Abstract
MHC I–restricted epitopes of chicken ovalbumin (OVA) were originally identified using CD8+ T cells as probes. Here, using bioinformatics tools, we identify 4 additional epitopes in OVA in addition to a cryptic epitope. Each additional epitope is presented in vivo, as deduced from the lack of CD8+ T cell response to it in OVA-transgenic mice. In addition, CD8 responses to the previously known epitopes and those identified in this study are examined in C57BL/6J mice exposed to the OVA-expressing tumor E.G7 in several ways. No responses to any epitope, including SIINFEKL, are detected in mice with growing E.G7 or mice immunized with the tumor. Only in E.G7-bearing mice treated with an anti–CTLA-4 antibody, which depletes tumor-infiltrating regulatory T cells, are CD8 responses to SIINFEKL and the epitope EKYNLTSVL identified in this study detected. Finally, all epitopes fail to treat mice with preexisting tumors. These observations force an important reconsideration of the common assumptions about the therapeutic value of neoepitopes detected by CD8 responses in tumor-bearing hosts.
Keywords: Immunology
Keywords: Antigen presentation, Cancer immunotherapy, Cellular immune response
Generation of a measurable CD8+ T cell response to an MHC class I–presented epitope does not predict that the epitope is a tumor rejection antigen.
Introduction
The antigen processing machinery enables surveillance of the intracellular protein milieu by CD8+ T cells. The intracellular proteins undergo partial proteolysis. The peptides, mostly 8 to 11 amino acids in length, generated by proteolysis, are loaded onto MHC class I molecules and presented on the cell surface (1). Recognition of the MHC-presented peptides by CD8+ T cells endows them with the status of an epitope. The characteristic of a peptide to serve as an epitope was termed by Sercarz as epitypicity (2, 3), and we use this term in that same meaning. Epitypicity is the same as immunogenicity, with the nuance that it is not whole antigens but fragments of antigens that are recognized by the immune system.
Tumors result from driver events deregulating cell division. With progression, tumors acquire additional driver or passenger mutations. The antigen-presenting machinery presents normal as well as mutated peptides (neoepitopes) on MHC I molecules to cognate CD8+ T cells (4–6). Current efforts at designing personalized cancer vaccines seek to exploit the inherent immunogenicity of tumors by identifying tumor-specific neoepitopes using high-throughput genomics and bioinformatics, and by directing CD8+ T cell responses in the patients against the identified neoepitopes. The success of these efforts depends on accurate definition of the epitopes that make tumors immunogenic, and immune response to the neoepitopes that can protect a host against tumor growth.
Here, we have used the model antigen ovalbumin (OVA) as a tumor-specific antigen. Because tumor-specific antigens are overwhelmingly mutated self-antigens, which are indeed non-self or foreign with respect to the germline, the choice of chicken OVA (which is also a foreign or non-self-antigen for mice) as a tumor neoantigen is reasonable. We have used the very same bioinformatics tools that are now used to predict potential cancer neoepitopes to identify the immunogenic epitopes of OVA; thus far, this has been accomplished purely through the use of anti-OVA CD8+ T cells. We have analyzed the immunogenicity of the 2 known and 4 newly identified epitopes of OVA by immunizing mice with individual peptides or whole OVA and testing the CD8 response on peptide-pulsed targets. We then analyzed the CD8+ T cell response to each individual epitope of OVA in mice with progressively growing as well as regressing OVA-expressing tumor E.G7 (7). Our studies reveal significant lessons for our current efforts to identify neoepitopes that may be used to immunize cancer patients with a view to elicit immunological protection from tumor growth.
Results
Immunogenicity of peptides of OVA identified in this study.
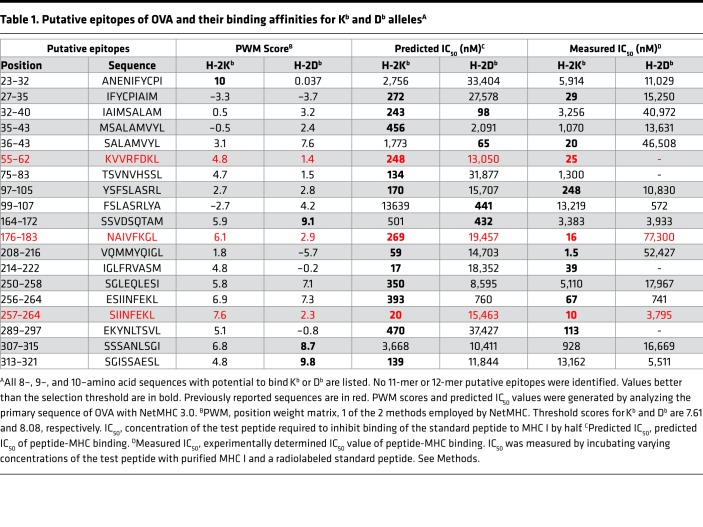
The primary sequence of OVA was analyzed using NetMHC (3.0). (NetMHC 4.0 identifies 4 additional peptides that were not tested.) Any 8–, 9–, or 10–amino acid–long sequence with a potential to bind Kb or Db (i.e., profile weight matrix or PWM score greater than the default threshold score 7.61 for Kb and 8.08 for Db, or a predicted IC50 less than the default threshold value 500 nM for either allele) was considered a putative epitope. Nineteen sequences fit the PWM, or IC50, or both criteria. All 3 previously reported epitopes (8–10) are included in this group of 19 and are predicted to have the potential to bind Kb, but not Db (Table 1). Thus, we identified 16 potential epitopes for Kb or Db. Of these, 10 putative epitopes were predicted to bind Kb alone, 4 to Db alone, and 2 to Kb as well as Db (Figure 1A).
Table 1. Putative epitopes of OVA and their binding affinities for Kb and Db allelesA.
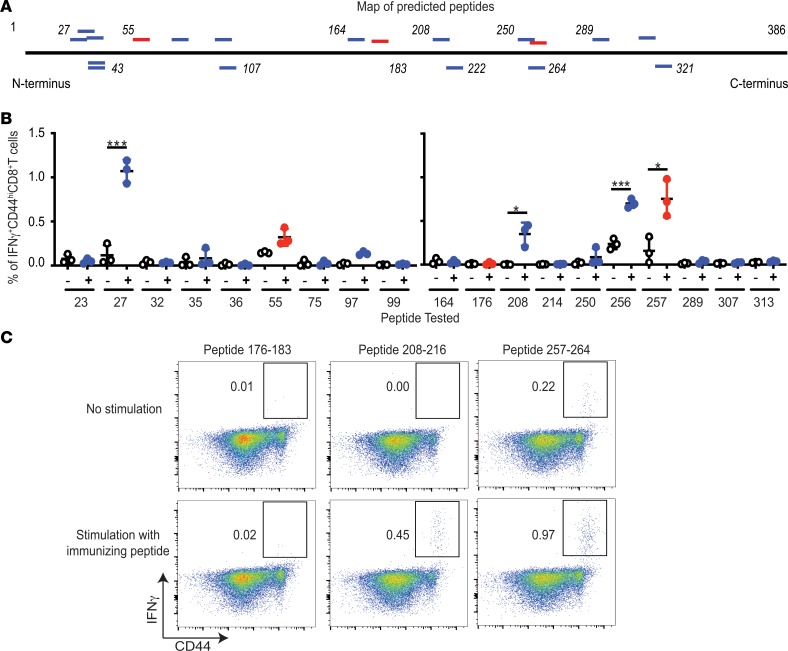
Figure 1. Antigenicity of putative and known epitypic peptides of OVA.
(A) Positions of putative (blue) and the previously reported (red) epitopes of OVA, as detailed in Table 1. Horizontal black line represents the primary structure of OVA. Italicized numbers above and below OVA mark various amino acid positions. (B) Immunogenicity of each putative epitypic (blue) and the previously known (red) peptides of OVA shown in Table 1. “–” Indicates no stimulation and “+” indicates stimulation of LN cells in vitro with the immunizing peptide for 12 hours. Tested peptides are identified on the x axis with the position of their N-terminal residue (n = 3; each peptide tested in 9 different mice over 4 independent experiments; Welch’s t test). (C) Representative assay of the ability of SIINFEKL (257–264), peptide 176–183 (also previously known), and 208–216 (potentially novel peptide) to elicit CD8+ T cell responses upon immunization of C57BL/6J mice, as described in Methods (each peptide tested in 9 different mice in several independent experiments). Flow cytometry plots of viable CD3+CD8+ cells from LNs of immunized mice are shown. *P ≤ 0.05, ***P ≤ 0.001.
Immunogenicity of peptides of OVA.
The ability of each of the 19 peptides within OVA (Table 1 and Figure 1A) to elicit CD8+ T cell responses in C57BL/6J mice was tested. In order to determine the appropriate dose of peptide for effective immunization, SIINFEKL (aa 257–264) was used as a guide. Naive C57BL/6J mice were immunized with doses of peptide 257–264 (emulsified in an adjuvant) varying from 1 μg to 100 μg per mouse. All doses of immunization elicited clear CD8 responses (data not shown). Subsequent immunizations were performed at a dose of 10 μg peptide per immunization, emulsified with TiterMax, injected in the footpad of naive C57BL/6J mice. Seven days later, the draining lymph nodes (dLNs) were harvested, and the single-cell suspensions generated were stimulated in vitro for 12 hours with the immunizing peptide or not stimulated. All 19 peptides were tested (Figure 1B). As expected, peptide 55–62 (9) and SIINFEKL were immunogenic (Figure 1B). Four out of the 16 potentially novel, predicted peptides of OVA (peptides 27–35, 97–105, 208–216, and 256–264), which to our knowledge have not previously been reported to be immunogenic, were noted to elicit significant levels of IFN-γ–secreting, CD44hi, CD8+ T cells (Figure 1B).
As typical examples of these experiments, expression of IFN-γ by the CD44hiCD8+ T cells from the immunized mice in response to peptide restimulation was tested using peptides 176–183 (previously reported), 208–216 (a potentially novel epitope), and the well-known SIINFEKL (Figure 1C). SIINFEKL was clearly immunogenic, but the peptide 176–183, which has previously been reported to be immunogenic by CD8 cytotoxicity assays (8, 9), was not observed to be immunogenic by the IFN-γ assay. The putative epitope, peptide 208–216, was significantly immunogenic (P < 0.05; Figure 1C).
Among all the predicted Kb-binding peptides, 214–222 has the strongest predicted affinity (Table 1) but this peptide was not observed to be immunogenic. SIINFEKL and peptide 208–216 have the next-strongest predicted affinities, and they are both immunogenic. The other known epitopes as well as the potentially novel peptides shown in Figure 1B have moderate predicted affinity for Kb (170–393 nM IC50). The remaining 12 peptides had a range of affinities for Kb (17–13,639 nM IC50), but were not immunogenic. None of the 4 peptides identified in this study have a significant affinity for Db.
Of the 4 immunogenic peptides identified in this study, one (peptide 256–264) is a single N-terminal amino acid extension of the peptide 257–264. In order to test whether 256–264 and 257–264 are immunologically distinct, mice were immunized with 256–264 or 257–264. CD8+ T cells from mice immunized with any one peptide recognized both peptides, indicating that 256–264 and 257–264 are cross-reactive and not immunologically distinct (data not shown).
Epitypicity of peptides in the context of immunization with whole OVA.
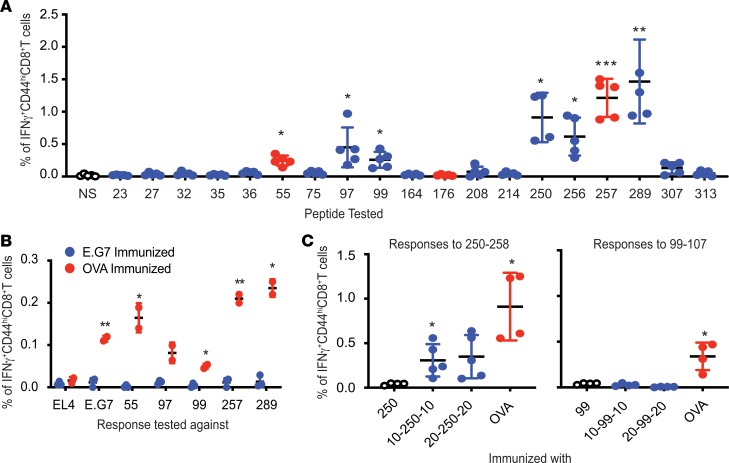
The epitypicity of all predicted epitopes was tested in the context of immunization with whole OVA protein, in contrast to the context of immunization with individual peptides, as tested in Figure 1. Naive mice were immunized with an emulsion containing an adjuvant and 450 μg of OVA, the approximate molar equivalent of 10 μg of peptide. Control mice were immunized with an emulsion lacking the antigen. The cells from the harvested dLNs of immunized mice were stimulated with each of the 19 individual peptides (Table 1) or not stimulated; the expression of IFN-γ by CD44hiCD8+ T cells in response to stimulation in vitro was tested (Figure 2A). As expected, peptides 55–62 and SIINFEKL successfully stimulated the CD8+ T cells from OVA-immunized mice (Figure 2A). Interestingly, peptide 176–183 (previously reported to be immunogenic; refs. 8, 9), which was nonimmunogenic upon peptide immunization (Figure 1B), was observed to be nonfunctional as an epitope upon OVA immunization as well. Among the potentially novel epitypic peptides, peptides 97–105, 99–107, 250–258, 256–264, and 289–297 stimulated OVA-primed CD8+ T cells. Mice immunized with the emulsion lacking the antigen (data not shown) had no CD8+ T cell responses to OVA. Peptide 256–264 was excluded from consideration in the following experiments because of its immunological similarity to SIINFEKL.
Figure 2. MHC I–restricted epitypicity of OVA.
(A) CD8+ T cell responses to OVA elicited in C57BL/6J mice immunized with OVA emulsified with TiterMax. Responses against each of the putative (blue) and the previously known (red) epitopes of OVA (as listed in Table 1) were tested as in Figure 1. NS indicates no restimulation. P values reflect the significance of difference in percentage IFN-γ–expressing CD44hiCD8+ T cells between NS and peptide-stimulated cultures (n = 5; experiment performed 3 times; Welch’s t test). (B) Inability of irradiated E.G7 cells to elicit CD8+ T cell responses against itself or OVA. CD8+ T cells from LNs of immunized mice were stimulated with splenocytes pulsed with indicated peptide or EL4 or E.G7 cells for 12 hours. P values indicate significance of difference between cultures stimulated with the indicated peptide or cells (n = 3 for E.G7-immunized mice, n = 2 for OVA-immunized mice; experiment performed 4 times; unpaired t test). (C) Extended variants of peptide 250–258 are immunogenic. Mice were immunized with precise peptide (labeled 250 or 99), extended peptides with 10 (10-250-10 or 10-99-10) or 20 (20-250-20 or 20-250-20) flanking amino acids on either termini, or whole OVA. Responses to 250–258 and 99–107 were tested as in Figure 1 (n = 4 for 250 and OVA, n = 5 for 10-250-10 and 20-250-20 in left panel; n = 4 for all sets in the right panel; experiment performed 2 times; Welch’s t test). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
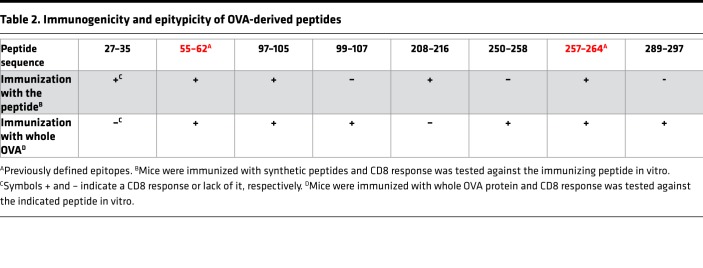
Table 2 summarizes the relationship of all 6 potentially novel sequences (and the 2 previously known epitopes) with their immunogenicity. Of the 4 peptides observed to be epitypic in the context of immunization with the whole OVA, one (peptide 97–105) had also been observed to be weakly immunogenic in the context of immunization with individual peptides (Figure 1B). The remaining 3 were not immunogenic by peptide immunization but were immunogenic by protein immunization (i.e., epitypic). In contrast, of the 3 peptides observed to be immunogenic by peptide immunization, two (27–35 and 208–216) are observed to be nonepitypic in the context of whole-OVA immunization (Figure 2A). Because peptides 27–35 and 208–216 are immunogenic as peptides but not recognized by the natural immune response to OVA, they are likely to be cryptic by definition (11).
Table 2. Immunogenicity and epitypicity of OVA-derived peptides.
Peptides must demonstrate sufficient binding affinity for the expressed MHC I alleles in order to be recognized by the CD8+ T cells. MHC I peptide binding with an IC50 value of 500 nM or less is considered strong and peptides capable of such interactions are more likely to be immunogenic as per the current consensus. Most of the 19 peptides tested here that are recognized by CD8+ T cells — 27–35, 55–62, 97–105, 208–216, 257–264, and 289–297 — have strong affinity for Kb as measured using competitive binding assays (Table 1, Figure 1B, and Figure 2A). Peptide 99–107 binds Db with an IC50 of 572 nM, which is very close to the threshold of 500 nM. However, peptide 250–258 does not adhere to the current paradigm and shows weak affinity for both Kb and Db (IC50 of 5,110 and 17,967 nM, respectively), despite being recognized by CD8+ T cells (Table 1 and Figure 2A).
CD8 responses to 55–62, 97–105, 99–107, 257–264, and 289–297 were also tested in mice immunized with irradiated E.G7-OVA (E.G7) cells. E.G7 is a murine thymoma line derived from EL4 and constitutively expresses whole OVA. Control mice were immunized with the protein. Surprisingly, responses to EL4 cells, E.G7 cells, or any of the tested epitopes of OVA were not detected in mice inoculated with irradiated E.G7 cells (Figure 2B; blue circles). As expected, CD8+ T cells from the mice immunized with OVA protein recognized E.G7 cells but not EL4 cells. Control mice also showed responses to OVA, as observed previously (Figure 2B; red circles).
Antigenicity of extended precursors of nonimmunogenic precise epitopes.
Peptides 99–107, 250–258, and 289–297 were noted to be epitypic (Figure 2A) but not immunogenic (Figure 1B). The possibility that longer precursors of peptides 99–107 and 250–258 may be antigenic was tested. C57BL/6J mice were immunized with long peptides in which the precise peptide was flanked by 10 (10-99-10 or 10-250-10) or 20 (20-99-20 or 20-250-20) naturally flanking amino acids on both termini. Control mice were immunized with the precise peptide or whole OVA. CD8+ T cell response was tested by stimulating the dLN cells with the corresponding precise peptide. It was observed that peptide 250–258, which was not immunogenic by itself as also seen in Figure 1B, was not immunogenic here as well, but its 10–amino acid and 20–amino acid extended variants were (Figure 2C). Interestingly, extended versions of peptide 99–107 did not elicit CD8+ T cell responses, and its even longer variants were not tested. Longer variants of peptide 289–297 were not tested.
Epitopes of OVA present within endogenous antigens induce immunological tolerance.
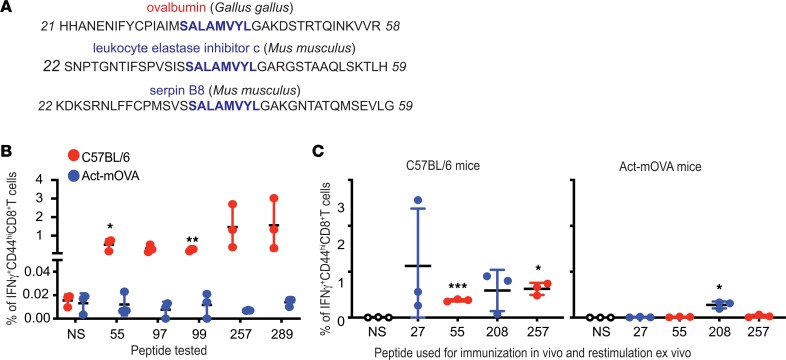
Peptides 36–43 and 214–222 (Table 1) were observed to be nonimmunogenic despite having strong affinities for Kb, with IC50 values of 20 nM and 39 nM, respectively. The possibility of their homology with sequences in the mouse proteome was considered. Peptides 36–43 and 214–222 were aligned with all the mouse (taxid:10090) nonredundant protein sequences using BLAST. Sequences identical to OVA peptide 36–43 but not peptide 214–222 were observed to be present in 2 mouse proteins, leukocyte elastase inhibitor c and serpin B8, both being members of the Serpin superfamily (Figure 3A). Indeed, OVA is also known to be a member of the Serpin family (12). This observation suggests that the lack of immunogenicity of peptide 36–43 may result from negative selection during thymic maturation. Lack of immunogenicity of peptide 214–222 may be due to a possible hole in the repertoire that does not result from negative selection.
Figure 3. Epitopes of OVA induce tolerance when expressed endogenously.
(A) Peptide 36–43 (SALAMVYL) is present in 2 proteins expressed by mice. Peptide 36–43 was aligned against all nonredundant mouse (taxid:10090) protein sequences. Thirty-nine–amino acid regions of OVA and 2 mouse proteins containing peptide 36–43 are shown. Numbers flanking the sequences indicate the position (from the N-terminus) of the first and the last depicted amino acid. (B) Act-mOVA mice are tolerized toward all epitopes of OVA. C57BL/6J mice (red) or Act-mOVA (blue) were immunized with OVA and CD8+ T cell responses against the indicated epitopes were tested (n = 3; experiment performed 2 times; unpaired t test). (C) Act-mOVA mice are not tolerized toward peptide 208–216. Wild-type C57BL/6J (left panel) or Act-mOVA (right panel) mice were immunized with peptides 27–35, 55–62, 208–216, and 257–264. CD8 responses were tested 7 days after immunizations (n = 3 for both panels; experiment performed 2 times; unpaired t test). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
We argued that expression of OVA by mice as an endogenous self-antigen should also induce tolerance towards all MHC I–restricted epitopes of OVA. Act-mOVA mice express a membrane-bound form of OVA ubiquitously (13). To test whether Act-mOVA mice are tolerized toward OVA, immunizations were performed with complete OVA. C57BL/6J mice were also immunized with complete OVA as positive controls. CD8+ T cell responses were tested in OVA-immunized mice against epitopes 55–62, 97–105, 99–107, 257–264, and 289–297. As hypothesized, CD8+ T cell responses were not observed against any of the tested epitopes of OVA in Act-mOVA (Figure 3B; blue bars). As observed previously, immunizations with OVA elicited CD8+ T cell responses against each of the tested peptides in C57BL/6J mice (Figure 3B; red bars). Presumably, the lack of response to any of the OVA epitopes in Act-mOVA mice derives from deletion of the T cells recognizing those “self-epitopes,” thus indicating that all the epitopes, including the epitopes identified by us here, are physiologically presented.
Cryptic epitopes, by definition, undergo intra-epitope proteolysis during the processing of the antigen. As a result, cryptic epitopes are not presented on MHC I and consequentially, cryptic epitopes should neither elicit CD8 responses upon immunization with exogenous antigens harboring the cryptic epitopes nor induce immunological tolerance when present within “self-antigens.” However, synthetic peptides mimicking cryptic epitopes, which do not require intracellular processing, elicit CD8 responses upon immunization. Immunization with OVA, which is an exogenous antigen to mice, does not elicit CD8 responses against peptides 27–35 and 208–216 (Figure 2A) but the synthetic peptides are immunogenic (Figure 1B). We hypothesized that peptides 27–35 and 208–216 are cryptic. Act-mOVA mice, which are tolerized toward all epitopes of OVA (Figure 3B), including epitopes 55–62 and 257–264, were immunized with peptides 27–35, 55–62, 208–216, and 257–264 emulsified in TiterMax; C57BL/6J mice were immunized as controls. All peptides elicited strong responses in C57BL/6J mice, as observed previously. In Act-mOVA mice, CD8 responses to peptides 55–62 and 257–264 were not observed, as expected (Figure 3C). As hypothesized, peptide 208–216 elicited significant responses in Act-mOVA mice, which is in accordance with the nature of cryptic epitopes.
Surprisingly, immunized Act-mOVA mice did not show CD8 responses against peptide 27–35. Two non–mutually exclusive possibilities, not tested here, might explain this observation. The epitope is obviously presented directly, but it may be unable to be cross-presented. It may be a subdominant epitope as classically defined; the reasons for subdominance are of course unclear.
Epitypicity of peptides in the context of an OVA-expressing tumor.
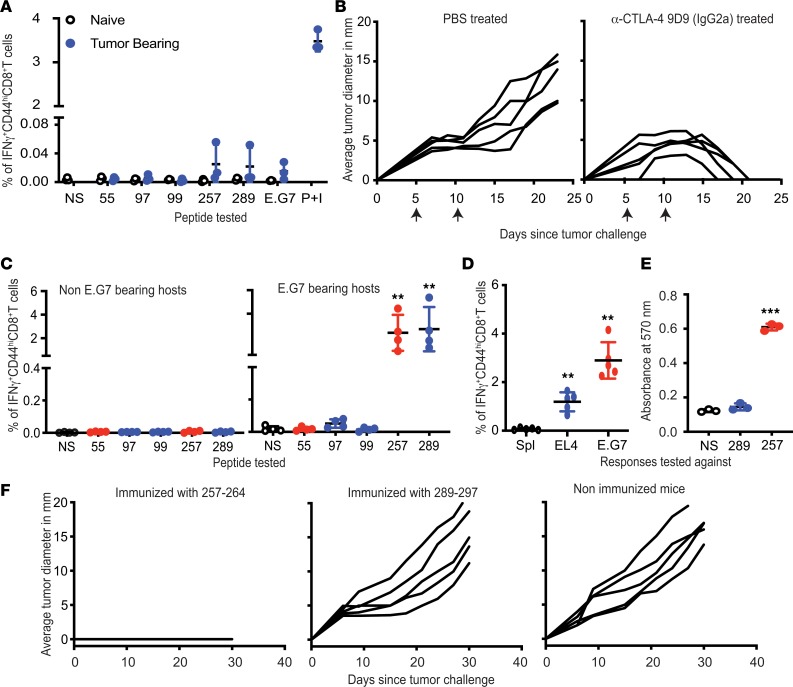
CD8+ T cell responses to 5 epitopes of OVA — 55–62, 97–105, 99–107, 257–264, and 289–297 — were tested in mice with progressively growing E.G7 tumors. CD8+ T cells from the inguinal nodes and spleens of the tumor-bearing and naive mice were isolated and stimulated with epitopes of OVA or E.G7 cells. Surprisingly, CD8+ T cells from E.G7-bearing mice also failed to detect E.G7 or the splenocytes pulsed with individual epitopes of OVA (Figure 4A). The CD8+ T cells, however, expressed IFN-γ and upregulated CD44 when stimulated nonspecifically with PMA and ionomycin (Figure 4A).
Figure 4. CD8+ T cell responses in E.G7-bearing mice.
(A) Lack of CD8+ responses against E.G7 or OVA in E.G7-bearing mice. CD8+ cells from tumor-bearing mice were stimulated with peptide-pulsed splenocytes, unpulsed splenocytes (NS), E.G7 cells, or PMA and ionomycin (P+I) (n = 3; experiment performed 3 times). (B) Treatment with antibody 9D9 IgG2a (α-CTLA-4) causes complete regression of E.G7. E.G7-bearing mice were treated with 9D9 or PBS on the days indicated with arrows (n = 5; experiment performed 3 times). (C) CD8+ responses to 257–264 and 289–297 are primed in mice rejecting E.G7. E.G7-bearing or naive mice were treated with 9D9. CD8+ cells were enriched after tumors regressed and stimulated with peptide-pulsed splenocytes or unpulsed splenocytes (NS; n = 4 for both panels; experiment performed 3 times; Welch’s t test). (D) CD8+ cells from mice rejecting E.G7 were stimulated with splenocytes (Spl), EL4 cells, or E.G7 cells (n = 5; experiment performed 2 times, Welch’s t test). (E) Splenocytes pulsed with 289–297 or SIINFEKL were cocultured with B3Z (n = 3; experiment performed 1 time; Welch’s t test). (F) Peptide 289–297 does not elicit protective immunity, while SIINFEKL does. Mice were immunized with SIINFEKL or 289–297 and challenged with E.G7 seven days later (n = 5 for each panel; experiment performed 2 times). **P ≤ 0.01, ***P ≤ 0.001.
Response to CD8+ T cell epitopes was tested in the context of depletion of tumor-infiltrating T regulatory cells (Tregs). E.G7 tumor–bearing C57BL/6J mice were treated with 9D9 (IgG2a), an anti–CTLA-4 monoclonal antibody, which inhibits the CTLA-4/B7 interaction but also depletes Tregs from the tumor microenvironment (14, 15). The tumors underwent complete regression in mice treated with this antibody (Figure 4B). Upon coculturing CD8+ T cells isolated from mice rejecting E.G7 tumors with splenocytes presenting individual epitopes of OVA, the CD8+ T cells were found to respond to peptides 257–264 (SIINFEKL) and 289–297 but not 55–62, 97–105, or 99–107 (Figure 4C). CD8+ T cells from non–tumor-bearing mice treated with antibody 9D9 did not recognize any epitopes of OVA (Figure 4C).
In addition to OVA, E.G7 tumor cells must express any neoepitopes that are present in the parental line EL4. CD8 responses against such neoepitopes of E.G7 cells were tested by coincubating CD8+ T cells from E.G7-rejecting mice with EL4 cells. EL4 cells were recognized by CD8+ T cells, indicating that responses against the neoepitopes of E.G7 were generated in 9D9 IgG2a–treated, E.G7-bearing mice (Figure 4D).
Because CD8+ responses to peptides SIINFEKL and 289–297 are always concordant, the possibility that they are cross-reactive was tested. Splenocytes pulsed with SIINFEKL but not peptide 289–297 were able to stimulate the B3Z hybridoma that specifically recognized SIINFEKL-Kb (Figure 4E). The ability of SIINFEKL and peptide 289–297 to protect naive C57BL/6J mice from an E.G7 challenge was tested. Mice immunized twice with either peptide, or whole OVA, or unimmunized mice were challenged with E.G7 cells. Mice immunized with SIINFEKL and those immunized with whole OVA (data not shown) were completely protected, but mice immunized with peptide 289–297 showed no inhibition of tumor growth (Figure 4F). Parenthetically, we tested peptide 289–297 for antitumor activity even though we had observed previously (Figure 1) that it does not elicit a CD8+ T cell response; we did this in order to consider the possibility that the CD8 response may be below the level of detection, and yet may be effective in slowing the tumor growth.
SIINFEKL as a therapeutic vaccine against E.G7 tumors.
The objective of identifying cancer neoepitopes is to treat cancer-bearing hosts by immunizing with such neoepitopes and obtaining a favorable change in the course of disease. Cancer neoepitopes are universally recognized based on their ability to elicit immunity in mice immunized before tumor challenge, or by their ability to elicit good CD8 responses. SIINFEKL meets both criteria. The efficacy of immunization with SIINFEKL in tumor-bearing mice was tested. Mice challenged with E.G7 cells were immunized with SIINFEKL admixed with an adjuvant on the day of (i.e., concurrent with) or 3 or 5 days after tumor challenge. Immunization with SIINFEKL on the day of the tumor challenge conferred mice with weak immunity against E.G7 tumors and resulted in the delayed onset of tumor growth in some mice (Supplemental Figure 1, left panel; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.127882DS1). However, the tumor burden in mice immunized 3 or 5 days after tumor challenge increased unabated (Supplemental Figure 1; middle and right panels).
Discussion
Our studies utilize bioinformatics and immunological approaches to reveal 6 peptides of immunological relevance in the well-studied model antigen OVA. The 6 peptides fall into 3 categories. (i) One of the 6 peptides is immunogenic by itself and is also recognized as an epitope upon immunization with OVA (one additional such peptide, 256–264, is a single amino acid extension of SIINFEKL, and is not considered a novel peptide). (ii) An additional 3 peptides are not immunogenic, i.e., do not elicit CD8+ T cell response upon immunization with peptides, but are perfectly epitypic, in that they are recognized by CD8+ T cells elicited upon immunization with whole OVA. The discordance between immunogenicity and epitypicity here must arise from instability of the immunizing peptides during immunization and processing within the dendritic cells. For 1 of these 3 peptides, we show that an extended version of the peptide can immunize effectively. (iii) One (peptide 27–35) is immunogenic, but is not seen as an epitope upon immunization with whole OVA, suggesting that this epitope is subdominant in the context of immunization. One additional peptide (peptide 208–216) cannot be naturally generated in vivo, and is thus cryptic, by the original definition of the term (3).
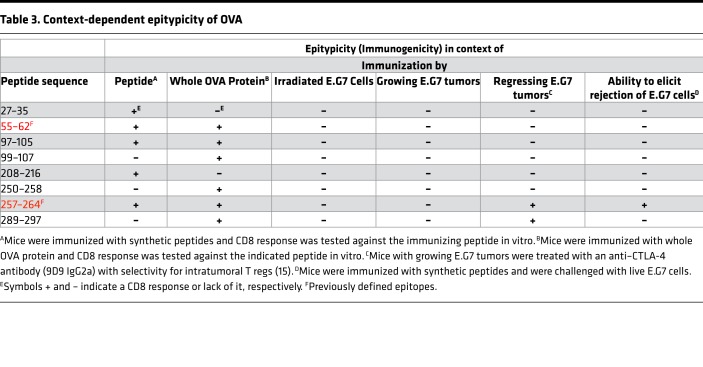
Viewed in the context of tumor antigenicity, the 4 epitopes (i.e., the 6 peptides identified minus 1 cryptic and 1 subdominant epitope, and also excluding the single amino acid extension of SIINFEKL) behave in unexpected ways. In mice immunized with irradiated E.G7 cells, no CD8+ T cell response to any of the OVA epitopes (including SIINFEKL) was detected. Mice with growing E.G7 cells too showed no response against any potentially novel epitope nor against SIINFEKL. Only in mice with regressing tumors (as a result of CTLA-4 blockade and FcγR-mediated depletion of Tregs) was a response detected, and it was confined to the known epitope SIINFEKL and an epitope reported here, peptide 289–297. No CD8+ T cell response to the previously reported epitope 55–62 was detected. These data indicate that epitopes of OVA besides SIINFEKL and 289–297 are subdominant to mutation-encoded neoepitopes of E.G7/EL4. Interestingly, while immunization with SIINFEKL can protect mice against a subsequent challenge with E.G7 tumor cells, immunization with 289–297 has no antitumor activity. The latter observation is consistent with our observation that 289–297 is not immunogenic in and of itself, even as it is perfectly epitypic. It is likely that immunization with an extended version of 289–297 may indeed elicit antitumor immunity; however, such extended peptides could not be synthesized due to technical difficulties. Parenthetically, immunization with 55–62 or 97–105 (both immunogenic and epitypic) also does not generate antitumor activity. Table 3 summarizes these data. Of note, the experiments with SIINFEKL and those that revealed the potentially novel MHC I–restricted epitopes of OVA were performed in non–tumor-bearing mice. Therapeutic immunization with SIINFEKL does not control tumor burden because of the possibility that responses elicited by SIINFEKL in tumor-bearing mice could be lower in magnitude than the responses observed in tumor-free mice. Additionally, the preestablished tumors could exclude SIINFEKL-specific T cells. Further, whether immunization with OVA elicits equally broad responses in mice with preexisting tumors is unclear and needs to be tested.
Table 3. Context-dependent epitypicity of OVA.
These results have a cautionary lesson for our current aspirations at neoepitope-based immunotherapy of human cancers. Neoepitopes that elicit CD8+ T cell responses in cancer patients are considered to be particularly useful indicators of a successful antitumor immune response, and also as potential antigens as cancer vaccines. If the results with OVA are broadly applicable, the antigens defined by the CD8+ T cells of cancer patients are unlikely to be effective in cancer therapy. And lest we dismiss OVA because it is OVA, it is useful to remember that everything that has been discovered with OVA, immunologically, has thus far been found to be true broadly.
Methods
Mice.
Naive C57BL/6J (stock number 00064) and Act-mOVA (stock number 005145) female mice were purchased from The Jackson Laboratory. Mice were maintained in the animal facility at UConn Health. Mice aged 6–10 weeks were used for all the experiments.
Cell lines and reagents.
B3Z hybridoma was a gift from Nilabh Shastri (Division of Immunology and Pathogenesis, Department of Molecular and Cell Biology, University of California, Berkeley, California, USA). EL4 (catalog ATCC TIB-38) and E.G7 (catalog ATCC CRL-2113) cell lines were purchased from American Type Culture Collection. PBS (catalog 10010023), Hank’s balanced salt solution (HBSS) (catalog 14025092), RPMI 1640 (catalog 11875085), sodium pyruvate (catalog 11360070), non-essential amino acids (catalog 11140050), penicillin-streptomycin-glutamine (catalog 10378016), and 2-mercaptoethanol (catalog 21985023) were purchased form Thermo Fisher Scientific. Fetal bovine serum (catalog F8067-500 ml), OVA (catalog A5503), and dimethyl sulfoxide (catalog D2650) were purchased from Sigma-Aldrich. All cells were cultured in RPMI containing 5% fetal bovine serum and the supplements mentioned above.
The fluorescently labeled antibodies anti-CD8-PerCP/Cy5.5 (catalog 100734), anti-CD3-Alexa Flour 488 (catalog 100210), anti-CD44-APC (catalog 103012), and anti-IFN-γ-PE (catalog 505808) were purchased from BioLegend. Fluorescent fixable viability dye (catalog 65-0865-14) was purchased from eBioscience.
The 9D9-IgG2a antibody against CTLA-4 was generated as described previously (15).
Synthetic peptides were purchased from the following vendors: Genemed Synthesis Inc., Advanced peptides, JPT Peptide Technologies, and Genscript.
Putative epitope prediction.
The primary structure of OVA was obtained from UniProt.org (entry number P01012). The primary structure of OVA was analyzed with NetMHC (3.0) available at http://www.cbs.dtu.dk/services/NetMHC-3.0/ to predict all 8–, 9–, 10–, and 11–amino acid–long sequences with the potential to bind H-2Kb or H-2Db. Both artificial neural network (ANN) and profile weight matrix (PWM) algorithms were utilized.
Testing peptide-MHC binding affinities.
The binding capacities of synthetic peptides were measured in classical competition assays using high-affinity radiolabeled ligands and purified MHC molecules, as detailed elsewhere (16). Six different concentrations of peptides were typically tested in at least 3 independent experiments. The IC50 value determined by this method approximately represents the dissociation constant value (17, 18).
Testing immunogenicity of peptides.
Synthetic peptides were dissolved in DMSO to prepare a master stock. Working stocks of peptides were prepared by diluting the master stocks in PBS. Required quantities of peptides from the working stocks were mixed with TiterMax, an adjuvant, at a 1:1 ratio and emulsified. Mice were anesthetized and immunized in the rear footpads with 10 μg of individual emulsified peptides. To test CD8+ T cell responses, mice were euthanized 7 days after immunization and the draining popliteal nodes were harvested. A single-cell suspension was generated from the LNs by crushing the nodes with butts of syringe plungers and then passing them through a 100-μm cell strainer (Thermo Fisher Scientific, 08-771-19). The LN cells were then restimulated with 10 μM immunizing peptide or PBS for 12 hours in the presence of brefeldin A. Cells were stained for CD8, CD3, CD44, and viability at the end of the incubation and fixed and permeabilized using Cytofix/Cytoperm (BD, 554714). Cells were finally stained for IFN-γ and analyzed by flow cytometry on a MACSQuant Analyzer (Miltenyi Biotec). The data generated through flow cytometry were analyzed using FlowJo software (FlowJo, LLC).
Testing MHC class I–restricted antigenicity of OVA.
OVA was dissolved in PBS and then emulsified with TiterMax. Mice were immunized with 450 μg (or as indicated) of emulsified OVA in rear footpads. Seven days later, the single-cell suspension generated from the dLNs was restimulated with individual peptide or not in the presence of brefeldin A for 12 hours. Expression of IFN-γ by the restimulated cells was tested using intracellular cytokine staining assay as described above.
Testing cross-reactivity of SIINFEKL and 289–297 with B3Z.
Splenocytes from naive C57BL/6J mice were pulsed with 10 μM indicated peptide. Forty thousand splenocytes were cocultured with 100,000 B3Z for 18 hours. Expression of β-galactosidase by the cultured B3Z cells was tested by adding a lysis buffer containing NP-40 and chlorophenol red-β-D-galactopyranoside (CPRG) was added to the cultures. Absorbance at 595 nm was measured 12 hours after addition of CPRG.
Testing homology between sequences within OVA and proteins endogenous to mouse.
Peptides 36–43 and 214–222 were aligned with all the mouse (taxid:10090) nonredundant protein sequences using the Basic Local Alignment Search Tool (pBLAST) available on the National Center for Biotechnology Information website.
Testing immunogenicity of E.G7.
E.G7 cells were subjected to 34 Gy of γ radiation. Cells were washed 3 times with HBSS to completely remove fetal bovine serum. Mice were subcutaneously inoculated with 2 × 107 irradiated cells twice, 7 days apart. Ten days after the second inoculation, the inguinal dLNs and spleens were harvested, and CD8+ T cells were enriched using a magnetic cell enrichment kit (STEMCELL Technologies, 19853). Enriched CD8+ T cells were cocultured with naive splenocytes pulsed with 10 μM indicated peptide or with E.G7 or EL4 cells for 12 hours in the presence of brefeldin A. Intracellular cytokine staining and flow cytometry were performed to test the expression of IFN-γ by the cultured CD8+ T cells.
Tumor challenges.
E.G7 cells were harvested from the cultures and washed 3 times with HBSS and maintained in HBSS after the final wash. Mice were challenged subcutaneously with 5 × 105 cells on the right flank. Growth of tumors was recorded as the measured average length of 2 perpendicular diameters. Mice carrying tumors with the average diameter of 20 mm were euthanized.
Antibody-mediated immunomodulation.
An anti–CTLA-4 monoclonal antibody 9D9 (isotype IgG2a) was used to apply checkpoint blockade in mice. E.G7 tumor–bearing C57BL/6J mice were administered 100 μg of the antibody admixed with PBS intraperitoneally on days 5 and 10 after tumor challenge. Non–tumor-bearing mice were subjected to a regimen identical to that used for the control.
Testing CD8+ T cell responses in tumor-bearing mice.
Mice were challenged with 5 × 105 E.G7 cells and then either treated with antibody 9D9 or not. The mice treated with 9D9 were euthanized after tumors were completely rejected, usually on day 20 after challenge. The untreated tumor-bearing mice were euthanized 9 days after the challenge. The inguinal dLNs and spleens were harvested and CD8+ T cells enriched from them. Responses against the epitopes of OVA or E.G7 or EL4 cells were tested by coculturing the isolated CD8+ T cells with the appropriate target cells.
Statistics.
Biological replicates were tested in each group in every experiment to enable statistical analysis. The statistical differences between experimental groups were determined by performing an unpaired t test (2-tailed) or t test (2-tailed) with Welch’s correction. A P value less than 0.05 was considered significant. Prism software (GraphPad Software) was used to perform the analysis.
Study approval.
All experimental protocols involving use of laboratory animals were approved by Institutional Animal Care and Use Committee at UConn Health (Farmington, Connecticut, USA).
Author contributions
SHK and PKS designed and performed experiments and wrote the manuscript. JS and AS measured MHC-peptide binding affinities and reviewed the manuscript. MJS and AJK generated the anti-CTLA-4 9D9 IgG2a antibody, discussed its application to the study, and reviewed the manuscript.
Supplementary Material
Acknowledgments
We acknowledge Sara Pan (UConn School of Medicine) for preliminary experiments, and Sreyashi Basu (University of Texas) and Ion Mandoiu (UConn) for valuable discussions.
Version 1. 03/14/2019
In-Press Preview
Version 2. 04/18/2019
Electronic publication
Footnotes
Conflict of interest: SHK has financial interest in Truvax Inc.
Copyright: © 2019, American Society for Clinical Investigation.
Reference information: JCI Insight. 2019;4(8):e127882. https://doi.org/10.1172/jci.insight.127882.
Contributor Information
Sukrut Hemant Karandikar, Email: sukrut.karandikar@gmail.com.
John Sidney, Email: jsidney@liai.org.
Alessandro Sette, Email: alex@liai.org.
Mark Joseph Selby, Email: mark_selbyphd@hotmail.com.
Alan Jerry Korman, Email: Alan.Korman@bms.com.
Pramod Kumar Srivastava, Email: srivastava@nso2.uchc.edu.
References
- 1.Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jerne NK. Immunological speculations. Annu Rev Microbiol. 1960;14:341–358. doi: 10.1146/annurev.mi.14.100160.002013. [DOI] [PubMed] [Google Scholar]
- 3.Sercarz EE, Lehmann PV, Ametani A, Benichou G, Miller A, Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annu Rev Immunol. 1993;11:729–766. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- 4.Duan F, et al. Genomic and bioinformatic profiling of mutational neoepitopes reveals new rules to predict anticancer immunogenicity. J Exp Med. 2014;211(11):2231–2248. doi: 10.1084/jem.20141308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gubin MM, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yadav M, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515(7528):572–576. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 7.Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54(6):777–785. doi: 10.1016/S0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 8.el-Shami K, et al. MHC class I-restricted epitope spreading in the context of tumor rejection following vaccination with a single immunodominant CTL epitope. Eur J Immunol. 1999;29(10):3295–3301. doi: 10.1002/(SICI)1521-4141(199910)29:10<3295::AID-IMMU3295>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 9.Lipford GB, Hoffman M, Wagner H, Heeg K. Primary in vivo responses to ovalbumin. Probing the predictive value of the Kb binding motif. J Immunol. 1993;150(4):1212–1222. [PubMed] [Google Scholar]
- 10.Rötzschke O, Falk K, Stevanović S, Jung G, Walden P, Rammensee HG. Exact prediction of a natural T cell epitope. Eur J Immunol. 1991;21(11):2891–2894. doi: 10.1002/eji.1830211136. [DOI] [PubMed] [Google Scholar]
- 11.Assarsson E, et al. A quantitative analysis of the variables affecting the repertoire of T cell specificities recognized after vaccinia virus infection. J Immunol. 2007;178(12):7890–7901. doi: 10.4049/jimmunol.178.12.7890. [DOI] [PubMed] [Google Scholar]
- 12.Benarafa C, Remold-O’Donnell E. The ovalbumin serpins revisited: perspective from the chicken genome of clade B serpin evolution in vertebrates. Proc Natl Acad Sci USA. 2005;102(32):11367–11372. doi: 10.1073/pnas.0502934102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3(11):1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 14.Waight JD, et al. Selective FcγR co-engagement on APCs modulates the activity of therapeutic antibodies targeting T cell antigens. Cancer Cell. 2018;33(6):1033–1047.e5. doi: 10.1016/j.ccell.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selby MJ, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1(1):32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 16.Sidney J, et al. Measurement of MHC/peptide interactions by gel filtration or monoclonal antibody capture. Curr Protoc Immunol. 2013;Chapter 18:Unit 18.3. doi: 10.1002/0471142735.im1803s100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gulukota K, Sidney J, Sette A, DeLisi C. Two complementary methods for predicting peptides binding major histocompatibility complex molecules. J Mol Biol. 1997;267(5):1258–1267. doi: 10.1006/jmbi.1997.0937. [DOI] [PubMed] [Google Scholar]
- 18.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.