Abstract
BACKGROUND. In sepsis, there may be dysregulation in programed cell death pathways, typified by apoptosis and necroptosis. Programmed cell death pathways may contribute to variability in the immune response. TRAIL is a potent inducer of apoptosis. Receptor-interacting serine/threonine protein kinase-3 (RIPK3) is integral to the execution of necroptosis. We explored whether plasma TRAIL levels were associated with in-hospital mortality, organ dysfunction, and septic shock. We also explored the relationship between TRAIL and RIPK3.
METHODS. We performed an observational study of critically ill adults admitted to intensive care units at 3 academic medical centers across 2 continents, using 1 as derivation and the other 2 as validation cohorts. Levels of TRAIL were measured in the plasma of 570 subjects by ELISA.
RESULTS. In all cohorts, lower (<28.5 pg/ml) versus higher levels of TRAIL were associated with increased organ dysfunction (P ≤ 0.002) and septic shock (P ≤ 0.004). Lower TRAIL levels were associated with in-hospital mortality in 2 of 3 cohorts (Weill Cornell-Biobank of Critical Illness, P = 0.012; Brigham and Women’s Hospital Registry of Critical Illness, P = 0.011; Asan Medical Center, P = 0.369). Lower TRAIL was also associated with increased RIPK3 (P ≤ 0.001).
CONCLUSION. Lower levels of TRAIL were associated with septic shock and organ dysfunction in 3 independent ICU cohorts. TRAIL was inversely associated with RIPK3 in all cohorts.
FUNDING. NIH (R01-HL055330 and KL2-TR002385).
Keywords: Immunology, Inflammation
Keywords: Apoptosis
Circulating levels of TRAIL, a potent inducer of apoptosis, are lower in cases of septic shock and inversely associated with organ dysfunction.
Introduction
Sepsis and septic shock remain an intractable source of lost life and prolonged morbidity across the world (1). Despite the burden of this syndrome and significant effort applied to basic and clinical investigation, there remains no successful therapy for sepsis (2). Several factors contribute to the difficulty in creating such a therapy. The current definition of sepsis identifies a heterogeneous population of individuals with diverse patterns of immune response, organ dysfunction, and outcomes (3, 4). This definition includes patients with a hyperinflammatory and others with an immunosuppressed phenotype at presentation to medical care (5). Host and pathogen responses evolve over the course of an illness. Currently, there is no reliable way to characterize the immune phenotype at the bedside (2, 6). Programmed cell death pathways, such as apoptosis, have been shown to kill immune and epithelial cells in studies of sepsis and may influence the immune response (7).
TRAIL is a member of the TNF super family and functions as an immune response regulator in sepsis (8, 9). TRAIL is conditionally expressed on human immune cells following cytokine stimulation and is a potent inducer of apoptosis in these activated cells (8, 10, 11). TRAIL recruits activated leukocytes to areas of infection and initiates their apoptosis to terminate the immune response (10, 12). Gene-targeted mice lacking TRAIL have demonstrated increased nonspecific necrosis and inflammatory phenotypes that can be rescued with exogenous TRAIL administration (13). TRAIL has been evaluated in a single-center study of sepsis and septic shock, in which lower levels of TRAIL were associated with lower leukocytes and poor outcomes (14).
In addition to apoptosis, which is immunologically silent, there are a number of nonapoptotic programmed cell death pathways that may be active in sepsis. Typified by necroptosis, these nonapoptotic programmed cell death pathways differ from apoptosis by the release of intracellular contents (15–17). Released intracellular contents can further stimulate an exuberant immune response by acting as damage-associated molecular patterns (DAMPs), which are associated with further inflammation and loss of homeostasis (15, 16, 18). Necroptosis occurs through the formation of the necrosome, a 3-protein complex, including receptor-interacting serine/threonine protein kinase-3 (RIPK3) (19). Inhibition of RIPK3 in experimental models of critical illness has been associated with improved outcomes (20, 21). In a large human study of patients with critical illness, we demonstrated that RIPK3 was elevated in parallel with increased organ dysfunction, and higher levels were associated with increased risk of in-hospital mortality (22).
The relationship between circulating levels of TRAIL and RIPK3 have not been evaluated. In this study we sought to characterize levels of TRAIL as they relate to the newest clinical criteria for sepsis, focusing on organ dysfunction (4), the severity of sepsis, and levels of RIPK3. We evaluated these biomarkers in 3 ICU cohorts. We hypothesized that the level of circulating TRAIL would be lower in septic shock and would be associated with in-hospital mortality. We also hypothesized that TRAIL would be inversely related to circulating RIPK3.
Results
Baseline characteristics.
A total of 570 subjects from 3 ICUs were included (Figure 1). From October of 2014 through July of 2016, 146 subjects were prospectively recruited for the derivation cohort from Weill Cornell-Biobank of Critical Illness (WC-BOCI). Subjects were admitted to the Medical ICU at the New York-Presbyterian Hospital/Weill Cornell Center (NYP/WMC) and were recruited within 48 hours of arrival. For the validation cohorts, 269 subjects were recruited from the Asan Medical Center (ASAN) prospective registry of critical illness from May 2011 through June 2015 and 155 subjects from the Brigham and Women’s Hospital Registry of Critical Illness (BWH-RoCI) cohort from June 2010 through September 2013. Both cohorts were drawn from the medical ICU at their respective hospitals.
Figure 1. Number of subjects and date ranges for each cohort and overall.
WCM, Weill Cornell Medicine; BWH, Brigham and Women’s Hospital; ASAN, Asan Medical Center.
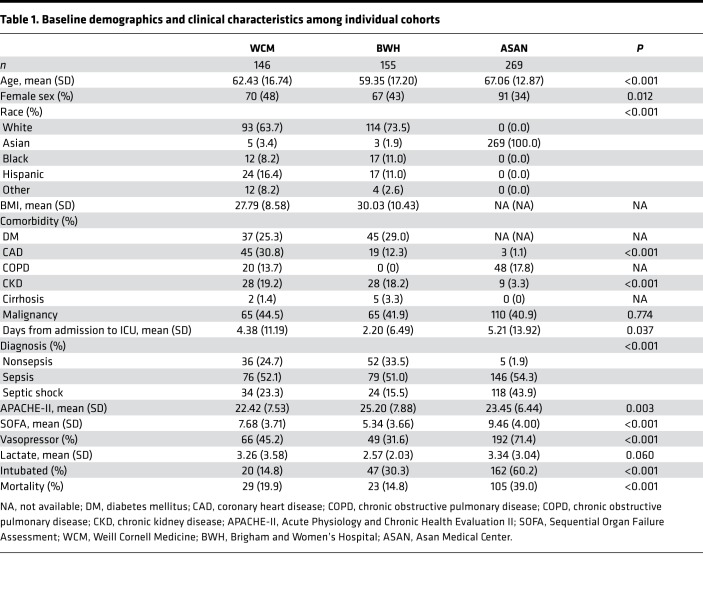
Baseline characteristics for the 3 cohorts are presented in Table 1. The BWH-RoCI cohort tended to be younger (ANOVA, P < 0.001), while ASAN recruited more male patients (P < 0.001). The distribution of comorbidities also varied across the cohorts, with chronic kidney disease and coronary artery disease more frequent in the BWH-RoCI and WC-BOCI cohorts (P < 0.001 for both conditions). A similar proportion of patients had malignancy across cohorts (P = 0.774). Patients in the ASAN cohort more frequently had sepsis and septic shock on admission. ASAN patients also had a higher mean Sequential Organ Failure Assessment (SOFA) score and were more likely to be intubated and on vasoactive agents.
Table 1. Baseline demographics and clinical characteristics among individual cohorts.
Before studying TRAIL in the validation cohorts, we analyzed the relationship between levels of TRAIL and in-hospital mortality in the WC-BOCI cohort. Youden’s index for the optimal discriminatory cut point was 28.5 pg/ml. The median interquartile range for TRAIL was 20.6 pg/ml (12.6–36.4 pg/ml, 31.7 pg/ml (20.0–59.1 pg/ml), and 21.6 pg/ml (13.0–36.0 pg/ml) for the WC-BOCI, BWH-RoCI, and ASAN cohorts, respectively.
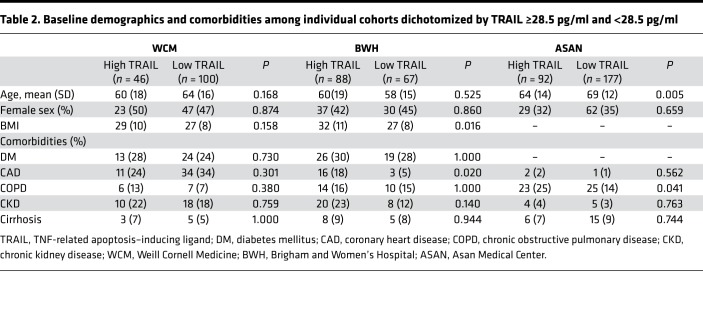
Using this cutoff, there were 46, 92, and 88 patients with high TRAIL levels and 100, 177, and 67 patients with low TRAIL levels in the WC-BOCI, ASAN, and BWH-RoCI cohorts, respectively. Baseline demographics and comorbidities, divided at TRAIL 28.5 pg/ml are presented in Table 2. Low TRAIL levels were associated with older age in the ASAN cohort but not in the BWH-RoCI or WC-BOCI cohorts. Low TRAIL levels were associated with lower BMI in the BWH-RoCI cohort but not in the other cohorts (WC-BOCI, P = 0.158; BWH-RoCI, P = 0.016; BMI not available in ASAN). The distribution of comorbidities between patients with lower and higher TRAIL levels was inconsistent across cohorts, although among patients with an active malignancy, TRAIL levels were lower than those without (WC-BOCI median 16.9 vs. 24.2 pg/ml, P = 0.009; BWH-RoCI 27.0 vs. 41.6 pg/ml, P = 0.006; ASAN 19.5 vs. 24.6 pg/ml, P = 0.006) (Supplemental Figure 2; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.127143DS1).
Table 2. Baseline demographics and comorbidities among individual cohorts dichotomized by TRAIL ≥28.5 pg/ml and <28.5 pg/ml.
Outcomes.
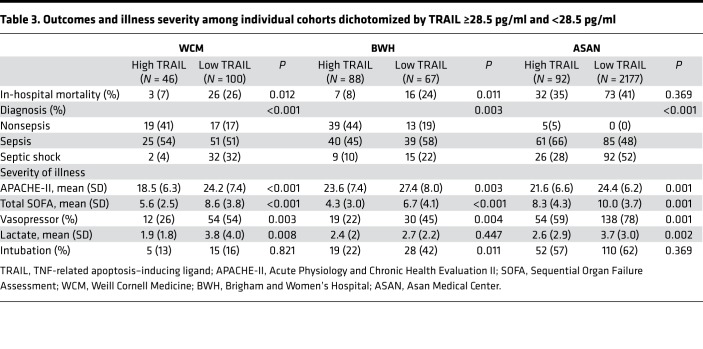
The primary endpoint of in-hospital mortality was more frequent in patients with lower TRAIL levels, as compared with higher TRAIL levels in the WC-BOCI (26% vs. 7%, P = 0.012) and BWH-RoCI (24% vs. 8%, P = 0.011) cohorts (Table 3). This relationship was consistent in the ASAN cohort, though it was not statistically significant (41% vs. 35%, P = 0.369).
Table 3. Outcomes and illness severity among individual cohorts dichotomized by TRAIL ≥28.5 pg/ml and <28.5 pg/ml.
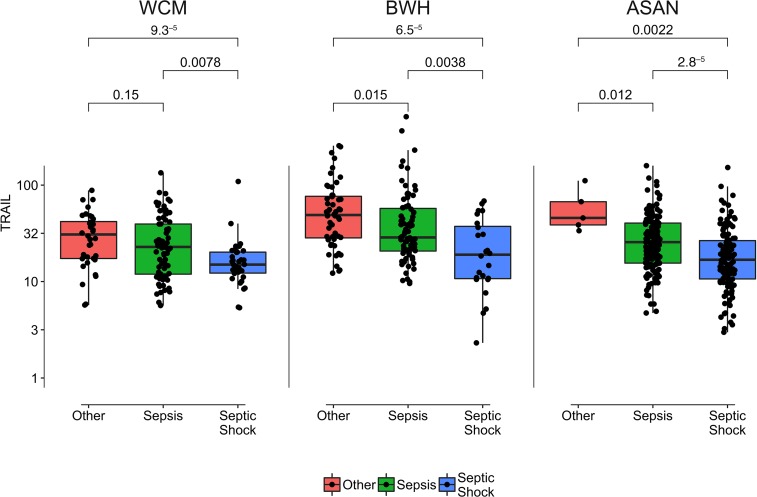
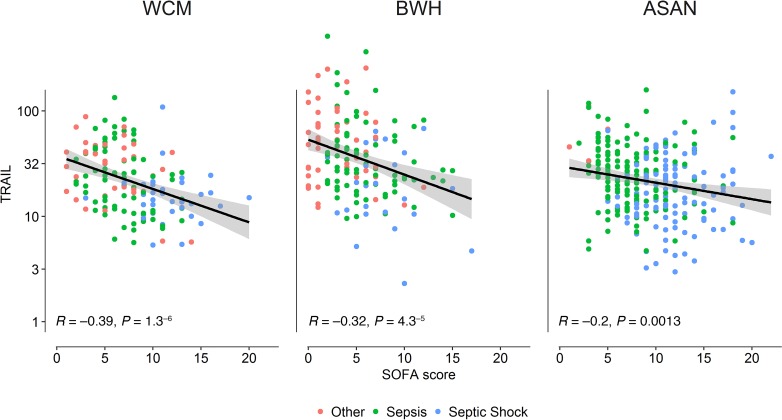
Additionally, lower TRAIL levels were consistently associated with a higher proportion of patients with septic shock as opposed to sepsis without shock in all cohorts. Median values of TRAIL by sepsis diagnosis are presented in Figure 2. Lower TRAIL levels were associated with the other secondary endpoints, with a higher baseline severity of illness by Acute Physiology and Chronic Health Evaluation II (APACHE-II) in all cohorts. Lower TRAIL levels were also associated with higher lactate in the WC-BOCI and Asan cohorts but not in the BWH-RoCI cohort. There was a negative linear relationship between TRAIL levels and SOFA scores in all cohorts, with R values of –0.39, –0.32, and –0.20 for the WC-BOCI, BWH-RoCI, and ASAN cohorts, respectively (Figure 3). The relationship between TRAIL and the individual SOFA subscores is displayed in Supplemental Figure 4. A negative linear relationship between TRAIL and RIPK3 was observed in all cohorts, with R values of –0.36, –0.44, and –0.27, respectively, for the WC-BOCI, BWH-RoCI, and ASAN cohorts (Supplemental Figure 1). Finally, there was a positive linear relationship between TRAIL and total white blood cell counts in each cohort, with R values of 0.34, 0.21, and 0.25 in the WC-BOCI, BWH-RoCI, and ASAN cohorts (Supplemental Figure 3).
Figure 2. TRAIL levels of patients with nonsepsis critical illness, sepsis, and septic shock.
TRAIL levels are presented as median value (black line), interquartile range (box), and 95% (whiskers). P values are Kruskal-Wallis nonparametric comparisons with Bonferroni adjustment for multiple comparisons. Nonsepsis critical illness is shown in pink, sepsis is shown in green, and septic shock is shown in blue. WCM, Weill Cornell Medicine; BWH, Brigham and Women’s Hospital; ASAN, Asan Medical Center.
Figure 3. Association between TRAIL level and organ failure, as measured by SOFA score.
Linear regression of TRAIL association with SOFA score, shown as a black line with the shaded area representing 95% pointwise CI, for patients with nonsepsis critical illness (pink dots), sepsis (green dots), and septic shock (blue dots). WCM, Weill Cornell Medicine; BWH, Brigham and Women’s Hospital; ASAN, Asan Medical Center.
Discussion
Using data from 570 patients in 3 cohorts across 2 continents, we described an inverse relationship between TRAIL, a potent inducer of apoptosis and immune modulator, and septic shock with in-hospital mortality. This report highlights the remarkably consistent association between lower circulating TRAIL and septic shock as compared with patients without septic shock. This supports findings from earlier animal models of sepsis (12) and a small single-center study using older criteria for sepsis and septic shock (14). Importantly, we have conducted our analysis using the Sepsis-3 definition, which highlights organ dysfunction rather than the systemic inflammatory response (3, 4). This finding adds to the growing literature suggesting that apoptosis-inducing members of the TNFA superfamily may be reduced in sepsis (23), which is in contrast to levels of TNFA, which can be increased (24).
A strength of our study comes from the evaluation of TRAIL in patients from 3 independent ICUs with differing practice patterns and baseline characteristics. Using the WC-BOCI cohort as the derivation cohort to establish a cutoff of 28.5 pg/ml, levels of TRAIL discriminated between in-hospital mortality in 2 of 3 cohorts. This cutoff for lower levels of circulating TRAIL was consistently associated with septic shock and more profound upfront severity of illness. In each cohort, we demonstrated a consistent inverse association between TRAIL and the total SOFA score. This relationship with organ dysfunction was seen despite varied admission diagnoses and comorbidities. Interestingly, the cardiovascular and platelet SOFA subscores were numerically more strongly associated with lower levels of circulating TRAIL across all cohorts than other markers of organ dysfunction (Supplemental Figure 4).
This study demonstrated a consistent association between TRAIL and septic shock; however, the pathogenic role of low levels of TRAIL in these cases is not clear. Differential TRAIL levels may be related to the dysregulation of the host immune response; however, they may also be affected by the virulence of infecting pathogens. Low TRAIL levels may also reflect appropriate apoptosis of immune cells in the setting of progressive systemic inflammation (12, 13) or may reflect low baseline immune cells numbers in these patients (Supplemental Figure 3). Additionally, low TRAIL levels may simply be related to the overall severity of illness or a significant delay in presentation. Due to these limitations in interpretation, we chose to present the differences between patients with a higher and lower levels of TRAIL and then analyze the relationship between mortality and TRAIL in an unadjusted manner. From our understanding of the biology of TRAIL, it is not clear whether severity of illness scores (APACHE-II, SOFA) are confounders or mediators of the relationship between TRAIL and mortality. Thus, we felt that including adjustments for these would interfere with the association and the evaluation of the epidemiology of TRAIL in this population (25).
This study explores the relationship between TRAIL, a potent inducer of apoptotic cell death, and RIPK3, a key protein in the pathway of necroptotic cell death. The results demonstrate that these biomarkers are inversely related to each other. Although this study does not offer direct evidence that lower TRAIL levels are associated with increased necroptotic cell death, it does suggest that septic shock may involve an imbalance among dominant types of cell death (26). Necroptosis, an immunologically activating subtype of cell death, may be maladaptive. Exuberant necroptotic activity may contribute to an excessive nonspecific host response and the loss of homeostasis in septic shock (17).
It should be emphasized that lower plasma levels of TRAIL do not directly imply a lower burden of apoptotic cell death and that an increased RIPK3 may not represent necroptosis (27). The relative increase in necroptotic machinery seen in this study may be due to other processes (18, 28). TRAIL binding to its canonical death receptors can lead to necroptotic cell death in pathologic situations where caspases are inhibited (29). Additionally, TRAIL and RIPK3 can lead to inflammasome release with subsequent inflammation (19, 27, 30). RIPK3 has been shown to be more important in the development of ventilator-induced lung injury than other molecules in the necrosome in a murine model (21). This highlights the potential role for TRAIL and RIPK3 outside of strictly apoptosis and necroptosis, respectively (31).
Our report has several additional limitations. We do not have data exploring the change in TRAIL over multiple time points in each patient. This is an important limitation, as each cohort had a different average length of time from hospital to ICU admission. Additionally, the recruitments practices varied across the cohorts. The WC-BOCI and BWH-RoCI cohorts recruited a broad group of patients from the medical ICU, while the ASAN cohort only focused on patients with a concern for infection. The distribution of ethnicities also varied. Each cohort used different criteria for admission to the ICU, and different treatments were carried out per the practice patterns of individual institutions and providers. However, this variation may be a strength to the analysis, highlighting the consistency of the relationship among TRAIL, major covariates, and outcomes, despite the analysis being conducted on an unselected population.
An additional limitation is the inconsistent relationship between lower TRAIL levels and mortality seen in our cohort at the designated cutoff point, despite the observed trend. One may speculate that this finding is related to differences in baseline unmeasured risk between cohorts or the variation in unmeasured processes of care (32, 33). However, the overall consistency of the relationships among TRAIL, septic shock, and organ dysfunction combine to make a strong association with a high chance of being replicated.
Conclusions.
Circulating TRAIL is associated with in-hospital mortality in 2 of 3 independent ICU cohorts and is lower in patients with septic shock. TRAIL is negatively associated with organ dysfunction and with a key protein related to necroptosis, RIPK3. Further work is needed to define whether lower TRAIL is definitively associated with altered cell death in sepsis and whether such a phenotype is maladaptive. Additional work exploring the kinetics of TRAIL during the course of sepsis and in different settings will be invaluable before considering TRAIL to be a robust biomarker offering potential insight to the disease course. Finally, associating TRAIL and RIPK3 with other circulating markers of apoptosis and necroptosis will be essential in improving our understanding of these processes.
Methods
Study design and patient population.
This study is an analysis of prospectively collected data from 570 subjects recruited from 3 prospective cohorts, 1 primary (n = 146) and 2 validation cohorts, admitted to ICUs from 2 continents (n = 155, n = 269). Subjects were recruited on the first or second day of their admission to the ICU. The primary cohort was derived from the WC-BOCI at the NYP/WMC, and the validation cohorts were drawn from the ASAN prospective Registry of Critical Illness and the BWH-RoCI. Protocols for recruitment, data collection, and sample processing have been described previously (22, 34–36), and further detail can be found in the Supplemental Methods. Briefly, both the WC-BOCI and BWH-RoCI cohorts recruited any patient admitted to the medical ICU, with exclusion for the lack of the ability to provide or the lack of a surrogate to provide informed consent and moribund state. At these institutions the noninfected patients have a range of diagnoses, ranging from acute liver failure and noninfectious respiratory failure to gastrointestinal hemorrhage. The ASAN investigators recruited patients who at the time of medical ICU admission had a concern for sepsis.
Clinical evaluation.
Clinical and laboratory data were collected from the electronic health records by trained research personnel, with clinical adjudication of the final diagnosis of sepsis confirmed by critical care board–certified attending physicians. Disease severity was assessed using APACHE-II (37) within the first 24 hours of ICU admission. Chronic comorbidities and immunosuppression were defined according to the APACHE definitions. Organ failure was defined by the SOFA scoring system (38), which quantifies the presence and severity of organ failure in 6 different organ systems. Missing individual organ system scores were designated as 0.
Definition of sepsis.
Subjects defined as having sepsis had a clinically documented or suspected infection that upon final adjudication was deemed to be the source of organ dysfunction. ICU subjects without a concern for infection or in whom infection was not thought to be the cause of the current admission were used as the control population. Among infected patients, subjects were further stratified based on the current Sepsis-3 definitions (4): sepsis was defined as a SOFA score ≥2, and septic shock was defined as a SOFA score ≥2, plasma lactate ≥2 mg/dl, and the need for vasopressor support to maintain a mean arterial pressure ≥65 mmHg.
Measurement of biomarkers.
The plasma isolation protocols for each individual cohort are discussed in detail in the Supplemental Methods. For all samples, blood was collected in EDTA-coated blood collection tubes. The collected blood samples were kept at 4°C and centrifuged within 4 hours. Plasma was subsequently divided into aliquots and kept at –80°C.
TRAIL concentration was measured using ELISA following the manufacturer’s protocol (DTRL00, Human TRAIL/TNFSF10 Quantikine Immunoassay, R&D Systems). RIPK3 concentration was measured previously (21, 22) using a commercially available ELISA kit as per the manufacturer’s instructions (CSB-EL019737HU, CUSABIO). For both biomarkers, the limits of detection, observed range, and intraindividual coefficients of variation are reported in the Supplemental Methods.
Outcomes.
The primary analysis for this study was discriminative ability of TRAIL related to overall in-hospital mortality. Secondary outcomes included the relationship between TRAIL and the diagnosis of sepsis and septic shock; initial organ dysfunction, as defined by the SOFA score; and levels of RIPK3. Further exploratory outcomes included the association between the level of TRAIL and circulating leukocytes and the level of TRAIL and other baseline laboratory and clinical values.
Statistics.
TRAIL concentration was analyzed after a log10 transformation. In-hospital survival was assessed in a binary fashion at time of discharge from the hospital. In the primary cohort, WC-BOCI, logistic regression was used to assess the discriminatory capability of TRAIL to differentiate between survivors and nonsurvivors. The optimal cutoff between levels of TRAIL and mortality was assessed using Youden’s index. Once established, this cutoff value was used to assess the differential associations among levels of TRAIL, baseline characteristics, and outcomes. Differences between baseline characteristics and outcomes were compared by χ2, Fisher’s exact, 2-tailed Student’s t test, and Wilcoxon rank-sum test, as appropriate. Comparisons across cohorts were done with ANOVA or χ2 tests. Associations with organ failure, as measured by SOFA score and with each SOFA component (platelets, bilirubin, creatinine, respiratory, Glasgow comma score, and cardiovascular) were studied using unadjusted linear regression. Additionally, linear regression was used to analyze the association between TRAIL and RIPK3 and TRAIL and white blood cell counts. All analyses were conducted in R (R Foundation for Statistical Computing). For all analyses, P < 0.05 was considered statistically significant, and 95% CIs are presented where appropriate.
Study approval.
Study protocols were approved by institutional review boards of Weill Cornell Medicine (1405015116A005), BWH (2008-P-000495), and ASAN (2011-0001). Written informed consent was obtained from all subjects or their surrogates.
Author contributions
KCM, AMKC, EJS, and IIS contributed to the conception and design of the study. KCM, EJS, ES, RMB, LEF, and JWH contributed to the collection of clinical data and biologic samples. KCM, ERG, and TN contributed to the performance of assays. EJS and CO performed all statistical analysis. EJS contributed to the drafting of the manuscript. KCM, EJS, IIS, CO, ERG, DRP, and AMKC contributed to the editing of manuscript. CO contributed to the creation of the figures. EJS, KCM, and IIS contributed to the editing of the figures. All authors contributed to the final approval of all submitted contents.
Supplementary Material
Acknowledgments
The authors would like to acknowledge valuable discussion and input from Kiichi Nakahira (Weill Cornell Medicine). This work was supported by NIH grants R01-HL055330 and KL2-TR002385. Portions of this work have previously been published in abstract form (39, 40).
Version 1. 05/02/2019
Electronic publication
Footnotes
Role of funding source: This study was funded by the NIH. The NIH had no role in study design, data collection, data analysis, data interpretation, or preparation of the manuscript.
Conflict of interest: AMKC is a cofounder and stockholder of and serves on the scientific advisory board for Proterris, which develops therapeutic uses for carbon monoxide. AMKC also has a use patent (7,678,390; carbon monoxide as a biomarker and therapeutic agent) on carbon monoxide. AMKC served as a consultant for Teva Pharmaceuticals in 2018.
Copyright: © 2019 American Society for Clinical Investigation
Reference information: JCI Insight. 2019;4(9):e127143. https://doi.org/10.1172/jci.insight.127143.
Contributor Information
Thomas Nicholson, Email: tnicho19@jhmi.edu.
Clara Oromendia, Email: mco2004@med.cornell.edu.
Elizabeth Sanchez, Email: els2314@med.cornell.edu.
Rebecca M. Baron, Email: rbaron@partners.org.
Ilias I. Siempos, Email: isiempos@yahoo.com.
References
- 1.Liu V, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312(1):90–92. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC. The search for effective therapy for sepsis: back to the drawing board? JAMA. 2011;306(23):2614–2615. doi: 10.1001/jama.2011.1853. [DOI] [PubMed] [Google Scholar]
- 3.Seymour CW, et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045. doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen J, et al. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15(5):581–614. doi: 10.1016/S1473-3099(15)70112-X. [DOI] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6(11):813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 8.Benedict CA, Ware CF. TRAIL: not just for tumors anymore? J Exp Med. 2012;209(11):1903–1906. doi: 10.1084/jem.20122235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janssen EM, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434(7029):88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 10.Condotta SA, Cabrera-Perez J, Badovinac VP, Griffith TS. T-cell-mediated immunity and the role of TRAIL in sepsis-induced immunosuppression. Crit Rev Immunol. 2013;33(1):23–40. doi: 10.1615/CritRevImmunol.2013006721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassatella MA, et al. Interferon-activated neutrophils store a TNF-related apoptosis-inducing ligand (TRAIL/Apo-2 ligand) intracellular pool that is readily mobilizable following exposure to proinflammatory mediators. J Leukoc Biol. 2006;79(1):123–132. doi: 10.1189/jlb.0805431. [DOI] [PubMed] [Google Scholar]
- 12.Cziupka K, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) improves the innate immune response and enhances survival in murine polymicrobial sepsis. Crit Care Med. 2010;38(11):2169–2174. doi: 10.1097/CCM.0b013e3181eedaa8. [DOI] [PubMed] [Google Scholar]
- 13.Steinwede K, et al. TNF-related apoptosis-inducing ligand (TRAIL) exerts therapeutic efficacy for the treatment of pneumococcal pneumonia in mice. J Exp Med. 2012;209(11):1937–1952. doi: 10.1084/jem.20120983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian Y, et al. Soluble tumor necrosis factor related apoptosis inducing ligand level as a predictor of severity of sepsis and the risk of mortality in septic patients. PLoS ONE. 2013;8(12):e82204. doi: 10.1371/journal.pone.0082204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schenck EJ, Ma KC, Murthy SB, Choi AMK. Danger Signals in the ICU. Crit Care Med. 2018;46(5):791–798. doi: 10.1097/CCM.0000000000003007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galluzzi L, Kepp O, Chan FK, Kroemer G. Necroptosis: Mechanisms and Relevance to Disease. Annu Rev Pathol. 2017;12:103–130. doi: 10.1146/annurev-pathol-052016-100247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;370(5):455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 19.Newton K, et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343(6177):1357–1360. doi: 10.1126/science.1249361. [DOI] [PubMed] [Google Scholar]
- 20.Duprez L, et al. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011;35(6):908–918. doi: 10.1016/j.immuni.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Siempos, et al. RIPK3 mediates pathogenesis of experimental ventilator-induced lung injury. JCI Insight. 2018;3(9):e97102. doi: 10.1172/jci.insight.97102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma KC, et al. Circulating RIPK3 levels are associated with mortality and organ failure during critical illness. JCI Insight. 2018;3(13):e99692. doi: 10.1172/jci.insight.99692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roderburg C, et al. Serum Levels of TNF Receptor Ligands Are Dysregulated in Sepsis and Predict Mortality in Critically Ill Patients. PLoS One. 2016;11(4):e0153765. doi: 10.1371/journal.pone.0153765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kothari N, et al. Tumor necrosis factor gene polymorphism results in high TNF level in sepsis and septic shock. Cytokine. 2013;61(2):676–681. doi: 10.1016/j.cyto.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Lederer DJ, et al. Control of Confounding and Reporting of Results in Causal Inference Studies. Guidance for Authors from Editors of Respiratory, Sleep, and Critical Care Journals. Ann Am Thorac Soc. 2019;16(1):22–28. doi: 10.1513/AnnalsATS.201808-564PS. [DOI] [PubMed] [Google Scholar]
- 26.Ma KC, Schenck EJ, Pabon MA, Choi AMK. The Role of Danger Signals in the Pathogenesis and Perpetuation of Critical Illness. Am J Respir Crit Care Med. 2018;197(3):300–309. doi: 10.1164/rccm.201612-2460PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kearney CJ, Martin SJ. An Inflammatory Perspective on Necroptosis. Mol Cell. 2017;65(6):965–973. doi: 10.1016/j.molcel.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 28.Jorgensen I, Rayamajhi M, Miao EA. Programmed cell death as a defence against infection. Nat Rev Immunol. 2017;17(3):151–164. doi: 10.1038/nri.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Karstedt S, Montinaro A, Walczak H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat Rev Cancer. 2017;17(6):352–366. doi: 10.1038/nrc.2017.28. [DOI] [PubMed] [Google Scholar]
- 30.Mandal P, et al. Caspase-8 Collaborates with Caspase-11 to Drive Tissue Damage and Execution of Endotoxic Shock. Immunity. 2018;49(1):42–55.e6. doi: 10.1016/j.immuni.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weng D, et al. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc Natl Acad Sci USA. 2014;111(20):7391–7396. doi: 10.1073/pnas.1403477111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson ME, Hopkins RO, Brown SM. Long-Term Functional Outcome Data Should Not in General Be Used to Guide End-of-Life Decision-Making in the ICU. Crit Care Med. 2019;47(2):264–267. doi: 10.1097/CCM.0000000000003443. [DOI] [PubMed] [Google Scholar]
- 33.Benoit DD, et al. Outcome in patients perceived as receiving excessive care across different ethical climates: a prospective study in 68 intensive care units in Europe and the USA. Intensive Care Med. 2018;44(7):1039–1049. doi: 10.1007/s00134-018-5231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finkelsztein EJ, et al. Comparison of qSOFA and SIRS for predicting adverse outcomes of patients with suspicion of sepsis outside the intensive care unit. Crit Care. 2017;21(1):73. doi: 10.1186/s13054-017-1658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dolinay T, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med. 2012;185(11):1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park J, et al. Plasma surfactant protein-D as a diagnostic biomarker for acute respiratory distress syndrome: validation in US and Korean cohorts. BMC Pulm Med. 2017;17(1):204. doi: 10.1186/s12890-017-0532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Vincent JL, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 39. Nicholson T, et al. Plasma level of TRAIL is associated with severity of sepsis and predicts survival after critical illness. Eur Respir J. 2016;48:OA3021. https://erj.ersjournals.com/content/48/suppl_60/OA3021 Accessed April 22, 2019.
- 40. Ma KC, et al. Plasma TRAIL levels correlate with organ injury and predict organ failure during critical illness. Am J Respir Crit Care Med. 2017;195:A6822. https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2017.195.1_MeetingAbstracts.A6822 Accessed April 22, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.