Abstract
Chronic pain is highly prevalent among older adults where it is associated with significant suffering, disability, social isolation, and greater costs and burden to health care systems. Pharmaceutical treatment of chronic pain in older adults is usually only partially effective and is often limited by side effects including urinary retention, constipation, sedation, cognitive impairment, and increased risk of falls. Since older adults are underrepresented in clinical trials testing treatments for chronic pain, the potential impacts of polypharmacy and frailty on reported outcomes and side effect profiles are largely unknown. Thus, for current treatments providers and patients must balance anticipated benefits of pain reduction with the known and unknown risks of treatment. Chronic pain is also a risk factor for premature death as well as accelerated cognitive decline, suggesting potential shared mechanisms between persistent pain (or its treatment) and dementia. Cognitive decline and dementia may also impact pain perception and the ability to report pain, complicating treatment decisions. Associations between persistent pain and the risks of premature death and accelerated cognitive decline make estimates for chronic pain in these populations particularly challenging. Future research is needed to improve estimates for chronic pain in older adults, to elucidate underlying mechanisms of pain with aging, and to develop and advance safer, more effective treatment options for chronic pain in older adults.
Keywords: chronic pain, aging, age-related disease, dementia, cognitive
1. Introduction
Chronic pain is among the most common, and consequential diseases in the United States and worldwide1–6. Chronic pain is defined as pain that persists past the normal time of healing (usually characterized as pain for at least 3 months), making it distinct from acute pain which is generally regarded as a local sensation7,8. In U.S. adults alone, estimated costs attributable to chronic pain—including disability, lost work, and treatments—exceed $600 billion annually8
Chronic pain is particularly common and problematic in older adults (≥age65) where it is associated with significant suffering, social isolation, disability, and greater costs and burden to health care systems1,2,9–12. Pharmaceutical treatment of chronic pain in older adults is usually only partially effective and is often limited by side effects. Older adults are underrepresented in clinical trials testing treatments for chronic pain13. As a consequence, the effectiveness, side effects, and potential impacts of polypharmacy and frailty on reported outcomes of many pain medications are largely unknown14. Thus, providers and patients must balance anticipated benefits of pain reduction with the known and unknown risks of treatment.
Chronic pain is also a risk factor for premature death15 as well as accelerated cognitive decline16 suggesting potential shared mechanisms between pain (or its treatment) and dementia. Cognitive decline and dementia may also impact pain perception and the ability to report pain, further complicating treatment decisions17. Associations between persistent pain and the risks of premature death and accelerated cognitive decline make estimates for chronic pain in these populations particularly challenging. Future research is needed to improve estimates and better appreciate the scope and impact of chronic pain in older adults, to elucidate underlying mechanisms of pain with aging and potential shared mechanisms with dementia, and to develop and advance safer, more effective treatment options for chronic pain in older adults.
In this topical review of chronic pain in older adults we summarize evidence from epidemiological studies reporting the prevalence and impact of chronic non-cancer pain, and describe the challenges and limitations of assessing and treating chronic pain in older adults. Specifically, we make the case that, chronic pain may be substantially underestimated in older adults and that the association between chronic pain and higher mortality may partially be responsible for this underestimation. We dedicate a significant portion of the manuscript to the relationship between chronic pain and Alzheimer’s Disease, two common and consequential diseases in older adults, and suggest that these diseases may be share common underlying mechanisms. We discuss limitations and risk of available pharmacological treatments for chronic pain in older adults and discuss, briefly, the evidence for nonpharmacological treatment strategies in this population. Finally, we make the case that future research is needed to improve estimates for chronic pain in older adults, to elucidate underlying mechanisms of pain with aging and dementia, and to develop and advance safer, more effective treatment options.
2. Epidemiology of Chronic Pain in Older Adults
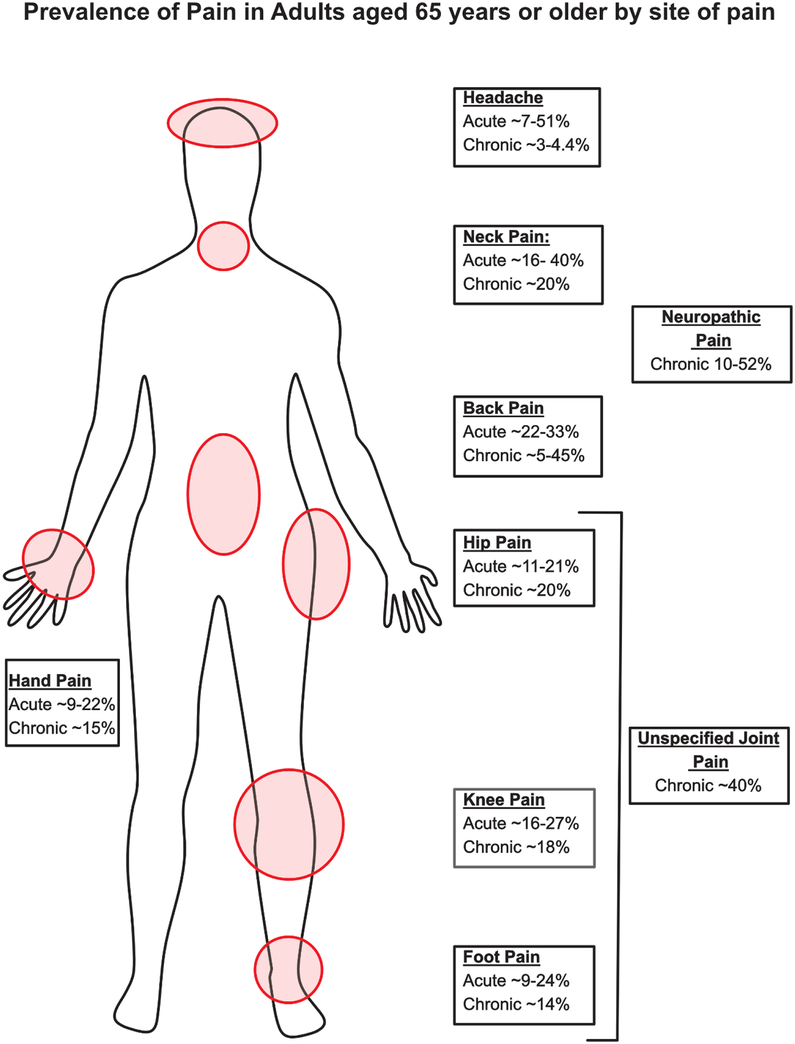
Epidemiological studies report that increased age is a risk factor for chronic pain2 and painful conditions such as chronic low back pain18, chronic neck pain19,20 as well as chronic pain in the hip and knee21,22. Population studies in both the US and globally reveal a higher prevalence of chronic pain in adults over the age of 65 as compared to the general adult population1,2,4; The most frequent chronic pain conditions in older adults are chronic unspecified joint pain23, chronic back pain18 and chronic neck pain24 affecting an estimated 40, 5–45, and 20%, respectively (figure 1). Notably, the literature is not clear as to the role of neuropathic pain in older adults, as epidemiological studies indicate that the prevalence of neuropathic pain in adults older than 65 ranges from 10–52%25–29. Some caution should be exercised when interpreting chronic pain prevalence, as results vary widely, likely due to differences in how chronic pain was assessed (i.e. self-report vs. diagnosis), definitions of chronic pain (i.e. duration, frequency, intensity), and other factors. Despite these limitations, studies reviewed here point to chronic pain being among the most common and consequential diseases in older adults6.
Figure 1: Prevalence of chronic pain in older adults by site.
Older adults suffer from a number of acute and chronic pain conditions including neuropathic pain25,26, unspecified joint pain23,33, back pain9,18,23,113,114, neck pain9,23,24,33, knee pain9,33,34,115, hip pain9,33,34,115, foot pain9,33,115,116, hand pain33,115 and headache23,117,118.
3. Chronic pain as a risk factor for early death
The previous section summarized the magnitude of the impact of chronic pain in older adults, indicating that the prevalence of people reporting chronic pain conditions increases almost linearly with increasing age2. Paradoxically, however, in cross-sectional studies chronic pain prevalence is often reported to peak at age 60 and possibly to decrease thereafter30–35. It was recently suggested, based on data from a large, longitudinal cohort study, that this apparent plateau of chronic pain prevalence at age 60 may be due to a markedly higher risk of death in chronic pain patients36. The concept that chronic pain is a risk factor for early death was corroborated in a large British longitudinal cohort and accompanying meta-analysis which found that chronic widespread pain was associated with a 57% increased risk of excess all-cause morality15. Obesity, lack of physical activity, smoking and diet not containing alcohol were identified as mediators accounting for some (but not all) of the increased risk of early death observed in chronic pain sufferers. It is possible that other factors associated with chronic pain such as increased prescription medication use37–39, illicit substance use40, dementia16, falls10,41,42 and/or shared biochemical mechanisms linking pain and death (i.e. inflammation or disease)42 could contribute to the early mortality observed in chronic pain sufferers. It should be noted that though the most recent meta-analysis found a link between pain and excess mortality, studies are not unanimous. In summary, the relationship between aging and pain is not fully understood and requires further study. However, available data suggest that chronic pain disproportionately affects older adults, and that the pain itself and possibly pain treatments are associated with increased risk of death.
4. Impact of chronic pain on quality of life and function in older adults
Some of this pain and its impact on daily life will reflect the specific pain location. For example, knee pain can often be eased by treatments directed at that specific pathology (e.g. joint replacement)43. However, chronic pain is also a condition-in-its-own-right, with features common across multiple locations and impacts on physical, psychological and social functioning. These general features may often require mixed approaches that address multiple impacts of living with pain. In patients of all ages, chronic pain is well-known to have adverse impacts on quality of life44–46, physical and mental function16, mood2,11, ability to work11, and participation in leisure activities11. Age has been reported as a risk factor for high impact chronic pain (HICP - chronic pain accompanied by activity/life participation limitations)1,11. HICP is associated with increased risk of depression11 a significant morbidity in older adults that is often comorbid with chronic pain. Moreover, older adults report greater levels of pain intensity as compared to younger adults (though, similar to pain prevalence data, not when compared with middle aged adults)47. Higher pain intensities and impacts, in older adult populations is of great societal importance as both pain intensity and high impact chronic pain have been linked to greater healthcare costs and resource utilization11,12. Additionally, less physical activity, as is observed in HICP sufferers, is believed to be one of the lifestyle factors that modulates the increased mortality that is seen in people suffering from chronic pain in genreal15 which makes HICP particularly troubling.
5. Differences in Pain Perception
Aging is associated with declines in sensations such as hearing and vision48,49. As pain likely includes a peripheral sensory component it is possible that it too declines, or is at least altered, with aging50. Could differences in pain perception or tolerance explain the increased complaints of chronic pain in older adults? One of the first investigations into pain perception with aging was conducted in 1944 where the authors demonstrated that pain threshold increases with age51. Since then a large number of cross-sectional studies have reported pain thresholds in young and old participants (a recent meta-analysis included 31 studies in healthy adults52). While results of studies assessing pain thresholds over the lifespan have been inconsistent52,53, many suggest an increased pain threshold associated with aging. However, studies assessing this are likely dependent on pain modality tested, site of pain, and testing paradigm54,55.
Increased pain threshold with aging is consistent with declines in other peripheral sensory systems with aging, however, it does not explain the increased prevalence, or overall increased pain complaints among older adults55. Several plausible explanations could account for these apparently contrasting observations. One likely explanation, is an overall increased tissue injury as evidenced by increased levels of degenerative arthritis in older adults1. Moreover, it is has been postulated that deficits in sensing pain that accompany aging, evidenced by increased pain thresholds in older adults, could lead to higher risk of injury in older adults50,52 although evidence for this hypothesis remains anecdotal.
However, chronic pain is more than the sensation of painful stimuli. While acute pain is a sensory experience that alerts the body of possible tissue damage, chronic pain, by definition, is quite different and may therefore be independent of pain thresholds. Indeed, pain threshold does not appear to predict susceptibility for developing chronic pain56,57 or chronic pain condition severity58, although mixed evidence exists for its ability to predict chronic pain severity58–60. Pain threshold tests are generally considered to be measures of peripheral sensory input, yet the subjective experience of pain is impacted by central nervous system (CNS) mechanisms such as descending inhibition and emotion and motivational circuits61. Therefore, it is possible that aging differentially affects CNS and PNS pain circuitry50. Indeed, neuroimaging studies have shown aging-associated decreases in activation of somatosensory cortex (S1), medial insular cortex and basal ganglia, however, the impact of these deficits on chronic pain are unknown62,63. The emotional and motivational component of pain perception is believed to be represented by pain tolerance measures50, which have been reported to decrease with increasing age50,52. Additionally, CNS mechanisms of pain can be measured by using different testing paradigms such as temporal summation of pain (increased pain response to repeated pain stimuli) and conditioned pain modulation. Similar to pain tolerance data, results from studies assessing the effect of aging on summation and conditioned pain modulation are mixed and may be dependent on testing factors (i.e. type of stimuli, intensity, location etc.)55,64–72. Therefore, the evidence of both PNS and CNS pain processing systems being altered with age indicates pain may be experienced differentially in older adults.
6. Dementia and Chronic Pain
Alzheimer’s disease (AD) and related dementias are significant sources of morbidity and mortality in older adults. A detailed review of dementia and chronic pain in older adults was recently published73; here we will briefly discuss the relationship of chronic pain in Alzheimer’s disease and dementia. Chronic pain has been linked to increased risk of cognitive decline and dementia16, suggesting that shared environmental exposures, genetics, or molecular mechanisms could play a role in both conditions. Notably, degenerative changes in brainstem regions that modulate descending pain inhibition—including the periaqueductal gray matter—have been reported in Alzheimer’s disease74. However, the potential role of neurodegeneration in chronic pain is largely unexplored.
Importantly, chronic pain has been associated with accelerated functional limitations16 and, in dementia patients, has been reported to be associated with neuropsychiatric symptoms such as aggression, anxiety and depression as well as worse overall quality of life44,45. Changes in pain have also been correlated, longitudinally, with neuropsychiatric symptoms and quality of life, highlighting the importance of treating pain in this population45.
Findings from epidemiological studies estimating analgesic usage and prevalence of pain in patients with AD and dementia are inconsistent. For example, it is often reported that pain is undertreated in AD17,75,76 which is in line with the results of a recent meta-analysis in patients in aged care facilities77. By contrast, several recent reports indicate that AD sufferers use more analgesics than older adults not suffering from AD78,79. Highlighting the complexity of this field, two reports from the MEDALZ cohort published in 2017 found that AD patients were more likely than non-AD patients to use strong opioid medications79 and less likely to be long-term opioid users80, thus, indicating differences in analgesic usage between these two groups may be dependent on analgesic type, strength, and duration of use. Epidemiological evidence of differences in pain prevalence among AD and dementia patients vs. age-matched controls is mixed and inconsistent73,81. For example, in a large, nationally representative sample of US, community dwelling adults, dementia was reported to be significantly associated with increased likelihood of pain9. On the contrary, a recent meta-analysis compiling studies from aged care facilities found patients with dementia were less likely to report or be observed to have pain77. Importantly, a recent review by Gagliese et al; assessed reported prevalence of pain in AD and dementia patients and concluded that these rates were at least comparable to rates of older adults without dementia73.
Patients with dementia may have impaired ability to report pain82 and there are a variety of new tools that rely on non-verbal pain responses (e.g. facial expressions, vocalizations, body movements) to assess pain in these patients that may not be able to communicate82. Neuroimaging studies have reported that AD patients have differences in neuronal connectivity between pain relevant brain regions as well as lower pain sensitivities as compared to age- matched controls17,83,84. Study authors proposed that these differences may have been due to AD patients being less able to accurately assess the experimental pain situation17,83. Importantly, these studies measured pain thresholds by self-report in response to a stimulus. A recent systematic review and meta-analysis found that pain thresholds (self-report) were not different between AD sufferers and healthy controls85. However, in AD patients, pain sensitivity increased when facial assessments of pain response was used as a measure85. Altogether, these data indicate that AD and dementia patients have an altered experience of pain that may not be captured by standard pain self-report measures.
The previous sections summarized the prevalence and impact of chronic pain in older adults. The final section of this review addresses some challenges associated with treating chronic pain in older adults.
7. Polypharmacy and other treatment complications
Polypharmacy is the phenomenon of a patient taking more than one medication simultaneously (most often defined as consumption 5 or more medications daily86). Polypharmacy is associated with many undesirable outcomes including: increased health costs, adverse drug events, drug interactions, functional and cognitive declines and falls87. Polypharmacy is prevalent in older adults regardless of their care setting (ambulatory, hospital or nursing home)87. For example, in the US general population adults greater than 65 years old take a median of 4 prescription meds and approximately 41% consume 5 or more medications per day. Concerningly, the prevalence rates of polypharmacy among older Americans increased approximately 3-fold since 198888,89.
Chronic pain is an independent risk factor for daily analgesic consumption in older adults90 and pain and analgesic use have previously been reported to be risk factors for polypharmacy91–94. An estimated 16% of older adults are taking prescription analgesics89, and approximately 70% of older adults consume non-prescription analgesics at least every other day95. Among arthritis sufferers, 40% of adults older than 65 experience polypharmacy compared to 29% of adults aged 50–6446. Among pain sufferers polypharmacy has been reported to be associated with decreased quality of life46.
The widespread polypharmacy among older adults is concerning as pharmacokinetics of many drugs is altered with age, and many trials testing pharmaceuticals exclude older adults limiting assessments of efficacy and side effects in this population13,14,96. An additional concern with older adults consuming pharmaceuticals is the potential impact of frailty (reviewed here14). Among older adults, analgesic use is most common in frail individuals, however, how frailty impacts analgesic drug toxicity is not fully understood14,97. Analgesics are involved in a significant proportion of drug related adverse events in older adults98–100. Additionally a significant proportion of older chronic pain sufferers report they need stronger pain medication101. In particular, anti-epileptic and anti-depressant drugs are commonly prescribed to treat chronic neuropathic pain, however, side effects associated with anticonvulsants—including cognitive impairment, sedation and increased falls—make prescribing these drugs to older adults risky and, in some instances, contraindicated102–104. While the benefits of properly treating chronic pain are well-established in older adults16, medical pain management must be balanced with the risks of side effects such as bleeding105,106, urinary and gastrointestinal side effects105,106, increased falls105,107 and cognitive symptoms105 associated with common pain medications.
In summary a complex balance between adequately treating pain while avoiding pitfalls associated with medication overuse in older adults needs to be achieved to enhance quality of life and function. Older adults are often excluded from clinical trials of analgesics making assessments of analgesics efficacy limited in this population13. In light of the complications with pharmacological interventions to treating pain and the limited ability to assess efficacy of pharmacological analgesics in older adults, non-pharmacological modalities may be especially suitable to treat pain in this patient population. In fact, many official bodies have recently highlighted the utility of multimodal/multidisciplinary approaches, that include non-pharmacological therapies, for the treatment of chronic pain108–110. Specific to older adults, in 2012 the American College of Rheumatology recommended a number of non-pharmacological approaches for management of osteoarthritis, including exercise, acupuncture and Tai Chi111. More recently, a randomized controlled trial found that a mindfulness based stress-reduction intervention significantly improved low back pain related function in older adults112. It should be noted that, though non-pharmacological approaches to treat chronic pain are recommended and low-risk relative to pharmacological interventions, more evidence from larger and more strictly controlled clinical trials are needed to determine efficacy of non-pharmacological treatments to chronic pain. Therefore, safer, more effective treatment options for chronic pain in older adults are needed. Until then, based on the limited evidence available, the best approach to treat pain in older adults is likely a multi-modal approach encompassing pharmacological and non-pharmacological approaches as well as lifestyle modifications, where the provider and patient attempt to balance the adequate pain control while limiting adverse side-effects.
8. Conclusions
In summary, chronic pain is highly prevalent among older adults where it is associated with significant suffering, disability, social isolation, and a burden to health care systems. Pharmaceutical options for treating chronic pain in older adults tend to be only partially effective and associated with important side effects. Future research is needed to improve estimates for chronic pain in older adults, to elucidate underlying mechanisms of pain with aging and dementia, and to develop and advance safer, more effective treatment options.
Highlights.
Chronic pain is prevalent and disabling in older adults
The experience of chronic pain may change with increased age
Chronic pain has been linked to increased mortality and dementia
Efficacy and safety of pharmacological treatments for chronic pain in older adults has not been fully evaluated
9. Acknowledgments
This work was supported by the intramural research program of the National Institute on Aging and National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health.
Abbreviations:
- HICP
High Impact Chronic Pain
- CNS
Central Nervous System
- S1
Primary Somatosensory Cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
10. References
- 1.Dahlhamer J et al. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults - United States, 2016. MMWR Morb. Mortal. Wkly. Rep 67, 1001–1006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsang A et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J. Pain 9, 883–891 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Elzahaf RA, Tashani OA, Unsworth BA & Johnson MI The prevalence of chronic pain with an analysis of countries with a Human Development Index less than 0.9: a systematic review without meta-analysis. Curr. Med. Res. Opin 28, 1221–1229 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Jackson T et al. A Systematic Review and Meta-Analysis of the Global Burden of Chronic Pain Without Clear Etiology in Low- and Middle-Income Countries: Trends in Heterogeneous Data and a Proposal for New Assessment Methods. Anesth. Analg 123, 739–748 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Jackson T et al. Prevalence of chronic pain in low-income and middle-income countries: a systematic review and meta-analysis. Lancet 385 Suppl 2, S10 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Vos T, Allen C & Arora M Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1545–1602 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ASP Task Force on Taxonomy in Classification of Chronic Pain, Second Edition (eds. Merskey H & Bogduk N) 209–214 (IASP Press, 1994). [Google Scholar]
- 8.Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in america: A blueprint for transforming prevention, care, education, and research (National Academies Press; (US: ), 2011). doi: 10.17226/13172 [DOI] [PubMed] [Google Scholar]
- 9.Patel KV, Guralnik JM, Dansie EJ & Turk DC Prevalence and impact of pain among older adults in the United States: findings from the 2011 National Health and Aging Trends Study. Pain 154, 2649–2657 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leveille SG et al. Chronic musculoskeletal pain and the occurrence of falls in an older population. JAMA 302, 2214–2221 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitcher MH, Von Korff M, Bushnell MC & Porter L Prevalence and Profile of High-Impact Chronic Pain in the United States. J. Pain (2018). doi: 10.1016/j.jpain.2018.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernfort L, Gerdle B, Rahmqvist M, Husberg M & Levin L-Å Severity of chronic pain in an elderly population in Sweden--impact on costs and quality of life. Pain 156, 521–527 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Paeck T et al. Are older adults missing from low back pain clinical trials? A systematic review and meta-analysis. Arthritis Care Res. (Hoboken) 66, 1220–1226 (2014). [DOI] [PubMed] [Google Scholar]
- 14.McLachlan AJ et al. Clinical pharmacology of analgesic medicines in older people: impact of frailty and cognitive impairment. Br. J. Clin. Pharmacol 71, 351–364 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macfarlane GJ, Barnish MS & Jones GT Persons with chronic widespread pain experience excess mortality: longitudinal results from UK Biobank and meta-analysis. Ann. Rheum. Dis 76, 1815–1822 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Whitlock EL et al. Association between persistent pain and memory decline and dementia in a longitudinal cohort of elders. JAMA Intern. Med 177, 1146–1153 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole LJ et al. Pain sensitivity and fMRI pain-related brain activity in Alzheimer’s disease. Brain 129, 2957–2965 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Husky MM, Ferdous Farin F, Compagnone P, Fermanian C & Kovess-Masfety V Chronic back pain and its association with quality of life in a large French population survey. Health Qual Life Outcomes 16, 195 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Côté P et al. The burden and determinants of neck pain in workers: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. J. Manipulative Physiol. Ther 32, S70–86 (2009). [DOI] [PubMed] [Google Scholar]
- 20.McLean SM, May S, Klaber-Moffett J, Sharp DM & Gardiner E Risk factors for the onset of non-specific neck pain: a systematic review. J. Epidemiol. Community Health 64, 565–572 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Miranda H, Viikari-Juntura E, Martikainen R & Riihimäki H A prospective study on knee pain and its risk factors. Osteoarthr. Cartil 10, 623–630 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Silverwood V et al. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthr. Cartil 23, 507–515 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Pleis JR, Ward BW & Lucas JW Summary health statistics for U.S. adults: National Health Interview Survey, 2009. Vital Health Stat 101–207 (2010). [PubMed] [Google Scholar]
- 24.Guez M, Hildingsson C, Nilsson M & Toolanen G The prevalence of neck pain. Acta Orthop Scand 73, 455–459 (2002). [DOI] [PubMed] [Google Scholar]
- 25.VanDenKerkhof EG et al. An epidemiological study of neuropathic pain symptoms in canadian adults. Pain Res. Manag 2016, 9815750 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rapo-Pylkkö S, Haanpää M & Liira H A one-year follow-up study of chronic pain in community-dwelling older adults with and without neuropathic pain. BMC Geriatr 17, 152 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouhassira D, Lantéri-Minet M, Attal N, Laurent B & Touboul C Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 136, 380–387 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Rapo-Pylkkö S, Haanpää M & Liira H Neuropathic Pain Among Community-Dwelling Older People: A Clinical Study in Finland. Drugs Aging 32, 737–742 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Rapo-Pylkkö S, Haanpää M & Liira H Chronic pain among community-dwelling elderly: a population-based clinical study. Scand. J. Prim. Health Care 34, 159–164 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy J, Roll JM, Schraudner T, Murphy S & McPherson S Prevalence of persistent pain in the U.S. adult population: new data from the 2010 national health interview survey. J. Pain 15, 979–984 (2014). [DOI] [PubMed] [Google Scholar]
- 31.QuickStats: Percentage of Adults Aged ≥18 Years Who Often Had Pain in the Past 3 Months,* by Sex and Age Group — National Health Interview Survey, United States, 2010–2011† at <https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6217a10.htm>
- 32.Wettstein M, Eich W, Bieber C & Tesarz J Pain Intensity, Disability, and Quality of Life in Patients with Chronic Low Back Pain: Does Age Matter? Pain Med 20, 464–475 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Picavet HSJ & Schouten JSAG Musculoskeletal pain in the Netherlands: prevalences, consequences and risk groups, the DMC(3)-study. Pain 102, 167–178 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Dawson J et al. Epidemiology of hip and knee pain and its impact on overall health status in older adults. Rheumatology 43, 497–504 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Helme RD & Gibson SJ The epidemiology of pain in elderly people. Clin Geriatr Med 17, 417–31, v (2001). [DOI] [PubMed] [Google Scholar]
- 36.Grol-Prokopczyk H Sociodemographic disparities in chronic pain, based on 12-year longitudinal data. Pain 158, 313–322 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shmagel A, Ngo L, Ensrud K & Foley R Prescription Medication Use Among Community-Based U.S. Adults With Chronic Low Back Pain: A Cross-Sectional Population Based Study. J. Pain 19, 1104–1112 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gwira Baumblatt JA et al. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern. Med 174, 796–801 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Krause JS, Cao Y & Clark JMR Pain intensity, interference, and medication use after spinal cord injury: association with risk of mortality after controlling for socioeconomic and other health factors. Arch. Phys. Med. Rehabil 98, 2464–2470 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Shmagel A, Krebs E, Ensrud K & Foley R Illicit substance use in US adults with chronic low back pain. Spine 41, 1372–1377 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munch T et al. Pain and falls and fractures in community-dwelling older men. Age Ageing 44, 973–979 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tosato M et al. Association of pain with behavioral and psychiatric symptoms among nursing home residents with cognitive impairment: results from the SHELTER study. Pain 153, 305–310 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Bannuru RR et al. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum 61, 1704–1711 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Sampson EL et al. Pain, agitation, and behavioural problems in people with dementia admitted to general hospital wards: a longitudinal cohort study. Pain 156, 675–683 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajkumar AP et al. Epidemiology of pain in people with dementia living in care homes: longitudinal course, prevalence, and treatment implications. J. Am. Med. Dir. Assoc 18, 453.e1–453.e6 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Meraya AM, Dwibedi N & Sambamoorthi U Polypharmacy and Health-Related Quality of Life Among US Adults With Arthritis, Medical Expenditure Panel Survey, 2010–2012. Prev Chronic Dis 13, E132 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nahin RL Estimates of pain prevalence and severity in adults: United States, 2012. J. Pain 16, 769–780 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gadkaree SK et al. Does Sensory Function Decline Independently or Concomitantly with Age? Data from the Baltimore Longitudinal Study of Aging. J. Aging Res 2016, 1865038 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nusbaum NJ Aging and sensory senescence. South Med. J 92, 267–275 (1999). [DOI] [PubMed] [Google Scholar]
- 50.Lautenbacher S Experimental approaches in the study of pain in the elderly. Pain Med 13 Suppl 2, S44–50 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Chapman WP & Jones CM Variations in cutaneous and visceral pain sensitivity in normal subjects. J. Clin. Invest 23, 81–91 (1944). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lautenbacher S, Peters JH, Heesen M, Scheel J & Kunz M Age changes in pain perception: A systematic-review and meta-analysis of age effects on pain and tolerance thresholds. Neurosci. Biobehav. Rev 75, 104–113 (2017). [DOI] [PubMed] [Google Scholar]
- 53.El Tumi H, Johnson MI, Dantas PBF, Maynard MJ & Tashani OA Age-related changes in pain sensitivity in healthy humans: A systematic review with meta-analysis. Eur. J. Pain 21, 955–964 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Edwards RR & Fillingim RB Age-associated differences in responses to noxious stimuli. J. Gerontol. A, Biol. Sci. Med. Sci 56, M180–5 (2001). [DOI] [PubMed] [Google Scholar]
- 55.Edwards RR, Fillingim RB & Ness TJ Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain 101, 155–165 (2003). [DOI] [PubMed] [Google Scholar]
- 56.Gupta A et al. Pressure pain thresholds and tender point counts as predictors of new chronic widespread pain in somatising subjects. Ann. Rheum. Dis 66, 517–521 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slade GD et al. Pressure pain thresholds fluctuate with, but do not usefully predict, the clinical course of painful temporomandibular disorder. Pain 155, 2134–2143 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neogi T et al. Sensitivity and sensitisation in relation to pain severity in knee osteoarthritis: trait or state? Ann. Rheum. Dis 74, 682–688 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arendt-Nielsen L et al. Sensitization in patients with painful knee osteoarthritis. Pain 149, 573–581 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Lauche R, Cramer H, Langhorst J, Dobos G & Gerdle B Neck pain intensity does not predict pressure pain hyperalgesia: re-analysis of seven randomized controlled trials. J Rehabil Med 46, 553–560 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Yarnitsky D Role of endogenous pain modulation in chronic pain mechanisms and treatment. Pain 156 Suppl 1, S24–31 (2015). [DOI] [PubMed] [Google Scholar]
- 62.Tseng M-T et al. Effect of aging on the cerebral processing of thermal pain in the human brain. Pain 154, 2120–2129 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Cole LJ, Farrell MJ, Gibson SJ & Egan GF Age-related differences in pain sensitivity and regional brain activity evoked by noxious pressure. Neurobiol. Aging 31, 494–503 (2010). [DOI] [PubMed] [Google Scholar]
- 64.Marouf R, Piché M & Rainville P Is temporal summation of pain and spinal nociception altered during normal aging? Pain 156, 1945–1953 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Riley JL, Cruz-Almeida Y, Staud R & Fillingim RB The effects of manipulating the inter-stimulus-interval on heat-evoked temporal summation of second pain across the age span. Pain (2018). doi: 10.1097/j.pain.0000000000001382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petersen KK et al. Age interactions on pain sensitization in patients with severe knee osteoarthritis and controls. Clin. J. Pain 33, 1081–1087 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Lautenbacher S, Kunz M, Strate P, Nielsen J & Arendt-Nielsen L Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain 115, 410–418 (2005). [DOI] [PubMed] [Google Scholar]
- 68.Khan J et al. Age and gender differences in mechanically induced intraoral temporal summation and conditioned pain modulation in healthy subjects. Oral Surg. Oral Med. Oral Pathol. Oral Radiol 126, 134–141 (2018). [DOI] [PubMed] [Google Scholar]
- 69.Naugle KM, Ohlman T, Naugle KE, Riley ZA & Keith NR Physical activity behavior predicts endogenous pain modulation in older adults. Pain 158, 383–390 (2017). [DOI] [PubMed] [Google Scholar]
- 70.Naugle KM, Cruz-Almeida Y, Vierck CJ, Mauderli AP & Riley JL Age-related differences in conditioned pain modulation of sensitizing and desensitizing trends during response dependent stimulation. Behav. Brain Res 289, 61–68 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skovbjerg S et al. Conditioned pain modulation and pressure pain sensitivity in the adult danish general population: the danfund study. J. Pain 18, 274–284 (2017). [DOI] [PubMed] [Google Scholar]
- 72.van Wijk G & Veldhuijzen DS Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. J. Pain 11, 408–419 (2010). [DOI] [PubMed] [Google Scholar]
- 73.Gagliese L, Gauthier LR, Narain N & Freedman T Pain, aging and dementia: Towards a biopsychosocial model. Prog. Neuropsychopharmacol. Biol. Psychiatry 87, 207–215 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Iseki E et al. Distribution and morphology of brain stem plaques in Alzheimer’s disease. Acta Neuropathol 78, 131–136 (1989). [DOI] [PubMed] [Google Scholar]
- 75.McDermott JH, Nichols DR & Lovell ME A case-control study examining inconsistencies in pain management following fractured neck of femur: an inferior analgesia for the cognitively impaired. Emerg. Med. J 31, e2–8 (2014). [DOI] [PubMed] [Google Scholar]
- 76.Scherder E et al. Recent developments in pain in dementia. BMJ 330, 461–464 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tan E et al. Prevalence of Analgesic Use and Pain in People with and without Dementia or Cognitive Impairment in Aged Care Facilities: A Systematic Review and Meta-Analysis. CCP 10, 194–203 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Bell JS et al. Use of strong opioids among community-dwelling persons with and without Alzheimer’s disease in Finland. Pain 152, 543–547 (2011). [DOI] [PubMed] [Google Scholar]
- 79.Hamina A et al. Differences in analgesic use in community-dwelling persons with and without Alzheimer’s disease. Eur. J. Pain 21, 658–667 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Hamina A et al. Long-term use of opioids for nonmalignant pain among community-dwelling persons with and without Alzheimer disease in Finland: a nationwide register-based study. Pain 158, 252–260 (2017). [DOI] [PubMed] [Google Scholar]
- 81.Corbett A et al. Assessment and treatment of pain in people with dementia. Nat. Rev. Neurol 8, 264–274 (2012). [DOI] [PubMed] [Google Scholar]
- 82.Hadjistavropoulos T et al. Pain assessment in elderly adults with dementia. Lancet Neurol 13, 1216–1227 (2014). [DOI] [PubMed] [Google Scholar]
- 83.Cole LJ et al. The impact of Alzheimer’s disease on the functional connectivity between brain regions underlying pain perception. Eur. J. Pain 15, 568.e1–11 (2011). [DOI] [PubMed] [Google Scholar]
- 84.Monroe TB et al. The impact of alzheimer’s disease on the resting state functional connectivity of brain regions modulating pain: A cross sectional study. J. Alzheimers Dis 57, 71–83 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stubbs B et al. Is pain sensitivity altered in people with Alzheimer’s disease? A systematic review and meta-analysis of experimental pain research. Exp. Gerontol 82, 30–38 (2016). [DOI] [PubMed] [Google Scholar]
- 86.Masnoon N, Shakib S, Kalisch-Ellett L & Caughey GE What is polypharmacy? A systematic review of definitions. BMC Geriatr 17, 230 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maher RL, Hanlon J & Hajjar ER Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf 13, 57–65 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Charlesworth CJ, Smit E, Lee DSH, Alramadhan F & Odden MC Polypharmacy Among Adults Aged 65 Years and Older in the United States: 1988–2010. J. Gerontol. A, Biol. Sci. Med. Sci 70, 989–995 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.National Center for Health Statistics. Health, United States, 2017: With special feature on mortality (Center for Disease Control, 2018). [PubMed] [Google Scholar]
- 90.Ersoy S & Engin VS Risk factors for polypharmacy in older adults in a primary care setting: a cross-sectional study. Clin Interv Aging 13, 2003–2011 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O’Dwyer M, Peklar J, McCallion P, McCarron M & Henman MC Factors associated with polypharmacy and excessive polypharmacy in older people with intellectual disability differ from the general population: a cross-sectional observational nationwide study. BMJ Open 6, e010505 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Niclós G, Olivar T & Rodilla V A cross-sectional evaluation of the prevalence and detection of predictors of polypharmacy amongst adult in Spain. Int J Pharm Pract 26, 242–249 (2018). [DOI] [PubMed] [Google Scholar]
- 93.Junius-Walker U, Theile G & Hummers-Pradier E Prevalence and predictors of polypharmacy among older primary care patients in Germany. Fam Pract 24, 14–19 (2007). [DOI] [PubMed] [Google Scholar]
- 94.Pokela N, Bell JS, Lihavainen K, Sulkava R & Hartikainen S Analgesic use among community-dwelling people aged 75 years and older: A population-based interview study. Am J Geriatr Pharmacother 8, 233–244 (2010). [DOI] [PubMed] [Google Scholar]
- 95.Paulose-Ram R et al. Prescription and non-prescription analgesic use among the US adult population: results from the third National Health and Nutrition Examination Survey (NHANES III). Pharmacoepidemiol Drug Saf 12, 315–326 (2003). [DOI] [PubMed] [Google Scholar]
- 96.McLachlan AJ, Hilmer SN & Le Couteur DG Variability in response to medicines in older people: phenotypic and genotypic factors. Clin. Pharmacol. Ther 85, 431–433 (2009). [DOI] [PubMed] [Google Scholar]
- 97.Koponen MPH et al. Analgesic use and frailty among community-dwelling older people: a population-based study. Drugs Aging 30, 129–136 (2013). [DOI] [PubMed] [Google Scholar]
- 98.Gurwitz JH et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA 289, 1107–1116 (2003). [DOI] [PubMed] [Google Scholar]
- 99.Shehab N et al. US Emergency Department Visits for Outpatient Adverse Drug Events, 2013–2014. JAMA 316, 2115–2125 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang M, Holman CDJ, Preen DB & Brameld K Repeat adverse drug reactions causing hospitalization in older Australians: a population-based longitudinal study 1980–2003. Br. J. Clin. Pharmacol 63, 163–170 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nawai A, Leveille SG, Shmerling RH, van der Leeuw G & Bean JF Pain severity and pharmacologic pain management among community-living older adults: the MOBILIZE Boston study. Aging Clin Exp Res 29, 1139–1147 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Savvas SM & Gibson SJ Overview of pain management in older adults. Clin Geriatr Med 32, 635–650 (2016). [DOI] [PubMed] [Google Scholar]
- 103.Nikolaus T & Zeyfang A Pharmacological treatments for persistent non-malignant pain in older persons. Drugs Aging 21, 19–41 (2004). [DOI] [PubMed] [Google Scholar]
- 104.Abdulla A et al. Guidance on the management of pain in older people. Age Ageing 42 Suppl 1, i1–57 (2013). [DOI] [PubMed] [Google Scholar]
- 105.O’Neil CK, Hanlon JT & Marcum ZA Adverse effects of analgesics commonly used by older adults with osteoarthritis: focus on non-opioid and opioid analgesics. Am J Geriatr Pharmacother 10, 331–342 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wehling M Non-steroidal anti-inflammatory drug use in chronic pain conditions with special emphasis on the elderly and patients with relevant comorbidities: management and mitigation of risks and adverse effects. Eur J Clin Pharmacol 70, 1159–1172 (2014). [DOI] [PubMed] [Google Scholar]
- 107.Musich S, Wang SS, Ruiz J, Hawkins K & Wicker E Falls-Related Drug Use and Risk of Falls Among Older Adults: A Study in a US Medicare Population. Drugs Aging 34, 555–565 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dowell D, Haegerich TM & Chou R CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016. MMWR Recomm. Rep 65, 1–49 (2016). [DOI] [PubMed] [Google Scholar]
- 109.Interagency Pain Research Coordinating Committee (IPRCC). National Pain Strategy - A Comprehensive Population Health-Level Strategy for Pain. (Department of Health and Human Services (HHS), 2016). [Google Scholar]
- 110.Pain Management Best Practices Inter-Agency Task Force. Draft Report onPain Management Best Practices: Updates, Gaps, Inconsistencies, and Recommendations (Health and Human Services (HHS), 2018). at https://www.hhs.gov/ash/advisory-committees/pain/reports/index.html [Google Scholar]
- 111.Hochberg MC et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. (Hoboken) 64, 465–474 (2012). [DOI] [PubMed] [Google Scholar]
- 112.Morone NE et al. A Mind-Body Program for Older Adults With Chronic Low Back Pain: A Randomized Clinical Trial. JAMA Intern. Med 176, 329–337 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Freburger JK et al. The rising prevalence of chronic low back pain. Arch. Intern. Med 169, 251–258 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Meucci RD, Fassa AG & Faria NMX Prevalence of chronic low back pain: systematic review. Rev Saude Publica 49, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Andersson HI, Ejlertsson G, Leden I & Rosenberg C Chronic pain in a geographically defined general population: studies of differences in age, gender, social class, and pain localization. Clin. J. Pain 9, 174–182 (1993). [DOI] [PubMed] [Google Scholar]
- 116.Thomas MJ et al. The population prevalence of foot and ankle pain in middle and old age: a systematic review. Pain 152, 2870–2880 (2011). [DOI] [PubMed] [Google Scholar]
- 117.Westergaard ML, Glümer C, Hansen EH & Jensen RH Prevalence of chronic headache with and without medication overuse: associations with socioeconomic position and physical and mental health status. Pain 155, 2005–2013 (2014). [DOI] [PubMed] [Google Scholar]
- 118.Stovner LJ & Andree C Prevalence of headache in Europe: a review for the Eurolight project. J Headache Pain 11, 289–299 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]