Abstract
Exercise can cause a decrease in serum ionized calcium (iCa) and increases in parathyroid hormone (PTH) and bone resorption. We used a novel intravenous iCa clamp technique to determine whether preventing a decline in serum iCa during exercise prevents increases in PTH and carboxy-terminal collagen crosslinks (CTX). Eleven cycling-trained men (aged 18 to 45 years) underwent two identical 60-min cycling bouts with infusion of Ca gluconate or saline. Blood sampling for iCa, total calcium (tCa), PTH, CTX, and procollagen type 1 amino-terminal propeptide (P1NP) occurred before, during, and for 4 hours after exercise; results are presented as unadjusted and adjusted for plasma volume shifts (denoted with subscript ADJ). iCa decreased during exercise with saline infusion (p = 0.01 at 60 min) and this was prevented by Ca infusion (interaction, p < 0.007); there were abrupt decreases in Ca content (iCaADJ and tCaADJ) in the first 15 min of exercise under both conditions. PTH and CTX were increased at the end of exercise (both p < 0.01) on the saline day, and markedly attenuated (−65% and −71%; both p < 0.001) by Ca. CTX remained elevated for 4 hours after exercise on the saline day (p<0.001), despite the return of PTH to baseline by 1 hour after exercise. P1NP increased in response to exercise (p < 0.001), with no difference between conditions, but the increase in P1NPADJ was not significant. Results for PTHADJ and CTXADJ were similar to unadjusted results. These findings demonstrate that bone resorption is stimulated early in exercise to defend serum iCa. Vascular Ca content decreased early in exercise, but neither the reason why this occurred, nor the fate of Ca, are known. The results suggest that the exercise-induced increase in PTH had an acute catabolic effect on bone. Future research should determine whether the increase in PTH generates an anabolic response that occurs more than 4 hours after exercise.
Keywords: BIOCHEMICAL MARKERS OF BONE TURNOVER, BONE MODELING AND REMODELING, EXERCISE
Introduction
Bone mineral density (BMD) is known to vary among athletes who participate in different sports. In general, BMD tends to be lower in those who participate in weight-supported (eg, cycling) or low-impact activities (eg, cross-country skiing) than in athletes who participate in higher-impact activities (eg, volley-ball).(1–3) This suggests that forces acting on bone in low-impact and non-impact sports are not sufficient to generate the anabolic skeletal response that occurs with high-impact activities. However, bone loss has been observed over time in some athletes, at an age when stable BMD would be expected, suggesting that exercise may sometimes have catabolic skeletal effects. For example, competitive male road cyclists followed over 1 year of training and competition had a 1.5% decrease in hip BMD and a 1.0% decrease in lumbar spine BMD.(4) College basketball players were also observed to have a 6% loss of total body bone mineral content (BMC) and a 10% loss of leg BMC over approximately 1 year.(5)
The reasons why exercise training may result in bone loss are not known. Our overarching hypothesis is that bone loss occurs under conditions in which the activation of bone resorption by exercise is not adequately countered by activation of bone formation. A number of factors could contribute to the stimulation of bone resorption during exercise; the current study focused on the disruption of calcium (Ca) homeostasis as a contributing factor. The working model portends that serum ionized Ca (iCa) decreases during vigorous exercise and stimulates the secretion of parathyroid hormone (PTH), which mobilizes Ca from bone to defend serum iCa concentration. We(4,6–9) and others(10–14) have demonstrated that this cascade of events occurs in both young and older, exercise trained and untrained, women and men, and that Ca supplementation before and/or during exercise may mitigate the PTH and bone resorption responses.
The aim of the current study was to determine whether the increases in PTH and bone resorption during exercise are prevented when serum iCa concentration is maintained. To test this, we developed the iCa clamp technique, whereby Ca gluconate is infused intravenously during exercise at a variable rate to maintain serum iCa above the pre-exercise serum concentration. The hypothesis was that the increase in PTH and bone resorption in response to exercise would be significantly attenuated by Ca infusion when compared with a volume-matched saline control.
Subjects and Methods
Participants
Men (n=11) aged 18 to 45 years who were accustomed to cycling exercise participated in the study. Activity status was evaluated by self-report and eligible volunteers were those who reported participating in moderate to vigorous cycling exercise at least 2 days per week for at least 1 hour at a time. Exclusion criteria included: history of type 1 or type 2 diabetes mellitus; active cardiovascular disease (evidence of ischemic heart disease or serious arrhythmias at rest or during the graded exercise test); use of medications known to affect bone metabolism in the previous 6 months (eg, osteoporosis medications, thiazide diuretics, oral glucocorticoids); BMD T-score ≤−2.5; moderate or severe renal impairment (estimated glomerular filtration rate of <60 mL/min/1.73m2); chronic hepatobiliary disease (aspartate transaminase or alanine transaminase concentration >1.5 times the upper limit of normal); uncontrolled hypertension (systolic blood pressure >150 mmHg or diastolic blood pressure >90 mmHg); serum Ca <8.5 or >10.3 mg/dL; abnormal thyroid function (ultrasensitive TSH <0.5 or >5.0mU/L); and serum 25(OH)D <20ng/mL. All participants provided written informed consent, and the study was approved by the Colorado Multiple Institutional Review Board and the Army Human Research Protection Office.
Screening visits
Participants completed a medical history questionnaire and underwent a physical examination, fasted blood draw, dual-energy X-ray absorptiometry (DXA) scans, and a maximal exercise test to evaluate eligibility criteria.
DXA
BMD at the lumbar spine (L1–L4), total hip, and femoral neck regions of the hip were measured during screening to verify eligibility. A total body scan was performed to determine fat-free mass and fat mass. All scans were performed using the Discovery W instrument (Hologic Inc., Waltham, MA, USA).
Maximal exercise test
An incremental cycling test was used to assess peak aerobic power (VO2peak) and screen for cardiovascular eligibility. During a 5-min warm-up, power output was adjusted to elicit a heart rate that was roughly 70% of age-predicted maximum. The test began at that power output and was increased by 25 W every 2 min until volitional fatigue. VO2 was measured using the Parvo Medics TruMax 2400 Metabolic Cart (Parvo Medics Inc, Sandy, UT, USA).
Exercise sessions
Participants performed two identical exercise sessions 1 to 5 weeks apart. Ca infusion always occurred during the first session to facilitate the volume-matched half-normal saline infusion during the second session. The exercise protocol was 60 min of vigorous cycling at ~80% of the maximal heart rate achieved during the maximal exercise test. There was a 3-min to 5-min warm-up before and a 3-min to 5-min cool-down after the exercise bout. The power output achieved during the first exercise session was replicated during the second session. Participants fasted overnight and then consumed a standardized meal 4 hours before both exercise sessions. The meal contained 25% of estimated daily caloric need based on sex-specific Mifflin equations adjusted for height, weight, sex, and an activity factor of 1.65.(15) Macronutrient content was 55%carbohydrate,25%fat, and 20% protein. Ca content was 90 to 100 mg. After the meal, participants fasted until after the final blood draw.
iCa clamp
Pilot testing was conducted to establish the iCa clamp during exercise. The following criteria defined a successful clamp: (i) the initial constant infusion rate during the 15 min before exercise should raise serum iCa by ~0.2 mg/dL; (ii) the variable infusion rate during the 60 min of exercise should prevent serum iCa from decreasing below the preinfusion baseline concentration; (iii) serum iCa should not exceed 5.7 mg/dL; and (iv) the iCa infusion should not exceed the maximal recommended rate (27 mg/min). We successfully completed pilot testing on four men before beginning the iCa clamp experiments.
The iCa clamp used intravenous infusion of Ca gluconate, at a concentration of 0.169 mg/mL of elemental Ca (CaELEM). The infusion was started 15 min before exercise at a rate that delivered 0.5 mg CaELEM per kg body weight over 15 min; the goal was to raise serum iCa by 0.1 to 0.2 mg/dL before exercise commenced. After the 15-min pre-exercise infusion, the infusion rate was set to deliver 2 mg CaELEM per minute. Blood samples were obtained every 5 min during exercise for measurement of iCa using the iSTAT analyzer (Abbot Point of Care, Princeton, NJ, USA) and results were used to adjust the infusion rate to maintain iCa above the preinfusion level, but not to exceed 5.7 mg/dL. The second exercise session was conducted at the same time of day as the first session, but with half-normal saline infusion. The volume and timing of the infusion and of water consumption were matched to the first exercise session.
Ca gluconate preparation
The Ca infusate was prepared by the University of Colorado Hospital Investigational Pharmacy. A hypertonic Ca gluconate solution was diluted approximately 50-fold to achieve a final concentration of 2g of Ca gluconate/1100 mL (186 mg CaELEM/1100 mL).
Urine Ca loss
Urine samples were collected at visit admission and immediately, 2 hours, and 4 hours after exercise. Total urine volume and weight of each void was recorded and 40-mL aliquots were sent for total Ca analysis by indirect ion selective electrode. (Beckman Coulter, Inc., Brea, CA, USA). Urine Ca concentration was multiplied by total void volume to calculate Ca loss for each time interval.
Blood collection
An indwelling intravenous catheter for serial blood sampling was positioned in an arm vein approximately 30 min before exercise. Blood samples (10 mL) were obtained before the start of the infusion, immediately before exercise, and every 15 min during exercise; blood sampling continued through 4 hours of recovery after exercise (Fig. 1). These samples were used for the measurement of intact PTH by Immulite two-site enzyme immunoassay (EIA) (Siemens, Erlangen, Germany), carboxy-terminal collagen crosslinks I (CTX) by chemiluminescence (Immunodiagnostic Systems, Boldon Business Park, UK), procollagen type 1 N-terminal propeptide (P1NP) by chemiluminescence (Immunodiagnostic Systems), and total Ca (tCa) by indirect ion sensitive electrode (Beckman Coulter, Inc.). Laboratory-specific intraassay and interassay coefficients of variation (CVs) are 2.4% and 3.7% for PTH; 3.4% to 7.7% (for concentrations ranging from 0.201 ng/mL to 2.05ng/mL) and 5.8% to 8.6% (for concentrations ranging from 0.196 ng/mL to 2.08 ng/mL) for CTX; 2.0% to 4.5% (for concentrations ranging from 2.77 ng/mL to 175.84 ng/mL) and 1.7% to 5.6% (for concentrations ranging from 20.36 ng/mL to 175.21 ng/mL) for P1NP; and 0.8% and 1.0% for tCa. Additional blood samples (3 mL) were obtained at 5-min intervals during exercise for measurement of serum iCa and hematocrit (i-STAT; Abbot Point of Care, Princeton, NJ, USA). Manufacturer-determined CVs for iCa are 1.1% and 1.4% for the 1.60 mmol/L and 0.84 mmol/L calibration standards, respectively. PTH, CTX, iCa, and tCa values during and after exercise were adjusted for shifts in plasma volume as described.(9) Both unadjusted and adjusted data are presented; adjusted values are designated with a subscript (ie, PTHADJ, CTXADJ, iCaADJ, and tCaADJ).
Fig. 1.
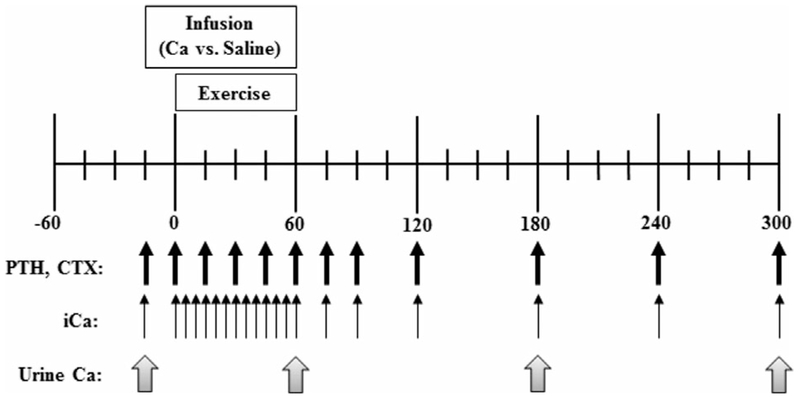
Experimental approach. Black arrows indicate blood sampling times for the measurement of serum iCa, PTH, and CTX. Gray arrows indicate urine collection times.
Statistical analysis
We hypothesized that increases in PTH and CTX during exercise would be attenuated when serum iCa concentration was maintained. The sample size required to evaluate the primary outcome, change in PTH from before to after exercise, was estimated from a previous study.(9) The previous study indicated that 10 evaluable participants would provide 91% power to detect a difference in the PTH response between the two experimental conditions of 74 ± 63 pg/mL based on a two-sided paired t test at an alpha level of 0.05.
The effects of Ca versus saline infusion on PTH and CTX responses were evaluated using linear contrasts in a repeated measures maximum likelihood model with all available data; one blood sample was missed at one time point for one participant. This approach is conceptually identical to repeated measures analysis of variance, but avoids the case-wise deletion of participants with missing assessments; estimates are unbiased under the assumption that missing data are missing at random. The data were not equally spaced over the 5 hours of collection. We accounted for that in the covariance structure; this was specified to allow heterogeneity by treatment condition (Ca versus saline). Linear contrasts were used to estimate within and between group differences over the 60-min exercise bout and the 4-hour recovery period. Secondary measures were evaluated in the same manner. Data are presented as mean ± SD, unless otherwise specified. All analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) and a p value of ≤0.05 defined statistical significance.
Results
The descriptive characteristics of the 11 participants are in Table 1. Exercise training status was reflected by a relative high cycling VO2peak. BMD was normal in all participants based on Z-score criteria for age and sex.
Table 1.
Descriptive Characteristics
| Characteristic | Value |
|---|---|
| Age (years) | 34.4 ± 5.1 |
| Weight (kg) | 74.1 ± 9.3 |
| Height (cm) | 176.4 ± 10.8 |
| Fat-free mass (kg) | 63.3 ± 7.4 |
| Fat mass (kg) | 10.8 ± 3.5 |
| VO2peak (mL/kg/min) | 53.0 ± 8.9 |
| Serum calcium (mg/dL) | 9.4 ± 0.3 |
| Serum 25-hydroxy vitamin D (ng/mL) | 33.7 ± 4.5 |
| Bone mineral density T-score | |
| Lumbar spine | −0.1 ± 0.9 |
| Proximal femur | −0.1 ± 0.6 |
| Femoral neck | −0.4 ± 0.5 |
| Trochanter | 0.0 0.7 |
Values are mean ± SD; n = 11.
iCa, tCa, PTH, CTX, and P1NP responses to exercise (immediately before to end of exercise)
iCa and iCaADJ
There was a condition-by-time interaction for iCa (p = 0.007) during exercise, and main effects of condition and time (both p < 0.001). The iCa clamp was successful in preventing a decline in serum iCa below the pre-exercise baseline level. The total amount of Ca infused before and during exercise was 156±20 mg, and the amount infused during exercise was 117±18 mg. Serum iCa concentration was significantly higher during Ca infusion than saline infusion, and was increased during Ca infusion relative to baseline at 15,30,45, and 60 min of exercise. In contrast, under the saline infusion, iCa was decreased relative to baseline at 45 and 60 min of exercise (Fig. 2A, B).
Fig. 2.
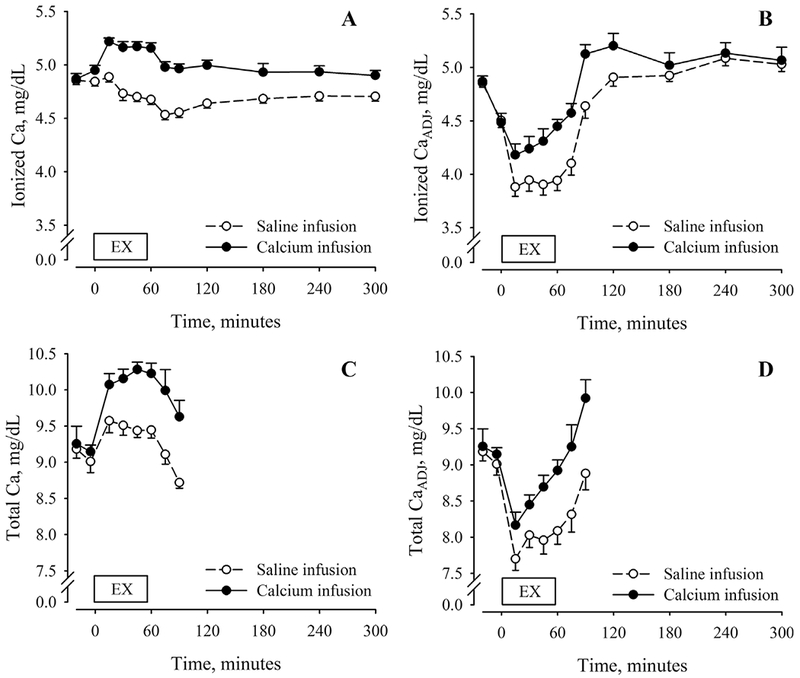
Serum ionized (A) and total calcium (C) concentrations before, during, and after 60 min of cycling exercise during conditions of saline (open symbols, dashed lines) and calcium infusion (closed symbols, solid lines). Adjustments for plasma volume shifts (iCaADJ, B; tCaADJ, D) reflect changes in vascular iCa and tCa content. Exercise and recovery data were analyzed separately. Statistical results for the condition-by-time interaction and main effects of condition and time for the exercise interval: (i) iCa: condition-by-time (p = 0.007), condition (p < 0.001), time (p < 0.001); (ii) iCaADJ: condition-by-time (p = 0.020), condition (p = 0.560), time (p = 0.060); (iii) tCa: condition-by-time (p = 0.010), condition (p = 0.001), time (p < 0.001); and (iv) tCaADJ: condition-by-time (p = 0.120), condition (p = 0.004), time (p < 0.001). Statistical results for the condition-by-time interaction and main effects of condition and time for the recovery interval: (i) iCa: condition-by-time (p = 0.100), condition (p < 0.001), time (p < 0.001); and (ii) iCaADJ: condition-by-time interaction (p = 0.040), condition (p = 0.010), time (p < 0.001). EX = exercise.
There was also a condition-by-time interaction for iCaADJ (p = 0.02), but not effects of condition (p = 0.56) or time (p = 0.06). The average serum iCaADJ concentration was higher during Ca infusion than saline infusion. Despite Ca infusion, iCaADJ was decreased relative to baseline at 15 and 30 min of exercise. During saline infusion, iCaADJ was less than the pre-exercise level at all time points.
tCa and tCaADJ
There was a condition-by-time interaction for tCa (p = 0.010) and main effects of condition (p = 0.001) and time (p < 0.001) over the exercise segment of the experiment. The average serum tCa concentration was higher during Ca infusion than saline infusion. For both the Ca infusion and saline infusion, tCa was increased relative to baseline at all time points during exercise (Fig. 2C, D).
There was a nonsignificant condition-by-time interaction for tCaADJ (p = 0.12) but significant main effects of condition (p = 0.004) and time (p < 0.001). The average serum tCaADJ concentration was higher during Ca infusion than saline infusion. Despite Ca infusion, tCaADJ was decreased relative to baseline at 15 and 30 min of exercise. During saline infusion, tCaADJ was less than the pre-exercise level at all time points.
PTH and PTHADJ
The condition-by-time interaction for PTH over the exercise segment was not significant (p = 0.130) but there were main effects of time (p < 0.001) and condition (p = 0.002), reflecting an increase in PTH during exercise in both conditions but a lower PTH response during Ca infusion than saline infusion. During the saline condition, PTH was nonsignificantly increased above the pre-exercise level after 15 min of exercise, and significantly increased after 30, 45, and 60 min. The Ca infusion attenuated, but did not prevent, the increase in PTH during exercise. When compared with the level immediately before exercise, PTH was increased at 15, 30, 45, and 60 min. The increase in PTH from immediately before to after exercise was 10.5 ± 3.2 pg/mL during Ca infusion compared with 30.1 ± 8.4 pg/mL during saline infusion. Results were similar after adjustment for PTHADJ (Fig. 3A, B).
Fig. 3.
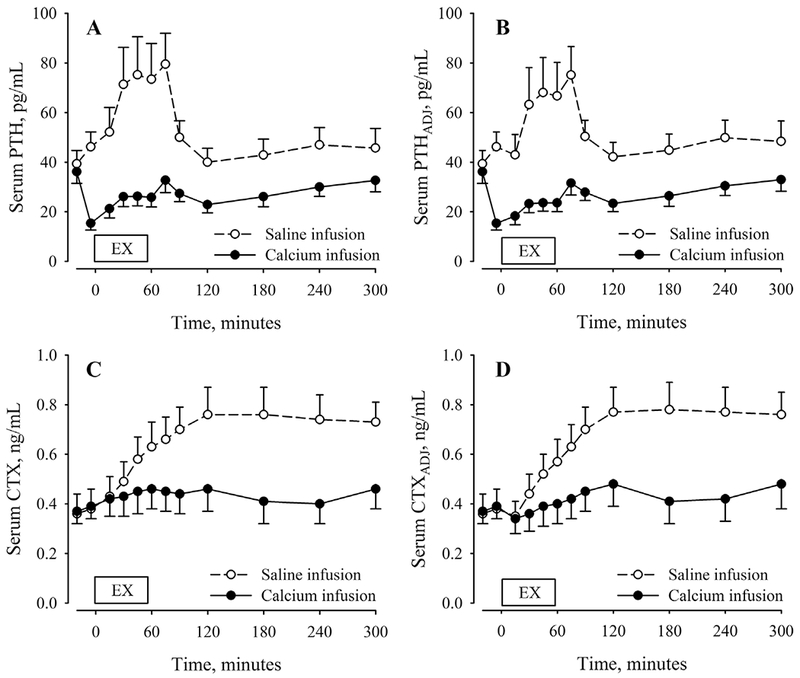
Serum PTH (A) and CTX (C) concentrations before, during, and after 60 min of cycling exercise during conditions of saline (open symbols, dashed lines) and calcium infusion (closed symbols, solid lines). Adjustments for plasma volume shifts (PTHADJ, B; CTXADJ, D) reflect changes in vascular PTH and CTX content. Exercise and recovery data were analyzed separately. Statistical results for the condition-by-time interaction and main effects of condition and time for the exercise interval: (i) PTH: condition-by-time (p = 0.130), condition (p = 0.002), time (p < 0.001); (ii) PTHADJ: condition-by-time (p = 0.070), condition (p = 0.004), time (p < 0.001); (iii) CTX: condition-by-time (p < 0.001), condition (p = 0.530), time (p < 0.001); and (iv) CTXADJ: condition-by-time (p < 0.001), condition (p = 0.480), time (p < 0.001). Statistical results for the condition-by-time interaction and main effects of condition and time for the recovery interval: (i) PTH: condition-by-time (p < 0.001), condition (p = 0.007), time (p < 0.001); (ii) PTHADJ: condition-by-time (p = 0.001), condition (p = 0.006), time (p < 0.001); (iii) CTX: condition-by-time (p < 0.001), condition (p = 0.040), time (p < 0.001); and (iv) CTXADJ: condition-by-time (p < 0.001), condition (p = 0.030), time (p < 0.001). PTH = parathyroid hormone; CTX = c-telopeptide cross-links of type 1 collagen.
CTX and CTXADJ
There was a condition-by-time interaction for CTX over the exercise segment of the experiment (p < 0.001) and a main effect of time (p < 0.001). During the saline condition, CTX was increased above the pre-exercise level at all time points during exercise. During the Ca infusion, CTX was increased above the pre-exercise level at 45 and 60 min of exercise. The increase in CTX from immediately before to after exercise was 0.07 ± 0.02 ng/mL during Ca infusion compared with 0.24 ± 0.03 ng/mL during saline infusion. Results were similar for CTXADJ (Fig. 3C, D).
P1NP and P1NPADJ
P1NP was measured only before, immediately after, and 4 hours after exercise. There was no condition-by-time interaction (p=0.57) or main effect of condition (p = 0.89). There was a main effect of time for P1NP (p < 0.001), but this was not significant after adjustment for plasma volume changes (p = 0.47). During the saline infusion, concentrations at these time points were 59.1 ± 23.1,71.1 ± 27.0, and 61.5 ± 21.1 ng/mL. During the Ca infusion, concentrations were 58.9 ± 17.2, 74.0 ± 28.6, and 64.4 ± 19.1 ng/mL. P1NPADJ concentrations at the three time points were 59.1 ± 23.1, 62.3 ± 24.0, and 65.8 ± 23.6 ng/mL for the saline condition and 58.9 ± 17.2, 65.8 ± 26.8, and 66.8 ± 21.4 ng/mL for the calcium condition.
iCa, PTH, and CTX responses during recovery (immediately after to 4 hours after exercise)
iCa and iCaADJ
The condition-by-time interaction for iCa during recovery was not significant (p = 0.100) but there were significant main effects of condition and time (both p < 0.001). The average iCa concentration during recovery was 0.31 ± 0.04mg/dL higher on the Ca day than the saline day. On the saline day, iCa remained below the pre-exercise concentration at 75, 90, 120, 180, 240, and 300 min. Results were similar for iCaADJ, with the exceptions that the condition-by-time interaction was significant and iCaADJ returned to the pre-exercise level by 1 hour after exercise on the saline day (Fig. 2A, B).
PTH and PTHADJ
There was a significant condition-by-time interaction for PTH during the 4 hours of recovery after exercise (p < 0.001) and main effects of condition and time (both p < 0.01). The average PTH concentration during recovery was higher on the saline infusion day than the Ca infusion day. The peak PTH concentration occurred 15 min after the end of exercise for both the saline (79.5 ± 41.2 pg/mL) and Ca (32.7 ± 16.4 pg/mL) conditions. By 30 min after exercise on the saline infusion day, PTH was no longer increased above the pre-exercise level. The average PTH concentration during recovery was 28.2 ± 12.4 pg/mL on the Ca day and 54.1 ± 26.5 pg/mL on the saline day. Results were similar for PTHADJ (Fig. 3A, B).
CTX and CTXADJ
There was a significant condition-by-time interaction for CTX during the 4 hours of recovery (p < 0.001). The average CTX concentration during recovery was higher on the saline day than the Ca day (p = 0.008). The peak CTX concentration occurred 60 min after the end of exercise for both the saline (0.76 ± 0.36 ng/mL) and Ca (0.46 ± 0.30 ng/mL) conditions. Despite the return of PTH to the pre-exercise level by 60 min after exercise on the saline day, CTX remained elevated above the pre-exercise level for 4 hours after exercise. The average CTX concentration during recovery was 0.44 ± 0.27 ng/mL on the Ca day and 0.71 ± 0.32 ng/mL on the saline day. Results were similar for CTXADJ (Fig. 3C, D).
Urinary Ca loss
There was a significant condition-by-time interaction for urinary Ca loss (p = 0.023). During saline infusion, urinary Ca loss during exercise was low and remained low during the 4 hours of recovery (Fig. 4). During Ca infusion, urinary Ca loss during exercise was approximately twofold higher than the saline day and remained higher during recovery. When compared with the saline day, the excess urinary Ca loss on the Ca day accounted for approximately 32% of the Ca infused.
Fig. 4.
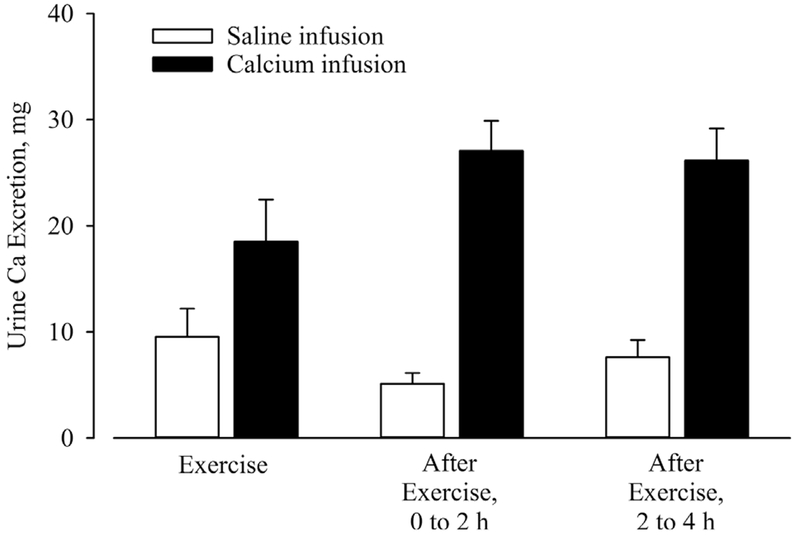
Urine Ca excretion during exercise and the first 2 hours and last 2 hours of recovery under conditions of saline (white bars) and calcium infusion (black bars). There was a condition-by-time interaction (p = 0.023) and a main effect of time (p < 0.001). Ca = calcium.
Discussion
Exercise can disrupt calcium homeostasis in a manner that stimulates PTH secretion and activates bone resorption in young and older, trained and untrained, women and men.(4,6–14) To isolate the decline in serum iCa as the trigger for these responses, we developed the iCa clamp technique, whereby intravenous Ca infusion during exercise prevents the decline in iCa. The major findings of the study were (i) Ca infusion markedly attenuated, but did not fully prevent, the increases in PTH and CTX in response to exercise when compared with volume-matched saline infusion; (ii) despite the return of PTH to the pre-exercise level by 60 min after exercise, serum CTX peaked 60 min after exercise and remained at that level for the remainder of the 4-hour recovery period; and (iii) after adjusting for shifts in plasma volume during exercise, serum iCaADJ decreased by ~0.7 mg/dL in the first 15 min of exercise on the saline infusion day; this was attenuated, but not prevented, by Ca infusion.
Ca and PTH responses to exercise: overview
Evidence is mixed as to whether exercise causes a decrease, no change, or increase in serum Ca and PTH.(16) Some of the discordance is related to differential reporting of results (ie, iCa versus tCa and adjustment or no adjustment for plasma volume changes). However, the discordance is also likely related to variability in the exercise stimulus, because the disruption of Ca homeostasis appears to be dependent on the duration and intensity of exercise. For example, in an early series of studies of young men by Ljunghall and colleagues,(17–20) brief high-intensity exercise caused an increase in serum iCa with no change in PTH, prolonged low-intensity exercise caused a decrease in serum iCa and increase in PTH, and less prolonged moderate-intensity exercise tended to increase PTH despite no decrease or an increase in iCa. A more recent series of studies of young men by Scott and colleagues(11) and Townsend and colleagues(21) confirmed that the PTH response to exercise is intensity dependent (ie, higher intensity, higher PTH response). The studies further suggested that the increase in PTH occurred with no decrease in serum tCa; iCa was not measured. Our series of studies of young men and older women(6–9) confirmed that the PTH response to exercise is intensity dependent. In contrast to some of the aforementioned studies, we found that iCa decreased in response to 60 min of vigorous cycling in young men(8,9) and 60 min of brisk walking in older women,(7) particularly after adjusting for the shift in plasma volume that occurs during exercise and causes hemoconcentration.
Collectively, these studies demonstrated that both prolonged and intensive exercise stimulates an increase in PTH secretion. When this occurred in the absence of a decrease in iCa or tCa it was likely provoked by metabolic acidosis, which is known to stimulate PTH secretion.(22) However, because even a small decrease in serum iCa triggers an increase in PTH within minutes,(23) it is possible that the PTH response to exercise is stimulated by small fluctuations in iCa during exercise, even in the absence of a change in iCa from before to after exercise. Thus, the dynamic regulation of iCa during exercise by PTH may not be apparent if blood samples are obtained only before and after exercise. Several studies have demonstrated that ingesting Ca before exercise attenuates the PTH response and, in some cases, the bone resorption response, highlighting the potential importance of disruption of Ca homeostasis during exercise as a mediator of bone metabolism.(7–9,13,14) In the current study, we isolated the contribution of a decrease in iCa during exercise to the stimulation of PTH secretion by preventing a decline in serum iCa (calcium infusion) and comparing responses to a control condition (volume-matched saline infusion).
iCa, tCa, PTH, and CTX responses to exercise during the control condition (saline infusion)
During the saline condition, serum iCa and tCa tended to increase during the first 15 min of exercise and then decrease slowly but steadily for the remainder of the exercise session (Fig. 2A, C). The early increase reflected hemoconcentration caused by the contraction of plasma volume, which occurs within minutes and is sustained during exercise.(18,24,25) Indeed, when adjusted for changes in plasma volume, iCaADJ and tCaADJ decreased by −0.7 and −1.3 mg/dL, respectively, in the first 15 min of exercise (Fig. 2B, D) and then plateaued. This observation corroborated previous findings that vascular Ca content decreases during exercise(24–26); this is discussed further in the next paragraph. However, because it is the serum iCa concentration, not content, that influences Ca-sensing receptor activity,(27) it is iCa and not iCaADJ that should drive the PTH response. In this context, the robust increase in PTH from 15 to 30 min of exercise was temporally synchronized with the decrease in iCa concentration during that time interval. The increase in CTX also steepened after only 15 min of exercise, indicating that bone resorption responded rapidly to the increase in PTH. It is not clear whether the rapid activation of bone resorption during exercise is achieved through increased osteoclast activity or osteocyte-mediated resorption. Support for the latter comes from the observation in mice that exercise provoked perilacunar (ie, space around an osteocyte) responses in bone, including a lower mineral-to-matrix ratio, when compared with sedentary mice.(28) Preclinical studies to address which bone cells respond to the exercise-induced increase in PTH would provide insight on how exercise influences bone quality and quantity.
We previously postulated that the decrease in iCa during exercise is provoked by dermal Ca loss (ie, sweating).(4,6) The current study was not designed to test this hypothesis, but the steep decrease in serum Ca content in the first 15 min of exercise, when the extent of dermal loss was likely small, suggests this is not the primary cause of the decrease in serum Ca during exercise. We are aware of one previous study(26) that also found a ~1 mg/dL decrease in serum iCa after 21 min of exercise. In that study serum tCa was unchanged during exercise, suggesting that the decrease in iCa was due to increased binding. However, it was not clear in that article whether the reported changes in serum iCa and tCa had both been adjusted for plasma volume contraction. In the current study, the parallel decreases in iCaADJ and tCaADJ early in exercise suggest that binding affinity was not markedly altered by exercise. Neither the reason for the rapid decline in serum Ca content early in exercise nor the fate of the Ca is clear. Urinary Ca loss during exercise (10 mg) could not account for the decrease in vascular Ca content. Because of the complex nature of Ca kinetics during exercise, including vascular flux, gut absorption, mobilization from bone, and urinary excretion, future studies should utilize stable isotopes to quantify some of these processes. Other factors involved in the regulation of calcium homeostasis (eg, phosphate, magnesium) should also be assessed.
iCa, tCa, PTH, and CTX responses to exercise during the experimental condition (Ca infusion)
Ca infusion was started 15 min before exercise with a goal of raising serum iCa ~0.2 mg/dL and then preventing a decrease below the baseline concentration. This was successfully achieved and required an average infusion of 117 mg Ca during exercise. Despite this magnitude of Ca delivery, vascular Ca content still decreased, as reflected by iCaADJ and tCaADJ. After the initial decreases in iCaADJ and tCaADJ of −0.3 and −1.0 mg/dL, respectively, in the first 15 min of exercise, both increased gradually and reached pre-exercise levels by the end of the exercise session.
Ca infusion to prevent the decline in serum iCa concentration during exercise markedly attenuated, but did not fully prevent, the increases in PTH and CTX (Fig. 3A, C). Starting Ca infusion 15 min before exercise caused the expected decrease in PTH. PTH then increased gradually from immediately before to the end of exercise, despite the maintenance of serum iCa above the pre-exercise concentration. This was likely related to changes in factors associated with vigorous exercise that are known to stimulate PTH secretion, such as an increase in epinephrine(29) or a decrease in pH.(30) However, Ca infusion attenuated the exercise-induced increase in serum PTH by −65% when compared with saline infusion, indicating that the PTH response to exercise is strongly influenced by the disruption of Ca homeostasis. The CTX response to exercise during Ca infusion paralleled the PTH response. Ca infusion diminished the increase in CTX during exercise by −71% when compared with saline infusion.
The approach of clamping iCa during exercise revealed the importance of the disruption of Ca homeostasis as a mediator of the PTH and CTX responses to exercise. Using this approach in future studies may be useful in advancing the understanding how factors like intensity, duration, and mode of exercise acutely alter bone metabolism, and in determining whether responses vary by age, sex, or fitness level. The results of this study may be specific to young, trained men during vigorous cycling exercise. However, we previously demonstrated that 60 min of brisk walking also stimulated increases in PTH and CTX in older, untrained women, suggesting these responses occur regardless of age, sex, fitness level, or mode of exercise.(7)
iCa, PTH, and CTX responses during recovery
The major findings during the 4-hour recovery period after exercise on the saline day were that (i) serum iCa remained below the pre-exercise concentration for 4 hours after exercise, (ii) PTH was no longer significantly elevated above the pre-exercise concentration by 30min after exercise, and (iii) CTX peaked 1 hour after exercise and remained at that level through the 4-hour recovery period. Because the half-life of CTX has been estimated to be approximately 1 hour,(31) the absence of a decline in CTX during recovery suggests the rate of bone resorption remained elevated for several hours after PTH had returned to the pre-exercise level. Urinary Ca loss during the 4 hours of recovery after exercise remained low (<4 mg/hour) on the saline day. This suggests that the sustained increase in mobilization of Ca from bone (ie, bone resorption) was necessary to restore Ca homeostasis. Indeed, iCa remained below the pre-exercise concentration for several hours after exercise and PTH remained slightly (nonsignificant) elevated. Further research will be necessary to better understand the temporal disruption in bone metabolism by exercise. Because participants remained fasted for the recovery period in the current study, it is possible this contributed to the persistent increase in bone resorption and that feeding, or specifically Ca ingestion, could attenuate the bone resorption response after exercise.
Anabolic versus catabolic actions of PTH
Bone formation (ie, P1NP) was assessed at only three time points during the experiment. The main finding was that P1NP increased in response to exercise, but this appeared to be due to plasma volume contraction because changes in P1NPADJ were not significant. PTH and CTX responses during exercise and recovery were markedly altered by Ca infusion, but P1NP was not, which suggests PTH had only catabolic effects on bone during exercise and the 4-hour recovery.
There is a paradoxical regulation of bone metabolism by PTH, such that a sustained elevation of PTH is catabolic but transient increases are anabolic.(32) Indeed, some of the drugs used to treat osteoporosis (ie, teriparatide, abaloparatide) are analogs of PTH or PTH-related protein that stimulate bone formation when given daily.(33,34) The main factor that determines whether the skeletal response to PTH is anabolic versus catabolic has been suggested to be the duration of time PTH is elevated rather than the magnitude of increase.(35) In rodents, the transition to catabolism occurs when PTH has been elevated for more than 4 or 5 hours.(35) In the current study, PTH was transiently elevated for only ~2 hours on the saline day, suggesting it should have generated an anabolic response, but this was not observed. It is possible that the anabolic response to an increase in PTH is delayed (ie, not apparent 4 hours after exercise) or may require more than a single “dose” of exercise. In postmenopausal women treated with a single dose of teriparatide, P1NP decreased during the 12 hours after injection, but was increased 2 days after injection.(36) In premenopausal women treated with teriparatide or placebo after incurring a stress fracture, 4 weeks of daily teriparatide treatment resulted in a 180% increase in P1NP compared with a 20% increase with placebo treatment; the acute response to the first dose was not measured.(37) Participants in the current study were regular exercisers and had presumably been exposed to previous “doses” of exercise that stimulated increases in PTH. Because this was not controlled, the potential impact on the pre-exercise P1NP level, as an indicator of a delayed increase in bone formation, could not be determined. However, the average pre-exercise P1NP level was in the normal range, which has been reported as 27.7 to 127.6 ng/mL, per the manufacturer (Immunodiagnostic Systems).
We are aware of only one study of rodents that evaluated how the exercise-induced increase in PTH influenced skeletal adaptations to exercise.(38) In that study, inhibition of PTH signaling attenuated the increase in trabecular bone volume in response to exercise, suggesting an anabolic role of PTH. However, the inhibition of PTH signaling during exercise attenuated changes in structure-level mechanical properties, but not tissue-level properties, suggesting PTH has a potentially complex role in mediating bone adaptations to exercise. Because the current study demonstrated that the exercise-induced increase in PTH stimulated a relatively prolonged increase in bone resorption, with no effect on formation during the same time interval, future studies should be expanded to determine whether there is a delayed activation of bone formation by PTH. If the exercise-induced increase in PTH does not have similar anabolic actions as pharmacologic PTH therapy, strategies to minimize the increase in PTH, such as pre-exercise Ca supplementation, may be effective in improving the effectiveness of exercise to increase BMD.
Conclusion
Preventing the decline in serum iCa during 60 min of vigorous cycling exercise via intravenous Ca infusion markedly attenuated, but did not prevent, the increase in PTH and the activation of bone resorption. During the saline infusion, vascular iCa and tCa decreased in the first 15 min of exercise, and PTH and CTX increased steadily during exercise. By 1 hour after exercise, PTH had returned to the pre-exercise level, but CTX peaked and remained at that level during recovery. These findings suggest that the rate of bone resorption is accelerated during vigorous exercise to mobilize Ca from bone and defend serum Ca concentration. There was no indication that the transient exercise-induced increase in PTH stimulated bone formation, but it is possible this could be a delayed response that would not be apparent 4 hours after exercise. Thus, there is clear evidence that the exercise-induced increase in PTH has an acute catabolic effect on bone, but further research is needed to determine whether PTH has a more chronic anabolic effect. Until such evidence is available, it is not clear whether strategies to minimize the increase in PTH during exercise, such as pre-exercise Ca supplementation, will have favorable or unfavorable effects on skeletal adaptations to exercise.
Acknowledgments
This research was supported by Department of Defense award W81XWH-12–1 −0364, NIH awards U01 TR001082 and P30 DK092718, and the Eastern Colorado VA Geriatric Research, Education, and Clinical Center. SJW was supported by T32 AG000279, VDS was supported by T32 DK007658, and CMS was supported by K23 AR070275. We thank the staff of the Clinical and Translational Research Center, the Energy Balance Assessment Lab, and Dr. Karen Shea for their assistance in conducting the study.
Authors’ roles: Study design: WMK and CMW. Study conduct and data collection: SJW, VDS, TW, and RSB. Data analysis and interpretation: WMK, PW, and SJW. Drafting manuscript: WMK, SJW, and PW. Revising manuscript content: WMK, SJW, PW, VDS, TW, CMS, CMW, and RSB. Approving final version of manuscript: WMK, SJW, PW, VDS, TW, CMS, CMW, and RSB. WMK takes responsibility for the integrity of the data analysis.
Footnotes
Disclosures
CMS was a consultant for Radius Health. WMK, SJW, PM, VDS,TW, CMW, and RSB have no conflicts of interest to disclose.
References
- 1.Nikander R, Sievanen H, Heinonen A, Kannus P. Femoral neck structure in adult female athletes subjected to different loading modalities. J Bone Miner Res. 2005;20(3):520–8. [DOI] [PubMed] [Google Scholar]
- 2.Stanforth D, Lu T, Stults-Kolehmainen MA, Crim BN, Stanforth PR. Bone mineral content and density among female NCAA Division I athletes across the competitive season and over a multi-year time frame. J Strength Cond Res. 2016;30(10):2828–38. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson M, Ohlsson C, Mellstrom D, Lorentzon M. Sport-specific association between exercise loading and the density, geometry, and microstructure of weight-bearing bone in young adult men. Osteoporos Int. 2013;24(5):1613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry DW, Kohrt WM. BMD decreases over the course of a year in competitive male cyclists. J Bone Miner Res. 2008;23(4):484–91. [DOI] [PubMed] [Google Scholar]
- 5.Klesges RC, Ward KD, Shelton ML, et al. Changes in bone mineral content in male athletes. Mechanisms of action and intervention effects. JAMA. 1996;276(3):226–30. [PubMed] [Google Scholar]
- 6.Barry DW, Kohrt WM. Acute effects of 2 hours of moderate-intensity cycling on serum parathyroid hormone and calcium. Calcif Tissue Int. 2007;80(6):359–65. [DOI] [PubMed] [Google Scholar]
- 7.Shea KL, Barry DW, Sherk VD, Hansen KC, Wolfe P, Kohrt WM Calcium supplementation and parathyroid hormone response to vigorous walking in postmenopausal women. Med Sci Sports Exerc. 2014;46(10):2007–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherk VD, Wherry SJ, Barry DW, Shea KL, Wolfe P, Kohrt WM. Calcium supplementation attenuates disruptions in calcium homeostasis during exercise. Med Sci Sports Exerc. 2017;49(7):1437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barry DW, Hansen KC, van Pelt RE, Witten M, Wolfe P, Kohrt WM. Acute calcium ingestion attenuates exercise-induced disruption of calcium homeostasis. Med Sci Sports Exerc. 2011;43(4):617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott JP, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD. The effect of training status on the metabolic response of bone to an acute bout of exhaustive treadmill running. J Clin Endocrinol Metab. 2010;95(8):3918–25. [DOI] [PubMed] [Google Scholar]
- 11.Scott JP, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD. The role of exercise intensity in the bone metabolic response to an acute bout of weight-bearing exercise. J Appl Physiol. 2011;110(2):423–32. [DOI] [PubMed] [Google Scholar]
- 12.Scott JP, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD. Effect of recovery duration between two bouts of running on bone metabolism. Med Sci Sports Exerc. 2013;45(3):429–38. [DOI] [PubMed] [Google Scholar]
- 13.Haakonssen EC, Ross ML, Knight EJ, et al. The effects of a calcium-rich pre-exercise meal on biomarkers of calcium homeostasis in competitive female cyclists: a randomised crossover trial. PLoS One. 2015;10(5): e 0123302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guillemant J, Accarie C, Peres G,Guillemant S Acute effects of an oral calcium load on markers of bone metabolism during endurance cycling exercise in male athletes. Calcif Tissue Int. 2004;74(5):407–14. [DOI] [PubMed] [Google Scholar]
- 15.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–7. [DOI] [PubMed] [Google Scholar]
- 16.Bouassida A, Latiri I, Bouassida S, et al. Parathyroid hormone and physical exercise: a brief review. J Sports Sci Med. 2006;5(3):367–74. [PMC free article] [PubMed] [Google Scholar]
- 17.Ljunghall S, Joborn H, Benson L, Fellstrom B, Wide L, Akerstrom G. Effects of physical exercise on serum calcium and parathyroid hormone. Eur J Clin Invest. 1984;14(6):469–73. [DOI] [PubMed] [Google Scholar]
- 18.Ljunghall S, Joborn H, Roxin LE, Rastad J, Wide L, Akerstrom G. Prolonged low-intensity exercise raises the serum parathyroid hormone levels. Clin Endocrinol (Oxf). 1986;25(5):535–42. [DOI] [PubMed] [Google Scholar]
- 19.Ljunghall S, Joborn H, Roxin LE, Skarfors ET, Wide LE, Lithell HO. Increase in serum parathyroid hormone levels after prolonged physical exercise. Med Sci Sports Exerc. 1988;20(2):122–5. [DOI] [PubMed] [Google Scholar]
- 20.Salvesen H, Johansson AG, Foxdal P, Wide L, Piehl-Aulin K, Ljunghall S. Intact serum parathyroid hormone levels increase during running exercise in well-trained men. Calcif Tissue Int. 1994;54(4):256–61. [DOI] [PubMed] [Google Scholar]
- 21.Townsend R, Elliott-Sale KJ, Pinto AJ, et al. Parathyroid hormone secretion is controlled by both ionized calcium and phosphate during exercise and recovery in men. J Clin Endocrinol Metab. 2016;101(8):3231–9. [DOI] [PubMed] [Google Scholar]
- 22.Lopez I, Aguilera-Tejero E, Estepa JC, Rodriguez M, Felsenfeld AJ. Role of acidosis-induced increases in calcium on PTH secretion in acute metabolic and respiratory acidosis in the dog. Am J Physiol Endocrinol Metab. 2004;286(5):E780–5. [DOI] [PubMed] [Google Scholar]
- 23.Schmitt CP, Schaefer F, Bruch A, et al. Control of pulsatile and tonic parathyroid hormone secretion by ionized calcium. J Clin Endocrinol Metab. 1996;81(12):4236–43. [DOI] [PubMed] [Google Scholar]
- 24.Ljunghall S, Joborn H, Lundin L, Rastad J, Wide L, Akerstrom G. Regional and systemic effects of short-term intense muscular work on plasma concentration and content of total and ionized calcium. Eur J Clin Invest. 1985;15(5):248–52. [DOI] [PubMed] [Google Scholar]
- 25.Convertino VA, Morey ER, Greenleaf JE. Reduction in plasma calcium during exercise in man. Nature. 1982;299(5884):658. [DOI] [PubMed] [Google Scholar]
- 26.Bouassida A, Zalleg D, Zaouali Ajina M, et al. Parathyroid hormone concentrations during and after two periods of high intensity exercise with and without an intervening recovery period. Eur J Appl Physiol. 2003;88(4–5):339–44. [DOI] [PubMed] [Google Scholar]
- 27.Cianferotti L, Gomes AR, Fabbri S, Tanini A, Brandi ML. The calciumsensing receptor in bone metabolism: from bench to bedside and back. Osteoporos Int. 2015;26(8):2055–71. [DOI] [PubMed] [Google Scholar]
- 28.Gardinier JD, Al-Omaishi S, Morris MD, Kohn DH. PTH signaling mediates perilacunar remodeling during exercise. Matrix Biol. 2016; 52–54: 162–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joborn H, Hjemdahl P, Larsson PT, et al. Effects of prolonged adrenaline infusion and of mental stress on plasma minerals and parathyroid hormone. Clin Physiol. 1990;10(1):37–53. [DOI] [PubMed] [Google Scholar]
- 30.Lopez I, Aguilera-Tejero E, Felsenfeld AJ, Estepa JC, Rodriguez M. Direct effect of acute metabolic and respiratory acidosis on parathyroid hormone secretion in the dog. J Bone Miner Res. 2002;17(9):1691–700. [DOI] [PubMed] [Google Scholar]
- 31.Bjarnason NH, Henriksen EE, Alexandersen P, Christgau S, Henriksen DB, Christiansen C. Mechanism of circadian variation in bone resorption. Bone. 2002;30(1):307–13. [DOI] [PubMed] [Google Scholar]
- 32.Silva BC, Costa AG, Cusano NE, Kousteni S, Bilezikian JP. Catabolic and anabolic actions of parathyroid hormone on the skeleton. J Endocrinol Invest. 2011;34(10):801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dobnig H, Sipos A, Jiang Y, et al. Early changes in biochemical markers of bone formation correlate with improvements in bone structure during teriparatide therapy. J Clin Endocrinol Metab. 2005;90(7):3970–7. [DOI] [PubMed] [Google Scholar]
- 34.Miller PD, Hattersley G, Riis BJ, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016;316(7):722–33. [DOI] [PubMed] [Google Scholar]
- 35.Frolik CA, Black EC, Cain RL, et al. Anabolic and catabolic bone effects of human parathyroid hormone (1–34) are predicted by duration of hormone exposure. Bone. 2003;33(3):372–9. [DOI] [PubMed] [Google Scholar]
- 36.Shiraki M, Sugimoto T, Nakamura T. Effects of a single injection of teriparatide on bone turnover markers in postmenopausal women. Osteoporos Int. 2013;24(1):219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Almirol EA, Chi LY, Khurana B, et al. Short-term effects of teriparatide versus placebo on bone biomarkers, structure, and fracture healing in women with lower-extremity stress fractures: a pilot study. J Clin Transl Endocrinol. 2016;5:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gardinier JD, Mohamed F, Kohn DH. PTH signaling during exercise contributes to bone adaptation. J Bone Miner Res. 2015;30(6): 1053–63. [DOI] [PMC free article] [PubMed] [Google Scholar]


