Abstract
Recent seminal studies have revealed that laboratory mice differ from adult humans with regard to the frequency, number, and distribution of memory T cells. Because our data show that memory T cells are more susceptible to sepsis-induced death than naive T cells, in this study we developed a model in which mice possess a memory T cell compartment more similar to that of adult humans, to better study immune responses during sepsis in the more physiologically relevant context of high frequencies of memory T cells. Using this model, we found that CD44hi memory T cells significantly upregulated the coinhibitory molecule 2B4 during sepsis, and 2B4+ memory T cells coexpressed markers of both activation and exhaustion. Genetic deficiency in 2B4 resulted in decreased mortality during sepsis. Mechanistically, this decreased mortality was associated with reduced caspase-3/7+ apoptotic T cells in 2B4–/– relative to WT, septic hosts. These results were corroborated by analysis of PBMCs isolated from human patients with sepsis, which showed increased frequencies of caspase-3/7+ apoptotic cells among 2B4+ relative to 2B4– T cells. Thus, 2B4 plays a critical role in sepsis-induced apoptosis in both murine memory T cells and those isolated from human patients with sepsis.
Keywords: Immunology, Infectious disease
Keywords: T cells
Coinhibitory molecule 2B4 plays a critical role in sepsis-induced apoptosis in both murine memory T cells and those isolated from human septic patients.
Introduction
Sepsis is the leading cause of death among critically ill patients in the US, with between 270,000 and 370,000 people dying from the disease annually (1). Outside of antibiotics, treatment of sepsis is nonspecific, aimed at early cardiopulmonary resuscitation to minimize the adverse systemic effects of infection (2). Although this approach is frequently effective (3), there are no approved therapeutics available for sepsis once antibiotics and supportive therapy fail (4). As the third most common cause of death in the US following heart disease and cancer (5–8), sepsis represents a prevalent and deadly public health issue.
T cells play a crucial role in host immune response during sepsis (4, 9, 10). Moreover, it is well known that a significant T cell loss occurs during sepsis both in animal and in human studies (9–14). We recently showed that sepsis results in the preferential attrition of memory phenotype CD8+ T cells relative to naive CD8+ T cells via apoptosis (15), suggesting that the frequency of CD8+ memory T cells in a given individual before the onset of sepsis could be an important factor in sepsis pathogenesis. In normal adult human subjects, the peripheral T cell compartment consists of about 30%–70% memory T cells. In contrast, laboratory mice housed under specific pathogen–free conditions possess only about 10%–20% memory T cells (16, 17). A recent seminal study by Beura and colleagues showed that exposure of laboratory animals to pet store or feral mice resulted in the rapid generation of CD8+ memory T cell populations in these animals, at levels that approximate those observed in adult humans (18). Thus, to better model the antigen-experienced immune system of human patients, in this study we have developed a model to generate mice that possess a memory T cell compartment more similar to that of adult humans, to study the T cell response to sepsis in the more physiologically relevant context of high frequencies of CD8+ memory T cells.
The ultimate outcome of T cell activation is determined by the balance of costimulatory and coinhibitory molecule signals received during T cell priming or recall (19). In addition to naive precursors, memory T cells are potently regulated by ligation of coinhibitory receptors on their cell surfaces (20–22). Furthermore, numerous studies have demonstrated that coinhibitory signals play a critical role in regulating T cell responses during sepsis (23–28). For example, increased expression of coinhibitory molecules, including programmed cell death 1 (PD-1) and B and T lymphocyte attenuator, has been identified on the surface of T cells isolated from patients with sepsis as opposed to those obtained from controls without sepsis (14), and blockade of these pathways may represent a therapeutic strategy for the amelioration of morbidity and mortality in individuals with sepsis (24, 26, 27, 29–34). In particular, many published studies demonstrate that anti–PD-1 and anti–programmed cell death ligand 1 (anti–PD-L1) improve survival in murine models of sepsis (25, 26, 32, 35–41), and a clinical trial of anti–PD-L1 in sepsis has been conducted (https://clinicaltrials.gov NCT02576457). In addition, we recently found that 2B4 signals are significantly associated with the outcome of sepsis pathogenesis (42). 2B4-knockout mice had a significantly better survival rate after cecal ligation and puncture (CLP) compared with WT mice. However, the role of these coinhibitory molecules on memory CD8+ T cell responses during sepsis is not well understood. In this study, we used a potentially novel “memory mouse” model to interrogate the role of the 2B4 coinhibitory pathway in sepsis pathogenesis in antigen-experienced hosts, and this model may better recapitulate the mature immune systems of adult patients with sepsis.
Results
Generation of “memory mice” for use in studies of sepsis pathogenesis.
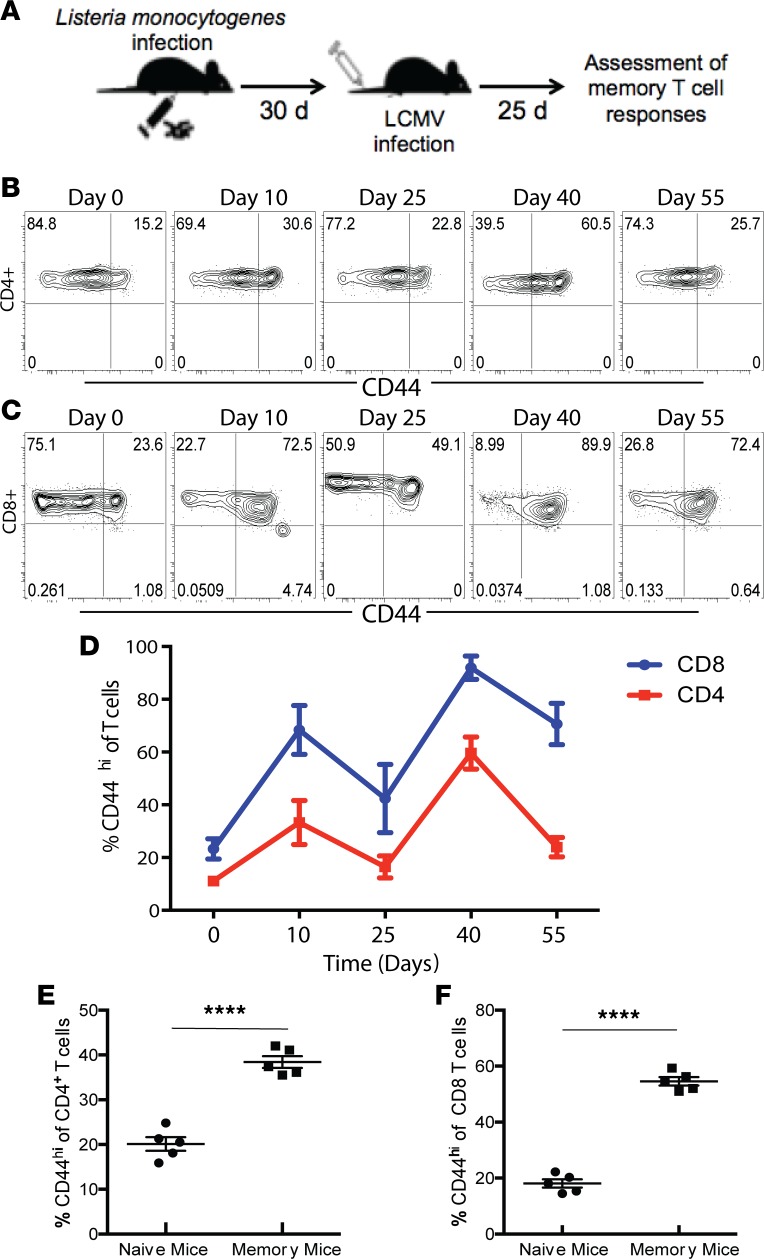
Naive B6 animals were first infected with a Listeria monocytogenes bacterial infection followed by an lymphocytic choriomeningitis virus (LCMV) viral infection as described in Methods to generate an effector T cell response that would resolve and generate memory T cells (Figure 1A). We assessed the magnitude and kinetics of the CD4+ and CD8+ T cell responses during the expansion and contraction of the immune response following these infections. The frequency of CD44hi cells among both CD4+ and CD8+ T cell populations significantly increased on day 10 after Listeria infection and then significantly decreased by day 25 after Listeria infection (Figure 1, B–D). Mice were then infected with LCMV, and 10 days later the frequency of CD44hi cells among both CD4+ and CD8+ T cell populations significantly increased again (day 40, Figure 1, B–D) and then decreased on day 55 after Listeria infection (day 25 after LCMV infection). In sum, results indicate that our model resulted in the generation of animals that possess a significantly increased frequency of CD44hi memory T cells in both the CD4+ and CD8+ T cell compartments (CD4: 38.4% ± 1.3% versus 20.1% ± 1.5%, P < 0.0001; CD8: 54.6% ± 1.5% versus 18.1% ± 1.4%, P < 0.0001) (Figure 1, E and F). As shown in Supplemental Figure 1 (supplemental material available online with this article; https://doi.org/10.1172/jci.insight.126030DS1), the increase in memory T cells shown in Figure 1, E and F, was due almost entirely to an increase in CD44hiCD62Llo effector memory cells (Tem) in both the CD4+ and CD8+ T cell compartments.
Figure 1. Generation and characterization of polyclonal memory T cells following infection.
Naive B6 mice were infected with Listeria and allowed to develop a population of memory T cells. Mice were infected with LCMV 30 days later. Establishment of memory was assessed by flow cytometry 25 days after LCMV infection. (A) Schematic of experimental design. (B and C) Representative flow plots depicting CD44 expression on splenic CD4+ T cells following infection. (D) Expansion of CD44+ T cells in the spleen over time following antigenic challenge (n = 20/group). (E and F) Summary of frequency of CD44hi cells among CD4+ and CD8+ T cell compartments in memory mice on day 55 following infection (per schematic in A) in the blood, compared with the frequency of CD44hi cells in the blood of naive uninfected animals. Groups (n = 5) were compared with the Mann-Whitney nonparametric test. ****P < 0.0001. All data expressed as mean ± SEM.
“Memory mice” exhibit significantly increased T cell loss during sepsis compared with naive hosts.
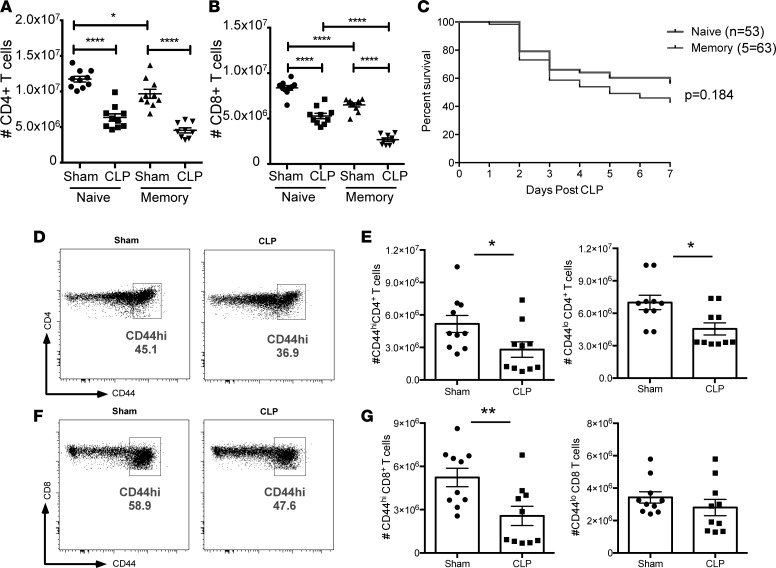
Memory mice, as well as age- and housing-matched naive controls, were then subjected to CLP. Animals were sacrificed at 24 hours after CLP to assess the impact of sepsis on the magnitude of the T cell compartment. We found that antigen-experienced memory mice exhibited a statistically significant increase in loss of CD8+ (but not CD4+) T cells following sepsis compared with naive septic animals (2.68 × 106 ± 1.85 × 105 versus 5.28 × 106 ± 3.01 × 105, P < 0.0001) (Figure 2, A and B). Based on the results that CLP led to a greater loss of CD8+ T cells in memory mice compared with naive septic control animals, we hypothesized that memory mice would exhibit increased mortality during sepsis. However, analysis of 7-day survival revealed no statistically significant difference between naive and memory mice after CLP (Figure 2C).
Figure 2. “Memory mice” exhibit significantly increased T cell loss during sepsis compared with naive hosts.
(A and B) Summary quantification of total number of CD4+ and CD8+ T cells in the spleen following sham or CLP surgery in either naive mice or memory mice generated as described in Figure 1. Groups (n = 10) were compared using a nonparametric, 2-way ANOVA. (C) Kaplan-Meier survival analysis of mortality in animals that had sepsis induced via CLP in either the presence or absence of memory T cells. (D) Representative flow cytograms indicating CD44 expression on CD4+ T cells after sham or CLP surgery. (E) Summary of total CD44hiCD4+ and CD44loCD4+ T cells, respectively, after sham or CLP surgery. (F) Representative flow cytograms indicating CD44 expression on CD8+ T cells after sham or CLP surgery. (G) Summary of total CD44hiCD4+ and CD44loCD8+ T cells, respectively, after sham or CLP surgery. In E and G, groups (n = 10) were compared with the Mann-Whitney nonparametric test. *P < 0.05, **P < 0.01, and ****P < 0.0001.
Given the findings that memory mice exhibited increased attrition of CD8+ T cell populations relative to naive controls following sepsis, we next sought to determine whether sepsis has a differential effect on naive (CD44lo) versus memory (CD44hi) T cell populations. Memory mice, generated as described above, were subjected to CLP or sham operation and splenocytes were harvested 24 hours after surgery. Results indicated that compared with the sham operation, both CD44hiCD4+ (2.81 × 106 ± 7.07 × 105 versus 5.17 × 106 ± 7.88 × 105, P = 0.038) and CD44loCD4+ (5.56 × 106 ± 5.60 × 105 versus 7.00 × 106 ± 6.64 × 105, P = 0.011) T cells were significantly reduced following sepsis (Figure 2, D and E), indicating that sepsis-induced T cell attrition affects both naive and memory CD4+ T cells. In contrast, for CD8+ T cells, only the CD44hi population was significantly reduced following CLP (2.57 × 106 ± 6.68 × 105 versus 5.23 × 106 ± 6.37 × 105, P = 0.010) (Figure 2, F and G), while the CD44loCD8+ T cell population was preserved. These data indicate that sepsis-induced T cell attrition preferentially affects memory CD8+ T cell populations compared with naive CD8+ T cell populations.
2B4 coinhibitory receptor is preferentially upregulated on CD44hi memory T cells during sepsis.
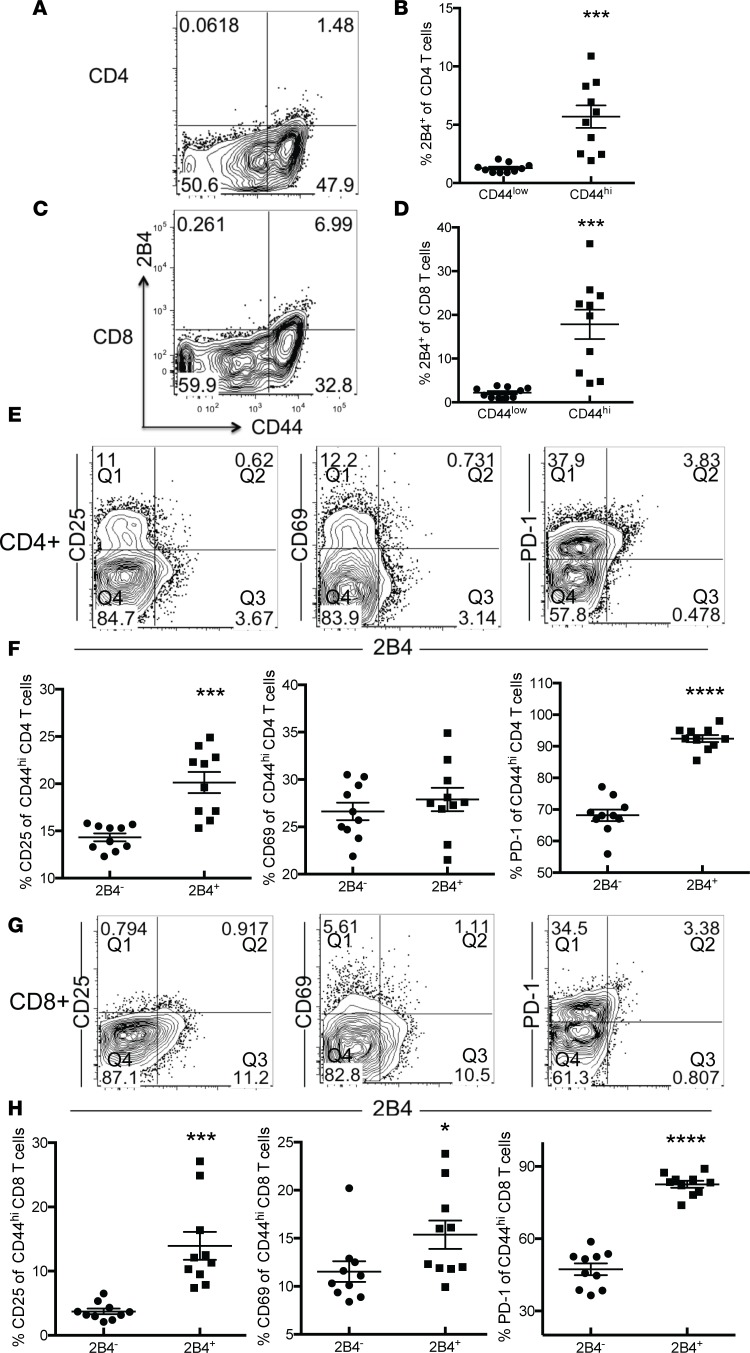
Because we found that CD44hiCD8+ T cells were preferentially lost relative to CD44loCD8+ T cells during sepsis, we queried the mechanism underlying increased CD8+ memory T cell sensitivity to cell loss during sepsis. Because we previously found that 2B4 expression was significantly upregulated on total CD4+ and CD8+ T cell populations following sepsis, we posited that it may play a crucial role in CD8+ memory T cell loss in these memory mice. To test this, we first assessed expression of the 2B4 coinhibitory receptor on naive CD44lo and memory CD44hiCD4+ and CD44hiCD8+ T cells in memory mice at 24 hours after CLP. The results showed that the frequency of 2B4-expressing T cells increased in both the CD44hiCD4+ (Figure 3, A and B) and CD44hiCD8+ (Figure 3, C and D) subsets following sepsis but not in the CD44loCD4+ and CD44loCD8+ subsets.
Figure 3. 2B4 coinhibitory receptor is preferentially upregulated on CD44hi memory T cells during sepsis.
Naive B6 mice were infected with Listeria. Mice were infected with LCMV 30 days later. CLP was performed 25 days after LCMV infection, and expression of 2B4 on CD44lo and CD44hi CD4+ and CD8+ T cells in the spleen was assessed via flow cytometry. (A) Frequency of 2B4 expression on total CD4+ T cells. (B) Summary data depicting frequency of 2B4 expression on CD44loCD4+ and CD44hiCD4+ T cells. (C) Frequency of 2B4 expression on total CD8+ T cells. (D) Summary data depicting frequency of 2B4 expression on CD44lo and CD44hi CD8+ T cells. (E) Representative plots depicting frequency of CD25, CD69, and PD-1 within 2B4+CD4+ and 2B4–CD4+ T cell compartments. (F) Summary of data presented in E. Percentages of CD25+, CD69+, and PD-1+ of 2B4– cells were calculated by dividing Q1 in the plot shown by Q4. Percentages of CD25+, CD69+, and PD-1+ of 2B4+ cells were calculated by dividing Q2 in the plot shown by Q3. (G) Representative plots depicting frequency of CD25, CD69, and PD-1 within 2B4+CD4+ and 2B4–CD8+ T cell compartments. (H) Summary of data presented in G. Percentages of CD25+, CD69+, and PD-1+ of 2B4– cells were calculated by dividing Q1 in the plot shown by Q4. Percentages of CD25+, CD69+, and PD-1+ of 2B4+ cells were calculated by dividing Q2 in the plot shown by Q3. Groups (n = 10) were compared with the Mann-Whitney nonparametric test. *P < 0.05, ***P < 0.001, and ****P < 0.0001.
To understand the impact of 2B4 expression on memory T cells during sepsis, we evaluated differences in T cell activation between 2B4hiCD44hi and 2B4loCD44hi memory T cells in both the CD4+ and CD8+ T cell compartments. Compared with 2B4–CD4+ T cells, CD25 and PD-1 were significantly upregulated on 2B4+CD4+ T cells (CD25: 20.13% ± 1.11% versus 14.33% ± 0.42%, P = 0.0001; PD-1: 92.44% ± 1.12% versus 68.16% ± 1.84%, P < 0.0001). There was no difference in expression of CD69 between 2B4+CD4+ and 2B4–CD4+ T cells (Figure 3, E and F). In contrast, expression of CD25, CD69, and PD-1 were all significantly higher on 2B4+CD8+ T cells relative to 2B4–CD8+ T cells (CD25: 13.94% ± 2.17% versus 3.72% ± 0.43%, P = 0.0002; CD69: 15.37% ± 1.48% versus 11.53% ± 1.07%, P = 0.0495; PD-1: 82.60% ± 1.41% versus 47.33% ± 2.41%, P < 0.0001) (Figure 3, G and H).
2B4 deficiency rescues memory mice from sepsis mortality.
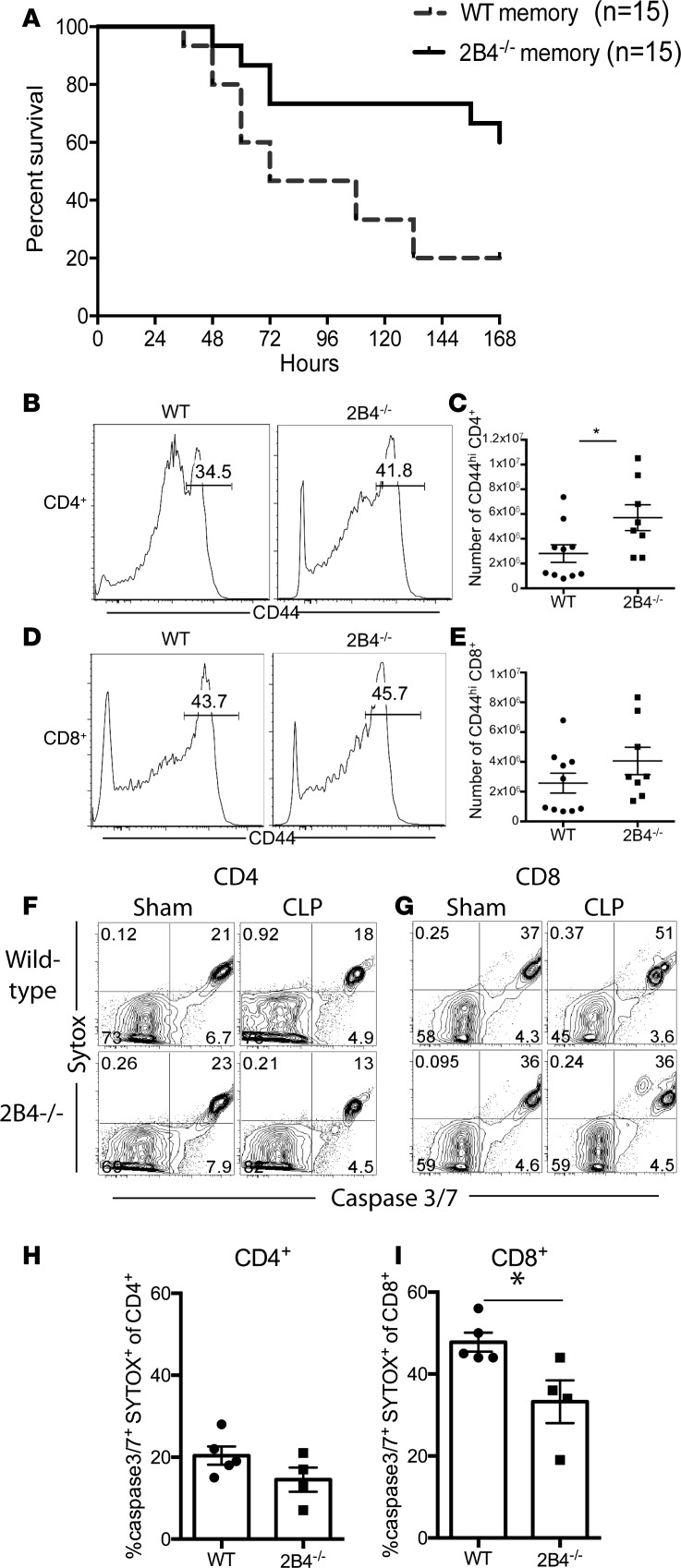
Because we found that 2B4+ memory T cells exhibited markers of both increased activation (CD25, CD69) and exhaustion (PD-1) compared with 2B4– T cells during sepsis, we queried the functional impact of 2B4 signaling during sepsis in memory mice by interrogating overall mortality in WT versus 2B4–/– memory mice following CLP. WT and 2B4–/– memory mice were generated using the method described above. We observed no difference in the frequency of CD4+ or CD8+ memory T cells generated following Listeria and LCMV infections in 2B4–/– compared with WT animals before sepsis induction (data not shown). Animals were then subjected to CLP. Importantly, results demonstrated a significant improvement in the 7-day survival of 2B4–/– memory mice compared with WT memory mice (Figure 4A).
Figure 4. 2B4 deficiency rescues memory mice from sepsis mortality.
(A) Kaplan-Meier survival curve comparing mortality of WT memory (n = 15) and 2B4–/– memory mice (n = 15) after CLP. P < 0.0001. (B–E) Analysis of absolute numbers of CD44hiCD4+ (B and C) and CD44hiCD8+ (D and E) T cells obtained from the spleens of WT versus 2B4–/– T cells at 24 hours after CLP. (F–I) Analysis of caspase-3/7+ and SYTOX+ cells among CD4+ and CD8+ T cell subsets obtained from the spleens of WT and 2B4–/– animals at 24 hours after CLP. Groups (n = 4–5/group, representative of 2 independent experiments with a total of n = 10/group) were compared with the Mann-Whitney nonparametric test. *P < 0.05.
To interrogate the cellular changes associated with improved mortality in the 2B4–/– memory mice, we assessed the magnitude of the CD4+ and CD8+ T cell compartments in 2B4–/– and WT memory mice following CLP. Results indicated that the absolute numbers of CD44hiCD4+ T cells were significantly higher in 2B4–/– memory mice with sepsis compared with WT memory mice with sepsis (5.71 × 106 ± 1.04 × 106 in 2B4–/– versus 2.81 × 106 ± 7.07 × 105 in WT, P = 0.030) (Figure 4, B and C). Absolute numbers of CD44hiCD8+ T cells in 2B4–/– memory mice with sepsis compared with WT memory mice with sepsis were not significantly different but showed a trend toward being increased (Figure 4, D and E).
To determine whether a difference in induction of apoptosis might underlie these alterations in cell numbers and mortality in 2B4–/– mice during sepsis, the frequency of cells expressing active caspase-3/7 and staining positively with SYTOX, a viability dye, was assessed. Results indicated that while the frequency of caspase-3/7+SYTOX+CD4+ T cells was not significantly different between WT and 2B4–/– mice following CLP (Figure 4, F and H), 2B4–/–CD8+ T cells contained significantly fewer caspase-3/7+SYTOX+ cells compared with WT CD8+ T cells following CLP (Figure 4, G and I).
Human 2B4+ T cells exhibit increased frequencies of active caspase-3/7+ cells compared with 2B4– T cells during sepsis.
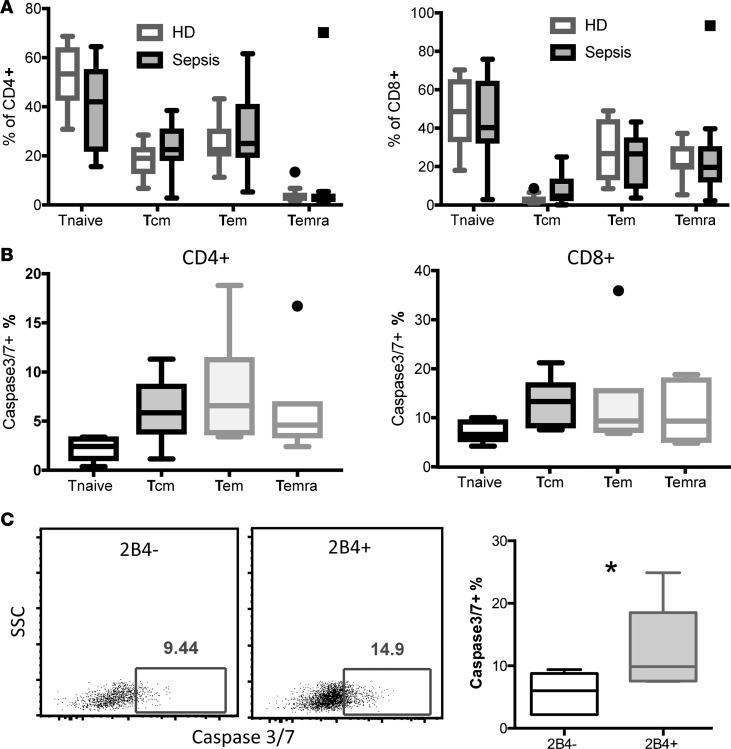
Given these data suggesting that 2B4 expression can promote mortality and immune dysregulation in antigen-experienced mice following sepsis, we next sought to understand the association of 2B4 expression with T cell death in human septic patients. To approach this, we collected PBMCs under an IRB-approved protocol from n = 10 healthy donors (HDs) or n = 14 patients who were hospitalized in the intensive care unit (ICU) and had received a diagnosis of sepsis within 24 hours. Patient demographic and clinical data are summarized in Supplemental Table 1. Results indicated that total frequencies of CD4+ and CD8+ naive T cells, central memory T cells, Tem, and terminal-differentiated effector memory cells (Temra) were not different between patients with sepsis versus HDs (Figure 5A). Moreover, frequencies of cells expressing active caspase-3/7, an indicator of apoptotic cell death, were not increased in memory T cell subsets compared with naive T cells in either the CD4+ or CD8+ T cell compartment of patients with sepsis (Figure 5B), indicating that in these patients, memory T cell status alone was insufficient to confer an increased frequency of apoptotic cells following sepsis. However, analysis of the 2B4+ subset within the CD8+ T cell compartment revealed an increased frequency of caspase-3/7+ apoptotic cells compared with the 2B4–CD8+ T cell subset (Figure 5C). In sum, these data suggest that 2B4 expression on CD8+ T cells is also associated with increased cell apoptosis in human patients with sepsis.
Figure 5. 2B4+CD8+ T cells in patients with sepsis exhibited increased cell apoptosis.
PBMCs were isolated from n = 14 patients with sepsis under an IRB-approved protocol within 24 hours of a sepsis diagnosis and from n = 10 normal healthy controls. (A) Frequencies of memory T cell subsets in n = 10 HDs versus n = 7 patients with sepsis. Naive T cells (Tnaive) were identified by gating on CD45RA+CCR7+; central memory T cells (Tcm) were gated on CD45RA–CCR7+; Tem were gated on CD45RA–CCR7–; and Temra were gated on CD45RA+CCR7–. Cells from septic patients 1–7 as identified in Supplemental Table 1 were used in A. The box plots depict the minimum and maximum values (whiskers), the upper and lower quartiles, and the median. The length of the box represents the interquartile range. (B) Active caspase 3/7 staining on CD4+ and CD8+ T cell subsets isolated from n = 7 patients with sepsis was determined by flow cytometry. Summary data of frequencies of active caspase-3/7+ T cells within memory T cell subsets of CD4+ T cells (left) and CD8+ T cells (right) are displayed. (C) CD8+ T cells were further divided into 2B4– and 2B4+ populations and caspase-3/7 activity was assessed. Representative flow plots for caspase-3/7 staining in CD8+2B4– and CD8+2B4+ T cells. Summary data of frequencies of caspase-3/7+ cells within 2B4–CD8+ and 2B4+CD8+ T cells. Cells from septic patients 8–14 as identified in Supplemental Table 1 were used in B and C. *P < 0.05. SSC, side scatter.
Discussion
The data presented here show that memory CD8+ T cells are more susceptible than naive CD8+ T cells to sepsis-induced attrition, highlighting the important concept that memory T cells may behave differently than naive T cell populations during sepsis-induced immune dysregulation. Because the T cell compartments of adult humans contain greater than 50% memory T cells (16, 17) while laboratory mice possess relatively few memory T cells, these data support the concept that analyzing the behavior of antigen-experienced immune systems during sepsis might yield novel insights closely mirroring the pathophysiology of human sepsis. This is potentially critically important for the field of sepsis, where numerous targets and therapeutics that looked promising in initial preclinical rodent models have failed when tested in human patients with sepsis. Some have posited that this indicates that murine hosts are a poor model for studying human sepsis (43). Our study instead suggests that generating mouse models that more closely recapitulate the antigen-experienced human immune system might help bridge this divide.
Numerous studies have shown that sepsis induces a significant increase in T cell apoptosis (9–13), including both antigen-dependent and -independent memory CD8+ T cells (15, 44). In our study, we identified a significant loss of T cells during sepsis in memory mice. Both CD44hiCD4+ and CD44loCD4+ T cells were reduced after CLP. However, only CD44hiCD8+ but not CD44loCD8+ T cells were decreased following sepsis, which was in line with a previous study in naive mice (15). It is also interesting to note that we observed a modest but statistically significant reduction in the number of T cells between the naive versus memory sham-operated mice, particularly within the CD8+ T cell compartment (Figure 2A). Because we know that memory T cells are more susceptible to sepsis-induced apoptosis (Figure 2G), we posit that the data shown in Figure 2A suggest that sterile inflammation induced by sham surgery could induce a weaker version of the same phenomenon (i.e., memory T cell apoptosis). This interesting idea warrants further investigation. However, despite this significant impact of memory status on CD8+ T cell attrition during sepsis, we did not observe a significant increase in mortality in memory mice using this model. It is possible that this is due to a limited breadth of memory T cells before sepsis in this model because only 2 infections were used to generate the CD44hi memory T cell compartment. Although several studies have demonstrated that memory T cells are cross-reactive and can respond not only to the priming antigen but also to a spectrum of related antigens (45–47), this memory T cell compartment may be narrower in its specificity compared with that of humans. Thus, this limited repertoire of the memory T cells generated here is a potentially important limitation of the model. Alternatively, there may be a “threshold effect” with regard to the impact of T cell apoptosis on mortality during sepsis: Although we observed an increase in T cell apoptosis in the memory mice, it may not have been a large enough effect to influence overall mortality. Nevertheless, our results indicate that rescuing the loss of memory T cell number and functionality (in this case via deletion of 2B4) may be beneficial for increasing sepsis survival.
Indeed, in addition to our group, 3 other groups are currently studying the impact of antigen experience or infectious history on sepsis physiology. The Lederer and Badovinac/Griffiths labs have used pet store–derived “dirty mice” to generate T cell memory before sepsis induction (48, 49). Griffiths and Badovinac showed that B6 mice that had been cohoused with pet store mice exhibited significantly worsened survival following sepsis (49), and Deutschman’s group showed that animals exposed to anti-CD3–mediated T cell activation and memory T cell generation demonstrated increased immune dysregulation following sepsis induction 30 days later (50). Each model has its own distinct advantages, in particular the fact that working with naturally colonized “dirty mice” may represent the most physiologically relevant way to generate T cell memory. Results from our model will synergize with data obtained from these related studies, in that in our system we can be certain that the observed effects are the result of immunologic memory and not the result of persistent, chronic infection in the “dirty mouse” models, as might occur following the transmission of murine cytomegalovirus, γ-herpesvirus 68 (an Epstein-Barr virus homolog), or murine polyomavirus (a persistent viral infection endemic to almost all wild rodents and humans). Overall, these studies have together illuminated the fact that the actions of memory lymphocytes significantly influence immune dysregulation during sepsis and that a significant knowledge gap exists in understanding the molecular pathways that mediate this effect.
In a previous study, we reported that 2B4 signals play a crucial role in mediating T cell dysregulation and mortality during sepsis in naive animals (42). Here, using a memory model to better approximate the antigen-experienced immune systems of adult humans, we also found that 2B4–/– memory mice exhibited a significantly higher survival rate following sepsis compared with WT memory CLP controls. Although our study focused on the role of 2B4 on T cells, it is worth noting that 2B4 is also highly expressed on NK cells. Although we have previously determined that 2B4 deficiency on NK cells did not contribute to the overall survival benefit observed in 2B4–/– mice, “memory” NK cells have been shown to be generated following exposure to IL-12 or IL-18 (in the presence or absence of IL-15) (51). As such, future research should determine whether cytokine-driven memory NK cells are generated in this model and whether 2B4 deficiency plays a differential role on those memory NK cells relative to the negligible role we found it to play on “naive” NK cells during sepsis.
Our previous report on naive animals indicated that 2B4 coinhibitory signaling on CD4+ T cells functioned in a cell-intrinsic manner to mediate immune dysfunction and mortality during sepsis (42). In the current study, we found that loss of 2B4 reversed attrition of CD4+CD44hi T cell populations. 2B4 deficiency was associated with a decrease in active caspase-3/7+SYTOX+CD4+ T cells. Moreover, studies of cells isolated from human patients with sepsis indicated that the frequency of caspase-3/7+ cells was higher in 2B4+CD8+ T cells during sepsis relative to 2B4–CD8+ T cells. Taken together, these complementary mouse and human data strongly suggest that expression of 2B4 promotes memory CD4+ T cell apoptosis during sepsis and raise the possibility that blockade of this signal could limit this deleterious effect and promote immune competence. To understand why the loss of 2B4 coinhibitory signaling can prevent memory T cell attrition during sepsis, we assessed the phenotype of 2B4+ versus 2B4– memory T cells during sepsis and found that the 2B4+CD44hi subset contained a higher frequency of activated CD25+CD69+ cells within both the CD4+ and CD8+ compartments compared with the 2B4–CD44hi subsets during sepsis. In sum, our results indicate that targeting 2B4 coinhibitory signaling may be a potential immunomodulatory therapy to improve outcomes in antigen-experienced human patients with sepsis.
Methods
Mice.
We purchased 6-week-old male B6 mice from The Jackson Laboratory. 2B4-knockout mice on a B6 background, a gift of C. Terhorst (Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts, USA), were bred and maintained in an approved animal facility at Emory University. We used age-matched 2B4–/– mice as B6 mice. All mice were maintained according to the Emory University Institutional Animal Care and Use Committee guidelines (protocol number 2003238-082518).
Generation of “memory mice.” Both WT and 2B4–/– mice were infected with 1 × 104 CFU of Listeria monocytogenes in 500 μl PBS intraperitoneally. On days 10 and 25 after infection, we assessed the frequency of CD44hiCD4+ and CD44hiCD8+ T cells. Mice were infected with 2 × 105 PFU LCMV intraperitoneally 30 days after infection, and the frequency of CD44hi memory CD4+ and CD44hi memory CD8+ T cells were assessed on days 40 and 55 following the first infection. Both of these infections are acutely cleared, allowing us to assess the impact of T cell memory in the absence of active infection. Two months after Listeria infection, groups of mice underwent CLP or sham surgery as indicated.
Sepsis model.
Animals were subjected to the CLP model of septic peritonitis on day 60 following the first infection. Mice were anesthetized via inhaled isoflurane, and a midline abdominal incision was made. The cecum was exteriorized, ligated, and punctured twice with a 25-gauge needle. The bowel was then returned to the abdominal cavity, and the incision was closed. All mice received buprenorphine (0.1 mg/kg) to relieve pain before the CLP procedure. Mice undergoing sham surgery received only the operation without ligation and puncture. Mice received a 1-ml subcutaneous injection of sterile saline immediately after surgery, as well as antibiotics (50 mg/kg of ceftriaxone and 35 mg/kg metronidazole) designed to mimic the early bundle treatment of patients with sepsis. Antibiotics were injected subcutaneously at 12, 24, and 36 hours after surgery. Mice were euthanized using CO2 asphyxiation at designated time points.
Flow cytometry.
Spleens were harvested after mice were sacrificed at 24 hours after CLP, and then spleens were processed to single-cell suspensions in a 70-μm filter placed over a 50-ml conical tube. Samples were rinsed with 10 ml cold PBS, and 200 μl from each spleen was aliquoted in a 96-well plate for staining. Alexa Fluor 700 anti-CD3 (BD Biosciences), anti-CD4-PB (BD Biosciences), anti-CD8-PO (Biolegend), and anti-CD44-PerCP (Biolegend) were used to stain to distinguish the naive and memory CD4+ and CD8+ T cells. Anti-CD25-FITC (Biolegend), anti-CD69-PE (Biolegend), anti-CD62L-PE Cy7 (BD Biosciences), anti-2B4-APC, anti-PD-1-APC-Cy7, anti-LAG-3-FITC (all from eBioscience) were used for surface staining to determine the T cell phenotype. Anti–active caspase-3/7 and SYTOX were used per manufacturer’s instructions to detect apoptotic cells. Accucheck Counting Beads (Thermo Fisher Scientific) were added during staining to calculate the absolute number of T cells per spleen. Human PBMCs were stained with anti-CCR7, anti-CD45RA, anti-CD4, anti-CD8, and anti–caspase-3/7 (Invitrogen). All the samples were run on an LSR II flow cytometer (BD Biosciences). FlowJo software (Tree Star Inc.) was used to analyze the data.
Human patients.
Blood was collected from patients with sepsis within the first 24 hours of meeting the consensus clinical definition of sepsis. Average age of the cohort with sepsis analyzed here was 49.4 ± 18.2 years and was not significantly different from the average age of the HDs. Similarly, the sex distributions within the sepsis versus HD cohorts were not different. In terms of severity of sepsis, the mean sequential organ failure assessment score was 7.6 ± 2.3, and the mean acute physiology, age, and chronic health evaluation score was 21.1 ± 10.4. The mean length of hospital stay for this cohort was 23.7 ± 16.4 days, and mean length of ICU stay was 9.1 ± 7.5 days. Additional demographic and clinical data are summarized in Supplemental Table 1. Values listed above represent the mean ± SD.
Statistics.
Statistical analyses were conducted using GraphPad Prism 6.0 software. Survival studies were analyzed using the log-rank test. Other variables were compared between the groups using 2-way ANOVA or the Mann-Whitney nonparametric test. All the data were expressed as mean ± SEM. A P value of less than 0.05 was considered statistically significant.
Study approval.
Animal experiments were approved by the Emory University Institutional Animal Care and Use Committee, under protocol number 2003238-082518. Approval for procurement of HD and septic patient PBMCs was provided by the Emory University IRB, under protocol number 00002503. Informed consent was received from all human subjects.
Author contributions
MLF and CMC designed research studies; JX, CWC, YS, WZ, and SO conducted experiments and acquired data; JX, CWC, YS, SJL, CMC, and MLF analyzed data; MLF and JX wrote the manuscript; and all authors edited the manuscript.
Supplementary Material
Acknowledgments
This work was funded by R01s GM113228, GM104323, and GM109779 to MLF and CMC and AI104699 to MLF. The authors would like to thank Jennifer Robertson (Emory Transplant Center) and Leona Wells (Emory University Hospital) for technical assistances.
Version 1. 05/02/2019
Electronic publication
Footnotes
JX’s present address is: Department of Critical Care Medicine, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, China, 210009
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2019 American Society for Clinical Investigation
Reference information: JCI Insight. 2019;4(7):e126030. https://doi.org/10.1172/jci.insight.126030.
Contributor Information
Jianfeng Xie, Email: xie820405@126.com.
Ching-wen Chen, Email: ching-wen.chen@emory.edu.
Yini Sun, Email: yini.sun@emory.edu.
Wenxiao Zhang, Email: zhang.wenxiao@emory.edu.
Shunsuke Otani, Email: otani-shun@umin.net.
Craig M. Coopersmith, Email: cmcoop3@emory.edu.
Mandy L. Ford, Email: mandy.ford@emory.edu.
References
- 1.Rhee C, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA. 2017;318(13):1241–1249. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 3.Seymour CW, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(21):2063. doi: 10.1056/NEJMc1312359. [DOI] [PubMed] [Google Scholar]
- 5.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41(5):1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 6.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 8.Carrico CJ, Meakins JL, Marshall JC, Fry D, Maier RV. Multiple-organ-failure syndrome. Arch Surg. 1986;121(2):196–208. doi: 10.1001/archsurg.1986.01400020082010. [DOI] [PubMed] [Google Scholar]
- 9.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13(3):260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 12.Hotchkiss RS, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166(11):6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 13.Hotchkiss RS, et al. Role of apoptosis in Pseudomonas aeruginosa pneumonia. Science. 2001;294(5548):1783. doi: 10.1126/science.294.5548.1783a. [DOI] [PubMed] [Google Scholar]
- 14.Boomer JS, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306(23):2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serbanescu MA, et al. Attrition of memory CD8 T cells during sepsis requires LFA-1. J Leukoc Biol. 2016;100(5):1167–1180. doi: 10.1189/jlb.4A1215-563RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cossarizza A, et al. CD45 isoforms expression on CD4+ and CD8+ T cells throughout life, from newborns to centenarians: implications for T cell memory. Mech Ageing Dev. 1996;86(3):173–195. doi: 10.1016/0047-6374(95)01691-0. [DOI] [PubMed] [Google Scholar]
- 17.Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev. 2006;127(3):274–281. doi: 10.1016/j.mad.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Beura LK, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532(7600):512–516. doi: 10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crompton PD, Pierce SK. PD-L2 elbows out PD-L1 to rescue T cell immunity to malaria. Immunity. 2016;45(2):231–233. doi: 10.1016/j.immuni.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 22.McKinney EF, Lee JC, Jayne DR, Lyons PA, Smith KG. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. 2015;523(7562):612–616. doi: 10.1038/nature14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue S, Bo L, Bian J, Unsinger J, Chang K, Hotchkiss RS. Dose-dependent effect of anti-CTLA-4 on survival in sepsis. Shock. 2011;36(1):38–44. doi: 10.1097/SHK.0b013e3182168cce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shubin NJ, Chung CS, Heffernan DS, Irwin LR, Monaghan SF, Ayala A. BTLA expression contributes to septic morbidity and mortality by inducing innate inflammatory cell dysfunction. J Leukoc Biol. 2012;92(3):593–603. doi: 10.1189/jlb.1211641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang KC, et al. Blockade of the negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Crit Care. 2013;17(3):R85. doi: 10.1186/cc12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukoc Biol. 2010;88(2):233–240. doi: 10.1189/jlb.0110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shubin NJ, Monaghan SF, Heffernan DS, Chung CS, Ayala A. B and T lymphocyte attenuator expression on CD4+ T-cells associates with sepsis and subsequent infections in ICU patients. Crit Care. 2013;17(6):R276. doi: 10.1186/cc13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao R, Li CS, Fang Y, Zhao L, Hang C. Low B and T lymphocyte attenuator expression on CD4+ T cells in the early stage of sepsis is associated with the severity and mortality of septic patients: a prospective cohort study. Crit Care. 2015;19:308. doi: 10.1186/s13054-015-1024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang K, et al. Targeting the programmed cell death 1: programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Crit Care. 2014;18(1):R3. doi: 10.1186/cc13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang KC, et al. Blockade of the negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Crit Care. 2013;17(3):R85. doi: 10.1186/cc12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guignant C, et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care. 2011;15(2):R99. doi: 10.1186/cc10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang X, et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A. 2009;106(15):6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monaghan SF, et al. Mechanisms of indirect acute lung injury: a novel role for the coinhibitory receptor, programmed death-1. Ann Surg. 2012;255(1):158–164. doi: 10.1097/SLA.0b013e31823433ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monaghan SF, et al. Programmed death 1 expression as a marker for immune and physiological dysfunction in the critically ill surgical patient. Shock. 2012;38(2):117–122. doi: 10.1097/SHK.0b013e31825de6a3. [DOI] [PubMed] [Google Scholar]
- 35.Young JS, et al. Effect of PD-1: PD-L1 in invariant natural killer T-cell emigration and chemotaxis following sepsis. Shock. 2016;45(5):534–539. doi: 10.1097/SHK.0000000000000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang F, Huang X, Chung CS, Chen Y, Hutchins NA, Ayala A. Contribution of programmed cell death receptor (PD)-1 to Kupffer cell dysfunction in murine polymicrobial sepsis. Am J Physiol Gastrointest Liver Physiol. 2016;311(2):G237–G245. doi: 10.1152/ajpgi.00371.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patera AC, Drewry AM, Chang K, Beiter ER, Osborne D, Hotchkiss RS. Frontline Science: defects in immune function in patients with sepsis are associated with PD-1 or PD-L1 expression and can be restored by antibodies targeting PD-1 or PD-L1. J Leukoc Biol. 2016;100(6):1239–1254. doi: 10.1189/jlb.4HI0616-255R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monneret G, Gossez M, Venet F. Sepsis in PD-1 light. Crit Care. 2016;20(1):186. doi: 10.1186/s13054-016-1370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao DN, Yang ZX, Qi QH. Roles of PD-1, Tim-3 and CTLA-4 in immunoregulation in regulatory T cells among patients with sepsis. Int J Clin Exp Med. 2015;8(10):18998–19005. [PMC free article] [PubMed] [Google Scholar]
- 40.Tang L, et al. Active players in resolution of shock/sepsis induced indirect lung injury: immunomodulatory effects of Tregs and PD-1. J Leukoc Biol. 2014;96(5):809–820. doi: 10.1189/jlb.4MA1213-647RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goyert SM, Silver J. Editorial: PD-1, a new target for sepsis treatment: better late than never. J Leukoc Biol. 2010;88(2):225–226. doi: 10.1189/jlb.0410240. [DOI] [PubMed] [Google Scholar]
- 42.Chen CW, et al. Cutting edge: 2B4-mediated coinhibition of CD4+ T cells underlies mortality in experimental sepsis. J Immunol. 2017;199(6):1961–1966. doi: 10.4049/jimmunol.1700375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stortz JA, Raymond SL, Mira JC, Moldawer LL, Mohr AM, Efron PA. Murine models of sepsis and trauma: Can we bridge the gap? ILAR J. 2017;58(1):90–105. doi: 10.1093/ilar/ilx007. doi:10.1093/ilar/ilx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duong S, Condotta SA, Rai D, Martin MD, Griffith TS, Badovinac VP. Polymicrobial sepsis alters antigen-dependent and -independent memory CD8 T cell functions. J Immunol. 2014;192(8):3618–3625. doi: 10.4049/jimmunol.1303460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adams AB, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111(12):1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selin LK, et al. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunol Rev. 2006;211:164–181. doi: 10.1111/j.0105-2896.2006.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adams AB, Pearson TC, Larsen CP. Heterologous immunity: an overlooked barrier to tolerance. Immunol Rev. 2003;196:147–160. doi: 10.1046/j.1600-065X.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 48.Guo F, et al. Comparing the phenotype betwen naturally-colonized “dirty mice” and laboratory “clean” mice by a CYTOF-based systems immunology approach. Shock. 2018;49(suppl 1):128 [Google Scholar]
- 49.Griffiths TS, et al. Physiology microbial exposure substantially influences de novo inflammatory responses in vivo. Shock. 2018;49(suppl 1):122 [Google Scholar]
- 50.Taylor MD, Abraham MN, Deutschamn CS. Induction of nonspecific memory T cells alters the immune response to cecal ligation and puncture (CLP) Shock. 2018;49(suppl 1):132 [Google Scholar]
- 51.Geary CD, Sun JC. Memory responses of natural killer cells. Semin Immunol. 2017;31:11–19. doi: 10.1016/j.smim.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.