Abstract
Type 1 diabetes (T1D) is an autoimmune disease in which pancreatic β-cell destruction can be mediated by dysbiosis, infiltration of pro-inflammatory immune cells, and cytokines/chemokines. Exposure to bisphenol A (BPA), an endocrine disruptor (ED), can lead to aberrant immunity and gut microbiota. We determined whether BPA had age-dependent effects on T1D by modulating immune homeostasis following various windows of exposure in non-obese diabetic (NOD) mice. Juvenile NOD females were orally exposed to 0 or 30 μg BPA/kg BW from postnatal day (PND) 28 to PND56. Adult NOD females were exposed to 0 or 300 μg BPA/kg BW. Female and male NOD offspring were exposed to 0 or 300 μg BPA/kg BW perinatally from gestation day 5 to PND28 by dosing the dams. It was found that BPA increased T1D risk in juvenile females with gut microbiota shifted towards pro-inflammation (e.g. increased Jeotgalicoccus). In agreement with our previous study, adult females had a trend of increased T1D and a general increase in immune responses. However, female offspring had a reduced T1D development. Consistently, female offspring had a shift towards anti-inflammation (e.g. decreased pro-inflammatory F4/80+Gr1+ cells). In contrast, BPA had minimal effects on immunity and T1D in male offspring. Thus, it was concluded that BPA had age- and sex-dependent effects on T1D with the alteration of gut microbiota and inflammation being the primary mechanisms for T1D exacerbation in juvenile exposure and decreases of inflammation being responsible for attenuated T1D in perinatally exposed females.
Keywords: Bisphenol A, type 1 diabetes, NOD mice, window of exposure, immunomodulation, microbiome
Introduction
Bisphenol A (BPA), an endocrine disruptor (ED), has been found in more than 90% of human urine samples due to its wide use in a variety of polycarbonate plastics and epoxy resins (e.g., water bottles and plastic food containers; (Lang et al. 2008)). Because of BPA’s ability to bind estrogen receptors (ERs) in various immune cells, BPA can modulate immune function and increase cytokine production leading to an increased risk for inflammatory diseases (Xu et al. 2016). Furthermore, estrogen and estrogen-like compounds (e.g. BPA) can cross-talk with gut microbiota (GMB) to alter microbiota, which influence various diseases including diabetes (Chen and Madak-Erdogan 2016). BPA has recently been shown to alter GMB and cause dysbiosis (Lai et al. 2016; Liu et al. 2016; Xu et al. 2019), which further suggests BPA may be contributing to type 1 diabetes (T1D). T1D is an autoimmune disease in which destruction of pancreatic β-cells results from pro-inflammatory immune cell infiltrates and cytokines/chemokines (Jörns et al. 2014). In addition to the genetic risk factors, environmental exposures are contributing to the increasing incidence of T1D (Tuomilehto 2013). GMB can also have a critical role in T1D development (Brown et al. 2011), since GMB promote immune cell development and function, while immunity regulates GMB composition (Belkaid and Hand 2014).
Animal studies have shown that BPA exposure during adulthood or throughout life can increase T1D incidence (Bodin et al. 2013; Bodin et al. 2015; Cetkovic-Cvrlje et al. 2017). However, only one animal study has examined whether perinatal BPA exposure can alter T1D (Bodin et al. 2014), and much is still uncertain about whether developmental exposure affects T1D development. In humans, the first study that measured BPA levels in children with T1D has recently been published; however, the authors stated problems with their small sample size prevented them from establishing a possible relationship between BPA and T1D (İnce et al. 2018). The present study aimed to determine BPA’s effect on the immune system, GMB and T1D outcome using different exposure windows. Due to the 40% increased risk of death in women with T1D compared to men (Huxley et al. 2015), emphasis was put on females in this study in different exposure windows, e.g. juvenile, perinatal and adult periods. Additionally, to our knowledge, this is also the first study to examine the effect of BPA exposure on T1D in perinatally exposed males.
Sex-biased changes have previously been reported for non-obese diabetic (NOD) mice in immune dysregulation and T1D development (Bao et al. 2002; Young et al. 2009) and for BPA exposure in human glucose homeostasis (Beydoun et al. 2014). The NOD mouse spontaneously develops T1D with many similarities in autoimmunity and genetics that are relevant to human T1D, which makes them an ideal model for examining the roles of environmental exposures and GMB in T1D risk (Chen et al. 2018b; Jörns et al. 2014). We hypothesized that BPA differentially affects T1D susceptibility in NOD mice depending on exposure windows and sex through alteration of immune responses and/or microbiota. Therefore, we have tested the whether NOD mice exposed to BPA during either juvenile, adult or developmental life stages have an altered T1D development.
Materials and Methods
Animal Husbandry
Specific pathogen free (SPF) NOD mice were from a breeding colony maintained in our animal facility and initially purchased from Taconic Biosciences (Hudson, NY). NOD mice were housed in polysulfone cages with irradiated laboratory animal bedding and Bed-r’Nest for enrichment (The Andersons Inc., Maumee, Ohio) in the Animal Facility at University of Georgia (UGA). Negligible amounts of BPA have been reported to leach from new or used polysulfone cages maintained at room temperature (Delclos et al. 2014; Johnson et al. 2016). The animal room was maintained at 22–25°C with relative humidity 50±20 and 12-h light/dark cycle (7:00AM lights on). An automatic watering system was provided to give mice free access to water, and either PicoLab diet (soy based, LabDiet, St. Louis, MO) or 5K96 diet (phytoestrogen free, TestDiet, St. Louis, MO) was provided ad libitum. Animals were treated humanely and with regard for alleviating suffering. All procedures were conducted under an approved animal protocol by the UGA Institutional Animal Care and Use Committee (IACUC).
Bisphenol A Exposure
Exposure of juvenile NOD females to BPA.
Juvenile female NOD mice (6/group) were randomized into vehicle (VH) or BPA groups, and then Student’s t-test was performed to ensure background body weight (BW) and blood glucose levels (BGLs) were not statistically different before dosing. Mice were fed with the soy-based PicoLab diet and gavaged with either VH or 30 μg BPA/kg BW using micropietting from postnatal day (PND) 28 to PND56, and they were euthanized at PND134. Dosing solution was prepared by dissolving BPA in 100% ethanol and then further diluting this solution in corn oil at a final concentration of 0.05% ethanol. We chose this exposure level and the soy-based PicoLab diet in juvenile females to compare to our previous study in female adults that showed 30 μg BPA/kg BW accelerated T1D development (Xu et al. 2019).
Exposure of adult NOD females to BPA.
We have reported our findings in female adults following BPA exposure when they were maintained on the soy-based PicoLab diet (Xu et al. 2019). To further determine the age-dependent effects of BPA exposure, a total of 30 adult female NOD mice (8–12 wks old; 15/group) on the phytoestrogen-free 5K96 diet were randomized into VH or BPA groups according to BW and BGLs, and administered either VH or 300 μg BPA/kg BW as described above. The dose was chosen because similar amounts to 300 μg BPA/kg were previously shown to alter the immunity in adult exposed female mice and may provide levels of uncongugated BPA in mice closer to what is found in humans (Taylor et al. 2011; Yoshino et al. 2003).
Exposure of NOD female and male offspring to BPA.
Sixteen time-mated adult female NOD mice on 5K96 phytoestrogen-free diet were dosed as described above from gestation day (GD) 5 to PND28 with VH or 300 μg BPA/kg BW. Mice were not dosed from the start of mating (GD0), since BPA exposure starting before GD4 has been shown to impair embryo implantation (Yuan et al. 2018). As in adults this dose was chosen also because this amount was previously shown to alter the immunity in perinatally exposed mice (Bodin et al. 2014; Yoshino et al. 2004). Offspring were weaned on PND28 to different cages to prevent further exposure to BPA from their mothers. Weaning was done on PND28 since BW was low on PND21 due to the 5K96 phytoestrogen-free diet (Ruhlen et al. 2008). Half of the VH and BPA treated female and male offspring were switched to the soy-based PicoLab diet on PND35 to determine dietary effects.
Body Weight, Blood Glucose Measurement And Diabetic Incidence
BW and non-fasting BGLs were measured every 1–2 weeks. Accu-Chek Diabetes monitoring kit (Roche Diagnositics, Indianapolis, IN) or Contour Blood Glucose Meter (Ascensia Diabetes Care, Parsippany, NJ) were used to measure BGLs from a small sample of venous blood (tail nick). Mice were considered diabetic once 2 consecutive non-fasting BGL measurements of ≥250 mg/dL were observed (Guo et al. 2014). At the end of the study or after detecting BGLs ≥600 mg/dL, mice were humanely euthanized. For male offspring on the PicoLab diet, body composition was measured on PND215 in a Minispec LF110 BAC Analyzer (Bruker Corporation, The Woodlands, TX) with a mouse probe, which measured the body composition with linearity and reproducibility in non-anesthetized rodents using magnetic resonance relaxometry.
Glucose And Insulin Tolerance Tests
For the glucose tolerance test (GTT), mice were fasted overnight for 15 h, and background fasting BW and BGLs were measured before injecting (i.p.) mice with glucose (2 g/kg BW; Sigma; (Huang et al. 2017)). For the insulin tolerance test (ITT), baseline BW and BGLs were determined, and then, mice were injected (i.p.) with insulin (1.5 IU/kg BW; Sigma; (Cui et al. 2015)). BGLs were measured at 15, 30, 60 and 120 min after injection for the GTT and ITT.
Organ Collection, Histopathology And Flow Cytometry
Organ collection and histopathology were done as previously described (Xu et al. 2019). Spleens were mashed in 3 mL PBS solution on ice. Flow cytometric analysis was performed for quantifying leukocyte populations with different combinations of fluorochrome-labeled antibodies (diluted 1:80; BD PharMingen, San Diego, CA) with a Becton Dickinson LSRII Flow Cytometer (BD Biosciences, San Jose, CA) as described previously (Xu et al. 2019). The cluster of differentiation (CD) 4-CD8-CD25 (V450-APCH7-APCA), CD40L-B220 (PE-FITC) and F4/80-Gr1 (PE-FITC) were used in female offspring and PicoLab diet male offspring, while male offspring on the phytoestrogen-free diet used CD4-CD8-CD25 (V450-APCH7-APCA), CD40L-B220 (PEFITC) and F4/80-CD40 (PE-FITC). CD4-CD8-CD25-Mac3-CD45R (V450-APCH7-APCA-FITCPE), CD40L-B220 (PE-FITC), CD5-CD24 (PE-FITC) and CD44-CD40 (PE-FITC) were used in juvenile females. CD4-CD8-CD25 (PE-PerCP-FITC), CD40L-B220 (PE-FITC) and Mac3-Gr1 (PE-FITC) were used in adult females. Isotype matched irrelevant antibodies were used as controls. Analysis was done using FlowJo software (FlowJo LLC).
Cytokine/chemokine Measurement
Thirty-two cytokines and chemokines were measured from sera collected at euthanasia using the Milliplex MAP Mouse Cytokine/Chemokine Magnetic Bead Panel (Cat. No. MCYTOMAG-70K, EMD Millipore, Billerica, MA) according to manufacturer protocols. Quality controls provided in the kit were also run on the same plate. Bio-rad Bio-Plex bead-based multiplexing (Bio-Rad, Inc., Hercules, California) with Bio-Plex Manager™ Software 6.1 was used to run the plates and measure the concentration (pg/ml).
Antibody Measurement
ELISA kits (eBioscience, San Diego, CA) were used to measure IgG subclasses (IgG1, IgG2c, IgG2b) and IgM levels in sera obtained at euthanasia as previously described (Huang et al. 2017) with either 3,3’,5,5’-tetramethylbenzidine (TMB) or 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) substrate (Sigma). Plates were then read using a microplate fluorescence reader (Synergy 4 Hybrid Microplate Reader, BioTek, Winooski, VT) either at a wavelength of 405 nm or at 450 nm following adding 100 μL/well stop solution (2N sulfuric acid).
GMB Preparation, Sequencing And Bioinformactics
Mice receiving the different treatments were not housed together to prevent coprophagy between groups. Individual mice had their cecal contents collected immediately after euthansia and kept at −20°C until extraction. Approximately 150 mg were weighed, and a DNeasy PowerLyzer PowerSoil Kit (Qiagen, Valencia, CA) was used following manufacturer protocols. For the bead beating step, two 5 minute cycles at 30 hertz with a Tissue Lyser II were used to homogenize the samples. Library preparation and bioinformactics analysis have been described previously (Xu et al., 2019), and are also included in the supplemental methods.
Statistical Analysis
Likelihood ratio and Logrank test were performed to analyze the rate of diabetes development and total diabetes incidence over time, respectively. Correlational analysis was assessed using Spearman’s correlation test. Two-way ANOVA and Tukey’s test were used for sex × treatment interactions. For all other data sets, one-tailed Student’s t-test were used for comparisons when the equal variance assumption was met (using Bartlett’s test); otherwise, Wilcoxon test was performed for non-parametric data. A treatment was considered statistically significant if p < 0.05. JMP Pro 12 (SAS Inc., Cary, NC) and GraphPad Prism 7 (GraphPad Software Inc., La Jolla, CA) were used for statistical analysis and data visualization.
Results
BPA Exposure Increased BGLs In Juvenile Females.
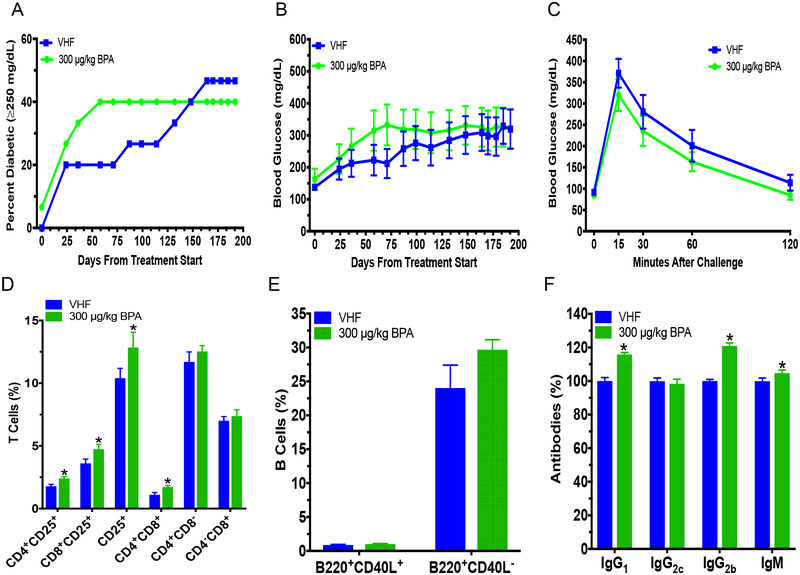
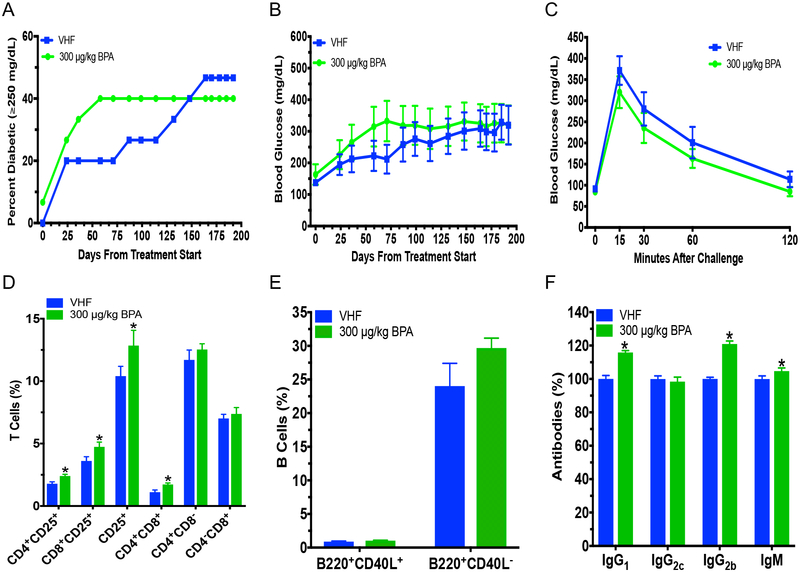
Since we previously found that BPA accelerated T1D in adult NOD females fed a soy-based diet (Xu et al. 2019), juvenile exposure was conducted by exposing NOD females to BPA during PND28 to PND56 using the soy-based PicoLab diet. Juvenile BPA exposure appeared to increase T1D incidence, although this did not reach the level of statistical significance (e.g. P = 0.054 on day 80 and 85; Fig. 1a). Non-fasting BGLs initially showed a slight decrease on PND80 from BPA exposure, but were increased later on with significant changes observed on PND108 and 113 (Fig. 1b). The GTT on PND120 also showed a trend of increased BGLs from BPA exposure (Fig. 1c). No significant effects were determined from the ITT or insulitis (Fig. S1a; Table S1). BPA exposure decreased CD4+CD8+ T cells and B220+CD40L− B cells (Fig. 1d–e). However, no significant changes were found for the other cell populations (Fig. S1b–d). In addition, juvenile BPA exposure did not affect serum IgG1, IgG2c, IgG2b, IgM antibody levels or organ weights (Fig. S1e; Table S2). Measurements of cytokines and chemokines suggested that G-CSF, IL-5 and LIX were increased from BPA exposure, while EOTAXIN and IL-15 were decreased by BPA (Fig. 1f; Table S3). These significantly altered cytokines/chemokines and CD4+CD8+ T cells were found to correlate with BGLs (Fig. 1g).
Fig. 1.
Diabetic incidence, changes in BGLs, alterations in immunity and immune-BGLs correlational analysis in juvenile BPA (30 μg/kg) exposed NOD females that were fed with the soy-based PicoLab diet. (a) T1D incidence. Blood glucose ≥250 mg/dL was considered diabetic. (b) Time course of BGLs. (c) GTT was conducted at 4 mo. old on non-diabetic mice. (d) %T cells in spleen. (e) %B cells in spleen. (f) Heat map of serum cytokine/chemokine changes at time of euthanasia (PND134). Values of VHF are shown as mean pg/mL. ND, not detected. (g) Correlation between significant immune endpoints and BGLs represented on a heatmap with Spearman rho’s correlation coefficients with red showing significantly positive and blue significantly negative correlation using Spearman correlation test (p < 0.05). Blank boxes with an X indicate no significant correlation. The values are presented as mean ± SEM. *p< 0.05 as compared to the respective vehicle female (VHF) control group. N = 5–6, except (c) where N = 4–6
On PND134 (4 mo. old) or 2.6 mo. after the last BPA exposure, GMB composition was determined, and it was significantly altered from juvenile BPA exposure. At the phylum level, BPA significantly increased Verrucomicrobia and decreased Nitrospirae, OD1, AD3, and Gemmatimonadetes (Fig. 2a–b). At the class level, Verrucomicrobiae and TA18 were increased by BPA, while Nitrospira, JG37 AG 4, Acidobacteriia, Gemmatimonadetes and Gitt GS 136 were all reduced in abundance compared to the VH control (Fig. 2c). BPA alteration of bacteria at the genus level included increased abundances of Lachnospiraceae (listed as Other), unclassified C114, Jeotgalicoccus, Ruminococcus, Akkermansia, Oscillospira, Rhodospirillales (listed as Other), unclassified RF32, Anaerofustis and Turicibacter, and decreased abundances of unclassified bacteria from Sinobacteraceae, N1423WL, SC I 84, Koribacteraceae, 0319 6A21, Ellin329, Gitt GS 136, EB1017 and JG37 AG 4 (Fig. 2d). Significantly different weighted beta diversity was also found between the VH and BPA groups (Fig. 2e). However, unweighted beta diversity, alpha diversity and Bacteroidetes:Firmicutes ratio were not significantly altered from BPA exposure (Fig. S2). Many of the bacteria significantly altered at the phylum, class and genus levels by BPA correlated with BGLs, the significantly altered cytokines/chemokines, and the decreased %CD4+CD8+ T cells and %B220+CD40L− B cells (Fig. 2f–g).
Fig. 2.
Gut microbiome composition alteration at PND134 in juvenile BPA (30 μg/kg) exposed NOD females that were fed with the soy-based PicoLab diet. (a) Taxonomy of gut microbiome is shown at the phylum level. LEfse results for phylum level (b), class level (c) and genus level (d) are shown. (e) Weighted UniFrac beta diversity. Correlation between significant microbiota (phylum, class and genus levels) and either BGLs (f) or significant immune endpoints (g) in juvenile exposed NOD females. Heatmaps show Spearman rho’s correlation coefficients with red showing significantly positive and blue significantly negative correlations using Spearman correlation test (p < 0.05). Blank boxes with an X were not significantly different. N = 5–6. VHF, vehicle females
Adult BPA Exposed Females.
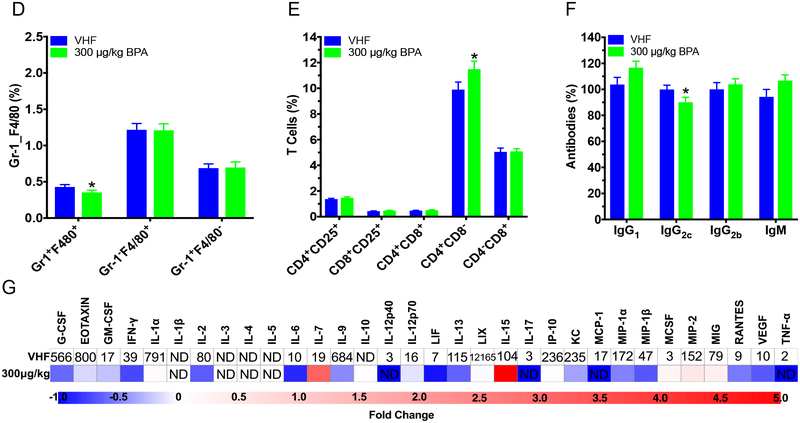
Our previous studies in adult females have shown that BPA could exacerbate T1D when the animals were maintained on the soy-based PicoLab diet (Xu et al. 2019). To determine if diet affected BPA’s alteration of T1D, adult female mice on the phytoestrogen-free 5K96 diet were exposed to BPA. Although not statistically significant, a shift towards accelerated T1D development was observed (Fig. 3a). No statistically significant differences in the non-fasting BGLs, GTT, ITT or BW were observed (Fig. 3b–c; Fig. S3). However, % CD4+CD25+ cells, CD8+CD25+ cells, CD4+CD8+ cells and CD25+ cells were increased from BPA exposure (Fig. 3d), although BPA exposure did not affect splenic neutrophils, macrophages and B cells (Fig. 3e; Fig. S4). In addition, antibody levels of IgG1, IgG2b and IgM were increased from BPA exposure, but IgG2c was not significantly altered (Fig. 3f). Adult females also had increased absolute spleen weight, while the absolute and % pancreas weights were decreased by BPA (Table S4).
Fig. 3.
Diabetic incidence, BGLs, glucose tolerance test (GTT) and immunity in adult female NOD mice on the phytoestrogen-free 5K96 diet. Time course for T1D incidence (a) and BGLs (b). Blood glucose ≥250 mg/dL was considered diabetic (N = 15). (c) GTT of non-diabetic adult females at 4 mo. after initial dose (N = 9–10). (d) %T cell populations. (e) B cell populations. (f) IgG1, IgG2c, IgG2b and IgM were measured at dilutions of 1:2,000, 1:2,000, 1:25 and 1:1,000, respectively, following titration by serial dilution from serum collected at euthanasia (day 198 after treatment start; N = 9). The values are presented as mean ± SEM. *, p< 0.05 using Dunnett’s test or Wilcoxin based on whether equal variance assumption was met as compared to the vehicle (VH) control group. VHF, vehicle females
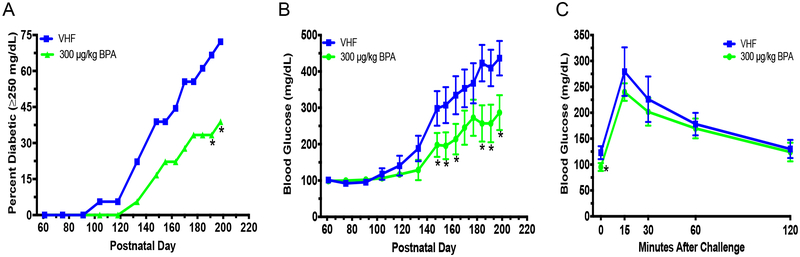
BPA Decreased BGLs And Diabetic Incidence In Female Offspring.
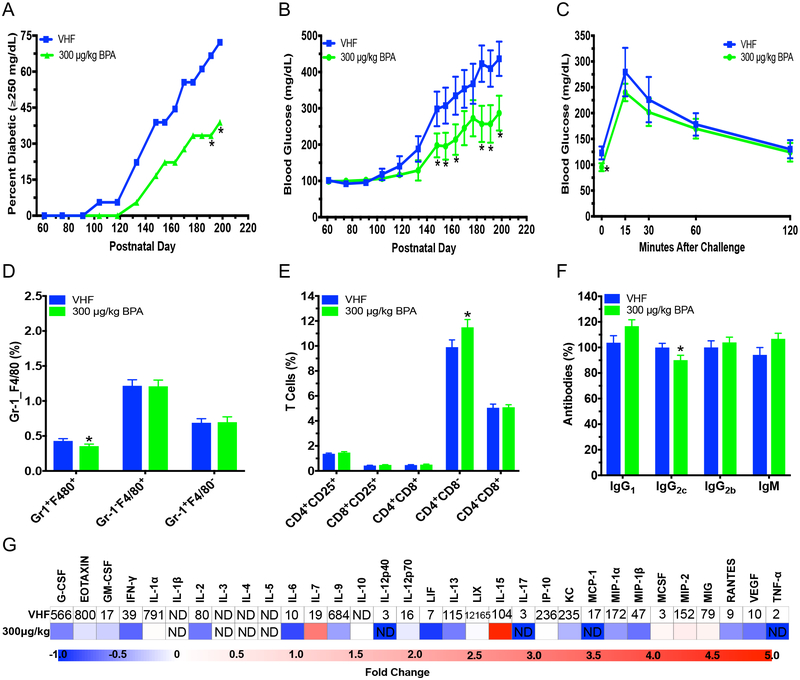
To ascertain the effect of BPA exposure during development on female offspring, mice were exposed in utero and during lactation to either VH or BPA by treating dams orally from GD5 to PND28. Diabetes incidence on PND191 and 198 and total diabetes incidence over time was significantly decreased in female offspring exposed to BPA on the phytoestrogen-free diet (P = 0.040; Fig. 4a). Additionally, BPA significantly decreased non-fasting BGLs between PND148–198 and fasting glucose during the GTT in these female offspring (Fig. 4b–c). However, no statistically significant effect was observed in the ITT (Fig. S5a). F4/80+Gr-1+ monocyte cells were decreased in BPA exposed female offspring; however, no changes in neutrophils were observed (Fig. 4d). Additionally, CD4+CD8− T cells and B220+CD40L− B cells were increased, while IgG2c and the chemokine RANTES was decreased (Fig. 4e–g; Table S3; Fig. S5b). However, IgG2c was the only significantly altered immune endpoint that significantly correlated with BGLs, and no significant changes in organ weights or BW were observed (Table S5 and Fig. S5c–d).
Fig. 4.
Diabetic incidence, changes in BGLs over time, glucose tolerance test (GTT) and immune cell alterations in female NOD offspring exposed to BPA (300 μg/kg) perinatally on phytoestrogen-free 5K96 diet. (a) T1D incidence over time. Blood glucose ≥250 mg/dL was considered diabetic (N = 18). (b) Time course of BGLs (N = 18). (c) GTT was conducted on non-diabetic mice at 5 mo. old (N = 7–12). (d) %Gr-1+F4/80+, Gr-1−F4/80+ and Gr-1+F4/80− spleen cells (N = 11–13). (e) %T cell populations (N = 11–13). (f) IgG1, IgG2c, IgG2b and IgM were measured at dilutions of 1:2,000, 1:2,000, 1:25 and 1:1,000, respectively, following titration by serial dilution from serum collected at euthanasia (PND204; N = 6). (g) Heat map of serum cytokine/chemokine changes at time of euthanasia (PND204). Values of VHF are shown as mean pg/mL (N = 6). ND, not detected. The values are presented as mean ± SEM. *p< 0.05 as compared to the vehicle female (VHF) control group
In female offspring switched to the soy-based PicoLab diet on PND35, no statistically significant effect was found in the development of diabetes, non-fasting BGLs or GTT (Fig. S6ac). The ITT showed increased BGLs at 120 min after the initial injection (Fig. S6d). No changes in any immune cell populations from BPA exposure were observed in soy-based diet female offspring (Fig. S7a–c). However, IgG2b was increased, and several cytokines/chemokines were decreased by BPA including G-CSF, IL-6, MIP-2 and RANTES (Fig. S7d–e; Table S3). BW was increased on PND148 and 208 in soy-based diet female offspring, but no significant differences were found in organ weights from perinatal BPA exposure in soy-based diet female offspring (Fig. S6e; Table S6). Overall, when compared to the phytoestrogen-free diet, the soy-based diet seemed to have attenuated BPA’s effects.
Perinatal BPA Exposure Had Minimal Effect On Male Offspring.
To determine if perinatal BPA exposure had any sex-dependent effects, male offspring were monitered for alteration of T1D development and immunity. In the phytoestrogen-free diet male offspring, no significant changes were found in time course BGLs, T1D, the 7 mo. old (except for reduced BGLs at the 15 min timepoint) and 9 mo. old GTT, ITT or organ weights, and minimal effect was seen on BW (Fig. S8; Table S7). No immune cells were altered other than decreased CD40 mean fluorescence intensity (MFI; expression levels of CD40 on cells) of CD40+F4/80− cells (Fig. S9).
Similarly, male offspring shifted to the soy-based PicoLab diet had no significant difference in their BGLs, T1D development or tolerance tests, but a reduced BW until PND216 was observed from BPA exposure (Fig. S10). However, this decrease in body mass was not due to a change in fat mass (Table S8). Interestingly, %fluid was increased from BPA treatment on PND215 (Table S8). Soy-based diet male offspring had increased CD4+CD8− T cells and B220+CD40L− B cells from perinatal BPA exposure, but no changes in their innate immune cell populations (Fig. S11). Absolute and %pancreas weights were found to be decreased from BPA exposure in soy-based diet male offspring (Table S9).
Discussion
With T1D incidence increasing globally, one of the biggest knowledge gaps is what environmental exposures are contributing to T1D development, and there is a great need for animal studies targeting various windows of exposures including the early developmental stages (Atkinson et al. 2014). We have found that females exposed during the juvenile period had a non-significant trend (P = 0.054) for increased T1D incidence and significantly increased BGLs, which indicated this might be a sensitive window of exposure. Adult females on the phytoestrogen-free diet were evaluated for possible interactions between BPA and age of exposure. BPA treated adult females had a nonsignificant trend of increased T1D incidence, which was consistent with our results in adult females on a soy-based diet where a significant acceleration of T1D was observed (Xu et al. 2019) and another study that exposed adult females on a phytoestrogen-free diet (Bodin et al. 2013). In contrast, perinatally exposed female offspring on a phytoestrogen-free diet were found to have reduced T1D as seen from the decreased diabetic incidence, BGLs and fasting glucose, while minimal effect on male offspring were observed. Together with our previous reports (Xu et al., 2019), it was concluded that BPA had age- and sex-dependent effects on T1D.
We found BPA alteration of GMB could play an important role in the increased BGLs from juvenile BPA exposure, since BGLs significantly correlated with microbiota changes. At the phylum level, BPA decreased many bacteria that were lower in type 2 diabetes (T2D) and/or anti-inflammatory, including Gemmatimonadetes, OD1 and Nitrospirae (Liu et al. 2017; Primec et al. 2018). Most of the genus level bacteria, including Turicibacter, Oscillospira, Ruminococcus, Jeotgalicoccus and Lachnospiraceae (other), that were increased from BPA exposure promote T1D pathogenesis and/or inflammation (Chen et al. 2018a; Gülden et al. 2018; Krych et al. 2015). Increased abundances of Ruminococcus (species level), Jeotgalicoccus (species level) and Akkermansia from BPA treatment have also previously been found in dogs, rabbits and perinatally exposed male mice, respectively (Javurek et al. 2016; Koestel et al. 2017; Reddivari et al. 2017). However, some increased bacteria following juvenile BPA exposure, including Verrucomicrobia, unclassified RF32, Akkermansia, Anaerofustis and Rhodospirillales (other), might also be anti-inflammatory and/or protective of T1D (Mao et al. 2012; Marietta et al. 2013; Sheng et al. 2018; Zhong et al. 2015; Brown et al. 2011). Although at the species level, Akkermansia and Oscillospira have also been found decreased from perinatal and adult BPA exposure, respectively, in rabbits (Reddivari et al. 2017). Previously, we found Lachnospiraceae (other) and unclassified RF32 were also increased in adult females within 1 mo. of BPA exposure, but unlike juvenile females, BPA exposure in adults after 4 mo. exposure altered different bacteria from that observed in juveniles (Xu et al. 2019). This suggests the age and duration of BPA exposure may affect which microbiota are altered.
Following juvenile BPA exposure, the cytokine/chemokines that were still altered on PND134 included increased levels of LIX, suggesting a pro-inflammation, while increased GCSF and IL-5, and decreased IL-15 and EOTAXIN are more supportive of an anti-inflammatory shift (Bobbala et al. 2017; Chao et al. 2014; Haller et al. 2016; Tuller et al. 2013; Zhang et al. 2014). In terms of immune changes, both B220+CD40L− B cells and CD4+CD8+ T cells, which can have either autoreactive or suppressive roles in immunity (Overgaard et al. 2015), were decreased after juvenile BPA exposure. CD4+CD8+ T cells negatively then positively correlated with BGLs, indicating these cells might have initially had an autoreactive role followed by a suppressive role when T1D developed. The increased CD4+CD8+ cells may be extrathymic autoreactive T cells that promote inflammation and autoimmune disease, but are still not fully understood (Overgaard et al. 2015). Additionally, increases of %CD25 cells, CD8+CD25+ cells and CD4+CD8+ cells by BPA were seen in adult females on both the soy-free diet in this study and soy-based diet previously (Xu et al. 2019). Although in the NOD mice these cells have a reduced ability to suppress autoreactive CD4+ T cells (D’Alise et al. 2008), the increase in these cell populations and negative correlations of these cells with BGLs may be a reason the increase in diabetes incidence did not reach the level of statistical significance. BPA has previously been indicated to upregulate both Th1 and Th2 responses (Kharrazian 2014; Yoshino et al. 2003). Together with the increased IgG1, IgG2b and IgM antibody levels, these results suggest that adult BPA exposure may cause a general increase in immune response.
In agreement with our studies, a study found BPA exposure from GD6 to PND21 increased β-cell mass and proliferation in C57BL6 female mice, which suggests some protective effects on β-cells from BPA during this exposure window; however, no changes in GTT, ITT or insulin production were observed (Liu et al. 2013). In our study, immune cell changes in these perinatally exposed females on a phytoestrogen-free diet showed an anti-inflammatory profile consistent with the decreased T1D incidence: (a) decreased pro-inflammatory F4/80+Gr1+ cells (Ghosn et al. 2010), (b) increased CD4+CD8− cells, which have previously been found to decrease during NOD T1D development (Young et al. 2009), and (c) decreased pro-inflammatory IgG2c, which may have adverse effects on glucose metabolism (Winer et al. 2011). Reduced serum RANTES, which activates T cells and monocytes in localized inflammatory cites, has also been shown to provide protection from T1D development (Zhernakova et al. 2006). Interestedly, the above mentioned immunomodulation from perinatal BPA exposure were detected on PND204, which indicates long lasting anti-inflammatory effects may result from perinatal BPA exposure. This was in contrast to a previous study in NOD female offspring on a phytoestrogen-free diet where 10 mg/L (~3000 μg/kg) BPA significantly exacerbated T1D, and their lower dose of 1 mg/L showed a nonsignificant trend of exacerbation (Bodin et al. 2014). This difference was probably due to different window of exposure, i.e., starting from the beginning of the preimplantation period (GD1–6), which affects glucose and insulin differently from fetal BPA exposure (GD6-PND0) (Bodin et al. 2014; Liu et al. 2013).
In conclusion, we have identified T1D development altered by BPA depending on exposure window and sex. Juvenile BPA exposure increased the risk for T1D development, but did not produce a clear shift in the immune system towards pro- or anti-inflammation, and alteration of GMB was found to be the likely mechanism of increased T1D risk. Adult female exposure showed a general increase of immune responses with a nonsignificant trend for increased T1D development. Perinatal BPA exposure on the phytoestrogen-free diet showed protective effects from T1D risk. In addition, T1D effects from peritnatal exposure was attenuated from switching to a soy-based diet and in male offspring, which has previously been observed in other studies that there existed sex-dependent differences and soy-dependent interactions with BPA (see Supplemental Material, Supplementary Discussion). Future studies using a wider range of doses and more time points examining immune effects are needed to determine the cause of these sex-dependent effects using additional exposure windows such as juvenile BPA exposure in males. Additionally, potential health consequences from juvenile and adult exposure should be further evaluated.
Supplementary Material
Acknowledgements:
We would like to thank Dr. Krzysztof Czaja’s lab for helping measure the male offspring body fat with their Minispec LF110 BAC Analyzer. This study was supported by National Institutes of Health (NIH) R21ES24487, and in part by NIH R41AT009523 and Interdisciplinary Toxicology Program at University of Georgia (UGA).
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
References
- Atkinson MA, Eisenbarth GS, Michels AW (2014) Type 1 diabetes The Lancet 383:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao M, Yang Y, Jun H-S, Yoon J-W (2002) Molecular mechanisms for gender differences in susceptibility to T cell-mediated autoimmune diabetes in nonobese diabetic mice The Journal of Immunology 168:5369–5375 [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Hand TW (2014) Role of the microbiota in immunity and inflammation Cell 157:121–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun HA, Khanal S, Zonderman AB, Beydoun MA (2014) Sex differences in the association of urinary bisphenol-A concentration with selected indices of glucose homeostasis among US adults Annals of epidemiology 24:90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC (2005) TCR stimulation with modified anti-CD3 mAb expands CD8+ T cell population and induces CD8+ CD25+ Tregs The Journal of clinical investigation 115:2904–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobbala D, Mayhue M, Menendez A, Ilangumaran S, Ramanathan S (2017) Trans-presentation of interleukin-15 by interleukin-15 receptor alpha is dispensable for the pathogenesis of autoimmune type 1 diabetes Cellular & molecular immunology 14:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodin J, Bolling AK, Becher R, Kuper F, Lovik M, Nygaard UC (2014) Transmaternal bisphenol A exposure accelerates diabetes type 1 development in NOD mice Toxicol Sci 137:311–323 doi: 10.1093/toxsci/kft242 [DOI] [PubMed] [Google Scholar]
- Bodin J, Bolling AK, Samuelsen M, Becher R, Lovik M, Nygaard UC (2013) Long-term bisphenol A exposure accelerates insulitis development in diabetes-prone NOD mice Immunopharmacol Immunotoxicol 35:349–358 doi: 10.3109/08923973.2013.772195 [DOI] [PubMed] [Google Scholar]
- Bodin J et al. (2015) Exposure to bisphenol A, but not phthalates, increases spontaneous diabetes type 1 development in NOD mice Toxicology Reports 2:99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CT et al. (2011) Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes PloS one 6:e25792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetkovic-Cvrlje M, Thinamany S, Bruner KA (2017) Bisphenol A (BPA) aggravates multiple low-dose streptozotocin-induced Type 1 diabetes in C57BL/6 mice Journal of immunotoxicology 14:160–168 [DOI] [PubMed] [Google Scholar]
- Chao GY, Wallis RH, Marandi L, Ning T, Sarmiento J, Paterson AD, Poussier P (2014) Iddm30 controls pancreatic expression of Ccl11 (Eotaxin) and the Th1/Th2 balance within the insulitic lesions The Journal of Immunology:1302383. [DOI] [PubMed] [Google Scholar]
- Chen C, You L-J, Huang Q, Fu X, Zhang B, Liu R-H, Li C (2018a) Modulation of gut microbiota by mulberry fruit polysaccharide treatment of obese diabetic db/db mice Food & function 9:3732–3742 [DOI] [PubMed] [Google Scholar]
- Chen KL, Madak-Erdogan Z (2016) Estrogen and Microbiota Crosstalk: Should We Pay Attention? Trends in Endocrinology & Metabolism 27:752–755 [DOI] [PubMed] [Google Scholar]
- Chen Y-G, Mathews CE, Driver JP (2018b) the role of NOD Mice in type 1 Diabetes research: Lessons from the Past and recommendations for the Future Frontiers in endocrinology 9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X-B, Luan J-N, Ye J, Chen S-Y (2015) RGC32 deficiency protects against high-fat diet-induced obesity and insulin resistance in mice Journal of Endocrinology 224:127–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alise AM, Auyeung V, Feuerer M, Nishio J, Fontenot J, Benoist C, Mathis D (2008) The defect in T-cell regulation in NOD mice is an effect on the T-cell effectors Proceedings of the National Academy of Sciences 105:19857–19862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delclos KB et al. (2014) Toxicity evaluation of bisphenol A administered by gavage to Sprague-Dawley rats from gestation day 6 through postnatal day 90 Toxicological Sciences:kfu022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosn EEB et al. (2010) Two physically, functionally, and developmentally distinct peritoneal macrophage subsets Proceedings of the National Academy of Sciences 107:2568–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gülden E et al. (2018) TRIF deficiency protects non-obese diabetic mice from type 1 diabetes by modulating the gut microbiota and dendritic cells Journal of autoimmunity 93:57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo TL, Wang Y, Xiong T, Ling X, Zheng J (2014) Genistein modulation of streptozotocin diabetes in male B6C3F1 mice can be induced by diet Toxicology and applied pharmacology 280:455–466 doi: 10.1016/j.taap.2014.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller MJ et al. (2016) Anti-thymocyte globulin+ G-CSF combination therapy leads to sustained immunomodulatory and metabolic effects in a subset of responders with established type 1 diabetes Diabetes:db160823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Xu J, Lefever DE, Glenn TC, Nagy T, Guo TL (2017) Genistein prevention of hyperglycemia and improvement of glucose tolerance in adult non-obese diabetic mice are associated with alterations of gut microbiome and immune homeostasis Toxicology and applied pharmacology doi: 10.1016/j.taap.2017.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley RR, Peters SA, Mishra GD, Woodward M (2015) Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis The lancet Diabetes & endocrinology 3:198–206 doi: 10.1016/s2213-8587(14)70248-7 [DOI] [PubMed] [Google Scholar]
- İnce T, Balcı A, Yalçın SS, Özkemahlı G, Erkekoglu P, Kocer-Gumusel B, Yurdakök K (2018) Urinary bisphenol-A levels in children with type 1 diabetes mellitus Journal of Pediatric Endocrinology and Metabolism 31:829–836 [DOI] [PubMed] [Google Scholar]
- Javurek AB, Spollen WG, Johnson SA, Bivens NJ, Bromert KH, Givan SA, Rosenfeld CS (2016) Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model Gut Microbes 7:471–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA et al. (2016) Effects of developmental exposure to bisphenol A on spatial navigational learning and memory in rats: A CLARITY-BPA study Hormones and behavior 80:139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörns A et al. (2014) Islet infiltration, cytokine expression and beta cell death in the NOD mouse, BB rat, Komeda rat, LEW. 1AR1-iddm rat and humans with type 1 diabetes Diabetologia 57:512–521 [DOI] [PubMed] [Google Scholar]
- Kharrazian D (2014) The potential roles of bisphenol A (BPA) pathogenesis in autoimmunity Autoimmune diseases 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koestel ZL et al. (2017) Bisphenol A (BPA) in the serum of pet dogs following short-term consumption of canned dog food and potential health consequences of exposure to BPA Science of The Total Environment 579:1804–1814 [DOI] [PubMed] [Google Scholar]
- Krych Ł, Nielsen DS, Hansen AK, Hansen CHF (2015) Gut microbial markers are associated with diabetes onset, regulatory imbalance, and IFN-γ level in NOD Mice Gut microbes 6:101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K-P, Chung Y-T, Li R, Wan H-T, Wong CK-C (2016) Bisphenol A alters gut microbiome: Comparative metagenomics analysis Environmental Pollution 218:923–930 [DOI] [PubMed] [Google Scholar]
- Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D (2008) Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults Jama 300:1303–1310 [DOI] [PubMed] [Google Scholar]
- Liu F et al. (2017) Dysbiosis of urinary microbiota is positively correlated with type 2 diabetes mellitus Oncotarget 8:3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J et al. (2013) Perinatal bisphenol A exposure and adult glucose homeostasis: identifying critical windows of exposure PloS one 8:e64143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yao Y, Li H, Qiao F, Wu J, Du Z-y, Zhang M (2016) Influence of Endogenous and Exogenous Estrogenic Endocrine on Intestinal Microbiota in Zebrafish PloS one 11:e0163895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao S, Zhang R, Wang D, Zhu W (2012) The diversity of the fecal bacterial community and its relationship with the concentration of volatile fatty acids in the feces during subacute rumen acidosis in dairy cows BMC Veterinary Research 8:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marietta EV et al. (2013) Low incidence of spontaneous type 1 diabetes in non-obese diabetic mice raised on gluten-free diets is associated with changes in the intestinal microbiome PloS one 8:e78687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard NH, Jung JW, Steptoe RJ, Wells JW (2015) CD4+/CD8+ double‐positive T cells: more than just a developmental stage? Journal of leukocyte biology 97:31–38 [DOI] [PubMed] [Google Scholar]
- Primec M et al. (2018) Clinical intervention using Bifidobacterium strains in celiac disease children reveals novel microbial modulators of TNF-< alpha> and short-chain fatty acids Clinical Nutrition [DOI] [PubMed] [Google Scholar]
- Reddivari L et al. (2017) Perinatal Bisphenol A Exposure Induces Chronic Inflammation in Rabbit Offspring via Modulation of Gut Bacteria and Their Metabolites MSystems 2:e00093–00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhlen RL et al. (2008) Low phytoestrogen levels in feed increase fetal serum estradiol resulting in the” fetal estrogenization syndrome” and obesity in CD-1 mice Environmental health perspectives 116:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Y, Zheng S, Zhang C, Zhao C, He X, Xu W, Huang K (2018) Mulberry leaf tea alleviates diabetic nephropathy by inhibiting PKC signaling and modulating intestinal flora Journal of Functional Foods 46:118–127 [Google Scholar]
- Taylor JA et al. (2011) Similarity of bisphenol A pharmacokinetics in rhesus monkeys and mice: relevance for human exposure Environmental health perspectives 119:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuller T, Atar S, Ruppin E, Gurevich M, Achiron A (2013) Common and specific signatures of gene expression and protein–protein interactions in autoimmune diseases Genes and immunity 14:67. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J (2013) The emerging global epidemic of type 1 diabetes Current diabetes reports 13:795–804 [DOI] [PubMed] [Google Scholar]
- Winer DA et al. (2011) B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies Nature medicine 17:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Huang G, Guo TL (2016) Developmental Bisphenol A Exposure Modulates Immune-Related Diseases Toxics 4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Huang G, Nagy T, Teng Q, Guo TL (2019) Sex-dependent effects of bisphenol A on type 1 diabetes development in non-obese diabetic (NOD) mice Archives of toxicology:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino S et al. (2004) Prenatal exposure to bisphenol A up‐regulates immune responses, including T helper 1 and T helper 2 responses, in mice Immunology 112:489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino S, Yamaki K, Yanagisawa R, Takano H, Hayashi H, Mori Y (2003) Effects of bisphenol A on antigen‐specific antibody production, proliferative responses of lymphoid cells, and TH1 and TH2 immune responses in mice British journal of pharmacology 138:1271–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EF, Hess PR, Arnold LW, Tisch R, Frelinger JA (2009) Islet lymphocyte subsets in male and female NOD mice are qualitatively similar but quantitatively distinct Autoimmunity 42:678–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Hu M, Lou Y, Wang Q, Mao L, Zhan Q, Jin F (2018) Environmentally relevant levels of bisphenol A affect uterine decidualization and embryo implantation through the estrogen receptor/serum and glucocorticoid-regulated kinase 1/epithelial sodium ion channel α-subunit pathway in a mouse model Fertility and sterility 109:735–744. e731 [DOI] [PubMed] [Google Scholar]
- Zhang L, Londono P, Yu L, Grimes S, Blackburn P, Gottlieb P, Eisenbarth GS (2014) MAS-1 adjuvant immunotherapy generates robust Th2 type and regulatory immune responses providing long-term protection from diabetes in late-stage pre-diabetic NOD mice Autoimmunity 47:341–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhernakova A et al. (2006) Genetic variants of RANTES are associated with serum RANTES level and protection for type 1 diabetes Genes and immunity 7:544. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Nyman M, Fåk F (2015) Modulation of gut microbiota in rats fed high‐fat diets by processing whole ‐ grain barley to barley malt Molecular nutrition & food research 59:2066–2076 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.