Introduction
Idiopathic acquired aplastic anemia (aAA) is a rare, life threatening bone marrow failure syndrome characterized by cytopenias and a hypocellular bone marrow. Aplastic anemia (AA) is classified as either inherited or acquired. Inherited bone marrow failure syndromes (IBMFS) are rare genetic diseases that are well characterized clinically and usually associated with an identified germline mutation. They typically present in childhood, though they can be diagnosed later in life and are associated with an increased risk of developing malignancy. AA is considered acquired (aAA) if no inherited syndrome is identified. The incidence of aAA is low, ranging from 2 to 14 per million per year, with higher incidences reported in Asia than Europe 1–3. There is a bimodal age of peak presentation, with the first occurring in patients aged 15–25 years and the second in patients over 60 years old4. The prognosis for severe or very severe aAA with supportive care only is dismal, with mortality rates exceeding 80% at two years5. Overall survival rates at 2 years after treatment with immune suppressive therapy (IST) with cyclosporine and ATG are typically greater than 80%6,7. However, event free survival, with events defined as death or requirement for a subsequent therapy, decreases over time and is as low as 58% at 60 months in a recent pediatric study8.
The pathophysiology of aAA is unknown, though the most favored model is that of a dysregulated immune system leading to autoreactive T cell destruction of hematopoietic stem and progenitor cells (HSPC) in a genetically susceptible host. In addition to the immune component, HSPC intrinsic defects and bone marrow microenvironment dysfunction are thought to contribute to the disease process. Here we summarize the current concepts regarding the pathophysiology of aAA and propose that destruction of HSPCs by a combination of intrinsic HSPC genetic defects and an inappropriately activated immune response to viral infection is the major driver of aAA.
Dysregulated Immune System
An immunologic mechanism in aAA was suspected when lymphocyte infusions in mice resulted in a hypocellular bone marrow9. It was further supported in humans when identical twin bone marrow transplantation without conditioning was initially unsuccessful in 15 of 23 patients, but hematopoiesis was restored with conditioning and repeat transplantation10. And finally, this model is largely accepted given favorable responses to immune suppressive therapy (IST) with equine antithymocyte globulin (ATG) and cyclosporine, two entirely nonspecific agents11.
Destruction or dysfunction of HPSC by activated cytotoxic T cells in the setting of infection, drug, or another unidentified environmental trigger is thought to occur via recognition of an auto-antigen(s) presented via class I or II HLA molecules11. However, there is no definitive evidence of this mechanism as an inciting auto antigen has not been identified. Furthermore, there have been very few demonstrations of HSPC specific in-vivo cytotoxic T cell (CTL) repertoires. There are many studies delineating immunologic changes seen at diagnosis and during treatment. It’s unclear if these immunologic changes result in the disease process of aAA, or are secondary to other concurrent, unidentified processes such as a preceding hepatitis, EBV, or other viral infection.
T cells
A number of studies have demonstrated increased percentages of activated CD8+ cytotoxic T cells in patients with AA in both peripheral blood and bone marrow8–10. In vitro co-culture of CD8+ T cells from untreated AA patients enhances apoptosis of CD3- bone marrow cells from normal individuals12 and inhibits colony formation of CD34+ cells13. Putative HSPC death from T cells expanded in-vivo has been demonstrated in two patients. In one patient, CD8+ cells induced cell death in 75% of autologous bone marrow mononuclear cells14, and in the second patient, a CD4+ T cell clone demonstrated cytotoxicity against autologous CD34+ cells in an HLA-DRB1 restricted manner13.
Abnormalities in the number and/or function of all types of CD4+ cells are reported, with increased Th1 and Th2 helper cells, and decreased Treg cells15. T regulatory cells play an important role in suppressing autoreactive T cells, and are thus believed to influence autoimmune diseases. Treg cells present in aAA are functionally impaired and unable to suppress normal effector T cells15,16. A reduction in the number of Treg cells in aAA correlates with disease severity, and conversely increased numbers of Treg cells predict better response to IST15,17. Since Treg depletion and dysfunction are regularly observed following viral infections18 and viral infection often precedes aAA, the Treg findings in aAA are of unclear pathophysiological significance. However, in general Treg cells tend to be depressed in autoimmunity.
A subset of patients with aAA at diagnosis have restricted CD8+ and CD4+ cytotoxic cell receptor diversity, ie., oligoclonal expansion identified by flow cytometry analysis for T cell receptor (TCR) subfamilies14,19–21. The presence and degree of oligoclonal T-cell expansion predicts response to IST, with strongly skewed TCR repertoires predicting good or partial response to IST in children20. Oligoclonal expansion is also quantitatively related to the disease course. Patients with good response to IST typically have waning or resolution of their immune dominant clones and restoration of TCR variability22. At the time of relapse, patients may have recurrence of original clones or expansion, perhaps indicating further putative antigen presentation23.
Limited heterogeneity of the T cell repertoire in aAA supports the hypothesis that an antigen (presumably on an HSPC) may be driving a pathologic lymphocyte response. However, controls derived from patients with recent viral infections are needed to determine the clinical significance of any these findings, as T cell clonal expansion is characteristic of such infections24. Furthermore in-vitro growth of T-cells may artificially skew representation of clones, and perfectly healthy individuals of all ages may exhibit skewing and nonpathologic expansion of T cell clones24.
Myelosuppressive Cytokines
T cells likely play a role in the pathogenesis of AA via the release of myelosuppressive cytokines. Elevated interferon gamma and TNF alpha levels are found in the serum and bone marrow of aAA patients25,26. INF-gamma alone inhibits murine myeloid progenitors and their differentiation and leads to aplasia27. TNF alpha and INF-gamma induce HSPC death via the Fas/FasL pathway28 and TRAIL expression29. In mice, anti- INF-gamma antibody can partially rescue hematopoiesis in a model of infusion induced bone marrow failure30. Thus, T cells, activated by either autoimmune response or viral infection may cause aplasia, particularly in patients with mutated HPSCs or overactive immune cells (vide infra).
Immune Cell dysregulation
That viral infection or other insult may lead to an inappropriate immune response in certain patients is illustrated by hemophagocytic lymphohistiocytosis (HLH). Mutations in the perforin gene, PRF1, cause some forms of familial HLH, and heterozygous mutations have been reported in aAA31. Polymorphisms in TNF2, the gene forTNF-alpha32,33, INF-gamma 34 and IL-6 genes 33 result in excessive myleosuppresive cytokine gene expression and are associated with an increased immune response and reported in aAA.
HLA genes
A number of studies have reported the epidemiologic association between certain class I and class II HLA alleles and increased or decreased risk of aAA. These studies are limited by effect size and reporting of statistically significant data that may have little clinical relevance. A recent meta-analysis demonstrated an increased risk of aAA associated with HLA-A and HLA-DRB1 polymorphisms and protective effect of other HLA-DRB polymorphisms35. HLA-DRB1* 1501 predicts response to cyclosporine therapy36. HLA allelic variations are hypothesized to be involved in the pathogenesis of aplastic anemia by two mechanisms; activation of autoreactive T cells and failure to protect with decreased production of auto regulatory (Treg) cells37. However, the mechanistic link of HLA alleles and AA remains unclear.
Antibodies
As mentioned above, T-cells, not antibodies, are the usual suspects as the inciters of an autoimmune mechanism of aAA. Therapies including plasmapheresis and anti-CD20 antibodies have been fruitlessly attempted and are rarely effective. While an autoantigen has not been identified in aAA, multiple antibodies have been observed. Their clinical significance is ill defined.
Kinectin is an antigen widely expressed and present in all hematopoietic cell lines. Kinectin antibodies are present in many patients with AA and in vitro are capable of suppressing granulocyte-macrophage colony forming units (CFU-GM). However, anti-kinectin focused T cells were not identified in these patients38. Anti-moesin antibodies are also found in patients with AA. In vitro, they stimulate peripheral mononuclear cells to secrete TNF alpha and IFN gamma. But, serum TNF-alpha levels are not influenced by anti-moesin antibody levels observed in aAA patients39. Other antibodies, including heterogenous nuclear ribonucleoprotein (hnRNP) antibodies are associated with good response to IST but their mechanistic link to aAA is unknown40.
Surprising lessons from clinical trials
Despite the immune dysfunctions outlined above, there remain some puzzling results of clinical trials. If cytotoxic T cells play a key role, one would expect further inhibition of T cells to result in improved clinical outcomes. However, Alemtuzumab, a humanized CD52 antibody that produces a more profound and durable lymphopenia than ATG, was unsuccessful as a single agent in treatment of naïve aAA patients. In fact, the response rate was so low in the clinical trial, that the arm was closed for safety concerns. In relapse or refractory settings, there were only modest response rates of ~30% to Alemtuzumab41. Rabbit ATG also more effectively depletes lymphocytes in vivo and is more cytotoxic on a weight basis42. But in a clinical trial, there was an inferior clinical response to rabbit ATG compared to horse ATG, 37% versus 68%, respectively43.
Perhaps horse ATG (which is really anti-human globulin) and cyclosporine (a drug with protean effects) exert changes beyond immune suppression and thereby may contribute to a clinical response in aAA by mechanisms that are not yet determined. For example, ATG stimulates CD34 dependent colony growth in normal, myelodysplastic, and aplastic anemia bone marrow44,45 and it reduces the expression of FAS on aplastic CD34 cells, an antigen that signals apoptosis44.
Stem Cell Abnormalities
There is evidence that groups of patients with aAA may have intrinsic abnormalities of their hematopoietic stem cells and progenitors. Hematopoietic stem and progenitor cells are measured immunophenotypically by the presence of CD34, and progenitors are assayed as myeloid colony-forming cells. Patients with aAA have a decreased number of immature hematopoietic cells at diagnosis, and those that are present demonstrate a poor plating efficiency for colony formation46,47. Decreased progenitor cells persist in many patients even when peripheral blood counts improve with treatment, perhaps suggesting an underlying stem cell abnormality47–49. Treatment of aplasia with simple infusion of stem cells from an identical twin donor without conditioning was successful in a small number of patients10,50, further supporting a primary stem cell etiology. Finally, there is a growing body of literature identifying germline mutations using next generation and whole exome sequencing in patients with a previously negative work up. This suggests that perhaps what is being called aAA, particularly in young people, may be a disease with an unidentified germline mutation in the hematopoietic stem cell.
Telomeres
About 1/3 of patients with AA have significantly short telomeres in their leukocytes51. Shorten telomeres are associated with poor response to IST51,52. Telomeres are structures that stabilize the ends of each chromosome to prevent excessive shortening with replication. Telomeres shorten until they reach a critical length when they signal cessation of division to prevent chromosomal rearrangements. Patients with mutations in the telomere complex (TERT or TERC) have increased chromosome end to end fusions and aneuploidy, suggesting that telomeres play an important role in the prevention of myelodysplasias and leukemias53,54.
Dyskeratosis congenita (DC) is an inherited bone marrow failure syndrome with genetic mutations that impair telomere length maintenance and is typically associated with other clinical features. A subset of patients with only aplastic anemia were found to harbor mutations in some of the same genes found in dyskeratosis congenita including TERC55,56 and TERT57. However, the majority of patients with aplastic anemia and short telomeres do not share these identifiable mutations. Thus, it is unclear whether telomeres are short secondary to increased stem cell turnover of a few remaining “over demanded” stem cells or if there are unidentified environmental, genetic or epigenetic modifiers causing erosion of telomeres, which then contribute to disease.
In addition to shortened telomeres, HSPCs from patients with aAA display downregulation of cell cycle check point genes including cyclin-dependent kinase 6 (CDK6), CDK2, MYB, MYC, and a Fanconi anemia (FA) complementation member (FANCG)37. Taken together, all of these changes in HSPCs in aAA may contribute to the inability of hematopoietic stem cells to compensate and replicate in the setting of insult.
Clonal Hematopoiesis
The detection of clonal hematopoiesis in over 50 percent of patients with aAA is perhaps the strongest indication that accumulated mutations play an important role in the disorder58–62. Early evidence suggesting a link between aAA, paroxysmal nocturnal hemoglobinuria (PNH), myelodysplastic syndrome (MDS) and AML was noted in the 1960s and has puzzled hematologists for some time63. High incidences of subsequent MDS or AML were noted prior to the use of IST, and are particularly evident after successful treatment with IST. Approximately 10% of patients with AA will later develop MDS or AML64, and as many as 25% will develop PNH65–67.
Cytogenetic abnormalities are reported in 4–11% of aAA68–71. However, given the difficulty of obtaining sufficient numbers of metaphases from a failing marrow, this could be an underestimate. Some common abnormalities are shared with myeloid malignancies including +8, −7, del(5q). Others, including +6 and +15 are rarely seen in the AML/MDS.
Somatic Mutations in AA
In a study of 156 patients by Yoshizato et al, 36% of patients with aAA had a somatic mutation; the most common was BCOR/BCORL172. Other clonal expansions of mutated cells fall in several large categories; paroxysmal nocturnal hemoglobinuria like cells or loss of glycophosphotiylinositol anchored proteins, loss of human leukocyte antigen alleles (6pLOH), and those commonly seen in myelodysplastic syndrome and/or myeloid malignancies.
PNH Like Cells
Paroxysmal nocturnal hemoglobinuria (PNH) is a bone marrow failure syndrome clinically characterized by acquired hemolytic anemia and thrombosis. Such patients have clonal expansion of cells derived from a HSPC carrying a somatic mutation in the PIGA gene. PIGA-mutated cells have defective cell surface expression of glcosylphosphatidylinositol (GPI)- anchored proteins including CD55 and CD59, making them vulnerable to complement mediated hemolysis. PNH and aAA are closely related with multiple reports of the complication of clinically relevant PNH in patients with aAA and vice versa.
Regardless of clinical manifestations of PNH, GPI deficient, “PNH like” cells are detectable in more than half of aAA patients when assessed by sensitive flow cytometry using antibodies to CD55 or CD59 or fluorescence aerolysin (FLAER) in aAA73–75. Clonal PNH expansion is strongly linked to HLA-DR276 and is a predictor of IST response77. This, in combination with an absence of these cell surface proteins in PNH has led to the hypothesis that the deficient cells have escaped immune destruction. However, there is no direct evidence supporting this particular mechanism. They may have survived for any one of many reasons.
6p Loss of Heterozygosity
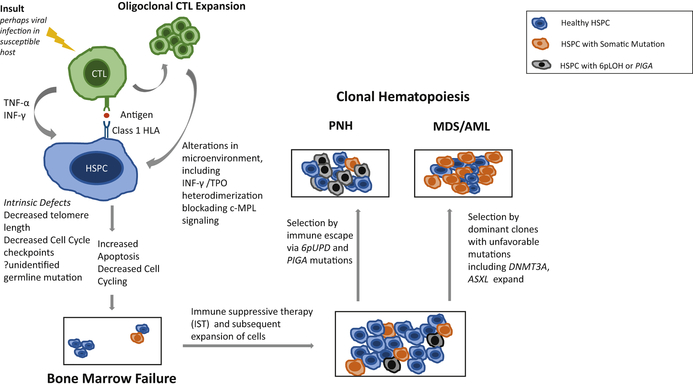
An acquired copy number neutral loss of heterozygosity has been identified in approximately 11–13% of patients with AA, usually involving the 6p locus and this finding is the second most common mutation detected in aAA36,78. Acquired 6pLOH is characteristic and relatively specific to aAA, as it is exceedingly rare in the general population (prevalence ~0.09%)36. It is also not commonly identified in other bone marrow failure syndromes or MDS. The 6pLOH involves the HLA locus, leading to the loss of expression of one HLA haplotype79. Missing HLA alleles were biased to particular alleles including HLA-A*02:01, A*02:06, A*31:01 ad B*40:0279. The hypothesis is that auto- antigens are expressed via these class I HLAs, and that HSPC loss of expression of this HLA via 6pLOH might allow escape from immune attack36,79. Thus, clones with PIGA and 6pLOH link clonality in aAA with the model of auto-directed CTL destruction of healthy HSPC (Figure 1).
Figure 1:
Pathophysiology of Acquired Aplastic Anemia (aAA), and the link between PNH, MDS and AML. An antigen is presented to a CTL via a Class 1 HLA molecule, and autoreactive T cells are activated, perhaps by a virus in a susceptible host, then begin to expand. T cells release cytokines including TNF-α and INF-γ, which have direct apoptotic effects on the HPSC, ultimately resulting in bone marrow failure. Clonal hematopoiesis may occur either in the setting of immune escape, as evidenced by PIG-A, which can subsequently lead to clinical PNH, or 6pLOH mutations. Alternatively, clonal hematopoiesis may occur via selection of dominant clones with somatic mutations that are identified in both the normal/aging population and those with aAA. Progressive proliferation of these clonal HPSC can then lead to progression of MDS/AML.
MDS/AML
Whole gene sequencing by Yoshizato et al demonstrated that mutations typically found in myeloid leukemias can be found in up to one third of patients with aAA72. DNMT3A and ASXL1 mutations are common to both aAA and MDS. In aAA, these clones tended to increase their clone size over years, although variability was high between individuals. Presence of these mutations is associated with faster progression to MDS/AML, shorter overall survival and poor response to IST. In contrast, PIGA and BCOR/BCORL1 mutations are underrepresented in MDS and AML compared to patients with aAA and clone sizes tend to stay stable or decrease over time. Compared to DNMT3A and ASXL2, these “favorable mutations” are associated with decreased mortality72. Of note, these data must be interpreted carefully, as somatic DNMT3A and ASXL1 are also found in healthy aged populations and are considered clonal hematopoiesis of indeterminate potential (CHIP)80.
These findings suggest a second theoretical model of aAA linking MDS and aAA. The immune surveillance controlling these abnormal clones may result in “bystander” destruction of HSPC. With IST, suppression of the malignant clone is removed. Then clone unchecked may ultimately lead to MDS/AML. Additionally, it’s possible that clonal hematopoiesis occurs in the setting of unidentified germline mutations as described below.
Germline Mutations
By definition, aAA may only be diagnosed after exclusion of an IBMFS through a detailed history, physical examination and functional assays for FA and DC or genetic testing for Shwachman Diamond syndrome and congenital amegakaryocytic thrombocytopenia (CAMT) to exclude these classic inherited syndromes. To more rigorously diagnose IBMFS and MDS, a single center study used next generation sequencing (NGS) targeted BMF/MDS gene capture on patients diagnosed with aAA who underwent stem cell transplantation from 1990–2012. Pathologic mutations in known BMF genes were identified in 5/98 (5.1%) of patients; 3/5 of these patient had no previous diagnosis, and family history and physical exam failed to distinguish them from other patients with aAA and no identified mutation81. In a more select population with suspected IBMFS, given family history, congenital abnormalities and young age but no previously identified mutation, whole exome sequencing identified pathologic mutations in 86/179 (48%) of patients82. Mutations included those well described in IBMFS as well as new mutations rarely described, including SAMD9/SAMD9L, MECOM/EVI1, and ERCC6L2. Mutations in these genes frequently had a distinct clinical phenotype; transient aplasia and monosomy 7 in SAMD9/SAMD9L, severe infantile aplastic anemia in MECOM/EVI1, and mild pancytopenia with myelodysplasia in ERCC6L2.
It is well known that stem cell depletion leads to aplasia and subsequent MDS/AML in IBMFS, just as it does is aAA. These studies as well as those summarized above support the idea that intrinsic abnormalities of hematopoietic stem cells and progenitors are a key component of aAA. Additionally, a cohort of patients diagnosed with aAA, particularly the young, may have unidentified germline mutations.
Alterations in Hematopoietic Stem Cell Environment
Mesenchymal stromal cells from AA patients are said to fail to form adherent layers in vitro and have reduced ability to sustain hematopoiesis as assessed by total cell proliferation83. Alterations in fibroblastic colony forming units and the stromal-derived cytokines ANG1, VEGF and VCAM-1 have also been reported84. Transforming growth factor (TGF-β) is a cytokine implicated in the regulation of hematopoietic stem cell cycling. In vitro and in vivo bone marrow stromal cells of AA express significantly less TGF- β than normal stroma85. While these alterations are provocative, they do not establish a clear link to the pathophysiology of AA.
Niches
Bone marrow niches are anatomically distinct spaces in the bone marrow that provide signals that maintain and support HSC. The osteoblastic niche is one of the best studied and is composed of osteoblasts in close proximity to quiescent HSC86. Destruction of osteoblasts in mice results in decreased HSC numbers and impaired hematopoiesis87. Examination of bone marrow samples in AA patients demonstrates fewer endosteal, vascular and perivascular cells indicating possible impairment of these niches in patients with aplastic anemia88.
Hematopoietic Growth Factors
Patients with AA have markedly elevated serum hematopoietic growth factors including erythropoietin (EPO), granulocyte colony stimulating factor (G-CSF), granulocyte macrophage colony stimulating factor (GM-CSF) and thrombopoietin (TPO)89,90. The use of hematopoietic growth factors in bone marrow failure syndromes has a long but frustrating history. Trials have included erythropoietin, GM-GCSF, G-CSF, interleukin 1, 3, and 6. They demonstrated no effect and except for GCSF, were associated with increased side effects91.
Thrombopoetin (TPO) is a potent endogenous cytokine that acts via the TPO-receptor, also known as MPL, to stimulate platelet production. MPL is present on HSPC and in vitro, TPO has been shown to play an important role in hematopoietic stem cell survival and expansion92,93,94. In vivo patients with MPL associated congenital amegakaryocytoic thrombocytopenia typically develop aplastic anemia early in life95,96.
Eltrombopag, a small molecule TPO mimetic, was studied initially as monotherapy97, and then in combination with ATG and cyclosporine. The addition of Eltrombopag improves response rates in aAA98. While Eltrombopag is clinically effective, the result gives little insight to the pathophysiology of AA. Eltrombopag binds MPL at a site distinct from that of endogenous TPO, so it may be exerting additional effects. In fact, recent unpublished work (Alvarado et al, ASH abstract 2017) indicates that INF-γ and TPO form heteromeric complexes that hinder binding to the MPL receptor at both high and low affinity99. Eltrombopag may evade this process, thus explaining its role on the HSPC and in aAA.
Concluding Remarks
aAA has a complex pathophysiology. Lymphocyte subsets are altered; however similar alterations are seen in viral infections. Functional assays demonstrate lymphocyte effects on the growth of HSPC, but very few published studies demonstrate T-cell clones expanded in-vivo with cytotoxic effects on HSPCs. Immune dysregulation likely occurs in patients with underlying genetic susceptibility in the setting of a second insult. Intrinsic deficits of the hematopoietic stem cell itself are also important in the disease process, as evidenced by shortened telomeres and somatic mutations ultimately leading to clonal hematopoiesis and increased risk of MDS and AML. The hematopoietic stem cell microenvironment may also contribute to disease. While immune suppressive therapy with equine ATG, cyclosporine and Eltrombopag have improved clinical outcomes, we have much to learn about the underlying mechanisms in acquired aplastic anemia and why these agents are effective. The potential role of upfront matched unrelated donor stem cell transplant as first-line treatment for aAA, particularly for younger patients, is currently under investigation.
Synopsis.
Idiopathic acquired aplastic anemia (aAA) is a rare, life threatening bone marrow failure syndrome characterized by cytopenias and a hypocellular bone marrow. The pathophysiology of aAA is unknown, though the most favored model is that of a dysregulated immune system leading to autoreactive T cell destruction of hematopoietic stem and progenitor cells (HSPC) in a genetically susceptible host. The authors review the literature and propose that the major driver of aAA is a combination of HSPC intrinsic defects and an inappropriately activated immune response in the setting of a viral infection. Alterations in bone marrow microenvironment may also contribute to the disease process.
Key Points.
The pathophysiology of acquired aplastic anemia (aAA) is unknown. The leading hypothesis is cytotoxic T cell destruction of hematopoietic stem cells, but no inciting auto-antigen has been identified.
There is evidence of immune dysregulation with abnormal lymphocyte subsets, including increased CD8+ cells, decreased T regulatory cells, and an expansion of Th1 and Th2 helper cells compared with normal individuals. Similar changes are observed in patients with autoimmune diseases and a viral infection.
The most striking evidence supporting the role of auto-reactive T cells in aAA is the presence of leukocytes with acquired copy number neutral (CNN) loss of heterozygosity (6pLOH) involving an HLA allele which might allow escape from immune attack.
Clonal hematopoiesis is common in aAA. The most frequent somatic mutations are BCOR/BCOR1, PIG-A and 6pLOH, followed by those shared with MDS/AML including DNMT3A and ASXL1.
That hematopoietic stem cells have other non-immune related genetic defects is further evidenced by shortened telomeres in leukocytes and recently described germline mutations in cohorts of patients with aAA.
While equine ATG, cyclosporine and eltrombopag have improved aAA clinical outcomes, we do not understand their mechanisms.
Acknowledgements
We thank Drs. Joseph Antin, Akiko Shimamura and Blanche Alter for their critical and constructive review of this manuscript.
Footnotes
disclosure statement
No disclosures from either author.
References
- 1.Incidence of aplastic anemia: the relevance of diagnostic criteria. By the International Agranulocytosis and Aplastic Anemia Study. Blood. 1987;70(6):1718 LP–1721. http://www.bloodjournal.org/content/70/6/1718.abstract. [PubMed] [Google Scholar]
- 2.McCahon E, Tang K, Rogers PC, McBride MLSK. The impact of Asian descent on the incidence of acquired severe aplastic anaemia in childrentle. Br J Haemotol. 2003;121(1):170–172. [DOI] [PubMed] [Google Scholar]
- 3.Issaragrisil S, Sriratanasatavorn C, Piankijagum A, et al. Incidence of aplastic anemia in Bangkok. The Aplastic Anemia Study Group. Blood. 1991;77(10):2166–2168. http://www.ncbi.nlm.nih.gov/pubmed/2029577. [PubMed] [Google Scholar]
- 4.Young NS, Kaufman DW. The epidemiology of acquired aplastic anemia. Haematologica. 2008;93(4):489 LP–492. http://www.haematologica.org/content/93/4/489.abstract. [DOI] [PubMed] [Google Scholar]
- 5.Brodsky RA, Jones RJ. Aplastic anaemia. 2005;365. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld S, Follmann D, Nunez O, NS Y. Antithymocyte globulin and cyclosporine for severe aplastic anemia: Association between hematologic response and long-term outcome. JAMA. 2003;289(9):1130–1135. 10.1001/jama.289.9.1130. [DOI] [PubMed] [Google Scholar]
- 7.Dufour C, Pillon M, Sociè G, et al. Outcome of aplastic anaemia in children. A study by the severe aplastic anaemia and paediatric disease working parties of the European group blood and bone marrow transplant. Br J Haematol. 2015;169(4):565–573. doi: 10.1111/bjh.13297. [DOI] [PubMed] [Google Scholar]
- 8.Rogers Z, Nakano TA Olson TS, Bertuch AA, Chawla A, Castillo P, Wang W, Gillio A, Nalepa GKP, Bennett CM, Hanna R, Gamper C, Kupfer GM, Rothman JA, Boklan J, Thompson AA, Geddis AE GB, Huang JN, Coates TD, Cada M, Walkovich KJ, Joshi S, Vlachos A, Weller EA, Williams DA and SA. Outcomes of Immunosuppressive Therapy for Pediatric Aplastic Anemia: A North American Pediatric Aplastic Anemia Consortium (NAPAAC) Study.; 2017.
- 9.Barnes DMR. Aplastic anaemia in sublethally irradiated mice given allogeneic lymph node cells. Br J Haematol. 1967;13(4):482–491. [DOI] [PubMed] [Google Scholar]
- 10.Hinterberger Wolfgang, Rowlings Philip A., Margareta Hinterberger-Fischer John Gibson, Jacobsen Niels,Klein John P., Kolb Hans-Jochem, Stevens Don A., Mary M. Horowitz RPG. Results of Transplanting Bone Marrow from Genetically Identical Twins into Patients with Aplastic Anemia. Ann Intern Med. 1997;126(2):116–122. [DOI] [PubMed] [Google Scholar]
- 11.Young NS, Calado RT, Scheinberg P. Review in translational hematology Current concepts in the pathophysiology and treatment of aplastic anemia. Bloodjournal. 2006;108(8):2509–2519. doi: 10.1182/blood-2006-03-010777.Supported. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing L, Liu C, Fu R, et al. CD8+HLA-DR+ T cells are increased in patients with severe aplastic anemia. Mol Med Rep. 2014;10(3):1252–1258. doi: 10.3892/mmr.2014.2344. [DOI] [PubMed] [Google Scholar]
- 13.Nakao S, Takami a, Takamatsu H, et al. Isolation of a T-cell clone showing HLA-DRB1*0405-restricted cytotoxicity for hematopoietic cells in a patient with aplastic anemia. Blood. 1997;89(10):3691–3699. http://www.ncbi.nlm.nih.gov/pubmed/9160674. [PubMed] [Google Scholar]
- 14.Risitano AM, Maciejewski JP, Green S, Plasilova M, Zeng W, Young NS. In-vivo dominant immune responses in aplastic anaemia: Molecular tracking of putatively pathogenetic T-cell clones by TCR β-CDR3 sequencing. Lancet. 2004;364(9431):355–364. doi: 10.1016/S0140-6736(04)16724-X. [DOI] [PubMed] [Google Scholar]
- 15.Kordasti S, Marsh J, Al-khan S, et al. Functional characterization of CD4 + T cells in aplastic anemia. 2012;119(9):2033–2043. doi: 10.1182/blood-2011-08-368308. [DOI] [PubMed] [Google Scholar]
- 16.Solomou EE, Rezvani K, Mielke S, et al. Brief report Deficient CD4 ϩ CD25 ϩ FOXP3 ϩ T regulatory cells in acquired aplastic anemia. 2007;110(5):1603–1606. doi: 10.1182/blood-2007-01-066258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutton KS, Shereck EB, Nemecek ER, Kurre P. Immune markers of disease severity and treatment response in pediatric acquired aplastic anemia. Pediatr Blood Cancer. 2013;60(3):455–460. doi: 10.1002/pbc.24247. [DOI] [PubMed] [Google Scholar]
- 18.Veiga-Parga T, Sehrawat S, Rouse BT. Role of Regulatory T Cells during Virus Infection. Immunol Rev. 2013;255(1):182–196. doi: 10.1111/imr.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng W, Maciejewski JP, Chen G, Young NS. Limited heterogeneity of T cell receptor BV usage in aplastic anemia. J Clin Invest. 2001;108(5):765–773. doi: 10.1172/JCI200112687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuster FR, Hubner B, Führer M, et al. Highly skewed T-cell receptor V-beta chain repertoire in the bone marrow is associated with response to immunosuppressive drug therapy in children with very severe aplastic anemia. Blood Cancer J. 2011;1(3):e8. doi: 10.1038/bcj.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moebius U, Herrmann F, Hercend T, Meuer SC. Clonal analysis of CD4+/CD8+ T cells in a patient with aplastic anemia. J Clin Invest. 1991;87(5):1567–1574. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC295239/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kochenderfer JN, Kobayashi S, Wieder ED, Su C, Molldrem JJ. Loss of T-lymphocyte clonal dominance in patients with myelodysplastic syndrome responsive to immunosuppression. Blood. 2002;100(10):3639–3645. doi: 10.1182/blood-2002-01-0155. [DOI] [PubMed] [Google Scholar]
- 23.Wlodarski MW, Gondek LP, Nearman ZP, et al. Molecular strategies for detection and quantitation of clonal cytotoxic T-cell responses in aplastic anemia and myelodysplastic syndrome Molecular strategies for detection and quantitation of clonal cytotoxic T-cell responses in aplastic anemia and myelod. Blood. 2006;108(8):2632–2641. doi: 10.1182/blood-2005-09-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batliwalla Franak, Monteiro Joanita, Davide Serrano PJG. Oligoclonality of CD8 + T cells in health and disease: Aging, infection, or immune regulation? Hum Immunol. 1996;48(1–2):68–76. [DOI] [PubMed] [Google Scholar]
- 25.Sloand E, Kim S, Maciejewski JP, Tisdale J, Follmann D, Young NS. Intracellular interferon-gamma in circulating and marrow T cells detected by flow cytometry and the response to immunosuppressive therapy in patients with aplastic anemia. Blood. 2002;100(4):1185–1191. doi: 10.1182/blood-2002-01-0035. [DOI] [PubMed] [Google Scholar]
- 26.Wu Q, Zhang J, Shi J, et al. Increased bone marrow (BM) plasma level of soluble CD30 and correlations with BM plasma level of interferon (IFN)-γ, CD4/CD8 T-cell ratio and disease severity in aplastic anemia. PLoS One. 2014;9(11). doi: 10.1371/journal.pone.0110787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin F, Karwan M, Saleh B, et al. IFN- g causes aplastic anemia by altering hematopoietic stem / progenitor cell composition and disrupting lineage differentiation. 2015;124(25):3699–3709. doi: 10.1182/blood-2014-01-549527.J.R.K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu CY, Fu R, Wang HQ, et al. Fas/FasL in the immune pathogenesis of severe aplastic anemia. Genet Mol Res. 2014;13(2):4083–4088. doi: 10.4238/2014.May.30.3. [DOI] [PubMed] [Google Scholar]
- 29.Kakagianni T, Giannakoulas NC, Thanopoulou E, et al. A probable role for trail-induced apoptosis in the pathogenesis of marrow failure. Leuk Res. 2017;30(6):713–721. doi: 10.1016/j.leukres.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Lipovsky K, Ellison FM, Calado RT, Young NS. Bystander destruction of hematopoietic progenitor and stem cells in a mouse model of infusion-induced bone marrow failure. 2004;104(6):1671–1678. doi: 10.1182/blood-2004-03-1115.Reprints. [DOI] [PubMed] [Google Scholar]
- 31.Solomou EE, Gibellini F, Stewart B, et al. Perforin gene mutations in patients with acquired aplastic anemia. Blood. 2007;109(12):5234–5237. doi: 10.1182/blood-2006-12-063495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng J, Liu C, Zhu K, Zhu Y, Yu Y, Li J, Hou M, Chen X, Xu CZM. The TNF2 allele is a risk factor to severe aplastic anemia independent of HLA-DR. Hum Immunol. 2003;64(9):896–901. [DOI] [PubMed] [Google Scholar]
- 33.Gidvani VK, Ramkissoon SH, Wong EW, Mainwaring L, Sloand EM, Young NS. Tumor Necrosis Factor-Alpha and Interleukin-6 Promoter Gene Polymorphisms in Acquired Bone Marrow Failure Syndromes. Blood. 2004;104(11):3707 LP–3707. http://www.bloodjournal.org/content/104/11/3707.abstract. [Google Scholar]
- 34.Dufour C, Capasso M, Svahn J, Marrone A, Haupt R, Bacigalupo A, Giordani L, Longoni D, Pillon M, Pistorio A, Di Michele P, Iori AP, Pongiglione C, Lanciotti MIA. Homozygosis for (12) CA repeats in the first intron of the human IFN-gamma gene is significantly associated with the risk of aplastic anaemia in Caucasian population. Br J Haematol. 2004;126(5):682–685. [DOI] [PubMed] [Google Scholar]
- 35.Deng XZ, Du M, Peng J, Long JX, Zheng CJ, Tan Y, Li LJ, Chen HY, Qing C, Pang YY, Lan Y ZH. Associations between the HLA-A/B/DRB1 polymorphisms and aplastic anemia: evidence from 17 case-control studies. Hematology. 2017;[Epub ahea. doi: 10.1080/10245332.2017.1375064. [DOI] [PubMed] [Google Scholar]
- 36.Zaimoku Y, Takamatsu H, Hosomichi K, et al. Identification of an HLA class I allele closely involved in the autoantigen presentation in acquired aplastic anemia. Blood. 2017;129(21):2908–2916. doi: 10.1182/blood-2016-11-752378. [DOI] [PubMed] [Google Scholar]
- 37.Zeng Y, Katsanis E. The complex pathophysiology of acquired aplastic anaemia. Clin Exp Immunol. 2015;180(3):361–370. doi: 10.1111/cei.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirano N, Butler MO, Von Bergwelt-Baildon MS, et al. Autoantibodies frequently detected in patients with aplastic anemia. Blood. 2003;102(13):4567–4575. doi: 10.1182/blood-2002-11-3409. [DOI] [PubMed] [Google Scholar]
- 39.Takamatsu H, Espinoza JL, Lu X, Qi Z, Okawa K, Nakao S. Anti-moesin antibodies in the serum of patients with aplastic anemia stimulate peripheral blood mononuclear cells to secrete TNF-α and IFN-γ. J Immunol. 2009;182(1):703–710. doi: 10.4049/jimmunol.182.1.703. [DOI] [PubMed] [Google Scholar]
- 40.Qi Z, Takamatsu H, Espinoza JL, Lu X, Sugimori N, Yamazaki H, Okawa KNS. Autoantibodies specific to hnRNP K: a new diagnostic marker for immune pathophysiology in aplastic anemia. Ann Hematol. 2010;89(12):1255–1263. [DOI] [PubMed] [Google Scholar]
- 41.Scheinberg P, Nunez O, Weinstein B, Scheinberg P, Wu CO, Young NS. Activity of alemtuzumab monotherapy in treatment-naive, relapsed, and refractory severe acquired aplastic anemia. Blood. 2012;119(2):345–354. doi: 10.1182/blood-2011-05-352328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scheinberg P, Fischer SH, Li L, et al. Distinct EBV and CMV reactivation patterns following antibody-based immunosuppressive regimens in patients with severe aplastic anemia. Blood. 2007;109(8):3219 LP–3224. http://www.bloodjournal.org/content/109/8/3219.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365(5):430–438. doi: 10.1056/NEJMoa1103975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Killick SB, Marsh JCW, Gordonsmith EC, Sorlin L, Gibson FM. Effects of antithymocyte globulin on bone marrow CD34+cells in aplastic anaemia and myelodysplasia [Full text delivery]. Br J Haematol. 2000;108:582–591. [DOI] [PubMed] [Google Scholar]
- 45.Flynn J, Cox CV, Rizzo S, et al. Direct binding of antithymoctye globulin to haemopoietic progenitor cells in aplastic anaemia. Br J Haematol. 2003;122(2):289–297. doi:4400 [pii]. [DOI] [PubMed] [Google Scholar]
- 46.Marsh JC, Chang J, Testa NG, Hows JMDT. In vitro assessment of marrow “stem cell” and stromal cell function in aplastic anaemia. Br J Haematol. 1991;78(2):258–267. [DOI] [PubMed] [Google Scholar]
- 47.Manz CY, Nissen C, Wodnar-filipowicz A. Deficiency of CD34 + c = kW and CD34 + 38- Hematopoietic Precursors in Aplastic Anemia After Immunosuppressive Treatment. 1996;274(1 996):264–274. [DOI] [PubMed] [Google Scholar]
- 48.Maciejewski JP, Selleri C, Sato T, Anderson S, Young NS. A severe and consistent deficit in marrow and circulating primitive hematopoietic cells (long-term culture-initiating cells) in acquired aplastic anemia. Blood. 1996;88(6):1983–1991. [PubMed] [Google Scholar]
- 49.Marsh JC, Chang J, Testa NG, Hows JM, Dexter TM. The hematopoietic defect in aplastic anemia assessed by long-term marrow culture. Blood. 1990;76(9):1748–1757. http://www.ncbi.nlm.nih.gov/pubmed/2224124. [PubMed] [Google Scholar]
- 50.Lu D Syngeneic bone marrow transplantation for treatment of aplastic anaemia: report of a case and review of the literature. Exp Hematol. 1981;9(3):257–263. [PubMed] [Google Scholar]
- 51.Brümmendorf TH, Maciejewski JP, Mak J, Young NS, Lansdorp PM. Telomere length in leukocyte subpopulations of patients with aplastic anemia. Blood. 2001;97(4):895 LP–900. http://www.bloodjournal.org/content/97/4/895.abstract. [DOI] [PubMed] [Google Scholar]
- 52.Sakaguchi H, Nishio N, Hama A, et al. Peripheral blood lymphocyte telomere length as a predictor of response to immunosuppressive therapy in childhood aplastic anemia. Haematologica. 2014;99(8):1312–1316. doi: 10.3324/haematol.2013.091165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calado RT, Regal JA, Yewdell WT, et al. Constitutional Loss-of-Function Mutations in Telomerase Are Genetic Risk Factors for Acute Myeloid Leukemia. Blood. 2015;110(11):16 LP–16. http://www.bloodjournal.org/content/110/11/16.abstract. [Google Scholar]
- 54.Calado RT, Cooper JN, Padilla-Nash HM, et al. Short telomeres result in chromosomal instability in hematopoietic cells and precede malignant evolution in human aplastic anemia. Leukemia. 2012;26(4):700–707. doi: 10.1038/leu.2011.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calado RT, Young NS. Review article Telomere maintenance and human bone marrow failure. Bone. 2008;111(9):4446–4455. doi: 10.1182/blood-2007-08-019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamaguchi H, Baerlocher GM, Lansdorp PM, et al. Mutations of the human telomerase RNA gene TERC in aplastic anemia and myelodysplastic syndrome. Blood. 2003;102(3):916 LP–918. http://www.bloodjournal.org/content/102/3/916.abstract. [DOI] [PubMed] [Google Scholar]
- 57.Yamaguchi H, Calado RT, Ly H, et al. Mutations in TERT, the Gene for Telomerase Reverse Transcriptase, in Aplastic Anemia. N Engl J Med. 2005;352(14):1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 58.Lane AA, Odejide O, Kopp N, et al. Low Frequency Clonal Mutations Recoverable by Deep Sequencing in Patients with Aplastic Anemia. Leukemia. 2013;27(4):968–971. doi: 10.1038/leu.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heuser M, Schlarmann C, Dobbernack V, et al. Genetic characterization of acquired aplastic anemia by targeted sequencing. Haematologica. 2014;99(9):e165 LP–e167. http://www.haematologica.org/content/99/9/e165.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kulasekararaj AG, Jiang J, Smith AE, et al. Somatic mutations identify a subgroup of aplastic anemia patients who progress to myelodysplastic syndrome. Blood. 2014;124(17):2698 LP–2704. http://www.bloodjournal.org/content/124/17/2698.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Babushok DV, Perdigones N, Perin JC, et al. Emergence of Clonal Hematopoiesis in the Majority of Patients with Acquired Aplastic Anemia. Cancer Genet. 2015;208(4):115–128. doi: 10.1016/j.cancergen.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ogawa S CME Article Clonal hematopoiesis in acquired aplastic anemia. Blood. 2016;128(3):337–348. doi: 10.1182/blood-2016-01-636381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DAMESHEK W Editorial: <em>Riddle:</em> What Do Aplastic Anemia, Paroxysmal Nocturnal Hemoglobinuria (PNH) and "Hypoplastic" Leukemia Have in Common? Blood. 1967;30(2):251 LP–254. http://www.bloodjournal.org/content/30/2/251.abstract. [PubMed] [Google Scholar]
- 64.Tichelli A, Gratwohl A, Würsch A, Nissen C and Speck B Late haematological complications in severe aplastic anaemia. Br J Haematol. 1988;69:413–418. [DOI] [PubMed] [Google Scholar]
- 65.Narita A, Muramatsu H, Okuno Y, et al. Development of Paroxysmal Nocturnal Hemoglobinuria in Children with Aplastic Anemia. Blood. 2016;128(22):1499 LP–1499. http://www.bloodjournal.org/content/128/22/1499.abstract. [Google Scholar]
- 66.Wanachiwanawin W, Siripanyaphinyo U, Piyawattanasakul NKT. A cohort study of the nature of paroxysmal nocturnal hemoglobinuria clones and PIG-A mutations in patients with aplastic anemia. Eur J Haematol. 2006;76(6):502–509. [DOI] [PubMed] [Google Scholar]
- 67.Tichelli A, Gratwohl A, Nissen C, Speck B. Late Clonal Complications in Severe Aplastic Anemia. Leuk Lymphoma. 1994;12(3–4):167–175. doi: 10.3109/10428199409059587. [DOI] [PubMed] [Google Scholar]
- 68.Appelbaum FR, Barrall J, Storb R, Ramberg R, Doney K, Sale GE TE. Clonal cytogenetic abnormalities in patients with otherwise typical aplastic anemia. Exp Hematol. 1987;15(11):1134–1139. [PubMed] [Google Scholar]
- 69.Socié G, Rosenfeld S, Frickhofen N, Gluckman ETA. Late clonal diseases of treated aplastic anemia. Semin Hematol. 2000;37(1):91–101. [PubMed] [Google Scholar]
- 70.Keung YK, Pettenati MJ, Cruz JM, Powell BL, Woodruff RDBD. Bone marrow cytogenetic abnormalities of aplastic anemia. Am J Hematol. 2001;66(3):167–171. [DOI] [PubMed] [Google Scholar]
- 71.Maciejewski JP, Risitano A, Sloand EM, Nunez O, Young NS. Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood. 2002;99(9):3129 LP–3135. http://www.bloodjournal.org/content/99/9/3129.abstract. [DOI] [PubMed] [Google Scholar]
- 72.Yoshizato T, Dumitriu B, Hosokawa K, et al. Somatic Mutations and Clonal Hematopoiesis in Aplastic Anemia. N Engl J Med. 2015;373(1):35–47. doi: 10.1056/NEJMoa1414799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schubert J, Vogt HG, Zielinska-Skowronek M, et al. Development of the glycosylphosphatitylinositol-anchoring defect characteristic for paroxysmal nocturnal hemoglobinuria in patients with aplastic anemia. Blood. 1994;83(8):2323 LP–2328. http://www.bloodjournal.org/content/83/8/2323.abstract. [PubMed] [Google Scholar]
- 74.Azenishi Y, Ueda E, Machii T, Nishimura J, Hirota T, Shibano M, Nakao S, Kinoshita T, Mizoguchi HKT. CD59-deficient blood cells and PIG-A gene abnormalities in Japanese patients with aplastic anaemia. Br J Haematol. 1999;104(3):523–529. [DOI] [PubMed] [Google Scholar]
- 75.Kawaguchi K, Wada H, Mori A, Takemoto Y, Kakishita EKA. Detection of GPI-anchored protein-deficient cells in patients with aplastic anaemia and evidence for clonal expansion during the clinical course. Br J Haematol. 1999;105(1):80–84. [PubMed] [Google Scholar]
- 76.Maciejewski JP, Follmann D, Nakamura R, et al. Increased frequency of HLA-DR2 in patients with paroxysmal nocturnal hemoglobinuria and the PNH/aplastic anemia syndrome. Blood. 2001;98(13):3513 LP–3519. http://www.bloodjournal.org/content/98/13/3513.abstract. [DOI] [PubMed] [Google Scholar]
- 77.Zhao X, Zhang L, Jing L, Zhou K, Li Y, Peng G, Ye L, Li Y, Li J, Fan H, Song L, Yang WZF. The role of paroxysmal nocturnal hemoglobinuria clones in response to immunosuppressive therapy of patients with severe aplastic anemia. Ann Hematol. 2015;94(7):1105–1110. [DOI] [PubMed] [Google Scholar]
- 78.Wlodarski MW, Hirabayashi S, Strahm B, et al. Recurrent 6pLOH Is the Most Common Somatic Lesion in Refractory Cytopenia of Childhood and Occurs Very Infrequently in Severe Aplastic Anemia. Blood. 2012;120(21):644 LP–644. http://www.bloodjournal.org/content/120/21/644.abstract. [Google Scholar]
- 79.Katagiri T, Sato-Otsubo A, Kashiwase K, et al. Frequent loss of HLA alleles from hematopoietic stem cells in patients with hepatitis-associated aplastic anemia. Blood. 2011;118 (21)(December):6601–6610. doi: 10.1182/blood-2011-07-365189. [DOI] [PubMed] [Google Scholar]
- 80.Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126(1):9–16. doi: 10.1182/blood-2015-03-631747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Keel SB, Scott A, Bonilla MS, et al. Genetic features of myelodysplastic syndrome and aplastic anemia in pediatric and young adult patients. Haematologica. 2016;101(11):1343–1350. doi: 10.3324/haematol.2016.149476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bluteau O, Sebert M, Leblanc T, et al. A landscape of germline mutations in a cohort of inherited bone marrow failure patients. Blood. January 2017. http://www.bloodjournal.org/content/early/2017/11/16/blood-2017-09-806489.abstract. [DOI] [PubMed]
- 83.Hamzic E, Whiting K, Gordon Smith E, Pettengell R. Characterization of bone marrow mesenchymal stromal cells in aplastic anaemia. Br J Haematol. 2015;169(6):804–813. doi: 10.1111/bjh.13364. [DOI] [PubMed] [Google Scholar]
- 84.Shipounova IN, Petrova TV., Svinareva DA, Momotuk KS, Mikhailova EA, Drize NI. Alterations in hematopoietic microenvironment in patients with aplastic anemia. Clin Transl Sci. 2009;2(1):67–74. doi: 10.1111/j.1752-8062.2008.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rizzo S, Killick SB, Patel S, et al. Reduced TGF-beta1 in patients with aplastic anaemia in vivo and in vitro. Br J Haematol. 1999;107(4):797–803. http://www.ncbi.nlm.nih.gov/pubmed/10606887. [DOI] [PubMed] [Google Scholar]
- 86.Wu JY, Scadden DT, Kronenberg HM. Role of the osteoblast lineage in the bone marrow hematopoietic niches. J Bone Miner Res. 2009;24(5):759–764. doi: 10.1359/jbmr.090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103(9):3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 88.Wu L, Mo W, Zhang Y, et al. Impairment of hematopoietic stem cell niches in patients with aplastic anemia. Int J Hematol. 2015;102(6):645–653. doi: 10.1007/s12185-015-1881-2. [DOI] [PubMed] [Google Scholar]
- 89.Kojima S, Matsuyama T, Kodera Y, et al. Measurement of endogenous plasma granulocyte colony-stimulating factor in patients with acquired aplastic anemia by a sensitive chemiluminescent immunoassay. Blood. 1996;87(4):1303–1308. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8608218. [PubMed] [Google Scholar]
- 90.Schrezenmeier H, Griesshammer M, Hornkohl A, Nichol JL, Hecht T, Heimpel H, Kubanek BRA. Thrombopoietin serum levels in patients with aplastic anaemia: correlation with platelet count and persistent elevation in remission. Br J Haematol. 1998;100(3):571–576. [DOI] [PubMed] [Google Scholar]
- 91.Marsh JC, Ganser ASM. Hematopoietic growth factors in the treatment of acquired bone marrow failure states. Semin Hematol. 2007;44(3):138–147. [DOI] [PubMed] [Google Scholar]
- 92.Kimura S, Roberts AW, Metcalf D, Alexander WS. Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci U S A. 1998;95(February):1195–1200. doi: 10.1073/pnas.95.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zeigler FC, de Sauvage F, Widmer HR, et al. In vitro megakaryocytopoietic and thrombopoietic activity of c-mpl ligand (TPO) on purified murine hematopoietic stem cells. Blood. 1994;84(12):4045–4052. [PubMed] [Google Scholar]
- 94.Yoshihara H, Arai F, Hosokawa K, et al. Thrombopoietin/MPL Signaling Regulates Hematopoietic Stem Cell Quiescence and Interaction with the Osteoblastic Niche. Cell Stem Cell. 2007;1(6):685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 95.King S, Germeshausen M, Strauss G, Welte K, Ballmaier M. Congenital amegakaryocytic thrombocytopenia: A retrospective clinical analysis of 20 patients. Br J Haematol. 2005;131(5):636–644. doi: 10.1111/j.1365-2141.2005.05819.x. [DOI] [PubMed] [Google Scholar]
- 96.Walne AJ, Dokal A, Plagnol V, et al. Exome sequencing identifies MPL as a causative gene in familial Aplastic anemia. Haematologica. 2012;97(4):524–528. doi: 10.3324/haematol.2011.052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and Improved Hematopoiesis in Refractory Aplastic Anemia. N Engl J Med. 2012;367(1):11–19. doi: 10.1056/NEJMoa1200931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag Added to Standard Immunosuppression for Aplastic Anemia. N Engl J Med. 2017;376(16):1540–1550. doi: 10.1056/NEJMoa1613878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alvarado Luigi J., Andreoni Alessio, Huntsman Heather D., Hai Cheng JRK and AL. 4 Heterodimerization of TPO and IFNγ Impairs Human Hematopoietic Stem/Progenitor Cell Signaling and Survival in Chronic Inflammation. In: American Society of Hematology.; 2017:https://ash.confex.com/ash/2017/webprogram/Paper10. [Google Scholar]