Abstract
Metabolic alterations are established as a hallmark of cancer. Such hallmark changes in cancer metabolism are characterized by reprogramming of energy-producing pathways and increases in the generation of biosynthetic intermediates to meet the needs of rapidly proliferating tumor cells. Various metabolic phenotypes such as aerobic glycolysis, increased glutamine consumption, and lipolysis have also been associated with the process of metastasis. However, in addition to the energy and biosynthetic alterations, a number of secondary functions of enzymes and metabolites are emerging that specifically contribute to metastasis. Here, we describe atypical intracellular roles of metabolic enzymes, extracellular functions of metabolic enzymes, roles of metabolites as signaling molecules, and epigenetic regulation mediated by altered metabolism, all of which can affect metastatic progression. We highlight how some of these mechanisms are already being exploited for therapeutic purposes, and discuss how others show similar potential.
Keywords: epigenetic, mutant isocitrate dehydrogenase, phosphohexose isomerase, glutaminase, ATP citrate lyase
Introduction
Despite recent exciting developments in cancer treatments, it is evident that metastatic disease is still a fundamental barrier to improved outcomes for the majority of patients. Indeed, metastatic disease is the main cause of cancer deaths. Metastatic progression is a multi-step process, that recent work suggest may actually begin early in cancer development, although this is still being investigated [1]. In order to establish metastatic colonies, cancer cells must alter themselves in a number of ways, including acquisition of a motile phenotype; transition from an epithelial to mesenchymal phenotype; acquisition of ability to enter, survive in, and exit the vasculature; and development of mechanisms to enable survival and ultimately outgrowth at distant sites. Cellular metabolism underlies many of those alterations and dysregulation of energy metabolism has been well established as a hallmark of cancer biology [2]. For example, the Warburg-effect, or the observation that highly proliferative cells such as tumor cells are largely dependent on glycolysis to meet their energetic needs even in the presence of oxygen, is frequently associated with cancer development. Outside of the bioenergetic consequences of altered metabolism however, recent research has established non-canonical functions of metabolic enzymes and metabolites that contribute to cancer progression. Understanding these novel pathways could reveal innovative ways to specifically target the metastatic process. Here we describe evidence for novel mechanisms that link metabolic alterations in cancer and metastatic progression, and highlight some potential therapeutic strategies that can arise as a result.
Atypical Intracellular Roles of Metabolic Enzymes in Metastasis
Metabolic alterations observed in cancer are often accompanied by dysregulation of the expression of metabolic enzymes. Secondary functions of these metabolic enzymes have been shown to contribute to metastatic progression through altering the signaling and genetic landscape of cancer cells. Glycolysis is a basic process that links glucose uptake with the initial steps of energy production as well as biosynthesis. However, several glycolytic enzymes also have a number of effects outside of their classical enzymatic activity. For example, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) can form a complex with the transcription factor Sp1 that binds to the SNAIL minimal promoter to drive its expression [3]. The expression of SNAIL contributes to metastasis through induction of epithelial to mesenchymal transition (EMT) and a more stem-like phenotype. EMT is an important process in metastasis that can cause epithelial cells to lose E-cadherin mediated cell-cell adhesion and gain an invasive phenotype allowing them to move away from the primary tumor into surrounding stroma and potentially into the vasculature. Suppression of GAPDH resulted in loss of stem cell markers that was linked to decreased tumor-forming ability in a colorectal cancer model [3]. Another example is Pyruvate kinase M2 (PKM2), which is an isoform of the enzyme that catalyzes the conversion of phosphoenolpyruvate to pyruvate. This isoform is frequently upregulated in cancers and has many pro-tumorigenic roles [4]. One way that PKM2 can contribute to metastatic progression is through EMT. PKM2 is able to translocate to the nucleus and form complexes with TGIF2, a repressor of Transforming Growth Factor (TGF)-β signaling, and histone deacetylase 3 (HDAC3) [5]. The PKM2 nuclear complex was shown to bind to the CDH1 promoter and deacetylate it leading to repression of E-cadherin expression.
Although not strictly acting non-canonically, an atypical role of glycolysis important for invasive phenotypes relates to the finding of glycolytic enzymes in abundance in invadopodia [6]. Glycolysis appears to be the primary energetic pathway for cytoskeleton remodeling in several breast and prostate cancer models [7]. When glycolysis was inhibited through treatment with 2-deoxy-D-glucose, there was a decrease in focal adhesions and motility in PC3 prostate cancer cells. In contrast, inhibition of oxidative phosphorylation with oligomycin had no effect on the motility of prostate cancer cell lines. In addition, the association of glycolytic enzymes with the cytoskeleton is important for the viability of cancer cells [8–10]. Treatment of Lewis lung carcinoma, colon carcinoma, or breast cancer cells with clotrimazole, a calmodulin antagonist, resulted in decreased cell viability [9]. The decrease in cell viability was preceded by dissociation of glycolytic enzymes from the cytoskeleton, resulting in a reduction of local adenosine triphosphate (ATP) supply to the cytoskeleton and subsequent altered morphology. Citric acid cycle enzymes are also associated with increased metastasis. Enhanced expression of ATP citrate lyase, which catalyzes the conversion of citrate to acetyl-CoA and oxaloacetate, is linked to increased lipogenesis. Normally, the majority of lipids used for cellular functions including lipid membranes are obtained from the diet or produced in the liver. The biosynthesis requirements of rapidly proliferating cancer cells can result in alternative mechanisms including generation of acetyl-CoA through the activity of ATP citrate lyase. The acetyl-CoA is then a substrate for fatty acid synthase, ultimately leading to membrane lipid production. Inhibition of fatty acid synthase and ATP citrate lyase slowed tumor growth and inhibited metastasis in non-small cell lung cancer, cervical cancer, and prostate cancer [11–13]. Concordantly, expression of microRNA 22 (miR-22), which inhibits ATP citrate lyase expression, is downregulated in a number of cancers [14,15]. The ectopic expression of miR-22 was shown to decrease de novo lipogenesis and metastatic ability in breast, lung, osteosarcoma, cervical, and prostate cancer [14]. ATP citrate lyase is a prospective therapeutic target and there are several novel inhibitors under investigation [12,16].
Dysregulation of succinate dehydrogenase activity, which normally catalyzes the conversion of succinate to fumarate, is associated with a number of cancers including pheochromocytoma, renal cell carcinoma, and paragangliomas [17–19]. Decreased succinate dehydrogenase activity leads to accumulation of succinate which inhibits prolyl-hydroxylase (PDH) [20]. This inhibition of PDH stabilizes hypoxia-inducible factor 1-alpha (HIF-1α), thus activating pro-angiogenic HIF-1α signaling. Additionally, succinate dehydrogenase 5 (SDH5) has been shown to regulate glycogen synthase kinase (GSK)-3β signaling in lung cancer [21]. SDH5 forms complexes with GSK-3β, and PP2A, a phosphatase that regulates activity of GSK- 3β. Loss of SDH5 results in increased β-catenin signaling and subsequent EMT in lung cancer. Evidence also suggests that genetic ablation of succinate dehydrogenase subunit b (SDHB), increases TGF-β signaling and activates a complex of the transcription factors SNAIL and SMAD3/4 leading to a metastatic phenotype in colorectal cancer cell lines [22]. Indeed, lack of SDHB expression is associated with invasive and metastatic disease in colorectal patient samples.
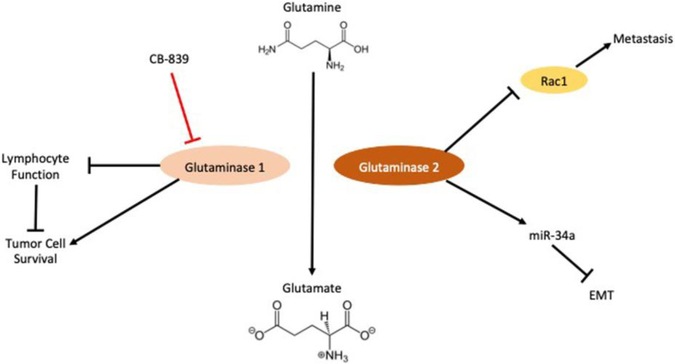
Glutamine addiction is another emerging metabolic hallmark of cancer cells [23]. The glutamine hydrolyzing enzyme, glutaminase has multiple isoforms that have differing effects on disease progression in cancer [Figure 1]. Increased expression of glutaminase 1 in triple- negative breast cancer is associated with poor disease-free survival, and decreased tumor infiltrating leukocytes [24]. The enhanced uptake and utilization of glutamine by the tumor cells results in decreased availability of this carbon source in the tumor microenvironment. The lack of environmental glutamine, which is important for lymphocyte function [25,26], may explain the decrease of tumor infiltrating lymphocytes and poor prognosis associated with glutaminase expression in triple negative breast cancer. Glutaminase 2, the liver isoform of glutaminase, appears to have an opposing role to glutaminase 1, as it is able to inhibit metastasis through protein binding instead of its classical catalytic functions. Glutaminase 2 was shown to bind the small GTPase Rac1, a pleiotropic regulator of multiple cellular processes [27]. The binding of Rac1 by glutaminase 2 blocks interactions with guanine exchange factors resulting in Rac1 inhibition. Glutaminase 2 is also known to stabilize Dicer, which results in the maturation of miR- 34a [28]. MiR-34a can repress the EMT transcription factor SNAIL and inhibit metastasis in hepatocellular carcinoma.
Figure 1. Isoforms of glutaminase have opposing roles in cancer metastasis.
Glutaminase 1 increases tumor cell survival via its canonical catalytic activity. Opposingly glutaminase 2 inhibits tumor metastasis and EMT via its secondary functions as a binding protein (Details in text). EMT (Epithelial-Mesenchymal Transition); Rac1 (Ras-related C3 botulinum toxin substrate 1)
Enzymes associated with nucleotide metabolism are also able to affect metastatic progression. Guanosine 5’-monophosphate synthase (GMPS) was shown to regulate p53 function through altering deubiquitylation complex [29]. GMPS is an enzyme normally involved in de novo purine biosynthesis, and is usually sequestered in the cytosol by TRIM21. A complex between USP7, MDM2, and p53 is formed in the nucleus that results in the ubiquitylation and degradation of p53. However, upon genotoxic stress GMPS is imported into the nucleus. When in the nucleus GMPS replaces MDM2 in the complex, and induces USP7 mediated deubiquitylation and stabilization of p53, resulting in increased transcription of p53 target genes. Loss of normal p53 function has been associated with metastasis [30]. Understanding how to target enzymes such as TRIM21 to promote this secondary function of GMPS and thus induce p53 activity has potential as a therapy for metastasis.
Extracellular Roles of Metabolic Enzymes in Metastasis
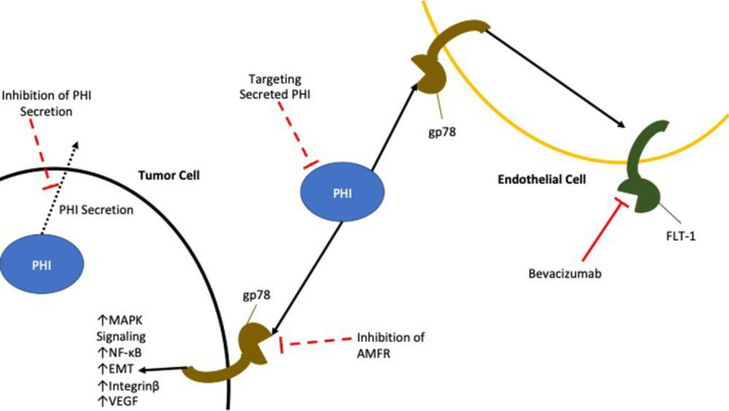
A number of metabolic enzymes can actually be secreted and drive cancer progression through alternative roles as signaling molecules. The most studied example of a secreted metabolic enzyme acting as a signaling molecule is phosphohexose isomerase (PHI), also known as autocrine motility factor (AMF), neuroleukin, or maturation factor. The canonical role of PHI is early in glycolysis where it catalyzes the conversion of glucose-5-phosphate to fructose-6-phosphate. The expression of PHI is under the control of HIF-1α, and phosphoinositide 3-kinase (PI3K) [31,32]. When secreted from cells, PHI exhibits functions outside of its normal enzymatic role by binding and signaling through its cognate receptor gp78 [33] [Figure 2]. PHI expression enhances metastasis in pancreatic and colorectal cancer [34,35]. One of the ways that PHI contributes to metastasis is through induction of EMT. Ectopic expression of PHI is sufficient to drive EMT in a number of cancers including breast cancer [36] and endometrial cancer via mitogen-activated protein kinase (MAPK) signaling [37]. PHI can also signal through NF-κB resulting in increased expression of the mesenchymal transcription factors ZEB1, and ZEB2 in addition to decreasing expression of miR-200, a microRNA that represses expression of ZEB1 and ZEB2 [38].
Figure 2. Secreted PHI has autocrine and paracrine signaling roles.
PHI drives pro-tumorigenic MAPK signaling, EMT, and secretion of angiogenic factors. PHI sensitizes endothelial cells to angiogenic signaling by increasing expression of VEGF receptors. PHI (Phosphohexose Isomerase); MAPK (Mitogen-activated protein kinase); EMT (Epithelial-Mesenchymal Transition); AMFR (Autocrine motility factor receptor); VEGF (Vascular Endothelial Growth Factor)
Expression of PHI was shown to increase motility of cancer cells by regulating expression of microtubule associated proteins such as kinesin-like protein KIF3A [39]. PHI also plays a role in cytoskeletal dynamics by modulating expression of Rho GTPases and Rac1 [40]. Expression of PHI in melanoma cells leads to the formation of stress fibers that are importance for cell migration. Additionally, PHI can increase the invasiveness of hepatoma cells by increasing expression of integrin β1, which is important for cellular adhesion to extracellular matrix, and secretion of MMP2, which plays a role in degrading the surrounding extracellular matrix and allowing tumor cells to invade [41]. In all these cases, PHI is thought to act in an autocrine manner, binding to the same cells from which it is secreted. Outside of intrinsic cancer cell signaling PHI has also demonstrated paracrine activity. Expression of the PHI receptor, gp78, was reporting in normal endothelial cells [42]. PHI secreted by tumor cells signaled in an autocrine manner to increase expression of vascular endothelial growth factor (VEGF) in cancer cells. Simultaneously, PHI acted in a paracrine manner on endothelial cells in order to increase expression of VEGF receptor FLT-1 and endothelial cell motility. Together these events increased the permeability of endothelial vessels and contributed to formation of ascites in a mouse mode l[43].
A number of other metabolic enzymes may contribute to cancer progression upon their secretion. Secreted phospholipase A2 (sPLA2) has been shown to have differing roles dependent on its localization. When expressed intracellularly, sPLA2 can inhibit Wnt signaling through activation of Yap in intestinal tissue [44]. Upon inflammation however sPLA2 is secreted into the lumen where it increases Wnt signaling, and prostaglandin E2 synthesis via the sPLA2 receptor Plar2r1 which is associated with increased susceptibility to colon cancer [44]. Increased Wnt signaling is also associated with metastasis, and EMT in cancer [45]. In addition, sPLA2 has been shown to confer protection against lipotoxic stress, and nutrient deprivation in breast cancer cell lines [46]. Peroxredoxin 4 (PRDX4), the only secreted member of a family of peroxidase enzymes, was shown to induce osteoclastogenesis in a RANKL independent manner [47]. The secretion of PRDX4 led to increased ERK, and calcium/NFATc1 signaling which is mediated by the IgG like receptors OSCAR and TREM-2. Genetic ablation of PRDX4 expression led to decreased oseteoclastogenesis in vitro, and decreased osteolytic lesions in mice in the setting of breast-prostate-to-bone metastasis. Changes in metabolism commonly observed in cancer often result in the accumulation of metabolites. These metabolites can act as intracellular or extracellular signaling molecules that have multiple effects, which are sometimes contrary to each other. For example, incubation of metastatic prostate cancer cell lines with citrate has been shown to enhance motility and invasion, as well as inhibit cell adhesion [11]. Moreover, in lung adenocarcinoma and squamous cell lung cancer, expression of SLC25A1, a transporter responsible for transporting citrate out of the mitochondria into the cytosol, could drive cancer cells to a stem-cell like phenotype, and increase colony formation [48]. In contrast, there is some evidence that treatment with citrate can slow tumor growth in a number of tumor models including breast, lung and pancreatic cancer [49]. The treatment with citrate was shown to inhibit glycolysis, and insulin-like growth factor 1 receptor phosphorylation. This corresponds with evidence of decreased citrate levels being a biomarkers in prostate cancer[50].
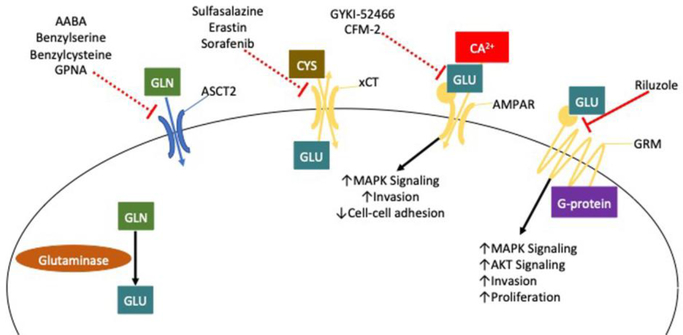
Glutamine is imported into cells through various transporters, including ASCT2 (also known as SLC1A5) [51] [Figure 3]. Blocking glutamine uptake by ablation of ASCT2 expression causes decreased proliferation, and activation of mTORC1 signaling in prostate cancer cells [52], as well as decreased migration in osteosarcoma, and triple-negative breast cancer [53]. Pharmacological blockade of ASCT2 mediated glutamine uptake by GPNA and benzylserine was shown to have anti-tumor effects in endometrial carcinoma [54]. A novel class of 2-amino-4-bis(aryloxybenzyl)amino butanoic acid (AABA) derived drugs designed to target ASCT2, such as V-9302, have been demonstrated to decrease proliferation, and increase cell death, and oxidative stress [55]. Recent evidence however, suggests that V-9302 may instead block glutamine uptake mediated by redundant glutamine transporters that show increased expression in some cancers such as SNAT2 [56]. In melanoma cells, glutamine can inhibit platelet-activating factor-induced MAPK signaling [57], resulting in decreased metastasis and angiogenesis downstream of platelet-activating factor signaling.
Figure 3. Glutamate is a pro-metastatic signaling molecule.
Glutamate produced by the hydrolyzation of glutamine is able to be exported from tumor cells via transporters like xCT. This secreted glutamate is able to drive pro-tumorigenic signaling by binding to ionotropic and metabotropic glutamate receptors AMPAR, and GRM. GRM (Metabotropic Glutamate Receptor); AMPAR (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor); GLN (Glutamine); GLU (Glutamate); MAPK (Mitogen-activated protein kinase); AKT (Protein kinase B)
The first step in the catabolism of glutamine is the conversion from glutamine to glutamate. Glutamate can also play an important role in regulating the metastasis of cancer by acting as a signaling molecule [Figure 3]. Disruption of the glutamate-cysteine antiporter xCT (also known as SLC7A11) leading to the retention of cellular glutamate and reduction of cysteine consumption, results in decreased proliferation, and decreased invasion in non-small cell lung cancer [58]. In addition, inhibition of xCT leads to decreased viability in glucose deprived states [59]. When xCT is functional and glutamate is exported, glutamate can signal through multiple types of receptors. The first class of glutamate receptors are metabotropic glutamate receptors [60]. G protein-coupled receptors that are able to activate multiple pro-tumorigenic signaling pathways such as MAPK and AKT signaling. Genetic manipulation of metabotropic glutamate receptor 1 (GRM1) to reduce its expression led to decreased proliferation of ER positive breast cancer cells [61]. Treatment of the oral cancer cell line B88-SDF-1 with an antagonist of metabotropic glutamate receptor 5 resulted in decreased metastasis and invasion in vivo and in vitro, respectively. Another class of glutamate receptor is the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, which are ionotropic receptors that act as ion channels. Activation of AMPA receptor was shown to drive invasion in pancreatic cancer cells via MAPK signaling [62]. Knockdown of the glutamate receptor AMPA4 reduced expression of genes associated with adhesion, and invasion [63].
Another important metabolic fate of glutamate is its conversion to gamma-aminobutyric acid (GABA) by glutamate decarboxylase. Expression of glutamate decarboxylase 1 increased the ability of breast cancer metastases to utilize glutamine [64]. GABAergic signaling in breast cancer was also found to increase migration and invasion in breast cancer [65]. The increase in migration, and invasion was mediated through ERK1/2 signaling. GABA receptor activation also affected the survival of the chondrosarcoma cell line OUMS-27 [66]. When the cells were exposed to the GABA antagonist CGP, the activities of apoptotic proteins caspase 3 and caspase 9 were elevated.
Along with alterations in cancer cell metabolism, perturbations in the metabolism of tumor stroma are emerging as a key driver of metastatic progression. The interactions between cancer-associated fibroblasts, and tumor cells is described as a ‘reverse Warburg effect’ where cancer cells induce metabolic reprogramming of fibroblasts leading to increased aerobic glycolysis [67], as well as increased expression of monocarboxylate transporter-4 (MCT4) [68] resulting in release of lactate into the tumor microenvironment. This is correlated with upregulated expression of monocarboxylate transporter-1 (MCT1) mediated lactate uptake in cancer cells which has been shown to contribute to survival and growth [68], as well as tumor migration [69]. The role of secreted lactate in disrupting innate and adaptive immune responses has been comprehensively reviewed elsewhere [70]. Novel roles of lactate in modulating immune response are also constantly emerging such as its ability to activate NF-κB in CD4+ T-cells and drive their polarization to the immunosuppressive Treg subtype driving prostate carcinoma progression [71]. Metabolic symbiosis has been shown to work both ways with fibroblasts reprogramming cancer cell metabolism to increase glycolytic metabolism and secretion of lactate to support metastasis [72] as well drive secretion of hepatocyte growth factor (HGF) from fibroblasts inducing resistance to tyrosine kinase inhibitor therapy [73].
Metabolism and Epigenetic Regulation in Metastasis
An emerging area of research in cancer is alterations in epigenetic activity that are controlled by metabolic changes [74,75]. A striking example is the association of epigenomic reprogramming and metabolism with distance metastases in pancreatic cancer [76]. The development of distant metastases was associated with global epigenetic changes including increases in histone acetylation, and decreased histone methylation. Metastatic lesions with these epigenetic changes frequently exhibited increased oxidative pentose phosphate pathway activity driven by overexpression of 6-phosphogluconate dehydrogenase (PGD). Inhibition of PGD in distant metastases reversed the epigenetic changes, indicating that increased oxidative pentose phosphate pathway activity is essential for disease progression in pancreatic cancer. Further work from the same authors showed that the metastatic capable cells evolved a pentose conversion pathway to provide substrate for PGD thus maintaining its hyperactivity [77]. This conversion pathway is distinct from the rate-limiting pentose phosphate pathway and is evidence of a novel metabolic program that appears to especially promote the metastatic phenotype via regulating the epigenome.
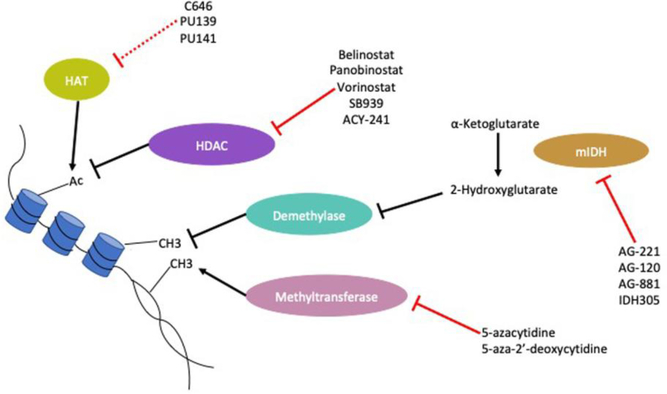
Altered metabolism can contribute to changes in the epigenomic state of cancer cells by providing cells with substrates for epigenetic enzymes [Figure 4]. ATP citrate-lyase (ACLY), which catalyzes the conversion of citrate to acetyl-CoA has been identified as an important enzyme for producing the nuclear pools of acetyl-CoA used by enzymes that control histone acetylation such as histone acetyltransferases [78]. Such as pathways has been suggested as necessary for polarization of macrophages to an ‘M2’ or alternatively activated tumor-promoting phenotype [79], although there is some question as to whether this is relevant in human macrophages [80]. Alternatively, activated macrophages are clearly associated with tumor progression and metastasis [81,82], however it is not yet clear if those dependent on ACLY activity are a true metastasis-promoting subtype [83]. In hepatocellular carcinoma, acetyl-CoA increases and associated histone acetylation were demonstrated to be downstream of Acyl-CoA thioesterase 12 (ACOT12) activity [84]. This increased histone acetylation was shown to drive expression of the transcription factor Twist2 which induced EMT.
Figure 4. Altered metabolism impacts regulation of cancer epigenome via production of substrates and allosteric regulators of epigenetic enzymes.
IDH mutations can produce 2-hydroxyglutarate which alter the function of demethylase enzymes. In addition, altered metabolism has been linked to changes in production of Acetyl-CoA the substrate of histone acetyltransferases. HAT (Histone Acetyltransferase); HDAC (Histone Deacetylase); mIDH (mutant IDH); Ac (Acetylation)
Metabolites may also act as competitive inhibitors of epigenetic enzyme activity. Dysregulation of the citric acid cycle in cancer has been associated with accumulation of a number of metabolites that can affect methylation of the epigenome, such as the oncometabolite 2-hydroxyglutarate. During normal metabolism, isocitrate is converted to α-ketoglutarate by the enzyme isocitrate dehydrogenase (IDH). Mutations in IDH are common in acute myeloid leukemia where they may play a role in pathogenesis, and in gliomas [85,86]. IDH R132, IDH2 r140, and IDH2 R172 mutations result in increased production of 2-hydroxyglutarate, via the action of the mutated enzymes on α-ketoglutarate [87]. 2-hydroxyglutarate can inhibit the function of α-ketoglutarate dependent dioxygenases, such as the demethylase KDM4C [88], and dysregulate the methylation status of cancer cells [89]. Mutant IDH was also shown to lower expression of ATM and interfere with DNA repair in acute myeloid leukemia [90]. IDH mutations that produce 2-hydroxyglutarate can cause EMT in colorectal cancer cells through driving the expression of the transcription factor ZEB1 [89,91]. Conversely, IDH mutant gliomas can have better prognosis due to promotion of methylation and thereby suppression of invasion-promoting genes such as G0S2 [92].
Deficiency and inhibition of succinate dehydrogenase causes hypermethylation in ovarian cancer, pheochromocytomas, and paragangliomas [93,94]. Fumarate has also been associated with progression in a number of cancers including renal cell carcinomas, paragangliomas, and nasopharyngeal cancers [95,96]. Loss of fumarate hydratase expression stabilized HIF-1α and HIF-2α, leading to EMT and upregulation of an anti-oxidant response in renal cancer [97,98]. In addition, fumarate can inhibit TET demethylases and cause a hypermethylation phenotype in renal cancer [99]. Finally, fumarate may cause senescence through oxidative stress [100].
Implications and Future Directions
Altered metabolism is well established as a hallmark of cancer biology [2] and associated with multiple aspects of cancer progression including metastasis [101,102]. The clear tumor-promoting roles of dysregulated metabolic pathways have led to a development of a number of therapies [103]. However, these therapies present particular challenges in their utilization in the clinic. Most critically, many of the metabolic pathways that may be dysregulated in cancer cells are still used by other cell types, thus identifying a reasonable therapeutic index has proven difficult. For example, inhibition of the key cancer associated metabolic phenotype aerobic glycolysis, using 2-deoxy-D-glucose (2DG) results in toxic effects similar to hypoglycemia [104]. However, understanding and targeting non-canonical functions of enzymes related to aerobic glycolysis, such as PHI, may present promising therapeutic strategies that lack the unintended toxicity of targeting ubiquitous metabolic pathways [Table 1]. On the other hand, there are a number of therapies in development for mutant IDH isoforms, which have the advantage of being distinct from the normal enzyme [105].
Table 1.
Current and potential therapeutic targets based on non-canonical metabolic roles.
| Target | Agent | Mechanism to Target | References |
|---|---|---|---|
| Glutaminase 1 | CB-839 | NCT02071927 | |
| NCT02071888 | |||
| NCT02071862 | |||
| GRM1 | Riluzole | Pro-tumorigenic glutamate signaling. | NCT00903214 |
| NCT01018836 | |||
| NCT01303341 | |||
| NCT00866840 | |||
| HDAC | Belinostat | Catalytic activity resulting in hypoacetylation. | NCT00993642 |
| Panobinostat | NCT01075308 | ||
| Vorinostat | NCT02635061 | ||
| SB939 | NCT01528501 | ||
| ACY-241 | NCT00274651 | ||
| mIDH | AG-221 | Production of on co metabolite 2-hydroxyglutarate. | NCT01915498 |
| AG-120 | NCT02073994 | ||
| AG-881 | NCT02492737 | ||
| IDH305 | NCT02987010 | ||
| Methyltransferase | 5-azacytidine, 5-aza-2’-deoxycytidine | Catalytic activity resulting in hypermethylation. | NCT03019003 |
| NCT03182894 | |||
| NCT02159820 | |||
| NCT00084981 | |||
| ACLY | 2,2-difluorocitrate Sulfoximine | Catalytic activity producing Acetyl-CoA which induces hyperacetylation. | [109,110] |
| AMF | ERI4P G6P |
Pro-metastatic, autocrine and paracrine signaling via gp78. | [111] |
| SLC1A5 (ASCT2) | AABA Benzylserine Benzylcysteine GPNA |
Import of glutamine, which is an important carbon source for a number of pro-metastatic processes. | [112,55,56,54] |
| SLC7A11 (xCT) | Sulfasalazine Erastin Sorafenib |
Release of glutamate which can act as a pro-metastatic signaling molecule. | [113–115] |
| AMPAR | GYKI-52466 CFM-2 |
Glutamate signaling which can drive metastasis. | [116] |
| HAT | C646 PU139 PU141 |
Increased activity resulting in hyperacetylation. | [117,118] |
| SPLA2 | Varespladib PLIs |
Secretion and binding to receptors that drive Wnt signaling. | [119,120] |
| GAPDH (Potential) | N/A | GAPDH binding to EMT transcription factor sp1 | |
| PKM2 (Potential) | N/A | PKM2 binding to HDAC3 and TGIF2. | |
| PRDX4 (Potential) | N/A | Secretion and function as an osteoclastogenic signaling factor. |
The opposing roles of GLS1 and GLS2 in metastasis [24,27,28] highlight the importance of understanding and targeting the isoform specific effects of metabolic enzymes. While pharmacologic inhibition, and genetic ablation of GLS1 slows cancer growth [106] the expression of GLS2 attenuates metastasis. These opposing roles might suggest that specificity of glutaminase inhibitors is critically important. However, the fact that the metastasis-promoting effects of glutaminase 1 are dependent on its catalytic activity while the metastasis-suppressing effects of glutaminase 2 are catalysis-independent enables possible methods of differential modulation.
The significance of changes in metabolism is not just limited to cancer cells, but also extends to the tumor stroma. As illustrated by interactions between cancer-associated fibroblasts and tumor cells, altered metabolism in the stroma can directly support metastatic progression by supplying tumors with high energy metabolites such as lactate [68,69]. In addition, lactate has been shown to affect the signaling of immune cells and produce an immunosuppressive tumor microenvironment [71]. Changes in cancer cell metabolism can also contribute to changes in signaling in the stroma that enhance metastasis, such as PHI paracrine signaling promoting angiogenesis [42]. These changes emphasize the importance of understanding changes in metabolism in the context of the tumor microenvironment and not just considering the cancer cells.
Finally changes in metabolism can sensitize cancer cells to other forms of therapy. Mutant IDH has been shown to sensitize glioma cells to inhibition of glutaminase [107]. Inhibited glutaminolysis results in decreased accumulation of α-ketoglutarate, the substrate of mutant IDH and a resultant slowed growth phenotype. In addition, gliomas with mutant IDH have been shown to be especially sensitive to treatment with inhibitors of DNA methyltransferases [108] since altered methylation is a key effect of mutant IDH. These examples depict novel targets induced by altered tumor metabolism that can be exploited for treatment.
Conclusion
Dysregulation of metabolism is a hallmark of cancer is commonly associated with metastasis. The increased consumption of carbon sources such as glucose, glutamine, and fatty acids commonly occur in multiple types of cancer leading to enhanced metabolic pathway activation. However, non-canonical functions of these metabolic pathways can influence metastatic progression in ways that diverge from their usual roles in regulating bioenergetics and biosynthesis. Here, we have particularly highlighted the ability of metabolic enzymes such as PHI to alter cancer cell signaling independent of their normal enzymatic functions, as well as the ability of metabolites to act as signaling molecules or change the epigenome of cancer cells. The challenge is to convert this knowledge of novel capabilities to new therapeutic approaches for patients with metastatic disease.
Acknowledgements:
The authors are grateful to the Fingleton lab members for reading and providing constructive criticism. This work was supported by the Office of the Assistant
Secretary of Defense for Health Affairs, through the Breast Cancer Research Program under award number W81XWH-16–1–0559 (to BF). Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. DW was also supported by T32 CA119925.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Bibliography
- 1.Friberg S, Nystrom A (2015) Cancer Metastases: Early Dissemination and Late Recurrences. Cancer Growth Metastasis 8:43–49. doi: 10.4137/CGM.S31244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144 (5):646–674. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 3.Liu K, Tang Z, Huang A, Chen P, Liu P, Yang J, Lu W, Liao J, Sun Y, Wen S, Hu Y, Huang P (2017) Glyceraldehyde-3-phosphate dehydrogenase promotes cancer growth and metastasis through upregulation of SNAIL expression. Int J Oncol 50 (1):252–262. doi: 10.3892/ijo.2016.3774 [DOI] [PubMed] [Google Scholar]
- 4.Yang W, Lu Z (2015) Pyruvate kinase M2 at a glance. J Cell Sci 128 (9):1655–1660. doi: 10.1242/jcs.166629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamabe A, Konno M, Tanuma N, Shima H, Tsunekuni K, Kawamoto K, Nishida N, Koseki J, Mimori K, Gotoh N, Yamamoto H, Doki Y, Mori M, Ishii H (2014) Role of pyruvate kinase M2 in transcriptional regulation leading to epithelial-mesenchymal transition. Proc Natl Acad Sci U S A 111 (43):15526–15531. doi: 10.1073/pnas.1407717111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attanasio F, Caldieri G, Giacchetti G, van Horssen R, Wieringa B, Buccione R (2011) Novel invadopodia components revealed by differential proteomic analysis. Eur J Cell Biol 90 (2–3):115–127. doi: 10.1016/j.ejcb.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 7.Shiraishi T, Verdone JE, Huang J, Kahlert UD, Hernandez JR, Torga G, Zarif JC, Epstein T, Gatenby R, McCartney A, Elisseeff JH, Mooney SM, An SS, Pienta KJ (2015) Glycolysis is the primary bioenergetic pathway for cell motility and cytoskeletal remodeling in human prostate and breast cancer cells. Oncotarget 6 (1):130–143. doi: 10.18632/oncotarget.2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glass-Marmor L, Beitner R (1997) Detachment of glycolytic enzymes from cytoskeleton of melanoma cells induced by calmodulin antagonists. Eur J Pharmacol 328 (2–3):241–248. doi: 10.1016/s0014-2999(97)83051-8 [DOI] [PubMed] [Google Scholar]
- 9.Penso J, Beitner R (2002) Detachment of glycolytic enzymes from cytoskeleton of Lewis lung carcinoma and colon adenocarcinoma cells induced by clotrimazole and its correlation to cell viability and morphology. Mol Genet Metab 76 (3):181–188. doi: 10.1016/s1096-7192(02)00046-x [DOI] [PubMed] [Google Scholar]
- 10.Meira DD, Marinho-Carvalho MM, Teixeira CA, Veiga VF, Da Poian AT, Holandino C, de Freitas MS, Sola-Penna M (2005) Clotrimazole decreases human breast cancer cells viability through alterations in cytoskeleton-associated glycolytic enzymes. Mol Genet Metab 84 (4):354–362. doi: 10.1016/j.ymgme.2004.11.012 [DOI] [PubMed] [Google Scholar]
- 11.Mycielska ME, Broke-Smith TP, Palmer CP, Beckerman R, Nastos T, Erguler K, Djamgoz MB (2006) Citrate enhances in vitro metastatic behaviours of PC-3M human prostate cancer cells: status of endogenous citrate and dependence on aconitase and fatty acid synthase. Int J Biochem Cell Biol 38 (10):1766–1777. doi: 10.1016/j.biocel.2006.04.008 [DOI] [PubMed] [Google Scholar]
- 12.Tyszka-Czochara M, Konieczny P, Majka M (2017) Caffeic Acid Expands Anti-Tumor Effect of Metformin in Human Metastatic Cervical Carcinoma HTB-34 Cells: Implications of AMPK Activation and Impairment of Fatty Acids De Novo Biosynthesis. Int J Mol Sci 18 (2). doi: 10.3390/ijms18020462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Migita T, Narita T, Nomura K, Miyagi E, Inazuka F, Matsuura M, Ushijima M, Mashima T, Seimiya H, Satoh Y, Okumura S, Nakagawa K, Ishikawa Y (2008) ATP Citrate Lyase: Activation and Therapeutic Implications in Non-Small Cell Lung Cancer. Cancer Research 68 (20):8547–8554. doi: 10.1158/0008-5472.Can-08-1235 [DOI] [PubMed] [Google Scholar]
- 14.Xin M, Qiao Z, Li J, Liu J, Song S, Zhao X, Miao P, Tang T, Wang L, Liu W, Yang X, Dai K, Huang G (2016) miR-22 inhibits tumor growth and metastasis by targeting ATP citrate lyase: evidence in osteosarcoma, prostate cancer, cervical cancer and lung cancer. Oncotarget 7 (28):44252–44265. doi: 10.18632/oncotarget.10020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Huang X, Ye T (2018) MiR-22 down-regulates the proto-oncogene ATP citrate lyase to inhibit the growth and metastasis of breast cancer. Am J Transl Res 10 (3):659–669 [PMC free article] [PubMed] [Google Scholar]
- 16.Koerner SK, Hanai JI, Bai S, Jernigan FE, Oki M, Komaba C, Shuto E, Sukhatme VP, Sun L (2017) Design and synthesis of emodin derivatives as novel inhibitors of ATP-citrate lyase. Eur J Med Chem 126:920–928. doi: 10.1016/j.ejmech.2016.12.018 [DOI] [PubMed] [Google Scholar]
- 17.Astuti D, Douglas F, Lennard TWJ, Aligianis IA, Woodward ER, Evans DGR, Eng C, Latif F, Maher ER (2001) Germline SDHD mutation in familial phaeochromocytoma. The Lancet 357 (9263):1181–1182. doi: 10.1016/s0140-6736(00)04378-6 [DOI] [PubMed] [Google Scholar]
- 18.Baysal BE, Maher ER (2015) 15 YEARS OF PARAGANGLIOMA: Genetics and mechanism of pheochromocytoma-paraganglioma syndromes characterized by germline SDHB and SDHD mutations. Endocr Relat Cancer 22 (4):T71–82. doi: 10.1530/ERC-15-0226 [DOI] [PubMed] [Google Scholar]
- 19.Ricketts C, Woodward ER, Killick P, Morris MR, Astuti D, Latif F, Maher ER (2008) Germline SDHB mutations and familial renal cell carcinoma. J Natl Cancer Inst 100 (17):1260–1262. doi: 10.1093/jnci/djn254 [DOI] [PubMed] [Google Scholar]
- 20.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E (2005) Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 7 (1):77–85doi: 10.1016/j.ccr.2004.11.022 [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Gao L, Zhang H, Wang D, Wang M, Zhu J, Pang C, Wang C (2013) Succinate dehydrogenase 5 (SDH5) regulates glycogen synthase kinase 3beta-beta-catenin-mediated lung cancer metastasis. J Biol Chem 288 (41):29965–29973. doi: 10.1074/jbc.M113.450106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Chen Y, Wu G (2016) SDHB deficiency promotes TGFbeta-mediated invasion and metastasis of colorectal cancer through transcriptional repression complex SNAIL1-SMAD3/4. Transl Oncol 9 (6):512–520. doi: 10.1016/j.tranon.2016.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Still ER, Yuneva MO (2017) Hopefully devoted to Q: targeting glutamine addiction in cancer. Br J Cancer 116 (11):1375–1381. doi: 10.1038/bjc.2017.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JY, Heo SH, Choi SK, Song IH, Park IA, Kim YA, Park HS, Park SY, Bang WS, Gong G, Lee HJ (2017) Glutaminase expression is a poor prognostic factor in node-positive triple-negative breast cancer patients with a high level of tumor-infiltrating lymphocytes. Virchows Arch 470 (4):381–389. doi: 10.1007/s00428-017-2083-5 [DOI] [PubMed] [Google Scholar]
- 25.Carr EL, Kelman A, Wu GS, Gopaul R, Senkevitch E, Aghvanyan A, Turay AM, Frauwirth KA (2010) Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol 185 (2):1037–1044. doi: 10.4049/jimmunol.0903586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinclair LV, Rolf J, Emslie E, Shi YB, Taylor PM, Cantrell DA (2013) Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat Immunol 14 (5):500–508. doi: 10.1038/ni.2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C, Liu J, Zhao Y, Yue X, Zhu Y, Wang X, Wu H, Blanco F, Li S, Bhanot G, Haffty BG, Hu W, Feng Z (2016) Glutaminase 2 is a novel negative regulator of small GTPase Rac1 and mediates p53 function in suppressing metastasis. Elife 5:e10727. doi: 10.7554/eLife.10727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuo TC, Chen CK, Hua KT, Yu P, Lee WJ, Chen MW, Jeng YM, Chien MH, Kuo KT, Hsiao M, Kuo ML (2016) Glutaminase 2 stabilizes Dicer to repress Snail and metastasis in hepatocellular carcinoma cells. Cancer Lett 383 (2):282–294. doi: 10.1016/j.canlet.2016.10.012 [DOI] [PubMed] [Google Scholar]
- 29.Reddy BA, van der Knaap JA, Bot AG, Mohd-Sarip A, Dekkers DH, Timmermans MA, Martens JW, Demmers JA, Verrijzer CP (2014) Nucleotide biosynthetic enzyme GMP synthase is a TRIM21-controlled relay of p53 stabilization. Mol Cell 53 (3):458–470. doi: 10.1016/j.molcel.2013.12.017 [DOI] [PubMed] [Google Scholar]
- 30.Powell E, Piwnica-Worms D, Piwnica-Worms H (2014) Contribution of p53 to metastasis. Cancer Discov 4 (4):405–414. doi: 10.1158/2159-8290.CD-13-0136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Funasaka T, Yanagawa T, Hogan V, Raz A (2005) Regulation of phosphoglucose isomerase/autocrine motility factor expression by hypoxia. FASEB Journal Sep 19 (11):1422–1430. doi: 10.1096/fj.05-3699com [DOI] [PubMed] [Google Scholar]
- 32.Niizeki H, Kobayashi M, Horiuchi I, Akakura N, Chen J, Wang J, Hamada JI, Seth P, Katoh H, Watanabe H, Raz A, Hosokawa M (2002) Hypoxia enhances the expression of autocrine motility factor and the motility of human pancreatic cancer cells. Br J Cancer 86 (12):1914–1919. doi: 10.1038/sj.bjc.6600331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe H, Carmi P, Hogan V, Raz T, Silletti S, Nabi IR, Raz A (1991) Purification of human tumor cell autocrine motility factor and molecular cloning of its receptor. J Biol Chem 266 (20):13442–13448 [PubMed] [Google Scholar]
- 34.Martins SF, Amorim R, Viana-Pereira M, Pinheiro C, Costa RF, Silva P, Couto C, Alves S, Fernandes S, Vilaca S, Falcao J, Marques H, Pardal F, Rodrigues M, Preto A, Reis RM, Longatto-Filho A, Baltazar F (2016) Significance of glycolytic metabolism-related protein expression in colorectal cancer, lymph node and hepatic metastasis. BMC Cancer 16:535. doi: 10.1186/s12885-016-2566-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsutsumi S, Yanagawa T, Shimura T, Kuwano H, Raz A (2004) Autocrine motility factor signaling enhances pancreatic cancer metastasis. Clin Cancer Res 10 (22):7775–7784. doi: 10.1158/1078-0432.CCR-04-1015 [DOI] [PubMed] [Google Scholar]
- 36.Funasaka T, Hogan V, Raz A (2009) Phosphoglucose isomerase/autocrine motility factor mediates epithelial and mesenchymal phenotype conversions in breast cancer. Cancer Res 69 (13):5349–5356. doi: 10.1158/0008-5472.CAN-09-0488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Che Q, Bian Y, Zhou Q, Jiang F, Tong H, Ke J, Wang K, Wan XP (2015) Autocrine motility factor promotes epithelial-mesenchymal transition in endometrial cancer via MAPK signaling pathway. Int J Oncol 47 (3):1017–1024. doi: 10.3892/ijo.2015.3091 [DOI] [PubMed] [Google Scholar]
- 38.Ahmad A, Aboukameel A, Kong D, Wang Z, Sethi S, Chen W, Sarkar FH, Raz A (2011) Phosphoglucose isomerase/autocrine motility factor mediates epithelial-mesenchymal transition regulated by miR-200 in breast cancer cells. Cancer Res 71 (9):3400–3409. doi: 10.1158/0008-5472.CAN-10-0965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanagawa T, Watanabe H, Takeuchi T, Fujimoto S, Kurihara H, Takagishi K (2004) Overexpression of autocrine motility factor in metastatic tumor cells: possible association with augmented expression of KIF3A and GDI-beta. Lab Invest 84 (4):513–522. doi: 10.1038/labinvest.3700057 [DOI] [PubMed] [Google Scholar]
- 40.Tsutsumi S, Gupta SK, Hogan V, Collard JG, Raz A (2002) Activation of small GTPase Rho is required for autocrine motility factor signaling. Cancer Research 62 (15):4484–4490 [PubMed] [Google Scholar]
- 41.Torimura T, Ueno T, Kin M, Harada R, Nakamura T, Kawaguchi T, Harada M, Kumashiro R, Watanabe H, Avraham R, Sata M (2001) Autocrine motility factor enhances hepatoma cell invasion across the basement membrane through activation of beta1 integrins. Hepatology 34 (1):62–71. doi: 10.1053/jhep.2001.25546 [DOI] [PubMed] [Google Scholar]
- 42.Funasaka T, Haga A, Raz A, Nagase H (2002) Autocrine motility factor secreted by tumor cells upregulates vascular endothelial growth factor receptor (Flt-1) expression in endothelial cells. Int J Cancer 101 (3):217–223. doi: 10.1002/ijc.10617 [DOI] [PubMed] [Google Scholar]
- 43.Funasaka T, Haga A, Raz A, Nagase H (2002) Tumor autocrine motility factor induces hyperpermeability of endothelial and mesothelial cells leading to accumulation of ascites fluid. Biochem Biophys Res Commun 293 (1):192–200. doi: 10.1016/S0006-291X(02)00202-4 [DOI] [PubMed] [Google Scholar]
- 44.Schewe M, Franken PF, Sacchetti A, Schmitt M, Joosten R, Bottcher R, van Royen ME, Jeammet L, Payre C, Scott PM, Webb NR, Gelb M, Cormier RT, Lambeau G, Fodde R (2016) Secreted Phospholipases A2 Are Intestinal Stem Cell Niche Factors with Distinct Roles in Homeostasis, Inflammation, and Cancer. Cell Stem Cell 19 (1):38–51. doi: 10.1016/j.stem.2016.05.023 [DOI] [PubMed] [Google Scholar]
- 45.Zhan T, Rindtorff N, Boutros M (2017) Wnt signaling in cancer. Oncogene 36 (11):1461–1473. doi: 10.1038/onc.2016.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jarc E, Kump A, Malavasic P, Eichmann TO, Zimmermann R, Petan T (2018) Lipid droplets induced by secreted phospholipase A2 and unsaturated fatty acids protect breast cancer cells from nutrient and lipotoxic stress. Biochim Biophys Acta Mol Cell Biol Lipids 1863 (3):247–265. doi: 10.1016/j.bbalip.2017.12.006 [DOI] [PubMed] [Google Scholar]
- 47.Rafiei S, Tiedemann K, Tabaries S, Siegel PM, Komarova SV (2015) Peroxiredoxin 4: a novel secreted mediator of cancer induced osteoclastogenesis. Cancer Lett 361 (2):262–270. doi: 10.1016/j.canlet.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 48.Fernandez HR, Gadre SM, Tan M, Graham GT, Mosaoa R, Ongkeko MS, Kim KA, Riggins RB, Parasido E, Petrini I, Pacini S, Cheema A, Varghese R, Ressom HW, Zhang Y, Albanese C, Uren A, Paige M, Giaccone G, Avantaggiati ML (2018) The mitochondrial citrate carrier, SLC25A1, drives stemness and therapy resistance in non-small cell lung cancer. Cell Death Differ 25 (7):1239–1258. doi: 10.1038/s41418-018-0101-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ren JG, Seth P, Ye H, Guo K, Hanai JI, Husain Z, Sukhatme VP (2017) Citrate Suppresses Tumor Growth in Multiple Models through Inhibition of Glycolysis, the Tricarboxylic Acid Cycle and the IGF-1R Pathway. Sci Rep 7 (1):4537. doi: 10.1038/s41598-017-04626-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giskeodegard GF, Bertilsson H, Selnaes KM, Wright AJ, Bathen TF, Viset T, Halgunset J, Angelsen A, Gribbestad IS, Tessem MB (2013) Spermine and citrate as metabolic biomarkers for assessing prostate cancer aggressiveness. PLoS One 8 (4):e62375. doi: 10.1371/journal.pone.0062375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhutia YD, Ganapathy V (2016) Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim Biophys Acta 1863 (10):2531–2539. doi: 10.1016/j.bbamcr.2015.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Q, Hardie RA, Hoy AJ, van Geldermalsen M, Gao D, Fazli L, Sadowski MC, Balaban S, Schreuder M, Nagarajah R, Wong JJ, Metierre C, Pinello N, Otte NJ, Lehman ML, Gleave M, Nelson CC, Bailey CG, Ritchie W, Rasko JE, Holst J (2015) Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J Pathol 236 (3):278–289. doi: 10.1002/path.4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Broer A, Gauthier-Coles G, Rahimi F, van Geldermalsen M, Dorsch D, Wegener A, Holst J, Broer S (2019) Ablation of the ASCT2 (SLC1A5) gene encoding a neutral amino acid transporter reveals transporter plasticity and redundancy in cancer cells. J Biol Chem 294 (11):4012–4026. doi: 10.1074/jbc.RA118.006378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marshall AD, van Geldermalsen M, Otte NJ, Lum T, Vellozzi M, Thoeng A, Pang A, Nagarajah R, Zhang B, Wang Q, Anderson L, Rasko JEJ, Holst J (2017) ASCT2 regulates glutamine uptake and cell growth in endometrial carcinoma. Oncogenesis 6 (7):e367. doi: 10.1038/oncsis.2017.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schulte ML, Fu A, Zhao P, Li J, Geng L, Smith ST, Kondo J, Coffey RJ, Johnson MO, Rathmell JC, Sharick JT, Skala MC, Smith JA, Berlin J, Washington MK, Nickels ML, Manning HC (2018) Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat Med 24 (2):194–202. doi: 10.1038/nm.4464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Broer A, Fairweather S, Broer S (2018) Disruption of Amino Acid Homeostasis by Novel ASCT2 Inhibitors Involves Multiple Targets. Front Pharmacol 9:785. doi: 10.3389/fphar.2018.00785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim HA, Kim KJ, Yoon SY, Lee HK, Im SY (2012) Glutamine inhibits platelet-activating factor-mediated pulmonary tumour metastasis. Eur J Cancer 48 (11):1730–1738. doi: 10.1016/j.ejca.2011.07.013 [DOI] [PubMed] [Google Scholar]
- 58.Ji X, Qian J, Rahman SMJ, Siska PJ, Zou Y, Harris BK, Hoeksema MD, Trenary IA, Heidi C, Eisenberg R, Rathmell JC, Young JD, Massion PP (2018) xCT (SLC7A11)-mediated metabolic reprogramming promotes non-small cell lung cancer progression. Oncogene 37 (36):5007–5019. doi: 10.1038/s41388-018-0307-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shin CS, Mishra P, Watrous JD, Carelli V, D’Aurelio M, Jain M, Chan DC (2017) The glutamate/cystine xCT antiporter antagonizes glutamine metabolism and reduces nutrient flexibility. Nat Commun 8:15074. doi: 10.1038/ncomms15074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu LJ, Wall BA, Wangari-Talbot J, Chen S (2017) Metabotropic glutamate receptors in cancer. Neuropharmacology 115:193–202. doi: 10.1016/j.neuropharm.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mehta MS, Dolfi SC, Bronfenbrener R, Bilal E, Chen C, Moore D, Lin Y, Rahim H, Aisner S, Kersellius RD, Teh J, Chen S, Toppmeyer DL, Medina DJ, Ganesan S, Vazquez A, Hirshfield KM (2013) Metabotropic glutamate receptor 1 expression and its polymorphic variants associate with breast cancer phenotypes. PLoS One 8 (7):e69851. doi: 10.1371/journal.pone.0069851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herner A, Sauliunaite D, Michalski CW, Erkan M, De Oliveira T, Abiatari I, Kong B, Esposito I, Friess H, Kleeff J (2011) Glutamate increases pancreatic cancer cell invasion and migration via AMPA receptor activation and Kras-MAPK signaling. Int J Cancer 129 (10):2349–2359. doi: 10.1002/ijc.25898 [DOI] [PubMed] [Google Scholar]
- 63.Luksch H, Uckermann O, Stepulak A, Hendruschk S, Marzahn J, Bastian S, Staufner C, Temme A, Ikonomidou C (2011) Silencing of selected glutamate receptor subunits modulates cancer growth. Anticancer Res 31 (10):3181–3192 [PubMed] [Google Scholar]
- 64.Schnepp PM, Lee DD, Guldner IH, O’Tighearnaigh TK, Howe EN, Palakurthi B, Eckert KE, Toni TA, Ashfeld BL, Zhang S (2017) GAD1 Upregulation Programs Aggressive Features of Cancer Cell Metabolism in the Brain Metastatic Microenvironment. Cancer Res 77 (11):2844–2856. doi: 10.1158/0008-5472.CAN-16-2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang D, Li X, Yao Z, Wei C, Ning N, Li J (2014) GABAergic signaling facilitates breast cancer metastasis by promoting ERK1/2-dependent phosphorylation. Cancer Lett 348 (1–2):100–108. doi: 10.1016/j.canlet.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 66.Kanbara K, Otsuki Y, Watanabe M, Yokoe S, Mori Y, Asahi M, Neo M (2018) GABAB receptor regulates proliferation in the high-grade chondrosarcoma cell line OUMS-27 via apoptotic pathways. BMC Cancer 18 (1):263. doi: 10.1186/s12885-018-4149-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang E, Xu Z, Wang M, Yan T, Huang C, Zhou X, Liu Q, Wang L, Chen Y, Wang H, Liu K, Shao Z, Shang Z (2019) Tumoral microvesicle-activated glycometabolic reprogramming in fibroblasts promotes the progression of oral squamous cell carcinoma. FASEB J 33 (4):5690–5703. doi: 10.1096/fj.201802226R [DOI] [PubMed] [Google Scholar]
- 68.Diehl K, Dinges LA, Helm O, Ammar N, Plundrich D, Arlt A, Rocken C, Sebens S, Schafer H (2018) Nuclear factor E2-related factor-2 has a differential impact on MCT1 and MCT4 lactate carrier expression in colonic epithelial cells: a condition favoring metabolic symbiosis between colorectal cancer and stromal cells. Oncogene 37 (1):39–51. doi: 10.1038/onc.2017.299 [DOI] [PubMed] [Google Scholar]
- 69.Wu W, Zaal EA, Berkers CR, Lemeer S, Heck AJR (2018) CTGF/VEGFA-activated Fibroblasts Promote Tumor Migration Through Micro-environmental Modulation. Mol Cell Proteomics 17 (8):1502–1514. doi: 10.1074/mcp.RA118.000708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morrot A, da Fonseca LM, Salustiano EJ, Gentile LB, Conde L, Filardy AA, Franklim TN, da Costa KM, Freire-de-Lima CG, Freire-de-Lima L (2018) Metabolic Symbiosis and Immunomodulation: How Tumor Cell-Derived Lactate May Disturb Innate and Adaptive Immune Responses. Front Oncol 8 (81):81. doi: 10.3389/fonc.2018.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Comito G, Iscaro A, Bacci M, Morandi A, Ippolito L, Parri M, Montagnani I, Raspollini MR, Serni S, Simeoni L, Giannoni E, Chiarugi P (2019) Lactate modulates CD4(+) T-cell polarization and induces an immunosuppressive environment, which sustains prostate carcinoma progression via TLR8/miR21 axis. Oncogene. doi: 10.1038/s41388-019-0688-7 [DOI] [PubMed] [Google Scholar]
- 72.Curtis M, Kenny HA, Ashcroft B, Mukherjee A, Johnson A, Zhang Y, Helou Y, Batlle R, Liu X, Gutierrez N, Gao X, Yamada SD, Lastra R, Montag A, Ahsan N, Locasale JW, Salomon AR, Nebreda AR, Lengyel E (2019) Fibroblasts Mobilize Tumor Cell Glycogen to Promote Proliferation and Metastasis. Cell Metab 29 (1):141–155 e149. doi: 10.1016/j.cmet.2018.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Apicella M, Giannoni E, Fiore S, Ferrari KJ, Fernandez-Perez D, Isella C, Granchi C, Minutolo F, Sottile A, Comoglio PM, Medico E, Pietrantonio F, Volante M, Pasini D, Chiarugi P, Giordano S, Corso S (2018) Increased Lactate Secretion by Cancer Cells Sustains Non-cell-autonomous Adaptive Resistance to MET and EGFR Targeted Therapies. Cell Metab 28 (6):848–865 e846. doi: 10.1016/j.cmet.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 74.Wong CC, Qian Y, Yu J (2017) Interplay between epigenetics and metabolism in oncogenesis: mechanisms and therapeutic approaches. Oncogene 36 (24):3359–3374. doi: 10.1038/onc.2016.485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miranda-Goncalves V, Lameirinhas A, Henrique R, Jeronimo C (2018) Metabolism and Epigenetic Interplay in Cancer: Regulation and Putative Therapeutic Targets. Front Genet 9:427. doi: 10.3389/fgene.2018.00427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McDonald OG, Li X, Saunders T, Tryggvadottir R, Mentch SJ, Warmoes MO, Word AE, Carrer A, Salz TH, Natsume S, Stauffer KM, Makohon-Moore A, Zhong Y, Wu H, Wellen KE, Locasale JW, Iacobuzio-Donahue CA, Feinberg AP (2017) Epigenomic reprogramming during pancreatic cancer progression links anabolic glucose metabolism to distant metastasis. Nat Genet 49 (3):367–376. doi: 10.1038/ng.3753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bechard ME, Word AE, Tran AV, Liu X, Locasale JW, McDonald OG (2018) Pentose conversions support the tumorigenesis of pancreatic cancer distant metastases. Oncogene 37 (38):5248–5256. doi: 10.1038/s41388-018-0346-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB (2009) ATP-Citrate Lyase Links Cellular Metabolism to Histone Acetylation. Science 324 (5930):1076–1080. doi: 10.1126/science.1164097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Covarrubias AJ, Aksoylar HI, Yu J, Snyder NW, Worth AJ, Iyer SS, Wang J, Ben-Sahra I, Byles V, Polynne-Stapornkul T, Espinosa EC, Lamming D, Manning BD, Zhang Y, Blair IA, Horng T (2016) Akt-mTORC1 signaling regulates Acly to integrate metabolic input to control of macrophage activation. Elife 5. doi: 10.7554/eLife.11612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Namgaladze D, Zukunft S, Schnutgen F, Kurrle N, Fleming I, Fuhrmann D, Brune B (2018) Polarization of Human Macrophages by Interleukin-4 Does Not Require ATP-Citrate Lyase. Front Immunol 9:2858. doi: 10.3389/fimmu.2018.02858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aras S, Zaidi MR (2017) TAMeless traitors: macrophages in cancer progression and metastasis. Br J Cancer 117 (11):1583–1591. doi: 10.1038/bjc.2017.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Komohara Y, Fujiwara Y, Ohnishi K, Takeya M (2016) Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy. Adv Drug Deliv Rev 99 (Pt B):180–185. doi: 10.1016/j.addr.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 83.Yang M, McKay D, Pollard JW, Lewis CE (2018) Diverse Functions of Macrophages in Different Tumor Microenvironments. Cancer Res 78 (19):5492–5503. doi: 10.1158/0008-5472.CAN-18-1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lu M, Zhu WW, Wang X, Tang JJ, Zhang KL, Yu GY, Shao WQ, Lin ZF, Wang SH, Lu L, Zhou J, Wang LX, Jia HL, Dong QZ, Chen JH, Lu JQ, Qin LX (2019) ACOT12-Dependent Alteration of Acetyl-CoA Drives Hepatocellular Carcinoma Metastasis by Epigenetic Induction of Epithelial-Mesenchymal Transition. Cell Metab. doi: 10.1016/j.cmet.2018.12.019 [DOI] [PubMed] [Google Scholar]
- 85.Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, Koboldt DC, Fulton RS, Delehaunty KD, McGrath SD, Fulton LA, Locke DP, Magrini VJ, Abbott RM, Vickery TL, Reed JS, Robinson JS, Wylie T, Smith SM, Carmichael L, Eldred JM, Harris CC, Walker J, Peck JB, Du F, Dukes AF, Sanderson GE, Brummett AM, Clark E, McMichael JF, Meyer RJ, Schindler JK, Pohl CS, Wallis JW, Shi X, Lin L, Schmidt H, Tang Y, Haipek C, Wiechert ME, Ivy JV, Kalicki J, Elliott G, Ries RE, Payton JE, Westervelt P, Tomasson MH, Watson MA, Baty J, Heath S, Shannon WD, Nagarajan R, Link DC, Walter MJ, Graubert TA, DiPersio JF, Wilson RK, Ley TJ (2009) Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med 361 (11):1058–1066. doi: 10.1056/NEJMoa0903840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360 (8):765–773. doi: 10.1056/NEJMoa0808710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, Cross JR, Fantin VR, Hedvat CV, Perl AE, Rabinowitz JD, Carroll M, Su SM, Sharp KA, Levine RL, Thompson CB (2010) The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 17 (3):225–234. doi: 10.1016/j.ccr.2010.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O, Edwards CR, Khanin R, Figueroa ME, Melnick A, Wellen KE, O’Rourke DM, Berger SL, Chan TA, Levine RL, Mellinghoff IK, Thompson CB (2012) IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 483 (7390):474–478. doi: 10.1038/nature10860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grassian AR, Lin F, Barrett R, Liu Y, Jiang W, Korpal M, Astley H, Gitterman D, Henley T, Howes R, Levell J, Korn JM, Pagliarini R (2012) Isocitrate dehydrogenase (IDH) mutations promote a reversible ZEB1/microRNA (miR)-200-dependent epithelial-mesenchymal transition (EMT). J Biol Chem 287 (50):42180–42194. doi: 10.1074/jbc.M112.417832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Inoue S, Li WY, Tseng A, Beerman I, Elia AJ, Bendall SC, Lemonnier F, Kron KJ, Cescon DW, Hao Z, Lind EF, Takayama N, Planello AC, Shen SY, Shih AH, Larsen DM, Li Q, Snow BE, Wakeham A, Haight J, Gorrini C, Bassi C, Thu KL, Murakami K, Elford AR, Ueda T, Straley K, Yen KE, Melino G, Cimmino L, Aifantis I, Levine RL, De Carvalho DD, Lupien M, Rossi DJ, Nolan GP, Cairns RA, Mak TW (2016) Mutant IDH1 Downregulates ATM and Alters DNA Repair and Sensitivity to DNA Damage Independent of TET2. Cancer Cell 30 (2):337–348doi: 10.1016/j.ccell.2016.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Colvin H, Nishida N, Konno M, Haraguchi N, Takahashi H, Nishimura J, Hata T, Kawamoto K, Asai A, Tsunekuni K, Koseki J, Mizushima T, Satoh T, Doki Y, Mori M, Ishii H (2016) Oncometabolite D-2-Hydroxyglurate Directly Induces Epithelial-Mesenchymal Transition and is Associated with Distant Metastasis in Colorectal Cancer. Sci Rep 6:36289. doi: 10.1038/srep36289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fukunaga T, Fujita Y, Kishima H, Yamashita T (2018) Methylation dependent down-regulation of G0S2 leads to suppression of invasion and improved prognosis of IDH1-mutant glioma. PLoS One 13 (11):e0206552. doi: 10.1371/journal.pone.0206552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aspuria PP, Lunt SY, Varemo L, Vergnes L, Gozo M, Beach JA, Salumbides B, Reue K, Wiedemeyer WR, Nielsen J, Karlan BY, Orsulic S (2014) Succinate dehydrogenase inhibition leads to epithelial-mesenchymal transition and reprogrammed carbon metabolism. Cancer Metab 2:21. doi: 10.1186/2049-3002-2-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Loriot C, Domingues M, Berger A, Menara M, Ruel M, Morin A, Castro-Vega LJ, Letouze E, Martinelli C, Bemelmans AP, Larue L, Gimenez-Roqueplo AP, Favier J (2015) Deciphering the molecular basis of invasiveness in Sdhb-deficient cells. Oncotarget 6 (32):32955–32965. doi: 10.18632/oncotarget.5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.He X, Yan B, Liu S, Jia J, Lai W, Xin X, Tang CE, Luo D, Tan T, Jiang Y, Shi Y, Liu Y, Xiao D, Chen L, Liu S, Mao C, Yin G, Cheng Y, Fan J, Cao Y, Muegge K, Tao Y (2016) Chromatin Remodeling Factor LSH Drives Cancer Progression by Suppressing the Activity of Fumarate Hydratase. Cancer Res 76 (19):5743–5755. doi: 10.1158/0008-5472.CAN-16-0268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, Leigh I, Gorman P, Lamlum H, Rahman S, Roylance RR, Olpin S, Bevan S, Barker K, Hearle N, Houlston RS, Kiuru M, Lehtonen R, Karhu A, Vilkki S, Laiho P, Eklund C, Vierimaa O, Aittomaki K, Hietala M, Sistonen P, Paetau A, Salovaara R, Herva R, Launonen V, Aaltonen LA, Multiple Leiomyoma C (2002) Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet 30 (4):406–410. doi: 10.1038/ng849 [DOI] [PubMed] [Google Scholar]
- 97.Ooi A, Wong JC, Petillo D, Roossien D, Perrier-Trudova V, Whitten D, Min BW, Tan MH, Zhang Z, Yang XJ, Zhou M, Gardie B, Molinie V, Richard S, Tan PH, Teh BT, Furge KA (2011) An antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinoma. Cancer Cell 20 (4):511–523. doi: 10.1016/j.ccr.2011.08.024 [DOI] [PubMed] [Google Scholar]
- 98.Sudarshan S, Shanmugasundaram K, Naylor SL, Lin S, Livi CB, O’Neill CF, Parekh DJ, Yeh IT, Sun LZ, Block K (2011) Reduced expression of fumarate hydratase in clear cell renal cancer mediates HIF-2alpha accumulation and promotes migration and invasion. PLoS One 6 (6):e21037. doi: 10.1371/journal.pone.0021037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sciacovelli M, Goncalves E, Johnson TI, Zecchini VR, da Costa AS, Gaude E, Drubbel AV, Theobald SJ, Abbo SR, Tran MG, Rajeeve V, Cardaci S, Foster S, Yun H, Cutillas P, Warren A, Gnanapragasam V, Gottlieb E, Franze K, Huntly B, Maher ER, Maxwell PH, Saez-Rodriguez J, Frezza C (2016) Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition. Nature 537 (7621):544–547. doi: 10.1038/nature19353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zheng L, Cardaci S, Jerby L, MacKenzie ED, Sciacovelli M, Johnson TI, Gaude E, King A, Leach JD, Edrada-Ebel R, Hedley A, Morrice NA, Kalna G, Blyth K, Ruppin E, Frezza C, Gottlieb E (2015) Fumarate induces redox-dependent senescence by modifying glutathione metabolism. Nat Commun 6:6001. doi: 10.1038/ncomms7001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Payen VL, Porporato PE, Baselet B, Sonveaux P (2016) Metabolic changes associated with tumor metastasis, part 1: tumor pH, glycolysis and the pentose phosphate pathway. Cell Mol Life Sci 73 (7):1333–1348. doi: 10.1007/s00018-015-2098-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Porporato PE, Payen VL, Baselet B, Sonveaux P (2016) Metabolic changes associated with tumor metastasis, part 2: Mitochondria, lipid and amino acid metabolism. Cell Mol Life Sci 73 (7):1349–1363. doi: 10.1007/s00018-015-2100-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Luengo A, Gui DY, Vander Heiden MG (2017) Targeting Metabolism for Cancer Therapy. Cell Chem Biol 24 (9):1161–1180. doi: 10.1016/j.chembiol.2017.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Raez LE, Papadopoulos K, Ricart AD, Chiorean EG, Dipaola RS, Stein MN, Rocha Lima CM, Schlesselman JJ, Tolba K, Langmuir VK, Kroll S, Jung DT, Kurtoglu M, Rosenblatt J, Lampidis TJ (2013) A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol 71 (2):523–530. doi: 10.1007/s00280-012-2045-1 [DOI] [PubMed] [Google Scholar]
- 105.Dang L, Yen K, Attar EC (2016) IDH mutations in cancer and progress toward development of targeted therapeutics. Ann Oncol 27 (4):599–608. doi: 10.1093/annonc/mdw013 [DOI] [PubMed] [Google Scholar]
- 106.Gross MI, Demo SD, Dennison JB, Chen L, Chernov-Rogan T, Goyal B, Janes JR, Laidig GJ, Lewis ER, Li J, Mackinnon AL, Parlati F, Rodriguez ML, Shwonek PJ, Sjogren EB, Stanton TF, Wang T, Yang J, Zhao F, Bennett MK (2014) Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther 13 (4):890–901. doi: 10.1158/1535-7163.MCT-13-0870 [DOI] [PubMed] [Google Scholar]
- 107.Seltzer MJ, Bennett BD, Joshi AD, Gao P, Thomas AG, Ferraris DV, Tsukamoto T, Rojas CJ, Slusher BS, Rabinowitz JD, Dang CV, Riggins GJ (2010) Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res 70 (22):8981–8987. doi: 10.1158/0008-5472.CAN-10-1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Turcan S, Fabius AW, Borodovsky A, Pedraza A, Brennan C, Huse J, Viale A, Riggins GJ, Chan TA (2013) Efficient induction of differentiation and growth inhibition in IDH1 mutant glioma cells by the DNMT Inhibitor Decitabine. Oncotarget 4 (10):1729–1736. doi: 10.18632/oncotarget.1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gribble AD, Ife RJ, Shaw A, McNair D, Novelli CE, Bakewell S, Shah VP, Dolle RE, Groot PH, Pearce N, Yates J, Tew D, Boyd H, Ashman S, Eggleston DS, Haltiwanger RC, Okafo G (1998) ATP-Citrate lyase as a target for hypolipidemic intervention. 2. Synthesis and evaluation of (3R,5S)-omega-substituted-3-carboxy-3, 5-dihydroxyalkanoic acids and their gamma-lactone prodrugs as inhibitors of the enzyme in vitro and in vivo. J Med Chem 41 (19):3582–3595. doi: 10.1021/jm980091z [DOI] [PubMed] [Google Scholar]
- 110.Gribble AD, Dolle RE, Shaw A, McNair D, Novelli R, Novelli CE, Slingsby BP, Shah VP, Tew D, Saxty BA, Allen M, Groot PH, Pearce N, Yates J (1996) ATP-citrate lyase as a target for hypolipidemic intervention. Design and synthesis of 2-substituted butanedioic acids as novel, potent inhibitors of the enzyme. J Med Chem 39 (18):3569–3584. doi: 10.1021/jm960167w [DOI] [PubMed] [Google Scholar]
- 111.Gallardo-Perez JC, Rivero-Segura NA, Marin-Hernandez A, Moreno-Sanchez R, Rodriguez-Enriquez S (2014) GPI/AMF inhibition blocks the development of the metastatic phenotype of mature multi-cellular tumor spheroids. Biochim Biophys Acta 1843 (6):1043–1053. doi: 10.1016/j.bbamcr.2014.01.013 [DOI] [PubMed] [Google Scholar]
- 112.Grewer C, Grabsch E (2004) New inhibitors for the neutral amino acid transporter ASCT2 reveal its Na+-dependent anion leak. J Physiol 557 (Pt 3):747–759. doi: 10.1113/jphysiol.2004.062521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dahlmanns M, Yakubov E, Chen D, Sehm T, Rauh M, Savaskan N, Wrosch JK (2017) Chemotherapeutic xCT inhibitors sorafenib and erastin unraveled with the synaptic optogenetic function analysis tool. Cell Death Discov 3:17030. doi: 10.1038/cddiscovery.2017.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nagane M, Kanai E, Shibata Y, Shimizu T, Yoshioka C, Maruo T, Yamashita T (2018) Sulfasalazine, an inhibitor of the cystine-glutamate antiporter, reduces DNA damage repair and enhances radiosensitivity in murine B16F10 melanoma. PLoS One 13 (4):e0195151. doi: 10.1371/journal.pone.0195151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cobler L, Zhang H, Suri P, Park C, Timmerman LA (2018) xCT inhibition sensitizes tumors to gamma-radiation via glutathione reduction. Oncotarget 9 (64):32280–32297. doi: 10.18632/oncotarget.25794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stepulak A, Sifringer M, Rzeski W, Brocke K, Gratopp A, Pohl EE, Turski L, Ikonomidou C (2014) AMPA antagonists inhibit the extracellular signal regulated kinase pathway and suppress lung cancer growth. Cancer Biology & Therapy 6 (12):1908–1915. doi: 10.4161/cbt.6.12.4965 [DOI] [PubMed] [Google Scholar]
- 117.Gajer JM, Furdas SD, Grunder A, Gothwal M, Heinicke U, Keller K, Colland F, Fulda S, Pahl HL, Fichtner I, Sippl W, Jung M (2015) Histone acetyltransferase inhibitors block neuroblastoma cell growth in vivo. Oncogenesis 4:e137. doi: 10.1038/oncsis.2014.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Santer FR, Hoschele PP, Oh SJ, Erb HH, Bouchal J, Cavarretta IT, Parson W, Meyers DJ, Cole PA, Culig Z (2011) Inhibition of the acetyltransferases p300 and CBP reveals a targetable function for p300 in the survival and invasion pathways of prostate cancer cell lines. Mol Cancer Ther 10 (9):1644–1655. doi: 10.1158/1535-7163.MCT-11-0182 [DOI] [PubMed] [Google Scholar]
- 119.Fraser H, Hislop C, Christie RM, Rick HL, Reidy CA, Chouinard ML, Eacho PI, Gould KE, Trias J (2009) Varespladib (A-002), a secretory phospholipase A2 inhibitor, reduces atherosclerosis and aneurysm formation in ApoE−/− mice. J Cardiovasc Pharmacol 53 (1):60–65. doi: 10.1097/FJC.0b013e318195bfbc [DOI] [PubMed] [Google Scholar]
- 120.Higashino Ki K, Yokota Y, Ono T, Kamitani S, Arita H, Hanasaki K (2002) Identification of a soluble form phospholipase A2 receptor as a circulating endogenous inhibitor for secretory phospholipase A2. J Biol Chem 277 (16):13583–13588. doi: 10.1074/jbc.M108752200 [DOI] [PubMed] [Google Scholar]