Summary
B cells and the antibodies they produce have a deeply penetrating influence on human physiology. Here we review current understanding of how B cell responses are initiated, the different paths to generate short- and long-lived plasma cells, germinal center cells, and memory cells, and how each path impacts antibody diversity, selectivity and affinity. We discuss how basic research is informing efforts to generate vaccines that induce broadly neutralizing antibodies against viral pathogens, revealing the special features associated with allergen-reactive IgE responses, and uncovering the antibody-independent mechanisms by which B cells contribute to health and disease.
Introduction
The importance of B cells to human health is hard to overstate. Vaccines capable of eradicating disease activate B cells, cancer checkpoint blockade therapies are produced using B cells, and B cell deficiencies have devastating impacts (Fig. 1). B cells have been a subject of fascination since at least the 1800s, when early microscopists observed foci of mitotic figures in lymphoid tissues that they named germinal centers (Flemming, 1885). The notion of a humoral branch to immunity emerged from the work of Ehrlich and contemporaries studying B cells in the early 1900’s (Ehrlich, 1908). Efforts to understand how we could make antibodies from B cells against almost any foreign surface while usually avoiding making them against self, led to Burnet’s clonal selection theory (Burnet, 1960). This was followed by the molecular definition of how a diversity of immunoglobulins can arise by gene rearrangement in developing B cells (Tonegawa, 1987). Recombination Activating Gene (RAG)-dependent processes of V-(D)-J rearrangement of immunoglobulin (Ig) gene segments in developing B cells are now known to be able to generate an enormous amount of antibody diversity (theoretically at least 1016 possible variants) (Briney et al., 2019; Schatz et al., 1989). With so much already known, B cell biology might be considered ‘done’ with only incremental advances still to be made, but instead there is great activity in the field today with numerous major challenges that remain. For example, efforts are underway to develop vaccines that induce broadly neutralizing antibody responses, to understand how autoantigen- and allergen-reactive antibodies arise, and to harness B cell-depletion therapies to correct non-autoantibody mediated diseases, making it evident that there is still an enormous amount we do not know about B cells and much work to be done. In this review, we give an overview of how B cells travel throughout the body surveying for antigen, how they respond upon antigen encounter, how they go on to become IgM, IgG, IgA or IgE secreting plasma cells (PCs) or memory B (Bmem) cells, and how they contribute to a variety of disease states including autoimmunity, allergy and cancer. We highlight newer findings in each area while guiding the reader to recent reviews for deeper assessment of each topic.
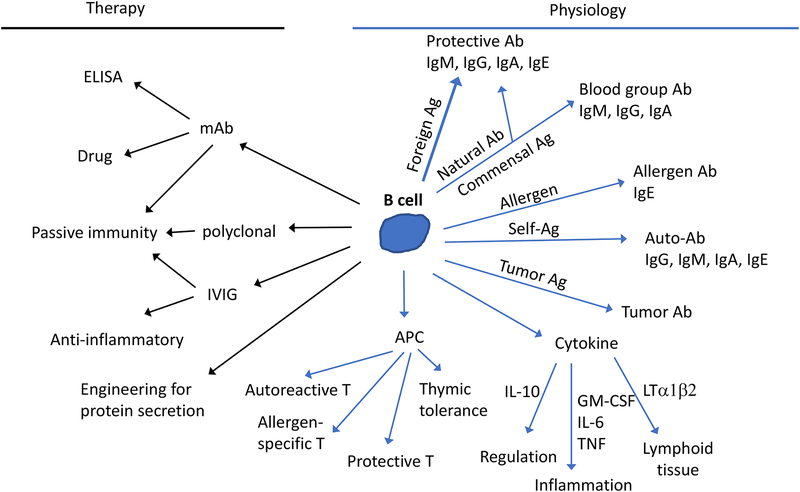
Figure 1. The many possible functions of a B cell.
Right side (blue arrows) shows physiological actions and left side (black arrows) shows therapeutic applications. APC, antigen presenting cell; ELISA, enzyme linked immunosorbent assay; IVIG, intravenous immunoglobulin; mAb, monoclonal antibody. Passive immunity refers to, for example, protection provided by horse serum against snake venom or from viral infection with transferred mAbs. IVIG is used to provide passive immunity against pathogens in immune-deficient patients but also has anti-inflammatory activities, dependent on antibody glycosylation and binding of inhibitory receptors, and can be used to treat some inflammatory and autoimmune diseases. B cells may be engineered (e.g. using CRISPR-Cas9 based gene targeting) to secrete pre-defined antibodies or other proteins such as plasma clotting factors. As antigen presenting cells, B cells may prime T cells that have protective, autoreactive, or allergy-promoting effector functions beyond providing help to B cells. For details about other parts of the diagram, see main text.
B cell trafficking and antigen encounter
The initiation of humoral immune responses requires that rare antigen-reactive B cells come in contact with antigen (Fig. 2). These encounters predominantly occur in secondary (or peripheral) lymphoid tissues – including the spleen, lymph nodes (LNs), and Peyer’s patches (PPs) – and are promoted by two core processes. First, the lymphoid tissues are specialized to filter body fluids – blood, lymph and mucosal contents – and to capture and display foreign antigens for B cells to ‘see’. Second, these tissues support the continual recruitment of lymphocytes from the blood across specialized lymphocyte-binding endothelial cells, and their movement into compartments (lymphoid follicles) where incoming antigens are focused (Cyster, 2010; Schulz et al., 2016). The initial cell types handling incoming antigens differs between the lymphoid tissue types, but the overall principle of making intact antigen available for B cell encounter is similar. In LNs, for example, specialized macrophages exist in a subcapsular location that capture particulate antigens from the lymph and transport them in a directional manner along their length to tail processes that contact follicular B cells (Cyster, 2010). Embedded within the center of lymphoid follicles are follicular dendritic cells (FDCs), specialized stromal cells that are highly efficient at capturing and displaying opsonized (antibody and/or complement coated) antigens on their extensive network of dendritic processes (Allen and Cyster, 2008; Heesters et al., 2014). The gene expression profile of FDCs has long been elusive due to their rarity and difficulty to isolate but has recently been obtained using single-cell RNAseq technology (Rodda et al., 2018). In contrast to most antigen-capturing cells, FDCs are non-phagocytic and can retain and display intact antigens on their cell surface for periods of weeks, though this may involve endocytic recycling (Heesters et al., 2014).
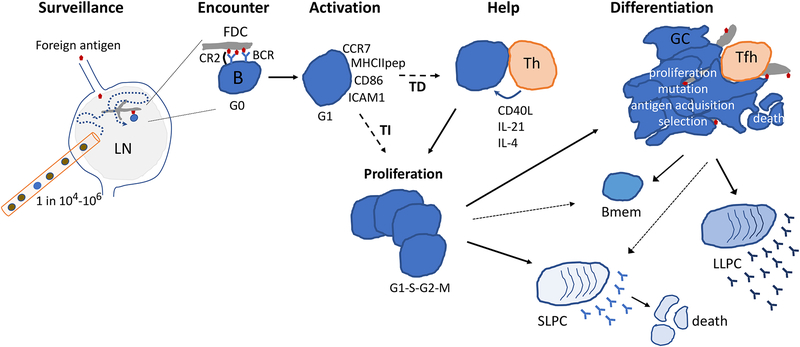
Figure 2. Cellular dynamics underlying a B cell response.
Circulating antigen specific B cells (blue) that are as rare as 1 in 104 to 1 in 106 enter and survey an antigen draining lymph node (LN). A B cell encounters an opsonized (complement coated) antigen displayed on a follicular dendritic cell (FDC) process and receives B cell receptor (BCR) and complement receptor-2 (CR2) signals. Activation involves upregulation of surface molecules, antigen internalization processing and (in the case of protein-containing antigens) presentation as MHC class II peptide (MHCIIpep) complexes, and entry into G1 of cell cycle. If the antigen engages multiple BCRs and/or coreceptors on the B cell, a T-independent (TI) proliferative response ensues. Lower valency protein-containing antigens drive T-dependent (TD) responses, where the B cell depends on signals from helper T cells to undergo proliferation. The proliferative phase is followed by differentiation into short-lived plasma cells (SLPCs), germinal center (GC) B cells, and/or memory B cells (Bmem). The relative differentiation to these distinct states varies and depends on the integration of signals received by the B cell including via the BCR, co-receptors, and T cell help. GC B cells take on a dendritic morphology that may facilitate antigen encounter and affinity discrimination. GCs give rise to more SLPCs, to memory B cells and to long-lived plasma cells (LLPCs).
B cell migration to follicles is guided by the chemokine CXCL13, the ligand for CXCR5, that is made by FDCs and by other follicle-associated stromal cells. Within the follicle, B cells migrate continuously along the follicular stroma in a non-directional fashion, at ~6μm per minute, surveying subcapsular macrophages and FDCs for surface-displayed antigen or for soluble antigens in their environment (Batista and Harwood, 2009; Cyster, 2010; Qi et al., 2014). Lymphoid stromal cells within follicles as well as in other parts of lymphoid tissues are a source of BAFF (TNFRSF13b), a critical B cell survival factor (Cremasco et al., 2014; Rodda et al., 2018). B cell access to the outer parts of the follicle is promoted by a second guidance factor, the oxysterol 7α,25-HC, that is produced by stromal cells in these regions and acts via the chemoattractant receptor EBI2 (GPR183) (Cyster et al., 2014; Gatto and Brink, 2013).
If, after several hours of surveillance, antigen is not encountered, B cells exit lymphoid tissues in response to sphingosine-1-phosphate (S1P) sensing via their S1PR1 receptor (Cyster and Schwab, 2012). From the spleen, B cells exit into blood vessels and, from LNs and PPs, into lymphatic vessels. Once in the circulatory fluid, B cells can travel to another lymphoid tissue in a matter of minutes to continue their surveillance program. The exact mechanisms controlling the times that B cells spend in a lymphoid tissue have not been fully delineated, though a modeling study suggested that dwell time might be accounted for by the migration properties of the B cells and their propensity to exit versus remain in the tissue upon each lymphatic sinus encounter (Grigorova et al., 2010). Recent work has shown that lymphocyte entry into and exit from lymphoid tissues has a circadian component; more lymphocytes accumulate in lymphoid tissues during the period of higher physical activity (Druzd et al., 2017). Neuronal activity may contribute to this circadian behavior since lymphocyte adrenergic receptor signaling can promote CXCR4-mediated retention in LNs (Suzuki et al., 2016).
Early B cell activation
Upon encounter with antigen, signaling via the B cell receptor (BCR) initiates B cell activation (Fig. 2). The actual mechanism by which antigen binding activates the BCR remains an area of active investigation. One model proposes that antigen binding leads to clustering of BCRs on the membrane to initiate signaling (Liu et al., 2016). Conversely, an alternative model is that BCR clusters preexist before antigen encounter and that antigen binding dissociates these clusters enabling signaling to occur (Yang and Reth, 2016). A further variant on these models is that the mobility of the BCR, relative to coreceptor molecules, may be altered by antigen binding (Liu et al., 2016; Yang and Reth, 2016). Naïve B cells express membrane-bound immunoglobulin of the IgM and/or IgD isotypes, which lack significant intracellular domains, thus signal transduction relies on associated molecules. In particular, Igα and Igβ are pre-associated with IgM and IgD and contain intracellular ITAM motifs that can be phosphorylated by tyrosine kinases (Kurosaki et al., 2010; Yang and Reth, 2016). The Syk tyrosine kinase is essential for BCR signaling while Src-family kinases (SFK) may be most critical for responses to monovalent antigens (Noviski and Zikherman, 2018). Tyrosine phosphorylation leads to a cascade of downstream signaling events including the activation of multiple pathways. In addition, CD19 associates with IgM and IgD and is involved primarily in triggering the PI3 kinase – Akt pathway (Kurosaki et al., 2010). Together, signals transduced downstream of the BCR complex lead to changes in the expression of numerous genes, including upregulation of costimulatory molecules (CD86, CD80), adhesion molecules (ICAM1), migration receptors, pro-survival molecules, and cell cycle related genes.
Most complex antigens will engage other receptors on the B cell in addition to the BCR. The ligation of some co-receptors, such as toll-like receptors (TLRs) or complement receptors (CR2), leads to amplification and possibly qualitative modification in the BCR signal (Carroll and Isenman, 2012; Suthers and Sarantopoulos, 2017). Co-receptor engagement may also reduce the threshold of antigen needed to activate B cells. Conversely, B cells express multiple ITIM-containing receptors that recruit negative regulatory phosphatases such as SHIP1 and SHP1, and these dampen the BCR signal (Tsubata, 2018). For example, FcγRIIb contributes to antibody-mediated negative feedback of B cell activation by recruiting SHIP1 to the BCR. CD22, a member of the sialic acid binding Ig-type lectin (Siglec) family, restrains tonic and antigen-induced BCR signaling in a gene-dosage sensitive and SHP1-dependent manner. CD72 recognizes the lupus self-antigen Sm/RNP and limits autoreactive B cell activation by recruitment of SHP1 to the BCR (Tsubata, 2018).
BCR triggering also prompts internalization of the BCR and bound antigen in a predominantly clathrin-dependent manner. Internalization requires tyrosine-containing motifs in Igα and Igβ and involves SFK activity (Hoogeboom and Tolar, 2016). Uptake of membrane bound antigens can involve an actin-dependent membrane ‘spreading and gathering’ process prior to internalization. Forces generated by myosin during invagination can lead to rupture of weak bonds between BCR and antigen and may serve as a form of affinity discrimination (Hoogeboom and Tolar, 2016). After internalization, antigen travels via endosomes to lysosomes for enzymatic processing, and peptides are docked on MHC class II for delivery to the cell surface and presentation to T cells.
For the B cell response to progress, proliferation has to occur (Fig. 2). This is necessary for the generation of a clonal population of daughter cells, but also for differentiation. Antigens of low valency trigger B cell transition from G0 to G1 of the cell cycle, poising the cell for rapid proliferation in response to helper T cell-derived signals in T-dependent responses. In some cases, when signaling via co-receptors (such as TLRs) is strong, the need for T cell help may be overcome. This is designated a T-independent type 1 response. Alternatively, highly multivalent antigens alone may trigger sufficiently strong BCR signaling for cells to enter into cycle, in a T-independent type 2 response. These T-independent events can be augmented by cytokines such as BAFF and APRIL (TNFSF13), derived from innate sensor-engaged accessory cells (Balazs et al., 2002). Following BCR engagement, co-receptor activation, T cell help, and/or cytokine signals, cell division is coupled with subsequent differentiation events. For example, in vitro studies have established that class switch recombination (CSR) to other BCR isotypes and B cell differentiation into plasma cells require a minimum number of cell divisions (Nutt et al., 2015).
Requirements for B-T interactions
In T-dependent immune responses, rare antigen-engaged B cells must encounter rare cognate antigen-specific T cells (Fig. 2). One early consequence of BCR triggering is the upregulation of CCR7 and EBI2, with these chemoattractant receptors acting together to guide B cells to the interface of the B cell follicle and the T cell zone (Cyster et al., 2014). Another effect is downregulation of S1PR1 to ensure that the activated cells are retained in the responding tissue. At the follicle-T zone interface, B cells interact with CD4 helper T cells, the latter often (but not always) pre-activated by encounter with antigen-presenting dendritic cells (DCs), and they engage in interactions that last 10’s of minutes and sometimes more than an hour (Allen et al., 2007; Hong et al., 2018; Qi et al., 2014).
Multiple interaction partners guide and shape the nature of cognate B-T interactions. Integrins are important players in most leukocyte cell-cell interactions and migration, and contribute to the stability of B-T interactions. The integrin LFA1 on helper T cells engages both ICAM1 and ICAM2 on B cells and augments the ability of lower affinity B cells to enter into responses (Zaretsky et al., 2017). The amount of MHC-peptide presented on the B cell also contributes to the stability of these interactions (Zaretsky et al., 2017). Actin regulatory proteins are important and some of the B cell response defects arising from deficiency in Wiskott-Aldrich syndrome protein (WASp), WASp interacting protein (WIP), various Rho GTPases, or the guanidine exchange factors (GEFs) DOCK8 and intersectin-2 (ITSN2), might be a consequence of reduced B-T conjugate stability (Burbage et al., 2018). Costimulatory signaling from CD86 to CD28 is also crucial in the early phase of B cell responses.
B cells that have received T cell help may undergo a variety of differentiation states described in Fig. 2, including the formation of germinal centers (GCs). Ongoing B-T interactions are critical for the maintenance of GCs. In a GC response, ICOSL engagement of ICOS, a relative of CD28, becomes more important. SLAM family molecules (in particular SLAM, CD84, and Ly108) undergo homotypic interactions between T follicular helper (Tfh) cells and B cells. When T cells lack the SLAM-associated protein, SAP, SLAM molecules recruit SHP1 in an unrestrained way and this antagonizes the ability of Tfh cells to support the GC response (Cannons et al., 2011; Crotty, 2014). The function of SLAM family homotypic interactions under SAP replete conditions have been difficult to unravel (Chen et al., 2017; Hu et al., 2016; Huang et al., 2016), but the pathway can contribute to IL-4 induction in Tfh cells (Yusuf et al., 2010). PD1 is highly expressed on Tfh cells and both PDL1 and PDL2 can be expressed on B cells, with PDL1 being constitutively present. The PDL1-PD1 interaction appears to exert a restraining influence on GC B cells since intrinsic deficiency in PDL1 gives GC B cells a competitive advantage (Shi et al., 2018).
A crucial component of T cell help is the expression of CD40L and engagement of B cell CD40. CD40L, a member of the TNF family, is expressed on T cells as a membrane homotrimer and its surface expression is upregulated following T cell activation. CD40 is constitutively expressed on mature B cells, including naïve and GC B cells. Within GCs, the surface area of interaction (or entanglement) of GC B cells and Tfh cells is increased by ICOSL-ICOS signaling. ICOSL is upregulated on mouse GC B cells by CD40 signaling in a positive feedback loop (Liu et al., 2015). In humans, CD40 was not found to augment ICOSL expression on GC B cells. Instead, dopamine was discovered as a novel Tfh cell secreted factor that augments ICOSL on human GC B cells via dopamine receptor 1 (DRD1). Imaging studies using supported lipid bilayers revealed that ICOS ligation augmented CD40L accumulation at the synaptic cleft in human T cells (Papa et al., 2017). The extent of CD40L-CD40 engagement is thought to impact on the fate decisions of B cells at various stages of differentiation in the context of other extrinsic signals (Ise et al., 2018; Zhou et al., 2018).
Tfh cells are also known to secrete cytokines that profoundly influence B cells. Two of the major cytokines secreted by Tfh cells are IL-4 and IL-21 (Crotty, 2014; Vinuesa et al., 2016). These cytokines promote B cell proliferation, CSR, and differentiation into plasma cells or GC B cells (Moens and Tangye, 2014; Vinuesa et al., 2016). Both IL-4 and IL-21 receptors signal through the common cytokine γ chain, yet are coupled to different STAT proteins which enables the regulation of distinct subsets of genes (Lin and Leonard, 2018). Indeed, studies in gene-targeted mice indicate that IL-4 and IL-21 have non-redundant roles, although the magnitude of the phenotypes of deficiencies in each of these cytokine pathways alone seems to be variable depending on the types of immune responses studied and assessments performed. For example, IL-4 is more critical for the formation of GCs in type 2 immune responses compared with type 1 immune responses (Turqueti-Neves et al., 2014). While mice with targeted mutations in either the IL-4 or IL-21 pathways can form GCs, the GC B cells show reductions in the expression of key genes such as Bcl6 and Aicda (Gonzalez et al., 2018; Moens and Tangye, 2014; Vinuesa et al., 2016). Combined deficiency in both IL-4 and IL-21 cytokine pathways leads to severe defects in B cell responses (Gonzalez et al., 2018; Moens and Tangye, 2014). How these cytokines may influence divergent outcomes remains unclear; for example, IL-21 promotes the expression of Blimp-1 in addition to Bcl-6 (Moens and Tangye, 2014), yet since these transcription factors repress each other their expression is mutually exclusive during plasma cell vs GC B cell differentiation, respectively. The regulation of B cell differentiation by IL-4 and IL-21 may depend on other signals received by the B cells as well as the timing in which the B cells are exposed to these cytokines during B-T interactions. Interestingly, recent studies indicate that the temporal expression of transcripts for IL-4 versus IL-21 in Tfh cells may differ over the course of the immune response (Gonzalez et al., 2018; Weinstein et al., 2016). There is also evidence in some types of immune responses that Tfh cells can secrete other cytokines, such as IL-10 and interferon gamma (IFNγ) (Luthje et al., 2012; Vinuesa et al., 2016). The production of IFNγ is associated with CSR to IgG2a/IgG2c (Vinuesa et al., 2016).
In humans, CXCL13 is a marker of Tfh cells (in addition to being made by follicular stromal cells) and a correlation has been observed between CXCL13 levels in plasma following immunization and the Tfh cell and GC response in the lymphoid tissue (Havenar-Daughton et al., 2016). The function of CXCL13 in Tfh cells is not yet defined but it may contribute to the stability of contacts between GC B cells and Tfh cells.
Isotype switching
After activation, B cells may undergo CSR to change the isotype of their BCR from IgM/IgD to IgG, IgA, or IgE (Fig. 3). Ultimately, the production of secreted antibodies of these different isotypes enables differential functional roles. Binding to Fc receptors is an important mechanism by which antibodies mediate effector functions. IgG can bind to multiple types of activating FcγRs that can promote numerous effector functions, such as antibody-dependent cytotoxicity and opsonization, as well as an inhibitory FcγR, FcγRIIb, that plays an immunomodulatory role (Bournazos and Ravetch, 2017). FcRn is responsible for endosomal recycling of IgG and thereby ensuring that IgG has a long (~3 week) serum half-life (Ward and Ober, 2018). A large fraction of IgE is bound to FcεRI, a high affinity receptor abundant on mast cells and basophils; engagement of IgE by cognate antigen activates FcεRI and promotes degranulation of these cells leading to the rapid release of inflammatory mediators in immediate-type hypersensitivity (Kinet, 1999). IgA and IgM binding to the polymeric Ig receptor on epithelial cells is critical for the delivery of these isotypes to lumenal secretions (Lycke and Bemark, 2017). IgA also exerts effector functions through FcαRI which is expressed in humans but not in mice (Aleyd et al., 2015). Complement activation is an important feature of IgM and IgG. The specific subclasses of IgG: IgG1, IgG2, IgG3, and IgG4 (in humans) differ in various properties including complement activation and their relative affinities for particular FcγRs, enabling functional specialization (Bournazos and Ravetch, 2017; Vidarsson et al., 2014). Overall, the IgG isotypes are the most predominant in serum, followed by IgA. In mucosal secretions, IgA is most abundant. In contrast, IgE is typically the least abundant isotype, most likely because IgE-producing cells are rare and secreted IgE has a short half-life (Yang et al., 2014).
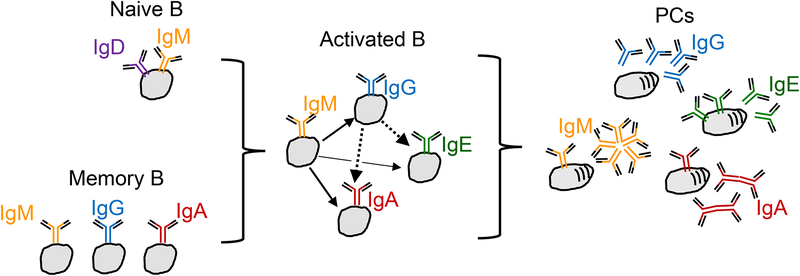
Figure 3. Class switch recombination at multiple stages.
Upon receiving appropriate activation signals, B cells undergo a DNA rearrangement of their Ig Hc gene leading to class switch recombination (CSR, also known as isotype switching). In this process, DNA encoding the constant region of the antibody is replaced with a downstream gene sequence to encode a different isotype. Naïve B cells express IgD and/or IgM, and CSR leads to the expression of IgG, IgE, or IgA isotypes. CSR may occur directly (for example, from IgM/IgD to IgE), or sequentially (for example, from IgM/IgD to IgG to IgE). Memory B cells may also undergo CSR upon re-activation (for example, from IgG to IgE). Once cells differentiate into PCs, CSR is extinguished. There are thus multiple paths giving rise to PCs expressing particular antibody isotypes. As well as secreting antibody, IgM, IgA and IgE PCs retain membrane forms of the antibody.
Transcription of distinct promoter regions within the Ig heavy chain (HC) locus, leading to the induction of so-called ‘germline transcripts,’ is a prerequisite for CSR (He et al., 2015). These distinct promoters contain elements responsive to a variety of transcription factors, enabling B cells to integrate signals from multiple inputs (including the BCR, CD40, TLRs, and cytokines) to determine the isotype to which they will ‘switch.’ CSR depends on expression of activation-induced cytidine deaminase (AID) and induction of mutations and double strand breaks in switch regions (Hwang et al., 2014). When CSR occurs, the intervening DNA regions are excised, such that subsequent CSR events can only occur to downstream genes. For example, the IgE gene is located downstream of the genes encoding the IgG isotypes, meaning that once a B cell switches to an IgG isotype it can subsequently switch to IgE, but conversely, an IgE+ B cell cannot switch to IgG. The genes encoding IgA in mice and one of the two IgA genes in humans are the last in the sequence and thus such IgA+ cells cannot undergo further CSR events.
Once B cells undergo CSR, this changes the BCR expressed on the cell. Unlike IgM/IgD which only have a short three amino-acid intracellular component, the IgG, IgE, and IgA isotype BCRs have significant intracellular tails (Wienands and Engels, 2016). Through gene targeting, these tails have been shown to have a physiological role in the responses of B cells of these isotypes (Wienands and Engels, 2016). In particular, a conserved tyrosine motif in the tails of the IgG and IgE BCRs is thought to provide signal amplification. Recently, a polymorphism was identified in IgG1 that potentiates the phosphorylation of the tyrosine motif and is associated with systemic lupus erythematosus (SLE) (Chen et al., 2018). However, not all of the unique signaling properties of these isotypes are due to the tail. Recent studies indicate that the IgE BCR has distinct signaling properties from the IgG1 BCR; in the absence of antigen, the IgE BCR seems to have increased basal signaling activity, and this was most highly associated with the extracellular membrane proximal domain, also known as the membrane Ig isotype-specific (migis) region, of IgE versus IgG1 (Haniuda et al., 2016; Yang et al., 2016). Evidence has been provided that this region associates with Igα/β and/or CD19 (Haniuda et al., 2016; Yang et al., 2016). The surface expression of distinct BCR isotypes is also not identical; for example, in GC B cells, IgE surface BCR expression is substantially lower than IgG1 surface BCR expression (He et al., 2015; Yang et al., 2016). This differential expression may be due to differences in the efficiency of polyadenylation of membrane IgE versus IgG1 transcripts (He et al., 2015; Yang et al., 2014), due to differences in intracellular molecule trafficking to the cell surface (Vanshylla et al., 2018), or due to internalization of the IgE BCR (Laffleur et al., 2015). The low surface IgE BCR expression reduces the ability of IgE+ GC B cells to capture and present antigen (Yang et al., 2016), which, together with the unique signaling properties of the IgE BCR, likely contributes to the poor competitivity of IgE+ B cells within GCs (He et al., 2015; Yang et al., 2014).
Plasma cell responses
As B cells terminally differentiate into plasma cells (Fig. 2), they initially continue proliferating, and are referred to as plasmablasts (Nutt et al., 2015). Once these cells cease dividing and fully mature, they become plasma cells (PCs). Since most studies do not actually assess the proliferative state of these cell types, they have been referred to by various names in the literature, in some cases simply as antibody-secreting cells. We generally refer to these as PCs in this review, but in some cases will note distinctions from plasmablasts.
The factors that determine whether a B cell undergoes PC differentiation, becomes a GC B cell, or a Bmem are being actively investigated. These differentiation states are influenced by a variety of signals, such as those from the BCR, co-receptors, and cytokines. B cells with higher affinity for antigen give rise to a stronger PC response than B cells responding to a lower affinity antigen and this reflects the strength of the plasmablast proliferative response (Krautler et al., 2017). The isotype of the BCR also seems to highly influence PC differentiation. A particularly striking case is that of the IgE BCR, which in mouse studies was shown to have an antigen-independent constitutive activity leading to increased PC differentiation, reduced GC B cell responses, and virtually undetectable Bmems (Haniuda et al., 2016; He et al., 2015; Yang et al., 2014; Yang et al., 2016). Recent evidence from cultured human B cells (Ramadani et al., 2017) and single-cell RNAseq of blood samples from allergic patients (Croote et al., 2018) provides further evidence for a bias of IgE B cells to differentiate into PCs. Another factor that can influence the propensity to become a PC versus a GC cell is the chronic exposure to low avidity autoantigen. B cells so exposed can take on an IgM low ‘anergic’ state and are poorly responsive in vitro to antigen stimulation. However, when exposed to a cross-reactive multivalent antigen and T cell help, such anergic B cells preferentially enter the GC response where they can then undergo somatic hypermutation to shift away from self-reactivity and develop increased ability to bind the foreign antigen in a process referred to as clonal redemption (Burnett et al., 2018; Reed et al., 2016; Sabouri et al., 2014). This propensity to favor the GC path appears to in part reflect a lower intrinsic signal strength from IgD versus IgM (Noviski et al., 2018).
The efficiency of entry into the GC response is also influenced by the amount of T cell help received (Schwickert et al., 2011; Woodruff et al., 2018; Zhang et al., 2017). A model has been proposed where regulation of division time by signal strength is a controlling feature of B cell fate decisions. Weaker stimulation via CD40 in vitro was concluded to enhance plasmablast generation by slowing times to divide (Zhou et al., 2018) though these findings need to be reconciled with evidence that within the GC, deletion of a single copy of the CD40 gene led to reduced plasmablast differentiation (Ise et al., 2018).
The PCs arising in the early phases of B cell responses, independently of GCs, typically remain within the peripheral lymphoid tissue and are short-lived PCs (SLPCs) (Nutt et al., 2015). Due to this short lifespan, most of these early cells are still actively proliferating as plasmablasts and the response has been referred to as ‘extrafollicular’ to distinguish from predominantly GC responses in the follicles. Some IgA+ PCs are short-lived but migrate to the intestinal lamina propria (Lycke and Bemark, 2017). In mouse studies, most IgE+ PCs seem to reside in lymphoid tissues, are short-lived and are derived by a GC-independent pathway, perhaps due to the relatively poor participation of IgE+ B cells in GCs (Yang et al., 2014). There is evidence in humans for IgE+ PCs in peripheral tissues, though the relative lifespan and abundance of these cells compared with those in lymphoid tissues has not been determined (He et al., 2015).
In contrast, GCs give rise to long-lived plasma cells (LLPCs), many of which have a BM tropism and can live for months (Nutt et al., 2015). The transcription factor Blimp-1 is a primary determinant of the PC fate and reporter mice have shown that plasmablasts have intermediate Blimp1 expression and PCs are Blimp1hi. The ER stress response factor, XBP1, is crucial in PCs, helping them reach their remarkable capacity of secreting thousands of antibody molecules per second (Nutt et al., 2015). Despite their importance for vaccinology, the factors determining whether PCs are short versus long-lived are not yet well understood. The transcription factor Zbtb20 has a role in establishing a LLPC pool and durable antibody responses after some immunization types but not others (Lam and Bhattacharya, 2018). PCs require large amounts of amino acids as well as sugars for antibody glycosylation and other intermediates for ER biogenesis. Metabolic studies have revealed that LLPCs import more glucose and express higher amounts of the amino acid transporter CD98 than SLPCs (Lam and Bhattacharya, 2018).
PCs home to supportive niches within spleen and LNs, the red pulp and medullary cords, respectively, using CXCR4 the receptor for CXCL12 (Cyster, 2003). PC exit from lymphoid tissues requires S1PR1 and homing to the BM requires CXCR4 while trafficking to the intestine involves the integrin α4β7 and the chemokine receptors CCR9 and CCR10 responding to CCL25 and CCL28 (Brandtzaeg and Johansen, 2005; Masahata et al., 2014; Matsuo et al., 2018). The PC supportive medullary niche in LNs contains stromal cells that produce high levels of IL-6 and APRIL, two PC trophic factors (Huang et al., 2018). In the BM, CXCL12+ VCAM1+ stromal cells and APRIL-producing hematopoietic cells are thought to be important (Nutt et al., 2015). BM mesenchymal stromal cell secreted factors allow prolonged LLPC survival in vitro and proteomic analysis recently identified roles for fibronectin and YWHAZ (14–3-3zeta/delta) as well as IL-6 (Nguyen et al., 2018). A hypoxic environment may also be an important feature of the BM niche (Nguyen et al., 2018). The degree to which the longevity of LLPCs is due to their environmental niche versus cell intrinsic properties remains an area of investigation (Lam and Bhattacharya, 2018; Wilmore and Allman, 2017). A relatively low abundance of IgE PCs in the BM may be in part due to expression of the IgE BCR which leads to reduced CXCR4 upregulation (Achatz-Straussberger et al., 2008). The IgA producing cells in the lamina propria can also be long lived (Lycke and Bemark, 2017). Gut stromal cells, dendritic cells and eosinophils have been suggested to produce APRIL, IL-6 and other supportive factors for these cells (Gommerman et al., 2014; Lycke and Bemark, 2017) though a role for eosinophils in the PC niche has not been supported by recent work (Bortnick et al., 2018; Haberland et al., 2018).
Germinal center responses
Basic mechanisms
A major challenge in understanding humoral immunity is to understand how B cells affinity mature during responses to infections, vaccine antigens and, undesirably, to autoantigens and allergens. Antibody V-region somatic hypermutation and selection events occur most prominently within GCs. Positive selection of GC B cells could occur through either of two mechanisms. Higher affinity cells could receive stronger BCR signals that favor their survival compared to low affinity cells. Alternatively, by acquiring and processing more antigen in a short period of time, higher affinity cells could be more effective in engaging Tfh cells and receiving helper signals (Allen et al., 2007). Strong support for the latter model comes from an antibody-based approach of loading B cells with increased amounts of antigen independently of BCR engagement. Antigen-loaded B cells achieve clonal dominance through accelerated cell division and increased biomass accumulation that is mTORC1-dependent (Ersching et al., 2017; Gitlin et al., 2015; Victora et al., 2010). However, recent work has continued to provide evidence that BCR signaling contributes to selection events (Krautler et al., 2017; Luo et al., 2018). While BCR signaling in GC B cells appears attenuated compared to naïve B cells based on some (but not all) in vitro studies (Khalil et al., 2012; Kwak et al., 2018; Nowosad et al., 2016), receptor recoupling occurs such that the BCR strengthens PI3K and weakens NFκB signaling while CD40 signals via NFκB but not PI3K (Luo et al., 2018; Nowosad et al., 2016). Internally, GCs are organized into a light zone (LZ), containing LZ GC B cells, antigen-bearing FDCs and Tfh cells, and a dark zone (DZ) containing DZ GC B cells and CXCL12-expressing reticular cells (or DZ-FDCs). LZ and DZ GC B cells (historically known as centrocytes and centroblasts, respectively) differ in their transcriptional program with LZ cells distinguished by higher CD83 and CD86 expression and DZ cells by higher AID and CXCR4 expression (Victora and Nussenzweig, 2012). BCR signaling to Akt is suggested to cause phosphorylation of Foxo1, a transcription factor required for the DZ state and that needs to be repressed in the LZ state. CD40 and BCR signaling act synergistically to induce c-Myc, a critical transcription factor involved in GC B cell growth and proliferation (Luo et al., 2018; Mesin et al., 2016). An important target of c-Myc is the transcription factor AP4 that promotes GC B cell division (Chou et al., 2016).
Thus, there may be an integration of BCR signal and T cell help signals that determine the clonal winners in GCs or the likelihood of a cell undergoing differentiation to a PC. Secreted antibody limits the availability of free antigen epitopes, which has also been shown to enhance GC B cell selection (Toellner et al., 2018). Other inputs, such as from TLR receptors, are likely to have an impact on selection dynamics, though whether these effects predominantly occur during GC seeding versus within GCs needs further investigation (Clingan and Matloubian, 2013; Rookhuizen and DeFranco, 2014; Tian et al., 2018).
GC B cells have increased metabolic activity compared to naïve B cells, taking up larger amounts of glucose and having greater mitochondrial content. GSK3 acts within B cells to restrain metabolic activity and prevent ROS-induced apoptosis (Jellusova et al., 2017). The highly proliferative GC microenvironment is hypoxic and this leads to HIF transcription factor induction and changes in gene expression that influence GC selection events (Cho et al., 2016). It is not known whether the hypoxic state of GCs varies across different types of responses or in different lymphoid tissues.
GC B cells have a markedly altered shape compared to naïve B cells (Fig. 2), exhibiting a dendritic, probing morphology, perhaps leading to a more efficient search for surface displayed antigen (Allen et al., 2007). When studied ex vivo, GC B cells interact with antigen using small peripheral clusters of BCRs that do not show a central gathering behavior before internalization. Importantly, stronger tugging forces are exerted by BCRs in GC B cells compared to naïve B cells, suggesting the altered morphology, cytoskeleton and BCR signaling properties of GC B cells contribute to more stringent affinity discrimination following binding of membrane-associated antigen (Kwak et al., 2018; Nowosad et al., 2016).
GC B cells are confined within the central region of lymphoid follicles and it is possible that each GC can function for some period as an ‘island’ and support the evolution of distinct B cell clones, thereby contributing to the overall diversity of the antigen-specific response (Fig. 4). GC B cell confinement is achieved by the migration inhibitory receptors S1PR2 and, in humans, P2RY8 that couple to Gα13 containing heterotrimeric G-proteins (Muppidi et al., 2014). GC B cells bind antigen and receive T cell help signals in the LZ and travel to the DZ in a CXCR4 dependent manner where they undergo one or more rounds of cell division (the number being determined by the strength of T cell help received in the LZ) and AID-induced mutation of their Ig Hc VDJ and Lc VJ regions (Bannard and Cyster, 2017; Mesin et al., 2016).
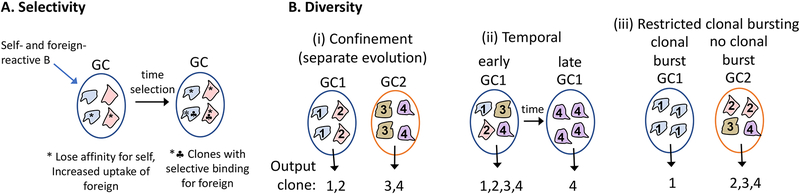
Figure 4. Germinal centers (GCs) promote selectivity and diversity in foreign antigen-reactive B cells.
(A) A self-reactive B cell that has bound a cross-reactive higher valency foreign antigen undergoes somatic hypermutation in a GC, leading to the emergence of foreign antigen-selective clones. This process is referred to as ‘clonal redemption’. (B) Models to explain how multiple properties of GCs may support induction of a diversity of antigen-reactive clones. (i) Confinement of cells to individual GCs for some period of clonal evolution may help ensure distinct clones emerge (as memory cells and PCs) from the separate GCs. (ii) GCs output a diversity of clones early in the response (mostly as memory B cells) followed by more restricted clones (mostly as PCs) after longer periods of selection. (iii) Some GCs undergo clonal bursting and are taken over by one or a few clones while others in the same lymphoid tissue refrain from clonal bursting and produce a diversity of output clones over a sustained period.
Tfh cell positioning within GCs is promoted by high CXCR5 expression and by downregulation of EBI2 (Suan et al., 2015; Vinuesa and Cyster, 2011). High PD1 expression on Tfh cells impedes their access to lymphoid follicles due to engagement of PDL1 on naïve B cells (Shi et al., 2018). This migration block is overcome by Tfh cell ICOS binding to ICOSL on naïve B cells to trigger PI3K activity that presumably cooperates with that induced by CXCL13 to promote follicle access (Liu et al., 2015). A number of additional Tfh cell-GC B cell ligand-receptor systems, including Semaphorin 4C-Plexin B2 and EphB4-EphrinB1, act to regulate Tfh cell access to or distribution within GCs (Yan et al., 2017)(Laidlaw et al., 2017; Lu et al., 2017). Precise control on the number of Tfh cells within GCs is important for desired B cell selection outcomes to occur, as perhaps most dramatically exemplified in Roquin mutant mice that have greatly exaggerated Tfh cell responses, exuberant GCs, and suffer from autoantibody production (Vinuesa and Cyster, 2011). T follicular regulatory (Tfr) cells are a Bcl6-expressing subset of FoxP3+ T regulatory cells that influences multiple events occurring during GC seeding and within GCs. Tfr cells have modulatory effects on affinity maturation, Tfh cell cytokine production, and PC output, and they restrain autoantibody production (Sage and Sharpe, 2015; Wing et al., 2018). There is also evidence that Tfr cells are involved in the contraction of GC responses (Wollenberg et al., 2011). Precisely how Tfr cells mediate these diverse effects and how closely the mouse models human Tfr cell behavior are areas of intense investigation (Wing et al., 2018).
GCs are the predominant site of antibody gene somatic hypermutation (SHM). AID deaminates cytidine residues in transcribed VDJ and VJ regions. Base excision repair (BER) and mismatch repair (MMR) mechanisms convert AID cytidine deamination lesions to point mutations and a small frequency of insertions and deletions (Hwang et al., 2014). The precise mechanisms acting to target AID to VDJ and VJ regions in GC B cells are not yet defined but the higher activity of AID in complementarity determining regions (CDRs) is partially explained by the enrichment ofa consensus AID motif in these regions. SHM requires higher amounts of AID than CSR, and this may explain why SHM is largely restricted to the AIDhi GC B cell state (Yeap et al., 2015). Following SHM the BCR needs to be replaced by newly translated HC and LC. Recent work shows that GC B cells replace their antigen receptors in the DZ (Stewart et al., 2018). MHC-peptide turnover also occurs in the DZ, promoted by the ubiquitin ligase March1, and the turnover is blocked in the LZ by the March1 antagonist, CD83 (Bannard et al., 2016). B cells with damaged BCRs undergo apoptosis in the DZ and cells failing to receive helper signals undergo cell deathin the LZ (Mayer et al., 2017). The overall rate of cell death is such that up to half of all GC B cells die every 6 hours. Apoptotic GC B cells are rapidly cleared by tingible body macrophages.
While the emphasis on GC studies has long been to understand how they support maturation towards the highest affinity antibodies, there has been an increasing realization that these responses also support the development of a diverse population of antigen-specific B cells (Bannard and Cyster, 2017; Finney et al., 2018). This is important in allowing sufficient breadth in the response to achieve protection from a rapidly evolving pathogen that might quickly escape recognition if all the response was committed towards the highest affinity cells (Baumgarth, 2013). Mouse studies have shown that Bmem responses to West Nile virus (WNV) retain the ability to engage variant viruses whereas antibody derived from LLPC is poorly able to inhibit variant virus (Purtha et al., 2011). Studies of human Zika and Dengue virus responses have shown that Bmem responses are more diverse than those of the serum (LLPC) derived antibody (Andrade et al., 2019).
The selection of a diversity of antigen-specific cells may be promoted in multiple ways in addition to the separate GC ‘island’ hypothesis mentioned above (Fig. 4). At one level, in GC responses to some immunogens, Bmems are preferentially generated early and are therefore more diverse than the LLPCs generated later in the response (Weisel and Shlomchik, 2017). At another level, GCs have been found capable of supporting a diversity of clones across time without necessarily being taken over by a high affinity ‘winner’ (Kuraoka et al., 2016; Tas et al., 2016). GC diversity may be promoted by antibody-mediated feedback, as dominant GC clones with BCRs specific for particular antigen epitopes give rise to PCs that secrete antibody that masks these epitopes, thereby enhancing the selection of clones that bind distinct epitopes (Toellner et al., 2018). Overall, the mechanisms allowing sustained diversity in the GC need further investigation as they have wide implications, for example, in the context of efforts to generate broadly neutralizing antibody responses by iterative immunizations with related, but distinct ‘shepherding’ immunogens (Abbott et al., 2018). Highlighting our incomplete understanding of the selection mechanism, recent work has revealed that B cells can participate in GCs for sustained periods after only a transient early period of antigen presentation to T cells (Turner et al., 2017; Zhang et al., 2017).
While the benefit of having a diversity of Bmems and antibodies specific for a foreign antigen is clear, this cannot be at the exclusion of having selectivity for the antigen (Fig. 4). Indeed, a central role of GC B cell somatic hypermutation and selection may be to increase the relative binding of foreign-versus self-antigen. Many B cells in the pre-immune repertoire have measurable autoreactivity and these cells have reduced surface IgM and are less responsive to in vitro BCR engagement (Goodnow et al., 2010). As noted above, when strongly engaged in vivo by cross-reactive foreign antigen and T cell help, IgMlo B cells preferentially enter the GC response. Tracking mutation paths of such cells has revealed that they are initially selected for mutations that diminish self-reactivity while preserving foreign-antigen reactivity; in some cases, this can involve a paradoxical decrease in overall affinity for the foreign antigen. This state can plausibly be selected for because the large decrease in receptor occupancy by self-antigen permits increased uptake of the foreign antigen and increased receipt of T cell help. The loss of chronic engagement by ubiquitous self-antigen may also be beneficial in allowing recovery of BCR signaling. Subsequent mutations can increase the affinity – and selectivity – for the foreign antigen. Thus, the maintenance of self-reactive B cells in an anergic state may provide a pool of cells that can subsequently undergo mutation in GCs to achieve selectivity for foreign antigen. This clonal redemption in GCs is postulated to limit the number of ‘holes in the repertoire’ caused by self-antigens that might otherwise become opportunities for pathogen mimicry and escape from antibody responses (Burnett et al., 2018; Reed et al., 2016; Sabouri et al., 2014).
Broadly neutralizing antibody-induction requirements
With large scale research and translational efforts ongoing to develop a vaccine that induces a broadly neutralizing Ab (bnAb) response to HIV-1, a new standard in vaccine development is emerging that draws upon fundamental B cell research (Andrabi et al., 2018; Bonsignori et al., 2017). These efforts involve studying the response of long term non-progressors and other individuals who show protection from the virus, with isolation of HIV-1 specific peripheral blood B cells and identification of rare bnAbs. Studies of the inferred germline (iGL) precursors of these antibodies, also known as the unmutated common ancestor, often reveals no detectable reactivity to the immunogen, suggesting the original responding B cell had a remarkably low affinity and was possibly induced by a distinct form of the immunogen. Mice encoding the iGL HC and LC are generated and B cells from these mice transferred to non-transgenic mice to achieve frequencies similar to those in the naïve human repertoire. The latter frequency is estimated by sampling blood from healthy individuals and performing flow cytometry with the immunogen – typical frequencies have been in the 1/300,000 range (Havenar-Daughton et al., 2018). Studies in iGL knockin mouse models have shown that the efficiency of B cell participation in the GC response is determined by both the frequency and affinity of the available B cells, and by the valency of the inducing immunogen (Abbott et al., 2018; Dosenovic et al., 2018). Such mouse models are used to study the efficiency of ‘germ-line targeting’ vaccine candidates in bringing rare precursor B cells into the response, and to test the ability of subsequent ‘shepherding’ immunogens to guide the SHM trajectory of the responding cells towards making bnAbs.
Another important factor in determining the quality of the B cell response is the duration of antigen exposure. With standard vaccinations of antigen in alum adjuvant, this exposure period is short (a few days) whereas with many infections the exposure period will be much longer and may, for a time, be escalating. Natural infections typically induce stronger and longer-lasting antibody responses than vaccinations. Studies in mice and non-human primates (NHPs) have found that simply extending the period of antigen delivery (using osmotic pumps or repeated and escalating dosing) is sufficient to augment the GC, Tfh and antibody response (Hu et al., 2015; Pauthner et al., 2017; Tam et al., 2016). There has also been a recognition that some adjuvant formulations cause denaturation of antigens and this can lead to the induction of antibodies that lack reactivity against the native pathogen (Ozorowski et al., 2018). These observations highlight how more work needs to be done to fully understand what determines how long antigen is displayed to B cells within GCs, and to develop vaccine delivery systems that achieve more prolonged exposure of antigens in their native form.
The factors determining whether B cells stay in the GC versus differentiate in to a LLPC or Bmem are being actively investigated. In models where GC B cells received elevated T cell help, a PC fate was favored (Victora and Nussenzweig, 2012). In another study, acutely blocking access to FDC-displayed antigen reduced PC formation suggesting that BCR signaling was required (Krautler et al., 2017). Cbl ubiquitin ligases in GC B cells were found to promote degradation of IRF4 to restrain PC differentiation. Strong BCR and CD40 signals trigger Cbl degradation in LZ cells, providing an explanation for how these signals can foster PC differentiation (Li et al., 2018). In vitro, combined BCR, CD40 and cytokine (IL-4, IL-21) signaling led to the most robust IRF4 induction in human GC B cells, with high-affinity antigen being more effective than low-affinity (Kwak et al., 2018). GC PC precursors have been identified as Bcl6loCD69hiIRF4+ LZ cells and induction of these cells was dependent on stable interactions with Tfh cells and was sensitive to the amount of CD40 signaling (Ise et al., 2018).
Memory B cells
In B cell responses, memory may be maintained in two forms, first through the long-term production of antibody by LLPCs (Nutt et al., 2015), and second by the generation of a pool of relatively quiescent Bmems that can be reactivated by subsequent antigen exposures (Kurosaki et al., 2015; Weisel and Shlomchik, 2017). Historically, Bmems were thought to have undergone isotype switching, particularly to IgG isotypes, which in part may have been due to easier technical detection: an isotype-switched cell that was maintained long after antigen exposure was considered a Bmem since naïve B cells express IgM/IgD. Recent evidence with improved methodology has revealed a population of IgM Bmems (Weisel and Shlomchik, 2017). Upon re-exposure to antigen, Bmems can differentiate into GC B cells or PCs, and specific subsets of IgM versus IgG Bmems, defined by surface markers such as CD73, CD80, and PDL2, show different propensities to undergo particular differentiation programs (Weisel and Shlomchik, 2017). GC B cells are thought to give rise to a large fraction of the Bmem pool, yet GC-independent Bmems have also been described that appear very early in the immune response (Kurosaki et al., 2015; Weisel and Shlomchik, 2017). The generation of Bmems is influenced by the BCR isotype as well as extrinsic signals such as ligation of CD40 or cytokines, with recent work suggesting a role for IL-9 in Bmem generation (Takatsuka et al., 2018; Wang et al., 2017). Within the GC, cells that express higher amounts of the transcription factor Bach2 may preferentially give rise to Bmems rather than LLPCs (Kurosaki et al., 2015). The likelihood of a Bmem becoming activated by subsequent antigen exposure depends on its BCR affinity as well as epitope specificity, since Bmems compete with each other as well as antibody secreted by LLPCs (Kurosaki et al., 2015). Many studies of Bmems have been in the context of adoptive transfers to recipient mice that lack this competition, although this approach has been useful for elucidating fundamental properties of specific Bmem subsets (Weisel and Shlomchik, 2017). The location of Bmems with respect to antigen encounter is also likely important; a majority of Bmems appear to be recirculating, but some are tissue resident, such as in the lung (Allie et al., 2018; Weisel and Shlomchik, 2017). Within LNs, Bmems appear to be positioned in a subcapsular niche enabling rapid encounter with antigen-bearing subscapsular sinus macrophages and Tfh cells (Moran et al., 2018).
One of the features of immunological memory is an enhanced speed of the response to antigen exposure. A major subset of Bmems rapidly differentiates into PCs that migrate via the bloodstream to the BM and become LLPCs (Nutt et al., 2015; Weisel and Shlomchik, 2017). This transient migration of PCs in the blood can be readily detected in humans that have received vaccine boosters (Tarlinton et al., 2008). While this rapid response may be facilitated by the maintenance of increased numbers of antigen-specific B cells and T cells, there is ample evidence that the Bmems themselves are poised to rapidly differentiate. In the case of IgG Bmems this may be due to amplification of signaling via the intracellular tail of the IgG BCR (Wienands and Engels, 2016). However, nuclear transfer experiments to generate ‘naïve’ IgG B cells showed that these differed from memory IgG B cells (Kurosaki et al., 2015), indicating a distinct transcriptional or epigenetic state of Bmems also accounts for their ability to rapidly respond to antigen. The predisposition of some IgG Bmems to become LLPCs seems to involve the downregulation of Bach2 (Kurosaki et al., 2015). Conversely, high expression of the transcriptional repressor Zbtb32 restrains the differentiation of Bmems into PCs (Weisel and Shlomchik, 2017).
Upon antigen re-exposure, Bmems may also undergo CSR (Fig. 3). In most studies in mice, IgE Bmems have not been detected, yet upon antigen re-exposure IgE can be rapidly produced, in some cases with higher affinity (He et al., 2015; Yang et al., 2014). This has led to a model in which memory is maintained in B cells of IgM or IgG isotypes that then switch to IgE and differentiate into PCs upon antigen re-exposure (He et al., 2015; Yang et al., 2014). Recent work has characterized particular subtypes of IgG Bmems that give rise to IgE PCs (He et al., 2017).
Natural antibody and ‘innate memory’
This review has focused on the behavior and function of the most prevalent B cells in the adult mouse and human body, known as follicular or B2 B cells. However, rodents have at least two additional B cell subsets, B1 cells and marginal zone B cells, that make unique contributions to the antibody response. Although less well defined, similar B cell subsets most likely exist in humans. B1 cells are produced in the fetal and neonatal period, become enriched in body cavities, and are self-renewing. Marginal zone B cells develop more slowly in a Notch-dependent manner in the spleen. These B cells share the feature of being ‘innate-like’, meaning they exist in a ‘pre-activated’ state and differentiate into antibody secreting cells very rapidly (within 1–2 days) following antigen encounter. They have a B cell repertoire enriched with specificities that recognize carbohydrate and lipid moieties present on various life-threatening microbes (Kearney et al., 2015). Body cavity B cells also make the dominant contribution to so-called ‘natural’ IgM antibody, which exists in circulation even without prior immunization (Kearney et al., 2015; Reynolds et al., 2015). Natural antibody of other isotypes, such as IgE, has also been described (Cahenzli et al., 2013), though the cellular origin is not well defined. Natural antibody can be produced in germ free contexts though its composition is shaped by the microbiota. Natural antibody provides a first line of defense against a range of pathogens and, through opsonization, augments the follicular B cell response (Fereidan-Esfahani et al., 2019; Kearney et al., 2015). It also contains antibodies against blood group antigens (Nydegger et al., 2005). Natural antibodies against oxidized lipids have a protective role in some atherosclerosis models and may be involved in clearance of apoptotic cells (Fereidan-Esfahani et al., 2019; Sage et al., 2018). Neonatal exposure to microbial antigens that stimulate B1 cells leads to life-long changes in the antibody repertoire that may influence the response to future encounters not only with the same microbe but with a range of other microbes and allergens that share glycan determinants (Kearney et al., 2015).
B cells in disease – autoantibodies, allergies, cancer and beyond
Multiple self-tolerance checkpoints exist to remove autoreactive specificities from the B cell repertoire or to limit the ability of such cells to secrete autoantigen-binding antibody. These include receptor editing and deletion in immature B cells developing in the BM, competitive elimination of chronically autoantigen binding B cells in the periphery, and a state of anergy that disfavors PC differentiation (Goodnow et al., 2010; Nemazee, 2017). Autoantibody production can occur due to failures in these checkpoints or in T cell self-tolerance mechanisms. Variants in multiple genes are implicated in increasing the likelihood of checkpoint failure and of autoantibody production occurring (Goodnow et al., 2010; Nemazee, 2017; Taher et al., 2017).
Autoantibodies are pathogenic in a number of human diseases including SLE, pemphigus vulgaris, Grave’s disease and myasthenia gravis. Pathology can be caused by immune complex formation and activation of the complement cascade or of phagocytes by engagement of activating FcγRs, or through direct modulation of signaling receptors on target cells (Townsend et al., 2010). B cell depletion therapy using anti-CD20 antibody has been protective in some of these diseases such as pemphigus vulgaris, but not others such as SLE, and this appears to reflect the contribution of SLPC versus LLPC to autoantibody production and the inability of even prolonged anti-CD20 treatment to eliminate the latter (Hale et al., 2018). These clinical findings have added to the importance of understanding what factors drive SLPC versus LLPC development and what the requirements are to support LLPCs.
B cell depletion therapy has been efficacious in several diseases that are not though to be autoantibody mediated including MS, type I diabetes and RA (Smith et al., 2017; Townsend et al., 2010). Autoantigen presentation has often been posited as a mechanism for B cell disease-promoting activity. A recent study has provided strong evidence that this can be a contributing factor with the discovery that Bmems present Rasgrp2-derived epitopes that are also targeted on neurons by CD4 T cells (Jelcic et al., 2018). In this case it appears that the shared high expression of Rasgrp2 by the B cells and the neurons is a key feature along with the strong ability of Bmems to promote T cell activation.
In addition to autoimmunity, B cells play an important role in allergic diseases. IgE antibodies specific for allergen components sensitize mast cells and basophils for rapid degranulation in response to allergen exposures at various sites, such as in the intestine (food allergy), nose (allergic rhinitis), and lung (allergic asthma). The events that lead to IgE production in allergic diseases remain poorly defined, though CSR to IgE has been extensively studied in cell culture. For example, while IL-4 is a critical cytokine for the induction of CSR to IgE (He et al., 2015), the robust production of IL-4 by Tfh cells in many types of immune responses (Crotty, 2014) suggests that other factors physiologically determine whether or not IgE CSR occurs. Recent evidence indicates that IgE B cells are poorly competitive within GCs (He et al., 2015; Yang et al., 2014). This may have important implications for IgE production in allergy. The generation of high affinity IgE antibodies was reported to require precursor cells that express IgG1 to first undergo affinity maturation in GCs and become memory cells, and then subsequently switch to IgE upon allergen re-exposure (He et al., 2015) (Fig. 3). Allergic responses do not necessarily require high affinity IgE antibodies, however, as revealed in a mouse model of food allergy (Jimenez-Saiz et al., 2017), suggesting the predominance of a GC-independent IgE PC response may also be relevant to allergic disease. There is evidence that in aeroallergen sensitization, the antigens that induce IgE responses induce relatively poor IgG responses (Aalberse and Platts-Mills, 2004). This may in part be due to very low quantitative exposures to aeroallergens through the respiratory tract. IgE production may thus be favored under conditions that induce weak B cell responses and minimal GC activity, thereby enabling IgE+ B cells and/or PCs to avoid being outcompeted by IgG+ cells. Aside from IgE antibodies, B cells may also contribute to allergic inflammation through their interactions with T cells (Ballesteros-Tato et al., 2016; Lindell et al., 2008).
B cells have also emerged as an important source of the immunosuppressive cytokine IL-10. Mouse studies revealed that B cell-derived IL-10 can promote recovery from EAE and can be protective in models of arthritis and type-1 diabetes (Shen and Fillatreau, 2015; Vonberg et al., 2018) and IL-10 production from B cells restrains T cell responses during some viral and bacterial infections (Madan et al., 2009; Shen and Fillatreau, 2015; Yu et al., 2018). IL-10 producing B cells, sometimes called Bregs, may be derived from several types of B cells (including CD1dhi and CD5+ cells) with recent work converging on evidence that a subset of PCs secretes IL-10 (Fillatreau, 2018). Correlative studies have suggested that B cells are a source of IL-10 in some human disease settings (Sakkas et al., 2018; Shen and Fillatreau, 2015). B cells may make additional immunosuppressive cytokines (Shen et al., 2014), but they can also contribute to response severity by producing pro-inflammatory cytokines such as GM-CSF, IL-6 and TNF (Barr et al., 2012; Shen and Fillatreau, 2015), with a study identifying such inflammatory B cells in MS patients (Li et al., 2015). Overall, these findings indicate that the influence of B cells on the cytokine milieu will be context dependent. Another B cell-derived cytokine is the TNF family member, LTα1β2, that has important roles in promoting NFκB-dependent events within lymphoid stromal cells, including induction of CXCL13-expression (Cyster, 2010). In addition to its roles in supporting normal lymphoid tissue function, B cell-derived LTα1β2 is likely involved in tertiary lymphoid tissue formation at sites of chronic inflammation such as in the synovium of RA patients and in the lungs after viral infection (Browning, 2008).
The presence of B cells in a variety of solid tumor types, including breast cancer, ovarian cancer and melanoma, has been associated in some studies with a positive prognosis (Wouters and Nelson, 2018). The mechanism involved is unclear but could include antigen presentation to CD4 and CD8 T cells, antibody production and subsequent enhancement of presentation, or by promoting tertiary lymphoid tissue formation and local T cell accumulation. However, in some cases such as in prostate cancer and some forms of hepatocellular carcinoma (in mouse models) tumor-associated B cells are linked with a negative outcome and this may be a consequence of their expression of PDL1, IL-10 or LTα1β2 (Ammirante et al., 2010; Shalapour et al., 2015). More work is needed to determine what factors promote B cell association with tumors and how they influence immune responses in the tumor microenvironment, with the studies so far already indicating that the contribution of B cells will vary depending on the tumor type. It is also noteworthy that B cells frequently make antibody responses to cancer antigens and this has led to efforts to use antibodies from cancer patients as biomarkers of disease and to identify immunotherapy targets (Wu et al., 2017).
Malignancies of B cells themselves are a common form of hematopoietic cancer. This predilection arises because the gene modifications that B cells undergo during development and in immune responses are not perfect in their fidelity and because antibody responses require extensive B cell proliferation. RAG-mediated gene recombination occasionally results in chromosomal translocations, prominent examples being t(14;18) and t(8:14) that place Bcl-2 and c-Myc, respectively, under control of the Ig locus. AID can have off-target activity, especially in genes that are highly transcribed in AID+ cells. A diverse group of B cell lymphomas, including Follicular Lymphoma, Burkitt Lymphoma and Diffuse Large B Cell Lymphoma (DLBCL) are thought to be of GC origin, and comprehensive gene expression studies have provided evidence that these malignancies derive from different stages of the response. DLBCL has been split into two main subtypes based on gene-expression studies, Activated B Cell (ABC)-type and GC B-type, corresponding to a post-GC early plasmablast stage and a LZ GC B cell stage, respectively (Basso and Dalla-Favera, 2015; Schmitz et al., 2018). Burkitt Lymphoma is characterized by chromosomal translocations leading to c-Myc over-expression and has been suggested to correspond to a DZ stage (Schmitz et al., 2014). Follicular Lymphoma, typified by Bcl-2 over-expression, shows evidence of both BCR and T cell dependence and has a LZ resemblance (Huet et al., 2018). Chronic Lymphocytic Leukemia (CLL), the most common adult leukemia in the Western world, is also thought to be GC-derived based on the presence of somatic mutations in the VH regions. A constellation of large-scale RNA and genome sequencing studies have revealed that GC-derived lymphomas carry heavy mutation burdens with alterations in transcription and chromatin regulatory factors, survival factors, BCR and TLR signaling components, cell cycle proteins, and confinement receptors, among others (Basso and Dalla-Favera, 2015; Schmitz et al., 2018). Other B cell malignancies can be of follicular (Mantle Zone Lymphoma), marginal zone (MZ Lymphoma) or plasma cell (Multiple Myeloma) origin. The study of B cell lymphomas and their associated genetic derangements continues to be illuminating about requirements for normal B cell differentiation and signaling while also leading to the development of targeted therapies (Dalla-Favera, 2017; Jerkeman et al., 2017). For example, the finding that CLL cells depend on BCR signaling for survival has led to inhibitors of Bruton’s tyrosine kinase (BTK) and PI3Kδ being developed as treatments for this cancer (Fabbri and Dalla-Favera, 2016). Similarly, the finding of frequent mutations that augment BCR signaling in ABC-DLBCL has led to trials of BTK and PI3K inhibitors as new treatments of this disease (Schmitz et al., 2018).
Conclusions
In this review we have attempted to capture some of the advances in the understanding of B cell biology that have occurred since the turn of the century. These include important steps forward in understanding how B cells encounter antigens, the costimulatory and cytokine requirements for their proliferation and differentiation, and how properties of the BCR, the antigen and helper T cells influence B cell responses. Many advances continue to transform the field including the impact of deep sequencing technologies on understanding B cell repertoires, the IgA-inducing microbiome, and the genetic defects in humans that compromise or exaggerate B cell responses or give rise to B cell malignancies. Other advances that are providing insight include single cell approaches to define B cell heterogeneity, glycomic approaches to study effector sugars on antibodies, new methods to study human B cell responses including CRISPR-based manipulation, and the use of systems biology to study changes at the whole organism level. With the recognition that B cells and antibodies are involved in most types of immune response and the realization that inflammatory processes contribute to a wider range of diseases than previously believed, including for example metabolic syndrome and neurodegeneration, we can be sure that further basic research-driven discovery about B cell biology will lead to more and improved approaches to maintain health and fight disease in the future.
Acknowledgements
The authors thank their lab members for helpful discussions. JGC is a HHMI investigator. CDCA is a Pew Scholar in the Biomedical Sciences, supported by The Pew Charitable Trusts. Research related to this review was supported in part by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Numbers R01AI130470 R01AI40098 and R01AI45073, as well as the Cardiovascular Research Institute and the Sandler Asthma Basic Research Center at UCSF. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aalberse RC, and Platts-Mills TA (2004). How do we avoid developing allergy: modifications of the TH2 response from a B-cell perspective. J Allergy Clin Immunol 113, 983–986. [DOI] [PubMed] [Google Scholar]
- Abbott RK, Lee JH, Menis S, Skog P, Rossi M, Ota T, Kulp DW, Bhullar D, Kalyuzhniy O, Havenar-Daughton C, et al. (2018). Precursor Frequency and Affinity Determine B Cell Competitive Fitness in Germinal Centers, Tested with Germline-Targeting HIV Vaccine Immunogens. Immunity 48, 133–146 e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achatz-Straussberger G, Zaborsky N, Konigsberger S, Luger EO, Lamers M, Crameri R, and Achatz G (2008). Migration of antibody secreting cells towards CXCL12 depends on the isotype that forms the BCR. Eur J Immunol 38, 3167–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleyd E, Heineke MH, and van Egmond M (2015). The era of the immunoglobulin A Fc receptor FcalphaRI; its function and potential as target in disease. Immunol Rev 268, 123–138. [DOI] [PubMed] [Google Scholar]
- Allen CD, and Cyster JG (2008). Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin Immunol 20, 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Okada T, and Cyster JG (2007). Germinal-center organization and cellular dynamics. Immunity 27, 190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allie SR, Bradley JE, Mudunuru U, Schultz MD, Graf BA, Lund FE, and Randall TD (2018). The establishment of resident memory B cells in the lung requires local antigen encounter. Nat Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammirante M, Luo JL, Grivennikov S, Nedospasov S, and Karin M (2010). B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature 464, 302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrabi R, Bhiman JN, and Burton DR (2018). Strategies for a multi-stage neutralizing antibody-based HIV vaccine. Curr Opin Immunol 53, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade P, Gimblet-Ochieng C, Modirian F, Collins M, Cárdenas M, Katzelnick L, Montoya M, Michlmayr D, Kuan G, Balmaseda A, et al. (2019). Impact of pre-existing dengue immunity on human antibody and memory B cell responses to Zika. Nat Comm in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs M, Martin F, Zhou T, and Kearney J (2002). Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity 17, 341–352. [DOI] [PubMed] [Google Scholar]
- Ballesteros-Tato A, Randall TD, Lund FE, Spolski R, Leonard WJ, and Leon B (2016). T Follicular Helper Cell Plasticity Shapes Pathogenic T Helper 2 Cell-Mediated Immunity to Inhaled House Dust Mite. Immunity 44, 259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannard O, and Cyster JG (2017). Germinal centers: programmed for affinity maturation and antibody diversification. Curr Opin Immunol 45, 21–30. [DOI] [PubMed] [Google Scholar]
- Bannard O, McGowan SJ, Ersching J, Ishido S, Victora GD, Shin JS, and Cyster JG (2016). Ubiquitin-mediated fluctuations in MHC class II facilitate efficient germinal center B cell responses. J Exp Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr TA, Shen P, Brown S, Lampropoulou V, Roch T, Lawrie S, Fan B, O’Connor RA, Anderton SM, Bar-Or A, et al. (2012). B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med 209, 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso K, and Dalla-Favera R (2015). Germinal centres and B cell lymphomagenesis. Nat Rev Immunol 15, 172–184. [DOI] [PubMed] [Google Scholar]
- Batista FD, and Harwood NE (2009). The who, how and where of antigen presentation to B cells. Nat Rev Immunol 9, 15–27. [DOI] [PubMed] [Google Scholar]
- Baumgarth N (2013). How specific is too specific? B-cell responses to viral infections reveal the importance of breadth over depth. Immunol Rev 255, 82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignori M, Liao HX, Gao F, Williams WB, Alam SM, Montefiori DC, and Haynes BF (2017). Antibody-virus co-evolution in HIV infection: paths for HIV vaccine development. Immunol Rev 275, 145–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortnick A, Chernova I, Spencer SP, and Allman D (2018). No strict requirement for eosinophils for bone marrow plasma cell survival. Eur J Immunol 48, 815–821. [DOI] [PubMed] [Google Scholar]
- Bournazos S, and Ravetch JV (2017). Diversification of IgG effector functions. Int Immunol 29, 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P, and Johansen FE (2005). Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol Rev 206, 32–63. [DOI] [PubMed] [Google Scholar]
- Briney B, Inderbitzin A, Joyce C, and Burton DR (2019). Commonality despite exceptional diversity in the baseline human antibody repertoire. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning JL (2008). Inhibition of the lymphotoxin pathway as a therapy for autoimmune disease. Immunol Rev 223, 202–220. [DOI] [PubMed] [Google Scholar]
- Burbage M, Gasparrini F, Aggarwal S, Gaya M, Arnold J, Nair U, Way M, Bruckbauer A, and Batista FD (2018). Tuning of in vivo cognate B-T cell interactions by Intersectin 2 is required for effective anti-viral B cell immunity. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet FM (1960). Immunological recognition of self. Nobel Lecture December 12. [Google Scholar]
- Burnett DL, Langley DB, Schofield P, Hermes JR, Chan TD, Jackson J, Bourne K, Reed JH, Patterson K, Porebski BT, et al. (2018). Germinal center antibody mutation trajectories are determined by rapid self/foreign discrimination. Science 360, 223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahenzli J, Koller Y, Wyss M, Geuking MB, and McCoy KD (2013). Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe 14, 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannons JL, Tangye SG, and Schwartzberg PL (2011). SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol 29, 665–705. [DOI] [PubMed] [Google Scholar]
- Carroll MC, and Isenman DE (2012). Regulation of humoral immunity by complement. Immunity 37, 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Cai C, Li Z, Liu G, Wang Y, Blonska M, Li D, Du J, Lin X, Yang M, et al. (2017). Dissection of SAP-dependent and SAP-independent SLAM family signaling in NKT cell development and humoral immunity. J Exp Med 214, 475–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Sun X, Yang W, Yang B, Zhao X, Chen S, He L, Chen H, Yang C, Xiao L, et al. (2018). An autoimmune disease variant of IgG1 modulates B cell activation and differentiation. Science 362, 700–705. [DOI] [PubMed] [Google Scholar]
- Cho SH, Raybuck AL, Stengel K, Wei M, Beck TC, Volanakis E, Thomas JW, Hiebert S, Haase VH, and Boothby MR (2016). Germinal centre hypoxia and regulation of antibody qualities by a hypoxia response system. Nature 537, 234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C, Verbaro DJ, Tonc E, Holmgren M, Cella M, Colonna M, Bhattacharya D, and Egawa T (2016). The Transcription Factor AP4 Mediates Resolution of Chronic Viral Infection through Amplification of Germinal Center B Cell Responses. Immunity 45, 570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clingan JM, and Matloubian M (2013). B Cell-intrinsic TLR7 signaling is required for optimal B cell responses during chronic viral infection. J Immunol 191, 810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremasco V, Woodruff MC, Onder L, Cupovic J, Nieves-Bonilla JM, Schildberg FA, Chang J, Cremasco F, Harvey CJ, Wucherpfennig K, et al. (2014). B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat Immunol 15, 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croote D, Darmanis S, Nadeau KC, and Quake SR (2018). High-affinity allergen-specific human antibodies cloned from single IgE B cell transcriptomes. Science 362, 1306–1309. [DOI] [PubMed] [Google Scholar]
- Crotty S (2014). T follicular helper cell differentiation, function, and roles in disease. Immunity 41, 529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster JG (2003). Homing of antibody secreting cells. Immunol Rev 194, 48–60. [DOI] [PubMed] [Google Scholar]
- Cyster JG (2010). B cell follicles and antigen encounters of the third kind. Nat Immunol 11, 989–996. [DOI] [PubMed] [Google Scholar]
- Cyster JG, Dang EV, Reboldi A, and Yi T (2014). 25-Hydroxycholesterols in innate and adaptive immunity. Nat Rev Immunol 14, 731–743. [DOI] [PubMed] [Google Scholar]
- Cyster JG, and Schwab SR (2012). Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol 30, 69–94. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R (2017). Molecular genetics of aggressive B-cell lymphoma. Hematol Oncol 35 Suppl 1, 76–79. [DOI] [PubMed] [Google Scholar]
- Dosenovic P, Kara EE, Pettersson AK, McGuire AT, Gray M, Hartweger H, Thientosapol ES, Stamatatos L, and Nussenzweig MC (2018). Anti-HIV-1 B cell responses are dependent on B cell precursor frequency and antigen-binding affinity. Proc Natl Acad Sci U S A 115, 4743–4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druzd D, Matveeva O, Ince L, Harrison U, He W, Schmal C, Herzel H, Tsang AH, Kawakami N, Leliavski A, et al. (2017). Lymphocyte Circadian Clocks Control Lymph Node Trafficking and Adaptive Immune Responses. Immunity 46, 120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich P (1908). Partial cell functions. Nobel Lecture December 11. [Google Scholar]
- Ersching J, Efeyan A, Mesin L, Jacobsen JT, Pasqual G, Grabiner BC, Dominguez-Sola D, Sabatini DM, and Victora GD (2017). Germinal Center Selection and Affinity Maturation Require Dynamic Regulation of mTORC1 Kinase. Immunity 46, 1045–1058 e1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri G, and Dalla-Favera R (2016). The molecular pathogenesis of chronic lymphocytic leukaemia. Nat Rev Cancer 16, 145–162. [DOI] [PubMed] [Google Scholar]
- Fereidan-Esfahani M, Nayfeh T, Warrington A, Howe CL, and Rodriguez M (2019). IgM Natural Autoantibodies in Physiology and the Treatment of Disease. Methods Mol Biol 1904, 53–81. [DOI] [PubMed] [Google Scholar]
- Fillatreau S (2018). Natural regulatory plasma cells. Curr Opin Immunol 55, 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney J, Yeh CH, Kelsoe G, and Kuraoka M (2018). Germinal center responses to complex antigens. Immunol Rev 284, 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming W (1885). Studien uber Regeneration der Gewebe. Arch f Mikr Anat 24, 50–91. [Google Scholar]
- Gatto D, and Brink R (2013). B cell localization: regulation by EBI2 and its oxysterol ligand. Trends Immunol 14, 446–453. [DOI] [PubMed] [Google Scholar]
- Gitlin AD, Mayer CT, Oliveira TY, Shulman Z, Jones MJ, Koren A, and Nussenzweig MC (2015). HUMORAL IMMUNITY. T cell help controls the speed of the cell cycle in germinal center B cells. Science 349, 643–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gommerman JL, Rojas OL, and Fritz JH (2014). Re-thinking the functions of IgA(+) plasma cells. Gut Microbes 5, 652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez DG, Cote CM, Patel JR, Smith CB, Zhang Y, Nickerson KM, Zhang T, Kerfoot SM, and Haberman AM (2018). Nonredundant Roles of IL-21 and IL-4 in the Phased Initiation of Germinal Center B Cells and Subsequent Self-Renewal Transitions. J Immunol 201, 3569–3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow CC, Vinuesa CG, Randall KL, Mackay F, and Brink R (2010). Control systems and decision making for antibody production. Nat Immunol 11, 681–688. [DOI] [PubMed] [Google Scholar]
- Grigorova IL, Panteleev M, and Cyster JG (2010). Lymph node cortical sinus organization and relationship to lymphocyte egress dynamics and antigen exposure. Proc Natl Acad Sci U S A 107, 20447–20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland K, Ackermann JA, Ipseiz N, Culemann S, Pracht K, Englbrecht M, Jack HM, Schett G, Schuh W, and Kronke G (2018). Eosinophils are not essential for maintenance of murine plasma cells in the bone marrow. Eur J Immunol 48, 822–828. [DOI] [PubMed] [Google Scholar]
- Hale M, Rawlings DJ, and Jackson SW (2018). The long and the short of it: insights into the cellular source of autoantibodies as revealed by B cell depletion therapy. Curr Opin Immunol 55, 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniuda K, Fukao S, Kodama T, Hasegawa H, and Kitamura D (2016). Autonomous membrane IgE signaling prevents IgE-memory formation. Nat Immunol 17, 1109–1117. [DOI] [PubMed] [Google Scholar]
- Havenar-Daughton C, Abbott RK, Schief WR, and Crotty S (2018). When designing vaccines, consider the starting material: the human B cell repertoire. Curr Opin Immunol 53, 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenar-Daughton C, Lindqvist M, Heit A, Wu JE, Reiss SM, Kendric K, Belanger S, Kasturi SP, Landais E, Akondy RS, et al. (2016). CXCL13 is a plasma biomarker of germinal center activity. Proc Natl Acad Sci U S A 113, 2702–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JS, Narayanan S, Subramaniam S, Ho WQ, Lafaille JJ, and Curotto de Lafaille MA (2015). Biology of IgE production: IgE cell differentiation and the memory of IgE responses. Current topics in microbiology and immunology 388, 1–19. [DOI] [PubMed] [Google Scholar]
- He JS, Subramaniam S, Narang V, Srinivasan K, Saunders SP, Carbajo D, Wen-Shan T, Hidayah Hamadee N, Lum J, Lee A, et al. (2017). IgG1 memory B cells keep the memory of IgE responses. Nat Commun 8, 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesters BA, Myers RC, and Carroll MC (2014). Follicular dendritic cells: dynamic antigen libraries. Nat Rev Immunol 14, 495–504. [DOI] [PubMed] [Google Scholar]
- Hong S, Zhang Z, Liu H, Tian M, Zhu X, Zhang Z, Wang W, Zhou X, Zhang F, Ge Q, et al. (2018). B Cells Are the Dominant Antigen-Presenting Cells that Activate Naive CD4(+) T Cells upon Immunization with a Virus-Derived Nanoparticle Antigen. Immunity. [DOI] [PubMed] [Google Scholar]
- Hoogeboom R, and Tolar P (2016). Molecular Mechanisms of B Cell Antigen Gathering and Endocytosis. Curr Top Microbiol Immunol 393, 45–63. [DOI] [PubMed] [Google Scholar]
- Hu JK, Crampton JC, Cupo A, Ketas T, van Gils MJ, Sliepen K, de Taeye SW, Sok D, Ozorowski G, Deresa I, et al. (2015). Murine Antibody Responses to Cleaved Soluble HIV-1 Envelope Trimers Are Highly Restricted in Specificity. J Virol 89, 10383–10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JK, Crampton JC, Locci M, and Crotty S (2016). CRISPR-Mediated Slamf1Delta/Delta Slamf5Delta/Delta Slamf6Delta/Delta Triple Gene Disruption Reveals NKT Cell Defects but Not T Follicular Helper Cell Defects. PLoS One 11, e0156074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Gomez-Rodriguez J, Preite S, Garrett LJ, Harper UL, and Schwartzberg PL (2016). CRISPR-Mediated Triple Knockout of SLAMF1, SLAMF5 and SLAMF6 Supports Positive Signaling Roles in NKT Cell Development. PLoS One 11, e0156072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HY, Rivas-Caicedo A, Renevey F, Cannelle H, Peranzoni E, Scarpellino L, Hardie DL, Pommier A, Schaeuble K, Favre S, et al. (2018). Identification of a new subset of lymph node stromal cells involved in regulating plasma cell homeostasis. Proc Natl Acad Sci U S A 115, E6826–E6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet S, Sujobert P, and Salles G (2018). From genetics to the clinic: a translational perspective on follicular lymphoma. Nat Rev Cancer 18, 224–239. [DOI] [PubMed] [Google Scholar]
- Hwang JK, Alt FW, and Yeap LS (2014). Related Mechanisms of Antibody Somatic Hypermutation and Class Switch Recombination. Microbiology Spectrum 3, MDNA3–0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ise W, Fujii K, Shiroguchi K, Ito A, Kometani K, Takeda K, Kawakami E, Yamashita K, Suzuki K, Okada T, et al. (2018). T Follicular Helper Cell-Germinal Center B Cell Interaction Strength Regulates Entry into Plasma Cell or Recycling Germinal Center Cell Fate. Immunity 48, 702–715 e704. [DOI] [PubMed] [Google Scholar]
- Jelcic I, Al Nimer F, Wang J, Lentsch V, Planas R, Jelcic I, Madjovski A, Ruhrmann S, Faigle W, Frauenknecht K, et al. (2018). Memory B Cells Activate Brain-Homing, Autoreactive CD4(+) T Cells in Multiple Sclerosis. Cell 175, 85–100 e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellusova J, Cato MH, Apgar JR, Ramezani-Rad P, Leung CR, Chen C, Richardson AD, Conner EM, Benschop RJ, Woodgett JR, et al. (2017). Gsk3 is a metabolic checkpoint regulator in B cells. Nat Immunol 18, 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerkeman M, Hallek M, Dreyling M, Thieblemont C, Kimby E, and Staudt L (2017). Targeting of B-cell receptor signalling in B-cell malignancies. J Intern Med 282, 415–428. [DOI] [PubMed] [Google Scholar]
- Jimenez-Saiz R, Chu DK, Mandur TS, Walker TD, Gordon ME, Chaudhary R, Koenig J, Saliba S, Galipeau HJ, Utley A, et al. (2017). Lifelong memory responses perpetuate humoral TH2 immunity and anaphylaxis in food allergy. J Allergy Clin Immunol 140, 1604–1615 e1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney JF, Patel P, Stefanov EK, and King RG (2015). Natural antibody repertoires: development and functional role in inhibiting allergic airway disease. Annu Rev Immunol 33, 475–504. [DOI] [PubMed] [Google Scholar]
- Khalil AM, Cambier JC, and Shlomchik MJ (2012). B cell receptor signal transduction in the GC is short-circuited by high phosphatase activity. Science this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinet JP (1999). The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol 17, 931–972. [DOI] [PubMed] [Google Scholar]
- Krautler NJ, Suan D, Butt D, Bourne K, Hermes JR, Chan TD, Sundling C, Kaplan W, Schofield P, Jackson J, et al. (2017). Differentiation of germinal center B cells into plasma cells is initiated by high-affinity antigen and completed by Tfh cells. J Exp Med 214, 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraoka M, Schmidt AG, Nojima T, Feng F, Watanabe A, Kitamura D, Harrison SC, Kepler TB, and Kelsoe G (2016). Complex Antigens Drive Permissive Clonal Selection in Germinal Centers. Immunity 44, 542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T, Kometani K, and Ise W (2015). Memory B cells. Nat Rev Immunol 15, 149–159. [DOI] [PubMed] [Google Scholar]
- Kurosaki T, Shinohara H, and Baba Y (2010). B cell signaling and fate decision. Annu Rev Immunol 28, 21–55. [DOI] [PubMed] [Google Scholar]
- Kwak K, Quizon N, Sohn H, Saniee A, Manzella-Lapeira J, Holla P, Brzostowski J, Lu J, Xie H, Xu C, et al. (2018). Intrinsic properties of human germinal center B cells set antigen affinity thresholds. Sci Immunol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffleur B, Duchez S, Tarte K, Denis-Lagache N, Peron S, Carrion C, Denizot Y, and Cogne M (2015). Self-Restrained B Cells Arise following Membrane IgE Expression. Cell reports 10, 900–909. [DOI] [PubMed] [Google Scholar]
- Laidlaw BJ, Schmidt TH, Green JA, Allen CD, Okada T, and Cyster JG (2017). The Eph-related tyrosine kinase ligand Ephrin-B1 marks germinal center and memory precursor B cells. J Exp Med 214, 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam WY, and Bhattacharya D (2018). Metabolic Links between Plasma Cell Survival, Secretion, and Stress. Trends Immunol 39, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Rezk A, Miyazaki Y, Hilgenberg E, Touil H, Shen P, Moore CS, Michel L, Althekair F, Rajasekharan S, et al. (2015). Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Science translational medicine 7, 310ra166. [DOI] [PubMed] [Google Scholar]
- Li X, Gadzinsky A, Gong L, Tong H, Calderon V, Li Y, Kitamura D, Klein U, Langdon WY, Hou F, et al. (2018). Cbl Ubiquitin Ligases Control B Cell Exit from the Germinal-Center Reaction. Immunity 48, 530–541 e536. [DOI] [PubMed] [Google Scholar]
- Lin JX, and Leonard WJ (2018). The Common Cytokine Receptor gamma Chain Family of Cytokines. Cold Spring Harbor perspectives in biology 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindell DM, Berlin AA, Schaller MA, and Lukacs NW (2008). B cell antigen presentation promotes Th2 responses and immunopathology during chronic allergic lung disease. PLoS One 3, e3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Xu H, Shih C, Wan Z, Ma X, Ma W, Luo D, and Qi H (2015). T-B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature 517, 214–218. [DOI] [PubMed] [Google Scholar]
- Liu W, Wang H, and Xu C (2016). Antigen Receptor Nanoclusters: Small Units with Big Functions. Trends Immunol 37, 680–689. [DOI] [PubMed] [Google Scholar]
- Lu P, Shih C, and Qi H (2017). Ephrin B1-mediated repulsion and signaling control germinal center T cell territoriality and function. Science 356. [DOI] [PubMed] [Google Scholar]
- Luo W, Weisel F, and Shlomchik MJ (2018). B Cell Receptor and CD40 Signaling Are Rewired for Synergistic Induction of the c-Myc Transcription Factor in Germinal Center B Cells. Immunity 48, 313–326 e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthje K, Kallies A, Shimohakamada Y, GT TB, Light A, Tarlinton DM, and Nutt SL (2012). The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat Immunol 13, 491–498. [DOI] [PubMed] [Google Scholar]
- Lycke NY, and Bemark M (2017). The regulation of gut mucosal IgA B-cell responses: recent developments. Mucosal Immunol 10, 1361–1374. [DOI] [PubMed] [Google Scholar]
- Madan R, Demircik F, Surianarayanan S, Allen JL, Divanovic S, Trompette A, Yogev N, Gu Y, Khodoun M, Hildeman D, et al. (2009). Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol 183, 2312–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masahata K, Umemoto E, Kayama H, Kotani M, Nakamura S, Kurakawa T, Kikuta J, Gotoh K, Motooka D, Sato S, et al. (2014). Generation of colonic IgA-secreting cells in the caecal patch. Nature communications 5, 3704. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Nagakubo D, Yamamoto S, Shigeta A, Tomida S, Fujita M, Hirata T, Tsunoda I, Nakayama T, and Yoshie O (2018). CCL28-Deficient Mice Have Reduced IgA Antibody-Secreting Cells and an Altered Microbiota in the Colon. J Immunol 200, 800–809. [DOI] [PubMed] [Google Scholar]
- Mayer CT, Gazumyan A, Kara EE, Gitlin AD, Golijanin J, Viant C, Pai J, Oliveira TY, Wang Q, Escolano A, et al. (2017). The microanatomic segregation of selection by apoptosis in the germinal center. Science 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesin L, Ersching J, and Victora GD (2016). Germinal Center B Cell Dynamics. Immunity 45, 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens L, and Tangye SG (2014). Cytokine-Mediated Regulation of Plasma Cell Generation: IL-21 Takes Center Stage. Frontiers in immunology 5, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran I, Nguyen A, Khoo WH, Butt D, Bourne K, Young C, Hermes JR, Biro M, Gracie G, Ma CS, et al. (2018). Memory B cells are reactivated in subcapsular proliferative foci of lymph nodes. Nature communications 9, 3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muppidi JR, Schmitz R, Green JA, Xiao W, Larsen AB, Braun SE, An J, Xu Y, Rosenwald A, Ott G, et al. (2014). Loss of signalling via Galpha13 in germinal centre B-cell-derived lymphoma. Nature 516, 254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemazee D (2017). Mechanisms of central tolerance for B cells. Nat Rev Immunol 17, 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DC, Garimalla S, Xiao H, Kyu S, Albizua I, Galipeau J, Chiang KY, Waller EK, Wu R, Gibson G, et al. (2018). Factors of the bone marrow microniche that support human plasma cell survival and immunoglobulin secretion. Nature communications 9, 3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noviski M, Mueller JL, Satterthwaite A, Garrett-Sinha LA, Brombacher F, and Zikherman J (2018). IgM and IgD B cell receptors differentially respond to endogenous antigens and control B cell fate. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noviski M, and Zikherman J (2018). Control of autoreactive B cells by IgM and IgD B cell receptors: maintaining a fine balance. Curr Opin Immunol 55, 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowosad CR, Spillane KM, and Tolar P (2016). Germinal center B cells recognize antigen through a specialized immune synapse architecture. Nat Immunol 17, 870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt SL, Hodgkin PD, Tarlinton DM, and Corcoran LM (2015). The generation of antibody-secreting plasma cells. Nat Rev Immunol 15, 160–171. [DOI] [PubMed] [Google Scholar]
- Nydegger UE, Tevaearai H, Berdat P, Rieben R, Carrel T, Mohacsi P, and Flegel WA (2005). Histo-blood group antigens as allo-and autoantigens. Ann N Y Acad Sci 1050, 40–51. [DOI] [PubMed] [Google Scholar]
- Ozorowski G, Cupo A, Golabek M, LoPiccolo M, Ketas TA, Cavallary M, Cottrell CA, Klasse PJ, Ward AB, and Moore JP (2018). Effects of Adjuvants on HIV-1 Envelope Glycoprotein SOSIP Trimers In Vitro. J Virol 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa I, Saliba D, Ponzoni M, Bustamante S, Canete PF, Gonzalez-Figueroa P, McNamara HA, Valvo S, Grimbaldeston M, Sweet RA, et al. (2017). TFH-derived dopamine accelerates productive synapses in germinal centres. Nature 547, 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauthner M, Havenar-Daughton C, Sok D, Nkolola JP, Bastidas R, Boopathy AV, Carnathan DG, Chandrashekar A, Cirelli KM, Cottrell CA, et al. (2017). Elicitation of Robust Tier 2 Neutralizing Antibody Responses in Nonhuman Primates by HIV Envelope Trimer Immunization Using Optimized Approaches. Immunity 46, 1073–1088 e1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purtha WE, Tedder TF, Johnson S, Bhattacharya D, and Diamond MS (2011). Memory B cells, but not long-lived plasma cells, possess antigen specificities for viral escape mutants. J Exp Med 208, 2599–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Kastenmuller W, and Germain RN (2014). Spatiotemporal basis of innate and adaptive immunity in secondary lymphoid tissue. Annu Rev Cell Dev Biol 30, 141–167. [DOI] [PubMed] [Google Scholar]
- Ramadani F, Bowen H, Upton N, Hobson PS, Chan YC, Chen JB, Chang TW, McDonnell JM, Sutton BJ, Fear DJ, et al. (2017). Ontogeny of human IgE-expressing B cells and plasma cells. Allergy 72, 66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JH, Jackson J, Christ D, and Goodnow CC (2016). Clonal redemption of autoantibodies by somatic hypermutation away from self-reactivity during human immunization. J Exp Med 213, 1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AE, Kuraoka M, and Kelsoe G (2015). Natural IgM is produced by CD5-plasma cells that occupy a distinct survival niche in bone marrow. J Immunol 194, 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda LB, Lu E, Bennett ML, Sokol CL, Wang X, Luther SA, Barres BA, Luster AD, Ye CJ, and Cyster JG (2018). Single-Cell RNA Sequencing of Lymph Node Stromal Cells Reveals Niche-Associated Heterogeneity. Immunity 48, 1014–1028 e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rookhuizen DC, and DeFranco AL (2014). Toll-like receptor 9 signaling acts on multiple elements of the germinal center to enhance antibody responses. Proc Natl Acad Sci U S A 111, E3224–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabouri Z, Schofield P, Horikawa K, Spierings E, Kipling D, Randall KL, Langley D, Roome B, Vazquez-Lombardi R, Rouet R, et al. (2014). Redemption of autoantibodies on anergic B cells by variable-region glycosylation and mutation away from self-reactivity. Proc Natl Acad Sci U S A 111, E2567–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage AP, Tsiantoulas D, Binder CJ, and Mallat Z (2018). The role of B cells in atherosclerosis. Nat Rev Cardiol. [DOI] [PubMed] [Google Scholar]
- Sage PT, and Sharpe AH (2015). T follicular regulatory cells in the regulation of B cell responses. Trends Immunol 36, 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakkas LI, Daoussis D, Mavropoulos A, Liossis SN, and Bogdanos DP (2018). Regulatory B cells: New players in inflammatory and autoimmune rheumatic diseases. Semin Arthritis Rheum. [DOI] [PubMed] [Google Scholar]
- Schatz DG, Oettinger MA, and Baltimore D (1989). The V(D)J recombination activating gene, RAG-1. Cell 59, 1035–1048. [DOI] [PubMed] [Google Scholar]
- Schmitz R, Ceribelli M, Pittaluga S, Wright G, and Staudt LM (2014). Oncogenic mechanisms in Burkitt lymphoma. Cold Spring Harb Perspect Med 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, Roulland S, Kasbekar M, Young RM, Shaffer AL, et al. (2018). Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N Engl J Med 378, 1396–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz O, Hammerschmidt SI, Moschovakis GL, and Forster R (2016). Chemokines and Chemokine Receptors in Lymphoid Tissue Dynamics. Annu Rev Immunol 34, 203–242. [DOI] [PubMed] [Google Scholar]
- Schwickert TA, Victora GD, Fooksman DR, Kamphorst AO, Mugnier MR, Gitlin AD, Dustin ML, and Nussenzweig MC (2011). A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J Exp Med 208, 1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalapour S, Font-Burgada J, Di Caro G, Zhong Z, Sanchez-Lopez E, Dhar D, Willimsky G, Ammirante M, Strasner A, Hansel DE, et al. (2015). Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature 521, 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P, and Fillatreau S (2015). Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol 15, 441–451. [DOI] [PubMed] [Google Scholar]
- Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, Ries S, Dang VD, Jaimes Y, Daridon C, et al. (2014). IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507, 366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Hou S, Fang Q, Liu X, Liu X, and Qi H (2018). PD-1 Controls Follicular T Helper Cell Positioning and Function. Immunity 49, 264–274 e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Simmons KM, and Cambier JC (2017). B cells in type 1 diabetes mellitus and diabetic kidney disease. Nat Rev Nephrol 13, 712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart I, Radtke D, Phillips B, McGowan SJ, and Bannard O (2018). Germinal Center B Cells Replace Their Antigen Receptors in Dark Zones and Fail Light Zone Entry when Immunoglobulin Gene Mutations are Damaging. Immunity 49, 477–489 e477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suan D, Nguyen A, Moran I, Bourne K, Hermes JR, Arshi M, Hampton HR, Tomura M, Miwa Y, Kelleher AD, et al. (2015). T follicular helper cells have distinct modes of migration and molecular signatures in naive and memory immune responses. Immunity 42, 704–718. [DOI] [PubMed] [Google Scholar]
- Suthers AN, and Sarantopoulos S (2017). TLR7/TLR9- and B Cell Receptor-Signaling Crosstalk: Promotion of Potentially Dangerous B Cells. Front Immunol 8, 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Hayano Y, Nakai A, Furuta F, and Noda M (2016). Adrenergic control of the adaptive immune response by diurnal lymphocyte recirculation through lymph nodes. J Exp Med 213, 2567–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taher TE, Bystrom J, Ong VH, Isenberg DA, Renaudineau Y, Abraham DJ, and Mageed RA (2017). Intracellular B Lymphocyte Signalling and the Regulation of Humoral Immunity and Autoimmunity. Clin Rev Allergy Immunol 53, 237–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuka S, Yamada H, Haniuda K, Saruwatari H, Ichihashi M, Renauld JC, and Kitamura D (2018). IL-9 receptor signaling in memory B cells regulates humoral recall responses. Nat Immunol 19, 1025–1034. [DOI] [PubMed] [Google Scholar]
- Tam HH, Melo MB, Kang M, Pelet JM, Ruda VM, Foley MH, Hu JK, Kumari S, Crampton J, Baldeon AD, et al. (2016). Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc Natl Acad Sci U S A 113, E6639–E6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlinton D, Radbruch A, Hiepe F, and Dorner T (2008). Plasma cell differentiation and survival. Current opinion in immunology 20, 162–169. [DOI] [PubMed] [Google Scholar]
- Tas JM, Mesin L, Pasqual G, Targ S, Jacobsen JT, Mano YM, Chen CS, Weill JC, Reynaud CA, Browne EP, et al. (2016). Visualizing antibody affinity maturation in germinal centers. Science 351, 1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Hua Z, Hong S, Zhang Z, Liu C, Lin L, Chen J, Zhang W, Zhou X, Zhang F, et al. (2018). B Cell-Intrinsic MyD88 Signaling Promotes Initial Cell Proliferation and Differentiation To Enhance the Germinal Center Response to a Virus-like Particle. J Immunol 200, 937–948. [DOI] [PubMed] [Google Scholar]
- Toellner KM, Sze DM, and Zhang Y (2018). What Are the Primary Limitations in B-Cell Affinity Maturation, and How Much Affinity Maturation Can We Drive with Vaccination? A Role for Antibody Feedback. Cold Spring Harbor perspectives in biology 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S (1987). Somatic Generation of Immune Diversity. Nobel Lecture December 8. [DOI] [PubMed] [Google Scholar]
- Townsend MJ, Monroe JG, and Chan AC (2010). B-cell targeted therapies in human autoimmune diseases: an updated perspective. Immunol Rev 237, 264–283. [DOI] [PubMed] [Google Scholar]
- Tsubata T (2018). Ligand Recognition Determines the Role of Inhibitory B Cell Co-receptors in the Regulation of B Cell Homeostasis and Autoimmunity. Front Immunol 9, 2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JS, Benet ZL, and Grigorova I (2017). Transiently antigen primed B cells can generate multiple subsets of memory cells. PLoS One 12, e0183877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turqueti-Neves A, Otte M, Prazeres da Costa O, Hopken UE, Lipp M, Buch T, and Voehringer D (2014). B-cell-intrinsic STAT6 signaling controls germinal center formation. Eur J Immunol 44, 2130–2138. [DOI] [PubMed] [Google Scholar]
- Vanshylla K, Opazo F, Gronke K, Wienands J, and Engels N (2018). The extracellular membrane-proximal domain of membrane-bound IgE restricts B cell activation by limiting B cell antigen receptor surface expression. Eur J Immunol 48, 441–453. [DOI] [PubMed] [Google Scholar]
- Victora GD, and Nussenzweig MC (2012). Germinal centers. Annu Rev Immunol 30, 429–457. [DOI] [PubMed] [Google Scholar]
- Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, and Nussenzweig MC (2010). Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell 143, 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidarsson G, Dekkers G, and Rispens T (2014). IgG subclasses and allotypes: from structure to effector functions. Frontiers in immunology 5, 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa CG, and Cyster JG (2011). How T cells earn the follicular rite of passage. Immunity 35, 671–680. [DOI] [PubMed] [Google Scholar]
- Vinuesa CG, Linterman MA, Yu D, and MacLennan IC (2016). Follicular Helper T Cells. Annu Rev Immunol 34, 335–368. [DOI] [PubMed] [Google Scholar]
- Vonberg AD, Acevedo-Calado M, Cox AR, Pietropaolo SL, Gianani R, Lundy SK, and Pietropaolo M (2018). CD19+IgM+ cells demonstrate enhanced therapeutic efficacy in type 1 diabetes mellitus. JCI Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shi J, Yan J, Xiao Z, Hou X, Lu P, Hou S, Mao T, Liu W, Ma Y, et al. (2017). Germinal-center development of memory B cells driven by IL-9 from follicular helper T cells. Nat Immunol 18, 921–930. [DOI] [PubMed] [Google Scholar]
- Ward SE, and Ober RJ (2018). Targeting FcRn to Generate Antibody-Based Therapeutics. Trends Pharmacol Sci 39, 892–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JS, Herman EI, Lainez B, Licona-Limon P, Esplugues E, Flavell R, and Craft J (2016). TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol 17, 1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel F, and Shlomchik M (2017). Memory B Cells of Mice and Humans. Annu Rev Immunol 35, 255–284. [DOI] [PubMed] [Google Scholar]
- Wienands J, and Engels N (2016). The Memory Function of the B Cell Antigen Receptor. Current topics in microbiology and immunology 393, 107–121. [DOI] [PubMed] [Google Scholar]
- Wilmore JR, and Allman D (2017). Here, There, and Anywhere? Arguments for and against the Physical Plasma Cell Survival Niche. J Immunol 199, 839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing JB, Tekguc M, and Sakaguchi S (2018). Control of Germinal Center Responses by T-Follicular Regulatory Cells. Front Immunol 9, 1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenberg I, Agua-Doce A, Hernandez A, Almeida C, Oliveira VG, Faro J, and Graca L (2011). Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol 187, 4553–4560. [DOI] [PubMed] [Google Scholar]
- Woodruff MC, Kim EH, Luo W, and Pulendran B (2018). B Cell Competition for Restricted T Cell Help Suppresses Rare-Epitope Responses. Cell Rep 25, 321–327 e323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters MCA, and Nelson BH (2018). Prognostic Significance of Tumor-Infiltrating B Cells and Plasma Cells in Human Cancer. Clin Cancer Res 24, 6125–6135. [DOI] [PubMed] [Google Scholar]
- Wu J, Li X, Song W, Fang Y, Yu L, Liu S, Churilov LP, and Zhang F (2017). The roles and applications of autoantibodies in progression, diagnosis, treatment and prognosis of human malignant tumours. Autoimmun Rev 16, 1270–1281. [DOI] [PubMed] [Google Scholar]
- Yan H, Wu L, Shih C, Hou S, Shi J, Mao T, Chen W, Melvin B, Rigby RJ, Chen Y, et al. (2017). Plexin B2 and Semaphorin 4C Guide T Cell Recruitment and Function in the Germinal Center. Cell Rep 19, 995–1007. [DOI] [PubMed] [Google Scholar]
- Yang J, and Reth M (2016). Receptor Dissociation and B-Cell Activation. Curr Top Microbiol Immunol 393, 27–43. [DOI] [PubMed] [Google Scholar]
- Yang Z, Robinson MJ, and Allen CD (2014). Regulatory constraints in the generation and differentiation of IgE-expressing B cells. Curr Opin Immunol 28, 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Robinson MJ, Chen X, Smith GA, Taunton J, Liu W, and Allen CD (2016). Regulation of B cell fate by chronic activity of the IgE B cell receptor. Elife 5, `. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeap LS, Hwang JK, Du Z, Meyers RM, Meng FL, Jakubauskaite A, Liu M, Mani V, Neuberg D, Kepler TB, et al. (2015). Sequence-Intrinsic Mechanisms that Target AID Mutational Outcomes on Antibody Genes. Cell 163, 1124–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Duong VHH, Westphal K, Westphal A, Suwandi A, Grassl GA, Brand K, Chan AC, Foger N, and Lee KH (2018). Surface receptor Toso controls B cell-mediated regulation of T cell immunity. J Clin Invest 128, 1820–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf I, Kageyama R, Monticelli L, Johnston RJ, Ditoro D, Hansen K, Barnett B, and Crotty S (2010). Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150). J Immunol 185, 190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretsky I, Atrakchi O, Mazor RD, Stoler-Barak L, Biram A, Feigelson SW, Gitlin AD, Engelhardt B, and Shulman Z (2017). ICAMs support B cell interactions with T follicular helper cells and promote clonal selection. J Exp Med 214, 3435–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TT, Gonzalez DG, Cote CM, Kerfoot SM, Deng S, Cheng Y, Magari M, and Haberman AM (2017). Germinal center B cell development has distinctly regulated stages completed by disengagement from T cell help. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JHS, Markham JF, Duffy KR, and Hodgkin PD (2018). Stochastically Timed Competition Between Division and Differentiation Fates Regulates the Transition From B Lymphoblast to Plasma Cell. Front Immunol 9, 2053. [DOI] [PMC free article] [PubMed] [Google Scholar]