Abstract
Background and Purpose:
Computer Tomography Perfusion (CTP) is a useful tool in the evaluation of acute ischemic stroke, where it can provide an estimate of the ischemic core and the ischemic penumbra. The optimal CTP parameters to identify the ischemic core remain undetermined.
Methods:
We utilized Artificial Neural Networks (ANNs) to optimally predict the ischemic core in acute stroke patients, using diffusion-weighted imaging as the gold standard. We first designed an ANN based on CTP data alone and next designed an ANN based on clinical and CTP data.
Results:
The ANN based on CTP data predicted the ischemic core with a mean absolute error of 13.8 ml (SD 13.6 ml) compared to DWI. The area under the receiver operator characteristic curve (AUC) was 0.85. At the optimal threshold, the sensitivity for predicting the ischemic core was 0.90 and the specificity was 0.62. Combining CTP data with clinical data available at time of presentation resulted in the same mean absolute error (13.8 ml) but lower SD (12.4 ml). Furthermore, the AUC, sensitivity, and specificity were 0.87, 0.91, and 0.65, respectively. The maximal Dice coefficient was 0.48 in the ANN based on CTP data exclusively.
Conclusions:
An artificial neural network that integrates clinical and CTP data predicts the ischemic core with accuracy.
Keywords: stroke, CT perfusion, neural network
Introduction
Computer Tomography Perfusion (CTP) can visualize ischemic brain tissue in patients with acute stroke1. Numerous CTP parameters, such as CBV, CBF and Tmax, have been proposed for identifying the ischemic core, a critical predictor of outcome2–4. Typically, a threshold is applied to a single CTP parameter to identify the ischemic core. The use of a single perfusion parameter, however, may not capture all predictive information in the CTP acquisition.
Machine learning algorithms such as Artificial Neural Networks (ANN) may provide the ideal tool to uncover patterns based on multiple CTP parameters to predict the ischemic core. Moreover, ANNs could integrate data from CTP with clinical data to further improve their predictive power. In this study we aimed to design an ANN that integrates data from all CTP parameters as well as clinical data to outline the ischemic core.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Patient selection and CTP acquisition
We used data from two prospective studies that collected imaging and clinical data on acute stroke patients from three US sites and one Australian site5, 6. Each study was approved by an Institutional Review Board. Written informed consent from the patient or a relative was required for participation in the study. For inclusion and exclusion criteria, please refer to supplementary material at please see http://stroke.ahajournals.org. Images were acquired on scanners from all major CT and MRI manufacturers. Further details on CTP acquisition can be found at http://stroke.ahajournals.org.
ANN model
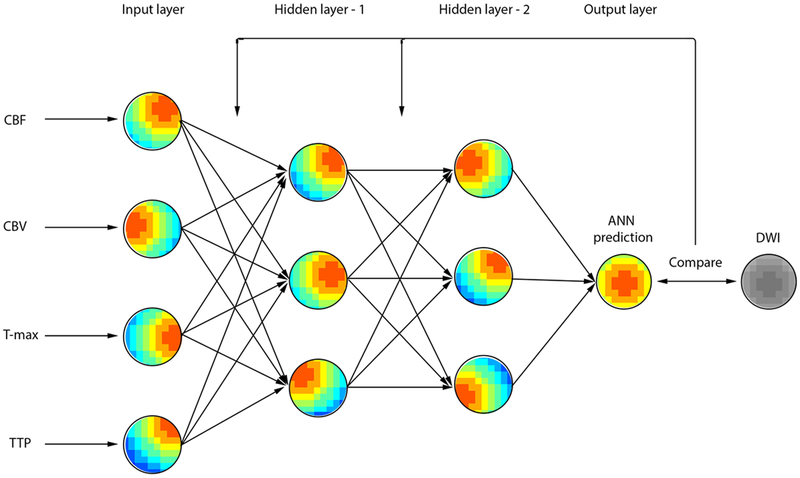
A feed-forward ANN with supervised training was designed, and back-propagation was employed to adjust the weights of the ANN connections in a supervised fashion. As in Figure-1, the nodes are organized in the input layer, hidden layers, and single-node output layer. Clinical parameters available at time of presentation integrated into the ANN were NIHSS, age, sex, and time from symptom onset to CTP. For further details on the ANN design, training, and validation as well as ANN prediction analysis, please refer to supplementary material at http://stroke.ahajournals.org.
Figure-1.
A model of a feedforward artificial neural network (ANN). Four CTP maps are used as input vectors for the ANN. Bias and weights were omitted for purposes of illustration.
Results
The dataset included 128 acute stroke patients who underwent back-to-back CTP and MRI between 2004 and 2012. Patients were excluded because more than 50% of the DWI lesion had normal perfusion at the time of CTP (n=18), severe motion artifact resulted in degradation of the image data not amenable to correction with motion correction algorithms (n=3), and there was insufficient quality of the baseline CTP data (n=4). For patients who met the eligibility criteria, the mean age was 68.7 years (SD 14.6), the median time from stroke onset to CT was 190.9 min (IQR 138.8–231.3), the median baseline NIHSS score was 15 (IQR 10–19), and the median time between CT completion and commencement of MR was 40.5 min (IQR 25.0–82.8).
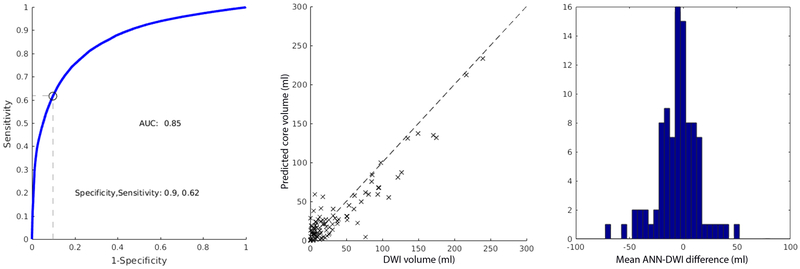
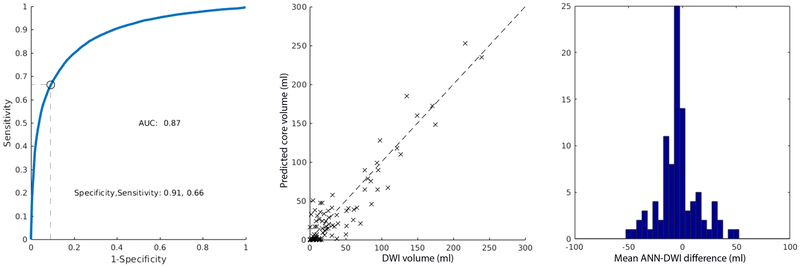
The ANN, based exclusively on CTP data, that minimized the mean absolute error between CTP and DWI was a 2-layered network with 3 nodes in both the first and second hidden layer. The threshold that minimized the mean absolute volumetric error between the ischemic core predicted by the ANN and the DWI lesion was 0.52 (mean absolute error 13.8 ml; SD 13.6 ml). The AUC was 0.85. The sensitivity and specificity were 0.90 and 0.62, respectively (Figure-2A). The optimal ANN, based on both CTP and clinical data, was a 2-layered network with 6 and 5 nodes in the first and second layers, respectively. For the ANN that included CTP and clinical data, the threshold with the smallest mean absolute error between predicted and DWI lesion volumes was 0.56 (mean absolute error of 13.8 ml; SD 12.4 ml). The AUC at this threshold was 0.87. The sensitivity and specificity were 0.91 and 0.65, respectively (Figure-2B). The maximal Dice coefficient occurred at the threshold 0.32 (Dice coefficient 0.48, IQR 0.23–0.70). ANN predictions were generated in less than a second. The mean ANN-DWI differences of were −4.8 ml (SD 13.6) and −4.8 ml (SD 18.0) for the ANN without and with clinical data, respectively. A Wilcoxon signed rank test was performed between the absolute errors that demonstrated no statistically significant difference between the two ANN designs (p = 0.56), suggesting no significant improvement by adding clinical variables. No statistically significant difference in the absolute errors between the previous model7 and the current model was found (p = 0.81).
Figure-2.
ROC curves, scatter plot of CTP and DWI ischemic core volumes, and mean ANN-DWI difference histogram for ANN using CTP data (A) and CTP and clinical data (B). The optimal sensitivity and specificity are determined at the threshold that minimizes the mean absolute CTP-DWI volumetric error.
A correlation analysis was performed between the CTP-to-MR times and the absolute volume error, and the Pearson correlation coefficient was 0.03 (p = 0.87) suggesting there was no correlation between these two variables.
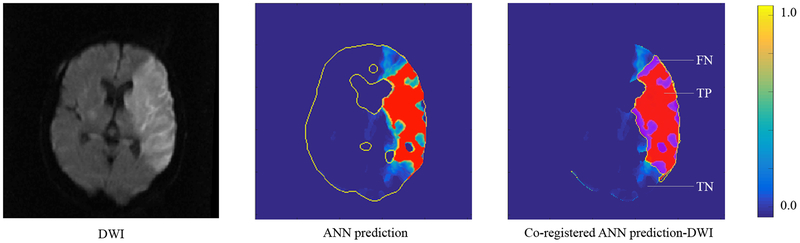
A representative case demonstrating volumetric and spatial correspondence of the voxel-wise ANN ischemic core prediction at the optimal threshold and the DWI is shown in Figure-3.
Figure-3.
CTP-ANN infarct voxel-level prediction. DWI (yellow outline), ANN prediction (red region), and the co-registered ANN prediction-DWI are shown. FN: false negative; TP: true positive; TN: true negative. The color bar represents the ischemic probability maps’ corresponding voxel-wise values.
Discussion
This study shows that an ANN that incorporates CTP and baseline clinical data accurately predicts the ischemic core. To our knowledge, this is the largest study to investigate a machine learning algorithm for CTP prediction of ischemic core and the first to be performed on human subjects. The results of this study are valuable in the acute ischemic stroke setting where CT is the primary imaging modality. The mean absolute error was 13.8 ml. Direct comparisons between the current and previous models are difficult given differences in methodologies and lack of statistical validation of previous models. However, with these limitations in mind, no statistically significant difference in reported errors was found between the current model and the previous algorithm currently used by our group7.
Previous studies utilizing machine learning algorithms for ischemic core prediction used MR input data or a combination of MR and CT data on a small group of patients or small animals. Alternative models predicting ischemic tissue fate include a generalized linear model and a probability of infarct model. However, these linear algorithms may not adequately capture the heterogenous and dynamic nature of stroke pathophysiology8. Unlike these models, the ANN holds no a priori assumptions regarding the linearity of infarction risk with input parameters, an assumption that has been previously challenged.
The current algorithm, once trained, can be readily appended to automated post-processing software. This would create a prediction streamline for fully-automated, multiparametric, sub-second prediction of the ischemic core using the most widely available stroke imaging modality in a computationally inexpensive way. Such a standardized platform would reduce variability introduced by different customized CTP processing algorithms, ischemic core definitions, and other methodological differences.
This study has some limitations. First, CTP approximations of the ischemic core using ANN will remain imperfect as the gold-standard for the ischemic core remains elusive (see ‘Supplementary Material’). This limitation was addressed in our study by including patients who underwent CTP and MRI with little time delay (median 40.5 min). Inherent variability of DWI ROI delineation can add to the variability. Second, limitations of voxelwise analysis include reported overestimation of the ischemic core volume, possible dependency on the arbitrary subregion of the brain assessed (e.g. whole brain versus hypoperfusion region), and sensitivity to coregistration errors. The limited explanatory power of the ANN, and machine learning algorithms more broadly, remains an inherent limitation.
To our knowledge, this is the first study to use CTP exclusive data to train an ANN to predict the ischemic core in humans. Future work is focused on evaluation of additional input parameters and application of deep learning methods such as U-net convolutional networks that inherently integrate large scale features into the segmentation. With such studies, the ANN may evolve into a quantitative framework for ischemic core diagnosis, therapeutic decision making, and prognostic evaluation of therapeutic efficacy at an individual level.
Supplementary Material
Acknowledgments
Sources of funding
The study was funded by grants from the National Institute for Neurological Disorders and Stroke (NINDS). 1U10NS086487 (G. Albers) and 5 R01 NS075209 (M. Lansberg).
Footnotes
Disclosures
Drs. Gregory Albers and Søren Christensen are equity shareholders in iSchemaView and perform consulting work for iSchemaView.
Dr. Mark Parsons is a consultant for Toshiba Medical and Apollo Medical Imaging.
Bibliography
- 1.Campbell BC, Christensen S, Levi CR, Desmond PM, Donnan GA, Davis SM, et al. Cerebral blood flow is the optimal ct perfusion parameter for assessing infarct core. Stroke. 2011;42:3435–3440 [DOI] [PubMed] [Google Scholar]
- 2.Albers GW, Goyal M, Jahan R, Bonafe A, Diener HC, Levy EI, et al. Ischemic core and hypoperfusion volumes predict infarct size in swift prime. Ann Neurol. 2016;79:76–89 [DOI] [PubMed] [Google Scholar]
- 3.Bivard A, Spratt N, Levi C, Parsons M. Perfusion computer tomography: Imaging and clinical validation in acute ischaemic stroke. Brain. 2011;134:3408–3416 [DOI] [PubMed] [Google Scholar]
- 4.Padroni M, Bernardoni A, Tamborino C, Roversi G, Borrelli M, Saletti A, et al. Cerebral blood volume aspects is the best predictor of clinical outcome in acute ischemic stroke: A retrospective, combined semi-quantitative and quantitative assessment. PLoS One. 2016;11:e0147910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al. Mri profile and response to endovascular reperfusion after stroke (defuse 2): A prospective cohort study. Lancet Neurol. 2012;11:860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin L, Bivard A, Levi CR, Parsons MW. Comparison of computed tomographic and magnetic resonance perfusion measurements in acute ischemic stroke: Back-to-back quantitative analysis. Stroke. 2014;45:1727–1732 [DOI] [PubMed] [Google Scholar]
- 7.Cereda CW, Christensen S, Campbell BC, Mishra NK, Mlynash M, Levi C, et al. A benchmarking tool to evaluate computer tomography perfusion infarct core predictions against a dwi standard. J Cereb Blood Flow Metab. 2016;36:1780–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu O, Christensen S, Hjort N, Dijkhuizen RM, Kucinski T, Fiehler J, et al. Characterizing physiological heterogeneity of infarction risk in acute human ischaemic stroke using mri. Brain. 2006;129:2384–2393 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.