Abstract
Background:
Maternal consumption of alcohol produces abnormalities in the developing fetus and can contribute to an increased incidence of many cardiovascular-related diseases. The first goal of this study was to determine whether in utero exposure to alcohol influences reactivity of cerebral arterioles in adult (12–15-weeks old) rats. The second goal of this study was to examine whether in utero exposure to alcohol increased the susceptibility of the brain to damage following an ischemic event in adult rats.
Methods:
We fed Sprague-Dawley dams a liquid diet with or without alcohol (3% ethanol) for the duration of their pregnancy (21–23 days). In the first series of studies, we examined reactivity of cerebral arterioles to eNOS- (ADP), nNOS-dependent (NMDA) and NOS-independent agonists in adult rats before and during application of L-NMMA. In another series of studies, we examined infarct volume following middle cerebral artery occlusion in adult offspring exposed to alcohol in utero. In both series of studies, we also determined the role for an increase in oxidative stress by feeding dams apocynin for the duration of their pregnancy.
Results:
We found that in utero exposure to alcohol reduced responses of cerebral arterioles to ADP and NMDA, but not to nitroglycerin in adult rats. In addition, treatment of the dams with apocynin prevented this impairment in cerebral vascular function. We also found that in utero exposure to alcohol worsened brain damage following ischemia/reperfusion in adult rats and that treatment of dams with apocynin prevented this increase in brain damage following ischemia/reperfusion.
Conclusions:
We suggest that our findings may have important implications for the pathogenesis of brain abnormalities associated with fetal alcohol exposure.
Keywords: Fetal alcohol syndrome, nitric oxide, oxidative stress, cerebral vascular function, stroke
Introduction
Maternal consumption of alcohol during pregnancy is an established cause of fetal alcohol spectrum disorders (FASD) (May et al., 2018). Those affected by FASD experience an array of structural and developmental disorders of the brain and craniofacial regions, as well as abnormalities of the gastrointestinal, urinary, heart, liver, pancreas and vascular systems. In addition, those that have been exposed to alcohol in utero often suffer from cognitive decline, behavioral disorders, dementia, and seizures that manifest in early childhood and persist into adulthood (Daft et al., 1986; Coffin et al., 2005; Bell et al., 2010; Guerri et al., 2009). Studies that have applied the developmental origins of health and disease (DOHaD) approach reveal that adult-onset diseases (cardiovascular, diabetes, obesity, cognitive decline) appear to be programmed in utero in response to maternal exposure to many types of stimuli. Support for this concept can be found in studies suggesting that in utero exposure to a variety of agents and environmental stimuli can contribute to diseases in adulthood by targeting the endothelium and vascular function (Gray et al., 2015; Care et al., 2016; Jones et al., 2004; Sahna et al., 2000), suggesting mechanistic effects beyond toxicity-induced cell death. With regards to in utero exposure to alcohol, studies have shown a significant increase in cardiovascular abnormalities (atrial septal defects, ventricular septal defects and other malformations in blood vessels) in infants and children with FASD (Löser and Majewski, 1977; Jones et al., 1973; Davidson, 1989). However, there is a lack of information regarding the influence of in utero exposure to alcohol on the cerebral vasculature and on cerebral vascular diseases in humans. Although the precise mechanisms underlying intrauterine programming of adult diseases are not fully understood, they have been suggested to include alterations in the hypothalamo-pituitary-adrenal axis, cellular differentiation, gene expression and/or mitochondrial oxidative stress. In our previous study, we found that impaired responses of cerebral arterioles in adolescent rats (4–6 weeks old) exposed to alcohol in utero was related to an increase in oxidative stress (Cananzi and Mayhan, 2017). Unfortunately, there is a lack of information regarding the relationship between in utero exposure to alcohol and the prevalence of cerebral vascular disease in adulthood. Thus, the first goal of this study was to examine the influence of in utero exposure to alcohol in reactivity of cerebral arterioles in adult rats.
Ischemic stroke is a leading cause of mortality and long-term disability. While we and others have documented the effect of chronic alcohol consumption by adult animals on brain damage following cerebral ischemia/reperfusion (Ducroquet et al., 2013; Zhao et al., 2011; Zhao et al., 2010; Hillbom and Kaste, 1983), the effect of in utero exposure to alcohol on the susceptibility of the brain to ischemic injury during development has not been widely examined. One recent study (Bake et al., 2017) found that binge exposure of mice to alcohol (3 g/kg body weight twice daily) during GD12.5 through GD15.5 produced significant decrease in cranial-directed blood flow and a decrease capacity to compensate for brain injury (neurological deficits), but surprisingly these authors did not find an increase brain infarct volume following an ischemic event at 3 months of age. Thus, very limited exposure to alcohol in utero did not appear to alter the susceptibility of the brain to damage following cerebral ischemia/reperfusion in young animals. The second goal of the present study was to determine the effect of in utero exposure to alcohol on brain damage after ischemia/reperfusion in adulthood. Given that reactive oxygen species (ROS) are a critical mediator of neuronal death and have been linked to neuronal damage during FASD and dysfunction following ischemia/reperfusion (Brocardo et al., 2011; Kalogeris et al., 2014; Navarro-Yepes et al., 2014; Cohen-Kerem and Koren, 2003), we also examined the role for an increase in oxidative stress in brain damage following cerebral ischemia in adult animals exposed to alcohol in utero.
Materials and Methods
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental diets.
We used virgin adult male (16-week-old) and female (16-week-old) Sprague-Dawley rats for breeding. One male and one female rat were allowed to mate overnight. Dams were then housed singly and assigned randomly to one of four groups that were fed one of the following liquid diets for the entire gestation period (21–23 days): Control (0% alcohol) diet, 3% alcohol diet, control+apocynin diet, or 3% alcohol+apocynin diet. Liquid diets were prepared daily, as we have described previously (Zhao et al., 2010; Mayhan, 1992b). The control diets contained 1.0 kcal/ml of which 35% are derived from fat, 47% from carbohydrates, and 18% from protein. The 3% alcohol diets contained 1.0 kcal/ml of which 35% are derived from fat, 18% from protein, 29% from carbohydrate and 18% from alcohol. The total daily volume of diet fed to the control animals was based upon the daily consumption of diet by the alcohol animals. The alcohol diet resulted in a blood alcohol concentration (BAC) of 13.6 ±1 mM at hour and 14.6±1 mM at 2 hours in the dams (Abcam ethanol assay kit (ab65343)). These values are equivalent to a BAC between 0.06% and 0.07%, respectively. BACs were only determined in the rats fed the alcohol alone diet and not the alcohol+apocynin diet. The liquid diets fed to the dams were replaced by a normal chow/water diet within a day of birth of the pups. Rats were weaned at 3 weeks of age and placed in cages with those of the same sex. Both male and female rats were used for the experiments. For the control+apocynin and alcohol+apocynin groups, we added apocynin (10 mg/kg/day) to the liquid diets fed to the dams for the duration of pregnancy.
Measurement of cerebral vascular reactivity.
The rats were prepared for studies at 12–15 weeks of age and were obtained from multiple different litters. On the day of the experiment, the rats were anesthetized with thiobutabarbital sodium (Inactin, 100 mg/kg IP) and a tracheotomy was performed. The rats were ventilated mechanically with room air and supplemental oxygen. A catheter was placed into a femoral vein for injection of supplemental anesthesia (10–30 mg/kg; as necessary). A femoral artery was cannulated for the measurement of arterial blood pressure and to obtain a blood sample for the determination of blood gases.
A craniotomy was prepared over the left parietal cortex to visualize the microcirculation of the cerebrum. The cranial window was suffused with a bicarbonate buffer (2 ml/min) that was bubbled continuously with 95% nitrogen and 5% carbon dioxide. Temperature of the suffusate was maintained at 37 ± 1°C. The cranial window was connected via a three-way valve to an infusion pump, which allowed for infusion of agonists and antagonists into the suffusate. This method maintained a constant temperature, pH, pCO2, and pO2 of the suffusate during infusion of drugs. Arterial blood gases were monitored and maintained within normal limits throughout the experimental period. Diameter of cerebral arterioles was measured using a video image-shearing device. The cranial window was superfused with artificial cerebral spinal fluid for 30 min prior to testing responses of cerebral arterioles to the agonists.
Responses of cerebral arterioles were examined during superfusion of agonists that produce dilation dependent on the release of nitric oxide via activation of eNOS: ADP (10 and 100 μM); or nNOS: NMDA (30 and 100 μM). We also examined responses of cerebral arterioles to nitroglycerin (1.0 and 10 μM), which dilates cerebral arterioles independent of NOS. Diameter of arterioles was measured before, and at 1-minute intervals for 5 minutes during application of agonists. Baseline diameter of cerebral arterioles returned to control levels (before application of agonists) within 2–3 minutes after application of agonists was stopped. To determine the role of nitric oxide in ADP- and NMDA-induced dilation of cerebral arterioles, we suffused the cranial window with NG-monomethyl-L-arginine (L-NMMA; 10 μM). L-NMMA has been shown to be a nonspecific inhibitor of all isoforms of NOS, and we and several others have used L-NMMA to examine the role of NOS in responses of cerebral arterioles to many agonists (Zheng et al., 2006; Mayhan, 1992a; Moncada and Higgs, 2006; Faraci and Heistad, 1991). Thus, after initially measuring responses of cerebral arterioles to the agonists, we started a continuous suffusion with L-NMMA. One hour after starting suffusion with L-NMMA, we retested responses of cerebral arterioles to the agonists.
Middle cerebral artery occlusion.
Cerebral ischemia was induced in rats by middle cerebral artery occlusion (MCAO) at 12–15 weeks of age. Rats were anesthetized with ketamine/xylazine (100/15 mg/kg IP, respectively). The left common and external carotid arteries were exposed and ligated. Occlusion of the middle cerebral artery (MCA) was accomplished through the insertion of a filament from the basal part of the external carotid artery and advancing it in the internal carotid artery to the point toward the location where the MCA branched from the circle of Willis. Occlusion of the MCA induced a rapid drop in blood flow to the cerebral hemisphere. Cerebral blood flow changes were assessed using a laser Doppler (Periflux System 5000, Perimed) flow probe attached to the parietal bone during the placement of the suture. The MCA was occluded for 90 minutes and reperfusion (24 hours) was initiated by removing the suture from the internal carotid artery. Once the suture was removed, the internal carotid artery was ligated.
At the end of the recovery period (24 hours after reperfusion), rats were neurologically assessed on a 24-point scale using a sensorimotor testing grade that measured spontaneous activity, symmetry of movement, response to vibrissae touch, floor walking, beam walking, symmetry of forelimbs, climbing, and reaction to touch (Garcia et al., 1995; Zhao et al., 2011). After the neurological evaluation, the rats were anesthetized and killed with an overdose of Inactin followed by a thoracotomy. The brains were removed, placed in ice-cold saline for 5 minutes and cut into six 2-mm coronal sections. Then, 2,3,5 triphenyltetrazolium chloride (TTC; 2%) was used to stain the brain sections. Images of the ischemic lesion were evaluated using NIH Imaging Software (ImageJ; version 1.52a). The percentage lesion was calculated by measuring the infarct damage area in the ipsilateral hemisphere and dividing by the total area of the contralateral hemisphere. Total, cortical, and subcortical lesions were evaluated.
Superoxide levels.
We measured superoxide anion levels using lucigenin-enhanced chemiluminescence. After transcardially perfusing the heart with cold normal saline, the brain was removed and immersed in a modified Krebs-HEPES buffer. Tissue samples from the parietal cortex were weighed and placed in polypropylene tubes containing 5 μM lucigenin. The samples were read in a Berthold Sirius luminometer, which reports relative light units emitted integrated over 30 second intervals for 5 minutes. Data were corrected for background and normalized to tissue weight. We measured superoxide levels under basal conditions and during exposure to NADPH (10 and 100 μM) for 5 minutes.
Statistical analysis.
A three-way ANOVA was used to compare findings between groups of rats (Figures 1, 5 and 6). Bonferroni correction was used to test for significance between groups. Alcohol, apocynin and sex were factors. No difference was observed between male and female rats, and thus data from males and females were pooled. There were significant interactions of alcohol and apocynin. Paired t tests were used to compare responses of arterioles to the agonists before and following application of L-NMMA (Figures 2, 3 and 4). Values are mean±SEM. A p-value of 0.05 or less was considered to be significant.
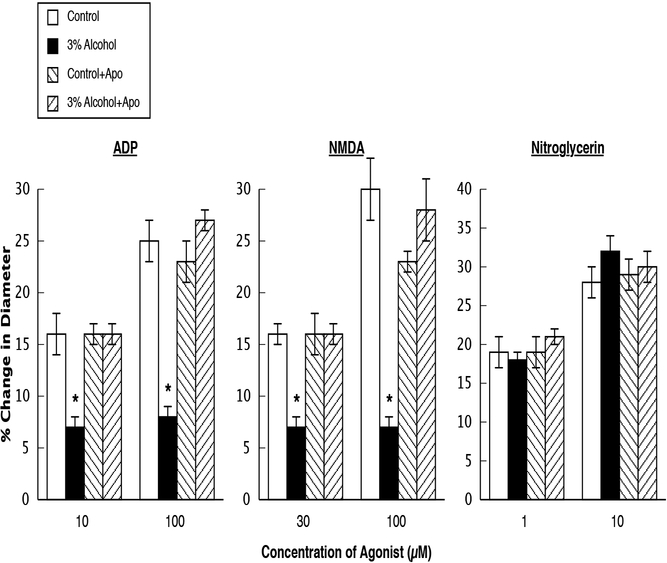
Figure 1.
Responses of cerebral arterioles to ADP, NMDA, and nitroglycerin in control rats, rats exposed to alcohol in utero, control+apocynin rats, and alcohol+apocynin rats. N=14 for each group of rats. Values are means ± SE. * p<0.05 versus control rats, control+apocynin rats and 3% alcohol+apocynin rats.
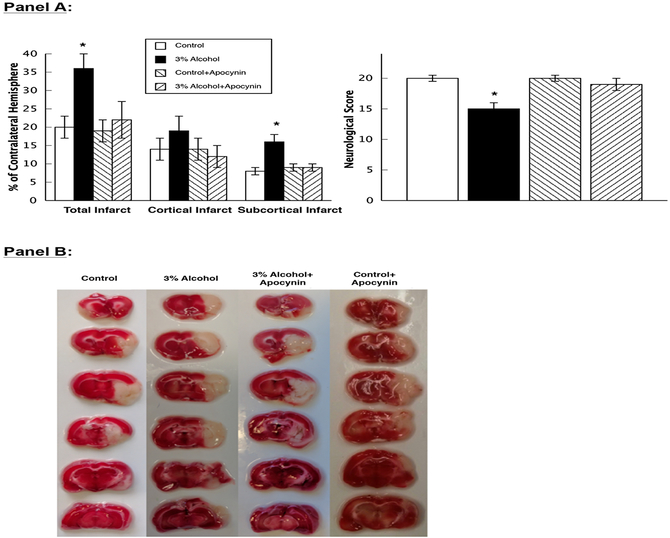
Figure 5.
Panel A: Mean data for total infarct, cortical infarct, and subcortical infarct volumes and neurological scores in control rats (n=13), rats exposed to alcohol in utero (n=12), control+apocynin rats (n=12), and alcohol+apocynin rats (n=13). Values are means ± SE. * p<0.05 versus control, control+apocynin and alcohol+apocynin rats. Panel B: Representative photograph of brain sections in control rats, 3% alcohol rats, control+apocynin rats and alcohol+apocynin rats after staining with TTC 24 hours following MCA occlusion.
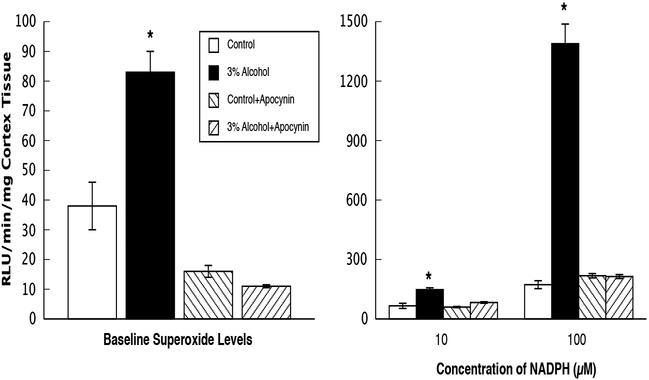
Figure 6.
Superoxide levels at baseline and during stimulation with NADPH (10 and 100 μM) in parietal cortex tissue from control rats, rats exposed to alcohol in utero, control+apocynin rats, and alcohol+apocynin rats. N=24 for each group of rats. Values are means ± SE.* p<0.05 versus responses in control, control+apocynin and alcohol+apocynin rats.
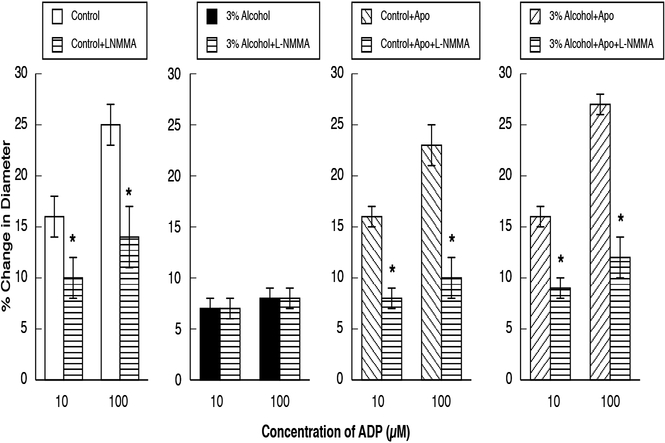
Figure 2.
Responses of cerebral arterioles to ADP in control rats, rats exposed to alcohol in utero, control+apocynin rats and alcohol+apocynin rats before and during suffusion of L-NMMA (10 μM). N=14 for each group of rats. Values are means ± SE. * p<0.05 versus response before L-NMMA.
Figure 3.
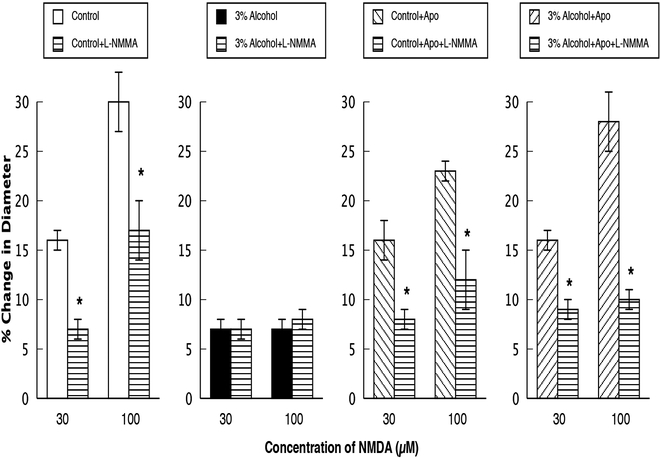
Responses of cerebral arterioles to NMDA in control rats, rats exposed to alcohol in utero, control+apocynin rats and alcohol+apocynin rats before and during suffusion of L-NMMA (10 μM). N=14 for each group of rats. Values are means ± SE. * p<0.05 versus response before L-NMMA.
Figure 4.
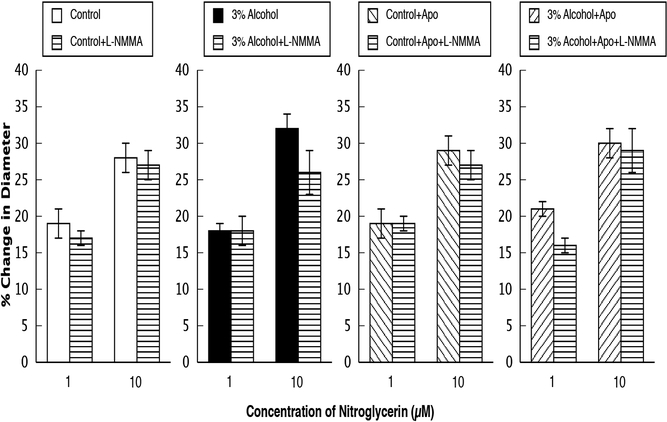
Responses of cerebral arterioles to nitroglycerin in control rats, rats exposed to alcohol in utero, control+apocynin rats and alcohol+apocynin rats before and during suffusion of L-NMMA (10 μM). N=14 for each group of rats. Values are means ± SE. *
Results
Responses to the agonists.
Baseline diameter of cerebral arterioles, mean arterial pressure, and body weight were similar in all groups at 12–15 weeks of age (Table 1).
Table 1.
Baseline parameters at 14–16-week old rats.
| Control | Alcohol | Control+Apo | Alcohol+Apo | |
|---|---|---|---|---|
| Baseline diameter (microns) | 36±3 | 44±4 | 37±2 | 38±3 |
| Mean arterial blood pressure (MAP) (mmHg) | 114±4 | 110±3 | 108±2 | 114±2 |
| Body weight (grams) | 361±21 | 337±22 | 340±34 | 378±28 |
Values are mean±SE. n=14 for all groups of rats.
Application of ADP, NMDA, and nitroglycerin dilated cerebral arterioles in all groups of rats (Figure 1). However, vasodilation in response to ADP and NMDA, but not to nitroglycerin, was reduced in rats exposed to alcohol in utero (Figure 1). In addition, treatment of the dams with the control+apocynin diet did not influence responses of cerebral arterioles to the agonists when compared to responses of arterioles in control rats not exposed to apocynin in utero (Figure 1). Treatment of the dams with the alcohol+apocynin diet prevented the impairment in cerebral vasodilation in response to ADP ([Low dose ADP=effect of ethanol: F(1, 52)=12.35, p<0.0009; effect of apocynin: F(1, 52)=14.06, p<0.0004; interaction of ethanol and apocynin: F(1, 52)=14.45, p<0.0004] and [High dose ADP=effect of ethanol: F(1, 52)=14.57, p<0.0004; effect of apocynin: F(1, 52)=31.30, 0.0001; interaction of ethanol and apocynin: F(1, 52)=46.57, p<0.0001]) and NMDA ([Low dose NMDA=effect of ethanol: F(1, 52)=10.65, p<0.0019; effect of apocynin: F(1, 52)=10.96, p<0.0017; interaction of ethanol and apocynin: F(1, 52)=10.76, p<0.0019] and [High dose NMDA=effect of ethanol: F(1, 52)=16.46, p<0.0002; effect of apocynin: F(1, 52)=10.00, p<0.0026; interaction of ethanol and apocynin: F(1, 52)=37.55, p<0.0001]) in 14–16-week-old adult rats (Figure 1). Thus, there were significant interactions of alcohol and apocynin.
Responses following L-NMMA.
Topical application of L-NMMA reduced baseline diameter in control, control+apocynin, and in alcohol+apocynin rats, but had no effect on baseline diameter of rats exposed to alcohol in utero (Table 2). In addition, L-NMMA reduced responses to ADP (Figure 2) and NMDA (Figure 3) in control, control+apocynin, and alcohol+apocynin rats, but had little effect in rats exposed to alcohol in utero. L-NMMA had no effect on responses of cerebral arterioles to nitroglycerin in any group (Figure 4).
Table 2.
Baseline diameter of cerebral arterioles before and after application of L-NMMA.
| Before L-NMMA | After L-NMMA | |
|---|---|---|
| Control rats | 45±3 | 38±3* |
| 3% Alcohol rats | 47±4 | 42±3 |
| Control+Apocynin rats | 46±3 | 36±2* |
| 3% Alcohol+Apocynin rats | 49±4 | 36±3* |
All values are given in microns (μm). Values are means ± SE.
* p<0.05 versus diameter before application of L-NMMA (10 μM). n= 14 for all groups of rats.
Middle cerebral artery occlusion.
MCAO produced a similar decrease in cerebral blood flow in control (61±5% reduction), alcohol (49±6% reduction), control+apocynin (53±5% reduction) and alcohol+apocynin (48±7% reduction) rats (p>0.05). However, we found a significant increase in total infarct ([effect of ethanol: F(1, 46)=5.987, p<0.0183; effect of apocynin: F(1, 46)=10.96, p<0.0519; interaction of ethanol and apocynin: F(1, 46)=3.066, p<0.0866]) and subcortical infarct ([effect of ethanol: F(1, 46)=10.34, p<0.0183; effect of apocynin: F(1, 46)=4.979, p<0.0306; interaction of ethanol and apocynin: F(1, 46)=8.985, p<0.0044]) volumes in rats exposed to alcohol in utero compared to control, control+apocynin and alcohol+apocynin rats (Figure 5). In addition, neurological scores were lower in alcohol rats ([effect of ethanol: F(1, 46)=10.44, p<0.0023; effect of apocynin: F(1, 46)=4.685, p<0.0357; interaction of ethanol and apocynin: F(1, 46)=3.897, p<0.0544]) when compared to control, control+apocynin and alcohol+apocynin rats (Figure 5) indicating a worse outcome following ischemia/reperfusion in rats exposed to alcohol in utero.
Superoxide.
We found that superoxide levels from cortex tissue were increased in rats exposed to alcohol in utero under basal conditions ([effect of ethanol: F(1, 88)=14.20, p<0.0003; effect of apocynin: F(1, 88)=76.58, p<0.0001; Effect of ethanol and apocynin: F(1, 88)=21.92, p<0.0001]) when compared to the other groups of rats (Figure 6). In addition, this basal increase in superoxide levels observed in rats exposed to alcohol in utero was decreased in rats from dams that were fed the alcohol+apocynin diet. Stimulation of cortex tissue with NADPH, the substrate for NADPH oxidase, produced a significant increase in superoxide levels in rats exposed to alcohol in utero when compared to all other groups ([effect of ethanol: F(1, 88)=40.42, p<0.0001; effect of apocynin: F(1, 88)=18.91, p<0.0001; interaction of ethanol X apocynin: F(1, 88)=12.31, p<0.0007 for low dose] and ([effect of ethanol: F(1, 88)=140.6, p<0.0001; effect of apocynin: F(1, 88)=121.8, p<0.0001; interaction of ethanol and apocynin: F(1, 88)=142.1, p<0.0001 for high dose]) (Figure 6).
Discussion
There are several new findings from this study. First, exposure to alcohol in utero impairs eNOS- and nNOS-dependent dilation of cerebral arterioles in adult (12–15-week-old rats). Second, brain infarct volume and neurological deficits following cerebral ischemia/reperfusion are increased in adult rats exposed to alcohol in utero. Third, in utero exposure to alcohol produced a sustained increase in basal levels of superoxide in the brain of adult rats and increased the ability of brain tissue to produce superoxide in response to NADPH (the substrate for NADPH oxidase). Fourth, treatment of the dams with apocynin to inhibit oxidative stress prevented in utero-induced impairment of cerebral vascular function, the increase in brain damage following cerebral ischemia/reperfusion and the basal increase in oxidative stress observed in adult rats. We suggest that our findings may have important implications for the pathogenesis of symptoms associated with FASD in adults, i.e., cognitive decline, behavioral disorders, dementia, and seizures (Daft et al., 1986; Coffin et al., 2005; Bell et al., 2010; Guerri et al., 2009), all of which may be influenced by the ability of the brain to maintain adequate cerebral blood flow in the face of changes in metabolic demand (neurovascular coupling).
We used ADP and NMDA as our eNOS- and nNOS-dependent agonists, respectively. We also used nitroglycerin as a NOS-independent agonist. Previous studies have shown that ADP binds to the P2Y12 G protein-coupled receptor, NMDA opens NMDA receptors allowing the inward passage of Ca2+ ions, while nitroglycerin releases free nitric oxide following enzymatic action by dehydrogenases (Lewis et al., 2000; Garthwaite et al., 1989; Harrison and Bates, 1993). We and others have shown that ADP dilates cerebral arterioles through a pathway involving eNOS (Faraci, 1991; Mayhan, 1992a; You et al., 1997). NMDA has been shown to dilate cerebral arterioles through a pathway involving nNOS (Faraci and Breese, 1993; Busija and Leffler, 1989; Faraci and Brian, 1995). Thus, it appears that ADP and NMDA are appropriate agonists to examine eNOS- and nNOS-dependent dilation in cerebral arterioles.
In the present study we found that L-NMMA produced modest constriction of cerebral arterioles in control, control+apocynin and alcohol+apocynin rats, but not in alcohol-exposed rats. This finding suggests that basal production of nitric oxide contributes to baseline diameter of cerebral arterioles in all groups except the alcohol-exposed rats. In light of our finding showing that basal levels of superoxide are increase in rats exposed to alcohol in utero, we suggest that these basal levels of superoxide may be sufficient to impair the ability of nitric oxide to influence baseline diameter of cerebral arterioles.
The influence of sex on reactivity of peripheral arteries has been examined, but less is known about the influence of sex on reactivity of cerebral arterioles. In a previous study, we found that dilation of cerebral arterioles to ADP and NMDA was elevated in female compared to male rats (Arrick et al., 2016). In contrast, we did not find sex-related differences in reactivity of cerebral arterioles in the present study. The discrepancy between the present study and our previous study is not clear, although the rats in the present study were older than in our previous study. One could suggest that feeding dams the liquid diets may have influenced reactivity to the agonists differently in male and female offspring, although this possibility seems unlikely. In any event, we are not certain as to why we did not see sex-related differences in vascular reactivity in the present study.
Several studies have examined the influence of in utero exposure to alcohol on reactivity of cerebral and peripheral blood vessels. Gleason et al., (Gleason et al., 1997) and Mayock et al., (Mayock et al., 2007) found that binge alcohol consumption in the second trimester impaired hypoxia-induced increases in cerebral blood flow in preterm and newborn (1–4 days old) sheep. However, underlying mechanisms were not examined. Likewise, Ngai et al., (Ngai et al., 2008) reported that alcohol consumption by pregnant ewes impaired responses of large cerebral arteries to vasoactive intestinal polypeptide (VIP) in adult (10–13 months old) offspring, but again mechanisms were not examined. Bake et al., (Bake et al., 2017) reported a decrease in blood acceleration (Doppler blood flow and pulse-wave recordings) through the carotid arteries of adult mice (3–12 months old) exposed to prenatal alcohol, but mechanisms were not examined. In recent studies, we found that in vivo responses of cerebral arterioles to eNOS- and nNOS-dependent agonists were impaired in young rats (4–6 weeks old) exposed to alcohol in utero (Cananzi and Mayhan, 2017). Finally, Turcotte et al., (Turcotte et al., 2002) reported that prenatal exposure to ethanol (6.4%) decreased relaxation of the aorta to carbamylcholine in rats 25 weeks after birth. Thus, limited evidence suggests that in utero exposure to alcohol can alter responses of cerebral and peripheral arteries in young, adolescent and adult animals. However, precise mechanisms and specific consequences to the brain have not been fully examined.
In the present study, we found that in utero exposure to alcohol specifically impaired eNOS- and nNOS-dependent reactivity of cerebral arterioles in adult rats exposed to alcohol in utero. We also found that this specific impairment in cerebral vascular function could be prevented by treatment of the dams with apocynin. These results extend and complement findings from our previous study (Cananzi and Mayhan, 2017) and from others (Bake et al., 2017; Gleason et al., 1997; Mayock et al., 2007; Ngai et al., 2008) by examining reactivity of cerebral resistance arterioles, by examining response in adult animals and by examining potential mechanisms that account for impaired responses of cerebral arterioles by in utero exposure to alcohol.
While many studies have examined the effect of chronic alcohol consumption by adult animals on brain damage following cerebral ischemia/reperfusion (Ducroquet et al., 2013; Zhao et al., 2011; Zhao et al., 2010; Hillbom and Kaste, 1983), the effect of in utero exposure to alcohol on the susceptibility of the brain to ischemic injury during development remains uncertain. One recent study (Bake et al., 2017) found that binge exposure of mice to alcohol (3 g/kg body weight twice daily) during gestational days 12.5 through 15.5 produced significant decrease in carotid artery blood flow and a decrease capacity to compensate for brain injury (neurological deficits), but surprisingly there was no increase in brain infarct volume following an ischemic event in these mice at 3 months of age. In the present study we found that exposure to alcohol in utero during the entire gestation period produced an increase in brain infarct volume in 12–15-week-old rats following cerebral ischemia/reperfusion. In addition, in agreement with the previous study (Bake et al., 2017), we also found an increase in neurological deficits in adult rats exposed to alcohol in utero. We also extend the findings of the previous study (Bake et al., 2017) by examining the influence of treatment of the dams with apocynin on brain infarct volume and neurological deficits in adult rats exposed to alcohol in utero. The discrepancy between the present study and the previous study (Bake et al., 2017) regarding the influence of in utero exposure to alcohol on brain infarct volume is not clear, but may be related to species differences, the degree of the reduction of cerebral blood flow during the occlusion, the time of exposure to alcohol and the timing of the exposure to alcohol during the development of the fetus.
A major source of superoxide is NADPH oxidase (Konior et al., 2014; Griendling et al., 2000). Activation of NADPH oxidase has been linked to brain damage following cerebral ischemia/reperfusion (Granger and Kvietys, 2015; Tang et al., 2007; Kahles and Brandes, 2012; Chen et al., 2009; Tang et al., 2008; Kusaka et al., 2004; Walder et al., 1997). NADPH oxidase is composed by two membrane bound proteins, gp91phox and p22phox, and three cytosolic proteins p67phox, p47phox, and p40phox. When activated, the cytosolic subunits reach the membrane and interact with membrane bound subunits, transferring electrons from NADPH to oxygen to form superoxide. We used apocynin to inhibit NOX-related proteins. Although the specificity of apocynin has been questioned in the past several years (Wind et al., 2010; Heumuller et al., 2008), others have suggested that apocynin prevents the formation of a working NADPH oxidase complex by inhibiting the association of subunit p47phox to the gp91phox/p22phox complex (Stolk et al., 1994). We found a decrease in eNOS- and nNOS-dependent responses of cerebral arterioles, an increase in superoxide levels and an increase in brain damage following ischemia/reperfusion in rats exposed to alcohol in utero. We also found that treatment of dams with apocynin reduced the magnitude of these abnormalities. This finding seems to suggest that an increase in oxidative stress, possibly via activation of NADPH oxidase, may contribute to cerebral vascular dysfunction in rats exposed to alcohol in utero.
In summary, we examined the effects of in utero exposure to alcohol on eNOS- and nNOS-dependent reactivity of cerebral arterioles, the susceptibility of the brain to damage following an ischemic event and superoxide levels in adult rats. We found that in utero exposure to alcohol impaired responses of cerebral arterioles to eNOS- and nNOS-dependent agonists and increased the susceptibility of the brain to damage following cerebral ischemia/reperfusion in adult rats. In addition, we found that treatment of dams with apocynin prevented impaired reactivity in cerebral arterioles and the increase in brain damage following cerebral ischemia/reperfusion in adult rats exposed to alcohol in utero, suggesting an important role for an increase in oxidative stress. We speculate that our findings may have important implications for those that suffer from symptoms associated with FASD, i.e., cognitive decline, behavioral disorders, dementia and seizures (Daft et al., 1986; Coffin et al., 2005; Bell et al., 2010; Guerri et al., 2009). Although there are no longitudinal studies that have examined the risk of stroke in individuals that suffer from FASD, there are studies that have shown that the prevalence of epileptic seizures is much higher in individuals affected by FASD compared to the general population (Bell et al., 2010). Given that there appears to be a relationship between seizures/epilepsy and the onset of stroke (Zelano et al., 2017), we speculate that individuals with FASD could be at a greater risk for the pathogenesis of cerebral vascular disorders, perhaps including stroke.
Acknowledgements
Sources of Support: These studies were supported by funds from a Malcolm Feist Fellowship from LSU Health Sciences Center-Shreveport (SGC), funds from the Sanford School of Medicine at the University of South Dakota (WGM) and a grant from the National Institute on Alcohol Abuse and Alcoholism (WGM; 1 R01 AA027206–01).
Footnotes
Conflict of Interest
None.
References
- Arrick DM, Li C, Mayhan WG (2016) Sex-related differences in reactivity of cerebral arterioles during moderate exercise training. Microcirculation 23: 549–557. [DOI] [PubMed] [Google Scholar]
- Bake S, Gardner R, Tingling JD, Miranda RC, Sohrabji F (2017) Fetal Alcohol Exposure Alters Blood Flow and Neurological Responses to Transient Cerebral Ischemia in Adult Mice. Alcohol Clin Exp Res. 41: 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SH, Stade B, Reynolds JN, Rasmussen C, Andrew G, Hwang PA, Carlen PL (2010) The remarkably high prevalence of epilepsy and seizure history in fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 34: 1084–1089. [DOI] [PubMed] [Google Scholar]
- Brocardo PS, Gil-Mohapel J, Christie BR (2011) The role of oxidative stress in fetal alcohol spectrum disorders. Brain Res Rev. 67: 209–225. [DOI] [PubMed] [Google Scholar]
- Busija DW, Leffler CW (1989) Dilator effects of amino acid neurotransmitters on piglet pial arterioles. American Journal of Physiology. 257: H1200–H1203. [DOI] [PubMed] [Google Scholar]
- Cananzi SG, Mayhan WG (2017) In utero exposure to alcohol alters reactivity of cerebral arterioles. J Cereb Blood Flow Metab. 271678X17728163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Care AS, Sung MM, Panahi S, Gragasin FS, Dyck JR, Davidge ST, Bourque SL (2016) Perinatal Resveratrol Supplementation to Spontaneously Hypertensive Rat Dams Mitigates the Development of Hypertension in Adult Offspring. Hypertension. 67: 1038–1044. [DOI] [PubMed] [Google Scholar]
- Chen H, Song YS, Chan PH (2009) Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J Cereb Blood Flow Metab. 29: 1262–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin JM, Baroody S, Schneider K, O’Neill J (2005) Impaired cerebellar learning in children with prenatal alcohol exposure: a comparative study of eyeblink conditioning in children with ADHD and dyslexia. Cortex. 41: 389–398. [DOI] [PubMed] [Google Scholar]
- Cohen-Kerem R, Koren G (2003) Antioxidants and fetal protection against ethanol teratogenicity. I. Review of the experimental data and implications to humans. Neurotoxicol Teratol. 25: 1–9. [DOI] [PubMed] [Google Scholar]
- Daft PA, Johnston MC, Sulik KK (1986) Abnormal heart and great vessel development following acute ethanol exposure in mice. Teratology. 33: 93–104. [DOI] [PubMed] [Google Scholar]
- Davidson DM (1989) Cardiovascular effects of alcohol. West J Med. 151: 430–439. [PMC free article] [PubMed] [Google Scholar]
- Ducroquet A, Leys D, Al Saabi A, Richard F, Cordonnier C, Girot M, Deplanque D, Casolla B, Allorge D, Bordet R (2013) Influence of chronic ethanol consumption on the neurological severity in patients with acute cerebral ischemia. Stroke. 44: 2324–2326. [DOI] [PubMed] [Google Scholar]
- Faraci FM (1991) Role of endothelium-derived relaxing factor in cerebral circulation: Large arteries vs. microcirculation. American Journal of Physiology. 261: H1038–H1042. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Breese KR (1993) Nitric oxide mediates vasodilatation in response to activation of N-methyl-D-aspartate receptors in brain. Circulation Research. 72: 476–480. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Brian JE (1995) 7-nitroindazole inhibits brain nitric oxide synthase and cerebral vasodilatation in response to N-methyl-D-aspartate. Stroke. 26: 2172–2176. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Heistad DD (1991) Regulation of cerebral blood vessels by humoral and endothelium-dependent mechanisms. Hypertension. 17: 917–922. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Wagner S, Liu KF, Hu XJ (1995) Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 26: 627–634. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Garthwaite G, Plamer RMJ, Moncada S (1989) NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. European Journal of Pharmacology. 172: 413–416. [DOI] [PubMed] [Google Scholar]
- Gleason CA, Iida H, Hotchkiss KJ, Northington FJ, Traystman RJ (1997) Newborn cerebrovascular responses after first trimester moderate maternal ethanol exposure in sheep. Pediatr Res. 42: 39–45. [DOI] [PubMed] [Google Scholar]
- Granger DN, Kvietys PR (2015) Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 6: 524–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C, Vickers MH, Segovia SA, Zhang XD, Reynolds CM (2015) A maternal high fat diet programmes endothelial function and cardiovascular status in adult male offspring independent of body weight, which is reversed by maternal conjugated linoleic acid (CLA) supplementation. PLoS One. 10: e0115994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M (2000) NAD(P)H oxidase. Role in cardiovascular biology and disease. Circulation Research. 86: 494–501. [DOI] [PubMed] [Google Scholar]
- Guerri C, Bazinet A, Riley EP (2009) Foetal Alcohol Spectrum Disorders and alterations in brain and behaviour. Alcohol Alcohol. 44: 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DG, Bates JN (1993) The nitrovasodilators. New ideas about old drugs. Circulation. 87: 1461–1467. [DOI] [PubMed] [Google Scholar]
- Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP (2008) Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 51: 211–217. [DOI] [PubMed] [Google Scholar]
- Hillbom M, Kaste M (1983) Ethanol intoxication: a risk factor for ischemic brain infarction. Stroke. 14: 694–699. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, Streissguth P (1973) Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1: 1267–1271. [DOI] [PubMed] [Google Scholar]
- Jones RD, Morice AH, Emery CJ (2004) Effects of perinatal exposure to hypoxia upon the pulmonary circulation of the adult rat. Physiol Res. 53: 11–17. [PubMed] [Google Scholar]
- Kahles T, Brandes RP (2012) NADPH oxidases as therapeutic targets in ischemic stroke. Cell Mol Life Sci. 69: 2345–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalogeris T, Bao Y, Korthuis RJ (2014) Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2: 702–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konior A, Schramm A, Czesnikiewicz-Guzik M, Guzik TJ (2014) NADPH oxidases in vascular pathology. Antioxid Redox Signal. 20: 2794–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka I, Kusaka G, Zhou C, Ishikawa M, Nanda A, Granger DN, Zhang JH, Tang J (2004) Role of AT1 receptors and NAD(P)H oxidase in diabetes-aggravated ischemic brain injury. Am J Physiol Heart Circ Physiol. 286: H2442–51. [DOI] [PubMed] [Google Scholar]
- Lewis CJ, Ennion SJ, Evans RJ (2000) P2 purinoceptor-mediated control of rat cerebral (pial) microvasculature; contribution of P2X and P2Y receptors. J Physiol. 527 Pt 2: 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löser H, Majewski F (1977) Type and frequency of cardiac defects in embryofetal alcohol syndrome. Report of 16 cases. Br Heart J. 39: 1374–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, Kopald D, Hasken JM, Xu R, Honerkamp-Smith G, Taras H, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Vaux K, Jewett T, Elliott AJ, Kable JA, Akshoomoff N, Falk D, Arroyo JA, Hereld D, Riley EP, Charness ME, Coles CD, Warren KR, Jones KL, Hoyme HE (2018) Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities. JAMA. 319: 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhan WG (1992a) Endothelium-dependent responses of cerebral arterioles to adenosine 5’-diphosphate. Journal of Vascular Research. 29: 353–358. [DOI] [PubMed] [Google Scholar]
- Mayhan WG (1992b) Responses of cerebral arterioles during chronic alcohol exposure. American Journal of Physiology. 262: H787–H791. [DOI] [PubMed] [Google Scholar]
- Mayock DE, Ness D, Mondares RL, Gleason CA (2007) Binge alcohol exposure in the second trimester attenuates fetal cerebral blood flow response to hypoxia. J Appl Physiol. 102: 972–977. [DOI] [PubMed] [Google Scholar]
- Moncada S, Higgs EA (2006) The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 147 Suppl 1: S193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Yepes J, Zavala-Flores L, Anandhan A, Wang F, Skotak M, Chandra N, Li M, Pappa A, Martinez-Fong D, Del Razo LM, Quintanilla-Vega B, Franco R (2014) Antioxidant gene therapy against neuronal cell death. Pharmacol Ther. 142: 206–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai AC, Mondares RL, Mayock DE, Gleason CA (2008) Fetal alcohol exposure alters cerebrovascular reactivity to vasoactive intestinal peptide in adult sheep. Neonatology. 93: 45–51. [DOI] [PubMed] [Google Scholar]
- Sahna E, Kurcer Z, Ozturk F, Cengiz N, Vardi N, Birincioglu M, Olmez E (2000) Effects of chronic ethanol consumption on alpha-adrenergic-induced contractions and endothelium-dependent relaxations in rat thoracic aorta. Pharmacol Res. 41: 629–633. [DOI] [PubMed] [Google Scholar]
- Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ (1994) Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 11: 95–102. [DOI] [PubMed] [Google Scholar]
- Tang LL, Ye K, Yang XF, Zheng JS (2007) Apocynin attenuates cerebral infarction after transient focal ischaemia in rats. J Int Med Res. 35: 517–522. [DOI] [PubMed] [Google Scholar]
- Tang XN, Cairns B, Cairns N, Yenari MA (2008) Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience. 154: 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte LA, Aberle NS, Norby FL, Wang GJ, Ren J (2002) Influence of prenatal ethanol exposure on vascular contractile response in rat thoracic aorta. Alcohol. 26: 75–81. [DOI] [PubMed] [Google Scholar]
- Walder CE, Green SP, Darbonne WC, Mathias J, Rae J, Dinauer MC, Curnutte JT, Thomas GR (1997) Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke. 28: 2252–2258. [DOI] [PubMed] [Google Scholar]
- Wind S, Beuerlein K, Eucker T, Müller H, Scheurer P, Armitage ME, Ho H, Schmidt HH, Wingler K (2010) Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br J Pharmacol. 161: 885–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J, Johnson TD, Childres WF, Bryan RM (1997) Endothelial-mediated dilations of rat middle cerebral arteries by ATP and ADP. American Journal of Physiology. 273: H1472–H1477. [DOI] [PubMed] [Google Scholar]
- Zelano J, Larsson D, Kumlien E, Åsberg S (2017) Pre-stroke seizures: A nationwide register-based investigation. Seizure. 49: 25–29. [DOI] [PubMed] [Google Scholar]
- Zhao H, Mayhan WG, Arrick DM, Xiong W, Sun H (2011) Dose-Related Influence of Chronic Alcohol Consumption on Cerebral Ischemia/Reperfusion Injury. Alcohol Clin Exp Res. 35: 1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Mayhan WG, Arrick DM, Xiong W, Sun H (2010) Alcohol-induced exacerbation of ischemic brain injury: Role of NAD(P)H oxidase. Alcohol Clin Exp Res. 34: 1948–1955. [DOI] [PubMed] [Google Scholar]
- Zheng H, Mayhan WG, Bidasee KR, Patel KP (2006) Blunted nitric oxide-mediated inhibition of sympathetic nerve activity within the paraventricular nucleus in diabetic rats. Am J Physiol Regul Integr Comp Physiol. 290: R992–R1002. [DOI] [PubMed] [Google Scholar]