Abstract
Introduction.
Metabolomics is a chemical process, involving the characterization of metabolites and cellular metabolism. Recent studies indicate that numerous metabolic pathways are altered in bladder cancer (BLCA), providing potential targets for improved detection and possible therapeutic intervention. We review recent advances in metabolomics related to BLCA and identify various metabolites that may serve as potential biomarkers for BLCA.
Areas covered.
In this review, we describe the latest advances in defining the BLCA metabolome and discuss the possible clinical utility of metabolic alterations in BLCA tissues, serum, and urine. In addition, we focus on the metabolic alterations associated with tobacco smoke and racial disparity in BLCA.
Expert commentary.
Metabolomics is a powerful tool which can shed new light on BLCA development and behavior. Key metabolites may serve as possible markers of BLCA. However, prospective validation will be needed to incorporate these markers into clinical care.
Keywords: BLCA, LC-MS, Metabolomics, Smoke, Racial Disparity
Introduction
Bladder Cancer (BLCA) is the ninth most common cancer worldwide: with more than 330,000 new cases and more than 130,000 deaths annually [1]. Early diagnosis of BLCA can reduce mortality and improve quality of life. The standard for initial diagnosis of BLCA is cystoscopy and histopathological analysis of biopsy specimens. However, these procedures are invasive, uncomfortable, and expensive [2, 3]. Furthermore, cystoscopy may miss certain lesions, particularly small areas of carcinoma in situ [4, 5, 6]. Therefore, the development of new biomarkers for early detection and prognosis of BLCA would benefit patients. Recent studies have suggested that metabolomics can act as a tool to facilitate the identification of biomarkers for this purpose. Metabolism is defined as the study of chemical transformations within a cell or organism that help in sustaining life. The main functions of metabolism are the conversion of food/fuel to building blocks such as proteins, lipids, nucleic acids, and carbohydrates which are essential for cellular processes. The end products or the downstream intermediate low molecular weight products of metabolic pathways are termed metabolites. The complete set of metabolites present in a biological sample can be referred to as the metabolome of the particular biological sample. Metabolomics is the identification and quantification of all (unbiased metabolomics profiling) or specified (targeted metabolomics profiling) metabolites in a biological sample (e.g., blood, urine) under a specified condition or disease and identification of metabolic pathways and genes associated with the measured metabolites [7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24]. Metabolomic analysis is less complex compared to genomics, transcriptomics, and proteomics due to fewer endpoints. As of 2018, the total number of human metabolites identified so far is around 114,000 according to the online platform The Human Metabolome Database (HMDB) [25]. These metabolites give rise to ~ 25000 unique metabolic pathways [25] that reflect the phenotype of a cell or tissue, which could be the result of different genetic or environmental influences. Metabolomics research is built on a platform of biochemistry, which lays the foundation to define the metabolic pathways that represent the translation profile data, which may not be possible with other ‘omics’ approaches.
BLCA is known to be associated with many risk factors [26, 27, 28, 29, 30]. The incidence of BLCA is higher in smokers [31, 32, 33, 34], people who are working in the textile [35, 36], or rubber [37, 38, 39] industry, painters [40, 41, 42], machinists, and truckers who are exposed to harmful chemicals. Studies also reveal ethnicity-specific disparities in bladder cancer stage, grade, treatment and survival between European American and African American (AA) BLCA [43, 44]. The major reason for increased risk with the above occupations is the accumulation of carcinogens in the urine. The amassment of these chemicals in the bladder is one of the major causes of developing BLCA. These carcinogens can also alter the xenobiotic pathways responsible for their removal from the body. Metabolomics facilitates the identification of these alterations by quantifying the levels of metabolites or the associated xenobiotics, thus providing insight into BLCA development and progression.
1. Body
2.1. Analytical Techniques for the detection of metabolites
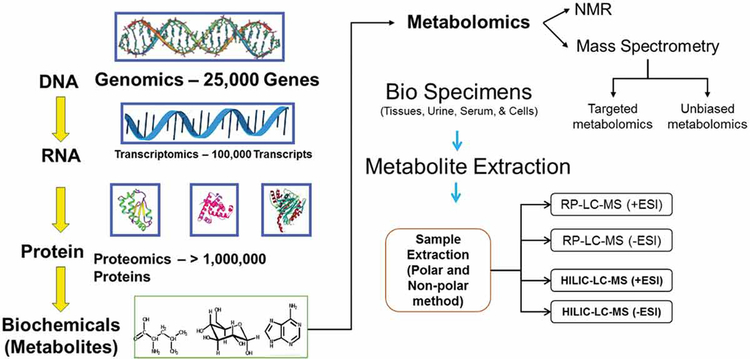
The separation and identification of metabolites in a biological specimen under specific conditions involves the implementation of chromatographic methods such as gas chromatography, high-performance liquid chromatography, or capillary electrophoresis for segregation of metabolites, followed by assessment using mass spectrometry (MS) or nuclear magnetic resonance (NMR) spectrometry [45, 46]. Both of these widely used methods have their strengths and limitations [45, 46, 47]. The major advantage of the MS-based methodology is its accuracy and specificity regarding metabolite detection. MS is more accurate compared to NMR spectrometry, but the analytes in a metabolome need to be separated for detection and assimilation [45, 46, 47]. In contrast, NMR based spectrometry is expensive and has lower sensitivity for metabolite detection, generally limited to less than 100 analytes in biological fluids, and does not entail segregation of analytes by chromatography or capillary electrophoresis for detection [45, 46, 47]. The major advantage of NMR is that samples are not destroyed and are reusable (Figure 1, Table 1).
Figure 1.
Overview of metabolomics workflow
Table 1:
Pros and Cons of commonly used mass-spectrometry techniques
| Technology | Advantage | Disadvantage |
|---|---|---|
| LC-MS | High sensitivity | Not quantitative |
| Simple sample preparation | Destructive | |
| Suitable to hydrophilic metabolites Eg: TCA, Glycolysis metabolites | Limited for the detection of hydrophilic metabolites | |
| High coverage of metabolites | ||
| Availability of open sources software for data analysis | ||
| Affordable Cost | ||
| Flexibility to play interplay with columns, mobile phase depends on interested metabolites | ||
| GC-MS | High coverage of metabolites | Not quantitative |
| Superior sensitivity | Destructive | |
| Suitable gaseous and volatile metabolites Eg: polyaromatic hydrocarbons, lipids | Not suitable for thermally sensitive molecules | |
| Availability of open sources software for data analysis | High variations due to conditions and instrument type | |
| Affordable Cost | ||
| NMR | Real-time monitoring and measurements | Low sensitivity |
| Structural information | Low metabolite coverage | |
| Simple/No sample preparation | High equipment cost and maintenance |
Detection and quantitation of the analytes using MS are dependent on the separation of analytes prior to mass analysis [45, 46, 47]. To isolate proteins from biological specimens or to separate small molecules or chemically diverse metabolites that are bound to the protein matrix, methanol is used prior to metabolomic analysis. Determination of different classes of metabolites requires the application of different solvents for extraction. Hence, aliquoted samples are prepared from the same biological specimen (for example plasma or urine samples from a cancer patient) and subjected to different extraction protocols to be analyzed under various chromatographic separations. Next, the analytes will be ionized for detection with the MS platform. Several ionization techniques have been described previously based on the chromatographic methodology (e.g., electron, cold or chemical ionization used in gas chromatography; electrospray ionization (ESI), atmospheric pressure chemical ionization used in liquid chromatography). Among these ionization techniques, ESI is the most widely used method.
The platform describing the best characteristics (i.e., least amount of nearby interference, ion suppression, etc.) is chosen for data analysis. The MS data is comprised of integrated peak areas that are quantified using the area under the receiver operating characteristic curves where the units are defined as ion counts. The metabolites are identified based on their retention times on liquid chromatography, and the mass-to-charge ratio (m/z) obtained on MS. Different normalization and transformation techniques are applied for transformation of obtained data to normal scaled data. Due to lower concentrations, the datasets may contain missing values that require imputation with the lowest detected metabolite value in the sample cohort.
2.2. Metabolic insights in BLCA
Diagnosis and prognosis of various diseases is enhanced by the identification of biomarkers which differentiate individuals with disease from healthy people. Ideal markers are easily detectable in tissue, serum, and urine and have high sensitivity and high specificity. Non-invasive markers are preferred to reduce morbidity of testing. Urine is ideal for testing BLCA since the cancer is in immediate proximity to urine. There are several potential applications of metabolomics in BLCA and other cancers including improving detection, providing prognostic information and impacting treatment. But there has been some limited research into the role of metabolomics in the detection of biomarkers specific for BLCA.
2.3. Metabolomic profiling in BLCA cell lines and tissues
BLCA has profound metabolic anomalies which play a central role in tumor progression [48]. Tissue metabolomic profiling provides in-depth insights into the steady state scenario of BLCA development. In-vitro cell culture models with BLCA cell lines provide a model for metabolic profiling that can mimic the conditions associated with BLCA carcinogenesis in patients. One of the earliest studies on metabolic profiling of normal and human BLCA cell lines revealed around 20 significantly differential metabolites between the two cell types [49]. Tissue-specific metabolic profiling in clinical specimens using high-throughput LC-MS identified 35 distinct metabolites specific to BLCA [50]. These metabolites are further validated in urine specimens showing a potential to improve diagnosis of BLCA and also in distinguishing between non-muscle invasive BLCA (NMIBC) from muscle-invasive BLCA (MIBC) [50], highlighting the alterations in xenobiotic metabolism (Table 2). This study provides the first clue that differences in xenobiotic metabolism can lead to the development of BLCA. Metabolic analysis in tissues by GC-MS and LC-MS-based approaches identified unique metabolic pathways that are altered in normal urothelium, non-muscle-invasive BLCA (NMIBC), and muscle-invasive BLCA (MIBC) [10]. Consistent with the Warburg effect, metabolic pathways such as the tricarboxylic acid (TCA) cycle, lipid synthesis, amino acid synthesis, nucleotide synthesis, glycolysis, and the sorbitol pathway are increased in cancer compared to adjacent benign tissue [10]. In addition, a stage wise comparison analysis revealed that cyclooxygenase (COX), lipoxygenase (LOX) signaling, heme catabolism, and nicotinamide adenine dinucleotide (NAD+) synthesis were enhanced in MIBC compared to NMIBC [10]. Consistent with these observations in amino acid metabolism, another study was able to quantify the levels of amino acids in BLCA tissues by application of targeted GC-MS analysis and observed significant changes in the levels of alanine, glutamate, glutamine, valine, isoleucine, leucine, phenylalanine, and tyrosine demonstrating alterations in amino acid metabolism [51]. Additionally, through metabolic approaches dysregulation of genetic pathways can also be identified. One such study on transitional carcinoma BLCA cell line T24 identified a distinct metabolic signature [52] in cells expressing abnormal Fanconi anemia (FA) pathway [53] which is involved in DNA repair [54]. Through LC-MS and GC-MS approaches, the authors were able to detect 206 metabolites and 72 differential metabolites between normal and abnormal FA pathway cells. The differential metabolites lead to the identification of canonical metabolomic pathways such as arginine biosynthesis, alanine biosynthesis, glutamate degradation, and renal cell carcinoma signaling, whichcan serve as prognostic tools in BLCA development [52].
Table 2.
Summary of included studies. All comparisons have determined altered metabolic pathways.
| Type of specimen | Metabolic pathways altered in BLCA | Metabolites altered | Reference |
|---|---|---|---|
| BLCA Tissue | TCA, Glycolysis, PPP, sorbitol pathway, Lipid metabolism, Nucleotide, amino acid metabolism, Eicosanoid, and arachidonic acid metabolism | Glucose, Glucose-6-phosphate, Fructose-6-phosphate, Fructose-1,6-biphosphate, Pyruvate, Lactate, Ribulose-5-phosphate, Sedoheptulosephosphate, Fumaricacid, Malic acid, Citrate, Succinate, Xanthine, Hypoxanthine, Adenine, Guanine, Xanthosine, Inosine, Adenosine −5- monophosphate,7-Methlguanine, Urate, Allontoin, Proline, Alanine, Asparagine, Leucine, Isoleucine, Valine, Glucosamine, N-acetyl glucosamine, N-acetyl glucosamine-6-phosphate, Glycerophopspholipids, Phosphosphingolipids. | [10] |
| BLCA Tissue | Lipid metabolism | Phosphatidyl serine, Phosphatidyl ethanolamine, Phosphocholines, Diacylglycerols. | [81] |
| BLCA Tissue | Tryptophan metabolism, Methionine pathway, Nicotinamide pathway, Amino acid metabolism, Purine metabolism, Nucleotide metabolism. | Tryptophan, S-Adenosyl homocysteine (SAH), Sarcosine, S- Adenosyl methionine (SAM), UDP-N-Acetyl glucosamine, Asparagine, Valine, Glycine- Leucine (Gly-Leu), N-acetyl lysine, Homoserine, Allo threonine, Proline, Nicotinamide, Glycerophosphocholine, N-acetyl glutamine, Methionine,4-Coumarate, Xanthine,Tyrosine,2-Aminobutyraldehyde,Thymine, Kynurenine. | [70] |
| BLCA Tissue | Amino acid metabolism, Glucose metabolism, Lipids and ketone bodies metabolism, | Tyrosine, Phenylalanine, Glucose, Citrate, Lactate, Very Low Density Lipoproteins (VLDL), Acetoacetate. | [65] |
| BLCA Tissue | Tryptophan metabolism, Methionine pathway, Amino acid metabolism | Serine, Asparagine, Valine, Tryptophan, Phenyl alanine, Histidine, 3-Hydroxykynurenine, 4-Hydroxyphenylacetic acid, 5-Hydroxyindoleacetic acid (5-HIAA), S- adenosyl methionine, Palmitic acid, Lauric acid, Oleic acid. | [50] |
| BLCA Serum | PPP, Nucleotide metabolism, Fatty acid metabolism, | Gluconic acid, 2-ketogluconic acid, Ribose, Xylitol, Arabitol, Maltose, D-cellobiose, Galactouronic acid, Myo-inositol, Glycolyic acid, Glyoxylic acid, Ethylene glycol, Lactose, D-mannitol, D-saccharic acid, D-Threitol, Erythritol, Ribonic acid, Cis- aconitic acid, Fumaric acid, Malic acid, Creatine, Hypo taurine, Kynurenine, Norleucine, Serine, N-acetyl serine, Hippuric acid, 2-Hg, 3-Indole propionic acid, 2,3,4-Trihydroxy butyric acid, Oleic acid, Hypoxanthine. | [67] |
| BLCA Serum | PPP, TCA, Glycolysis, Fatty acid metabolism. | S100A8, S100A9 (Proteomics analysis) | [66] |
| BLCA Urine | Glycolysis, TCA, Amino acid metabolism, Purine metabolism, PPP, Glutathione metabolism | D-Ribose, D-glucuronic acid, D-lyxose, Ribitol, Xylitol, Xylulose, D-cellobiose, D-rhamnose, L-fucose, D-allose, D-Sorbitol, 3-Phospho glyceric acid, Isocitric acid, Cis-aconitic acid, Succinic acid, 2-Hydroxyglutaric acid, 3-Hydroxypropionic acid, 5-hydroxyvaleric acid, Adenine, Inosine, Cholesterol, Lactic acid, 1, 3-Propanediol. | [12] |
| BLCA Urine | PPP, Lipid metabolism, TCA, Glycolysis, Amino acid metabolism | 3-Hydroxy butyrate, Lactate, 2-Hydroxybutyrate, Acetyl carnitine, Palmitoyl spingomyelin, Adipate, Gluconate, Betahydroxypyruvate, Phosphocholine, Isoleucine, Valine, Fructose, Pyridoxate, Succinate, Xanthurate, 2-Methylbutyrylglycine, Tyramine, Guandoacetate, Creatine. | [62] |
| BLCA Urine | TCA, Glycolysis, Fatty acid metabolism. | Succinate, Pyruvate, Oxoglutarate, Carnitine, Phosphoenolpyruvate, Trimethyllysine, Melatonin, Isovalerylcarnitine, Glutaryl carnitine, Octenoylcarnitne, Decanoyl carnitine, Acetyl coA. | [15] |
| BLCA Urine | Purine metabolism | Betaine, Leucine, Hypoxanthine, Histidine, Phenylalanine, Uric acid,1-Methyl histidine, N,N-dimethyl lysine, Tyrosine, Sorbitol, Mannitol, 3-Amino-2-napthoic acid, Acetylcarinitine, Tryptophan, Carnosine, Cystine, N-Acetyl tryptophan, Palmitic amide, Heptanoyl carnitine, Decanoyl carnitine, 6-Ketodecanoyl carnitine. | [17] |
| BLCA Urine | Tryptophan metabolism | Adipic acid, Anthranilic acid, Citrate, Coumaric acid derivative, Cyclopentane 1,2-diamine, Dihydroxy Acetone, Erythritol, Erythropentonic acid, Ethyltartarate, Ethylmalonic acid, Gluconic acid, Glycerol, Heptadecanoic acid, Hydroxybutyric acid, Itaconic acid, Lactic acid, Melibose, N-Acetyl anthranilic acid, P-Cresol, Pinene, Pseudouridine, Ribitol, Ribonic acid, Sebacic acid, Talonic acid, 2-Amino isobutyric acid. | [60] |
| BLCA Urine | TCA, Phenylalanine metabolism | Citrate, Phenylalanine, Taurine, Dimethylamine, Hippurate | [61] |
BLCA: Bladder Cancer; TCA: Tricarboxylic acid; PPP: Pentose Phosphate Pathway
2.4. Metabolomic profiling of BLCA patient urine samples.
The ability to indicate the presence, recurrence, and progression of a disease, as well as the appropriate treatment for a specific type of cancer, has been a major hub of research focus in recent years. Biomarkers present in liquid biopsies play a prominent role as they can record and monitor the real-time diseased state without invasive intervention. Liquid biopsy samples include urine, serum, plasma, saliva, cerebrospinal, and pleural fluids which allow the prognosis of the advanced disease. In BLCA, the most commonly used liquid biopsies are serum and urine [55]. Since cancers have altered metabolism, assessment of metabolites in biopsy specimens provides an initial review of the afflicted condition.
In BLCA, urine specimens are more widely used for surveillance and detection due to the intimate contact of the urine with the tumor. This characteristic feature of urine makes this body fluid an ideal platform for identification of metabolite biomarkers [56]. A pilot study with frozen urine from BLCA and healthy individuals using high-performance liquid chromatography showed that metabolomics platform has the potential to become an early detection method for diagnosis of BLCA [57]. Pasikanti et al. used a gas chromatography/time-of-flight mass spectrometry approach to perform metabolic profiling of BLCA patients and observed 100% sensitivity in BLCA detection in comparison to the traditional cytology approach that resulted in only in 33% sensitivity [58]. Similar studies revealed deregulation of the tryptophan-quinolonic metabolic axis, glycolysis, and beta-oxidation pathways in BLCA based on their metabolic profiling [15, 59]. The association of these metabolites with BLCA was corroborated by microarray results showing that carnitine acyl transferase and pyruvate dehydrogenase complex expressions are significantly altered in cancer [15]. Metabolites involved in tryptophan metabolism pathway such as kynurenine and tryptophan have been proposed as potential urinary candidate biomarkers and also as therapeutic targets for BLCA treatment [17, 60]. Srivastava et al., profiled the first-pass urine specimens from 33 BLCA, 33 benign, and 37 healthy controls using NMR platform and identified upregulation of taurine and downregulation of citrate and hippuric acid levels in BLCA specimens [61].
Wittmann et al. performed unbiased metabolomics on a set of urine samples from BLCA patients which revealed nearly 1000 distinct metabolic signatures of which 587 have a chemical identity. The authors chose a set of 25 potential biomarkers from this group and tested on a second independent cohort to validate their predictive power [62]. A new group of metabolites including lactate, adenosine, succinate, and palmitoyl sphingomyelin were proposed to have a role as urinary biomarkers, thus showing the involvement of lipid metabolism in BLCA progression [62]. As the field of metabolomics advanced, rapid methods were developed to identify urinary biomarkers. One such study utilized ultra performance liquid chromatography- high resolution mass spectrometry (UPLC-HRMS) method which is a 30minute method to detect and quantify nearly 9000 unique metabolites [63]. This range is four times higher than the previous studies which were able to report only 2000 metabolites [63] (Table 2).
Further studies on metabolomics profiling in non-muscle invasive bladder cancer (NMIBC) unraveled dysregulated metabolic pathways such as energy metabolism, anabolic metabolism, and redox states. These studies also confirmed the variability in tryptophan metabolism which was previously reported [7, 9, 12]. Urinary metabolomics shows a great clinical promise in the identification of biomarkers. However, the use of different platforms for metabolic profiling, food intake, and environmental exposures will strongly impact the composition of metabolome which alters the metabolic profiles obtained. These issues need to be taken into account to create an unequivocal metabolic profile that can serve as future biomarkers for estimating the prognostic state of BLCA.
2.5. Metabolomic profiling of BLCA patient serum
One of the first studies on serum metabolomic analysis focused on the classification of genitourinary cancers by using comprehensive hydrophilic interaction chromatography (HILIC) and reverse phase liquid chromatography (RPLC) in conjunction with LC-MS [64]. In this study, the authors found eicosatrienol, azaprostanoic acid, docosatrienol, retinol, and 14-apo-beta-carotenal as specific biomarkers for BLCA. The authors propose that these markers could differentiate between genitourinary cancers such as kidney cancer and BLCA [64]. A similar study based on (1)H nuclear magnetic resonance (NMR) metabolomics observed decreased levels of isoleucine/leucine, tyrosine, lactate, glycine, citrate, as well as increased levels of lipids and glucose (Table 2). The observations revealed disturbed metabolic pathways of aromatic amino acids, glycolysis and citrate cycle, as well as lipogenesis metabolism in BLCA patients [65]. Bansal et al., used (1)H NMR and Matrix Assisted Laser Desorption/Ionization-Time of Flight Mass Spectrometry approach to identify biomarkers that are shown to accurately distinguish 81% of BLCA cases compared to healthy controls [16, 66]. Further, the same group was able to put forward two additional biomarkers that could discriminate 92% of low-grade BLCA from high-grade BLCA [16, 66]. In high-grade BLCA plasma samples, using a pseudotargeted metabolomic method based on gas chromatography-mass spectrometry with selected ion monitoring (GC-MSSIM) revealed that metabolites specific to the pentose phosphate pathway (PPP), nucleotide, and fatty acid synthesis were found to be differential compared to healthy control [67]. In addition, evaluation of serum specimens in pre-operative and post-operative BLCA settings revealed aberrant downregulation of S10089, S100A9, S100A4, CA I and upregulation of Annexin V in post-operative BLCA. This genomic signature may distinguish BLCA based on the serum proteomics integration approach [68].
2.6. OMICS based integration approach to identify biomarkers
Friedrich-Carl von Rundstedt et al. used the OMICS based approach by integration of metabolite alterations with transcriptome data from TCGA (The Cancer Genome Atlas) [69] to identify the molecular signature of 30 metabolic genes [70]. In this type of approach, researchers utilize the publicly available BLCA transcriptomics databases such as TCGA [69], KIM [71], Lindgren [72], Sjodhal [73], and Riester [74] to understand the underlying mechanism of BLCA. Through integrative approaches, potential biomarkers identified with diagnostic, prognostic, or predictive value are proposed that may predict BLCA outcomes and improve early clinical diagnosis. With this approach, Rundstedt et al. conducted an LC-MS study and identified 31 differential metabolites. These were then used to identify 174 genes using the KEGG (Kyoto Encyclopedia of Genes and Genomes) database. By assimilating the metabolomic and transcriptomics signatures, they were able to develop a signature consisting of 30 genes that correlated with patient survival in the TCGA database [70].
2.7. Altered lipid metabolism in BLCA patient tissues and cell lines
Nearly all cancers share six common traits or hallmarks: (1) Stimulation of their own growth (i.e., self-sustainability in growth signals); (2) Development of resistance to inhibitory signals (Insensitivity to anti-growth signals); (3) Evasion of apoptotic signals; (4) Unlimited proliferative capacity; (5) Stimulation of blood vessels for continuous supply of nutrients to tumors (angiogenesis sustainability) (6) Invasion of local tissues and spreading to various organs (metastatic capabilities) [75, 76]. One of the major criteria for a tumor or cancer to grow and metastasize is the ability to synthesize new cells, which requires increased lipid synthesis. Lipid and phospholipid metabolism are known to play a key role in cell motility, cell invasion, and tumor metastasis which are trademarks of cancer cells. It was suggested that perturbations in phospholipid metabolism are associated with many cancers [77, 78, 79].
One possible lipid alteration is a change in citrate concentrations, which is found to be downregulated in urine and serum of BLCA patients [58, 60, 61, 65]. Tumor cells were observed to actively uptake nutrients from extracellular medium to synthesize citrate. The synthesized citrate is actively converted to acetyl-CoA that is utilized for fatty acid synthesis[80]. Similar to quantification and measurement of metabolites, lipids can also be measured using the mass spectrometry platform. Piyarathna et al. performed the first ever lipidomics study on pathologically confirmed BLCA tissues and identified alterations in different classes of lipids such as phosphocholines (PCs), phosphatidylethanolamines, plasmenyls PEs and triglycerides (TGs) between benign and BLCA [81]. Mapping of these altered lipids to corresponding genes and integrating with transcriptomics from TCGA resulted in a gene signature which is significantly associated with BLCA patient survival [81]. Two recent studies performed lipid profiling on BLCA cell lines and reported distinct cisplatin resistant alterations in lipidome [82, 83]. One of the studies used UPLC-MS platform and reported increased lipid production in a cisplatin-resistant cell line, indicating altered lipid metabolism can be taken into account for analyzing drug resistance [82].
Tracer studies using isotopically labeled compounds can be helpful in determining the source of lipid synthesis, and studies have demonstrated that glutamine or glucose mediated fatty acid synthesis plays a major role in cancer progression [84]. Lee et al. used an NMR based tracer metabolomics approach to report increased fatty acid production in cisplatin resistant BLCA cell lines [83] and this production utilizes glucose as the major carbon source. This study demonstrates a metabolomics approach can be utilized to pinpoint the perturbations induced in different metabolic pathways which are known to be involved in driving BLCA progression.
2.8. Effect of tobacco on BLCA metabolic pathways
BLCA has profound metabolic anomalies which play a central role in tumor progression [48]. Metabolomics helps in the understanding of relevant alterations of impaired metabolic processes in aggressive BLCA through the identification of smoke-specific metabolic biomarkers with potential diagnostic, prognostic or predictive value. BLCA smoker’s tissues were found to have 90 altered metabolites, which are completely different from nonsmokers and benign bladder tissues. Specifically, elevated levels of methylated metabolites, hexosamine biosynthetic pathway intermediates, acetylated metabolites, poly-cyclic aromatic hydrocarbons (PAH) and their aromatic counterparts and hydroxylated derivatives were reported compared to non-smokers. Further, intermediates of the methionine cycle, i.e., S-(5’-adenosyl)-L-methionine [51] and S-adenosyl-L-homocysteine [10] were significantly decreased and increased, respectively, in smokers compared to non-smokers with BLCA. Gene mapping of differential smoke specific metabolites by KEGG, reactome, and GOBP (Gene Ontology Biological Process) show the deregulation of DNA methylation, nicotine, glutathione, nucleotide metabolism, and methionine salvage pathway in smokers compared to nonsmokers. In addition to primary metabolites, a xenobiotic compound, aniline, which is known to be involved in BLCA [85, 86] was very high in smokers compared to non-smokers with BLCA. To identify potential metabolites to be used as urine biomarkers, we used urine specimens of both smokers and nonsmokers with BLCA to target the 90 tissue-specific metabolite signatures in smokers with BLCA, out of which 52 were detected in the urine samples. Out of the latter, 40 were differentially expressed in smokers and non-smokers and were further used to run individually as Receiver Operator Characteristic [86] curves and logistic regression models to identify risk prediction value of individual metabolites. A total of 23 metabolites (out of the 40 differential compounds) were found to have significant ROC [87] from which can be further delineated a set of highly specific metabolites that can be used as predictive/prognostic biomarkers to predict the risk of developing aggressive BLCA in smoker patients.
DNA adducts represent markers for exposure to aromatic compounds [88],[89]. Metabolic active carcinogens such as aminobiphenyl (ABP), methyl, NNK, BaP, hydroxy-hydroxymethyl propane 1,3-diyl (HMHP), and trihydroxybutyl (THB) adducts are found in higher levels in smokers than non-smokers with BLCA. Xenobiotic metabolism is often considered a major pathway associated with BLCA progression [50] and its associated enzymes N-acetyltransferase 2 (NAT2), cytochrome P450 1B1 (CYP1B1), aldehyde oxidase 1 (AOX1), and epoxide hydrolase 1 (EPHX1) were lower, whereas aromatic hydrocarbon receptor [90], glutathione S-transferase kappa 1 (GSTK1), glutathione S-transferase T1 (GSTT1), and ATP-binding cassette subfamily B member 1 (ABCB1) were higher in the tissues of smokers with BLCA.
2.9. Effect of smoking on DNA and epigenetic modification in BLCA
Genomic instability is a hallmark of all type of cancers[91]. In our study, smokers with BLCA, and BLCA cell lines treated with NNK and BaP showed increased expression of Ƴ-H2AX and check-point kinase 2 (Chk2) in comparison to non-smokers [87]. Hence, we postulate that smoking causes DNA damage when erroneous DNA repair causes mutations and chromosomal aberrations leading to activation of oncogenes and loss of tumor suppressor genes which in turn may cause more aggressive BLCA. NNK and BaP treatments in BLCA cell lines cause increased cell proliferation and upregulation of mesenchymal markers N- cadherin and vimentin. NNK and BaP treated cells also show higher levels of methylated nucleotides such as N-methylguanine, methyladenine, methyl adenosine, methyl cytosine, methyl guanosine, and methylcytidine. All of the above findings suggest that exposure to tobacco smoke leads to the formation of DNA adducts and damage which further leads to an aggressive form of BLCA. In addition, we have also found high turnover of the methionine cycle by observing low SAM levels and high SAH, homocysteine, and glutathione in NNK and BaP treated cells compared to control cells.
DNA methylation is the principal epigenetic mechanism to regulate many genes in eukaryotes [92]. DNA methyl transferases (DNMTs) transfer the methyl group from S-adenosyl methionine [51] to cytosine at CpG sites, which alters the chromatin structure and thereby regulates gene expression [93]. In our study, we found that BLCA smokers and BLCA cell lines treated with NNK and BaP also show very high DNMT1 expression compared to nonsmokers with BLCA which further supports the role of DNMT1 in BLCA development. DNMT1 inhibitors are currently being investigated as epigenetic therapeutics for the treatment of cancer [94], [95], and our findings further strengthen the evidence that DNMT inhibitors may serve as potential therapeutics for smokers with BLCA. The amino acid methionine is a principal source of a methyl group for the DNMT1 mediated epigenetic modification. Isotope-labeled methionine flux studies on DNMT1 knockdown BLCA cells treated with NNK, BaP show higher levels of methionine and SAM, lower levels of SAH, methylated metabolites, and DNA adducts which further confirms that tobacco smoke-induced DNMT1 plays a major role in inducing epigenetic changes that lead to more aggressive BLCA.
2.10. Racial disparity of BLCA
Cancer is a complex group of diseases that consist of anatomically distinct pathophysiologies and differ in their genesis and manifestation [96]. A large racial disparity in disease development and outcome has been observed in BLCA. Significant survival differences among race groups were found, and the lowest overall survival is observed in AA BLCA compared to EA BLCA [97]. Metabolic reprogramming plays a crucial role in tumor progression, invasion, and metastasis. Global metabolic profiles of paired tumor samples from liver, breast, and pancreas identified metabolites unique to each tissue and cancer type with significant differences observed in lipid and amino acid pathways [98]. With emerging statistics detailing the disparities in cancer prevalence among different races, particularly AA (the reasons for which are poorly defined) [99], it is critical to understand the underlying mechanisms for racial groups to best apply therapeutic interventions. A recent metabolic comparative study in serum from AA and EA BLCA identified differential metabolites and altered metabolic pathways. We have identified significant alterations in circulating hypoxanthine, uric acid, taurine, and the amino acids glutamine, serine, asparagine, valine, glycine, and lysine in AA compared to EA BLCA serum [100]. Pathways analyzed from altered metabolites revealed that one-carbon, tryptophan, and nucleotide metabolisms are significantly altered in AA BLCA patients. Further, the analysis of metabolites mapped genes in the TCGA cohort revealed potential disparity-driving genes for AA BLCA patients. Among those genes KDM2A, ME3, and P3H2 had proven to have a significant clinical correlation in AA BLCA patients [100].
2. Conclusion
Metabolomic evaluation of bladder cancer is in its early stages. This review systematically outlines the specific process of metabolomics and the use of metabolomics in BLCA studies in recent years. We have reviewed the bladder tumor tissue and biofluid (urine, plasma and serum) studies used for metabolomics analyses in BLCA. While there is a potential role in using metabolomics to improve detection and treatment of bladder cancer, most of the research is limited to small cohorts without validation. Improved understanding of metabolomics processes may help understand carcinogenesis and progression of BLCA. Furthermore, it can help elucidate processes by which carcinogens such as tobacco smoke result in bladder cancer and the differential outcomes of different racial groups. Based on the available studies, we have further described the aberrant metabolic pathways of BLCA and have suggested some metabolites that may be potential biomarkers for BLCA detection. The integrated study of metabolomic platforms of BLCA smokers vs. never smokers provided numerous novel insights into disease biology and delineated multiple potential opportunities for therapeutic intervention. In this metabolomics approach, there are still major challenges notably in data analysis that need to be overcome. Integration of metabolomics data with other data sets is also required. Based on this substantial data set, we discuss major current limitations in clinical data analysis to provide guidance to researchers in the field. Metabolomics presents a transformative new approach towards cancer biomarker discovery with high translational capacity to early cancer screening.
3. Expert opinion
A metabolomics focused approach to assist in the diagnosis of Bladder Cancer (BLCA) is promising. High sensitivity and specificity biomarkers should be investigated by a non-invasive method. Technological advances to date have allowed proof of principle studies with NMR and MS. This study is the first systematic review of the recent advances of metabolomic profiling as a diagnostic tool for BLCA. The main aim of this review was to summarize the use of urine, serum and tissue-based studies for defining the metabolome of BLCA. In this review, we have presented the methods of metabolite analysis. Currently, biofluids are analyzed using predominantly 2 analytic platforms: nuclear magnetic resonance (NMR) and mass spectrometry (MS). Metabolite analysis shows that BLCA tissue has profound metabolic anomalies which play a central role in tumor progression. The advantage of lipidomics profiling of BLCA was highlighted. Using a metabolomics approach, we have highlighted the signature associated with BLCA smokers. In particular, tobacco smoke carcinogens induced methylation, which plays a critical role in the accumulation of methylated metabolites with resultant DNA damage and altered metabolism in BLCA. We also highlighted the racial disparities in BLCA development and BLCA patient outcome. Metabolites whose expression levels have changed may be identified as potential biomarkers in BLCA patients. The essential hallmarks of cancer are associated with an altered cancer cell-intrinsic metabolism, either as a consequence or as a cause. Improvements in technology are likely to further advance this field. Using metabolomics as a screening tool for BLCA certainly warrants further instigation. Monitoring treatment and recurrence has not been investigated and should be pursued.
Article highlights.
Analytical methods used for BLCA metabolomics and their limitations are highlighted.
The metabolomic profiles of bladder cancer tissues, urine, and serum identify deregulated metabolites, which may improve detection and prognostication of disease.
Smoking which is a common risk factor for bladder cancer results in metabolic deregulations, which are responsible for bladder cancer development and progression.
Lipidomics is a rapidly emerging branch of metabolomics, which can be used to identify lipid species, which are by-products of altered lipid metabolism in BLCA tissues and cell lines.
Integration of OMICS offers the opportunity to expand the understanding of BLCA development and progression. Integration of metabolomics with transcriptomics from TCGA, KIM, Lindgren and Sjodhal identified potential biomarkers with diagnostic, prognostic, or predictive value which may predict BLCA outcomes and improve early clinical diagnosis
African American patients with bladder cancer have a higher mortality rate than European Americans. We attempted to understand metabolic differences behind racial disparity and key genes might be responsible for racial differences in survival.
Funding
This research was fully supported by the American Cancer Society (ACS) Award 127430-RSG-15–105–01-CNE (N.P.), NIH/NCI R01CA220297 (N.P.), and NIH/NCI R01CA216426 (N.P.), partially supported by the following grants: Agilent Technologies Center of Excellence (COE) in Mass Spectrometry at Baylor College of Medicine, Metabolomics Core, with funding from the NIH (P30 CA125123), CPRIT Proteomics and Metabolomics Core Facility (N.P.), (RP170005), and Dan L. Duncan Cancer Center.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Reference
Papers of special note have been highlighted as: * of interest ** of considerable interest
- 1.Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009. June;27(3):289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lotan Y, Svatek RS, Sagalowsky AI. Should we screen for bladder cancer in a high-risk population?: A cost per life-year saved analysis. Cancer. 2006. September 1;107(5):982–90. [DOI] [PubMed] [Google Scholar]
- 3.Vrooman OP, Witjes JA. Urinary markers in bladder cancer. Eur Urol. 2008. May;53(5):909–16. [DOI] [PubMed] [Google Scholar]
- 4.van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol. 2005. June;47(6):736–48. [DOI] [PubMed] [Google Scholar]
- 5.Han DS, Zhou W, Seigne JD, et al. Geographic Variation in Cystoscopy Rates for Suspected Bladder Cancer between Female and Male Medicare Beneficiaries. Urology. 2018. August 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daneshmand S, Bazargani ST, Bivalacqua TJ, et al. Blue light cystoscopy for the diagnosis of bladder cancer: Results from the US prospective multicenter registry. Urol Oncol. 2018. August;36(8):361 e1–361 e6. [DOI] [PubMed] [Google Scholar]
- 7.Cheng X, Liu X, Liu X, et al. Metabolomics of Non-muscle Invasive Bladder Cancer: Biomarkers for Early Detection of Bladder Cancer. Front Oncol. 2018;8:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yumba Mpanga A, Siluk D, Jacyna J, et al. Targeted metabolomics in bladder cancer: From analytical methods development and validation towards application to clinical samples. Anal Chim Acta. 2018. December 11;1037:188–199. [DOI] [PubMed] [Google Scholar]
- 9.Loras A, Trassierra M, Sanjuan-Herraez D, et al. Bladder cancer recurrence surveillance by urine metabolomics analysis. Sci Rep. 2018. June 15;8(1):9172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahu D, Lotan Y, Wittmann B, et al. Metabolomics analysis reveals distinct profiles of nonmuscle-invasive and muscle-invasive bladder cancer. Cancer Med. 2017. September;6(9):2106–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Extensive metabolomics profiling in non-muscle invasive (NMIBC) and muscle invasive bladder cancer (MIBC).
- 11.Shao CH, Chen CL, Lin JY, et al. Metabolite marker discovery for the detection of bladder cancer by comparative metabolomics. Oncotarget. 2017. June 13;8(24):38802–38810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Song R, Ma C, et al. Discovery and validation of potential urinary biomarkers for bladder cancer diagnosis using a pseudotargeted GC-MS metabolomics method. Oncotarget. 2017. March 28;8(13):20719–20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi H, Li X, Zhang Q, et al. Discovery of urine biomarkers for bladder cancer via global metabolomics. Biomarkers. 2016. November;21(7):578–88. [DOI] [PubMed] [Google Scholar]
- 14.Cheng Y, Yang X, Deng X, et al. Metabolomics in bladder cancer: a systematic review. Int J Clin Exp Med. 2015;8(7):11052–63. [PMC free article] [PubMed] [Google Scholar]
- 15.Jin X, Yun SJ, Jeong P, et al. Diagnosis of bladder cancer and prediction of survival by urinary metabolomics. Oncotarget. 2014. March 30;5(6):1635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bansal N, Gupta A, Mitash N, et al. Low- and high-grade bladder cancer determination via human serum-based metabolomics approach. J Proteome Res. 2013. December 6;12(12):5839–50. [DOI] [PubMed] [Google Scholar]
- 17.Alberice JV, Amaral AF, Armitage EG, et al. Searching for urine biomarkers of bladder cancer recurrence using a liquid chromatography-mass spectrometry and capillary electrophoresis-mass spectrometry metabolomics approach. J Chromatogr A. 2013. November 29;1318:163–70. [DOI] [PubMed] [Google Scholar]
- 18.Atala A Re: NMR-based metabolomics study of canine bladder cancer. J Urol. 2013. August;190(2):808. [DOI] [PubMed] [Google Scholar]
- 19.Wood DP. Re: metabolomics study on the biochemical profiles of odor elements in urine of human with bladder cancer. J Urol. 2013. April;189(4):1288. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Wei S, Liu L, et al. NMR-based metabolomics study of canine bladder cancer. Biochim Biophys Acta. 2012. November;1822(11):1807–14. [DOI] [PubMed] [Google Scholar]
- 21.Jobu K, Sun C, Yoshioka S, et al. Metabolomics study on the biochemical profiles of odor elements in urine of human with bladder cancer. Biol Pharm Bull. 2012;35(4):639–42. [DOI] [PubMed] [Google Scholar]
- 22.Hyndman ME, Mullins JK, Bivalacqua TJ. Metabolomics and bladder cancer. Urol Oncol. 2011. Sep-Oct;29(5):558–61. [DOI] [PubMed] [Google Scholar]
- 23.Bansal N, Gupta A, Sankhwar SN. Proteometabolomics of bladder cancer: current and future prospects. Cancer Biomark. 2015;15(4):339–48. [DOI] [PubMed] [Google Scholar]; * One of the first reviews of proteometabolomics of bladder cancer.
- 24.Chan EC, Pasikanti KK, Hong Y, et al. Metabonomic profiling of bladder cancer. J Proteome Res. 2015. February 6;14(2):587–602. [DOI] [PubMed] [Google Scholar]
- 25.Wishart DS, Feunang YD, Marcu A, et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2018. January 4;46(D1):D608–D617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huguet J, Monllau V, Sabate S, et al. Diagnosis, risk factors, and outcome of urethral recurrences following radical cystectomy for bladder cancer in 729 male patients. Eur Urol. 2008. April;53(4):785–92 discussion 792–3. [DOI] [PubMed] [Google Scholar]
- 27.Hayes RB, Friedell GH, Zahm SH, et al. Are the known bladder cancer risk-factors associated with more advanced bladder cancer? Cancer Causes Control. 1993. March;4(2):157–62. [DOI] [PubMed] [Google Scholar]
- 28.Schulte PA, Ringen K, Hemstreet GP, et al. Risk factors for bladder cancer in a cohort exposed to aromatic amines. Cancer. 1986. November 1;58(9):2156–62. [DOI] [PubMed] [Google Scholar]
- 29.Mommsen S, Aagaard J. Susceptibility in urinary bladder cancer: acetyltransferase phenotypes and related risk factors. Cancer Lett. 1986. August;32(2):199–205. [DOI] [PubMed] [Google Scholar]
- 30.Rebelakos A, Trichopoulos D, Tzonou A, et al. Tobacco smoking, coffee drinking, and occupation as risk factors for bladder cancer in Greece. J Natl Cancer Inst. 1985. September;75(3):455–61. [PubMed] [Google Scholar]
- 31.van Osch FHM, Jochems SHJ, Wesselius A, et al. A Stratified Meta-Analysis of the Association between Exposure to Environmental Tobacco Smoke during Childhood and Adulthood and Urothelial Bladder Cancer Risk. Int J Environ Res Public Health. 2018. March 22;15(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun X, Deng Q, Liang Z, et al. Cigarette smoke extract induces epithelial-mesenchymal transition of human bladder cancer T24 cells through activation of ERK1/2 pathway. Biomed Pharmacother. 2017. February;86:457–465. [DOI] [PubMed] [Google Scholar]
- 33.Tao L, Xiang YB, Wang R, et al. Environmental tobacco smoke in relation to bladder cancer risk--the Shanghai bladder cancer study [corrected]. Cancer Epidemiol Biomarkers Prev. 2010. December;19(12):3087–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alberg AJ, Kouzis A, Genkinger JM, et al. A prospective cohort study of bladder cancer risk in relation to active cigarette smoking and household exposure to secondhand cigarette smoke. Am J Epidemiol. 2007. March 15;165(6):660–6. [DOI] [PubMed] [Google Scholar]
- 35.Serra C, Kogevinas M, Silverman DT, et al. Work in the textile industry in Spain and bladder cancer. Occup Environ Med. 2008. August;65(8):552–9. [DOI] [PubMed] [Google Scholar]
- 36.Gonzales CA, Riboli E, Lopez-Abente G. Bladder cancer among workers in the textile industry: results of a Spanish case-control study. Am J Ind Med. 1988;14(6):673–80. [DOI] [PubMed] [Google Scholar]
- 37.Cavallo D, Casadio V, Bravaccini S, et al. Assessment of DNA damage and telomerase activity in exfoliated urinary cells as sensitive and noninvasive biomarkers for early diagnosis of bladder cancer in ex-workers of a rubber tyres industry. Biomed Res Int. 2014;2014:370907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Checkoway H, Smith AH, McMichael AJ, et al. A case-control study of bladder cancer in the United States rubber and tyre industry. Br J Ind Med. 1981. August;38(3):240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parkes HG. Screening for bladder cancer in the rubber industry. Proc R Soc Med. 1973. March;66(3):289. [PMC free article] [PubMed] [Google Scholar]
- 40.Vineis P Bladder cancer risk in painters. Occup Environ Med. 2010. August;67(8):505–6. [DOI] [PubMed] [Google Scholar]
- 41.Golka K, Weistenhofer W, Jedrusik P, et al. N-acetyltransferase 2 phenotype in painters with bladder cancer and controls. Ann Acad Med Singapore. 2001. September;30(5):464–7. [PubMed] [Google Scholar]
- 42.Golka K, Kempkes M, Flieger A, et al. Overrepresentation of the slow acetylator phenotype in painters suffering from urinary bladder cancer. Med Lav. 1997. Sep-Oct;88(5):425–6. [PubMed] [Google Scholar]
- 43.Lee CT, Dunn RL, Williams C, et al. Racial disparity in bladder cancer: trends in tumor presentation at diagnosis. J Urol. 2006. September;176(3):927–33; discussion 933–4. [DOI] [PubMed] [Google Scholar]
- 44.Gild P, Wankowicz SA, Sood A, et al. Racial disparity in quality of care and overall survival among black vs. white patients with muscle-invasive bladder cancer treated with radical cystectomy: A national cancer database analysis. Urol Oncol. 2018. October;36(10):469 e1–469 e11. [DOI] [PubMed] [Google Scholar]
- 45.Weiss RH, Kim K. Metabolomics in the study of kidney diseases. Nat Rev Nephrol. 2011. October 25;8(1):22–33. [DOI] [PubMed] [Google Scholar]
- 46.Emwas AH. The strengths and weaknesses of NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. Methods Mol Biol. 2015;1277:161–93. [DOI] [PubMed] [Google Scholar]
- 47.Kalim S, Rhee EP. An overview of renal metabolomics. Kidney Int. 2017. January;91(1):61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Massari F, Ciccarese C, Santoni M, et al. Metabolic phenotype of bladder cancer. Cancer Treat Rev. 2016. April;45:46–57. [DOI] [PubMed] [Google Scholar]
- 49.Pasikanti KK, Norasmara J, Cai S, et al. Metabolic footprinting of tumorigenic and nontumorigenic uroepithelial cells using two-dimensional gas chromatography time-of-flight mass spectrometry. Anal Bioanal Chem. 2010. October;398(3):1285–93. [DOI] [PubMed] [Google Scholar]
- 50.Putluri N, Shojaie A, Vasu VT, et al. Metabolomic profiling reveals potential markers and bioprocesses altered in bladder cancer progression. Cancer Res. 2011. December 15;71(24):7376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Earlier study of metabolomics profiling in bladder cancer.
- 51.Tripathi P, Somashekar BS, Ponnusamy M, et al. HR-MAS NMR tissue metabolomic signatures cross-validated by mass spectrometry distinguish bladder cancer from benign disease. J Proteome Res. 2013. July 5;12(7):3519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Panneerselvam J, Xie G, Che R, et al. Distinct Metabolic Signature of Human Bladder Cancer Cells Carrying an Impaired Fanconi Anemia Tumor-Suppressor Signaling Pathway. J Proteome Res. 2016. April 1;15(4):1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panneerselvam J, Park HK, Zhang J, et al. FAVL impairment of the Fanconi anemia pathway promotes the development of human bladder cancer. Cell Cycle. 2012. August 1;11(15):2947–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kottemann MC, Smogorzewska A. Fanconi anaemia and the repair of Watson and Crick DNA crosslinks. Nature. 2013. January 17;493(7432):356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lodewijk I, Duenas M, Rubio C, et al. Liquid Biopsy Biomarkers in Bladder Cancer: A Current Need for Patient Diagnosis and Monitoring. Int J Mol Sci. 2018. August 24;19(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bauca JM, Martinez-Morillo E, Diamandis EP. Peptidomics of urine and other biofluids for cancer diagnostics. Clin Chem. 2014. August;60(8):1052–61. [DOI] [PubMed] [Google Scholar]
- 57.Polec-Pawlak K, Abramski JK, Ferenc J, et al. Application of capillary electrophoresis-inductively coupled plasma mass spectrometry to comparative studying of the reactivity of antitumor ruthenium(III) complexes differing in the nature of counter-ion toward human serum proteins. J Chromatogr A. 2008. May 30;1192(2):323–6. [DOI] [PubMed] [Google Scholar]
- 58.Pasikanti KK, Esuvaranathan K, Ho PC, et al. Noninvasive urinary metabonomic diagnosis of human bladder cancer. J Proteome Res. 2010. June 4;9(6):2988–95. [DOI] [PubMed] [Google Scholar]
- 59.Lian W, Upadhyaya P, Rhodes CA, et al. Screening bicyclic peptide libraries for protein-protein interaction inhibitors: discovery of a tumor necrosis factor-alpha antagonist. J Am Chem Soc. 2013. August 14;135(32):11990–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pasikanti KK, Esuvaranathan K, Hong Y, et al. Urinary metabotyping of bladder cancer using two-dimensional gas chromatography time-of-flight mass spectrometry. J Proteome Res. 2013. September 6;12(9):3865–73. [DOI] [PubMed] [Google Scholar]
- 61.Srivastava S, Roy R, Singh S, et al. Taurine - a possible fingerprint biomarker in non-muscle invasive bladder cancer: A pilot study by 1H NMR spectroscopy. Cancer Biomark. 2010;6(1):11–20. [DOI] [PubMed] [Google Scholar]; * Used two different approaches to highlight the metabolic changes in bladder cancer.
- 62.Wittmann BM, Stirdivant SM, Mitchell MW, et al. Bladder cancer biomarker discovery using global metabolomic profiling of urine. PLoS One. 2014;9(12):e115870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shen C, Sun Z, Chen D, et al. Developing urinary metabolomic signatures as early bladder cancer diagnostic markers. OMICS. 2015. January;19(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin L, Huang Z, Gao Y, et al. LC-MS-based serum metabolic profiling for genitourinary cancer classification and cancer type-specific biomarker discovery. Proteomics. 2012. August;12(14):2238–46. [DOI] [PubMed] [Google Scholar]
- 65.Cao M, Zhao L, Chen H, et al. NMR-based metabolomic analysis of human bladder cancer. Anal Sci. 2012;28(5):451–6. [DOI] [PubMed] [Google Scholar]
- 66.Bansal N, Gupta A, Sankhwar SN, et al. Low- and high-grade bladder cancer appraisal via serum-based proteomics approach. Clin Chim Acta. 2014. September 25;436:97–103. [DOI] [PubMed] [Google Scholar]
- 67.Zhou Y, Song R, Zhang Z, et al. The development of plasma pseudotargeted GC-MS metabolic profiling and its application in bladder cancer. Anal Bioanal Chem. 2016. September;408(24):6741–9. [DOI] [PubMed] [Google Scholar]
- 68.Bansal N, Gupta AK, Gupta A, et al. Serum-based protein biomarkers of bladder cancer: A pre- and post-operative evaluation. J Pharm Biomed Anal. 2016. May 30;124:22–25. [DOI] [PubMed] [Google Scholar]
- 69.Cancer Genome Atlas Research N. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014. March 20;507(7492):315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.von Rundstedt FC, Rajapakshe K, Ma J, et al. Integrative Pathway Analysis of Metabolic Signature in Bladder Cancer: A Linkage to The Cancer Genome Atlas Project and Prediction of Survival. J Urol. 2016. June;195(6):1911–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim WJ, Kim EJ, Kim SK, et al. Predictive value of progression-related gene classifier in primary non-muscle invasive bladder cancer. Mol Cancer. 2010. January 8;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lindgren D, Sjodahl G, Lauss M, et al. Integrated genomic and gene expression profiling identifies two major genomic circuits in urothelial carcinoma. PLoS One. 2012;7(6):e38863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sjodahl G, Lauss M, Lovgren K, et al. A molecular taxonomy for urothelial carcinoma. Clin Cancer Res. 2012. June 15;18(12):3377–86. [DOI] [PubMed] [Google Scholar]
- 74.Riester M, Taylor JM, Feifer A, et al. Combination of a novel gene expression signature with a clinical nomogram improves the prediction of survival in high-risk bladder cancer. Clin Cancer Res. 2012. March 1;18(5):1323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000. January 7;100(1):57–70. [DOI] [PubMed] [Google Scholar]
- 76.Lazebnik Y What are the hallmarks of cancer? Nat Rev Cancer. 2010. April;10(4):232–3. [DOI] [PubMed] [Google Scholar]
- 77.Cummings BS. Phospholipase A2 as targets for anti-cancer drugs. Biochem Pharmacol. 2007. October 1;74(7):949–59. [DOI] [PubMed] [Google Scholar]
- 78.Inazu M Choline transporter-like proteins CTLs/SLC44 family as a novel molecular target for cancer therapy. Biopharm Drug Dispos. 2014. November;35(8):431–49. [DOI] [PubMed] [Google Scholar]
- 79.Smith EL, Schuchman EH. The unexpected role of acid sphingomyelinase in cell death and the pathophysiology of common diseases. FASEB J. 2008. October;22(10):3419–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mycielska ME, Patel A, Rizaner N, et al. Citrate transport and metabolism in mammalian cells: prostate epithelial cells and prostate cancer. Bioessays. 2009. January;31(1):10–20. [DOI] [PubMed] [Google Scholar]
- 81.Piyarathna DWB, Rajendiran TM, Putluri V, et al. Distinct Lipidomic Landscapes Associated with Clinical Stages of Urothelial Cancer of the Bladder. Eur Urol Focus. 2017. April 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee MY, Yeon A, Shahid M, et al. Reprogrammed lipid metabolism in bladder cancer with cisplatin resistance. Oncotarget. 2018. March 2;9(17):13231–13243. [DOI] [PMC free article] [PubMed] [Google Scholar]; (*)
- 83.Wen H, Lee S, Zhu WG, et al. Glucose-derived acetate and ACSS2 as key players in cisplatin resistance in bladder cancer. Biochim Biophys Acta Mol Cell Biol Lipids. 2018. June 5. [DOI] [PubMed] [Google Scholar]; (*) * Studies showing distinct metabolomic profiles in drug resistant Bladder cancer cell lines.
- 84.Schug ZT, Vande Voorde J, Gottlieb E. The metabolic fate of acetate in cancer. Nat Rev Cancer. 2016. November;16(11):708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Excellent review on cancer metabolism
- 85.Shapiro SG, Knapp DW, Breen M. A cultured approach to canine urothelial carcinoma: molecular characterization of five cell lines. Canine Genet Epidemiol. 2015;2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brockmoller J, Kaiser R, Kerb R, et al. Polymorphic enzymes of xenobiotic metabolism as modulators of acquired P53 mutations in bladder cancer. Pharmacogenetics. 1996. December;6(6):535–45. [DOI] [PubMed] [Google Scholar]
- 87.Jin F, Thaiparambil J, Donepudi SR, et al. Tobacco-Specific Carcinogens Induce Hypermethylation, DNA Adducts, and DNA Damage in Bladder Cancer. Cancer Prev Res (Phila). 2017. October;10(10):588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Besaratinia A, Maas LM, Brouwer EM, et al. Comparison between smoking-related DNA adduct analysis in induced sputum and peripheral blood lymphocytes. Carcinogenesis. 2000. July;21(7):1335–40. [DOI] [PubMed] [Google Scholar]
- 89.Veglia F, Matullo G, Vineis P. Bulky DNA adducts and risk of cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2003. February;12(2):157–60. [PubMed] [Google Scholar]
- 90.Jensen OM, Wahrendorf J, Knudsen JB, et al. The Copenhagen case-referent study on bladder cancer. Risks among drivers, painters and certain other occupations. Scand J Work Environ Health. 1987. April;13(2):129–34. [DOI] [PubMed] [Google Scholar]
- 91.O’Connor MJ. Targeting the DNA Damage Response in Cancer. Mol Cell. 2015. November 19;60(4):547–60. [DOI] [PubMed] [Google Scholar]
- 92.Bird AP. CpG-rich islands and the function of DNA methylation. Nature. 1986. May 15–21;321(6067):209–13. [DOI] [PubMed] [Google Scholar]
- 93.Dhe-Paganon S, Syeda F, Park L. DNA methyl transferase 1: regulatory mechanisms and implications in health and disease. Int J Biochem Mol Biol. 2011;2(1):58–66. [PMC free article] [PubMed] [Google Scholar]
- 94.Jung Y, Park J, Kim TY, et al. Potential advantages of DNA methyltransferase 1 (DNMT1)-targeted inhibition for cancer therapy. J Mol Med (Berl). 2007. October;85(10):1137–48. [DOI] [PubMed] [Google Scholar]
- 95.Singh V, Sharma P, Capalash N. DNA methyltransferase-1 inhibitors as epigenetic therapy for cancer. Curr Cancer Drug Targets. 2013. May;13(4):379–99. [DOI] [PubMed] [Google Scholar]
- 96.Alizadeh AA, Aranda V, Bardelli A, et al. Toward understanding and exploiting tumor heterogeneity. Nat Med. 2015. August;21(8):846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mallin K, David KA, Carroll PR, et al. Transitional cell carcinoma of the bladder: racial and gender disparities in survival (1993 to 2002), stage and grade (1993 to 2007). J Urol. 2011. May;185(5):1631–6. [DOI] [PubMed] [Google Scholar]
- 98.Budhu A, Terunuma A, Zhang G, et al. Metabolic profiles are principally different between cancers of the liver, pancreas and breast. Int J Biol Sci. 2014;10(9):966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006. January 26;354(4):333–42. [DOI] [PubMed] [Google Scholar]
- 100.Vantaku V, Donepudi SR, Piyarathna DWB, et al. Large-scale profiling of serum metabolites in African American and European American patients with bladder cancer reveals metabolic pathways associated with patient survival. Cancer. 2019. January 2. [DOI] [PMC free article] [PubMed] [Google Scholar]; * First study on ethinic based metabolomic profiling in bladder cancer serum specimens.