Abstract
With almost 2 million new HIV infections worldwide each year, the prevention of HIV infection is critical for stopping the pandemic. The only approved form of pre-exposure prophylaxis is a costly daily pill, and it is recognized that several options will be needed to provide protection to the various affected communities around the world. In particular, many at-risk people would benefit from a prevention method that is simple to use and does not require medical intervention or a strict daily regimen. We show that silk fibroin protein can be formulated into insertable discs that encapsulate either an antibody (IgG) or the potent HIV inhibitor 5P12-RANTES. Several formulations were studied, including silk layering, water vapor annealing and methanol treatment to stabilize the protein cargo and impact the release kinetics over weeks. In the case of IgG, high concentrations were released over a short time using methanol treatment, with more sustained results with the use of water vapor annealing and layering during device fabrication. For 5P12-RANTES, sustained release was obtained for 31 days using water vapor annealing. Further, we show that the released inhibitor 5P12-RANTES was functional both in vitro and in ex vivo colorectal tissue. This work shows that silk fibroin discs can be developed into formidable tools to prevent HIV infection.
Keywords: Silk fibroin, controlled release, HIV prevention, HIV microbicide, broadly neutralizing antibody, 5P12-RANTES
1. Introduction
Currently there are almost 2 million new HIV infections worldwide per year, a number that remains stubbornly high due to limited prevention methods even as treatment options continue to expand. Several populations are at particularly high risk: in the developed world, men who have sex with men (MSM) are disproportionately affected; while in the developing world, young women are among the most vulnerable [1–3]. Indeed, worldwide, heterosexual women comprise the majority of adults living with HIV. Recent clinical trial results have revealed a particularly troubling trend, showing that even efficacious prevention methods are not adopted by young women and adolescents (25 years old and below), while their older peers show better compliance and attain some level of protection. For example, the VOICE (Vaginal and Oral Interventions to Control the Epidemic) trials included over 5,000 women and utilized oral prophylaxis (daily pills) as well as gel inserts. In these studies, low user compliance among young women led to a nearly 10% HIV infection rate at some sites for that group [4]. More recently, the vaginally inserted dapivirine ring has shown some success but requires user compliance for effectiveness and was found to not be effective for users under the age of 21 [5]. Overall, while these methods and current trials with intravenous/injectable antibodies [6] are all likely to provide good protection, the issue remains that user compliance is a key, often overlooked, factor in HIV prevention, particularly among adolescents. Therefore, a critical task in reducing new HIV infections is to provide alternatives that are attractive to the user so that they will be properly utilized.
HIV microbicides are generally envisioned as topically administered, user-controlled antiviral agents such as insertable gels or films. Ideally, these would not need refrigeration and could be obtained inexpensively off-the-shelf in the developing world for use as needed. Due to the low cost and lack of need for advanced planning or medical intervention, microbicides can fill an important niche in the fight against HIV. However, as mentioned, low compliance with gels and other modalities has led to the need for options that are more attractive to users. As such, a microbicide that is capable of providing sustained release over the course of days or weeks would be a critical tool for use by vulnerable populations that are unwilling or unable to comply with strict dosing schedules for protection.
Silk fibroin (hereinafter referred to as silk) is a natural protein derived from the silk cocoons of Bombyx mori silkworms, and has been shown to be biocompatible, biodegradable, non-inflammatory, and extremely versatile in its applications as it can be formed into nano/microparticles, microneedles, hydrogels, sponges, fibers, films, discs and tubes [7]. Silk fibroin, the core protein used in this work, does not cause an immune response or a significant inflammatory reaction as demonstrated in many publications over the past 20 years, as well as based on the FDA approval for silk-based medical devices. Thus, it can be safely applied via vaginal or rectal routes [8, 9]. In addition to being a Food and Drug Administration (FDA) approved biomaterial as medical sutures and soft tissue scaffolds [10], silk has shown the ability to successfully deliver a wide range of bioactive molecules including antineoplastic drugs [11–18], antibiotics [19], antiepileptics [20], genes [21, 22] and biological drugs such as growth factors [23] and antibodies [24]. Silk also increases the stability of drugs and biomacromolecules [25–27].
Protein HIV entry inhibitors are particularly valuable as potential microbicides, both because of their high potency and because they are not generally used in antiretroviral treatment and therefore would not be expected to promote viral escape. These proteins include broadly neutralizing antibodies (bnAbs) as well as the proteins 5P12-RANTES (5P12R) and griffithsin, all of which are highly potent (sub-nM effectiveness in vitro), and with a range of properties that are consistent with vaginal and rectal administration [28–31]. BnAbs have been effective in non-human primates and are currently in clinical trials as intravenous prevention agents [6, 32, 33] and have been incorporated into vaginal rings [34]. 5P12-RANTES a CCR5-binding protein which is derived from the human chemokine RANTES [35] is non-inflammatory, able to be made in clinical quantities, and is stable in both vaginal and rectal lavage [29, 36–38] and is being prepared for use in clinical trials.
Recently, we showed that silk discs could stabilize multiple HIV entry inhibitors such as 5P12-RANTES for over a year at 50°C, and that silk discs could mediate the extended release of small amounts of griffithsin for a month [39]. Our goal has been to develop silk for the sustained release of inhibitory amounts of several microbicidal candidate proteins, including bnAbs and 5P12-RANTES for use as vaginal inserts. Here we present the use of silk fibroin to mediate the sustained release of a model antibody (IgG1) and of 5P12-RANTES. We show that silk inserts can be loaded with substantial amounts of inhibitor, and that the protein is released over the course of a month. Ex vivo studies in blood and colorectal tissue, using released 5P12-RANTES, showed inhibition of HIV infection, demonstrating the feasibility of silk as a sustained release delivery vehicle for HIV microbicides.
2. Materials and Methods
2.1. Materials
Purified murine IgG1 monoclonal antibody was provided by Sanofi Genzyme Corporation (Framingham, MA). Sodium chloride (NaCl), disodium hydrogen phosphate dihydrate (Na2HPO4), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), lithium bromide (LiBr), sodium carbonate (Na2CO3) and methanol (MeOH) were purchased from Sigma Aldrich (St. Louis, MO). Phosphate buffered saline (PBS) was obtained from Gibco™ (Life Technologies, Carlsbad, CA). 15N-isotopically labelled ammonium chloride (15NH4Cl) was purchased from Cambridge Isotopes Lab (Tewksbury, MA).
2.2. Production of the 5P12-RANTES Protein Inhibitor
The protein 5P12-RANTES was produced recombinantly as described previously [40, 41], Briefly, the gene encoding 5P12-RANTES was subcloned into the pET32a expression vector, with N-terminal His6 and Thioredoxin fusion tags. The vector plasmid was transformed into BL21 (DE3) E. coli cells (Novagen) and cultured in M9 media with 15NH4Cl as the sole nitrogen source. Protein overexpression was induced by addition of isopropyl β-D-1-thiogalactopyranoside (IPTG) to 1 mM concentration and incubated with shaking at 22°C for 2 hours, followed by centrifugal harvest of cells. The bacterial pellets were resuspended in lysis buffer (8.0 M Urea, 500 mM NaCl, 10 mM sodium phosphate, pH 7.8) and cell membranes disrupted by homogenization (Avestin®). After lysate centrifugation (27,000 × g) for 1 hour, supernatants were collected and target protein purified using pre-packed Nickel(Ni2+)-charged HiTrap® IMAC Sepharose FF columns (GE Healthcare Life Sciences), followed by refolding using modified conditions identified from the Foldlt™ Screen (Hampton Research, Aliso Viejo, CA). During subsequent dialysis, enterokinase was added to the protein to cleave the N-terminal fusion tag [41]. The 5P12-RANTES protein was further purified by reversed-phase HPLC (RP-HPLC) using a C4 column (GraceVydac, Hesperia, CA), lyophilized and stored as powder until use. Protein purity and integrity were verified by SDS-PAGE and by 1H–15N heteronuclear single quantum coherence (HSQC) spectroscopy. Protein concentrations in solution were determined from sequence-based calculated molar extinction coefficients at 280 nm (http://web.expasy.org/protparam). To fully cyclizethe N-terminal glutamine residue (which spontaneously cyclizes in solution to form a pyroglutamate moiety) of 5P12-RANTES, the protein was prepared at >1 mM concentration in 20 mM sodium phosphate buffer, pH 2.5, and incubated at 37°C for at least 120 hours [41], and completion of cyclization was verified by NMR (1H–15N HSQC spectra, data not shown).
2.3. Lyophilization of Antibody
Antibody solutions were formulated in 0.02 M histidine buffer (pH 6.0) with 0.5% (w/v) sucrose at a 5 mg/mL concentration. To obtain powder IgG1, antibody solutions were lyophilized in a LyoStarll tray freeze dryer (FTS Systems, Stone Ridge, NY) as previously described [24]. Samples were frozen to −45°C and held for 8 h. Primary drying was performed at −20°C, 100 mTorr for 40 h, followed by secondary drying at 35°C, 100 mTorr for 11 h. Lyophilized IgG1 samples were stored at 4°C until use.
2.4. Silk Fibroin Isolation
Silk fibroin was isolated from B. mori cocoons as previously described [7]. Briefly, cocoons were cut into approximately 1cm2 pieces and degummed by boiling in 0.02 M Na2CO3 for 30 minutes (30 MB). Obtained fibers were rinsed thoroughly in deionized water to remove sericin proteins and air-dried overnight. Following overnight air-drying, silk fibroin fibers were dissolved of 9.3M lithium bromide (1 g in 4 mL) for 4 h at 60°C, then the solution was dialyzed (3.4 kDa MWCO, Thermo Fisher Scientific, Waltham, MA) against ultrapure water at room temperature for 3 days to remove LiBr. The resulting silk solution (~7%) was centrifuged to remove residual debris and stored at 4°C until use in production of IgG1-loaded devices. The silk solution used in production of 5P12-RANTES discs underwent the additional step of sterilization by autoclave and the resultant (−5.4% wt./vol.) solution was stored at 4°C until use.
2.5. Preparation of Silk Discs
Six percent, silk solution was used to prepare three types of discs; methanol treated (MT), water vapor annealed (WVA) and silk coated (SC). One mL silk solution was placed in 24-well plates and lyophilized to form blank silk discs, which were then soaked in 1 mL of 80% MeOH for an hour to reduce solubility. Insoluble discs were then soaked into 1 mL of IgG1 aqueous solution (2.5 mg/mL)for 3 days and then air-dried to obtain IgG1 loaded MT discs. To obtain WVA discs, IgG1 was weighed (2.5 and 5 mg) and dissolved in silk solution and then 1 mL of the mixture was placed in 24-well plates and lyophilized. The resulting discs were water vapor annealed [42] for 24 hours at room temperature to induce crystalline structure. Some WVA discs were then dip coated with 10 layers of silk to form SC discs.
Solutions of 5P12-RANTES (159 μg) were prepared in 20 mM HEPES buffer (pH 8.0) with or without 150 mM NaCl. The protein solutions were then combined with the aqueous silk solution to produce mixtures containing a final concentration of 20 μM 5P12-RANTES, 1.5% or 2% silk, and either 80 mM NaCl or no Salt. Sets of controls were prepared using ‘blank’ solutions containing only the HEPES buffer (and no 5P12-RANTES) that were combined with silk to create discs with the desired silk percentage and salt content. Sets of 1.0 mL solution aliquots were pipetted into sterile 24-well plates (with 1.7 cm well diameter = ~2.3 cm2 bottom surface area), frozen and lyophilized. Silk discs were then prepared for sustained release by water vapor annealing (WVA) at 37°C with ≥75% relative humidity (RH) for various amounts of time [42]. Trays of discs were then transferred to a 37°C forced-air incubator to allow the discs to dry, and were then stored in room-temperature desiccators until use (or else were stored in a 50°C forced-air incubator for 1 month prior to commencement of drug release experiments). All stock solutions used in the creation of the 5P12-RANTES/silk discs were assayed for endotoxin levels using the ToxinSensor Gel Clot Endotoxin Assay Kit (Genscript, Piscataway, NJ) and showed less than 0.25 EU/mL.
2.6. Characterization of Silk Discs
All discs were washed in PBS to remove free IgG1 and PBS was analyzed to calculate the antibody loading in WVA and SC silk discs. MT disc antibody loading was calculated based on the IgG1 concentration left in the loading solution. IgG1 was quantified chromatographically, using a 1200 series HPLC (Agilent Technologies, Santa Clara, CA) and a protein G ID cartridge (Applied Biosystems, Carlsbad, CA) [24, 27]. The binding mobile phase was 0.01M sodium phosphate, 0.15M sodium chloride, pH 7.3 and the elution buffer was 0.012M hydrochloric acid, 0.15M sodium chloride, pH 2.0 with a flow rate of 1.5 mL/min. Column temperature was kept at 5°C during the analysis and the detection wavelength was 220 nm. Antibody concentrations were calculated using a standard curve generated under the same conditions.
Scanning Electron Microscopy (SEM) imaging was performed to evaluate the morphology of the IgG1-containing silk discs using a Zeiss EVO-10MA microscope (Zeiss, Oberkochen, Germany) at 5 kV accelerating voltage. Prior to imaging, the disc samples were coated with ~10 nm gold using a SC7620 sputter coater (Quorum Technologies, UK). Sets of silk discs for 5P12-RANTES release experiments were sputter-coated with ~20 nm gold using a Polaron SEM Coating Unit E5000 (Bio-Rad Microscience Ltd.) and SEM imaging was performed using a Zeiss GeminiSEM-500 microscope with 3 kV accelerating voltage.
The structural features and crystalline structure of the silk discs were evaluated using Fourier Transform Infrared (FTIR) Spectroscopy(JASCO FTIR 6200 spectrometer, Jasco, USA ora Bruker Vertex 70 spectrometer, Germany). The Amide I region (1605–1705 cm-1) of silk fibroin structure was deconvoluted using OPUS 5.0 software (Bruker Optics, USA) to determine β-sheet content as described previously [43]. Fourier Self Deconvolution was performed using a Lorentzian peak with a half bandwidth of 27 cm−1 and a noise reduction factor of 0.3. One Way ANOVA was performed for statistical analysis (p < 0.05) of the FTIR results using IBM SPSS Statistics 22 Software (New York, USA).
2.7. In Vitro Release Studies
Sustained release of IgG from silk discs
In vitro release studies were performed in 1 mL PBS solution and incubated at 37°C. All of the PBS was collected at the sampling times and replaced with fresh PBS. The amount of released IgG1 was quantified using the HPLC method described in section 2.6. Blank disc samples were also tested as a control group.
Sustained release of 5P12-RANTES from silk discs
The WVA-treated silk discs containing 5P12-RANTES (and silk-only control discs) were kept in sterile 24-well plates with 1.0 mL of PBS added to each disc, and incubated at 37°C with relative humidity ≥ 85%. To account for the initial “burst” effect often seen in drug release devices, the incubation solutions in each well were removed after the first hour and fresh release media (PBS) was added to each disc. Thereafter, time points were taken daily for the first week (7 days) and every other day until Day 31. For each time point, the extracted volume of release medium was measured and fresh solution (sterile PBS) was added to continue the incubation. For the control set silk-only discs, time points were taken in this same manner. The concentration of 5P12-RANTES in each time point sample was quantified by ELISA. In brief, time point samples were diluted with a given volume of coating buffer (100 mM sodium carbonate/bicarbonate, pH 9.5) and mixtures were added 100 μL/well into a 96-well plate (Nunc, Thermo Fisher) and incubated at 4°C overnight. These solutions were subsequently removed, and the plate wells were blocked with 3% BSA in Tris-Buffered Saline (‘TBS’, 150 mM NaCl in 20 mM Tris, pH 7.6). After incubation and wash steps, Biotinylated anti-Human RANTES monoclonal antibody (clone VL-1, Thermo Scientific) was added to each well. Following incubation and wash steps, horseradish peroxidase (HRP)-conjugated streptavidin (R & D Systems) was added to each well. Subsequent to additional wash steps, the substrate 2,2’-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (‘ABTS’, Thermo Scientific) was added to each well and signal development was followed by measurement of absorbance at 405 nm. Absorbance readings for ‘silk-only’ control disc time points were subtracted from those measured in corresponding sets of 5P12-RANTES–loaded silk discs. The standard curve in each assay was generated from a 20 μM 5P12-RANTES stock solution used to construct a 10-point concentration ladder with points ranging from 5 nM to 120 nM. Concentrations down to 10 nM could be readily detected in this manner. Readings from the standard curve were fit to a four-parameter logistic (4PL) curve and sample concentrations were calculated relative to this fitted curve, with time point concentration readings being subsequently multiplied by the appropriate dilution factor.
2.8. IgG Recovery from Silk Discs
Recovery of IgG1 from silk discs was evaluated at 4°C, room temperature (RT) and 37°C for 4 weeks as a parameter of stability. After the incubation time, non-treated discs were dissolved in water and recovered IgG1 was calculated in comparison to IgG1 solutions that were incubated in the same storage conditions. IgG1 amounts in the samples were quantified using IgG (Total) Mouse Uncoated ELISA Kit (Invitrogen, Carlsbad, CA) as instructed by the manufacturer. Denatured antibody (heated at 90°C for 90 minutes) was used to confirm selectivity of ELISA method. IBM SPSS Statistics 22 Software was used to perform One Way ANOVA and Tukey HSD post hoc tests for statistical analysis (p<0.05).
2.9. Cells and virus
TZM-bl cells [44–46] were grown in Dulbecco’s Minimal Essential Medium (DMEM) (Sigma-Aldrich, Inc., St. Louis, MO) containing 10% fetal calf serum (FCS), 2mM L-glutamine and antibiotics (100 U of penicillin/ml, 100 μg of streptomycin/ml) at 37°C in an atmosphere containing 5% CO2.
Full-length, replication and infection-competent proviral HIV-1 clone, pYU.2 [47, 48] was provided by the NIH AIDS Research & Reference Reagent Program (http://www.aidsreagent.org/). The plasmid was transfected into 293FT cells and passaged through activated peripheral blood mononuclear cells [49] for 11 days.
2.10. Patients and tissue explants
Surgically-resected specimens of colorectal tissue were collected at St George’s Hospital, London and St Mary’s Hospital, Imperial College London, UK. All tissues were collected after receiving signed informed consent from all patients and under protocols approved by the Local Research Ethics Committee. All patients were HIV negative. On arrival in the laboratory, resected tissue was cut into 2-3 mm3 explants comprising both epithelial and muscularis mucosae as described previously [50]. Colorectal explants were maintained with DMEM containing 10% fetal calf serum, 2mM L-glutamine and antibiotics (100 U of penicillin/ml, 100 μg of streptomycin /ml, 80 μg of gentamicin /ml) at 37°C in an atmosphere containing 5% CO2.
2.11. HIV Inhibition assays
HIV Inhibition assays were performed using a standardized amount of virus culture supernatant normalized for infectivity. Cells or tissue explants were incubated with silk supernatant for 1 h at 37°C before addition of virus. For assays in TZM-bl cells, virus was left for 48 h. Alternatively, virus (103 TCID50) was added to tissue explants for 2 h and then explants were washed 4 times with PBS before transferring onto gelfoam rafts (Welbeck Pharmaceuticals, UK) as described previously [51]. On days 3, 7, 11 and 15, approximately 2/3 of the supernatant was harvested and explants were re-fed with fresh media without compound. The extent of virus replication was determined in TZM-bl cells by luciferase quantification of cell lysates (Promega, Madison, WI) and in tissue explants by measuring the p24 antigen concentration in supernatants (HIV-1 p24 ELISA, Launch Diagnostics Ltd, UK), as described previously [50].
Statistical and mathematical analysis of HIV inhibition
IC50 values were calculated from sigmoid curve-fits (GraphPad Prism). All IC50 data presented fulfill the criterion of R2 > 0.7.
3. Results and Discussion
3.1. Formulation and Characterization of IgG1 and 5P12-RANTES in Silk Discs
The model antibody IgG was incorporated into a 6% silk solution in three ways, as described in Methods. Methanol treated (MT) discs utilized lyophilized silk discs that were then treated with methanol and subsequently introduced to IgG1. Water vapor annealed (WVA) discs were prepared by first mixing the silk solution with IgG1, followed by lyophilization and annealing in a controlled humid environment. Finally, silk coated (SC) discs were prepared as WVA discs then subsequently coated with layers of soluble silk to minimize initial burst release and to allow for sustained release. IgG1 loading in the silk discs was calculated based on the IgG1 concentration in PBS used to rinse away residual the free antibody, as measured by HPLC. Average antibody loadings were 1425.7 ± 47.7, 4941.8 ± 49.2 and 2477.5 ± 6.0 μg for MT, WVA and SC discs respectively. The highest loading efficiency achieved with SC discs was 99.1±0.2% of the available antibody. WVA discs followed with 98.8±1.0%, whereas the loading efficiency for MT discs was 57.0±1.9%.
Morphology of the silk discs was evaluated using SEM. Micrographs (Figure 1) show the porous structure of the silk discs prepared for IgG release studies, where larger pores are present in the blank (no IgG1) MT discs (Figure 1A) compared to the blank WVA discs (Figure 1B). The smaller pore sizes of WVA discs were associated with the higher loading rate due to the larger surface area [52]. The effects of antibody and protein loading on porous structure were also investigated. Addition of the IgG1 protein to Silk Fibroin prior to casting and WVA produced discs with smaller average pore size (Figure 1C) compared to that of the blank discs.
Figure 1.
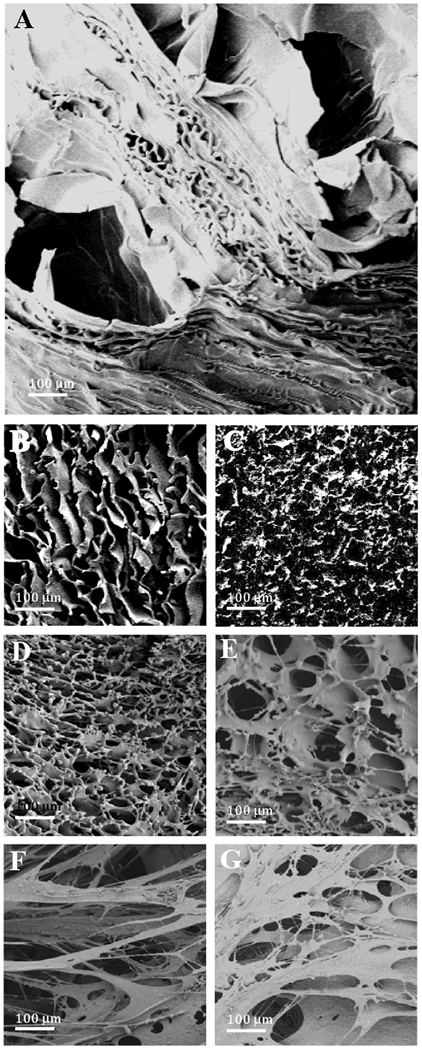
SEM micrographs showing the porous structure of A) MeOH treated, 6% silk, blank discs (MT), B) Watervapor annealed (WVA), 6% silk, blank discs, C) WVA, 6% silk, IgG1 loaded discs; (D and E) WVA-treated, 2% silk, blank control disks with or without salt in the formulation; (F and G) 5P12-RANTES-loaded WVA-treated, 2% silk discs with or without salt. (Magnification=100×, scale bar=100 μm)
FTIR analysis confirmed the insoluble structure of the IgG-related silk disc formulations following methanol treatment or water vapor annealing based on their crystallinity. All formulations had high crystalline structures where β-sheet fractions were between 33.9±1.0 to 39.0±0.2 percent (Figure 2A). The data showed MT discs have a significantly higher β-sheet ratio as methanol treatment induces the silk II structure, whilst water vapor annealing induces silk I structure, which is an organized crystalline form of fibroin which then transforms into the β-sheet structure of Silk II that makes the fibroin strong, tough, and resistant to release [53–55]. However, the IgG1 loaded MT discs showed similar β-sheet content to that of the WVA discs, which indicates that the difference in crystalline structure of the discs is not a significant factor in their release profiles.
Figure 2.
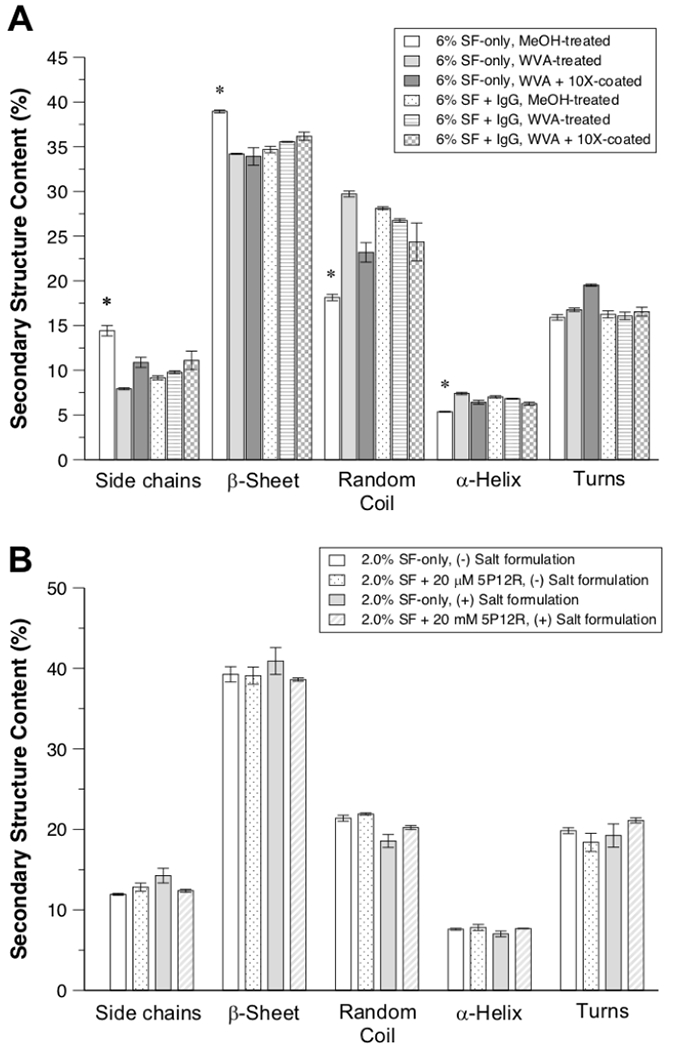
Structural properties of silk (SF) discs quantified by FTIR spectroscopy. Percentage of the secondary structural content in all formulations for IgG-related (A) and 5P12-RANTES-related (5P12-R) (B) silk discs. (+) Salt formulation indicates the presence of 80 mM NaCl to the silk solution prior to lyophilization. (All data represent mean ± SD for n=3, statistical significance within groups has shown with *(p < 0.05)).
In order to achieve sustained release of 5P12-RANTES from silk over a 1-month time period, different formulations were explored, including variation of parameters such as silk percentage, length of time used in the WVA process, and the salt (NaCl) content in the formulation. For 20 μM 5P12-RANTES concentration in a 1.0 mL final volume (corresponding to a total of 158.5 μg inhibitor loaded), it was experimentally determined (Figure 4) that reasonable 5P12-RANTES release profiles could be achieved with a formulation consisting of 2% (wt./vol.) silk and a WVA duration of 7 hours. The morphology of these disks (with or without 80 mM NaCl in the final prepared disc) was examined using SEM. The micrographs (Figure 1) reveal a highly porous architecture for the silk-only control disks, though inclusion of salt (with final in-silk concentration of 80 mM NaCl) resulted in smaller average pore size (Figure 1D vs. 1E). Inclusion of 5P12-RANTES protein in the silk discs significantly altered their porous structure, causing the formation of much larger pore size (Figure 1F,G) and a certain ‘stringy’ appearance for the formulation that included salt (80 mM NaCl in the 1.0 mL formula volume) (Figure 1F). There were also visible differences in the gross morphology (visible to the unaided human eye) of the discs: whereas the 5P12-RANTES-containing silk discs with no salt maintained a spongy and compressible form that could be readily lifted off the bottom of its slot in the 24-well tray after WVA treatment and overnight drying, the discs formulated with salt were much more brittle and friable, having a more ‘feathered-needlelike’ form that could not be readily removed from the bottom of the plastic well. Therefore, this formulation (WVA treated, 2% silk, formulated with salt) would likely need to be adjusted by increasing silk concentration or reducing salt for practical use.
Figure 4.
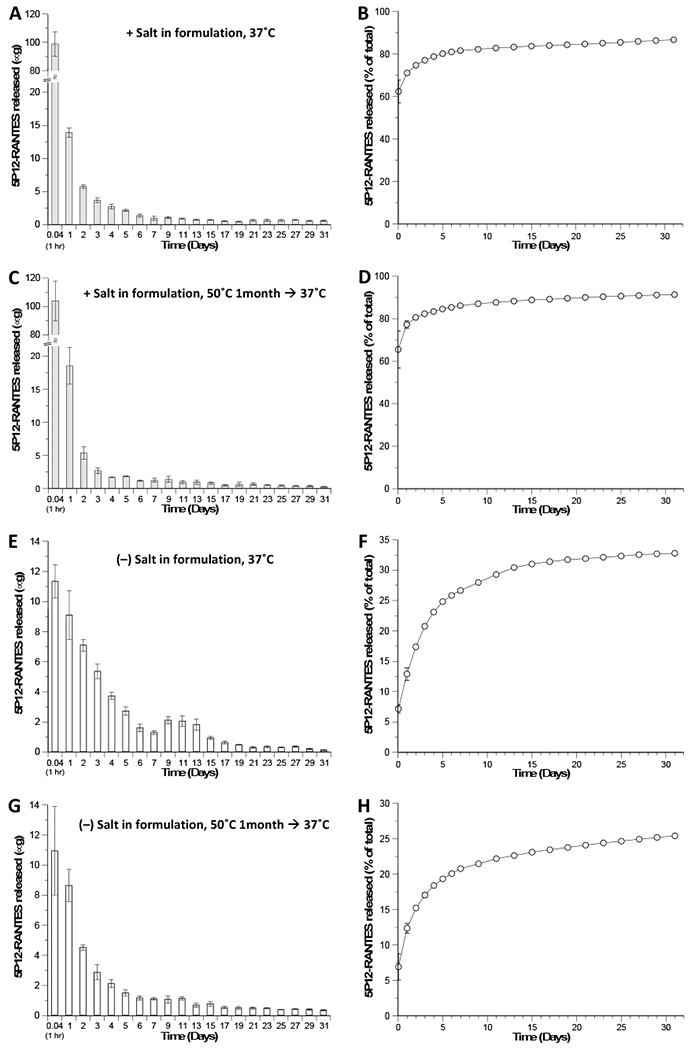
Periodic (left) and cumulative (right) in vitro release of 5P12-RANTES from WVA-treated 2% (wt./vol.) silk discs. One set of silk discs was formulated with final concentrations of 20 μM 5P12-RANTES and 80 mM Salt (NaCl) (A–D, gray bars and circles) and another set with 20 μM 5P12-RANTES and no Salt (E–H, white bars and circles). Incubations in PBS were carried out directly at 37°C (A–B, E–F)for 31 days; or after a set of discs had first been incubated under arid conditions at 50°C for 1 month (C–D, G–H) prior to addition of PBS and time point collection. The release medium (1.0 mL of PBS) was removed and replaced with fresh solution each day for the first 7 days, and subsequently every other day until Day 31. 5P12-RANTES concentrations in the release media were determined for each time point by ELISA (as per the Methods section). Data presented are the mean ± SD of a triplicate (n=3) of discs.
FT-IR analysis of secondary structural content was also performed for the 5P12-RANTES-related silk discs and revealed significant β-sheet content, ranging from 38.6 ± 0.2% to 40.9 ± 1.7% among all formulations examined (Figure 2B), consistent with the percentage β-sheet content previously reported for SF of 1 or 2% (wt./vol.) that had been WVA-treated at 37°C and for the same duration employed here [42]. The percent β-sheet content observed here was thus largely invariant to our inclusion of salt or 5P12-RANTES protein in the formulation, indicating that differences in gross morphology and porous architecture of the silk discs, as well as drug release profiles (Figure 4), are not correlated with differences in secondary structural content of the SF in these devices.
3.2. In Vitro Release Studies
Sustained release of IgG from silk discs
Phosphate-buffered saline (PBS) was incubated with each discat 37°C for various amounts of time to determine the release rate. MT, WVA and SC discs released 34.7±0.46 %, 10.4±0.41%, and 2.1±0.5% of the encapsulated IgG1 in the first 24 hours and 95.6±0.49 %, 18.9±0.01, 5.2±0.03% at the end of the 29 days, respectively (Figure 3). The released IgG1 was significantly different for all three formulations (p<0.05), where MT discs had a burst release and the highest release ratio in compliance with their large porous structure. Smaller pores create a larger surface area and provide a more controlled release from WVA discs [56]. SC discs released a smaller portion of the total loaded antibody where 10 layers of silk coating provided a sustained release profile without any burst. This showed that altering the layers of the coating to increase or decrease the release rate could modify release from the discs. Methanol treated discs were the most able to quickly release IgG, showing 34.7±0.46 % (494.3±6.6 μg) release in 1 day, with more material released at day 3 (1147.5±11.5 μg) and most released within a week (Figure 3). With the ability of releasing milligram levels of antibody, this formulation would therefore be suitable for so-called “weekend release” in which a person may be well protected for a few days but not for an extended period of time. On the other hand, SC discs are able to release smaller portion of the antibody over the course of a month with an average of 4.3 μg per day, which can be used to provide a long-term maintenance dose.
Figure 3.
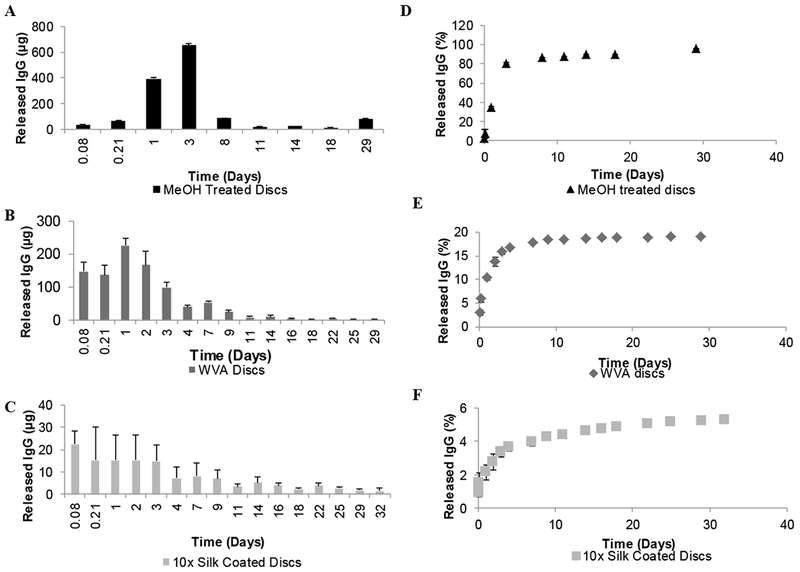
In vitro IgG1 release from silk discs. Released IgG1 amounts at each time point from A) MeOH treated discs, B) WVA discs, C) WVA 10× coated discs. Cumulative IgG1 release percentage from E) MeOH treated discs, F) WVA discs, G) WVA 10× coated discs. Incubations in PBS were carried out directly at 37°C for 32 days. The release medium (1.0 mL of PBS) was removed and replaced with fresh solution at every sampling time point. IgG1 concentrations in the release media were determined for each time point by HPLC (as per the Methods section). Data presented are the mean ± SD of a triplicate (n=3) of discs.
Sustained release of 5P12-RANTES from silk discs
As noted in the IgG release studies, WVA mediates a combination of traits in the silk disc to allow both a substantial burst of inhibitor release (to provide protection within minutes or hours of insertion), as well as a continuous release of inhibitor over the course of days and weeks. Therefore, silk discs prepared with 5P12-RANTES and WVA treatment were tested under several conditions to determine their stability and release capability. While extended duration of the WVA process results in a fully insoluble silk matrix with high β-sheet content, this can hamper drug release from the matrix; inversely, insufficient WVA processing can produce a silk scaffold that dissolves in aqueous solution and is unable to mediate sustained drug release [42]. For a formulation of 2% (wt./vol.) silk encapsulating 158.5 μg of 5P12-RANTES in a 1.0 mL volume (20 μM final in-silk concentration of the drug) a 7-hour WVA was determined to produce reasonable release profiles of the drug into PBS at 37°C (Figure 4).
Given the disparity in pl (isoelectric point) values of the drug (5P12-RANTES, pl ~9.5) and silk (pl ~4.5) [35, 57], it was hypothesized that, for near-neutral pH release medium (PBS, pH ~7.3), there might be a significant electrostatic component to the interaction between silk and 5P12-RANTES. In light of this, two different formulations were created—one containing a final 80 mM concentration of NaCl, and one with no salt. For the set of discs containing NaCl in the formulation (Figure 4A–B), the initial ‘burst’ time point (removed at T = 1 hour post-addition of PBS to the dried device) contained approximately 99 μg, corresponding to ~62% of the total amount of drug loaded, with a cumulative 71% release after the first 24 hours. After Day 7, a more linear release rate was observed with an average of ~0.34 μg per day between days 7 and 31, corresponding to maintenance of a ~43 nM concentration of inhibitor in the approximate 1 mL fluid volume used in this study, which is above the ex vivo IC50 of 5P12-RANTES measured in PBMCs and colorectal tissue explants [39], and orders of magnitude above the value in other in vitro tests [38, 40]. After 31 days, a cumulative total of 137.4 ± 11.9 μg of 5P12-RANTES had been released, corresponding to 86.7 ± 7.5% of the total amount of drug initially loaded into the device.
For the set of discs formulated without salt (Figure 4E–F), the initial (T = 1 hour) burst time point contained ~11.3 μg of inhibitor, corresponding to ~7.2% of total drug loading, and a cumulative total 12.9% released after the first 24 hours. For comparison, between days 7 and 31, an average of ~0.41 μg per day was released, corresponding to maintenance of a ~51 nM inhibitor concentration in a 1 mL volume. At the end of 31 days, a cumulative total 52 ± 5.9 μg or, 32.8 ± 3.7% of the total amount of drug loaded was released. Overall, we see that inclusion of NaCl in the formulation served to increase the initial ‘burst’ effect release of drug from the silk discs but did not significantly alter the rate of release between days 7 and 31, with the ‘no salt’ formulation having a just slightly higher release rate during that phase. Rather than having significant (noticeable?) effect on the hypothesized significant electrostatic contribution to the interaction between silk and 5P12-RANTES, the impact of salt included in the formulation may have merely served to alter the gross morphology of the silk discs (see Section 3.1 above), creating greater exposed surface area which, in turn, produced greater initial surface escape of the drug from the device.
Additional sets of silk-5P12-RANTES discs were stored for one month at 50°C to simulate unrefrigerated sub-Saharan conditions, then placed at 37°C and PBS release media added. As described before, 1.0 mL of PBS was incubated with the discs at 37°C and then removed and replaced with fresh buffer at various time points. As shown in Figure 4 (parts C–D and G–H), comparable release amounts and profiles were observed for these sets of discs as for the identically-formulated discs that were only ever exposed to temperatures of 37°C or less, demonstrating that 1 month of exposure to elevated temperature does not significantly alter the release kinetics of 5P12-RANTES from the silk. For all formulations and temperature exposure conditions tested, the WVA-treated silk discs maintained their general structure and appearance while gradually releasing 5P12-RANTES for 31 days. However, it should be noted that discs lacking salt in the formulation are likely to be more mechanically robust and better withstand handling and usage within the human body.
Overall, these results show that several silk formulations could be suitable for use as insertable anti-HIV microbicides, with perhaps the best combination of traits being observed in WVA-prepared discs. These show high release short term and significant release longer term of both the model antibody IgG and the HIV inhibitor 5P12-RANTES. Tuning the formulation with percentage of silk and amount of time for WVA may lead to more optimal results. The use of layering silk onto the discs to attenuate the initial burst in combination with WVA may also ultimately provide an optimal amount of short and long-term release. In our studies, substantially more protein was used in the IgG studies, demonstrating that silk can be loaded with the large amounts of protein that would be required for human studies. However, due to its potent inhibitory capability, 5P12-RANTES amounts (limited by quantity available) were consistent with full in vitro inhibition and are approaching the amount needed for in vivo macaque studies (preliminary work ongoing).
3.3. IgG1 Recovery from Silk Discs
In general silk stabilizes sensitive materials such as RNA, blood components and proteins, which remain functional even after extended periods at high temperatures [26, 39, 58, 59]. IgG1 was incubated at 4°C, room temperature (RT) and 37°C for 4 weeks, both in solution form and in a rapid-dissolve silk disc to evaluate the stabilization effect of silk.
The discs were dissolved in water on the day of preparation (D0) (controls) to test the immediate recovery of IgG1, and 87.0±3.9% of the IgG1 was recovered. This was used as the baseline level of IgG1 in the subsequent recovery calculations. In addition, the recovery of protein tends to decrease with time upon exposure of the silk matrix to heat and humidity resulting in silk crystallization, thus, making extraction of the IgG1 more difficult as the discs lose dissolution in water by 30 days [42].
After 3 days at 37°C, IgG1 in the extract had 62.8±15.2 % of the IgG1 was recovered from the silk discs. The percent recovered IgG1 was higher in the silk discs than in solution at all temperatures tested for 28 days (Figure 5) despite the losses in the extraction process due to the changes in the silk matrix with time. The recovered IgG1 loss in solution form was significant after 21 days at 4°C and 37°C (p<0.05), while no significant recovery loss was observed in silk discs except with the 37°C storage.
Figure 5.
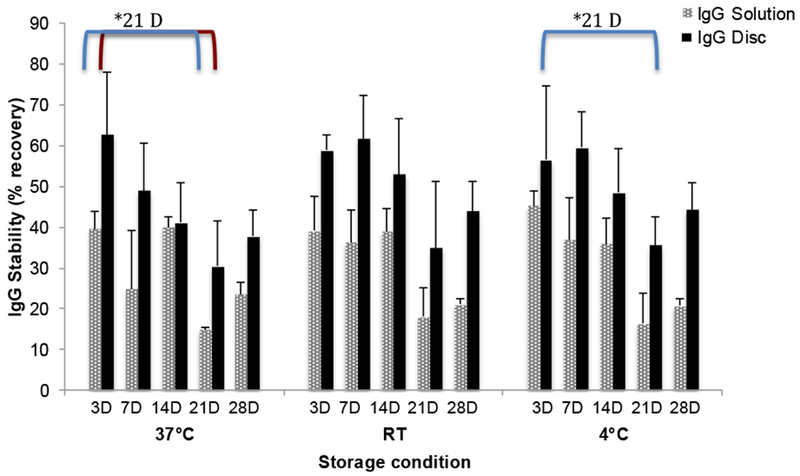
IgG1 recovery from silk discs after 4 weeks of incubation at various temperatures. Unbound and stable IgG1 amount extracted from silk discs was compared to IgG1 in solution following incubation at 4°C, RT (room temperature) and 37°C for 4 weeks. (All data represent mean ± SD for n=3, statistical significance within groups has shown with *(p< 0.05)).
3.4. Functional Protein released from silk discs
5P12-RANTES is functional when released from silk discs. 5P12-RANTES released from WVA-treated, salt containing discs, that had been incubated at 37°C (i,e, from Figure 4A and 4B), was tested against replication competent HIV as shown in Figure 6A. First, several time points were incubated in TZM-bl cells at various levels of dilution and then challenged with HIV strain cYU.2. Significant inhibition was observed for time points from 7, 21, and 31 days, including 100% inhibition up to three weeks for the undiluted sample. As shown in Figure 6B, significant inhibition over the silk-only control was also observed for inhibitor released from discs described in Figure 4C and 4D, that had first been incubated at 50°C then tested for sustained release at 37°C. In this case, near-full inhibition was observed for the 7-day sample, with less inhibition at later time points. Finally, silk discs formulated with lower salt content (from Figure 4 E and 4F) were fully inhibitory even when diluted 100-fold at the 7-day time point (Figure 6C). These discs were not tested past 7 days because our release studies showed essentially equivalent amounts of inhibitor released after one week for the discs formulated with and without salt. Further, osmolarity plays a large role in insertable formulations; particularly those that may be used in the digestive tract, so experiments proceeded largely with the silk discs containing moderate salt amounts [60–62],
Figure 6:
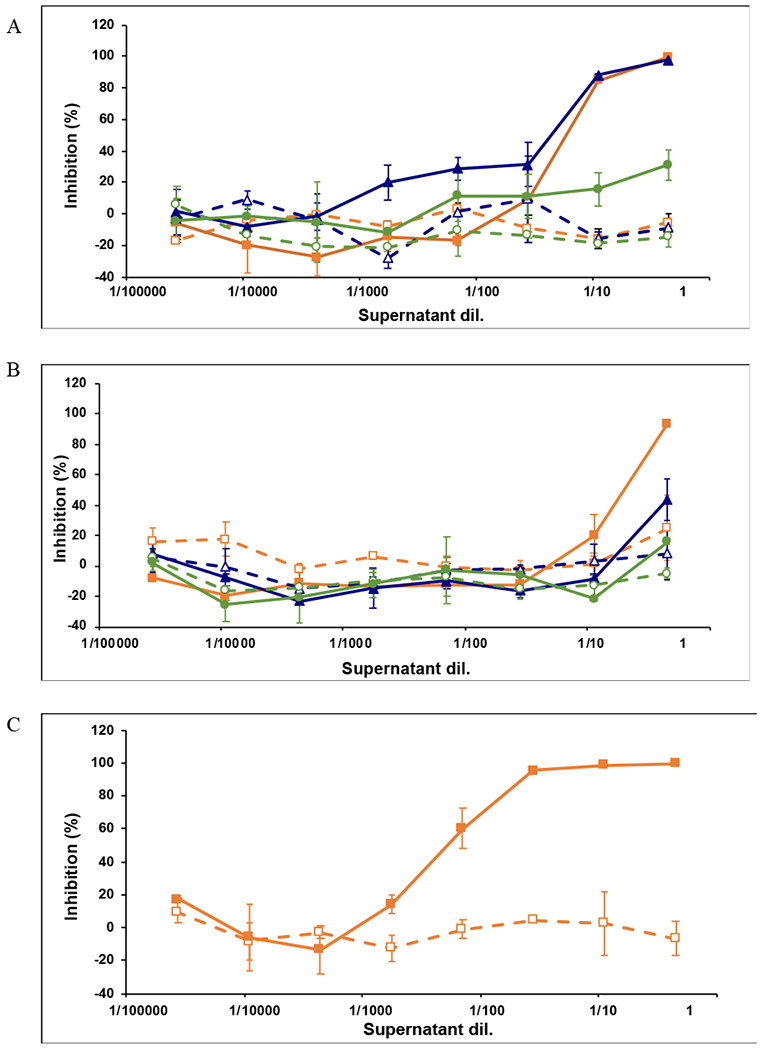
Inhibitory potency of 5P12-RANTES released at different time points from silk disks. TZM-bl cells were pre-incubated with serial dilutions of supernatants for 1 h prior to addition of virus, HIV-1 cYU.2. Luciferase expression (r.I.u. values) was determined after 48 h and the extent of inhibition by each drug was calculated. The percentage of inhibition was normalized relative to the r.I.u values obtained for cells grown in the absence of virus (100% inhibition) and for cells infected with virus in the absence of drug (0% inhibition). Inhibition by supernatants from control (open symbols) or 5P12-RANTES-containing silk discs (filled symbols) harvested at days 7 (□, ■), 21 (Δ, ▲) or 31 (○, ●) and containing A: 5P12-RANTES released from 2% silk, WVA discs formulated with 80 mM NaCl, incubated at 37°C; B: Inhibition of 5P12-RANTES released from 2% silk, WVA discs formulated with 80 mM NaCl, incubated at 50°C for one month, then stored at 37°C; C: Inhibition of 5P12-RANTES released from 2% silk, WVA discs formulated with no added salt, incubated at 37°C, release at Day 7. Data are the mean (± SD) of duplicates.
Functionality of the released 5P12-RANTES was also tested against human colorectal explant tissue. As shown in Figure 7, for all discs tested, supernatants harvested at day 7 were able to fully inhibit HIV-1 YU.2; and despite progressive loss of inhibition observed, supernatants harvested at day 31 still reach inhibitory levels above the IC50. As noted above, discs formulated without salt were inhibitory, but performed less well, possibly due to low osmolarity.
Figure 7:
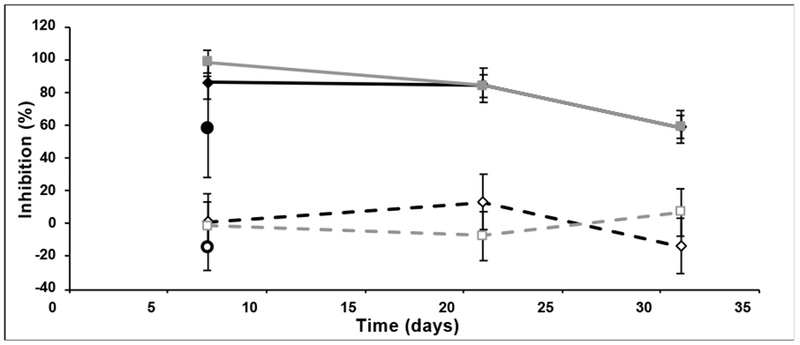
HIV Inhibitory function of 5P12-RANTES-containing supernatants in colorectal explants. Colorectal explants were treated for 1 h in the presence or absence of supernatants at a single dilution (3/5) prior to viral challenge with HIV-1 cYU.2. After 2 h of exposure to virus, explants were transferred on gelfoam rafts and cultured for 15 days. The levels of p24 in the harvested supernatants were quantified by ELISA and the extent of inhibition was calculated. The level of infection was normalized relative to the p24 values obtained for explants not exposed to virus (100% inhibition) and for explants infected with virus in the absence of compound (0% inhibition). Supernatants harvested at different time points from control (open symbols) or 5P12-RANTES-containing (filled symbols) 2% silk, WVA discs formulated with 80 mM NaCl incubated at 37°C (□, ■); incubated at 50°C for one month, then stored at 37°C (◇, ♦); or from 2% silk, WVA discs formulated with no added salt, incubated at 37°C (⦿, ●). Data shown are the mean (± SD) of triplicates at day 15.
Overall, this work shows that silk discs can encapsulate and release IgG, a model antibody that is expected to act similarly to broadly neutralizing antibodies against HIV. We also demonstrate that silk inserts can reliably release μg amounts of 5P12-RANTES daily over the course of weeks into PBS. Our current inserts are relatively small (1 mL) and loaded with 0.159 mg protein, resulting in concentrations that significantly larger than the IC50 of the protein. This level and even higher amounts are generally deemed necessary for in vivo use. These discs, loaded with higher protein amounts (experiments ongoing), are appropriate for macaque testing and would be scaled up for use in humans. Successful sustained release results are seen for discs that have been pre-incubated for 1 month at 50°C, demonstrating that these silk discs would not require refrigeration in developing countries such as those in sub-Saharan Africa, even if they are stored first for weeks in a warehouse or kept in an automobile.
The silk inserts, therefore, show several of the properties that are critical for use as HIV microbicides, such as the ability to use these without medical intervention, the stability after storage at high temperatures, and to be protective immediately upon insertion and potentially for several weeks. While this demonstrates the core of what is required for a vaginal microbicide, two key additional issues must be addressed. First, it has been repeatedly demonstrated that user acceptability is critically important, as clinical trials of effective materials have failed due to poor compliance, particularly among young women and adolescents [5]. Silk has some advantages in this regard, as it is a natural silk product that can be marketed as having properties associated with silk such as smoothness and even luxury. Further, silk can be formulated into a variety of shapes and colors, and the basic disc can be shaped by the user prior to insertion which is expected to be attractive to young women. A second major obstacle to a long-acting silk insert is the necessity of remaining in place in the cervico-vaginal area throughout menses and coitus. While studies are ongoing with macaques to determine in vivo distribution from silk in short time frames, the current formulation must clearly evolve to include muco-adhesives that would allow for success in placement for longer time periods [63–65].
Overall, we demonstrate that silk can be used to encapsulate antibodies and a potent anti-HIV protein, 5P12-RANTES, formulated to release inhibitory amounts of the protein over the course of weeks. This demonstrates that silk/protein combinations are uniquely capable of meeting numerous requirements for preventing HIV transmission in the real world situations where effectiveness and user acceptability are recognized as critical factors in successful outcomes. Here, the material can be formulated in either a quick dissolving format or in a sustained release format. Current protein inhibitors are used, as opposed to using small molecule drugs that are currently in the treatment regimen (and which may encourage HIV mutation making the small molecules less effective). Further, the material can be stored without refrigeration for improved distribution to users, can be easily inserted by the user; the silk insert can be prepared in a variety of shapes and even colors, and the material is a natural and safe product, leading to high user acceptability and demand. This unique combination of effectiveness over days or weeks and attractive formulations supports the assertion that silk/protein inserts could be a valuable component in an arsenal of HIV prevention choices.
4. Conclusions
We describe the formulation and characterization of silk discs that provide sustained release of two proteins that are relevant for HIV prevention. Using IgG as a model for broadly neutralizing antibodies, we demonstrate several formulations with silk and show release of protein up to a month. We further show that the potent HIV entry inhibitor 5P12-RANTES can be incorporated into silk discs, release protein for up to 31 days, and that the released protein can inhibit HIV infection in both blood and in human colorectal tissue. Therefore, silk is a highly promising material for use as a sustained release delivery vehicle in an HIV microbicide.
Acknowledgements
Authors acknowledge the support of the Imaging and Microscopy Facility (IMF) and Laura Showalter and Kennedy Nguyen at U.C. Merced.
Funding
The National Institutes of Health (R01AI112011) supported this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
Authors declare no conflict of interest.
References
- [1].Dellar RC, Dlamini S, Karim QA, Adolescent girls and young women: key populations for HIV epidemic control, J Int Aids Soc, 18 (2015) 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].HIV prevention among adolescent girls and young women, in, Unaids.
- [3].UNAIDS, UNAIDS Data in, 2018.
- [4].Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, Palanee T, Nakabiito C, van der Straten A, Noguchi L, Hendrix CW, Dai JY, Ganesh S, Mkhize B, Taljaard M, Parikh UM, Piper J, Masse B, Grossman C, Rooney J, Schwartz JL, Watts H, Marzinke MA, Hillier SL, McGowan IM, Chirenje ZM, Team VS, Tenofovir-Based Preexposure Prophylaxis for HIV Infection among African Women, New Engl J Med, 372 (2015) 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, Mgodi NM, Kiweewa FM, Nair G, Mhlanga F, Siva S, Bekker LG, Jeenarain N, Gaffoor Z, Martinson F, Makanani B, Pather A, Naidoo L, Husnik M, Richardson BA, Parikh UM, Mellors JW, Marzinke MA, Hendrix CW, van der Straten A, Ramjee G, Chirenje ZM, Nakabiito C, Taha TE, Jones J, Mayo A, Scheckter R, Berthiaume J, Livant E, Jacobson C, Ndase P, White R, Patterson K, Germuga D, Galaska B, Bunge K, Singh D, Szydlo DW, Montgomery ET, Mensch BS, Torjesen K, Grossman CI, Chakhtoura N, Nel A, Rosenberg Z, McGowan I, Hillier M--AS Team, Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women, New Engl J Med, 375 (2016) 2121–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mayer KH, Seaton KE, Huang YD, Grunenberg N, Isaacs A, Allen M, Ledgerwood JE, Frank I, Sobieszczyk ME, Baden LR, Rodriguez B, Tieu HV, Tomaras GD, Deal A, Goodman D, Bailer RT, Ferrari G, Jensen R, Hural J, Graham BS, Mascola JR, Corey L, Montefiori DC, Team HP, Network NHVT, Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: Results of a phase 1 randomized trial, Plos Med, 14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rockwood DN, Preda RC, Yucel T, Wang XQ, Lovett ML, Kaplan DL, Materials fabrication from Bombyx mori silk fibroin, Nat Protoc, 6 (2011) 1612–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Thurber AE, Omenetto FG, Kaplan DL, In vivo bioresponses to silk proteins, Biomaterials, 71 (2015) 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ge Z, Yang Q, Xiang X, Liu KZ, Assessment of silk fibroin for the repair of buccal mucosa in a rat model, Int J Oral Max Surg, 41 (2012) 673–680. [DOI] [PubMed] [Google Scholar]
- [10].Jewell M, Daunch W, Bengtson B, Mortarino E, The development of SERI (R) Surgical Scaffold, an engineered biological scaffold, Pharmaceutical Science to Improve the Human Condition: Prix Galien 2014, 1358 (2015) 44–55. [DOI] [PubMed] [Google Scholar]
- [11].Seib FP, Pritchard EM, Kaplan DL, Self-Assembling Doxorubicin Silk Hydrogels for the Focal Treatment of Primary Breast Cancer, Adv Funct Mater, 23 (2013) 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chiu B, Coburn J, Pilichowska M, Holcroft C, Seib FP, Charest A, Kaplan DL, Surgery combined with controlled-release doxorubicin silk films as a treatment strategy in an orthotopic neuroblastoma mouse model, Brit J Cancer, 111 (2014) 708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yucel T, Lovett ML, Giangregorio R, Coonahan E, Kaplan DL, Silk fibroin rods for sustained delivery of breast cancer therapeutics, Biomaterials, 35 (2014) 8613–8620. [DOI] [PubMed] [Google Scholar]
- [14].Kim SY, Naskar D, Kundu SC, Bishop DP, Doble PA, Boddy AV, Chan HK, Wall IB, Chrzanowski W, Formulation of Biologically-Inspired Silk-Based Drug Carriers for Pulmonary Delivery Targeted for Lung Cancer, Sci Rep-Uk, 5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Seib FP, Coburn J, Konrad I, Klebanov N, Jones GT, Blackwood B, Charest A, Kaplan DL, Chiu B, Focal therapy of neuroblastoma using silk films to deliver kinase and chemotherapeutic agents in vivo, Acta Biomaterialia, 20 (2015) 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Coburn J, Harris J, Zakharov AD, Poirier J, Ikegaki N, Kajdacsy-Balla A, Pilichowska M, Lyubimov AV, Shimada H, Kaplan DL, Chiu B, Implantable chemotherapy-loaded silk protein materials for neuroblastoma treatment, International Journal of Cancer, 140 (2017) 726–735. [DOI] [PubMed] [Google Scholar]
- [17].Yavuz B, Zeki J, Coburn JM, Ikegaki N, Levitin D, Kaplan DL, Chiu B, In vitro and in vivo evaluation of etoposide - silk wafers for neuroblastoma treatment, J Control Release, 285 (2018) 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zeki J, Taylor JS, Yavuz B, Coburn J, Ikegaki N, Kaplan DL, Chiu B, Disseminated injection of vincristine-loaded silk gel improves the suppression of neuroblastoma tumor growth, Surgery, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pritchard EM, Valentin T, Panilaitis B, Omenetto F, Kaplan DL, Antibiotic-Releasing Silk Biomaterials for Infection Prevention and Treatment, Adv Funct Mater, 23 (2013) 854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wilz A, Pritchard EM, Li T, Lan JQ, Kaplan DL, Boison D, Silk polymer-based adenosine release: therapeutic potential for epilepsy, Biomaterials, 29 (2008) 3609–3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Price R, Gustafson J, Greish K, Cappello J, McGill L, Ghandehari H, Comparison of silk-elastinlike protein polymer hydrogel and poloxamer in matrix-mediated gene delivery, Int J Pharm, 427 (2012) 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Megeed Z, Haider M, Li DQ, O’Malley BW, Cappello J, Ghandehari H, In vitro and in vivo evaluation of recombinant silk-elastinlike hydrogels for cancer gene therapy, Journal of Controlled Release, 94 (2004) 433–445. [DOI] [PubMed] [Google Scholar]
- [23].Uebersax L, Merkle HP, Meinel L, Insulin-like growth factor I releasing silk fibroin scaffolds induce chondrogenic differentiation of human mesenchymal stem cells, J Control Release, 127 (2008) 12–21. [DOI] [PubMed] [Google Scholar]
- [24].Guziewicz N, Best A, Perez-Ramirez B, Kaplan DL, Lyophilized silk fibroin hydrogels for the sustained local delivery of therapeutic mono clonal antibodies, Biomaterials, 32 (2011) 2642–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li AB, Kluge JA, Guziewicz NA, Omenetto FG, Kaplan DL, Silk-based stabilization of biomacromolecules, J Control Release, 219 (2015) 416–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].He JY, Yavuz B, Kluge JA, Li AB, Omenetto FG, Kaplan DL, Stabilization of RNA Encapsulated in Silk, Acs Biomater Sci Eng, 4 (2018) 1708–1715. [DOI] [PubMed] [Google Scholar]
- [27].Guziewicz NA, Massetti AJ, Perez-Ramirez BJ, Kaplan DL, Mechanisms of monoclonal antibody stabilization and release from silk biomaterials, Biomaterials, 34 (2013) 7766–7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Emau P, Tian B, O’Keefa BR, Mori T, McMahon JB, Palmer KE, Jiang Y, Bekele G, Tsai CC, Griffithsin, a potent HIV entry inhibitor, is an excellent candidate for anti-HIV microbicide, J Med Primatol, 36 (2007) 244–253. [DOI] [PubMed] [Google Scholar]
- [29].Cerini F, Offord R, McGowan I, Hartley O, Stability of 5P12-RANTES, A Candidate Rectal Microbicide, in Human Rectal Lavage, Aids Res Hum Retrov, 33 (2017) 768–777. [DOI] [PubMed] [Google Scholar]
- [30].Girard L, Birse K, Holm JB, Gajer P, Humphry’s MS, Garber D, Guenthner P, Noel-Romas L, Abou M, McCorrister S, Westmacott G, Wang L, Rohan LC, Matoba N, McNicholl J, Palmer KE, Ravel J, Burgener AD, Impact of the griffithsin anti-HIV microbicide and placebo gels on the rectal mucosal proteome and microbiome in non-human primates, Sci Rep-Uk, 8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].McCoy LE, The expanding array of HIV broadly neutralizing antibodies, Retrovirology, 15 (2018) 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gautam R, Nishimura Y, Gaughan N, Gazumyan A, Schoofs T, Buckler-White A, Seaman MS, Swihart BJ, Follmann DA, Nussenzweig MC, Martin MA, A single injection of crystallizable fragment domain-modified antibodies elicits durable protection from SHIV infection, Nat Med, 24 (2018) 610–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gautam R, Nishimura Y, Pegu A, Nason MC, Klein F, Gazumyan A, Golijanin J, Buckler-White A, Sadjadpour R, Wang KY, Mankoff Z, Schmidt SD, Lifson JD, Mascola JR, Nussenzweig MC, Martin MA, A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges, Nature, 533 (2016) 105–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhao CX, Gunawardana M, Villinger F, Baum MM, Remedios-Chan M, Moench TR, Zeitlin L, Whaley KJ, Bohorov O, Smith TJ, Anderson DJ, Moss JA, Pharmacokinetics and Preliminary Safety of Pod-Intravaginal Rings Delivering the Monoclonal Antibody VRC01-N for HIV Prophylaxis in a Macaque Model, Antimicrob Agents Ch, 61 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gaertner H, Cerini F, Escola JM, Kuenzi G, Melotti A, Offord R, Rossitto -Borlat I, Nedellec R, Salkowitz J, Gorochov G, Mosier D, Hartley O, Highly potent, fully recombinant anti-HIV chemokines: Reengineering a low-cost microbicide, P Natl Acad Sci USA, 105 (2008) 17706–17711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cerini F, Landay A, Gichinga C, Lederman MM, Flyckt R, Starks D, Offord RE, Le Gal F, Hartley O, Chemokine Analogues Show Suitable Stability for Development as Microbicides, Jaids-J Acq Imm Def, 49 (2008) 472–476. [DOI] [PubMed] [Google Scholar]
- [37].McBride JW, Dias N, Cameron D, Offord RE, Hartley O, Boyd P, Kett VL, Malcolm RK, Pharmacokinetics of the Protein Microbicide 5P12-RANTES in Sheep following Single-Dose Vaginal Gel Administration, Antimicrob Agents Ch, 61 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cerini F, Gaertner H, Madden K, Tolstorukov I, Brown S, Laukens B, Callewaert N, Harner JC, Oommen AM, Harms JT, Sump AR, Sealock RC, Peterson DJ, Johnson SK, Abramson SB, Meagher M, Offord R, Hartley O, A scalable low-cost cGMP process for clinical grade production of the HIV inhibitor 5P12-RANTES in Pichia pastoris, Protein Expres Purif, 119 (2016) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhang L, Herrera C, Coburn J, Olejniczak N, Ziprin P, Kaplan DL, LiWang PJ, Stabilization and Sustained Release of HIV Inhibitors by Encapsulation in Silk Fibroin Disks, Acs Biomater Sci Eng, 3 (2017) 1654–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhao B, Mankowski MK, Snyder BA, Ptak RG, LiWang PJ, Highly Potent Chimeric Inhibitors Targeting Two Steps of HIV Cell Entry, Journal of Biological Chemistry, 286 (2011) 28370–28381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nguyen AF, Schill MS, Jian M, LiWang PJ, The Effect of N-Terminal Cyclization on the Function of the HIV Entry Inhibitor 5P12-RANTES, Int J Mol Sci, 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hu X, Shmelev K, Sun L, Gil ES, Park SH, Cebe P, Kaplan DL, Regulation of Silk Material Structure by Temperature-Controlled Water Vapor Annealing, Biomacromolecules, 12 (2011) 1686–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hu X, Kaplan D, Cebe P, Determining beta-sheet crystallinity in fibrous proteins by thermal analysis and infrared spectroscopy, Macromolecules, 39 (2006) 6161–6170. [Google Scholar]
- [44].Derdeyn CA, Decker JM, Sfakianos JN, Wu XY, O’Brien WA, Ratner L, Kappes JC, Shaw GM, Hunter E, Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120, J Virol, 74 (2000) 8358–8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D, Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1, J Virol, 72 (1998) 2855–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wei XP, Decker JM, Liu HM, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu XY, Shaw GM, Kappes JC, Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy, Antimicrob Agents Ch, 46 (2002) 1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Li Y, Hui H, Burgess CJ, Price RW, Sharp PM, Hahn BH, Shaw GM, Complete nucleotide sequence, genome organization, and biological properties of human immunodeficiency virus type 1 in vivo: evidence for limited defectiveness and complementation, J Virol, 66 (1992) 6587–6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Li Y, Kappes JC, Conway JA, Price RW, Shaw GM, Hahn BH, Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes, J Virol, 65 (1991) 3973–3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gordon CJ, Muesing MA, Proudfoot AEI, Power CA, Moore JP, Trkola A, Enhancement of human immunodeficiency virus type 1 infection by the CC-chemokine RANTES is independent of the mechanism of virus-cell fusion, J Virol, 73 (1999) 684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Herrera C, Cranage M, McGowan I, Anton P, Shattock RJ, Reverse Transcriptase Inhibitors as Potential Colorectal Microbicides, Antimicrob Agents Ch, 53 (2009) 1797–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Fletcher PS, Elliott J, Grivel JC, Margolis L, Anton P, McGowan I, Shattock RJ, Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides, Aids, 20 (2006) 1237–1245. [DOI] [PubMed] [Google Scholar]
- [52].Bai L, Phua SZ, Lim WQ, Jana A, Luo Z, Tham HP, Zhao L, Gao Q, Zhao Y, Nanoscale covalent organic frameworks as smart carriers for drug delivery, Chemical communications, 52 (2016) 4128–4131. [DOI] [PubMed] [Google Scholar]
- [53].Lu Q, Hu X, Wang XQ, Kluge JA, Lu SZ, Cebe P, Kaplan DL, Water-insoluble silk films with silk I structure, Acta Biomaterialia, 6 (2010) 1380–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Jin HJ, Park J, Karageorgiou V, Kim UJ, Valluzzi R, Kaplan DL, Water-stable silk films with reduced beta-sheet content, Adv Funct Mater, 15 (2005) 1241–1247. [Google Scholar]
- [55].Cebe P, Partlow BP, Kaplan DL, Wurm A, Zhuravlev E, Schick C, Silk I and Silk II studied by fast scanning calorimetry, Acta Biomaterialia, 55 (2017) 323–332. [DOI] [PubMed] [Google Scholar]
- [56].Zhu W, Wan L, Zhang C, Gao Y, Zheng X, Jiang T, Wang S, Exploitation of 3D face - centered cubic mesoporous silica as a carrier for a poorly water soluble drug: influence of pore size on release rate, Materials science & engineering. C, Materials for biological applications, 34 (2014) 78–85. [DOI] [PubMed] [Google Scholar]
- [57].Yucel T, Lovett ML, Keplan DL, Silk-based biomaterials for sustained drug delivery, Journal of Controlled Release, 190 (2014) 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kluge JA, Li AB, Kahn BT, Michaud DS, Omenetto FG, Kaplan DL, Silk-based blood stabilization for diagnostics, P Natl Acad Sci USA, 113 (2016) 5892–5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Li AB, Kluge JA, Zhi M, Cicerone MT, Omenetto FG, Kaplan DL, Enhanced Stabilization in Dried Silk Fibroin Matrices, Biomacromolecules, 18 (2017) 2900–2905. [DOI] [PubMed] [Google Scholar]
- [60].Fuchs EJ, Lee LA, Torbenson MS, Parsons TL, Bakshi RP, Guidos AM, Wahl RL, Hendrix CW, Hyperosmolar sexual lubricant causes epithelial damage in the distal colon: Potential implication for HIV transmission, J Infect Dis, 195 (2007) 703–710. [DOI] [PubMed] [Google Scholar]
- [61].Dezzutti CS, Brown ER, Moncla B, Russo J, Cost M, Wang L, Uranker K, Ayudhya RPKN, Pryke K, Pickett J, LeBlanc MA, Rohan LC, Is Wetter Better? An Evaluation of Over-the-Counter Personal Lubricants for Safety and Anti-HIV-1 Activity, Plos One, 7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lacey CJ, Woodhall S, Qi Z, Sawant S, Cowen M, McCormack S, Jiang S, Unacceptable side-effects associated with a hyperosmolar vaginal microbicide in a phase 1 trial, Int J Std Aids, 21 (2010) 714–717. [DOI] [PubMed] [Google Scholar]
- [63].Valenta C, The use of mucoadhesive polymers in vaginal delivery, Adv Drug Deliver Rev, 57 (2005) 1692–1712. [DOI] [PubMed] [Google Scholar]
- [64].Perioli L, Ambrogi V, Venezia L, Pagano C, Ricci M, Rossi C, Chitosan and a modified chitosan as agents to improve performances of mucoadhesive vaginal gels, Colloid Surface B, 66 (2008) 141–145. [DOI] [PubMed] [Google Scholar]
- [65].Baloglu E, Senyigit ZA, Karavana SY, Bernkop-Schnurch A, Strategies to Prolong the Intravaginal Residence Time of Drug Delivery Systems, J Pharm Pharm Sci, 12 (2009) 312–336. [DOI] [PubMed] [Google Scholar]


