Abstract
The present study examined longitudinal associations between family member perceived burden and clinical correlates to understand potential covariation in change over time in the context of first-episode schizophrenia in the RAISE-ETP study (N=282). Across 24 months, family burden, patient quality of life, and positive symptoms improved. Findings from the present study suggest covariation in change over time in quality of life and family burden. As patient quality of life improved, family burden decreased. However, initial levels of quality of life were not significantly associated with changes in family burden and vice versa. Initial levels of positive symptoms were significantly associated with initial levels of family burden. These findings have treatment implications by suggesting the potential for interventions aimed at improving quality of life to have a spillover effect on family burden, or alternatively, that reducing perceived family burden may improve patient quality of life.
Keywords: Family Burden, First Episode Psychosis, Quality of Life, Positive Symptoms
Introduction
Family members often serve as primary caregivers for individuals with schizophrenia. Although the experience of caring for an adult family member with a major medical illness is not unique to schizophrenia, there is some evidence that family members experience more burden related to this disorder than other major illnesses (Magliano et al., 2005; Möller-Leimkühler, 2005). Caregiving burden is associated with stronger effects on mental and physical health functioning of family members of persons with schizophrenia than caregivers of patients with other disorders (Gupta et al., 2015).
In addition to the effects of caregiving on family members of people with schizophrenia, the family environment can influence both patient relapse and recovery. There is a rich body of research showing that emotionally charged family communication styles can increase the risk of relapse for people with schizophrenia and other psychiatric disorders (Butzloff and Hooley, 1998; Hooley, 2007). Furthermore, family psychoeducation is effective at reducing both caregiver burden (Yesufu-Udechuku et al., 2015) and relapse rates (Pitschel-Walz et al., 2001). Accordingly, understanding the dynamic interplay between family member burden and patient clinical status and functioning has important implications for improving the ability to involve families in treatment and, more generally, to improve quality of life in both patients and caregivers.
The extant literature on perceived family burden relies largely on cross-sectional studies to determine patient correlates of family burden. Both clinical symptoms, including positive and negative symptoms (Dyck et al., 1999; Magliano et al., 2002; Mantovani et al., 2016; Perlick et al., 2006; Provencher and Mueser, 1997; Schene et al., 1998; Webb et al., 1998; Wolthaus et al., 2002), and patient quality of life (Perlick et al., 2006) have been associated with subjective family burden. Although cross-sectional studies are useful for understanding family burden in the context of schizophrenia, they do not address how family burden and clinical characteristics change over time, which could have important implications for treatment.
Longitudinal studies evaluating changes in family burden in schizophrenia report conflicting directions of change which may be confounded by illness stage. For example, Roick and colleagues (2006) found decreases in family burden across five waves of assessments over 30 months in a sample with a wide range of ages and illness stages. Similarly, Perlick and colleagues (2010) noted reduced family burden levels 18-months after an initial assessment in a chronically ill sample. In contrast, Levene and colleagues (2009) noted increased family burden levels from 1-month post-discharge from a first hospitalization to 9 months later.
Prospective studies examining family burden as a predictor of subsequent clinical factors or clinical factors as a predictor of subsequent burden suggest associations between family burden and clinical factors over time. For example, Levene and colleagues (2009) found that higher perceived family burden at hospital discharge predicted more psychotic symptoms at follow-up 9 months later, controlling for baseline level of psychosis. In examining early predictors of later family burden, Möller-Leimkühler (2005) found that caregiver traits, rather than patient symptoms, at first hospitalization predicted family burden five years later. In examining changes in symptoms, Roick and colleagues (2006) found that worsening in patient negative symptoms predicted increases in family burden. Moreover, Levene and colleagues (2009) noted that higher levels of family burden at follow-up were particularly common in families of patients who relapsed. Although together these studies suggest that family burden and clinical factors influence one another over time, further work is needed to evaluate the associations between changes in family burden and clinical factors over time. Such a question requires assessing both family burden and clinical factors with repeated measures and evaluating trajectories of change over time in order to evaluate whether family burden and clinical factors are initially related and whether they change together over time.
There are two major gaps in the literature assessing longitudinal associations between family burden and clinical factors over time. First, prior research has not examined longitudinal associations between family burden and clinical factors in first-episode psychosis patients, despite this being a critical period of intervention (Marshall et al., 2005). As stated above, differences in course and severity of illness likely have confounded results in prior studies. Although Möller-Leimkühler (2005) utilized a first-episode sample, their emphasis was on early predictors of later family burden rather than repeated assessments of family burden and clinical factors in order to evaluate change and covariation of change in these constructs. In cross- sectional data, greater burden has been observed in families of patients in their first year of treatment than in families of patients who have been in treatment longer (Lowyck et al., 2004), further supporting the importance of examining associations between family burden and clinical factors over time among the first-episode population. Second, few longitudinal studies have examined the relationship between family burden and quality of life in patients—arguably a more important treatment target than clinical symptoms (Eack and Newhill, 2007; Eack et al., 2007). Moreover, these studies have focused on multi-episode rather than first-episode schizophrenia (Rhee and Rosenheck, 2018).
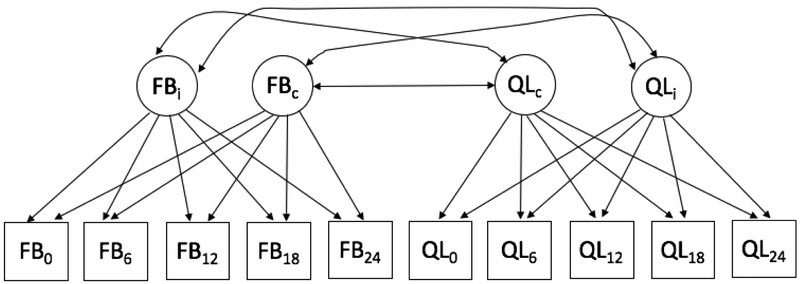
To fill these knowledge gaps, the present study examined the longitudinal associations between changes in perceived family burden and in clinical variables over time in individuals receiving treatment for a first-episode of psychosis. We hypothesized that family burden and patient clinical factors would be associated and change together over time (see Figure 1 for model with quality of life correlate). Given conflicting findings regarding direction of change over time, potentially due to differences in patient illness stage across studies (e.g., Levene et al., 2009; Roick et al., 2006), we sought to first evaluate longitudinal trajectories of change over time in order to identify the appropriate change patterns over time for family burden and clinical correlates prior to evaluating the associations between these change trajectories. Based on findings that duration of untreated psychosis (Marshall et al., 2005), medication adherence (Perlick et al., 2006; Robinson et al., 1999), depressive symptoms (Huppert et al., 2001), and family psychoeducation (Nasr and Kausar, 2009) may influence family burden and/or clinical factors, these variables were included as covariates along with age and gender, which have previously been associated with family burden (Ochoa et al., 2008; Perlick et al., 2006), in order to examine associations between family burden and clinical correlates not driven by these demographic or treatment factors.
Figure 1.
Multivariate Latent Growth Curve Model Path Diagram
Note: FB = Family Burden, QL = Quality of Life; Observed variable subscripts indicate assessment wave in months, Latent variable subscripts i and c indicate intercept level and change factors; Covariates and residual variances are excluded for figure simplicity
2. Methods
2.1. Participants and Procedure
Participants were drawn from a nationally representative sample of 404 individuals experiencing their first episode of psychosis in the NIMH-funded Recovery After an Initial Schizophrenia Episode-Early Treatment Program (RAISE-ETP) study (Kane et al., 2015, 2016). For the present study we selected a subsample of 282 participants who provided data on family burden during at least one time point in which family burden and clinical correlates were collected (baseline, 6, 12, 18, and 24 month assessments). Sample characteristics are presented in Table 1. We collapsed participants across treatment conditions (NAVIGATE coordinated specialty care program vs. community care treatment-as-usual control) for the present analyses because we were interested in associations between family burden and clinical correlates regardless of treatment type, an approach that has been used to study associations in change over time in other treatment studies (Luo et al., 2018), including longitudinal examinations of family burden (Rhee and Rosenheck, 2018; Roick et al., 2006). We controlled for whether or not families in either condition received family psychoeducation.
Table 1.
Demographic Variables (N=282)
| Mean (SD) | |
|---|---|
| Patient Age | 22.53 (4.87) |
| % (n) | |
| Patient Gender, Male | 75.5% (213) |
| Patient Race | |
| American Indian/Alaska Native | 5.3% (15) |
| Asian | 3.2% (9) |
| Black | 31.6% (89) |
| White | 59.9% (169) |
| Patient SCID DSM-PV Diagnosis | |
| Schizophrenia | 53.5% (151) |
| Schizoaffective Disorder | 16.7% (47) |
| Schizophreniform Disorder | 17.7% (50) |
| Brief Psychotic Disorder | 0.4% (1) |
| Psychotic Disorder NOS | 11.7% (33) |
| Patient Education | |
| No high school | 5.7% (16) |
| High school no diploma | 31.9% (90) |
| High school diploma | 27.3% (77) |
| Some college | 33.7% (95) |
| 4-year college degree and above | 4.3% (12) |
| Post-graduate experience | 1.1% (3) |
| Mother Education | |
| No high school | 6.0% (17) |
| High school no diploma | 8.5% (24) |
| High school diploma | 25.2% (71) |
| Some college | 21.6% (61) |
| 4-year college degree | 17.7% (50) |
| Post-graduate experience | 6.7% (19) |
| Missing | 14.2% (40) |
| Father Education | |
| No high school | 2.9% (8) |
| High school no diploma | 7.4% (21) |
| High school diploma | 28.7% (81) |
| Some college | 15.6% (44) |
| 4-year college degree and above | 12.8% (36) |
| Post-graduate experience | 6.7% (19) |
| Missing | 25.9% (73) |
| Family Psychoeducation | |
| No family psychoeducation | 28.4% (80) |
| Any family psychoeducation | 71.3% (201) |
| Long-Acting Injectable Medication | |
| Baseline | 8.9% (25) |
| 6-months | 9.2% (26) |
| 12-months | 11.0% (31) |
| 18-months | 9.6% (27) |
| 24-months | 9.2% (26) |
2.2. Measures
Data were collected with families and with patients via interview-based assessments at a baseline assessment and follow-up assessments at 6, 12, 18, and 24 months.
2.2.1. Burden Assessment Scale (BAS; Reinhard & Horwitz, 1995).
The BAS consists of 19 items measuring perceptions of burdens associated with providing support to a relative with mental illness, such as financial strain, shame, and worry. Burden items are rated on a 4-point Likert scale from not at all (0) to a lot (3), such that higher scores indicate greater burden. The BAS was completed by family members at the baseline and follow-up assessments.
2.2.2. Quality of Life Scale (QLS; Heinrichs et al., 1984).
The QLS is a measure of psychosocial functioning that includes 21 items tapping the domains of social relationships, role functioning, “intrapsychic foundations” (or motivation), and common objects and activities. QLS ratings are made on a 7-point Likert scale from 0 to 6, such that higher scores indicate better functioning or quality of life. We utilized the total score.
2.2.3. Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987).
Positive symptoms and negative symptoms were assessed using the PANSS. The positive subscale (4 items) and negative subscale (6 items) of a five factor model (Wallwork et al., 2012) were used to assess the symptom severity on a Likert scale from absent (1) to extreme (7), such that high scores reflect greater symptoms.
2.2.4. Calgary Depression Scale for Schizophrenia (CDSS; Addington et al., 1990).
The CDSS consists of 12 items measuring depressive symptoms in the past two weeks. Items are rated on a 4-point Likert scale from absent (0) to severe (3). We utilized the total score.
2.2.5. Oral Antipsychotic Medication Adherence Review.
Oral medication adherence was
assessed through a modified version of the Brief Adherence Rating Scale previously validated against electronic Medication Event Monitoring System caps (Byerly et al., 2008). Medication adherence was defined as the amount of missed oral medication over the past 60 days divided by the total amount of prescribed oral medication over the past 60 days.
2.2.6. Services Utilization Monthly (SURF-M; Rosenheck et al, 2003).
Data on receipt of family psychoeducation was collected via phone or in-person interviews during each month of the study period as part of the Service Utilization assessment. Receipt of psychoeducation was coded 1 for families who received at least one session of psychoeducation during the study and 0 for families with no reported psychoeducation.
2.3. Data Analytic Strategy
Latent growth curve models (LGCM) were used to assess within-person changes and between-person differences across 24 months and factor associations (e.g., level-level, slope- slope, level-slope) between family burden and clinical factors (Bollen and Curran, 2006). Data were modeled with Mplus version 8 (Muthén and Muthén 1998–2012). Model fit was assessed using multiple indices: χ2 index (Bollen, 1989), TLI (Tucker and Lewis, 1973), CFI (Bentler, 1990), and RMSEA (Hu and Bentler, 1999).
As recommended by Bollen and Curran (2006), we first fit a series of univariate models in order to identify the appropriate change over time pattern in each construct prior to examining multivariate associations. Thus, no-change and change models were compared for both family burden and quality of life. Data were first modeled with: 1) an intercept-only model with three parameters (intercept mean, intercept variance, and residual variance) representing stability over time; 2) a linear model with six parameters (intercept and slope means, intercept and slope variances and their covariance, and residual variance) representing a constant rate of linear change; and 3) a latent basis growth curve model with nine parameters (intercept and slope means, intercept and slope variances and their covariance, residual variance, and basis coefficients at 6, 12, and 18 months) representing a non-linear change pattern indicated by the data. In latent basis models, we set the basis coefficients for baseline and 24 months as 0 and 1, respectively, and freely estimated basis coefficients for 6, 12, and 18 months; therefore, the latent intercept is interpreted as the level of family burden at the baseline assessment and the latent change is interpreted as total change between baseline and 24 months.
A multivariate model was then fit to the data to examine hypotheses of factor associations between initial levels and changes in family burden and quality of life, positive symptoms, and negative symptoms, respectively; this model included covariates. Gender, age, family psychoeducation, and duration of untreated psychosis at the baseline assessment were included as time-invariant covariates. Gender was coded 0 for male and 1 for female. Age and duration of untreated psychosis were grand mean centered. Medication adherence and depressive symptoms were included as time-varying covariates.
3. Results
3.1. Univariate Models
Table 3 reports model fit of all univariate models. The latent basis model was selected as the final model for family burden, quality of life, and positive symptoms. Full parameter estimates for these models are presented in Table 4. The latent basis model for negative symptoms indicated a negative variance for the change factor mean and there was not significant variance in between-person change in the linear or latent basis models. Therefore, we did not move forward with fitting multivariate models with negative symptoms.
Table 3.
Fit Statistics for Univariate Models
| Family Burden Models | Quality of Life Models | ||
|---|---|---|---|
| No-Change | Fit Improvement | No-Change | Fit Improvement |
| χ2(17) = 212.08 | χ2(17) = 188.00 | ||
| RMSEA = 0.21 | RMSEA = 0.19 | ||
| CFI = 0.06 | CFI = 0.67 | ||
| TLI = 0.45 | TLI = 0.81 | ||
| Linear | Linear | ||
| χ2(14) = 88.36 | Δχ2(3) = 123.72 | χ2(14) = 56.13 | Δχ2(3) = 131.87 |
| RMSEA = 0.14 | p< .001 | RMSEA = 0.07 | p< .001 |
| CFI = 0.64 | CFI = 0.92 | ||
| TLI = 0.74 | TLI = 0.94 | ||
| Latent Basis | Latent Basis | ||
| X2(11) = 30.99 | Δχ2(3) = 57.37 | χ2(11) = 30.99 | Δχ2(3) = 28.08 |
| RMSEA = 0.08 | p< .001 | RMSEA = 0.08 | p< .001 |
| CFI = 0.90 | CFI = 0.90 | ||
| TLI = 0.91 | TLI = 0.91 | ||
| Positive Symptoms Models | Negative Symptoms Models | ||
|---|---|---|---|
| No-Change | Fit Improvement | No-Change | Fit Improvement |
| X2(17) = 181.81 | χ2(17) = 84.36 | ||
| RMSEA = 0.19 | RMSEA = 0.12 | ||
| CFI = 0.42 | CFI = 0.74 | ||
| TLI = 0.66 | TLI = 0.85 | ||
| Linear | Linear | ||
| χ2(14) = 85.34 | Δχ2(3) = 96.47 | χ2(14) = 32.24 | Δχ2(3) = 52.12 |
| RMSEA = 0.13 | p< .001 | RMSEA = 0.07 | p< .001 |
| CFI = 0.75 | CFI = 0.92 | ||
| TLI = 0.82 | TLI = 0.95 | ||
| Latent Basis | Latent Basis | ||
| χ2(11) = 11.97 | Δχ2(3) = 73.37 | χ2(11) = 21.03 | Δχ2(3) = 11.21 |
| RMSEA = 0.02 | p< .001 | RMSEA = 0.06 | p< .05 |
| CFI =1.00 | CFI = 0.96 | ||
| TLI = 1.00 | TLI = 0.97 | ||
Table 4.
Univariate Latent Growth Curve Models Unstandardized Estimates
| Family Burden Model | |||
|---|---|---|---|
| Parameters | Estimates | SE | p-value |
| Level factor mean | 44.86 | 0.90 | <.01 |
| Level factor variance | 144.52 | 18.72 | <.01 |
| Change factor mean | −11.13 | 1.25 | <.01 |
| Change factor variance | 139.24 | 30.57 | <.01 |
| Level and change covariance | −80.07 | 19.67 | <.01 |
| 6-month basis coefficient | 0.71 | 0.06 | <.01 |
| 12-month basis coefficient | 0.84 | 0.07 | <.01 |
| 18-month basis coefficient | 0.95 | 0.07 | <.01 |
| Indicator residual variance | 50.08 | 4.55 | <.01 |
| Quality of Life Model | |||
| Parameters | Estimates | SE | p-value |
| Level factor mean | 53.15 | 1.15 | <.01 |
| Level factor variance | 223.35 | 31.73 | <.01 |
| Change factor mean | 14.39 | 1.54 | <.01 |
| Change factor variance | 141.56 | 48.11 | <.01 |
| Level and change covariance | 27.77 | 30.81 | .37 |
| 6-month basis coefficient | 0.62 | 0.07 | <.01 |
| 12-month basis coefficient | 0.64 | 0.07 | <.01 |
| 18-month basis coefficient | 0.81 | 0.08 | <.01 |
| Indicator residual variance | 150.74 | 9.69 | <.01 |
| Positive Symptoms Model | |||
| Parameters | Estimates | SE | p-value |
| Level factor mean | 12.10 | 0.23 | <.01 |
| Level factor variance | 7.64 | 1.27 | <.01 |
| Change factor mean | −2.90 | 0.32 | <.01 |
| Change factor variance | 6.84 | 1.85 | <.01 |
| Level and change covariance | −.23 | 1.25 | .07 |
| 6-month basis coefficient | 0.90 | 0.08 | <.01 |
| 12-month basis coefficient | 0.70 | 0.07 | <.01 |
| 18-month basis coefficient | 0.91 | 0.08 | <.01 |
| Indicator residual variance | 6.79 | 0.44 | <.01 |
For family burden, the results indicated that, on average, family burden declined from baseline to 24-months. The basis coefficients (also called factor loadings) estimated for the 6-, 12-, and 18-month assessments indicate that of the total decrease between baseline and 24- months, for the average individual 71% occurred between baseline and 6 months, 13% occurred between 6 and 12 months, and 11% occurred between 12 and 18 months. The estimated covariance between the intercepts and the slopes indicates that individuals with higher levels of family burden at baseline declined more rapidly.
For quality of life, the results indicated that, on average, quality of life increased from baseline to 24-months. The basis coefficients estimated for the 6-, 12-, and 18-month assessments indicate that of the total increase in quality of life between baseline and 24-months, for the average individual 62% occurred between baseline and 6-months, 2% occurred between 6 and 12 months, and 17% occurred between 12 and 18 months. The estimated covariance between the intercepts and the slopes indicates that baseline quality of life was not significantly associated with increases in quality of life.
For positive symptoms, the results indicated that, on average, positive symptoms decreased from baseline to 24-months. The basis coefficients estimated for the 6-, 12-, and 18- month assessments indicate that, on average, 90% of the total increase in quality of life between baseline and 24-months occurred in the first six months. The estimated covariance between the intercepts and the slopes indicates that severity of baseline positive symptoms was not significantly associated with increases in positive symptoms.
3.2. Multivariate LGCM
Parameter estimates for the quality of life model are displayed in Table 5. Results indicated a significant negative association between family burden and quality of life change factors reflecting that, on average, steeper increases in quality of life are associated with steeper decreases in family burden. There was no significant association between baseline initial levels of family burden and quality of life. The associations between baseline family burden and changes in quality of life over time and between baseline quality of life and changes in family burden over time were also not significant. Gender was significantly associated with baseline quality of life, with females reporting better quality of life. Medication adherence at 12 months was significantly associated with quality of life at 12 months, such that better adherence was associated with higher quality of life at the 12-month assessment. Depressive symptoms were significantly associated with quality of life at all timepoints, with greater depression associated with lower quality of life. Greater severity of depression at 6 and 12 months was significantly associated with higher family burden at the same timepoints.
Table 5.
Multivariate Latent Growth Curve Model with Quality of Life Unstandardized Estimates
| Multivariate Covariances | |||
|---|---|---|---|
| Parameters | Estimates | SE | p-value |
| FB level and QoL level | −30.74 | 17.10 | 07 |
| FB level and QoL change | 35.37 | 21.69 | 10 |
| QoL level and FB change | 13.42 | 19.64 | 49 |
| FB change and QoL change | −64.08 | 25.43 | < .05 |
| FB and QoL Residuals | −10.73 | 5.34 | < .05 |
| Family Burden Model | Quality of Life Model | |||||
|---|---|---|---|---|---|---|
| Parameters | Estimates | SE | p-value | Estimates | SE | p-value |
| Latent Growth Curve | ||||||
| Level factor mean | 43.57 | 2.14 | <.01 | 54.11 | 2.57 | <.01 |
| Level factor residual | 140.12 | 17.93 | <.01 | 211.45 | 29.59 | <.01 |
| Change factor mean | −13.89 | 2.88 | <.01 | 16.37 | 3.49 | <.01 |
| Change factor residual | 122.11 | 27.80 | <.01 | 119.47 | 41.99 | <.01 |
| Level and change covariance | −75.18 | 18.40 | <.01 | 20.82 | 28.04 | .46 |
| Indicator residual variance | 47.31 | 4.35 | <.01 | 135.20 | 8.87 | <.01 |
| Time-Varying Covariate Effects | ||||||
| MA at Baseline | −2.04 | 3.97 | .61 | 6.57 | 4.28 | .12 |
| MA at 6 months | −0.72 | 2.94 | .81 | 2.02 | 3.91 | .61 |
| MA at 12 months | 1.93 | 3.48 | .58 | −12.45 | 3.63 | <.01 |
| MA at 18 months | 5.60 | 3.11 | .07 | −0.71 | 4.27 | .87 |
| MA at 24 months | 3.60 | 4.05 | .37 | −2.98 | 4.65 | .52 |
| DS at Baseline | −0.24 | 0.22 | .28 | −0.82 | 0.23 | <.01 |
| DS at 6 months | 0.61 | 0.22 | <.01 | −1.28 | 0.25 | <.01 |
| DS at 12 months | 0.69 | 0.26 | <.01 | −1.09 | 0.33 | <.01 |
| DSatl 8 months | 0.54 | 0.29 | .07 | −1.69 | 0.33 | <.01 |
| DS at 24 months | −0.04 | .46 | .93 | −1.66 | 0.39 | <.01 |
| Time-Invariant Covariate Effects | ||||||
| Gender → level factor | 1.93 | 2.08 | .35 | 8.09 | 2.61 | <.01 |
| Age → level factor | −0.22 | 0.19 | .24 | −0.25 | 0.24 | .30 |
| DUP → level factor | 0.01 | 0.01 | .92 | −0.01 | .01 | .29 |
| FP → level factor | 3.07 | 1.99 | .12 | −0.34 | 2.47 | .89 |
| Gender → change factor | 0.61 | 2.62 | .82 | −2.75 | 3.30 | .40 |
| Age → change factor | −0.07 | 0.23 | .76 | 0.03 | 0.28 | .92 |
| DUP → change factor | 0.01 | 0.01 | .71 | −0.01 | 0.01 | .30 |
| FP → change factor | −0.26 | 2.61 | .92 | 1.49 | 3.18 | .64 |
Note: Model fit: χ 2(170) = 266.32; RMSEA = 0.05 [CI: 0.034, 0.055]; TLI = 0.88; CFI = 0.89. FB = Family Burden, QoL = Quality of Life, MA = Medication Adherence, DS = Depressive Symptoms, FP = Family Psychoeducation, DUP = Duration of Untreated Psychosis; Gender
Parameter estimates for the positive symptoms model are displayed in Table 6. Results indicated a significant association between baseline initial levels of family burden and positive symptoms: on average, greater initial family burden was associated with greater initial positive symptoms. There was no significant association between changes in family burden and changes in positive symptoms. The associations between baseline family burden and changes in positive symptoms over time and between baseline positive symptoms and changes in family burden over time were also not significant. Depressive symptoms were significantly associated with positive symptoms at all timepoints except 24 months, with greater depression associated with more severe positive symptoms. Greater severity of depression at 6 and 12 months was also significantly associated with higher family burden at the same timepoints.
Table 6.
Multivariate Latent Growth Curve Model with Positive Symptoms Unstandardized Estimates
| Multivariate Covariances | |||
|---|---|---|---|
| Parameters | Estimates | SE | p-value |
| FB level and PS level | 7.32 | 3.52 | < .05 |
| FB level and PS change | −6.21 | 3.91 | .11 |
| PS level and FB change | −0.34 | 4.13 | .94 |
| FB change and PS change | 4.69 | 4.75 | .32 |
| FB and PS Residuals | 2.90 | 1.22 | < .05 |
| Family Burden Model | Positive Symptoms Model | |||||
|---|---|---|---|---|---|---|
| Parameters | Estimates | SE | p-value | Estimates | SE | p-value |
| Latent Growth Curve | ||||||
| Level factor mean | 43.30 | 2.16 | <.01 | 11.10 | 0.54 | <.01 |
| Level factor residual | 142.76 | 18.21 | <.01 | 7.14 | 1.21 | <.01 |
| Change factor mean | −13.67 | 2.90 | <.01 | −2.35 | 0.66 | <.01 |
| Change factor residual | 125.14 | 27.96 | <.01 | 5.33 | 1.67 | <.01 |
| Level and change covariance | −76.62 | 18.55 | <.01 | −2.42 | 1.13 | <.05 |
| Indicator residual variance | 46.71 | 4.26 | <.01 | 6.48 | 0.42 | <.01 |
| Time-Varying Covariate Effects | ||||||
| MA at Baseline | −2.15 | 3.99 | .59 | 0.32 | 0.95 | .74 |
| MA at 6 months | −0.28 | 2.98 | .93 | 0.37 | 0.84 | .66 |
| MA at 12 months | 1.68 | 3.46 | .63 | 1.30 | 0.76 | .09 |
| MA at 18 months | 5.30 | 3.04 | .08 | 0.10 | 0.92 | .92 |
| MA at 24 months | 5.05 | 4.14 | .22 | 0.09 | 0.98 | .93 |
| DS at Baseline | −0.22 | 0.22 | .32 | 0.18 | 0.05 | <.01 |
| DS at 6 months | 0.62 | 0.22 | <.01 | 0.25 | 0.05 | <.01 |
| DS at 12 months | 0.66 | 0.26 | <.05 | 0.23 | 0.07 | <.01 |
| DSatl 8 months | 0.57 | 0.29 | .05 | 0.29 | 0.07 | <.01 |
| DS at 24 months | −0.16 | 0.46 | .73 | 0.12 | 0.08 | .13 |
| Time-Invariant Covariate Effects | ||||||
| Gender → level factor | 1.64 | 2.09 | .43 | −0.60 | 0.52 | .25 |
| Age → level factor | −0.20 | 0.19 | .30 | 0.06 | 0.05 | .20 |
| DUP → level factor | 0.01 | 0.01 | .94 | 0.01 | 0.01 | .60 |
| FP → level factor | 3.35 | 2.00 | .09 | 0.45 | 0.50 | .37 |
| Gender → change factor | 1.15 | 2.66 | .67 | 0.37 | 0.60 | .54 |
| Age → change factor | −0.09 | 0.23 | .71 | −0.01 | 0.05 | .98 |
| DUP → change factor | 0.01 | 0.01 | .51 | 0.01 | 0.01 | <.05 |
| FP → change factor | −0.53 | 2.64 | .84 | −0.50 | 0.59 | .40 |
Note: Model fit: χ2(170) = 264.07; RMSEA = 0.04 [CI: 0.034, 0.054]; TLI = 0.83; CFI = 0.85. FB = Family Burden, PS = Positive Symptoms, MA = Medication Adherence, DS = Depressive Symptoms, FP = Family Psychoeducation, DUP = Duration of Untreated Psychosis
4. Discussion
The goal of the present study was to examine the longitudinal associations between changes in perceived family burden over time and changes in clinical correlates over time in a large sample entering treatment for a first episode of psychosis. Four major findings emerged. First, family burden, quality of life, and positive symptoms all improved over time, with the greatest changes in the first 6 months. Second, baseline levels of family burden were not associated with baseline levels of quality of life, but were associated with baseline severity levels of positive symptoms. Third, baseline levels of patient quality of life and positive symptoms were not significantly associated with changes in family burden, nor were baseline levels of family burden associated with changes in patient quality of life or changes in positive symptoms. However, and most importantly, changes in quality of life were associated with changes in family burden, indicating that improvements in one domain were accompanied by improvements in the other domain. Therefore, the present findings provide critical information about processes of family burden and clinical correlates in first-episode patients.
The emergence of significant covariation in change over time in quality of life and family burden over the course of the 24 months in first episode psychosis is particularly salient. Recent examination of this potential association in multi-episode persons with schizophrenia and their family members did not find this association (Rhee and Rosenheck, 2018). One possible explanation for these discrepant findings between multi-episode and first-episode patients rests on the supposition that family burden reflects, at least in part, reactions to a relatively long period of psychosocial impairment in a family member. With the long duration of untreated psychosis that typically precedes treatment initiation in first-episode patients, family members have likely witnessed a gradually worsening mental health symptoms and daily functioning over the course of many months (Addington et al., 2015), which subsequently improve relatively rapidly once treatment is initiated. However, in treated multi-episode patients, symptom exacerbations and functional declines likely receive a more rapid response, restoring the patient to a previous level of functional more rapidly. Thus, to the extent that perceived burden is in response to longer periods of impairment, one might expect changes in psychosocial functioning to be more strongly correlated with changes in family burden after the first episode (following more prolonged impairment) than after subsequent episodes (following briefer periods of impairment. This hypothesis remains to be tested, but at the very least, these findings underscore the importance of illness course for the longitudinal associations between family burden and quality of life.
Moreover, baseline levels of patient quality of life were not significantly associated with changes in family burden and vice versa (also see Möller-Leimkühler, 2005). The same was true for positive symptoms. Furthermore, we found that baseline levels of quality of life and family burden were not significantly associated during first-episode psychosis. In contrast, baseline levels of positive symptoms were significantly associated with family burden during first episode psychosis, but unlike quality of life, not coordinated longitudinally. Differences in longitudinal associations with burden may be attributable to how these symptoms change with treatment. Positive symptoms are generally more responsive to treatment than quality of life. Indeed, in this sample, 90% of the total reduction in positive symptoms occurred within six months of treatment initiation, whereas only 62% of the total improvement in quality of life occurred over the same period. Alternatively, these differential patterns of findings between quality of life and positive symptoms and associations with family burden may reflect the initial distress and alarm that accompany witnessing a loved one experiencing positive psychosis for the first time. In contrast to quality of life, the association between positive symptoms and family burden may not persist longitudinally because positive symptoms, unlike quality of life, are not so tightly intertwined with role functioning in the family (Bellack et al., 1990).
The finding that family burden decreased over a 24-month period extends prior research noting decreases in family burden across a 30-month period following inpatient hospitalization among a broader age range of patients diagnosed with schizophrenia, which likely included a mix of first-episode and multi-episode patients (18 to 64; Roick et al., 2006). Such a finding is important given that burden is expected to be greatest among families of those earliest in their course of illness and treatment (Lowyck et al., 2004). Moreover, quality of life increased most dramatically within the first 6 months while family burden and positive symptoms decreased most dramatically within the first 6 months, likely indicating that for first-episode patients treatment is rapidly effective in improving patient quality of life and reducing strain on families.
Although not a primary focus of the current paper, we found that depressive symptoms were associated with reductions in quality of life and positive symptoms, consistent with previous reports (Conley et al., 2007). There are increased efforts to treat depression in the early stages of schizophrenia, given its association with functioning and prediction of suicide in these individuals (Upthegrove et al., 2017). In addition, greater depressive symptoms in patients also predicted increased perceived burden in caregivers, at 6 and 12 months following the baseline measurement. These associations could reflect a contagion effect of living with someone with depression (Coyne et al., 1987). The observed relationship between perceived family burden and depressive symptoms warrant further investigation into the potentially therapeutic effects of family interventions on mood symptoms in individuals with first-episode schizophrenia.
4.1. Limitations
The findings should be interpreted in light of several limitations. First, these results address covariation in changes in quality of life and family burden rather than directional relationships. Second, we did not conduct factor analyses and test factorial invariance with this sample. Alternative factor structures for the BAS have been reported (e.g. Reinhard et al., 1994) and more factor analytic work is needed. Third, there was variability in family memberacross wavesreporting; however, restricting analyses to same-reporter data would have reduced the sample size substantially and made the present models infeasible. Therefore, we focused on assessment of family burden broadly and future work is needed to understand associations between individual family members’ perceptions of burden and clinical correlates.
4.2. Conclusions
The present analysis extends prior research to examine associations of longitudinal trajectories of family burden and patient quality of life among first-episode schizophrenia patients from the nationally representative RAISE-ETP study. Covariance in change over time in quality of life and family burden indicate that as quality of life improved, family burden decreased and vice versa, suggesting the potential for interventions aimed at improving quality life to have a spillover effect to reduce family burden, or alternatively, that reducing perceived family burden may improve patient quality of life.
Table 2.
Family Burden Item Endorsements Over Time
| Scale Item | Baseline | 6-Months | 12-Months | 18-Months | 24-Months |
|---|---|---|---|---|---|
| 1. Financial Problems | 73% (172) | 59% (98) | 45% (54) | 47% (52) | 43% (39) |
| 2. Missed work/school | 64% (132) | 33% (47) | 30% (31) | 31% (31) | 16% (12) |
| 3. Difficulty concentrating | 83% (201) | 65% (109) | 60% (74) | 58% (66) | 53% (48) |
| 4. Change personal plans | 63% (148) | 48% (77) | 32% (39) | 29% (32) | 32% (28) |
| 5. Reduced leisure time | 72% (173) | 53% (88) | 44% (54) | 41% (46) | 35% (32) |
| 6. Upset household routine | 78% (188) | 62% (104) | 52% (62) | 51% (57) | 42% (38) |
| 7. Less time for friends | 65% (156) | 42% (69) | 35% (42) | 28% (31) | 29% (26) |
| 8. Neglected family’s needs | 61% (147) | 40% (67) | 37% (45) | 33% (36) | 29% (26) |
| 9. Family Frictions | 68% (166) | 59% (99) | 49% (60) | 54% (61) | 41% (37) |
| 10. Frictions with others | 40% (97) | 24% (40) | 23% (27) | 21% (24) | 19% (17) |
| 11. Embarrassed | 43% (105) | 31% (51) | 33% (40) | 27% (31) | 30% (27) |
| 12. Guilty not helping enough | 73% (178) | 52% (86) | 49% (60) | 50% (56) | 48% (44) |
| 13. Guilty for causing illness | 49% (118) | 38% (63) | 31% (38) | 28% (32) | 26% (23) |
| 14. Resented demands | 37% (89) | 29% (49) | 32% (39) | 27% (31) | 30% (27) |
| 15. Felt trapped | 50% (120) | 48% (80) | 45% (55) | 38% (43) | 37% (33) |
| 16. Upset about relative’s change | 78% (189) | 57% (93) | 59% (71) | 54% (48) | 54% (48) |
| 17. Worry make illness worse | 73% (175) | 66% (109) | 59% (73) | 57% (64) | 51% (46) |
| 18. Worry about future | 97% (236) | 93% (156) | 91% (112) | 88% (100) | 84% (76) |
| 19. Stigma upsetting | 70% (169) | 60% (97) | 54% (66) | 53% (59) | 42% (37) |
Note: Percentages reflect the proportion of the valid sample at each wave who reported any burden (i.e., “little” “some” or “a lot”) and parentheses report the accompanying frequency.
Highlights.
Covariation in change over time occurs between family burden and patient quality of life
Family burden, quality of life, and positive symptoms improved over time
Baseline level of patient quality of life and positive symptoms were not associated with change in family burden
Baseline level of family burden was not associated with changes in patient quality of life or positive symptoms
Acknowledgements
This work has been funded in whole or in part with funds from the American Recovery and Reinvestment Act and from NIMH under contract HHSN271200900019C. The contents of this article are solely the responsibility of the authors and do not necessarily represent the views of NIMH or the U.S. Department of Health and Human Services. Clinical trials registration: NCT01321177: An Integrated Program for the Treatment of First Episode of Psychosis (RAISE ETP) (http://www.clinicaltrials.gov/ct2/show/NCT01321177)
Dr. Achtyes has received research support from Alkermes, AssurEx, Astellas, Avanir, Boehringer Ingelheim, Janssen, Neurocrine Biosciences, Novartis, Otsuka, Pfizer, Pine Rest Foundation, Priority Health, Network180, Takeda and the Vanguard Research Group and has served on advisory panels for Alkermes, Indivior, Janssen, Neurocrine Biosciences, Roche and the Vanguard Research Group.
Dr. Kane has received grant support from Otsuka, Lundbeck and Janssen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
Dr. Kane has been a consultant for or received honoraria from Alkermes, Forest (Allergan), Genentech, H. Lundbeck. Intracellular Therapies, Janssen Pharmaceutica, Johnson and Johnson, Merck, Neurocrine, Otsuka, Pierre Fabre, Reviva, Roche, Sunovion, Takeda and Teva.
Dr. Kane has participated in Advisory Boards for Alkermes, Intracellular Therapies, Lundbeck, Neurocrine, Otsuka, Pierre Fabre, Reviva, Roche, Sunovion, Takeda, Teva.
Dr. Kane is a Shareholder in Vanguard Research Group and LB Pharmaceuticals, Inc.
All other authors: None.
Literature Cited
- Addington D, Addington J, Schissel B, 1990. A depression rating scale for schizophrenics. Schizophr. Res 3, 247–251. 10.1016/0920-9964(90)90005-R [DOI] [PubMed] [Google Scholar]
- Addington J, Heinseen RK, Robinson DG, Schooler NR, Marcy P, Brunette MF, Correll CU, Estroff S, Mueser KT, Penn D, Robinson JA, Rosenheck RA, Azrin ST, Goldstein AB, Severe J, Kane JM (2015). Duration of untreated psychosis in communtiy treatment settings in the United States. Psychiatr. Serv 66, 753–756. 10.1175/appi.ps.201400124 [DOI] [PubMed] [Google Scholar]
- Bellack AS, Morrison RL, Wixted JT, Mueser KT (1990) An analysis of social competence in schizophrenia. BJP 156, 809–818. 10.1192/bjp.156.6.809 [DOI] [PubMed] [Google Scholar]
- Bentler P, 1990. Comparative Fit Indices in Structural Models. Psychol. Bull. 107, 238–246. [DOI] [PubMed] [Google Scholar]
- Bollen K, 1989. A New Incremental Fit Index for General Structural Equation Models . Sociol. Methods Res. 17, 303–316. [Google Scholar]
- Bollen KA, Curran PJ, 2006. Latent Curve Models A Structural Equation Perspective. John Wiley & Sons, Inc. 10.1002/0471746096 [DOI] [Google Scholar]
- Byerly MJ, Nakonezny PA, Rush AJ, 2008. The brief adherence rating scale validated against electronic monitoring in assessing the antipsychotic medication adherence of outpatients with schizophrenai and schizoaffective disorder. Schizophr. Res 110, 60–69 [DOI] [PubMed] [Google Scholar]
- Conley RR, Ascher-Svanum H, Zhu B, Faries DE, Kinon BJ, 2007. The burden of depressive symptoms in the long-term treatment of patients with schizophrenia. Schizophr. Res 90, 186–197. 10.1016/j.schres.2006.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JC, Kessler RC, Tal M, Turnbull J, Wortman CB, & Greden JF, 1987. Living with a depressed person. J. Consult. Clin. Psychol, 55, 347–52. [DOI] [PubMed] [Google Scholar]
- Dyck DG, Short R, Vitaliano PP, 1999. Predictors of burden and infectious illness in schizophrenia caregivers. Psychosom. Med 61, 411–419. 10.1097/00006842-199907000-00001 [DOI] [PubMed] [Google Scholar]
- Eack S, Newhill C, Anderson C, Rotondi A, 2007. Quality of Life For Persons Living with Schizophrenia: More Than Just Symptoms. Psychiatry Rehabil. J 30, 219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Newhill CE, 2007. Psychiatric symptoms and quality of life in schizophrenia: A meta-analysis. Schizophr. Bull 33, 1225–1237. 10.1093/schbul/sbl071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Isherwood G, Jones K, Van Impe K, 2015. Assessing health status in informal schizophrenia caregivers compared with health status in non-caregivers and caregivers of other conditions. BMC Psychiatry 15, 1–11. 10.1186/s12888-015-0547-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs D Hanlon TE Carpenter WT 1984. The Qu lity of Life Scale : An Instrument for Rating the Schizophrenic Deficit Syndrome 10, 388–398. [DOI] [PubMed] [Google Scholar]
- Hooley JM, 2007. Expressed Emotion and Relapse of Psychopathology. Annu. Rev. Clin. Psychol 3, 329–352. 10.1146/annurev.clinpsy.2.022305.095236 [DOI] [PubMed] [Google Scholar]
- Hu L-T, Bentler PM, 1999. Structural Equation Modeling: A Multidisciplinary Journal Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Multidiscip. J 6, 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Huppert JD, Weiss KA, Lim R, Pratt S, Smith TE, 2001. Quality of life in schizophrenia: Contributions of anxiety and depression. Schizophr. Res 51, 171–180. 10.1016/S0920-9964(99)00151-6 [DOI] [PubMed] [Google Scholar]
- Kane JM, Robinson DG, Schooler NR, Mueser KT, Penn DL, Rosenheck RA, Addington J, Brunette MF, Correll CU, Estroff SE, Marcy P, Robinson J, Meyer-Kalos PS, Gottlieb JD, Glynn SM, Lynde DW, Pipes R, Kurian BT, Miller AL, Azrin ST, Goldstein AB, Severe JB, Lin H, Sint KJ, John M, Heinssen RK, 2016. Comprehensive versus usual community care for first-episode psychosis: 2-Year outcomes from the NIMH RAISE early treatment program. Am. J. Psychiatry 173, 362–372. 10.1176/appi.ajp.2015.15050632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JM, Schooler NR, Marcy P, et al. , 2015. The RAISE early treatment program for first-episode psychosis: background, rationale, and study design. J Clin Psychiatry 76, 240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA, 1987. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13, 261–276. [DOI] [PubMed] [Google Scholar]
- Kretchy IA, Osafo J, Agyemang SA, Appiah B, Nonvignon J, 2018. Psychological burden and caregiver-reported non-adherence to psychotropic medications among patients with schizophrenia. Psychiatry Res. 259, 289–294. [DOI] [PubMed] [Google Scholar]
- Levene JE, Lancee W, Seeman MV, Skinner H, Freeman SJJ, 2009. Family and patient predictors of symptomatic status in schizophrenia. Can. J. Psychiatry 54, 446–451. 10.1177/070674370905400705 [DOI] [PubMed] [Google Scholar]
- Lowyck B, De Hert M, Peeters E, Wampers M, Gilis P, Peuskens J, 2004. A study of the family burden of 150 family members of schizophrenic patients. Eur. Psychiatry 19, 395–401. 10.1016/j.eurpsy.2004.04.006 [DOI] [PubMed] [Google Scholar]
- Luo X, Nuttall AK, Locke KD, Hopwood CJ, 2018. Dynamic longitudinal relations between binge eating symptoms and severity and style of interpersonal problems. J. Abnorm. Psychol 127, 30–42. 10.1037/abn0000321 [DOI] [PubMed] [Google Scholar]
- Magliano L, Fiorillo A, De Rosa C, Malangone C, Maj M, 2005. Family burden in long-term diseases: A comparative study in schizophrenia vs. physical disorders. Soc. Sci. Med 61, 313–322. 10.1016/j.socscimed.2004.11.064 [DOI] [PubMed] [Google Scholar]
- Magliano L, Marasco C, Fiorillo A, Malangone C, Guameri M, Maj M, 2002. The impact of professional and social network support.pdf 291–298. https://doi.org/https://doi-org.proxy1cl.msu.edu/10.1034/j.1600-0447.2002.02223.x [DOI] [PubMed] [Google Scholar]
- Mantovani LM, Ferretjans R, Marçal IM, Oliveira AM, Guimarães FC, Salgado JV, 2016. Family burden in schizophrenia: the influence of age of onset and negative symptoms. Trends Psychiatry Psychother. 38, 96–9. 10.1590/2237-6089-2015-0082 [DOI] [PubMed] [Google Scholar]
- Marshall M, Lewis S, Lockwood A, Drake R, Jones P, Croudace T, 2005. Relationship Between Duration of Untreated Psychosis and Outcome Am. Med. Assoc 62, 975–983. 10.1176/appi.ajp.162.10.1785 [DOI] [PubMed] [Google Scholar]
- Möller-Leimkühler AM, 2005. Burden of relatives and predictors of burden. Baseline results from the Munich 5-year-follow-up study on relatives of first hospitalized patients with schizophrenia or depression. Eur. Arch. Psychiatry Clin. Neurosci 255, 223–231. 10.1007/s00406-004-0550-x [DOI] [PubMed] [Google Scholar]
- Mueser KT, Sengupta A, Schooler NR, Bellack AS, Xie H, Glick ID, Keith SJ, 2001. Family treatment and medication dosage reduction in schizophrenia: Effects on patient social functioning, family attitudes, and burden. J. Consult. Clin. Psychol 69, 3–12. 10.1037/0022-006X.69.1.3 [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO, 1998–2014. Mplus 7.4. Los Angeles, CA: Author. [Google Scholar]
- Nasr T, Kausar R, 2009. Psychoeducation and the family burden in schizophrenia: A randomized controlled trial. Ann. Gen. Psychiatry 8, 1–6. 10.1186/1744-859X-8-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa S, Vilaplana M, Haro JM, Villalta-Gil V, Martínez F, Negredo MC, Casacuberta P, Paniego E, Usall J, Dolz M, Autonell J, 2008. Do needs, symptoms or disability of outpatients with schizophrenia influence family burden? Soc. Psychiatry Psychiatr. Epidemiol 43, 612–618. 10.1007/s00127-008-0337-x [DOI] [PubMed] [Google Scholar]
- Perlick DA, Rosenheck RA, Kaczynski R, Swartz MS, Canive JM, Lieberman JA, 2010. Impact of antipsychotic medication on family burden in schizophrenia: Longitudinal results of CATIE trial. Schizophr. Res 116, 118–125. 10.1016/j.schres.2009.09.026 [DOI] [PubMed] [Google Scholar]
- Perlick DA, Rosenheck RA, Kaczynski R, Swartz MS, Canive JM, Lieberman JA, 2006. Components and Correlates of Family Burden in Schizophrenia. Psychiatr. Serv 57, 1117–1125. 10.1176/appi.ps.57.8.1117 [DOI] [PubMed] [Google Scholar]
- Pitschel-Walz G, Leucht S, Bäuml J, Kissling W, Engel RR, 2001. The effect of family interventions on relapse and rehospitalization in schizophrenia - A meta-analysis. Schizophr. Bull 27, 73–92. 10.1093/oxfordjournals.schbul.a006861 [DOI] [PubMed] [Google Scholar]
- Provencher HL, Mueser KT, 1997. Positive and negative symptom behaviors and caregiver burden in the relatives of persons with schizophrenia. Schizophr. Res 26, 71–80. 10.1016/S0920-9964(97)00043-1 [DOI] [PubMed] [Google Scholar]
- Reinhard SC, Horwitz AV, 1995. Caregiver Burden: Differentiating the Content and Consequences of Family Caregiving. J. Marriage Fam. 57, 741 10.2307/353928 [DOI] [Google Scholar]
- Reinhard SC, Gubman GD, Horwitz AV, Minsky S, 1994. Burden assessment scale for families of the seriously mentally ill. Eval. and Prog. Plan 17, 261–269. [Google Scholar]
- Rhee TG, Rosenheck RA, 2018. Does improvement in symptoms and quality of life in chronic schizophrenia reduce family caregiver burden?, Psychiatry Res. 10.1016/j.psychres.2018.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D et al. , 1999. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 56, 241–247. [DOI] [PubMed] [Google Scholar]
- Roick C, Heider D,Toumi M,Angermeyer MC,2006. The impact of caregivers’ characteristics patients’ conditions and regional differences on family burden in schizophrenia: A longitudinal analysis. Acta Psychiatr. Scand 114, 363–374. 10.1111/j.1600-0447.2006.00797.x [DOI] [PubMed] [Google Scholar]
- Rosenheck RA, Kasprow W., et al. 2003. Cost-effectiveness of supported housing for homeless persons with mental illness. Arch Gen Psychiatry. 60, 940–951. [DOI] [PubMed] [Google Scholar]
- Schene AH, Van Wijngaarden B, Koeter MWJ, 1998. Family caregiving in schizophrenia: Domains and distress. Schizophr. Bull 24, 609–618. 10.1093/oxfordjournals.schbul.a033352 [DOI] [PubMed] [Google Scholar]
- Tucker LR, Lewis C, 1973. A reliability coefficient for maximum likelihood factor analysis. Psychometrika 38, 1–10. 10.1007/BF02291170 [DOI] [Google Scholar]
- Upthegrove R, Marwaha S, Birchwood M, 2017. Depression and Schizophrenia: Cause, Consequence, or Trans-diagnostic Issue? Schizophr. Bull 43, 240–244. 10.1093/schbul/sbw097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D, 2012. Searching for a consensus five-factor model of the Positive and Negative Syndrome for schizophrenia. Schizophr. Res 137, 246–250. 10.1016/j.schres.2012.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb C, Pfeiffer M, Mueser KT, Gladis M, Mensch E, DeGirolamo J, Levinson DF, 1998. Burden and well-being of caregivers for the severely mentally ill: The role of coping style and social support. Schizophr. Res 34, 169–180. 10.1016/S0920-9964(98)00089-9 [DOI] [PubMed] [Google Scholar]
- Wolthaus JED, Dingemans PMAJ, Schene AH, Linszen DH, Wiersma D, Van Den Bosch RJ, Cahn W, Hijman R, 2002. Caregiver burden in recent-onset schizophrenia and spectrum disorders: The influence of symptoms and personality traits. J. Nerv. Ment. Dis 190, 241–247. 10.1097/00005053-200204000-00005 [DOI] [PubMed] [Google Scholar]
- Yesufu-Udechuku A, Harrison B, Mayo-Wilson E, Young N, Woodhams P, Shiers D, Kuipers E, & Kendall T (2015). Interventions to improve the experience of caring for people with severe mental illness: Systematic review and meta-analysis. British Journal of Psychiatry, 206, 268–74. DOI: 10.1192/bjp.bp.114.147561 [DOI] [PubMed] [Google Scholar]