Abstract
Background:
HIV-1 set point viral load (SPVL) is a highly variable trait that influences disease progression and transmission risk. Men who are exclusively insertive (EI) during anal intercourse require more sexual contacts to become infected than exclusively receptive (ER) men. Thus, we hypothesize that EIs are more likely to acquire their viruses from highly infectious partners (i.e., with high SPVLs) and to have higher SPVLs than infected ERs. Methods: We used a one-generation Bernoulli model, a dynamic network model, and data from the Multicenter AIDS Cohort Study (MACS) to examine whether and under what circumstances MSM differ in SPVL by sexual role.
Results:
Both models predicted higher SPVLs in EIs than role versatile (RV) or ER men, but only in scenarios where longer-term relationships predominated. ER and RV men displayed similar SPVLs. EI men remained far less likely than ER men to become infected, however. When the MACS data were limited by some estimates of lower sex partner counts (a proxy for longer relationships), EI men had higher SPVLs; these differences were clinically relevant (> 0.3 log10 copies/mL) and statistically significant (p < 0.05).
Conclusions:
Mode of acquisition may be an important aspect of SPVL evolution in MSM, with clinical implications.
Keywords: HIV-1, Network modeling, Mathematical modeling, Men who have sex with men (MSM), Sexual role, MACS study, Set point viral load
1. Introduction
Viral load (VL)—the density of HIV-1 particles in an infected person’s peripheral blood plasma—varies over time and across individuals. Set point viral load (SPVL)—the average viral load during the period shortly after acute HIV-1 infection—also varies. Individuals with higher SPVLs average faster disease progression in the absence of treatment (Modjarrad et al., 2008), while higher plasma VL is associated with higher rates of transmitting HIV-1 (Fideli et al., 2001; Quinn et al., 2000). This viral evolutionary trade-off spawned the hypothesis that, after introduction of the virus into a treatment-naïve population, HIV-1 evolves to intermediate SPVL values; one model found that, for their proposed relationship between VL and transmission, SPVL evolved to a value (4.52) close to the mean observed within some empirical populations (Fraser et al., 2007).
Nonetheless, SPVL varies greatly among infected individuals, as influenced by host HLA and CCR5 genotypes (Blanpain et al., 2002; McLaren et al., 2015; Sabeti et al., 2005), age (Hollingsworth et al., 2010), sex (Gandhi et al., 2002), repeated exposure (Hasselrot et al., 2010; Pala et al., 2013), concurrent STI infection (Roberts et al., 2012), and viral genotype (Fraser et al., 2014; Touloumi et al., 2013). Intra-host meta-population dynamics may also play a role (Lythgoe et al., 2016). Heritability in SPVL between HIV-1 donor and recipient—that is, the proportion of the variation in SPVL among individuals that is attributable to variation in the viral genotype—is maintained despite the wide range of host responses (Bonhoeffer et al., 2015; Hollingsworth et al., 2010; Lingappa et al., 2013; Yue et al., 2013). Although individual genome studies have presented widely varying estimates of heritability, one meta-analysis (Fraser et al., 2014) demonstrated that much of this apparent discrepancy is explained by differences in reported metrics; a re-analysis of heritability estimates using a common definition for the classic h2 measure of heritability across serodiscordant couples studies obtained a pooled estimate of 33% (95% confidence interval, 20–46%).
One additional potential source of variation in individual SPVL is the route of HIV-1 acquisition—e.g. insertive vs. receptive role during anal intercourse (AI). These routes entail different mean acquisition probabilities (receptive much higher), while the probability for any individual act depends on the transmitting partner’s current viral load (VL) (Baggaley et al., 2010; Patel et al., 2014). Although the exact functional form linking VL to transmission probability is debated, there is consensus that transmission risks increase monotonically with VL (Fraser et al., 2007; Hughes et al., 2012). Following on a concept introduced in Carlson et al. (2014), we hypothesize that someone who is at high risk of HIV-1 acquisition (e.g., an exclusively receptive male) is more likely to get infected during one of their earliest exposures, and thus to acquire a subset of circulating viruses that is relatively unbiased with respect to fitness. A person engaging in sexual acts with lower transmission risk may require more contacts on average before getting infected, or be disproportionately infected during scenarios with higher transmission probability, i.e. during acute infection or from partners with very high SPVL (Ma et al., 2009; Wawer et al., 2005). The latter effect could theoretically cause sub-populations infected by different routes to have different mean SPVLs (MSPVL).
Indirect evidence for this hypothesis may come from heterosexual transmission. Analyses have found women to have higher per-act risk of acquisition than men during penile-vaginal sex (Patel et al., 2014), and average lower SPVL (Gandhi et al., 2002). One study found that viruses transmitted female-to-male contain amino acid variants reflecting higher mean fitness than those transmitted male-to-female (Carlson et al., 2014), demonstrating the transmission bottleneck’s evolutionary impact and suggesting the potential for SPVL in transmitted strains to differ by sex through similar mechanisms. However, alternative explanations for SPVL sex differences exist. These include higher estrogen concentrations in women, which may reduce TNFα concentration (Shanker and Adams, 1994) and thus viral expression (Folks et al., 1989; Mellors et al., 1991), and higher progesterone concentration, which could inhibit CCR5 expression (Portales et al., 2001), lowering VL (Jackson, 2015). Some studies of persons who inject drugs (PWIDs) show lower SPVL for women (Farzadegan et al., 1998), supporting endocrine-mediated mechanisms over transmission bottleneck selection. However, other studies found no significant sex differences in PWID’s SPVL (Moroni, 1999), and those found could be due to sexspecific cocaine and opiate usage, which upregulates HIV-1 replication (Peterson et al., 1991, 1990).
To our knowledge, the question of acquisition mode and SPVL has not been explored among men who have sex with men (MSM), even though the effects may be larger among MSM than heterosexuals, as the acquisition probability differential via receptive vs. insertive AI is greater than the same differential (receptive vs. insertive) for vaginal intercourse (Patel et al., 2014). Moreover, MSM are less constrained than heterosexuals in terms of transmission chains alternating by sexual role (Goodreau and Golden, 2007). For heterosexuals, males and females represent each other’s source viral pool, and evolution in one group should change the substrate for evolution in the other, holding divergence in check. For MSM, however, an individual man could be infected through receptive AI, allowing transmission of a viral genotype that maintains a relatively low SPVL, and then transmit through insertive AI, allowing his receptive partner to acquire the same low-SPVL genotype. The resulting dynamics are thus less intuitive than for heterosexual transmission. Teasing them apart may provide theoretical insight into SPVL dynamics, as well as provide an additional explanation for unexplained but clinically relevant SPVL variation. It might also help disentangle hypotheses about SPVL differences by sex for heterosexuals.
In this paper, we use a combination of mathematical modeling and empirical data analysis to explore differences in HIV-1 SPVL among exclusively insertive (EI), exclusively receptive (ER), and role versatile (RV) MSM.
2. Methods
Our methods comprise three parts: a basic one-generation model to gain insight about expectations under different assumptions; a more complex, data-driven dynamic network simulation to understand SPVL evolution in a linked system between groups; and an empirical analysis of SPVL measures in an HIV-1 incidence cohort, by self-reported sexual role.
2.1. One-generation model
Our first model was purposely simple in order to gain theoretical insight, and was not based on empirical data. It is a stochastic agent-based pair-formation Bernoulli model of one generation of HIV-1 transmission from a founder population. Code was written in R and is available at github/EvoNetHIV/RoleSPVL/OneGenModel.
We seeded an initially-infected population of 100,000 men with SPVLs (log10 copies/mL) distributed at random using a beta function with mean 4.5 and range 2–7. We then simulated an equally sized pool of HIV-1-negative men engaging in condomless AI acts with the HIV-1-positive men, comparing runs where the negative men were EI vs. ER. We varied the number of acts per negative man between 1 and 104, the order of magnitude we considered a reasonable upper limit. Values for numbers of acts were concentrated at the lower end of the scale to provide more insight into mean population SPVL where it was changing most quickly. We considered one scenario in which all acts for an HIV-1-negative man were with the same partner, and another in which the HIV-1-negative men randomly select a new partner for each act. Transmission probability depended on each partner’s sexual role, the number of acts in the relationship, and the HIV-1-positive partner’s SPVL using a modified version of the function in Fraser et al. (2007). Their function focused on vaginal intercourse (VI) and assumed equal risk for insertive (male) and receptive (female) VI. To convert this to AI, we added two relative risks from Patel et al. (2014), which estimated receptive and insertive AI to have 23.0 and 1.8 times higher acquisition risks per serodiscordant act than VI, when the latter is averaged by role. Fraser’s model was also per-time-period, and we back-calculated approximate per-act estimates following our earlier models (see Supplementary section S4). The population had no mortality, arrivals, or departures. As the population was large and the events independent, we did not repeat simulation runs.
2.2. Dynamic network model
The one-generation model fostered development of basic insight about the relationship between role and SPVL. However, it assumed 100% heritability and complete role segregation, i.e. it did not include RV men. It also ignored the stages of HIV-1 infection that are marked by changes in VL and transmission probability. Moreover, it could not account for the fact that transmissions are a linked system: changes in VL in one generation of transmission changes the source VLs for the next. To explicitly address these points in a more realistic and empirically-parameterized context, we extended a stochastic, dynamic, network-based model described previously (Herbeck et al., 2018). This code builds upon the EpiModel (Jenness et al., 2016a) and statnet (Handcock et al., 2008, 2016) R packages and is available at github/EvoNetHIV/RoleSPVL. Full methods for this model are found in Supplement Sections S1–Supplement Sections S8.
Model parameters governing sexual network structure, sexual role categories and other behavioral parameters, and agent attributes were obtained primarily from two modeling studies of HIV among US MSM, either directly from Jenness et al. (2016b) or by obtaining weighted means of race-specific values in Goodreau et al. (2017). Empirical data for these parameters came from two studies of Atlanta-area non-Hispanic Black and White MSM conducted 2010–2014 (Hernandez-Romieu et al., 2015; Sullivan et al., 2015).
Agents were defined by a variety of attributes (e.g. age, CD4+ cell count); key ones included sexual role (EI, ER, RV), SPVL, and current VL. Sexual network structure was modeled with separable temporal exponential random graph models (STERGM) (Krivitsky and Handcock, 2014), as implemented in statnet. We simulate a dynamic model that maintains the desired network features stochastically. Partnership formation occurred to preserve mean momentary degree (0.70, the average number of relationships a man is in at a cross-section of time), with partnerships between two EI men or two ER men forbidden. Coital acts occurred stochastically within partnerships with probability 0.4/day, but terminated at late-stage AIDS. The number of arrivals followed a Poisson distribution with mean set to give 1% population growth, while departures occurred through AIDS mortality, background mortality, and aging out. Relational dissolution occurred with a constant hazard, leading to geometrically distributed relationship durations. We explored mean relationship durations between 50 and 3000 days (corresponding roughly to 20–1200 coital acts/relationship given coital frequency). The transmission function matched that used in our one-generation model. Recipient SPVL had both inherited and non-inherited components, with heritability = 0.36 based on one study (Hollingsworth et al., 2010); we note this is very similar to the subsequent meta-analysis value of 0.33 described above (Fraser et al., 2014). We did not explicitly model host HLA or other host genetic factors, as we have no expectation that these would systematically differ between EI and ER men. However, we note that non-viral-genetic factors influencing SPVL are implicitly modeled within the non-inherited component of SPVL variation. MSPVL in the initial population was 4.5.
VL varied continuously through time, following a set of parameters described in the Supplement (section S6) and in Herbeck et al. (2018). We modeled four CD4+ cell categories, with transition times dependent on SPVL; CD4+ cell category governed progression and mortality. The model excluded treatment, as our goal was to understand the basic evolutionary dynamics more clearly. Not including treatment also allowed us to compare results to the MACS data, where infections in men in our analysis mainly predate the onset of treatment. The population had 1000 individuals aged 18–50, with 20% initial HIV-1 prevalence; population size was smaller than for the one-generation model given much higher computational burden. Simulations were run in one-day timesteps, with results compiled from years 2–5 of the simulation; we ran 50 simulations for each relationship duration value. All other parameters were fixed at the point estimates derived from our literature review.
2.3. MACS analysis
Finally, we conducted an empirical analysis of data from the Multicenter AIDS Cohort Study (MACS) (Kaslow et al., 1987), an ongoing prospective cohort study begun in 1984 among MSM in four US cities. Participants are asked to return every 6 months for HIV-1 testing (including VL measurement) and a behavioral survey. Our initial dataset had 712 individuals, comprising men who entered the study HIV-1-negative and who have seroconverted during the course of the study (Table 1).
Table 1.
Derivation of sample.
| Variable | N | % | |
|---|---|---|---|
| Conditions required for all analyses (nested) | MACS Cohort total | 7,087 | |
| Entered seronegative | 4,124 / 7,084 | 58.2% | |
| Seroconverted | 712 / 4,124 | 17.3% | |
| Seroconversion interval < 1 year | 535 / 712 | 75.1% | |
| Had SPVL information | 435 / 535 | 81.3% | |
| Had seroconversion interval role information | 374 / 435 | 86.0% | |
| Role distribution of sample | Exclusively insertive (El) | 46 / 374 | 12.3% |
| Role versatile (RV) | 278 / 374 | 74.3% | |
| Exclusively receptive (ER) | 50 / 374 | 13.4% |
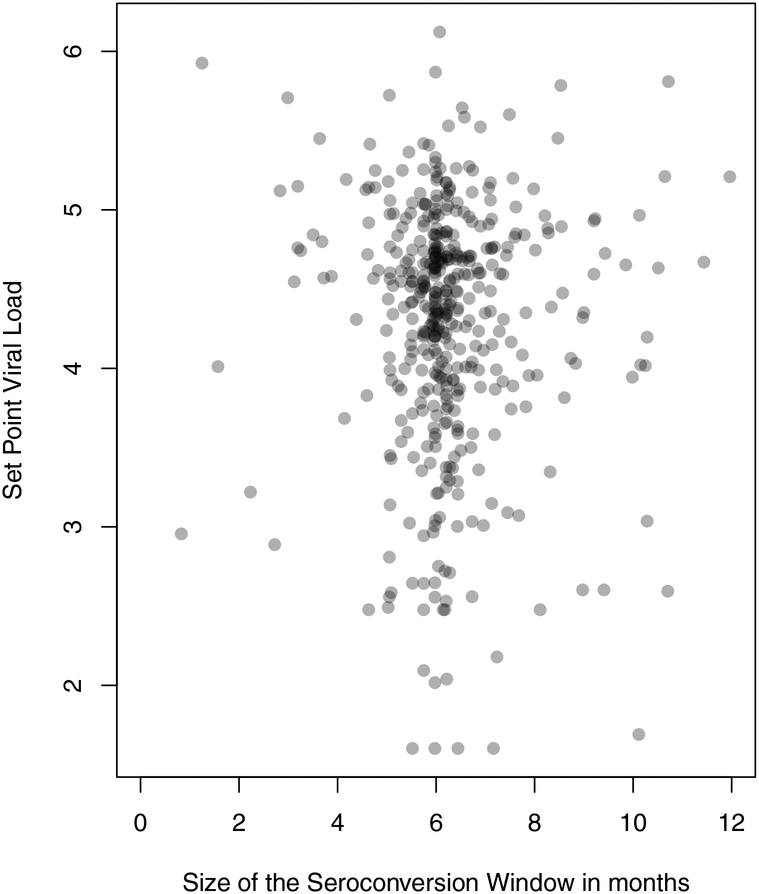
We defined the interval between last negative visit and first positive visit as the seroconversion interval. While the size of this interval is designed to be 6 months, it varies in practice, sometimes widely. We thus restricted our sample to men for whom a last HIV-1-negative and first HIV-1-positive visit were strictly less than 1 year apart (i.e. maximum 364 days), in order to limit the uncertainty in seroconversion timing; this reduced our sample size to 535. The distribution of the seroconversion interval size is shown in Fig. 1; this concentrates around 6 months given the study design, and maxes out at < 1 year given our inclusion criterion. Seroconversion could occur anywhere within the interval. We estimated SPVL by using VL measures for study visits between 6 and 18 months after the midpoint of the seroconversion interval (Herbeck et al., 2008). If two visits fell in the interval, their log10 measures were averaged. For men following the 6-month visit schedule perfectly, our method ensures that the VL measures fall approximately 9 and 15 months post seroconversion, since actual seroconversion could have occurred anywhere in the seroconversion interval. For men with more variable follow-up schedules, our SPVL could in theory fall quite early after seroconversion. We thus separately checked durations of the time between the first HIV-1-positive visit and first VL measure included in our SPVL calculation for each man in the analysis, and determined that only one of these was < 90 days (our estimate of acute phase infection duration). This man’s interval was 87 days, and his first viral load reading (5.02) was slightly less than his subsequent one (5.17), so we retained him in the analysis. Fig. 1 also shows a lack of clear relationship between seroconversion interval length and estimated SPVL (linear regression p = 0.559), further suggesting that our method is not systematically capturing VLs that are poor representations of SPVL for men with either short or long seroconversion intervals. We had at least one SPVL measure and information about role during seroconversion interval for 374 men; Table 2 shows demographic characteristics for this sub-sample.
Fig. 1.
Seroconversion window and SPVL distributions, MACS sample.
Size of the seroconversion window reflects the duration in months between the last visit with HIV-1-negative test results and first visit with HIV-1-positive test results for men in our sample. SPVL is measured at the subsequent one or two visits, as described in the text.
Table 2.
Demographic characteristics of the MACS sample, individuals with set point viral load data and seroconversion role data available.
| Categorical Variables | Category | n | % |
|---|---|---|---|
| Age at Seroconversion | < 30 | 106 | 28.3 |
| 30–39.9 | 174 | 46.5 | |
| 40–49.9 | 73 | 19.5 | |
| ≥50 | 21 | 5.6 | |
| Cohort (enrollment years) | 1984 (1984–1985) | 344 | 92.0 |
| 1987 (1987–1991) | 11 | 2.9 | |
| 2001 (2002–2003) | 19 | 5.1 | |
| Race/ethnicity | Black, non-Hispanic | 32 | 8.6 |
| Hispanic | 25 | 6.7 | |
| Asian or Pacific Islander | 2 | 0.5 | |
| White, non-Hispanic | 315 | 84.2 | |
| City | Baltimore | 101 | 27.0 |
| Chicago | 87 | 23.3 | |
| Los Angeles | 105 | 28.0 | |
| Pittsburgh | 81 | 21.7 |
| Continuous Variables | Measurement Time | n | Mean | Median | IQR | |
|---|---|---|---|---|---|---|
| Est. age at seroconversion | Seroconversion interval midpoint | 374 | 35.3 | 34.0 | 29.4 | 40.1 |
| Plasma log10 viral load | Visit 1 | 334 | 4.30 | 4.49 | 3.95 | 4.83 |
| Visit 2 | 304 | 4.28 | 4.40 | 3.90 | 4.76 | |
| 2-visit mean | 264 | 4.31 | 4.47 | 3.94 | 4.75 | |
| Overall | 374 | 4.29 | 4.45 | 3.92 | 4.76 |
Seroconversion sexual role was estimated from reported numbers of insertive or receptive partners at the first HIV-1-positive visit. We classified individuals reporting receptive AI with ≥1 partner and no insertive AI partners as ER, individuals reporting the reverse as EI, and individuals reporting each with ≥1 partner as RV. We use role behaviors in the seroconversion interval only since these are most directly related to virus acquisition, and, had we included role throughout the entire study, few men would have been anything other than role versatile.
Methods for obtaining and quantifying VL have been published previously (Mellors et al., 1996). Statistical comparisons of MSPVL by subgroup were completed in Stata 13.1 using both Mann-Whitney and t-tests for the empirical analyses. Based on our model findings (described below), we performed three analyses, one comparing the SPVLs of EI men and all others, and another two stratifying based on different metrics for numbers of partners. Because our final sample sizes were quite small, we kept our statistical analysis purposefully simple; that is, we did not consider additional predictors of SPVL beyond sexual (e.g. host genotype), assuming that these other predictors should be un-correlated with this behavioral attribute.
3. Results
3.1. One-generation model
Below we lay out the behavior observed in our simulations from this model. We note that key aspects of this behavior can also be demonstrated mathematically, and we include this derivation (provided to us by a reviewer) in Supplement section S9.
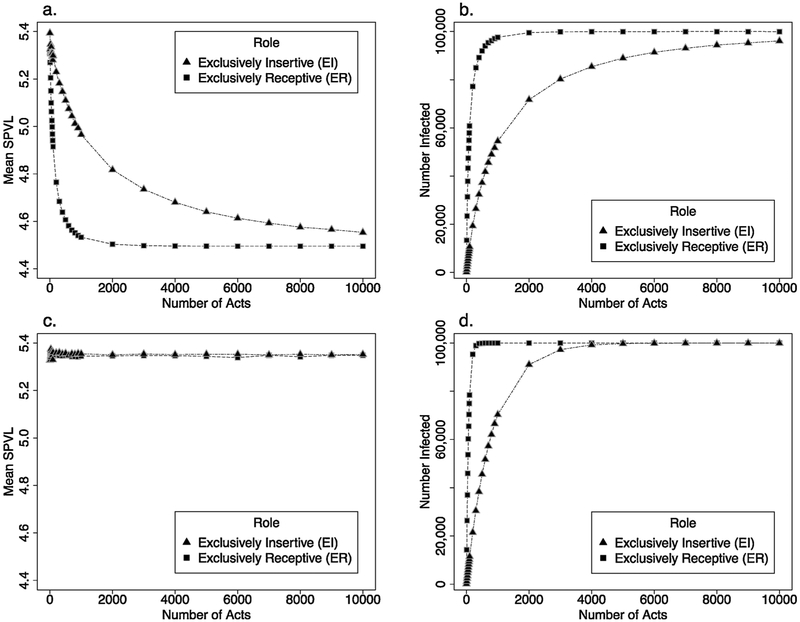
The model considering only one sex act demonstrated little, if any, difference in MSPVL between newly-infected EI and ER men (both values slightly below 5.4, Fig. 2a). These values represent a rapid shift away from the source population mean of 4.5; individuals with higher SPVL transmit more easily, and this effect is not counter-balanced here by the trade-off of shorter life expectancy. Many more ER than EI men become infected (n = 1493 vs. 128, Fig. 2b).
Fig. 2.
Results from one-generation model.
Mean SPVL and number of individuals infected changes based on role and number of sex acts in the one-generation model. In each panel, role signifies the role of the initially HIV-1-negative partner. In 2a and 2b, there was no partner change: individuals stayed in the same relationship for the duration of the modeled time. In 2c and 2d, individuals selected a new partner with every act. (a) Mean SPVL for each role group, without partner change; (b) Number of individuals infected for each role group, without partner change; (c) mean SPVL for each role group, with partner change; (d) number of individuals infected for each role group, with partner change.
At the other extreme, when negative men have 104 acts with one partner, there is also little difference in MSPVL of newly-infected EI and ER men (4.55 vs. 4.50). These values, approximately equal to the source population’s, reflect the fact that nearly every HIV-1-positive man transmits to his one partner eventually, although transmissions generally take longer when the negative partner is insertive. MSPVL for EI men is slightly higher because there remain a few non-transmitting pairs involving receptive source partners with very low SPVL even after many acts.
Intermediate counts of coital acts do, however, yield a difference by role, with ER men averaging markedly lower SPVL. This difference peaks at 400 acts, with a 0.508 log10 difference between the two groups.
When we consider the same scenarios, but allow HIV-1-negative men to re-select partners between acts, we see a different pattern: MSPVL equals ~5.35 for both groups, for any number of coital acts (Fig. 2c). However, the pattern of relative times until men become infected is similar to that of the previous model (Fig. 2d). Contrasting this set of scenarios with those above, then, we see examples in which the same number of partners but different number of acts per partner leads to different MSPVL values by sexual role; however, different number of partners but the same number of acts per partner do not.
3.2. Network model
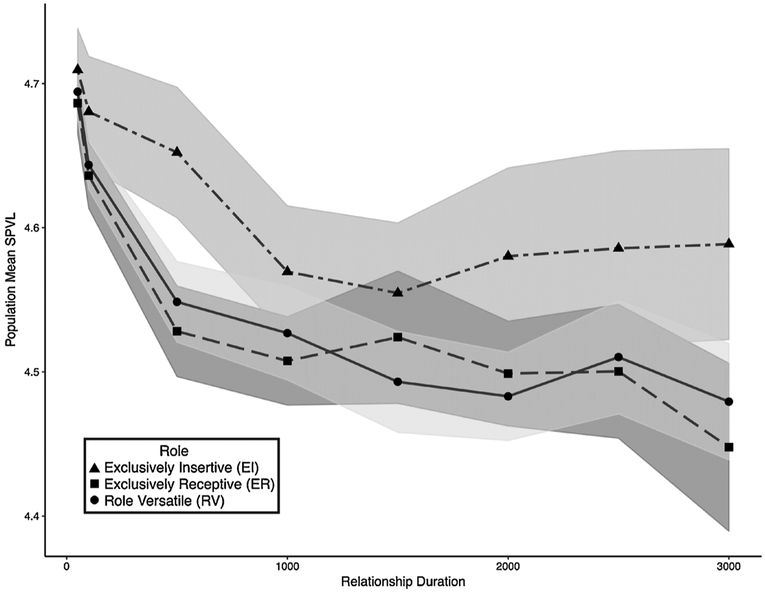
Fig. 3 shows results from our dynamic network model across different relationship lengths. Results show greater stochasticity than for the one-generation model; presumably this is at least in part because the explicit feedback added here (those newly infected are in the partner pool for other HIV-uninfected men) allows for small random differences to become compounded. Consistent with the one-generation model, we see little, if any, difference between EI and ER men at short relationship durations. Differences appear at longer durations, for example at 500 days (corresponding to roughly 200 acts per relationship; 0.124 MSPVL difference). Other durations also show noteworthy differences by role, although some with overlapping confidence intervals. In contrast, RV men display SPVL values similar to those of ER men, presumably because their greatest risk (and most of their infections) comes from their acts of receptive AI. Differences between EI and ER men are generally smaller here than in the Bernoulli model for the same number of acts, where those comparisons are possible; for example, at around 1000 acts per relationship, the Bernoulli model displayed a difference of ~0.4 log10 SPVL, while the network model showed only ~0.1. Presumably this is because the added realism of the latter, i.e. partial heritability and evolutionary feedback between role groups. As with the one-generation model, here EI men are by far the least likely to become infected.
Fig. 3.
Results from dynamic network model.
Data represent mean SPVL for each role group averaged across 50 simulations. Men are included if they are infected during years 2–5 of the simulation. Data are shown for 8 scenarios differing in mean relations durations (50, 100, 500, 1000, 1500, 2000, 2500 and 3000 days). There were on average 0.4 acts/day leading to roughly 20, 40, 200, 400, 600, 800, 1000, and 1200 acts per scenario. Shaded areas represent 95% confidence intervals around the mean values, using standard assumptions of normal variation across simulations.
3.3. MACS analysis
Since both models demonstrated that a difference in SPVL by sexual role was expected under some conditions, we proceeded with our empirical analyses. Given our model results, we hypothesized that EI men would have higher SPVL than either ER or RV men, combining the latter two for the analysis and switching to one-tailed tests given the directionality of our hypothesis.
The combined sample of ER and RV men has a MSPVL of 4.29 and the EI men had a nearly identical 4.28 (Mann-Whitney U test, p = 0.371). However, both of our models indicated that we should only expect a difference when considering men whose main risk comes from relationships containing many acts. We did not have access to data on acts per relationship, nor on relational duration. However, we had two different measures of numbers of AI partners reported by respondents, which we could use as proxies for acts per relationship, assuming that low partner counts correlate with higher acts per partnership. To add support to this supposition, we analyzed data from the two studies of MSM that formed the main sources for the behavioral parameters in our dynamic network model. Both yielded a strong and significant relationship in the expected direction; e.g. in one study (n = 949), men with 1 partner in the last 6 months had a 56% chance of reporting 10+ acts per partner and a 19% chance of 1 act; for those with 5+ partners, the corresponding numbers were 11% and 54%, respectively (details in Supplement section S10). An additional literature review indicated that existing reports on this topic focus on acts per partnership per unit time, not acts in total, while our interest was in the latter given our model.
Each of our two proxy measures is imperfect in different ways, but provides some insight. The first was the reported number of partners in the 6 months prior to the first HIV-1-positive study visit. Given the short time frame, this measure showed relatively little variation, and is likely less reflective of long-term behavior. The other (available for the 344 men in the 1984 cohort only) was the estimated number of receptive AI partners (i.e. individuals the respondent had receptive sex with) a respondent had in the 2 years before and 6 months after their baseline visit, a proxy for risk commonly used in analyses of MACS data (Detels et al., 1994; Herbeck et al., 2015). In this case, the longer duration gives a more stable estimate for partner acquisition rates; however, its limitation to receptive partners introduces some confounding with our main dependent variable, sexual role. We note that it is possible for a man we classify as EI (i.e. reporting no receptive partners) to have a value > 0 for this metric (i.e. reporting receptive partners), since the two measures represent different times, potentially years apart. Given this caveat, we conducted two sub-analyses. First, we compared ER/RV men with ≤3 estimated baseline receptive partners to EI men with an estimated 0 baseline receptive partners, under the assumption that the latter represent men who are consistently insertive in the long-term. Second, we tried a more symmetric model, considering a ≤3-partner cutoff for each group. Finally, we tried different cutoffs (≤ 3 vs. 10 partners) for each analysis to see how sensitive our results were to this arbitrary cutoff and to balance the desire to limit to those with very few, long relationships with a need for larger sample sizes. We used non-parametric one-tailed Mann-Whitney tests given the small sample sizes and likely violation of distributional assumptions, but also repeated analyses using one-sided t-tests, with qualitatively similar findings.
Table 3 and Fig. 4 show these results. When considering partners in the previous 6 months, MSPVL was nearly identical between EI men and others for both partnership count cutoffs; differences were not significant. However, when considering estimated number of receptive partners over the earlier 2.5-year span, EI men had significantly higher SPVL than others. For example, with the 10-partner cutoff for both groups, EI men had MSPVL of 4.51 and others had 4.19. This difference (~0.3 log10 copies/mL) is considered clinically significant; a meta-analysis found it to translate into a 25% higher annual risk of progression to an AIDS-defining illness (Modjarrad et al., 2008). Results were qualitatively insensitive to cutoff.
Table 3.
Results of MACS role analyses.
| Role | n | Mean SPVL | Pseudo-median SPVL (95% CI) | p-value | |
|---|---|---|---|---|---|
| All individuals | El | 46 | 4.28 | 4.36 (4.08–4.61) | 0.371 |
| " | ER or RV | 328 | 4.29 | 4.36 (4.28–4.44) | |
| 6 mo. partner count ≤ 3 | El | 30 | 4.20 | 4.28 (3.89–4.69) | 0.554 |
| " | ER or RV | 110 | 4.33 | 4.42 (4.29–4.53) | |
| 6 mo. partner count ≤ 10 | El | 39 | 4.22 | 4.31 (3.99–4.56) | 0.488 |
| " | ER or RV | 253 | 4.26 | 4.34 (4.24–4.43) | |
| 30 mo. receptive partner count = 0 | El | 7 | 4.65 | 4.82 (4.04–5.05) | 0.027 |
| 30 mo. receptive partner count ≤ 3 | ER or RV | 33 | 4.05 | 4.17 (3.79–4.45) | |
| 30 mo. receptive partner count = 0 | El | 7 | 4.65 | 4.82 (4.04–5.05) | 0.024 |
| 30 mo. receptive partner count ≤ 10 | ER or RV | 91 | 4.19 | 4.28 (4.10–4.43) | |
| 30 mo. receptive partner count ≤ 3 | El | 17 | 4.49 | 4.67 (4.02–4.93) | 0.039 |
| " | ER or RV | 33 | 4.05 | 4.17 (3.79–4.45) | |
| 30 mo. receptive partner count ≤ 10 | El | 20 | 4.51 | 4.68 (4.09–4.88) | 0.017 |
| " | ER or RV | 91 | 4.19 | 4.28 (4.10–4.43) |
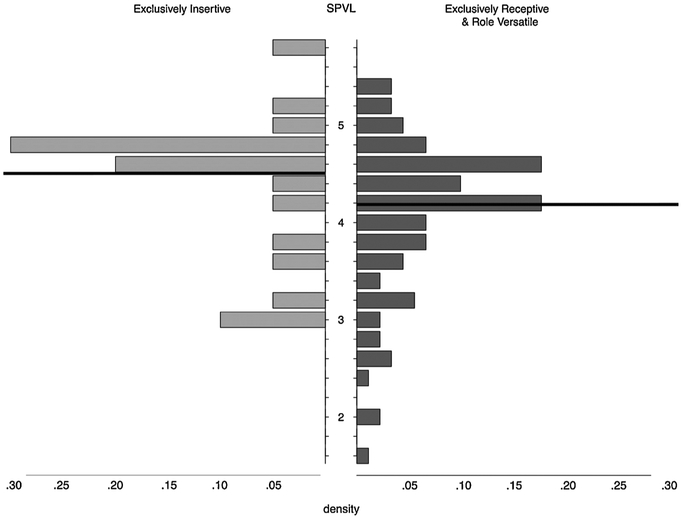
Fig. 4.
Distribution of SPVL by role at seroconversion, for men with lower partner numbers.
Results shown here are for the sub-analysis comparing EI men (n = 20) with ER or RV men (n = 91) who each have an estimated 10 or fewer receptive partners in the 2.5 years around baseline. Role category is based on behavior in the first HIV-1-positive visit. The exclusively insertive men’s mean = 4.51 and the exclusively receptive and role versatile men’s mean = 4.19 (shown with dark lines). The small clustering at 3 for EI men and lack of tail below that might suggest an issue with detection threshold; however, this is not present in the ER/RV men, and we know of no explanation why detection thresholds would differ by sexual role.
Bold = significant at p = 0.05. All distributions were compared using a two-sample Wilcoxon (a.k.a. Mann-Whitney) test with exact p-values, given the small sample sizes and potential violation of distributional assumptions in some cases. Means are those observed; pseudo-medians and confidence intervals were calculated using a one-sample Wilcoxon test. We also conducted a two-sample one-tailed t-test with Welch-Satterthwaite approximation (no assumption of equal variances) since this focuses more specifically on comparing means; results were qualitatively similar, although the fifth comparison became nonsignificant, at p = 0.055. All analyses were conducted using the stats package in R, using default settings for all parameters not mentioned. Note that pseudo-medians are all higher than observed means given that the underlying distributions are left-skewed. Using the internal definitions of the MACS data set, “receptive partner count” refers to the number of partners with whom the respondent had receptive anal intercourse.
4. Discussion
To our knowledge, no previous study has considered differences in SPVL by sexual role among MSM, despite the clinical and epidemiological significance of this virological measure, and numerous studies among heterosexuals. Our one-generation model demonstrated that we might expect exclusively insertive (EI) men to have higher MSPVL than others, but only when partnerships are sufficiently long to involve numerous sex acts. In our dynamic simulation, which included many additional forms of realism, the impact of role on SPVL was smaller but still present at sufficiently long relationship durations. In the one-generation model, the gap closed again at very high (2000+) mean numbers of acts per relationship. We considered such high numbers for the sake of insight-building; for the more data-driven and computationally intensive dynamic model, we did not explore this range, and the gap in SPVL between EI men and others remained wide. It is crucial to remember, however, that in each case EI men were the least likely to become infected overall, as would be expected given the difference in transmission probabilities by role.
The logic behind these findings is subtle, and relies on consideration of two extremes: when there is only one act per relationship, and when there are very many (e.g. 104). In the former, transmission is biased towards those with high SPVL, but is biased equally by role. In the latter, nearly all partnerships eventually transmit, so there is no bias by SPVL or role. At intermediate numbers, the level of bias differs by role, as Fig. 2 illustrated. Here all MSM acquire high SPVL strains disproportionately, but EI men do so more disproportionately than others (see also Supplement Section S9 for further elaboration).
Acute infection, with its increased transmission probability, complicates this idea. Short relationships mean greater opportunity to become infected and then infect others during a brief window, facilitating transmission from individuals with high VL because of acute infection, but not necessarily high SPVL. Notably, however, our models that did (dynamic) and did not (one-generation) include infection stages showed the same pattern at short relationships, indicating that acute infection is not the main driver of these patterns.
Results from the MACS data analysis provided partial confirmation for our models. The unstratified analysis failed to demonstrate a difference between groups. However, this included men with relationships of all lengths, while our model only predicted a difference for longer relationships; in the earliest cohort especially, many very short relationships likely predominated (Rotello, 1997). We conducted two subsets of stratified analyses focusing on men with few (and presumed longer) relationships, and these consistently demonstrated the predicted pattern of higher SPVL in EI men, with significance.
One early MACS study (Phair et al., 1992), although not considering role or SPVL, found that rapid progressors had more partners. We interpret this finding as consistent with our assumption that more partners suggests shorter relationships, and our model finding that shorter relationships should lead to higher SPVL and faster progression. However, we note that dual or superinfection could also generate this pattern (Gottlieb et al., 2004).
Our work provides indirect evidence to support the notion that some of the SPVL differences by sex in heterosexual transmissions might be influenced by differences in acquisition risk. This builds on recent findings that the transmission bottleneck leads to females being disproportionately infected by HIV-1 strains with amino acids residues associated with lower viral fitness (Carlson et al., 2014). It is worth noting that, even though some authors have found that the greater probability for male-to-female transmission than vice versa disappears after controlling for a variety of factors that foster greater acquisition probabilities in females (Hughes et al., 2012), it is the unadjusted probabilities that represent the full set of environmental conditions shaping viral evolution.
This analysis has several limitations. We lacked a direct measure for acts per partnership, and our proxies were both imperfect; this adds noise to our analysis, and potentially bias related to the fact that our measures of role and coital frequency were not independent, with ER men seeming to have more, and thus shorter, partnerships on average. Despite beginning with a large cohort, our final analyses involved small sample sizes. Ascertainment of sexual role was unlikely to have correctly classified everyone as it was based on reported partner counts instead of a direct survey question. Given the small sample sizes generated by our need for complete behavioral data and narrow seroconversion windows, we did not explore other predictors of SPVL that might have confounded our observed relationships; however, we note that, unlike analysis of SPVL in heterosexual transmission, where the insertive (male) and receptive (female) partners are expected to exhibit a host of systematic genetic and endocrinological differences, we have no such expectations for differences between MSM who exhibit different sexual roles. Our models did not consider that men likely have a mixture of relationship types (e.g. one long and many short), which might further complicate observed patterns. Furthermore, our models contained many parameters that were fixed at single values drawn from the literature, including the parameters governing the relationship between VL and transmission probability (Fraser et al., 2007). Our qualitative findings might vary under different values for any of these, and we did not conduct sensitivity analyses to explore this. Many factors that might vary across populations, such as the numbers of sexual partners, the use of condoms, the presence of co-circulating STIs, or the level of antiviral treatment or more recently pre-exposure prophylaxis, could alter the evolutionary landscape for HIV-1 considerably. However, the impact of this limitation is tempered by the fact that our models served primarily to build the insights used to guide the empirical analysis.
Our Bernoulli model contained two comparisons: one in which we compared scenarios with the same number of partners but different numbers of acts per partner; and one in which we compared scenarios with different numbers of partners but the same number of acts per partner. The former yielded differences in MSPVL by sexual role in some cases, while the latter did not. This led to us focusing on number of acts as our main explanatory variable for the empirical analysis, despite the fact that our actual measure was partner number, since we expect these to correlate (and demonstrated that they did in the main source studies that parametrized our network model). In reality, however, patterns of partner number and acts per partner are likely to be more complex than we fully explored, and interact with other network phenomena, e.g. through the presence of core groups—the tendency for those with many short-term partnerships to choose each other as partners disproportionately. We are in the process of conducting additional modeling work examining more complex relationships among these phenomena, and examining additional relationships between partner number and SPVL in the MACS data in light of these model findings.
Our results suggest that EI men, although less likely to become infected than men who engage in receptive AI, could be disproportionately infected by more virulent strains and might thus face worse outcomes, including as much as a 25% higher probability of progression to AIDS per year (Modjarrad et al., 2008). In the modern era, availability of treatment may mask these differences in many settings, although universal treatment is still far from a reality, even among MSM in developed settings (Singh et al., 2014). Analyses of VL measures from additional cohorts, especially cohorts with data on the number of both insertive and receptive acts per partnership in the period before seroconversion, could help confirm this finding, as would further work identifying viral selection at the transmission bottleneck (Carlson et al., 2014). Nonetheless, it appears that insertive men in long-term relationships now have an additional reason not to be complacent about their HIV risks.
Supplementary Material
Acknowledgments
We thank the researchers, staff and participants of the Multicenter AIDS Cohort Study, the members of the Evonet project (Neil Abernethy, Juandalyn Burke, Kathryn Peebles, Molly Reid), the EpiModel and statnet teams, especially Martina Morris and Sam Jenness, the members of the Network Modeling Group at the University of Washington, and Eli Rosenberg. We also thank Chris Wymant for an exceptionally detailed and helpful review, and an additional anonymous reviewer.
Funding
This work was supported by the National Institutes of Health [R01-AI108490, R21-HD075662, and R01-HD068395]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Partial support for this research came from a Eunice Kennedy Shriver National Institute of Child Health and Human Development research infrastructure grant, R24 HD042828, to the Center for Studies in Demography & Ecology at the University of Washington. Data in this article were collected by the MACS with centers (Principal Investigators) at Johns Hopkins Bloomberg School of Public Health (Joseph B. Margolick, Lisa P. Jacobson), Northwestern University (Steven Wolinsky), University of California, Los Angeles (Roger Detels), and University of Pittsburgh (Charles Rinaldo). The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional supplemental funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH) (UO1-AI-35042, UL1-RR025005, UM1-AI-35043, UO1-AI-35039, UO1-AI-35040, and UO1-AI-35041). The funding for this substudy was supported by the National Institute of Allergy and Infectious Diseases (R21-AI-109817). This work was facilitated though the use of advanced computational, storage, and networking infrastructure provided by the Hyak supercomputer system and funded by the STF at the University of Washington. The funding sources had no involvement in the study design, data interpretation, manuscript preparation, or submission decisions.
Abbreviations:
- VL
viral load
- SPVL
set point viral load
- MSPVL
mean set point viral load
- MSM
men who have sex with men
- EI
exclusively insertive
- ER
exclusively receptive
- RV
role versatile
- MACS
multicenter AIDS cohort study
- AI
anal intercourse
- VI
vaginal intercourse
- PWID
persons who inject drugs
- STERGM
separable temporal exponential random graph models
Footnotes
Conflict of interest
The authors have no conflicts of interest to declare.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.epidem.2018.08.006.
References
- Baggaley RF, White RG, Boily M-C, 2010. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int. J. Epidemiol 39, 1048–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Libert F, Vassart G, Parmentier M, 2002. CCR5 and HIV infection. Recept. Channels 8, 19–31. [PubMed] [Google Scholar]
- Bonhoeffer S, Fraser C, Leventhal GE, 2015. High heritability is compatible with the broad distribution of set point viral load in HIV carriers. PLoS Pathog. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Schaefer M, Monaco DC, Batorsky R, Claiborne DT, Prince J, Deymier MJ, Ende ZS, Klatt NR, DeZiel CE, Lin T-H, Peng J, Seese AM, Shapiro R, Frater J, Ndung’u T, Tang J, Goepfert P, Gilmour J, Price MA, Kilembe W, Heckerman D, Goulder PJR, Allen TM, Allen S, Hunter E, 2014. Selection bias at the heterosexual HIV-1 transmission bottleneck. Science 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detels R, Liu Z, Hennessey K, Kan J, Visscher BR, Taylor JM, Hoover DR, Rinaldo CR Jr., Phair JP, Saah AJ, et al. , 1994. Resistance to HIV-1 infection. Multicenter AIDS cohort study. J. Acquir. Immune Defic. Syndr 7, 1263–1269. [PubMed] [Google Scholar]
- Farzadegan H, Hoover DR, Astemborski J, Lyles CM, Margolick JB, Markham RB, Quinn TC, Vlahov D, 1998. Sex differences in HIV-1 viral load and progression to AIDS. Lancet 352, 1510–1514. [DOI] [PubMed] [Google Scholar]
- Fideli ÜS, Allen SA, Musonda R, Trask S, Hahn BH, Weiss H, Mulenga J, Kasolo F, Vermund SH, Aldrovandi GM, 2001. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res. Hum. Retroviruses 17, 901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks TM, Clouse KA, Justement J, Rabson A, Duh E, Kehrl JH, Fauci AS, 1989. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc. Natl. Acad. Sci. U. S. A 86, 2365–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser C, Hollingsworth TD, Chapman R, de Wolf F, Hanage WP, 2007. Variation in HIV-1 set-point viral load: epidemiological analysis and an evolutionary hypothesis. Proc. Natl. Acad. Sci. U. S. A 104, 17441–17446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser C, Lythgoe K, Leventhal GE, Shirreff G, Hollingsworth TD, Alizon S,Bonhoeffer S, 2014. Virulence and pathogenesis of HIV-1 infection: an evolutionary perspective. Science 343, 1243727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M, Bacchetti P, Miotti P, Quinn TC, Fulvia V, Greenblatt RM, 2002. DoesPatient Sex Affect Human Immunodeficiency Virus Levels? Clin. Infect. Dis 35, 313–322. [DOI] [PubMed] [Google Scholar]
- Goodreau SM, Golden MR, 2007. Biological and demographic causes of high HIV and sexually transmitted disease prevalence in men who have sex with men. Sex. Transm. Infect 83, 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodreau SM, Rosenberg ES, Jenness SM, Luisi N, Stansfield SE, Millett GA, Sullivan PS, 2017. Sources of racial disparities in HIV prevalence in men who have sex with men in Atlanta, GA, USA: a modelling study. Lancet HIV 4, e311–e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb GS, Nickle DC, Jensen MA, Wong KG, Grobler J, Li F, Liu SL, Rademeyer C, Learn GH, Karim SS, Williamson C, Corey L, Margolick JB, Mullins JI, 2004. Dual HIV-1 infection associated with rapid disease progression. Lancet 363, 619–622. [DOI] [PubMed] [Google Scholar]
- Handcock M, Hunter D, Butts C, Goodreau S, Morris M, 2008. Statnet: software tools for the representation, visualization, analysis and simulation of network data. J. Stat. Softw 24, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handcock M, Hunter D, Butts C, Goodreau S, Krivitsky P, Bender-deMoll S, Morris M, 2016. Statnet: Software Tools for the Statistical Analysis of Network Data. https://cran.r-project.org/web/packages/statnet/. [DOI] [PMC free article] [PubMed]
- Hasselrot K, Bratt G, Hirbod T, Säberg P, Ehnlund M, Lopalco L, Sandström E, Broliden K, 2010. Orally exposed uninfected individuals have systemic anti-HIV responses associating with partners’ viral load. AIDS 24, 35–43. [DOI] [PubMed] [Google Scholar]
- Herbeck JT, Gottlieb GS, Li X, Hu Z, Detels R, Phair J, Rinaldo C, Jacobson LP, Margolick JB, Mullins JI, 2008. Lack of evidence for changing virulence of HIV-1 in North America. PLoS One 3, e1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbeck J, Ghorai S, Chen L, Rinaldo CR, Margolick JB, Detels R, Jacobson L, Wolinsky S, Mullins JI, 2015. p21(WAF1/CIP1) RNA expression in highly HIV-1 exposed, uninfected individuals. PLoS One 10, e0119218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbeck JT, Peebles K, Edlefsen PT, Rolland M, Murphy JT, Gottlieb GS, Abernethy N, Mullins JI, Mittler JE, Goodreau SM, 2018. HIV population-level adaptation can rapidly diminish the impact of a partially effective vaccine. Vaccine 36, 514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Romieu AC, Sullivan PS, Rothenberg R, 2015. Heterogeneity of HIV Prevalence Among the Sexual Networks of Black and White Men Who Have Sex With Men in Atlanta: Illuminating a Mechanism for Increased HIV Risk for Young Black Men Who Have Sex With Men. Sex. Transm. Dis 42, 505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth TD, Laeyendecker O, Shirreff G, Donnelly CA, Serwadda D, Wawer MJ, Kiwanuka N, Nalugoda F, Collinson-Streng A, Ssempijja V, Hanage WP, Quinn TC, Gray RH, Fraser C, 2010. HIV-1 transmitting couples have similar viral load set-points in Rakai, Uganda. PLoS Pathog. 6, e1000876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JP, Baeten JM, Lingappa JR, Magaret AS, Wald A, de Bruyn G, Kiarie J, Inambao M, Kilembe W, Farquhar C, Celum C, Team, t.P.i.P.H.H.T.S, 2012. Determinants of per-coital-Act HIV-1 infectivity among african HIV-1–Serodiscordant couples. J. Infect. Dis 205, 358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MW, 2015. The Genealogy of a Gene: Patents, HIV/AIDS, and Race. The MIT Press, Cambridge. [Google Scholar]
- Jenness SM, Goodreau SM, Morris M, 2016a. EpiModel: Mathematical Modeling of Infectious Disease. https://cran.r-project.org/web/packages/EpiModel/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness SM, Goodreau SM, Rosenberg E, Beylerian EN, Hoover KW, Smith DK, Sullivan P, 2016b. Impact of the centers for disease control’s HIV preexposure prophylaxis guidelines for men who have sex with men in the United States. J. Infect. Dis 214, 1800–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CRJ, 1987. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am. J. Epidemiol 126, 310–318. [DOI] [PubMed] [Google Scholar]
- Krivitsky PN, Handcock MS, 2014. A separable model for dynamic networks. J. R. Stat. Soc. Ser. B 76, 29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa JR, Thomas KK, Hughes JP, Baeten JM, Wald A, Farquhar C, deBruyn G, Fife KH, Campbell MS, Kapiga S, Mullins JI, Celum C, 2013. Partner characteristics predicting HIV-1 set point in sexually acquired HIV-1 among african seroconverters. AIDS Res. Hum. Retroviruses 29, 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lythgoe KA, Blanquart F, Pellis L, Fraser C, 2016. Large variations in HIV-1 viral load explained by shifting-mosaic metapopulation dynamics. PLoS Biol. 14, e1002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z-M, Stone M, Piatak M, Schweighardt B, Haigwood NL, Montefiori D, Lifson JD, Busch MP, Miller CJ, 2009. High specific infectivity of plasma virus from the pre-ramp-Up and ramp-up stages of acute simian immunodeficiency virus infection. J. Virol 83, 3288–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren PJ, Coulonges C, Bartha I, Lenz TL, Deutsch AJ, Bashirova A, Buchbinder S, Carrington MN, Cossarizza A, Dalmau J, De Luca A, Goedert JJ, Gurdasani D, Haas DW, Herbeck JT, Johnson EO, Kirk GD, Lambotte O, Luo M, Mallal S, van Manen D, Martinez-Picado J, Meyer L, Miro JM, Mullins JI, Obel N, Poli G, Sandhu MS, Schuitemaker H, Shea PR, Theodorou I, Walker BD, Weintrob AC, Winkler CA, Wolinsky SM, Raychaudhuri S, Goldstein DB, Telenti A, de Bakker PI, Zagury JF, Fellay J, 2015. Polymorphisms of large effect explain the majority of the host genetic contribution to variation of HIV-1 virus load. Proc. Natl. Acad. Sci. U. S. A 112, 14658–14663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellors JW, Griffith BP, Ortiz MA, Landry ML, Ryan JL, 1991. Tumor necrosis Factor-α/Caehectin enhances human immunodeficiency virus type 1 replication in primary macrophages. J. Infect. Dis 163, 78–82. [DOI] [PubMed] [Google Scholar]
- Mellors JW, Rinaldo CR Jr., Gupta P, White RM, Todd JA, Kingsley LA, 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272, 1167–1170. [DOI] [PubMed] [Google Scholar]
- Modjarrad K, Chamot E, Vermund SH, 2008. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS 22, 2179–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni M, 1999. Sex differences in HIV-1 viral load and progression to AIDS. Lancet353, 589–590. [DOI] [PubMed] [Google Scholar]
- Pala P, Serwanga J, Watera C, Ritchie AJ, Moodie Z, Wang M, Goonetilleke N, Birabwa E, Hughes P, Senkaali D, Nakiboneka R, Grosskurth H, Haynes B, McMichael A, Kaleebu P, Immunology, C.f.H.A.V, 2013. Quantitative and qualitative differences in the t cell response to HIV in uninfected ugandans exposed or unexposed to HIV-Infected partners. J. Virol 87, 9053–9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J, 2014. Estimating per-act HIV transmission risk: a systematic review. AIDS 28, 1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson PK, Sharp BM, Gekker G, Portoghese PS, Sannerud K, Balfour HHJ, 1990. Morphine promotes the growth of HIV-1 in human peripheral blood mono-nuclear cell cocultures. AIDS 4, 869–874. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Chao CC, Schut R, Molitor TW, Balfour HH, 1991.Cocaine potentiates HIV-1 replication in human peripheral blood mononuclear cell cocultures. Involvement of transforming growth factor-beta. J. Immunol 146, 81–84. [PubMed] [Google Scholar]
- Phair J, Jacobson L, Detels R, Rinaldo C, Saah A, Schrager L, Munoz A, 1992. Acquired immune deficiency syndrome occurring within 5 years of infection with human immunodeficiency virus type-1: the Multicenter AIDS Cohort Study. J. Acquir. Immune Defic. Syndr 5, 490–496. [PubMed] [Google Scholar]
- Portales P, Clot J, Corbeau P, 2001. Sex differences in HIV-1 viral load due to sex difference in CCR5 expression. Ann. Intern. Med 134, 81–82. [DOI] [PubMed] [Google Scholar]
- Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan MO, Lutalo T, Gray RH, 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N. Engl. J. Med 342, 921–929. [DOI] [PubMed] [Google Scholar]
- Roberts L, Passmore J-AS, Mlisana K, Williamson C, Little F, Bebell LM, Walzl G, Abrahams M-R, Woodman Z, Karim QA, Karim SSA, 2012. Genital tract inflammation during early HIV-1 infection predicts higher plasma viral load set point in women. J. Infect. Dis 205, 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotello G, 1997. Sexual Ecology : AIDS and the Destiny of Gay Men. Dutton, New York, N.Y., U.S.A. [Google Scholar]
- Sabeti PC, Walsh E, Schaffner SF, Varilly P, Fry B, Hutcheson HB, Cullen M, Mikkelsen TS, Roy J, Patterson N, Cooper R, Reich D, Altshuler D, O’Brien S, Lander ES, 2005. The case for selection at CCR5-Δ32. PLoS Biol. 3, e378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanker G, Sorci-Thoma s.M., Adams MR, 1994. Estrogen modulates the expression of tumor necrosis factor alpha mRNA in phorbol ester- stimulated human monocytic THP-1 cells. Lymphokine Cytokine Res. 13, 377–382. [PubMed] [Google Scholar]
- Singh S, Bradley H, Hu X, Skarbinski J, Hall HI, Lansky A, Centers for Disease, C., Prevention, 2014. Men living with diagnosed HIV who have sex with men: progress along the continuum of HIV care–united States, 2010. MMWR Morb. Mortal. Wkly. Rep 63, 829–833. [PMC free article] [PubMed] [Google Scholar]
- Sullivan PS, Rosenberg ES, Sanchez TH, 2015. Explaining racial disparities in HIV incidence in black and white men who have sex with men in Atlanta, GA: a prospective observational cohort study. Ann. Epidemiol 25, 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touloumi G, Pantazis N, Pillay D, Paraskevis D, Chaix M-L, Bucher HC, Kücherer C, Zangerle R, Kran A-MB, Porter K, 2013. Impact of HIV-1 subtype on CD4 count at HIV seroconversion, rate of decline, and viral load set point in european seroconverter cohorts. Clin. Infect. Dis 56, 888–897. [DOI] [PubMed] [Google Scholar]
- Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, Kiwanuka N, Kigozi G, Kiddugavu M, Lutalo T, Nalugoda F, Wabwire-Mangen F, Meehan MP, Quinn TC, 2005. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J. Infect. Dis 191, 1403–1409. [DOI] [PubMed] [Google Scholar]
- Yue L, Prentice HA, Farmer P, Song W, He D, Lakhi S, Goepfert P, Gilmour J, Allen S, Tang J, Kaslow RA, Hunter E, 2013. Cumulative impact of host and viral factors on HIV-1 viral-load control during early infection. J. Virol 87, 708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.