Abstract
Background
Proton Magnetic Resonance Spectroscopy (1H-MRS) studies have demonstrated abnormal levels of a variety of neurometabolites in inpatients/outpatients with Alcohol Use Disorder (AUD) following acute alcohol withdrawal relative to healthy controls. In contrast, few studies have compared neurometabolite levels between less severe, treatment-naïve AUD individuals and light drinkers or related them to recent alcohol consumption. The present study compared neurometabolite levels between treatment-naïve AUD and LD individuals.
Methods
Twenty treatment-naïve individuals with AUD and 20 demographically-matched LD completed an 1H-MRS scan, approximately 2.5 days following their last reported drink. 1H-MRS data were acquired in dorsal anterior cingulate (dACC) using a Two-dimensional J-resolved Point Resolved Spectroscopy sequence. dACC neurometabolite levels, with a focus on glutamate, glutamine, and GABA, were compared between AUD and LD participants. Associations between metabolite levels and recent drinking were explored.
Results
AUD participants had significantly lower concentrations of GABA (Cohen’s D=0.79, p=0.017) and glutamine (Cohen’s D=1.12, p=0.005), but not glutamate (Cohen’s D=0.05, p=0.893), relative to LD. As reported in Prisciandaro et al. (2016), AUD participants’ glutamate and NAA concentrations were inversely associated with their number of heavy drinking days. In contrast, neither number of drinking (mean p=0.56) nor heavy drinking (mean p=0.47) days were associated with metabolite concentrations in LD.
Conclusions
The present study demonstrated significantly lower levels of prefrontal GABA and glutamine in treatment-naïve individuals with AUD relative to LD. Whether these findings reflect the neurotoxic consequence and/or neuroadaptive response of alcohol consumption versus a pre-drinking trait, and therefore a more durable neurochemical disturbance, awaits elucidation from longitudinal studies.
Keywords: magnetic resonance spectroscopy, alcohol use disorder, treatment naïve, GABA, glutamine
INTRODUCTION
Data from animal studies demonstrate that acute ethanol exposure augments the effects of ɣ-aminobutyric acid (GABA) on the GABAA receptor and inhibits the effects of glutamate on the N-methyl-Daspartate (NMDA) receptor (Gass and Olive 2008; Krystal et al., 2006). Conversely, prolonged ethanol exposure engenders compensatory downregulation of GABAA receptors and upregulation of NMDA receptors, culminating in the hypoGABAergic/ hyperglutamatergic state of alcohol withdrawal (Gass and Olive 2008; Krystal et al., 2006). Although glutamine, the critical amino acid precursor to both glutamate and GABA (Bak et al., 2006), has rarely been the subject of investigation of preclinical ethanol studies, the available literature suggests increased brain glutamine concentrations in ethanol-fed rats (Lee et al., 2012) and decreased brain glutamine concentrations during acute ethanol withdrawal (Hermann et al., 2012). Recent advances in proton magnetic resonance spectroscopy (1H-MRS) technology (e.g., increased magnetic field strength, development of spectral editing techniques) have enabled accurate, in-vivo measurement of concentrations of glutamate and GABA, as well as glutamine, in human brain tissue.
Notwithstanding a few notable exceptions (e.g., Bauer et al., 2013, Yeo et al., 2013), when cross-sectional 1H-MRS investigations of brain glutamate levels in individuals with Alcohol Use Disorder (AUD) are sorted by time since participants’ last drink, the literature as a whole demonstrates that brain glutamate levels in individuals with AUD are abnormally high during acute withdrawal (e.g., ≤ 72 hours after last alcohol consumption (Hermann et al., 2012), and abnormally low one-week after last alcohol consumption (Mon et al., 2012, Thoma et al., 2011). Short-term follow-up data from two studies have additionally demonstrated potential normalization of brain glutamate levels approximately 2–5 weeks from last alcohol consumption (Hermann et al., 2012, Mon et al., 2012). Further, glutamate levels in frontal white matter were significantly lower in active heavy drinkers who reported a loss-of-control over their drinking relative to both light and heavy drinkers who did not report loss-of-control over drinking (Ende et al., 2013).
1H-MRS studies investigating brain GABA and glutamine levels have been relatively rare, likely in part because measurement of these metabolites is more technically challenging relative to glutamate. Advances in 1H-MRS acquisition sequences have resulted in accessible methods (e.g., Two-Dimensional J-resolved 1H-MRS) for isolating GABA and glutamine signals from other, larger overlapping signals (Prescot & Renshaw, 2013). Nonetheless, while some research has supported decreased GABA (Behar et al., 1999) and increased glutamine (Thoma et al., 2011) in individuals with AUD relative to controls, other studies have failed to replicate these findings (Mason et al., 2006, Mon et al., 2012, Abe et al., 2013). Although not conducted in individuals with AUD, a recent study of “emerging adults” (aged 18–24 years) found significantly decreased ACC GABA levels in binge-drinking (with an average of 6 days since their last drink) vs. light drinking participants (Silveri et al., 2014).
Although the studies discussed above have provided a wealth of information concerning glutamate (and to a lesser extent, GABA and glutamine) disturbances in severe, treatment-seeking individuals with AUD, it is unclear whether and to what degree these disturbances are also present in less-severe, treatment-naïve individuals with AUD. The present study was designed to inform this gap in the extant literature by focusing on non-severe, relatively-young (i.e., 21–40 years old), non-treatment-seeking individuals with AUD. The present study extends Prisciandaro and colleagues (2016), which reported a significant inverse correlation between glutamate and recent heavy drinking in treatment-naïve individuals with AUD, through the addition of a demographically-matched light-drinking (LD) comparator group.
METHOD
Overview
The present study used the same methodology, as well as the same sample of AUD participants (with the exception of one additional participant that was added to the present sample), as Prisciandaro and colleagues (2016) while recruitment of the comparator light drinker sample temporally overlapped with recruitment of AUD sample, using the same scanner and personnel.
Participants
Twenty individuals with AUD and 20 demographically-matched light drinkers were recruited from community advertisements. All participants were required to be between the ages of 21 and 40. AUD individuals were also required to meet criteria for DSM-IV diagnostic criteria for alcohol dependence (including the “loss of control over drinking” and/or “inability to cut-down or stop drinking” criteria), to report consuming at least 20 total drinks per week, with at least one heavy drinking day (i.e., ≥ 5/4 drinks in a day for men/women) per week, in each of the two weeks preceding the study, and to not be actively seeking AUD treatment. Light-drinking individuals were required to not meet DSM-IV diagnostic criteria for alcohol dependence, to report drinking fewer than 14 drinks (7 for women) in each of the two weeks preceding the study, and to have a negative result (i.e., ≤ 1.6 IU) for disialo carbohydrate-deficient transferrin (%dCDT), a biomarker for recent (past 2-week) heavy drinking (Helander et al., 2016). For both groups, exclusion criteria included current DSM-IV Axis I disorder other than alcohol dependence or nicotine dependence, positive urine drug or alcohol breath screens on the day of the scan, and/or history of severe alcohol withdrawal (seizure, delirium tremens, need for inpatient or outpatient detoxification), or current alcohol withdrawal symptoms (i.e., Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised [CIWA-Ar; (Sullivan et al., 1989)] > 3). Additional MRI-related exclusion criteria included presence of non-MRI safe objects in the body, claustrophobia, history of traumatic brain injury, or pregnancy.
Procedure
Demographic and diagnostic (via the Structured Clinical Interview for DSM-IV; (First, 1998)) information, along with the Alcohol Dependence Scale (Skinner and Horn, 1984), was collected. For the current study, participants were administered a breathalyzer, urine drug screen, pregnancy test (females), and 90-day Time-Line Follow-Back interview on the day of the scan. Participants completed an MRI scan on a 3T Siemens TIM Trio including a two-dimensional j-resolved proton MRS acquisition (2D JPRESS; TR/TE=2400/31–229ms; ΔTE=2ms; 4 signal averages per TE step with online averaging; 2D spectral width=2000×500 Hz; 2D matrix size=2048×100; total acquisition time for 100 TE steps=13:28 min (Prescot and Renshaw, 2013)) from a 25×25×30mm3 voxel in the dorsal anterior cingulate cortex (dACC; as per Hermann et al., 2012); see Figure 1 for a sample voxel placement. The dACC was chosen, given its demonstrated role in brain response to alcohol cues, relapse to heavy alcohol drinking, and core neurobehavioral deficits associated with chronic alcoholism (e.g., loss of cognitive control), and because the majority of previous 1H-MRS studies of AUD have utilized this region (Schacht et al., 2013; Luijten et al., 2014; Zakiniaeiz et al., 2017). Water unsuppressed 2D 1H-MRS data were also acquired from the dACC voxel with 1 signal averages recorded for each TE step (total acquisition time for 100 TE steps=3:28 min). A structural scan (magnetization prepared rapid gradient echo) was acquired immediately before 2D JPRESS in order to facilitate voxel placement and tissue segmentation (TR/TE=1900/2.26ms; FOV=256mm2; flip angle=9°; spatial resolution=1.0 × 1.0 × 1.0 mm).
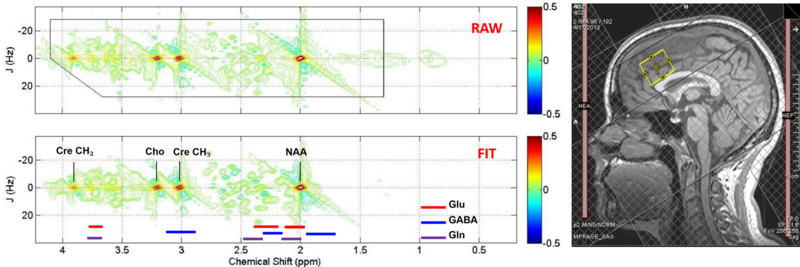
Figure 1.
Sample ACC voxel location (right) and raw (top) and fitted (bottom) two-dimensional j-resolved 1H-MRS spectra analyzed using Prior Knowledge Fitting (ProFit). Colored horizontal bars represent approximate primary spectral locations of Glu, GABA, and Gln. Gln=glutamine, Glu=glutamate, GABA=gamma-aminobutyric acid, NAA=n-acetyl aspartate, Cre=creatine, Cho=choline.
1H-MRS Post-processing
Skull stripping and whole brain tissue-type segmentation were performed on MP-RAGE images using the FSL BET and FAST tools (Smith et al., 2004). In-house MATLAB functions were used to extract the 3D volume corresponding to the positioned MRS voxel to obtain within-voxel gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) tissue content for each subject. Eddy currents and residual water were removed using in-house MATLAB functions. Subsequently, the ProFit algorithm was applied using software-supplied 2D basis sets (Schulte and Boesiger, 2006). Prior to Fourier transformation, the raw 2D matrix was zero-filled. Cramer-Rao Lower Bound (CRLB) values, which reflect the uncertainty of estimated model parameters, were provided by the ProFit software. Estimated metabolite peak areas were normalized to the unsuppressed water signal. Finally, metabolite/water ratios were corrected for within-voxel CSF fraction (Prescot and Renshaw, 2013).
Analytic Plan
Following characterization of demographic and clinical variables between groups, separate general linear models were evaluated for glutamate, glutamine, and GABA, with group (AUD vs. LD) included as the sole modeled between-subjects factor. Although our hypotheses concerned glutamate, glutamine, and GABA, we estimated supplementary models for the remaining estimable metabolites (NAA, choline, creatine, and myo-inositol) for completeness. Within-voxel GM tissue fraction, expressed as the ratio of gray matter to brain matter (i.e., GM + WM), was entered as a covariate in each model to adjust coefficients for individual differences in within-voxel tissue composition. Following these analyses, correlations were estimated between metabolite levels and TLFB-derived drinking variables (e.g., number of drinking days, number of heavy drinking days; past 14-day recall window). Alpha was set conservatively (i.e., given non-independence of dependent variables, mean r = 0.40) to p = 0.017 for primary comparisons of interest (i.e., between-group differences in glutamate, glutamine, and GABA; p = .05/3). Alpha was set to p < 0.05, uncorrected, for all additional analyses.
RESULTS
See Table 1 for demographic and alcohol use characteristics of the sample. AUD and LD participants did not significantly differ on any demographic variable, but, as expected, differed significantly on alcohol use characteristics (i.e., ADS scores, number of heavy drinking days, and drinks per drinking day, but not number of days since last drinking day). AUD participants reported an average of 2.45 (SD = 1.23, Range = ≤1–5) days since their last drinking day. CRLBs for metabolite concentration estimates were < 20% (M glutamate CRLB = 1.44%, M GABA CRLB = 6.56%, M glutamine CRLB = 6.36%), with the exception of one AUD participant with a glutamine CRLB of 21.58%. Raw, Voigt-fit water integrals did not differ between AUD and light drinker groups (t=1.41, p=0.166). There was a significant difference in average within-voxel GM to brain matter tissue fraction (i.e., GM / [GM / WM]) between AUD (61.08%) and LD (64.59%; t [38] = 4.49, p < 0.001).
Table 1.
Demographic and alcohol use characteristics of the sample (n = 40 [20 per group])
| Alcohol Use Disorder | Light Drinker | ||
|---|---|---|---|
| Variable | No. (%) of participants / Mean (SD) | No. (%) of participants / Mean (SD) | p |
| Gender (male) | 15 (75.0%) | 11 (65.0%) | 0.185 |
| Smoking Status (non-smoker) | 19 (95.0%) | 20 (100.0%) | 0.311 |
| Race (Caucasian) | 17 (85.0%) | 15 (75.0%) | 0.346 |
| Age | 26.75 (6.24) | 24.25 (3.19) | 0.122 |
| Education (# of years) | 14.35 (1.93) | 15.25 (1.21) | 0.085 |
| Alcohol Dependence Scale | 9.75 (4.60) | 3.10 (3.19) | <0.001 |
| # Heavy drinking days (past 14) | 4.75 (3.26) | 0.47 (0.77) | <0.001 |
| Drinks per drinking day | 7.22 (3.43) | 2.42 (1.70) | <0.001 |
| Days since last drink | 2.45 (1.23) | 5.20 (7.62) | 0.127 |
Note: “smoker” was defined as an individual who reports smoking ≥ 10 cigarettes/day. Significant p values (i.e., p < 0.05) have been bolded.
See Table 2 for general linear model results. GM to brain matter proportion was not significantly associated with metabolite concentrations in any of the general linear models (mean p = 0.35). Although not significantly different between groups, there was a trend towards differences in education between AUD and LD participants (p=0.085), so we additionally evaluated education as a potential covariate across models. Education was not significantly associated with metabolite concentrations in any of the general linear models (mean p = 0.81). Final models were estimated without GM to brain matter proportion or education included as covariates, and results did not change substantively from their removal. As detailed in Table 2, levels of GABA and glutamine, but not other metabolites, were significantly different between AUD and LD participants. Specifically, GABA (Cohen’s D = 0.79) and glutamine levels (Cohen’s D = 1.12) were significantly lower in AUD relative to LD participants.
Table 2.
Results from general linear models with dorsal anterior cingulate neurometabolite concentrations examined by group (i.e., Alcohol Use Disorder [AUD] vs. Light Drinker [LD]).
| M (SD) | Cohen’s D | F | p | ||
|---|---|---|---|---|---|
| Metabolite/water | AUD | LD | |||
| Glutamate | 4.13 (0.47) | 4.16 (0.70) | 0.05 | 0.02 | 0.893 |
| GABA | 0.52 (0.06) | 0.59 (0.11) | 0.79 | 6.25 | < 0.017 |
| Glutamine | 0.81 (0.18) | 0.99 (0.21) | 1.12 | 8.79 | 0.005 |
| N-acetylaspartate | 4.86 (0.61) | 4.94 (0.94) | 0.10 | 0.10 | 0.758 |
| Choline | 1.09 (0.12) | 1.01 (0.19) | 0.50 | 2.29 | 0.138 |
| Creatine | 3.60 (0.36) | 3.40 (0.67) | 0.37 | 1.37 | 0.249 |
| Myo-Inositol | 2.85 (0.41) | 2.80 (0.60) | 0.10 | 0.08 | 0.777 |
Note: Significant p values (i.e., p < 0.017) have been bolded.
As reported in Prisciandaro and colleagues (2016), number of heavy drinking days in the preceding 14 days was significantly inversely associated with glutamate (r = −0.55, p = 0.012) and NAA concentrations (r = −0.51, p = 0.022) in AUD participants (coefficients and p-values updated here to reflect the present, n+1, AUD sample). Conversely, neither number of heavy drinking days (mean p = 0.47, all correlations negative) nor number of drinking days (mean p = 0.56, all correlations negative) was significantly associated with concentrations of any of the measured metabolites in LD participants.
DISCUSSION
The present study demonstrated significantly lower dACC GABA and glutamine levels in actively-drinking, treatment-naïve individuals with AUD relative to demographically-matched light drinkers (LD), with each group reporting an average of approximately 2.5 days since their last drink. As published in Prisciandaro and colleagues (2016), dACC glutamate, but not GABA or glutamine, levels were inversely correlated with number of recent heavy drinking days in the present sample of AUD individuals. As an extension of that study, the present study found no correlation between recent drinking and levels of any of the measured neurometabolites in LD.
Most prior 1H-MRS studies of AUD have focused on more severe, treatment-seeking individuals reporting >1 week of abstinence from alcohol, and have found decreased levels of prefrontal glutamate relative to controls. Investigations of individuals with < 1 week of abstinence have been relatively rare, presumably due to the physiological instability and need for GABA/glutamate-altering (e.g., benzodiazepine) treatments associated with acute alcohol withdrawal. Indeed, the only published 1H-MRS study of acute alcohol withdrawal in AUD demonstrated large, transient elevations in prefrontal glutamate that would be expected to accompany alcohol withdrawal symptoms (e.g., autonomic hyperarousal; Hermann et al 2012). In contrast, by focusing on lower severity, frequent heavy episodic drinkers, two additional 1H-MRS studies have characterized glutamate and GABA levels, respectively, in drinkers with < 1 week of abstinence who did not have clinically significant alcohol withdrawal symptoms. Consistent with the present study, Silveri and colleagues (2014) reported lower levels of dorsal ACC GABA in 18–24-year-old binge drinkers relative to light drinkers, along with associations between ACC GABA and cognitive measures of response inhibition and attention; there were no between group differences in ACC glutamate. Demonstration of significantly lower ACC GABA levels in recently-drinking AUD individuals is consistent with the well-known compensatory downregulation of GABAA receptors following prolonged ethanol exposure, as well as results from a study that found decreased occipital GABA levels in response to an acute intravenous alcohol challenge 1H-MRS (Gomez et al., 2012). As reported by Silveri and colleagues, low ACC GABA levels in this population are particularly concerning as they have been linked with greater alcohol use consequences and worse response inhibition and mental flexibility in chronic heavy drinkers (Silveri et al., 2014).
In contrast to the absence of observed differences in prefrontal glutamate levels between AUD and LD from the present study, Ende and colleagues (2013) found that heavy drinkers who reported loss-of-control over their drinking had significantly lower levels of frontal white matter glutamate than did heavy drinkers reporting no loss of control or light drinkers. Several factors may account for differences between Ende and colleagues’ (2013) results and the present study. First, participants in Ende and colleagues’ (2013) study were substantially older than were participants in the present study (M age = 49 years v. 27 years). Furthermore, the heavy-drinking participants in Ende and colleagues’ (2013) sample reported drinking, on average, far more heavily than the AUD participants in the present study (i.e., approximately 75% of recent days classified as heavy drinking days versus approximately 35% in the present study). This speculative explanation is consistent with Prisciandaro and colleagues’ (2016) finding that AUD participants with a greater number of recent heavy drinking days had significantly lower levels of ACC glutamate relative to participants with fewer recent heavy drinking days.
Neither Silveri and colleagues (2014) nor Ende and colleagues (2013) reported participants’ brain glutamine levels, as reliably measuring glutamine in-vivo requires specialized 1H-MRS acquisition sequences, such as the 2D J-PRESS sequence used in the present study. Nonetheless, one previous study reported significantly higher dACC levels of glutamine, in a combined sample of AUD individuals with active (n=7) or remitted (n=6) drinking (many of whom were diagnosed with a variety of psychiatric disorders) relative to controls, using a standard PRESS acquisition sequence (Thoma et al., 2011). In contrast, the present study found evidence for significantly lower levels of dACC glutamine in AUD relative to LD participants. Given that glutamine acts as a metabolic precursor to both glutamate and GABA, the present study’s finding of significantly lower glutamine levels is consistent with its corollary finding of significantly lower GABA levels in AUD, as well as lower glutamate levels in AUD reporting a greater number of recent heavy drinking days. Differences in 1H-MRS acquisition may account for the difference between these findings and Thoma and colleagues’ (2011) results.
Due to the cross-sectional design of the present study, and most of the associated literature, what is most needed at this time are longitudinal studies designed to elucidate the interrelated dynamics of brain glutamate, GABA, and glutamine levels in individuals with AUD. Given the dearth of longitudinal 1H-MRS studies in individuals with AUD, it remains controversial whether the oft-observed neurochemical disturbances result from the acute neurotoxic consequences of, and/or neuroadaptive response to, heavy drinking on the brain and, if so, to what degree these disturbances normalize across periods of abstinence. Since evaluation of neurometabolism in non-abstinent individuals with severe AUD is greatly complicated by the acute, excitotoxic effects of alcohol withdrawal, or alternatively the acute, inhibitory effects of alcohol consumption/intoxication, it may prove fruitful to evaluate trajectories of neurochemical disturbances in non-abstinent individuals with less-severe AUD, who do not experience clinically-significant alcohol withdrawal symptoms upon ceasing alcohol intake. In any case, it is very important that 1H-MRS studies of AUD provide detailed descriptions of recent drinking levels and abstinence times in order to compare results across studies.
The results of the present study should be interpreted in light of several limitations. First, the study included a relatively modest-sized sample of AUD participants. Results should therefore be considered tentative until replicated. Second, the recent drinking variable relied entirely on subjective self-report; supplementation of subjective drinking data with objective biomarkers of recent alcohol consumption would strengthen confidence in our findings. Third, the present analytic methods available for our 2D J-PRESS 1H-MRS sequence did not allow for separation of co-edited macromolecules from the observed GABA signal. Fourth, because assessment for the present study was limited and the sample was selected to be homogeneous on a number of key alcohol diagnostic and drinking variables, we were not able to evaluate correlations between metabolite concentrations and additional clinically meaningful variables (e.g., age of onset). Fifth, as noted above, the cross-sectional design of the present study substantially limited the potential interpretability of findings. Sixth, the present study was not adequately powered to examine potential sex differences and did not include assessment of menstrual cycle phase in females. This is potentially problematic because females with a natural menstrual cycle (e.g., who are not taking hormonal contraceptives) have been shown to have small but significant variation in prefrontal GABA levels across menstrual cycle phases (De Bondt et al., 2015). Seventh, we cannot comment on the regional specificity of our findings given that we did not acquire data from brain regions other than ACC. Finally, we were unable to examine potential associations between blackout history and metabolite concentrations in AUD participants.
These limitations notwithstanding, the present study extends the literature by demonstrating significantly lower dorsal ACC GABA and glutamine levels in a sample of relatively young and less severe, non-treatment-seeking individuals with AUD. If these results are replicated, this could lead to enhanced knowledge of how the GABA and glutamate systems change with drinking and could lead to pharmacological interventional/preventative strategies, as well as allowing identification of groups at risk for accelerated AUD symptoms based on genetic or other demographic/diagnostic differences. The ability of MRS techniques to link brain neurochemistry to knowledge in other areas warrants further exploration.
ACKNOWLEDGMENTS
Funding Sources: This research was supported by NIAAA P50 AA010761 (PI: Becker) and K05 AA017435 (PI: Anton). Dr. Prisciandaro was supported by NIAAA K23 AA020842. The authors have no conflicts of interest to report.
REFERENCES
- ABE C, MON A, DURAZZO TC, PENNINGTON DL, SCHMIDT TP & MEYERHOFF DJ 2013. Polysubstance and alcohol dependence: unique abnormalities of magnetic resonance-derived brain metabolite levels. Drug Alcohol Depend, 130, 30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAK LK, SCHOUSBOE A & WAAGEPETERSEN HS 2006. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem, 98, 641–53. [DOI] [PubMed] [Google Scholar]
- BAUER J, PEDERSEN A, SCHERBAUM N, BENING J, PATSCHKE J, KUGEL H, HEINDEL W, AROLT V & OHRMANN P 2013. Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology, 38, 1401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEHAR KL, ROTHMAN DL, PETERSEN KF, HOOTEN M, DELANEY R, PETROFF OA, SHULMAN GI, NAVARRO V, PETRAKIS IL, CHARNEY DS & KRYSTAL JH 1999. Preliminary evidence of low cortical GABA levels in localized 1H-MR spectra of alcohol-dependent and hepatic encephalopathy patients. Am J Psychiatry, 156, 952–4. [DOI] [PubMed] [Google Scholar]
- DE BONDT T, DE BELDER F, VANHEVEL F, JACQUEMYN Y, & PARIZEL PM 2015. Prefrontal GABA concentration changes in women – Influence of menstrual cycle phase, hormonal contraceptive use, and correlation with premenstrual symptoms. Brain Research, 1597, 129–38. [DOI] [PubMed] [Google Scholar]
- ENDE G, HERMANN D, DEMIRAKCA T, HOERST M, TUNC-SKARKA N, WEBER-FAHR W, WICHERT S, RABINSTEIN J, FRISCHKNECHT U, MANN K & VOLLSTADT-KLEIN S 2013. Loss of control of alcohol use and severity of alcohol dependence in non-treatment-seeking heavy drinkers are related to lower glutamate in frontal white matter. Alcohol Clin Exp Res, 37, 1643–9. [DOI] [PubMed] [Google Scholar]
- FIRST MB 1998. Structured clinical interview for DSM-IV axis I disorders : patient edition (February 1996 final), SCID-I/P, New York, N.Y., Biometrics Research Dept., New York State Psychiatric Institute. [Google Scholar]
- GASS JT & OLIVE MF 2008. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol, 75, 218–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOMEZ R, BEHAR KL, WATZL J, WEINZIMER SA, GULANSKI B, SANACORA G, KORETSKI J, GUIDONE E, JIANG L, PETRAKIS IL, PITTMAN B, KRYSTAL JH & MASON GF 2012. Intravenous ethanol infusion decreases human cortical gamma-aminobutyric acid and N-acetylaspartate as measured with proton magnetic resonance spectroscopy at 4 tesla. Biol Psychiatry, 71, 239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELANDER A, WIELDERS J, ANTON R, ARNDT T, BIANCHI V, DEENMAMODE J, JEPPSSON JO, WHITFIELD JB, WEYKAMP C & SCHELLENBERG F 2016. Standardisation and use of the alcohol biomarker carbohydrate-deficient transferrin (CDT). Clin Chim Acta, 459, 19–24. [DOI] [PubMed] [Google Scholar]
- HERMANN D, WEBER-FAHR W, SARTORIUS A, HOERST M, FRISCHKNECHT U, TUNC-SKARKA N, PERREAU-LENZ S, HANSSON AC, KRUMM B, KIEFER F, SPANAGEL R, MANN K, ENDE G & SOMMER WH 2012. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol Psychiatry, 71, 1015–21. [DOI] [PubMed] [Google Scholar]
- KRYSTAL JH, STALEY J, MASON G, PETRAKIS IL, KAUFMAN J, HARRIS RA, GELERNTER J & LAPPALAINEN J 2006. Gamma-aminobutyric acid type A receptors and alcoholism: intoxication, dependence, vulnerability, and treatment. Arch Gen Psychiatry, 63, 957–68. [DOI] [PubMed] [Google Scholar]
- LEE DW, KIM SY, LEE T, NAM YK, JU A, WOO DC, YOU SJ, HAN JS, LEE SH, CHOI CB, KIM SS, SHIN HC, KIM HY, KIM DJ, RHIM HS & CHOE BY 2012. Ex vivo detection for chronic ethanol consumption-induced neurochemical changes in rats. Brain Res, 1429, 134–44. [DOI] [PubMed] [Google Scholar]
- LUIJTEN M, MACHIELSEN MWJ, VELTMAN DJ, HESTER R, DE HAAN L & FRANKEN IHA 2014. Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. J Psychiatry Neurosci, 39, 149–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASON GF, PETRAKIS IL, DE GRAAF RA, GUEORGUIEVA R, GUIDONE E, CORIC V, EPPERSON CN, ROTHMAN DL & KRYSTAL JH 2006. Cortical gamma-aminobutyric acid levels and the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biol Psychiatry, 59, 85–93. [DOI] [PubMed] [Google Scholar]
- MON A, DURAZZO TC & MEYERHOFF DJ 2012. Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Depend, 125, 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA. 2006. Focus on Young Adult Drinking. Alcohol Alert [Online]. [Google Scholar]
- PRESCOT AP & RENSHAW PF 2013. Two-dimensional J-resolved proton MR spectroscopy and prior knowledge fitting (ProFit) in the frontal and parietal lobes of healthy volunteers: assessment of metabolite discrimination and general reproducibility. J Magn Reson Imaging, 37, 642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRISCIANDARO JJ, SCHACHT JP, PRESCOT AP, RENSHAW PF, BROWN TR & ANTON RF 2016. Associations Between Recent Heavy Drinking and Dorsal Anterior Cingulate N-Acetylaspartate and Glutamate Concentrations in Non-Treatment-Seeking Individuals with Alcohol Dependence. Alcohol Clin Exp Res, 40, 491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHACHT JP, ANTON RF & MYRICK H (2013). Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol, 18, 121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTE RF & BOESIGER P 2006. ProFit: two-dimensional prior-knowledge fitting of J-resolved spectra. NMR Biomed, 19, 255–63. [DOI] [PubMed] [Google Scholar]
- SILVERI MM, COHEN-GILBERT J, CROWLEY DJ, ROSSO IM, JENSEN JE & SNEIDER JT 2014. Altered anterior cingulate neurochemistry in emerging adult binge drinkers with a history of alcohol-induced blackouts. Alcohol Clin Exp Res, 38, 969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKINNER HA & HORN JL 1984. The Alcohol Dependence Scale (ADS) User’s Guide. Toronto, Canada: Addiction Research Foundation. [Google Scholar]
- SMITH SM, JENKINSON M, WOOLRICH MW, BECKMANN CF, BEHRENS TE, JOHANSEN-BERG H, BANNISTER PR, DE LUCA M, DROBNJAK I, FLITNEY DE, NIAZY RK, SAUNDERS J, VICKERS J, ZHANG Y, DE STEFANO N, BRADY JM & MATTHEWS PM 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23 Suppl 1, S208–19. [DOI] [PubMed] [Google Scholar]
- SULLIVAN JT, SYKORA K, SCHNEIDERMAN J, NARANJO CA & SELLERS EM 1989. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict, 84, 1353–7. [DOI] [PubMed] [Google Scholar]
- THOMA R, MULLINS P, RUHL D, MONNIG M, YEO RA, CAPRIHAN A, BOGENSCHUTZ M, LYSNE P, TONIGAN S, KALYANAM R & GASPAROVIC C 2011. Perturbation of the glutamate-glutamine system in alcohol dependence and remission. Neuropsychopharmacology, 36, 1359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YEO RA, THOMA RJ, GASPAROVIC C, MONNIG M, HARLAAR N, CALHOUN VD, KALYANAM R, MAYER AR, DURAZZO TC & HUTCHISON KE 2013. Neurometabolite concentration and clinical features of chronic alcohol use: a proton magnetic resonance spectroscopy study. Psychiatry Res, 211, 141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAKINIAEIZ Y, SCHEINOST D, SEO D, SINHA R, & CONSTABLE T 2017. Cingulate cortex functional connectivity predicts future relapse in alcohol dependent individuals. Neuroimage: Clinical, 13, 181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]