawing olefin substituent, however, had an adverse effect on efficiency. tert-Butyl(dimethyl)silyl ether 2k was isolated in 42% yield (compared to 74% and 71% yield for 2i and 2j, respectively), and there was no conversion to allylic boronate 2l. This last finding underscores the greater difficulty associated with the formation of alkenyl nitrile products in comparison to alkenyl halides, since Mo-1a, despite bearing a bulkier 2,6-bis(2,4,6-triethylphenyl)phenoxy ligand, was effective in generating Z-γ-chloroallyl boronates (5.0 mol % loading, 22 °C, 4 h, 66% yield, >98:2 Z:E)50. β-Branched secondary homoallyl silyl ether 2m, and 2n, containing a benzylic substituent, were isolated in 41% and 46% yield, respectively. While 2o, a Z-alkenyl nitrile with an unprotected indole, was obtained in 69% yield, there was <2% conversion when styrene was used as the substrate. Complete Z selectivity was observed in all cases (<2% E; more on this later).
The more challenging Z-alkenyl nitriles.
To address the limitations in scope noted above, we turned to Mo monoaryloxide chloride (MAC) complexes55, recently demonstrated to exhibit greater reactivity than the MAP systems. Because MAC species decompose readily in the presence of a terminal alkene55, a Z-alkene must be used as the starting material. We have shown that many such substrates can be prepared readily and in high yield by single-vessel operations, often involving an efficient catalytic cross-coupling of an alkenyl boronate. Furthermore, a mixture of easily separable fumaronitrile and maleonitrile (E- and Z-3) can be obtained by treatment of the commercially available E isomer with 5.0 mol % iodine (160 °C, 6 h)55. Therefore, subjection of commercially available Z-crotyl–B(pin) to 1.5 equivalents of maleonitrile and 5.0 mol % Mo-2a afforded cyano-substituted Z-allyl–B(pin) product 2l (Fig. 3b) in 64% yield and >98:2 Z:E ratio after four hours at ambient temperature. When the same transformation was carried out with Mo-1a, under otherwise identical conditions, the major product was derived from self-metathesis of Z-crotyl-B(pin) (72% conv.) while 2l was the minor component (25% conv., >98:2 Z:E). This is likely because, unlike Mo-2a, Mo-1a is unable to react with the severely electron-deficient maleonitrile (Z-3). The approach is applicable to α-branched alkenes (2q-s), which are among the most challenging substrates in cross-metathesis.
Unlike when MAC complex Mo-2a was used, attempts to generate amine 2t, 1,3-diene 2u, and 1,4-diene 2v with a MAP species (Mo-1a) led to much less favorable results (<30% conv. to the desired product). Intramolecular N→Mo chelation may be responsible for the diminished conversion to 2t, whereas the MAC catalyst is probably reactive enough such that even a low concentration of the active four-coordinate alkylidene species can be sufficient for efficient cross-metathesis. When a MAP complex was used to prepare 1,3-diene 2u, there was <2% conversion to the desired product. In the case of diene 2v, significant amounts of byproducts from transformation at the substrate’s E-alkene could be observed. As noted previously55, MAC complexes react with Z alkene isomers preferentially.
Equally notable are the transformations that generate different aryl- and heteroaryl-substituted Z-alkenyl nitriles (2p-2ae; Fig. 3b). Thus, regardless of the position or the electronic attributes of the aryl substituent, the desired products were isolated in 55–98% yield and 92:8 to >98:2 Z:E ratio. In certain cases, slight heating to 40 °C led to a higher yield, but the duration of all transformations was just four hours. Two additional points merit note: 1) With a MAC species reaction with unprotected indole-containing substrate (cf. 2ad) did not lead to any significant conversion (<5%). 2) This set of products (Fig. 3b) is not in the purview of any existing cross-metathesis methods, where a more traditional Mo-41 or Ru-based42 complex is used. The case of ortho-tolyl-substituted alkenyl nitrile 2ac, which was secured in 98% yield (93:7 Z:E), is especially noteworthy, considering the steric pressure that probably exists within the corresponding metallacyclobutane intermediate.
Nevertheless, a set of substrates that we were unable to transform to their corresponding alkenyl nitriles efficiently were allylic ethers, regardless of the nature of the Mo complex used or the nature of the protecting unit (e.g., tert-butyldimethyl silyl, benzyl). This shortcoming is reflected in the yield with which primary allyl silyl ether 2k was obtained (42% yield); unlike other instances mentioned above (Fig. 3b), efficiency did not improve in the corresponding stereoretentive process involving a MAC complex. The steric hindrance imposed by the allylic substituent together with diminution of alkene Lewis basicity, caused by the adjacent C–O bond, and the relative stability of a CN-substituted Mo alkylidene (see Fig. 2), are likely responsible for the lack of reactivity.
E-Alkenyl nitriles.
Next, we investigated reactions that would generate an E-disubstituted alkenyl nitrile (Fig. 4). As in the past, we chose to focus on stereoretentive51 processes (vs. stereoselective). To identify an effective catalyst, we studied the reaction of aryl olefin E-4a with commercially available fumaronitrile (E-3; Fig. 4a). The transformation with pentafluorophenyl imido MAP complexes Mo-1a and Mo-1b, while highly stereoretentive (>98:2 E:Z), were moderately efficient, despite the elevated temperature (47% and 52% conv. to E-4a, respectively, at 80 °C). To improve efficiency, we again turned to MAC alkylidenes, mindful that this class of complexes were not formerly used for reactions that generate E alkenes. We began with Mo-2a, which proved effective for the processes with hindered alkyl- and aryl-substituted olefins and leading to Z-alkenyl nitriles (Fig. 3b). Although there was only 20% conversion to E-5a, we were encouraged for several reasons. Firstly, the reaction was completely stereoretentive. Secondly, the transformation was more efficient than when a MAP species was used, as a considerably greater portion of the product mixture consisted of the desired product (22% conv., 20% to E-5a compared to 82% conv., 52% to E-5a for Mo-1b). Thirdly, whereas Mo-1b is already a pentafluoro-imido complex, Mo-2a is an adamantyl imido derivative, leaving room for the possibility of achieving better efficiency through incorporation of an activating polyfluoroaryl imido ligand.
Figure 4. E-Disubstituted alkenyl nitriles.
a, Cross-metathesis between an E-disubstituted olefin and fumaronitrile was more efficient with Mo-2b, but E:Z ratios were low compared to when Mo-1b or Mo-2a were used (88:12 vs. >98:2, respectively). Control experiments indicated that this is probably due to isomerization of E-3 to Z-3, catalysed by the released PMe2Ph by Mo-2b. Thus, with Mo-2c (3-bromopyridine ligand), 4a was obtained with 96:4 E:Z selectivity. b, The approach can be used to access aryl-substituted E-alkenyl nitriles. c, E-β-Alkyl-styrenyl precursors can be converted to E-alkyl-substituted alkenyl nitriles. With a bulky aliphatic alkene higher efficiency was observed with Mo-2d (smaller aryloxide ligand). See the Supplementary Information Section 3 for experimental and analytical details. Bn, benzyl; Ts, para-toluenesulfonyl.
To promote cross-metathesis between E-3 and E-4a, we probed the ability of the pentafluoro-imido MAC alkylidenes derived from Mo-2b, most efficiently prepared and isolated as a dimethylphenylphosphine complex56. We used 15 mol % tris(pentafluorophenyl)borane as the additive to generate the active four-coordinate species (after loss of the phosphine) and to cap the hydroxy group of residual free 2,6-(2,4,6-triisopropyl)phenol (remainder from catalyst synthesis), a strategy that we would later use to address another important issue (see below). After 12 hours at 40 °C, there was 67% consumption of E-4a, with 49% conversion to E-5a, representing a notable boost in reactivity. Unexpectedly, though, there was significant diminution in the E:Z ratio (88:12). Usually, the reason for lower product stereoisomeric purity in stereoretentive olefin metathesis is adventitious isomerisation of the starting alkene. We surmised that E-3, an exceedingly electrophilic reagent, might interconvert with its similarly favored Z isomer (as noted above) through an addition/elimination sequence. The likely nucleophilic promoter for this event, considering the complete retention of stereochemistry with the acetonitrile complex Mo-2a, would be an uncoordinated dimethylphenylphosphine. This led us to subject E-3 to 5.0 mol % of tricyclohexylphosphine (easier to handle than PhMe2P) and 15 mol % (C6F5)3B (22 °C, 4 h), which resulted in just 3% isomerisation (i.e., from >98:2 to 97:3 E-3:Z-3). This might seem insignificant, but, considering that Z alkenes generally react faster with this catalyst class55, particularly with a larger aryloxide ligand, this could indeed be the source of the 12% loss in stereochemical purity. To confirm, we prepared Mo-2c, a complex that bears a less nucleophilic 3-bromopyridyl ligand, and, under otherwise identical conditions as was used for Mo-2b, we isolated 5a in 78% yield and 96:4 E:Z ratio after 12 hours at 40 °C [6.0 mol % (C6F5)3B was used as there was less contaminating phenol remaining from preparation of the Mo-2b]. Control experiments indicated that there is no post-metathesis alkene isomerisation.
The method is applicable to E-aryl-substituted and E-heteroaryl-substituted alkenes of disparate steric and/or electronic properties (5b-i, Fig. 4b); products were obtained in up to 87% yield, with stereoselectivity ranging from 90:10 to 97:3 E:Z ratio. In the case of o-tolyl-substituted 5c, with the substrate bearing a particularly hindered substituent, the reaction was much more efficient when the less sterically demanding Mo-2d was employed (50% yield, 96:4 E:Z; compared to 34% conv., 84:16 E:Z with Mo-2c).
E-Alkenyl nitriles with an n-alkyl substituent were most efficiently generated by catalytic stereoretentive reactions with E-β-alkyl styrenes (Fig. 4c)51; 6a-c were thus obtained in 69–75% yield and 93:7–96:4 E:Z ratio. As in the case of sterically hindered 5c, the reaction of α-branched alkene E-7 to generate 6d was more efficient with Mo-2d (60% yield, 97:3 E:Z compared to 20% conv., 54:46 E:Z with Mo-2c); the smaller aryloxide ligand might better accommodate the sizeable alkyl moiety, which would be projected towards it in the corresponding metallacyclobutane.
The stereoisomeric purity of the E-alkenyl nitrile products, although generally high, is slightly lower than the related Z isomers accessed through stereoretentive cross-metathesis (Fig. 3b); this difference may be attributed to increased steric pressure between an E-disubstituted alkene and the large aryloxide ligand of a Mo complex (e.g., Mo-2c). Consequently, fumaronitrile-to-maleonitrile isomerization (E-3 → Z-3), despite the lower nucleophilicity of the released 3-bromopyridine, can become more competitive, especially at 40 °C, and diminution in E:Z product ratios ensues. This scenario is supported by the finding that more Z-alkenyl nitrile is generated when the more sterically demanding ortho-substituted substrates are used (i.e., 84:16–90:10 E:Z for 5b-c when Mo-2c was used). In the case of less hindered alkyl-substituted alkenyl nitriles (6a-c), substrate self-metathesis and E-to-Z isomerization are probably more facile, and stereoisomeric purity suffers.
Trisubstituted E- and Z-alkenyl nitriles.
We then turned to determining whether a catalytic method for stereoretentive synthesis of trisubstituted alkenyl nitriles is feasible. What distinguishes this set of transformations, other than the involvement of a more congested metallacyclobutane, is that they probably involve a cyano-substituted alkylidene exclusively, as opposed to a 1,1-disubstituted variant arising from initial reaction with a trisubstituted olefin. This means that reaction with either Z- or E-3 should lead to the same degree of stereochemical purity, although, as already noted, reaction involving the former isomer would probably be more efficient.
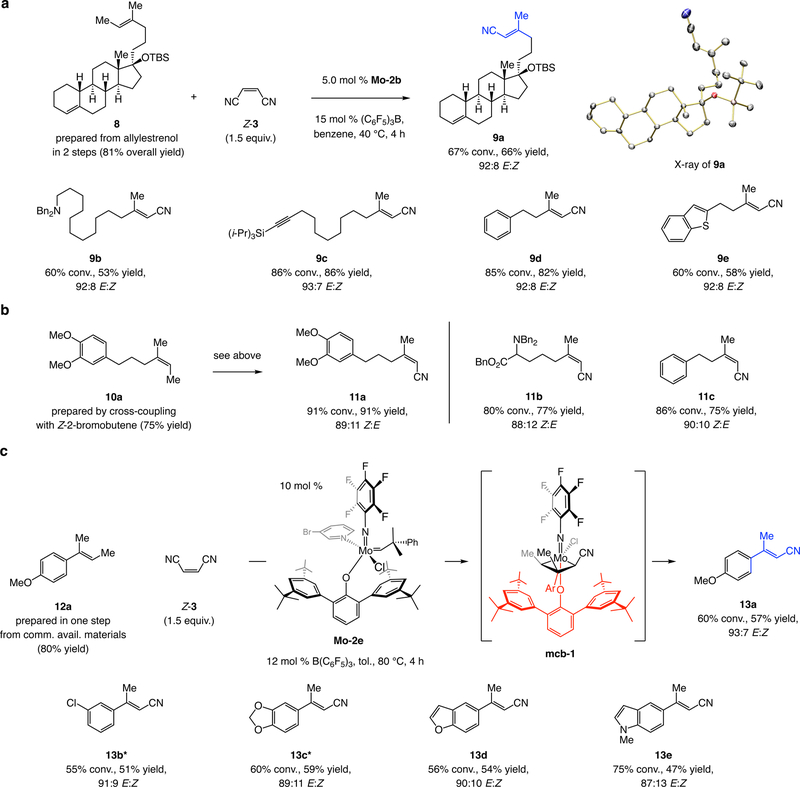
Trisubstituted alkene 8 was prepared by a single-vessel operation from a silyl ether of allylestrenol (see the Supplementary Information for details). Subjection of 8 to Mo-2b (5.0 mol %), 15 mol % (C6F5)3B and Z-3 (1.5 equiv.) afforded 9a in 66% yield and 92:8 E:Z selectivity (Fig. 5a). The E:Z ratio was the same with 3-bromopyridine-containing MAC complex Mo-2c, in line with predominant intermediacy of the cyano-substituted syn-alkylidene, regardless of whether E- or Z-3 is involved. Assorted aliphatic E-trisubstituted alkenyl nitriles were accessed similarly (9b-e, in 53–86% yield and 92:8–93:7 E:Z). The approach is applicable to preparation of Z-trisubstituted alkenyl nitriles (see 11a-c). A rationale for the lower stereochemical control in the formation of the Z isomers was provided recently in connection with the synthesis of trisubstituted alkenyl chlorides and bromides57.
Figure 5. E- and Z-Trisubstituted alkenyl nitriles.
a, Readily accessible stereochemically defined E-trisubstituted alkenes, bearing a relatively diminutive methyl group terminus, can be converted in the presence of Mo-2b and Z-3 to the corresponding E-alkenyl nitriles. The method is applicable to various alkyl-substituted olefins (9a-e). b, Z-Trisubstituted alkenyl nitriles can be obtained similarly. c, An even more difficult process is one that might deliver a trisubstituted alkenyl nitrile with a sizeable aryl unit. This may be accomplished with 10 mol % Mo-2e at 80 °C (via mcb-1 to give 13a-e). *15.0 mol % Mo-2e, 18 mol % B(C6F5)3 was used. See the Supplementary Information Section 3 for experimental and analytical details.
Perhaps the most challenging aspect of this study was designing efficient reactions between relatively stabilized cyano-substituted alkylidenes and hindered trisubstituted alkenes; particularly difficult would be processes involving an aryl olefin. Yet again, in the case of alkenyl chlorides, the corresponding products were obtained when MAP complex Mo-1b57 was used. However, the same strategy was ineffective when applied to reactions proceeding via a more stabilized/less reactive cyano-substituted alkylidene species (compare I to II, Fig. 2). There was <2% conversion to 13a with MAC complex Mo-2c or Mo-2d. To address this issue, we synthesized Mo-2e (Fig. 5c), which bears an aryloxide with 3,5-di-t-butylphenyl groups at its C2 and C6 sites. We expected reduced steric pressure in the corresponding metallacyclobutane (mcb-1). Through the use of 10 mol % Mo-2e and 12 mol % (C6F5)3B, and at 80 °C for four hours, we were able to isolate 13a in 57% yield and 93:7 E:Z selectivity. As indicated by the synthesis of 13b-e, the approach is applicable to different aryl alkenes. Reactions were slower with the more electron-withdrawing aryl alkenes, as synthesis of 13b-c required 15 mol % Mo-2e and 18 mol % (C6F5)3B to reach 55–60% conversion (with 10 mol% Mo-2e: 36% and 41% conv., 32% and 35% yield, 91:9 and 89:11 E:Z, respectively).
Under the same conditions and with a Z-trisubstituted aryl olefin, there was only ca. 20% conversion to the desired alkenyl nitrile, formed with minimal stereoisomeric purity (~60:40 Z:E). Development of a more effective solution to these important but difficult cross-metathesis reactions is a goal of future investigations.
Utility.
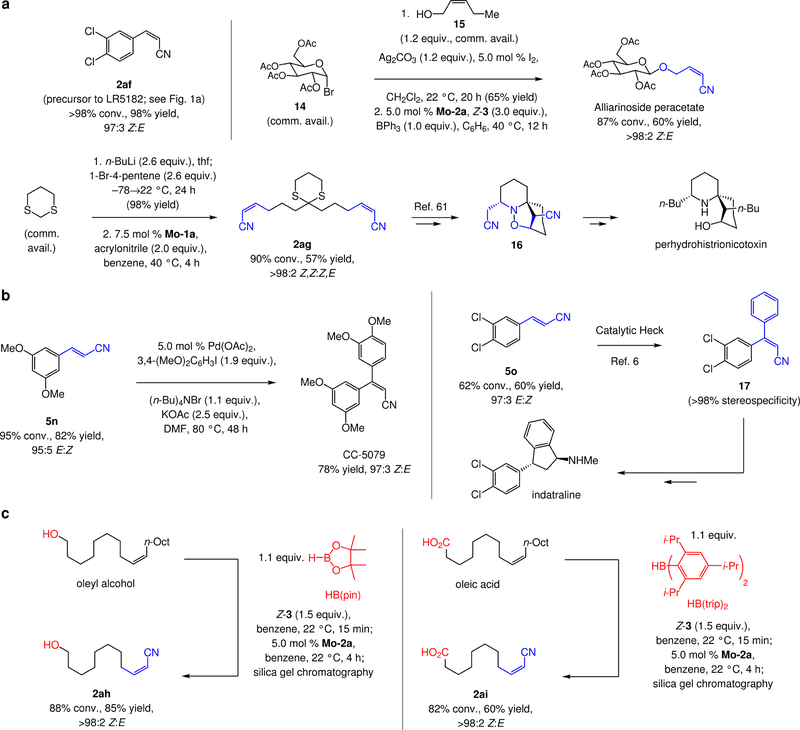
The present advance provides a convenient entry to many otherwise difficult-to-prepare stereochemically defined alkenyl nitriles, facilitating the synthesis of a large variety of biologically active compounds. 3,4-Dichloroaryl-substituted Z-alkenyl nitrile 2af (Fig. 6a), obtained in 98% yield and 97:3 Z:E ratio, is an intermediate en route to LR5182 (Fig. 1). The cross-metathesis approach is more efficient than the previously utilized Knoevenagel condensation (aryl aldehyde and cyano acetic acid)/decarboxylation at elevated temperature, which generated an 80:20 E:Z mixture9.
Figure 6. Utility of the method in chemical synthesis.
a, The possibility of synthesizing aryl- or alkyl-substituted Z-alkenyl nitriles by catalytic cross-metathesis is likely to have a notable impact on the efficiency with which many bioactive compounds can be prepared. Representative cases are agent for cocaine abuse treatment LR5182, agrochemical agent alliarinoside, and perhydrohistrionicotoxin (step 2ag to 1661), which has been used for probing mechanisms of neuromuscular impulses. b, E-Alkenyl nitriles are crucial for stereoselective generation of a variety of bio-active molecules. Anti-cancer agent CC-5079 and anti-depressant indatraline are two examples. 5o to 17 – catalytic Heck reaction6. c, In situ protection/deprotection of a neighbouring hydroxy and carboxylic acid group may be carried out, significantly enhancing the scope of the method. See the Supplementary Information Sections 4–9 for experimental and analytical details. pin, pinacolato; trip, 2,4,6-triisopropylphenyl; Ac, acetyl.
The union of glycosyl bromide 14 and allylic alcohol 15, both commercially available, followed by catalytic stereoretentive cross-metathesis delivered alliarinoside peracetate in 39% overall yield as a single olefin isomer (>98:2 Z:E) (Fig. 6a). Previously reported protocols either generate a near-equal mixture of alkene isomers (Horner-Wadsworth-Emmons-type processes)58,59, or demand initial generation of a Z-alkenyl iodide (catalytic cross-coupling), requiring at least two additional operations27. Also noteworthy is bis-alkenyl nitrile 2ag, accessed by a double-cross-metathesis in 57% yield and >98:2 Z,Z’:Z,E’ (Fig. 6a), and utilized in total synthesis of perhydrohistrionicotoxin (via 16)60. This compound was formerly accessed by a route that included synthesis of an alkene via the corresponding bis-aldehyde, the preparation of which necessitated an additional deprotection step (acetal removal) and highly toxic HMPA (hexamethylphosphoramide) was required to facilitate alkylation.
E-Alkenyl nitriles 5n and 5o were isolated in 82% and 60% yield, and 95:5 and 97:3 E:Z ratio, respectively (Fig. 6b). These compounds have been converted to anti-cancer agent CC-507913 by catalytic Heck reaction, and to anti-depressant indatraline61, via 17, by a similar process, followed by catalytic enantioselective hydrogenation6. The Z isomer of CC-507962 is more potent and must therefore be synthesized selectively. Furthermore, the cross-coupling processes are considerably more efficient with an E alkene6; in line with such findings, we were unable to detect any of the desired trisubstituted alkene when Z-5n was subjected to the conditions used for the reaction of the corresponding E isomer (Fig. 6b). In previous studies the requisite 1,2-disubstituted alkenes could only be generated as 80:20 E:Z mixtures by Wittig-type reactions13,6, and removal of stoichiometric amounts of phosphine–oxide side product often required difficult chromatographic procedures.
We then set out to address another major shortcoming, namely, the instability of such species to an alcohol or a carboxylic acid moiety. In the case of a substrate that bears a hydroxy group, we find that simply by treating the alkenes with 1.1 equivalents of commercially available HB(pin) (pin, pinacolato) at ambient temperature for 15 minutes, and then the requisite amount of the Mo complex for 4 hours, followed by silica gel chromatography, the desired alkenyl nitrile product can be obtained in high yield and stereochemical purity. The conversion of oleyl alcohol to 2ah is a case in point (85% yield, >98:2 Z:E; Fig. 6c). For a starting material containing a carboxylic acid moiety, the most effective approach is to use HB(trip)2 (trip, 2,4,6-triisopropylphenyl)63, a reagent that can be prepared easily on gram scale from commercially available materials in two steps (70–75% overall yield). Transformation of oleic acid to alkenyl nitrile 2ai is representative (60% yield, >98:2 Z:E vs. 47% yield, >98:2 Z:E with HB(pin)). This development promises to expand the practical utility of Mo-based catalysts considerably.
Conclusions
We have developed a broadly applicable set of catalytic methods for the preparation of Z- and E-disubstituted and trisubstituted alkenyl nitriles in high stereoisomeric purity. We have shown that by considering various attributes of the Mo-based complex (MAP or MAC), and the electronic and steric attributes of the intermediate alkylidenes and metallacyclobutanes, catalysts providing access to stereoisomerically enriched alkenyl nitriles, from those that bear a linear aliphatic substituent to those that contain a hindered α-branched or aryl moiety, can be identified. Similarly notable is that an equimolar amount of the two cross partners is not only sufficient, but is optimal, for achieving high efficiency in a cross-metathesis reaction. We introduce the use of easily accessible boron hydride compounds for in situ temporary protection of hydroxy and carboxylic acid groups, which can otherwise quickly deactivate a Mo-based catalyst. The ability to access an alkenyl nitrile isomer with high stereochemical purity allows for significant enhancement in the efficiency with which many biologically active entities are prepared.
Supplementary Material
Acknowledgements
This research was supported by a grant from the National Institutes of Health (GM-59426). T. T. N. was supported as a John LaMattina Graduate Fellows in Chemical Synthesis. We are grateful to Dr. Sebastian Torker for helpful discussions.
Footnotes
Data availability
X-ray crystallographic data for compound 9a, are freely available from the Cambridge Crystallographic Data Centre (CCDC 1861573). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other data that are in support of the findings of this study are available within the Article and its Supplementary Information, or from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
References
- 1.Fleming FF, Yao L, Ravikumar PC, Funk L & Shook BC Nitrile-Containing Pharmaceuticals: efficacious roles of the nitrile pharmacophore. J. Med. Chem 53, 7902–7917 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segura JL, Martín N & Hanack M Oligo-2,6-naphthylenevinylenes – New building blocks for the preparation of photoluminescent polymeric materials. Eur. J. Org. Chem 643–651 (1999).
- 3.Allgäuer DS et al. Quantification and theoretical analysis of the electrophilicities of Michael acceptors. J. Am. Chem. Soc 139, 13318–13329 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Serafimova IM et al. Reversible targeting of nanocatalytic cysteines with chemically tuned electrophiles. Nat. Chem. Biol 8, 471–476 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee D, Kim D & Yun J Highly enantioselective conjugate reduction of β,β-disubstituted α,β-unsaturated nitriles. Angew. Chem. Int. Ed 45, 2785–2787 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Yan Q, Kong D, Li M, Hou G & Zi G Highly efficient Rh-catalyzed asymmetric hydrogenation of α,β-unsaturated nitriles. J. Am. Chem. Soc 137, 10177–10181 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Müller M-A & Pfaltz A Asymmetric hydrogenation of α,β-unsaturated nitriles with base-activated iridium N,P ligand complexes. Angew. Chem. Int. Ed 53, 8668–8671 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Lee J-E & Yun J Catalytic asymmetric boration of acyclic α,β-unsaturated esters and nitriles. Angew. Chem. Int. Ed 47, 145–147 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Deutsch HM et al. Synthesis and pharmacology of site-specific cocaine abuse treatment agents: 2-(aminomethyl)-3-phenylbicyclo[2.2.2]- and –[2.2.1]alkane dopamine uptake inhibitors. J. Med. Chem 42, 882–895 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Janssen PA et al. In search of a novel anti-HIV drug: multidisciplinary coordination in the discovery of 4-[[4-[[4-[(1E)-2-cyanoethyenyl]-2,6-dimethylphenyl]amino]-2-pyrimidinyl]amino]benzonitrile (R278474, Rilpivirine). J. Med. Chem 48, 1901–1909 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Castellino S et al. Central nervous system disposition and metabolism of fosdevirine (GSK2248761), a non-nucleoside reverse transcriptase inhibitor: an LC-MS and matrix-assisted laser desorption/ionization imaging MS investigation into central nervous system toxicity. Chem. Res. Toxicol 26, 241–251 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Zhang L-H et al. The synthetic compound CC-5079 is a potent inhibitor of tubulin polymerization and tumor necrosis factor-a production with antitumor activity. Cancer Res 66, 951–959 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Ruchelman AL et al. 1,1-Diarylalkenes as anticancer agents: dual inhibitors of tubulin polymerization and phosphodiesterase 4. Bioorg. Med. Chem 19, 6356–6374 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Searle PA, Molinski TF, Brzezinski LJ & Leahy JW Absolute configuration of phorboxazoles A and B from the marine sponge Phorbs sp. 1. Macrolide and hemiketal rings. J. Am. Chem. Soc 118, 9422–9423 (1996). [Google Scholar]
- 15.Dalisay DS & Molinski TF Structure elucidation at the nanomole scale. 2. Hemi-phorboxazole A from Phorbas sp. Org. Lett. 11, 1967–1970 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Doyle MP, Buhro WE, Davidson JG, Elliott RC, Hoekstra JW & Oppenhuizen M Lewis acid promoted reactions of diazocarbonyl compounds. 3. Synthesis of oxazoles from nitriles through intermediate b-imidatoalkenediazonium salts. J. Org. Chem 45, 3657–3664 (1980). [Google Scholar]
- 17.Vedejs E, Piotrowski DW & Tucci FC Oxazolium-derived azomethine ylides. External oxazole activation and internal dipole trapping in the synthesis of aziridinomitosene. J. Org. Chem 65, 5498–5505 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Suganuma M et al. Calyculin A, an inhibitor of protein phosphatases, a potent tumor promoter on CD-1 mouse skin. Cancer Res 50, 3521–3525 (1990). [PubMed] [Google Scholar]
- 19.Jang H, Romiti F, Torker S & Hoveyda AH Catalytic diastereo- and enantioselective addition of versatile allyl groups to N-H ketimines. Nat. Chem 9, 1269–1275 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z & Liebeskind LS Palladium-catalyzed, copper(I)-mediated coupling of boronic acids and benzylthiocyanate. A cyanide-free cyanation of boronic acids. Org. Lett 8, 4331–4333 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powell KJ, Han L-C, Sharma P & Moses JE Chemoselective palladium-catalyzed cyanation of alkenyl halides. Org. Lett 16, 2158–2161 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Nakao Y, Yada A, Ebata S & Hiyama T A dramatic effect of Lewis-acid catalysts on nickel-catalyzed carbocyanation of alkynes. J. Am. Chem. Soc 129, 2428–2429 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Xie X, Liu Y Nickel-catalyzed highly regioselective hydrocyanation of terminal alkynes with Zn(CN)2 using water as the hydrogen source. J. Am. Chem. Soc 140, 7385–7389 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Qin C & Jiao N Iron-facilitated direct oxidative C–H transformation of allylarenes or alkenes to alkenyl nitriles. J. Am. Chem. Soc 132, 15893–15895 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Murai M, Hatano R, Kitabata S & Ohe K Gallium (III)-catalysed bromocyanation of alkynes: regio- and stereoselective synthesis of β-bromo-α,β-unsaturated nitriles. Chem. Commun 47, 2375–2377 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Wang Z & Chang S Copper-mediated transformation of organosilanes to nitriles with DMF and ammonium iodide. Org. Lett 15, 1990–1993 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Pradal A & Evano G A vinylic Rosenmund–von Braun reaction: practical synthesis of acrylonitriles. Chem. Commun 50, 11907–11910 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Gao D-W et al. Direct access to versatile electrophiles via catalytic oxidative cyanation of alkenes. J. Am. Chem. Soc 140, 8069–8073 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye F, Chen J & Ritter T Rh-catalyzed anti-Markovnikov hydrocyanation of terminal alkynes. J. Am. Chem. Soc 139, 7184–7187 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Zhang TY, O’Toole JC & Dunigan JM An efficient and practical synthesis of diphenyl cyanomethylenephosphonate: applications to the stereoselective synthesis of cis-α,β-unsaturated nitriles. Tetrahedron Lett 39, 1461–1464 (1998). [Google Scholar]
- 31.Fang F, Li Y & Tian S-K Stereoselective olefination of N-sulfonyl imines with stabilized phosphonium ylides for the synthesis of electron-deficient alkenes. Eur. J. Org. Chem 1084–1091 (2011).
- 32.Palomo C et al. A new version of the Peterson olefination using bis(trimethylsilyl)methyl derivatives and fluoride ion as catalyst. J. Org. Chem 55, 2498–2503 (1990). [Google Scholar]
- 33.Kojima S, Fukuzaki T, Yamakawa A & Murai Y Highly (Z)-selective synthesis of β-monosubstituted α,β-unsaturated cyanides using the Peterson reaction. Org. Lett 6, 3917–3920 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Chakraborty S, Das UK, Ben-David Y & Milstein D Manganese catalyzed α-olefination of nitriles by primary alcohols. J. Am. Chem. Soc 139, 11710–11713 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto Y, Asatani T & Kirai N Copper-catalyzed stereoselective hydroarylation of 3-aryl-2-propynenitrile with arylboronic acids. Adv. Synth. Catal 351, 1243–1249 (2009). [Google Scholar]
- 36.Barrado AG, Zielinski A, Goddard R & Alcarazo M Regio- and stereoselective chlorocyanation of alkynes. Angew. Chem. Int. Ed 56, 13401–13405 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Wang X & Studer A Metal-free direct C–H cyanation of alkenes. Angew. Chem. Int. Ed 57, 11792–11796 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han Y-P et al. Lewis acid mediated tandem reaction of propargylic alcohols with hydroxylamine hydrochloride to give α,β-unsaturated amides and alkenyl nitrile. J. Org. Chem 80, 9200–9207 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Su W, Gong T-J, Xiao B & Fu Y Rhodium(III)-catalyzed cyanation of vinylic C–H bonds: N-cyano-N-phenyl-p-toluensulfonamide as a cyanation reagent. Chem. Commun 51, 11848–11851 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Suginome M, Yamamoto A & Murakami M Palladium-catalyzed addition of cyanoboranes to alkynes: regio- and stereoselective synthesis of α,β-unsaturated β-boryl nitriles. Angew. Chem. Int. Ed 44, 2380–2382 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Crowe WE & Goldberg DR Acrylonitrile cross-metathesis: coaxing olefin metathesis reactivity from a reluctant substrate. J. Am. Chem. Soc 117, 5162–5163 (1995). [Google Scholar]
- 42.Randl S, Gessler S, Wakamatsu H & Blechert S Highly selective cross-metathesis with acrylonitrile using a phosphine free Ru-complex. Synlett 430–432 (2001).
- 43.Miao X, Dixneuf PH, Fischmeister C & Bruneau C A green route to nitrogen-containing groups: the acrylonitrile cross-metathesis and applications to plant oil derivatives. Green Chem 13, 2258–2271 (2011). [Google Scholar]
- 44.Gawin R et al. Cyclic alkyl amino ruthenium complexes – efficient catalysts for macrocyclization and acrylonitrile cross metathesis. ACS Catal 7, 5443–5449 (2017). [Google Scholar]
- 45.Michrowska A et al. Nitro-substituted Hoveyda–Grubbs ruthenium carbenes: enhancement of catalyst activity through electronic activation. J. Am. Chem. Soc 126, 9318–9325 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Bieniek M et al. Advanced fine-tuning of Grubbs/Hoveyda olefin metathesis catalysts: a further step toward an optimum balance between antinomic principles. J. Am. Chem. Soc 128, 13652–13653 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Bai C-X, Lu X-B, He R, Zhang W-Z & Feng X-J Lewis-acid assisted cross-metathesis of acrylonitrile with functionalized olefins catalysed by phosphine-free ruthenium carbene complex. Org. Biomol. Chem 3, 4139–4142 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Wiberg KB, Wang Y, Petersson GA & Bailey WF Intramolecular nonbonded attractive interactions: 1-substituted propenes. J. Chem. Theory Comput 5, 1033–1037 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Torker S, Koh MJ, Khan KM & Hoveyda AH Regarding a persisting puzzle in olefin metathesis with Ru complexes: why are transformations of alkenes with a small substituent Z-selective? Organometallics 35, 543–562 (2016). [Google Scholar]
- 50.Koh MJ, Nguyen TT, Zhang H, Schrock RR & Hoveyda AH Direct synthesis of Z-alkenyl halides through catalytic cross-metathesis. Nature 531, 459–465 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen TT, Koh MJ, Shen X, Romiti F, Schrock RR & Hoveyda AH Kinetically controlled E-selective catalytic olefin metathesis. Science 352, 569–575 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoveyda AH, Khan RKM, Torker S & Malcolmson SJ Catalyst-controlled stereoselective olefin metathesis in Handbook of Metathesis, Grubbs RH, Wenzel AG, O’Leary DJ, Khosravi E Eds (Wiley–VCH, Weinheim: ), 2014; pp. 503–562. [Google Scholar]
- 53.Xu C, Shen X & Hoveyda AH In situ methylene capping: a general strategy for efficient stereoretentive catalytic olefin metathesis. The concept, methodological implications, and applications to synthesis of biologically active compounds. J. Am. Chem. Soc 139, 10919–10928 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Ahmed TS, Grubbs RH Fast-initiating, ruthenium-based catalysts for improved activity in highly E-selective cross metathesis. J. Am. Chem. Soc 139, 1532–1537 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Ficken GE; Linstead RP, Stephen E & Whalley M Conjugated macrocycles. Part XXXI. Catalytic hydrogenation of tetraazaporphins, with a note on its stereochemical course. J. Chem. Soc 3879–3886 (1958).
- 56.Lam JK et al. Synthesis and evaluation of molybdenum and tungsten monoaryloxide halide alkylidene complexes for Z-selective cross-metathesis of cyclooctene and Z-1,2-dichloroethylene. J. Am. Chem. Soc 138, 15774–15783 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Nguyen TT, Koh MJ, Mann TJ, Schrock RR & Hoveyda AH Synthesis of E- and Z-trisubstituted alkenes by catalytic cross-metathesis. Nature 552, 347–354 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haribal M, Yang Z, Attygale AB, Renwick JAA & Meinwald J A cyanoallyl glucoside from Alliaria petiolata, as a feeding deterrent larvae of Pieris napi oleracea. J. Nat. Prod 64, 440–443 (2001). [DOI] [PubMed] [Google Scholar]
- 59.Olsen CE, MØller BL & Motawia MS Synthesis of the allelochemical alliarinoside present in garlic mustard (Alliaria petiolata), an invasive plant species in north America. Carbohydr. Res 394, 13–16 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Stockman RA, Sinclair A, Arini LG, Szeto P & Hughes DL A two-directional synthesis of (±)-Perhydrohistrionicotoxin. J. Org. Chem 69, 1598–1602 (2004). [DOI] [PubMed] [Google Scholar]
- 61.Walton JGA et al. Synthesis and evaluation of indatraline-based inhibitors of trypanothione reductase. ChemMedChem 6, 321–328 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.http://www.pipelinereview.com/index.php/2007041711056/Small-Molecules/EntreMed-Presents-Multi-Mechanism-Antitumor-Data-for-ENMD-1420-in-Preclinical-Models.html.
- 63.Pelter A, Smith K, Buss D & Norbury A Hindered organoboron groups in organic synthesis. 15. Preparation and properties of di(2,4,6-triisopropylphenyl)borane. Tetrahedron Lett 32, 6239–6242 (1991). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.