Abstract
Stressful and traumatic life events (STLEs) are common among HIV-infected individuals and may affect health behaviors such as adherence to antiretroviral (ARV) therapy, with important implications for treatment outcomes. We examined the association between STLEs and ARV adherence among 289 US-based participants enrolled between 7/½010 and 9/½013 in a study of depression treatment for HIV-infected patients. Participants received monthly telephone calls to assess STLEs and pill count-based ARV adherence. Inverse probability of observation weighting was combined with multiple imputation to address missing data. Participants were mostly male (71%) and black (63%), with a median age of 45 years. Median monthly adherence was 96% (interquartile range (IQR): 85–100%). Participants experienced a mean of 2.48 STLEs (range: 0–14) in the previous month. The presence of ≥2 STLEs was associated with a mean change in adherence of −3.67% (95% confidence interval (CI): −7.12%, −0.21%) and decreased likelihood of achieving ≥95% adherence (risk ratio (95% CI)=0.82 (0.71, 0.95)). For each additional STLE, the mean adherence change was −0.90% (95% CI: −1.79%, 0.00%). STLEs were associated with poorer ARV adherence, including decreased likelihood of adhering to ≥95% of ARV doses. This level of adherence has a critical role in regimen effectiveness and prevention of resistance.
Keywords: antiretroviral, HIV, medication adherence, mental health, stressful events
INTRODUCTION
Persons living with HIV/AIDS (PLWHA) face a number of challenges related to their HIV infection, including life-long use of antiretroviral (ARV) medications, HIV-related stigma, and opportunistic infections. Depression is also common, affecting upwards of 20% of PLWHA in the US,(Bing et al., 2001; Burack et al., 1993; Ickovics et al., 2001; Kilbourne, Justice, Rabeneck, Rodriguez-Barradas, & Weissman, 2001; Lyketsos et al., 1993; Lyketsos, Hutton, Fishman, Schwartz, & Treisman, 1996) in comparison to 12–13% of the general population.(Kessler et al., 2003) More attention has been paid recently to stressful and traumatic life events (STLEs) among PLWHA, which are common in this population,(Whetten et al., 2006; M. D. Wong, Sarkisian, Davis, Kinsler, & Cunningham, 2007) and may have important implications for psychological, behavioral, and other outcomes. STLEs include moderately or severely stressful or traumatic events such as death or illness of family or friends; difficulties with relationships, finances, or employment; legal trouble; threats to safety; housing instability; and physical or sexual assault. STLEs are experienced more often by PLWHA than by the general population, in part due to the predominance of HIV infection among populations experiencing economic and other difficulties that are often related to such events.(Mugavero et al., 2009)
Previous studies of STLEs have documented their high prevalence and association with negative clinical and behavioral outcomes. In the Coping with HIV/AIDS in the Southeast (CHASE) study, HIV-infected participants reported medians of nine incident stressful events and three incident severely stressful events over two years.(Mugavero et al., 2009) Increasing numbers specifically of traumatic events were associated with higher rates of unprotected sex, worse medication adherence, increased emergency department use and hospitalization, accelerated HIV disease progression, and increased mortality rates.(Leserman et al., 2007; Mugavero et al., 2009; Pence, Mugavero, et al., 2012) In a separate, cross-sectional study of HIV-uninfected young men who have sex with men, recent financial- and health-related stressful events were associated with increased risk of substance use, and recent health- and partner-related stressors were associated with sexual risk-taking.(C. F. Wong, Kipke, Weiss, & McDavitt, 2010)
One important HIV-related outcome that STLEs may affect is adherence to ARV medication. STLEs can cause disruptions to routines, and for PLWHA, such disruptions may result in decreased ARV adherence. Adequate ARV adherence is necessary to achieve viral suppression, maintain health, and avoid developing drug resistance.(Bangsberg, 2006; Bangsberg, Moss, & Deeks, 2004; Maggiolo et al., 2007; Shuter, Sarlo, Kanmaz, Rode, & Zingman, 2007) Two prior studies examined the association between STLEs and self-reported ARV adherence and found that increased frequency and greater severity of events were associated with poorer adherence.(Leserman, Ironson, O’Cleirigh, Fordiani, & Balbin, 2008; Mugavero et al., 2009) Both studies relied on self-reported ARV adherence and long recall periods for STLEs, which may have affected exposure and outcome measurement accuracy.
The current study examined the longitudinal association between STLEs and ARV adherence, measured monthly by pill counts, among adults in the Southeastern USA with HIV and depression. Unannounced, telephone-based pill counts have been shown to provide valid, accurate measures of adherence,(Kalichman et al., 2007) and are more sensitive than self-report to adherence changes over time.(Lee et al., 2007) Pill counts may, therefore, allow for a more accurate assessment of the relationship between STLEs and ARV adherence. Likewise, recent STLEs may be more strongly related to current ARV adherence than STLEs measured over longer recall periods. We hypothesized that higher numbers and severity of STLEs would be associated with poorer ARV adherence, and that improved measurements of both STLEs and adherence would yield stronger associations than those found in prior studies.
METHODS
Study sample
Data for this study came from the Strategies to Link Antidepressant and Antiretroviral Management at Duke University, the University of Alabama-Birmingham, Northern Outreach Clinic (Henderson, NC), and the University of North Carolina-Chapel Hill (SLAM DUNC) Study, a randomized, controlled trial testing the effect of evidence-based decision support for depression treatment on ARV adherence, which has been described previously.(Pence, Gaynes, et al., 2012) The study population comprises adult (age 18–65 years) HIV-infected patients with depression attending infectious disease clinics at the study sites. Participants enrolled between July 1, 2011 and September 30, 2013 were eligible for the current analysis; data on STLEs were collected during this period. Participants were followed for up to one year following enrollment, receiving up to 12 monthly pill count calls. The number of calls completed depended on attrition and timing of enrollment relevant to collection of STLE data and the end of the study. Study procedures were approved by the Institutional Review Boards at the University of North Carolina at Chapel Hill, Duke University, and the University of Alabama at Birmingham.
Measures
Stressful and Traumatic Life Events.
STLEs were assessed during monthly telephone calls with participants using the Life Events Survey (LES),(Leserman et al., 2008; Sarason, Johnson, & Siegel, 1978) modified to include those events considered moderately or severely stressful or traumatic based on prior research.(Leserman et al., 2002; Leserman et al., 2005) Participants were asked whether they had experienced any of 46 events, from within nine categories, during the prior month. STLE categories were: 1) romantic relationship changes; 2) estrangement from family; 3) death or serious illness of family member or close friend; 4) major illness, injury, or hospitalization; 5) employment difficulties; 6) financial difficulties; 7) legal difficulties; 8) life transitions; and 9) safety concerns (e.g. physical attacks, feeling unsafe). The total number of STLEs reported at each time point (monthly) was summed for each participant and coded discretely. The exposure was also dichotomized, to indicate experiencing at least the study population median number of STLEs vs. less than the median number, during the month prior. Additionally, the most potentially severe STLEs (divorce/separation, death/illness of immediate family member, major financial problems, time in jail, and sexual and/or physical assault) were combined, based on prior work,(Mugavero et al., 2009) to create a second discretely-coded variable. The severe-only total was dichotomized to indicate one or more severe STLEs vs. zero during the past month.
Antiretroviral Adherence.
ARV adherence was measured by monthly, unannounced, telephone-based pill counts and recorded as a continuous percentage. Adherence was calculated as the observed number of pills taken since the last count divided by the expected number of pills taken since the last count. The observed number of pills taken was the number of pills present at the current count, subtracted from the number present during the previous count, after accounting for pills gained (e.g., dispensed) and lost (e.g., thrown away) in the interim. The expected number of pills taken was the prescribed daily number of pills, multiplied by the number of days since the previous count. Both the observed and expected number of pills were summed across all ARV medications prior to dividing. Values of adherence <0% (n=20) or >200% (n=1) were discarded as outside the range of plausibility.
Secondary analyses explored 1) dichotomous coding of “adequate” (≥95%) vs. “inadequate” (<95%) adherence,(Lima et al., 2009; Lima et al., 2008) and 2) differences between pill count-based and self-reported adherence. Self-reported adherence was measured at baseline (in-person) and every three months thereafter (by telephone) during structured research interviews, with the following questions: 1) “Over the past month, how much of the time have you taken all of your HIV/AIDS medications?” and 2) “Over the past month, how much of the time have you missed or skipped taking your HIV/AIDS medications?” Responses, ranging from 0%−100%, were averaged (after reverse-coding the second question) to get a measure ranging from 0–100%. As self-reported adherence was measured every three months, while STLEs were measured every month, the average number of STLEs was calculated for each three-month time period. Self-reported adherence was also dichotomized at 95%, as above.
Additional Covariates.
Covariates used in the analysis were sex, age, depressive severity, HIV care self-efficacy, stress coping style, HIV-related physical symptoms, HIV status disclosure, employment status, and drug and/or alcohol abuse. Sex and age were assessed at study enrollment. Depressive severity, HIV care self-efficacy, stress coping style, HIV-related physical symptoms, HIV status disclosure, and employment status were measured at enrollment and every three months thereafter. Depressive severity was measured with the Hamilton Rating Scale for Depression (HAM-D),(Hamilton, 1960, 1980; Zimmerman, Chelminski, & Posternak, 2004) and coded discreetly (range: 0 to 50).(Hamilton, 1960) HIV-related self-efficacy was measured using the managing depression/mood, managing symptoms, communicating with health care provider, getting support/help, and managing fatigue subscales of the HIV Self-Efficacy questionnaire.(Shively, Smith, Bormann, & Gifford, 2002) Stress coping style was assessed with the Brief COPE instrument,(Carver, 1997; Carver, Scheier, & Weintraub, 1989) and physical symptoms were measured using the HIV Symptom Inventory.(Bing et al., 2001) Disclosure of HIV status to 1) close friends/family and 2) everyday acquaintances was measured separately with four-point Likert scales (“All,” “Most,” “Some,” or “None”) and was included as ordinal variables. Employment was dichotomized to indicate employed vs. not. Any abuse or dependence of drugs and/or alcohol was assessed at enrollment and at six- and twelve-month follow-up interviews with the alcohol/substance dependence and abuse sections of the Mini International Neuropsychiatric Interview.(Lecrubier et al., 1997)
Statistical analysis
The main exposure-outcome association was assessed using a linear model with robust variance to account for repeated observations on participants, yielding an estimate of the mean difference in past-month percent adherence. To address missing covariate values among completed contacts, multiple imputation by chained equations (MICE) was employed for all variables included in the analysis.(White, Royston, & Wood, 2011) Ten cycles of imputation were carried out per imputed dataset; 50 datasets were imputed and analyzed. To address potential selection bias arising from uncompleted contacts, inverse probability of observation weights (IPOWs) were calculated for the inverse probability of completing a given contact conditional on predictors of contact completion, using a logistic model.(Moodie, Delaney, Lefebvre, & Platt, 2008; Seaman, White, Copas, & Li, 2012) Baseline and time-updated variables found to be statistically significantly associated (at alpha=0.05) with completion in bivariable analyses were included in the model. The IPOWs were stabilized by the marginal probability of contact completion and used to weight the final exposure-outcome model.
In analyses with dichotomous coding of the ARV adherence outcome, a Poisson model with robust variance was used instead of a linear model (and in place of a log-binomial model due to model convergence issues(Yelland, Salter, & Ryan, 2011)), to yield an estimate of the risk ratio (RR) for ≥95% adherence. All other analyses followed the approach described above.
RESULTS
Study population
Two-hundred eighty-nine participants enrolled in the SLAM DUNC study while STLEs were being measured. The majority of the study sample (71%) was male, black (63%), non-Hispanic (96%), and single (78%). The median (interquartile range [IQR]) age was 45 (38–51), and participants reported contracting HIV, on average, 11 years prior to study enrollment. Eighty-five percent of participants had at least a high school-level education and 26% were employed at enrollment. Self-reported past-month ARV adherence was high at enrollment, with a median (IQR) of 98% (85–100%) adherence (Table 1).
Table 1.
Study sample characteristics, Southeastern USA, 2010–2013 (n = 289).
| Variable | n (%) | Median (IQR) | Missing |
|---|---|---|---|
| Sex | 0 | ||
| Male | 205 (71) | ||
| Female | 84 (29) | ||
| Age | 45 (38–51) | 0 | |
| Marital status | 2 | ||
| Married/cohabitating | 62 (21) | ||
| Single | 225 (78) | ||
| Race | 0 | ||
| White | 94 (33) | ||
| Black | 181 (63) | ||
| Other | 14 (5) | ||
| Ethnicity | 0 | ||
| Hispanic | 13 (4) | ||
| Non-Hispanic | 276 (96) | ||
| Education | 3 | ||
| Less than HS grad | 40 (14) | ||
| High school grad | 110 (38) | ||
| More than high school | 136 (47) | ||
| Monthly household income ($) | 1000 (674–1752) | 18 | |
| Log-10 income ($) | 3 (2.83–3.24) | ||
| Currently employed | 76 (26) | 3 | |
| Years since HIV diagnosis | 11 (4–17) | 11 | |
| Baseline log10-viral load | 1.67 (1.59–2.31) | 11 | |
| Baseline viral load 50+ | 90 (32) | ||
| Baseline CD4 count | 539 (326–789) | 14 | |
| Baseline depression score | 21 (15–25) | 36 | |
| Baseline self-reported adherence | 98 (85–100) | 26 | |
Participants completed, on average, five monthly telephone interviews (IQR: 1–8); the number of interviews completed ranged from zero to 12. Of the total possible 2,634 monthly interviews, 1,412 (54%) were completed and included in analyses. Among completed contacts, 3,373 STLEs were reported, for a mean of 2.48 events per month; the number of STLEs experienced in a month ranged from 0 to 14, and the median (IQR) was 2 (1–4). Six hundred seventy-eight severe STLEs were reported, or 0.50 events per month. Financial difficulties were most commonly reported, with 1.24 events/month, followed by illness/injury/hospitalization of the participant (0.31 events/months) and death/serious illness of family member/friend (0.25 events/month). Legal troubles were reported least often (0.04 events/month) (Table 2). Across all contacts, pill count-measured median (IQR) ARV adherence was 96% (83–100%).
Table 2.
Description of STLEs, Southeastern USA, 2010–2013 (n = 289).
| Incidence |
||
|---|---|---|
| Per person- month |
Per person- year |
|
| Total # STLEs | 2.48 | 29.76 |
| Financial difficulties | 1.24 | 14.88 |
| Major illness, injury, or hospitalization | 0.31 | 3.72 |
| Death/illness of family member/friend | 0.25 | 3 |
| Employment difficulties | 0.24 | 2.88 |
| Safety concerns | 0.12 | 1.44 |
| Romantic relationship changes | 0.11 | 1.32 |
| Estrangement from family | 0.1 | 1.2 |
| Life transitions | 0.08 | 0.96 |
| Legal difficulties | 0.04 | 0.48 |
| Total # severe STLEsa | 0.5 | 6 |
Note: STLEs = Stressful and traumatic life events.
Includes: divorce/separation, death/illness of immediate family member, major financial problems, time in jail, and sexual and physical assault.
Missing data
Among completed contacts, multiple imputation was used to impute missing baseline values for 0.4% of employment data, 4% viral load, 5% CD4 count, 2% self-efficacy, and 0.5% SF12 mental functioning score. There was more substantial missing data for post-baseline variables. Pill count-measured ARV adherence was imputed in 15% of observations, employment status in 14%, and HIV status disclosure in 74%. For drug/alcohol abuse, coping style, self-efficacy, and HIV symptoms, values were imputed in 11% of observations.
In calculating the IPOWs, baseline predictors of completing interviews that were statistically significant at alpha=0.05 were age, employment, viral load, CD4 count, self-efficacy, and SF12 mental functioning score. A time-updated variable indicating whether the previous month’s pill count was completed was also included in the weighting model. The IPOWs had a mean (standard deviation) of 1.06 (0.50), and ranged from 0.58 to 5.35. These weights were applied to the 1,412 completed observations, to account for the 46% of contacts that were not completed.
Primary outcomes
A greater number of past-month STLEs was associated with poorer past-month ARV adherence, after adjusting for confounding by drug/alcohol abuse, coping style, self-efficacy, employment, HIV symptoms, age, gender, and HIV status disclosure. One additional STLE experienced was associated with a mean difference (95% confidence interval (CI)) in ARV adherence of −0.99% (−1.89%, −0.09%), indicating that for each additional STLE, mean adherence decreased by nearly one percentage point (Table 3). A stronger association was observed when comparing participants with at least the median number of STLEs (2) to those with less than the median (mean difference=−3.67%, 95% CI=−7.12%, −0.21%). When limited to the most potentially severe STLEs, each additional event was associated with a more extreme mean difference of −2.43%; however, the 95% CI (−5.16%, 0.30%) spanned the null value of no difference. Experiencing any severe STLE vs. none was associated with a mean adherence difference (95% CI) of −3.03% (−6.41%, 0.35%). While these associations were modest in magnitude, they were relatively precisely estimated.
Table 3.
Associations between STLEs and ARV adherence, pill count-based and self-reported, Southeastern USA, 2010–2013.
| Pill count-based adherence | Self-reported adherence | |||
|---|---|---|---|---|
| Mean % adherence difference (95% CI) |
Adherence ≥95% RR (95% CI) |
Mean % adherence difference(95% CI) |
Adherence ≥95% RR (95% CI) |
|
| All STLEs | ||||
| Per 1-unit increase in number | −0.99% (−1.89%, −0.09%) | 0.95 (0.90, 0.99) | −0.63% (−1.61%, 0.35%) | 0.98 (0.94, 1.02) |
| ≥2 vs. <2a | −3.67% (−7.12%, −0.21%) | 0.82 (0.71, 0.95) | −1.63% (−5.16%, 1.91%) | 0.91 (0.79, 1.05) |
| Severe STLEs | ||||
| Per 1-unit increase in number | −2.43% (−5.16%, 0.30%) | 0.84 (0.74, 0.96) | −1.99% (−5.50%, 1.52%) | 0.89 (0.77, 1.04) |
| Any vs. none | −3.03% (−6.41%, 0.35%) | 0.80 (0.69, 0.94) | −3.45 (−6.33, −0.58) | 0.84 (0.74, 0.96) |
Note: STLEs = Stressful and traumatic life events; ARV = antiretroviral; RR = risk ratio; CI = confidence interval.
2 = population median monthly number of STLEs.
Secondary outcomes
When adherence was dichotomized, each additional past-month STLE was associated with decreased likelihood of ≥95% adherence in the past month, both overall (risk ratio (RR)=0.95, 95% CI=0.90, 0.99) and for severe STLEs (RR=0.84, 95% CI=0.74, 0.96). The decreases in likelihood of adherence were more marked for participants experiencing at least the median number vs. less than the median number of overall STLEs (RR=0.82, 95% CI=0.71, 0.95) and any severe STLE vs. none (RR=0.80, 95% CI=0.69, 0.94) (Table 3). The crude proportion of observations in which ≥95% adherence was achieved decreased with increasing number of STLEs (zero, one, two or more), both for overall and severe STLEs (Figure 1).
Figure 1a.
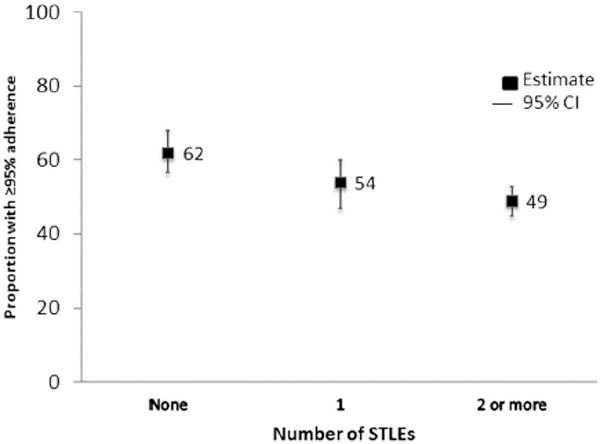
Proportion with ≥95% ARV Adherence by Number of STLEs, US, 2010–2013
ARV=antiretroviral, STLE=stressful or traumatic life event
Estimates of association between STLEs and self-reported ARV adherence were similar in direction and precision but attenuated compared to pill count-measured adherence (Table 3). All estimates using self-reported adherence were closer to the null than the estimates obtained using pill count-based adherence, except that of the mean difference in ARV adherence when comparing any severe STLE vs. none.
DISCUSSION
In our study population, representing HIV-infected patients with depression in the Southeastern USA, a higher number of STLEs was associated with poorer ARV adherence, and in particular, lower likelihood of achieving or maintaining ≥95% adherence. Participants experiencing at least the median number of STLEs in a given month had 18% lower likelihood of ≥95% adherence in that same month, compared to those experiencing less than the median number. The association between STLEs and reduced adherence was similar when limited to the most severe STLEs.
STLEs were common, with participants reporting, on average, more than two events per month during follow-up of up to 12 months; number of events reported per month ranged from zero to 14 (median (IQR): 2 (1–4)). This high burden of STLEs, similar to that found in other studies,(Leserman et al., 2008; Mugavero et al., 2009) emphasizes their potential impact on ARV adherence. The relatively modest difference in mean adherence of roughly 1% associated with one additional STLE implies that an individual experiencing ten STLEs in a given month could be expected to have 10% lower adherence than an individual experiencing no STLEs. Also, each additional STLE was associated with a 5% reduction in the likelihood of ≥95% adherence, and each additional severe STLE was associated with a 16% reduction. These results suggest that while the impact of STLEs on continuously measured adherence may be modest, it may be sufficient to reduce adherence below a critical threshold required for regimen effectiveness and prevention of resistance.(Lima et al., 2009; Lima et al., 2008) The mean differences in adherence and the reductions in likelihood of ≥95% adherence were even more pronounced when comparing at least the median number of STLEs to less than the median, or any severe STLE vs. none.
Our results add to the existing evidence that ongoing STLEs may interfere with ARV adherence among PLWHA. Prior studies have demonstrated this association, but have exclusively used self-report to measure adherence, and long recall periods for STLEs.(Leserman et al., 2008; Mugavero et al., 2009) This is the first study showing the association between STLEs measured monthly over time and pill count-based ARV adherence. Pill counts have been shown to be more sensitive to changes in adherence over time than self-reported measures,(Lee et al., 2007) and the measurement of both STLEs and adherence at the same point in time over short recall periods allowed us to capture more proximal associations of STLEs with adherence.
We found an attenuation of estimates of association when ARV adherence was measured by self-report, compared to pill counts. This might be due in part to reduced variability of the measurement, as self-reported adherence tends to be skewed upwards; however, it is likely also due to the improved accuracy of using the more objective measure of unnannouned pill counts. Telephone-based pill counts have been shown to be as accurate as researcher-performed pill counts.(Kalichman et al., 2007)
Previous studies have also used dichotomous outcomes to indicate adherent vs. non-adherent, based on report of missing ARV doses. The 5% reduction in risk of ≥95% adherence in our study is lower than both the 10% increase in non-adherence (OR=1.10, 95% CI=1.04, 1.16) in one study assessing missed doses over the past seven days,(Mugavero et al., 2009) and the 36% increase in non-adherence (OR=1.36, 95% CI=1.13, 1.63) in a study using missed doses in the prior two weeks as the outcome.(Leserman et al., 2008) The use of risk ratios instead of odds ratios in those studies may account for some of the difference. In our study there was an 11% reduction in odds of ≥95% adherence (data not shown). Our continuous pill count measure allowed us to additionally assess differences in adherence not tied to a somewhat arbitrary adherent/non-adherent cut-point.
Strengths of this study include the continuous pill count measure of adherence, monthly assessments of both adherence and STLEs, and the availability of a comprehensive set of potential confounders for inclusion in multivariable models. One important limitation was the low retention rate during follow-up and the resulting large amount of missing data. Rather than conduct a complete-case analysis, we used a combination of multiple imputation and inverse probability of observation weighting, based on observed predictors of missingness, to address the potential for selection bias arising from these missing data. We cannot exclude the possibility, however, that missingness could also be a function of unobserved characteristics, and in particular could be associated both with higher numbers of STLEs and with lower adherence. If this were the case, the present analysis would likely have underestimated the actual association of STLEs with poor adherence, but overestimation is possible as well. There is also the possibility of misspecification of the weighting model, the imputation model, or both.
Interventions should be targeted to increase provider awareness of STLEs, and to improve patient coping skills to mitigate negative effects of STLEs, in order to improve ARV adherence. One study found that participants receiving a quality-improvement intervention, aimed at increasing the proportion of patients receiving appropriate depression treatment, experienced fewer STLEs than those in usual care.(Sherbourne et al., 2008) Other studies found that expressive writing about STLEs being experienced was associated with greater cognitive engagement, fewer days of activity restriction, and improved health status.(Andersson & Conley, 2013; Smyth, Stone, Hurewitz, & Kaell, 1999) Other such interventions, focused on reducing the incidence of and/or developing skills to cope with STLEs, could be developed and potentially integrated with direct ARV adherence support strategies.
STLEs are common and potentially impactful, especially among persons managing both medical and mental health diagnoses. While some STLEs are unavoidable (e.g., death/illness), others (e.g., relationship problems, employment issues) might be mitigated through management of depressive symptoms and/or working with outside help, such as a social worker. In either case, raising awareness of the frequency and impact of STLEs among HIV providers, and developing interventions to prevent or alleviate their negative impacts could help in maximizing the clinical benefits of HIV treatment.
Figure 1b.

Proportion with ≥95% ARV adherence by number of severe STLEs
ARV=antiretroviral, STLE=stressful or traumatic life event
ACKNOWLEDGEMENTS
Funding sources: The SLAM DUNC study was supported by grant R01MH086362 of the National Institute of Mental Health and the National Institute for Nursing Research, National Institutes of Health, Bethesda, MD, USA. JKO was partially supported by National Institutes of Health training grant 5T32AI070114
Footnotes
Conflicts of interest: none
REFERENCES
- Andersson MA, & Conley CS (2013). Optimizing the perceived benefits and health outcomes of writing about traumatic life events. Stress Health, 29(1), 40–49. doi: 10.1002/smi.2423 [DOI] [PubMed] [Google Scholar]
- Bangsberg DR (2006). Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis, 43(7), 939–941. doi: 10.1086/507526 [DOI] [PubMed] [Google Scholar]
- Bangsberg DR, Moss AR, & Deeks SG (2004). Paradoxes of adherence and drug resistance to HIV antiretroviral therapy. J Antimicrob Chemother, 53(5), 696–699. doi: 10.1093/jac/dkh162 [DOI] [PubMed] [Google Scholar]
- Bing EG, Burnam A, Longshore D, Fleishman JA, Sherbourne CD, London AS, Shapiro M (2001). Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Archives of General Psychiatry, 58(8), 721–728. doi: DOI 10.1001/archpsyc.58.8.721 [DOI] [PubMed] [Google Scholar]
- Burack JH, Barrett DC, Stall RD, Chesney MA, Ekstrand ML, & Coates TJ (1993). Depressive symptoms and CD4 lymphocyte decline among HIV-infected men. JAMA, 270(21), 2568–2573. [PubMed] [Google Scholar]
- Carver CS (1997). You want to measure coping but your protocol’s too long: consider the brief COPE. Int J Behav Med, 4(1), 92–100. doi: 10.1207/s15327558ijbm0401_6 [DOI] [PubMed] [Google Scholar]
- Carver CS, Scheier MF, & Weintraub JK (1989). Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol, 56(2), 267–283. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. J Neurol Neurosurg Psychiatry, 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M (1980). Rating depressive patients. J Clin Psychiatry, 41(12 Pt 2), 21–24. [PubMed] [Google Scholar]
- Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, & Moore J (2001). Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA, 285(11), 1466–1474. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Amaral CM, Stearns H, White D, Flanagan J, Pope H, Kalichman MO (2007). Adherence to antiretroviral therapy assessed by unannounced pill counts conducted by telephone. J Gen Intern Med, 22(7), 1003–1006. doi: 10.1007/s11606-007-0171-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, National Comorbidity Survey R. (2003). The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA, 289(23), 3095–3105. doi: 10.1001/jama.289.23.3095 [DOI] [PubMed] [Google Scholar]
- Kilbourne AM, Justice AC, Rabeneck L, Rodriguez-Barradas M, & Weissman S (2001). General medical and psychiatric comorbidity among HIV-infected veterans in the post-HAART era. J Clin Epidemiol, 54 Suppl 1, S22–28. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Sheehan KH, Dunbar GC (1997). The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: Reliability and validity according to the CIDI. European Psychiatry, 12(5), 224–231. doi: Doi 10.1016/S0924-9338(97)83296-8 [DOI] [Google Scholar]
- Lee JK, Grace KA, Foster TG, Crawley MJ, Erowele GI, Sun HJ, Taylor AJ (2007). How should we measure medication adherence in clinical trials and practice? Ther Clin Risk Manag, 3(4), 685–690. [PMC free article] [PubMed] [Google Scholar]
- Leserman J, Ironson G, O’Cleirigh C, Fordiani JM, & Balbin E (2008). Stressful life events and adherence in HIV. AIDS Patient Care STDS, 22(5), 403–411. doi: 10.1089/apc.2007.0175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leserman J, Pence BW, Whetten K, Mugavero MJ, Thielman NM, Swartz MS, & Stangl D (2007). Relation of lifetime trauma and depressive symptoms to mortality in HIV. American Journal of Psychiatry, 164(11), 1707–1713. doi: DOI 10.1176/appi.ajp.2007.06111775 [DOI] [PubMed] [Google Scholar]
- Leserman J, Petitto JM, Gu H, Gaynes BN, Barroso J, Golden RN, Evans DL (2002). Progression to AIDS, a clinical AIDS condition and mortality: psychosocial and physiological predictors. Psychol Med, 32(6), 1059–1073. [DOI] [PubMed] [Google Scholar]
- Leserman J, Whetten K, Lowe K, Stangl D, Swartz MS, & Thielman NM (2005). How trauma, recent stressful events, and PTSD affect functional health status and health utilization in HIV-infected patients in the south. Psychosom Med, 67(3), 500–507. doi: 10.1097/01.psy.0000160459.78182.d9 [DOI] [PubMed] [Google Scholar]
- Lima VD, Harrigan R, Bangsberg DR, Hogg RS, Gross R, Yip B, & Montaner JS (2009). The combined effect of modern highly active antiretroviral therapy regimens and adherence on mortality over time. J Acquir Immune Defic Syndr, 50(5), 529–536. doi: 10.1097/QAI.0b013e31819675e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima VD, Harrigan R, Murray M, Moore DM, Wood E, Hogg RS, & Montaner JS (2008). Differential impact of adherence on long-term treatment response among naive HIV-infected individuals. AIDS, 22(17), 2371–2380. doi: 10.1097/QAD.0b013e328315cdd3 [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Hoover DR, Guccione M, Senterfitt W, Dew MA, Wesch J, . . . Morgenstern H. (1993). Depressive symptoms as predictors of medical outcomes in HIV infection. Multicenter AIDS Cohort Study. JAMA, 270(21), 2563–2567. [PubMed] [Google Scholar]
- Lyketsos CG, Hutton H, Fishman M, Schwartz J, & Treisman GJ (1996). Psychiatric morbidity on entry to an HIV primary care clinic. AIDS, 10(9), 1033–1039. [DOI] [PubMed] [Google Scholar]
- Maggiolo F, Airoldi M, Kleinloog HD, Callegaro A, Ravasio V, Arici C, . . . Suter F. (2007). Effect of adherence to HAART on virologic outcome and on the selection of resistance-conferring mutations in NNRTI- or PI-treated patients. HIV Clin Trials, 8(5), 282–292. doi: 10.1310/hct0805-282 [DOI] [PubMed] [Google Scholar]
- Moodie EE, Delaney JA, Lefebvre G, & Platt RW (2008). Missing confounding data in marginal structural models: a comparison of inverse probability weighting and multiple imputation. Int J Biostat, 4(1), Article 13. [DOI] [PubMed] [Google Scholar]
- Mugavero MJ, Raper JL, Reif S, Whetten K, Leserman J, Thielman NM, & Pence BW (2009). Overload: impact of incident stressful events on antiretroviral medication adherence and virologic failure in a longitudinal, multisite human immunodeficiency virus cohort study. Psychosom Med, 71(9), 920–926. doi: 10.1097/PSY.0b013e3181bfe8d2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence BW, Gaynes BN, Williams Q, Modi R, Adams J, Quinlivan EB, Mugavero MJ (2012). Assessing the effect of Measurement-Based Care depression treatment on HIV medication adherence and health outcomes: Rationale and design of the SLAM DUNC Study. Contemporary Clinical Trials, 33(4), 828–838. doi: DOI 10.1016/j.cct.2012.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence BW, Mugavero MJ, Carter TJ, Leserman J, Thielman NM, Raper JL, Whetten K (2012). Childhood trauma and health outcomes in HIV-infected patients: an exploration of causal pathways. J Acquir Immune Defic Syndr, 59(4), 409–416. doi: 10.1097/QAI.0b013e31824150bb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarason IG, Johnson JH, & Siegel JM (1978). Assessing the impact of life changes: development of the Life Experiences Survey. J Consult Clin Psychol, 46(5), 932–946. [DOI] [PubMed] [Google Scholar]
- Seaman SR, White IR, Copas AJ, & Li L (2012). Combining multiple imputation and inverse-probability weighting. Biometrics, 68(1), 129–137. doi: 10.1111/j.1541-0420.2011.01666.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherbourne CD, Edelen MO, Zhou A, Bird C, Duan N, & Wells KB (2008). How a therapy-based quality improvement intervention for depression affected life events and psychological well-being over time: a 9-year longitudinal analysis. Med Care, 46(1), 78–84. doi: 10.1097/MLR.0b013e318148478d [DOI] [PubMed] [Google Scholar]
- Shively M, Smith TL, Bormann J, & Gifford AL (2002). Evaluating Self-Efficacy for HIV Disease Management Skills. AIDS & Behavior, 6(4), 371–379. [Google Scholar]
- Shuter J, Sarlo JA, Kanmaz TJ, Rode RA, & Zingman BS (2007). HIV-infected patients receiving lopinavir/ritonavir-based antiretroviral therapy achieve high rates of virologic suppression despite adherence rates less than 95%. J Acquir Immune Defic Syndr, 45(1), 4–8. doi: 10.1097/QAI.0b013e318050d8c2 [DOI] [PubMed] [Google Scholar]
- Smyth JM, Stone AA, Hurewitz A, & Kaell A (1999). Effects of writing about stressful experiences on symptom reduction in patients with asthma or rheumatoid arthritis: a randomized trial. JAMA, 281(14), 1304–1309. [DOI] [PubMed] [Google Scholar]
- Whetten K, Leserman J, Lowe K, Stangl D, Thielman N, Swartz M, Van Scoyoc L (2006). Prevalence of childhood sexual abuse and physical trauma in an HIV-positive sample from the deep south. Am J Public Health, 96(6), 1028–1030. doi: 10.2105/AJPH.2005.063263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IR, Royston P, & Wood AM (2011). Multiple imputation using chained equations: Issues and guidance for practice. Stat Med, 30(4), 377–399. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- Wong CF, Kipke MD, Weiss G, & McDavitt B (2010). The impact of recent stressful experiences on HIV-risk related behaviors. J Adolesc, 33(3), 463–475. doi: 10.1016/j.adolescence.2009.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MD, Sarkisian CA, Davis C, Kinsler J, & Cunningham WE (2007). The association between life chaos, health care use, and health status among HIV-infected persons. J Gen Intern Med, 22(9), 1286–1291. doi: 10.1007/s11606-007-0265-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelland LN, Salter AB, & Ryan P (2011). Performance of the modified Poisson regression approach for estimating relative risks from clustered prospective data. American Journal of Epidemiology, 174(8), 984–992. doi: 10.1093/aje/kwr183 [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Chelminski I, & Posternak M (2004). A review of studies of the Hamilton depression rating scale in healthy controls: implications for the definition of remission in treatment studies of depression. J Nerv Ment Dis, 192(9), 595–601. [DOI] [PubMed] [Google Scholar]


