Abstract
A central role of the mechanistic target of rapamycin (mTOR) in regulation of fundamental cell processes is well-recognized. mTOR functions in two distinct complexes: rapamycin-sensitive mTOR complex (C) 1 and rapamycin-insensitive mTORC2. While the role of mTORC1 in shaping immune responses, including transplant rejection, and the influence of its antagonism in promoting allograft tolerance have been studied extensively using rapamycin, lack of selective small molecule inhibitors has limited understanding of mTORC2 biology. Within the past few years, however, intracellular localization of mTORC2, its contribution to mitochondrial fitness, cell metabolism, cytoskeletal modeling and cell migration, and its role in differentiation and function of immune cells have been described. Studies in mTORC2 knockdown/knockout mouse models and a new class of dual mTORC1/2 inhibitors, have shed light on the immune regulatory functions of mTORC2. These include regulation of antigen-presenting cell, NK cell, T cell subset and B cell differentiation and function. mTORC2 has been implicated in regulation of ischemia/reperfusion injury and graft rejection. Potential therapeutic benefits of antagonizing mTORC2 to inhibit chronic rejection have also been described, while selective in vivo targeting strategies using nanotechnology have been developed. We briefly review and discuss these developments and their implications.
1. Introduction
The mechanistic target of rapamycin (mTOR) is a conserved, nutrient-sensing, serine/threonine kinase that coordinates cell growth and metabolism with environmental input. It functions in two distinct complexes: rapamycin-sensitive mTOR complex (C) 1 and rapamycin-insensitive mTORC2. Extensive research has established a central role for mTOR signaling in regulating numerous fundamental cell processes including metabolism, protein synthesis and autophagy1. The role of mTORC1 in shaping immune responses, including allograft rejection, and the influence of its antagonism by rapamycin in promoting experimental transplant tolerance have been studied extensively. However, there is a relative paucity of information regarding the functional immunobiology of mTORC2. This is due, in part, to the lack of an mTORC2-specific pharmacological inhibitor and to the absence of a constitutively active mTORC2 model. Within the past three years, new fundamental observations at the cellular level, accompanied by studies in which mTORC2 has been deleted specifically in either antigen-presenting cells (APCs) or lymphocytes, together with the use of knockdown or knockout (KO) mouse models and the advent of a new class of adenosine triphosphate (ATP) competitive, dual mTORC1/2 inhibitors, have begun to shed light on the roles of mTORC2 in regulation of immune cell differentiation and function. mTORC2 has also been implicated in regulation of ischemia/reperfusion (I/R) injury, allograft rejection, tumor growth and aging.
2. Biology of mTORC2
The seminal discovery in 19912 of two related genes,- TOR1 and TOR2, in the yeast Saccharomyces cerevisiae led to demonstration of their kinase activity and their requirement for cell proliferation3. This was soon followed by identification of their mammalian counterparts and of upstream and downstream regulators of mTOR (reviewed in ref 1), that defined a signaling pathway4 fundamental to control of cell growth and metabolic homeostasis. The biology of the two distinct mTOR complexes is depicted in Figure 1. Structurally, mTORC2 consists of several components,- i.e. mTOR, the essential mTORC2 component rapamycin-insensitive companion of mTOR (Rictor), DEP domain-containing mTOR interacting protein (Deptor), protein observed with Rictor (Protor), mammalian lethal with SEC13 protein 8 (mLST8), and mammalian stress-activated MAP kinase-interacting protein 1 (mSIN1).
Figure 1.
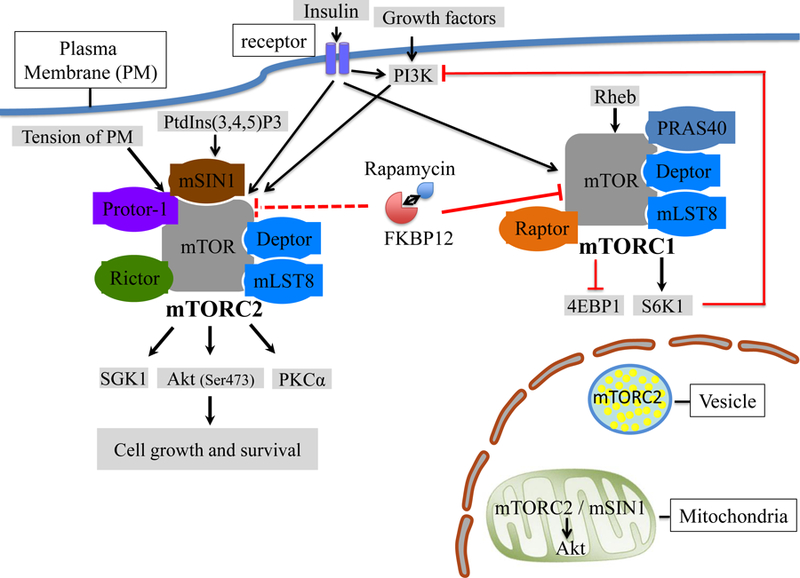
Biology of mTORC2. mTORC2 consists of several components, including mTOR, Rictor, Protor, Deptor, mSIN1 and mLST8. In contrast, mTORC1 consists of mTOR, Raptor, PRAS40, Deptor and mLST8. mTORC2 has been localized in both cell membrane and intracellular compartments, including mitochondria and endosomal vesicles. PtdIns(3,4,5) P3, plasma membrane tension and growth factors, including insulin, can activate mTORC2. Active mTORC2 phosphorylates multiple protein kinase (PK) PKA, PKC, and PKG family kinases, including Akt, PKCα and SGK1 to support cell growth and survival. Rapamycin inhibits mTORC1 through binding to the immunophilin FKBP12. mTORC2 has been described as insensitive to rapamycin (sirolimus). mTORC2 signaling is also regulated by mTORC1 through a negative feedback loop between mTORC1-S6K1 and insulin/PI3K signaling.
Unlike with mTORC1, the guanosine triphosphate-binding protein Rheb (Ras homolog enriched in brain) is not an upstream activator of mTORC2 and indeed, upstream regulators of mTORC2 have not been defined. Furthermore, how or even if mTORC2 is regulated by extracellular cues has remained unclear. Insulin can activate mTORC2, but only if the complex contains two specific SIN isoforms5. There is also evidence that, in human embryonic kidney 293 T cells, mTORC2 can be activated directly by phosphatidylinositol 3,4,5-trisphosphate (PtdIns(3,4,5) P3)6. In yeast, TORC2 is regulated in part by plasma membrane tension7 and this may also be the case in mammalian cells, e.g. epithelial and vascular smooth muscle cells, as cell stretching induces mTORC2-dependent phosphorylation of Akt at the Ser473 site. In neutrophils, increasing plasma membrane tension acts through a pathway containing the phospholipase D2 and mTORC2 to limit actin network assembly. Without this negative feedback loop, neutrophils display larger leading edges, greater membrane tension and markedly defective chemotaxis8. However, in addition to cell membrane localization, mTORC2 has also been localized to intracellular compartments9. By examining localization of the obligate mTORC2 component mSIN1 using imaging and biochemical approaches, Ebner et al10 have demonstrated that within mammalian cells, mTORC2 activity localizes to the plasma membrane, mitochondria and a subpopulation of endosomal vesicles. mTORC2 located to the plasma membrane and mitochondria can be activated toward Akt through the mSIN1 pleckstrin homology domain that is independent of PI3K and growth factor, suggesting that mTORC2 may exist in subpopulations that are not sensitive to PI3K inside cells10.
Active mTORC2 phosphorylates multiple protein kinase (PK) A, PKC, and PKG family kinases, including Akt, PKCα (a regulator of the actin cytoskeleton) and serum/glucocorticoid regulated kinase 1 (SGK1). Activation of Akt by mTORC2 couples extracellular growth and survival cues with pathways controlling cell growth and proliferation, but how growth factors target mTORC activity towards Akt is not known. Elucidation of the downstream effector pathways of mTORC2 remains difficult, since these phosphorylation events may not impact kinase activity as much as substrate specificity.
Canonically, mTORC2 has been described as insensitive to rapamycin11. However, prolonged exposure of many cell types to rapamycin reduces levels of mTORC2 needed to maintain Akt/PKB signaling12. It has also been shown13 that rapamycin-mediated mTORC2 inhibition is determined by the relative expression of FK506-binding proteins (FKBP),- primarily FKBP12 and FKBP51. Significantly, mTORC2 signaling is also regulated by mTORC1 due to a negative feedback loop between mTORC1 and insulin/PI3K signaling14. This has multiple implications for pharmacological targeting of mTOR and provides a rationale for developing mTORC2-specific inhibitors that do not perturb this feedback loop.
3. Regulation of mitochondrial fitness and cell metabolism
Conditional deletion of mTOR, Raptor or Rictor in primary hematopoietic cells has revealed that mTOR plays a developmental stage-specific role in regulation of mitochondrial fitness, that is independent of conventional mTOR complexes and kinase activity15. By comparing the effects of mTOR deletion with that of Raptor or Rictor on mitochondrial fitness of progenitor stage (lineage [Lin])− and lineage-committed (Lin+) hematopoietic cells, it has been shown15 that mTOR regulates glycolysis, mitochondrial mass and mitochondrial membrane potential in Lin− cells and mitochondrial mass and oxidative phosphorylation (OXPHOS) in Lin+ cells depending on mTORC1. By contrast, mTOR regulates OXPHOS in Lin− cells and glycolysis in Lin+ cells depending on both mTORC1 and mTORC2. Whether this phenomenon occurs in other cells or tissues, as well as its underlying mechanism, are uncertain.
The role of mTOR in cell metabolism in immunity has been reviewed extensively1, 16. Separately, both mTORC1 and mTORC2 are involved in the control of glucose metabolism. In contrast to mTORC1, the most important role of mTORC2 is likely as an effector of insulin/PI3K signaling17. mTORC2 inhibition disrupts the physiological response to insulin and liver-specific KO mice exhibit severe insulin resistance and glucose intolerance18.
4. Role of mTORC2 in innate immune cells (Table 1)
TABLE 1.
Functions of Rictor/mTORC2 in innate immune cells
| Cell type | Observation | Reference |
|---|---|---|
| Macrophages | Rictor/TORC2 signaling promotes macrophage activation, migration, M2 polarization | 50 |
| mTORC2 pathway activation is required for enhanced glucose metabolism during M2 activation | 19 | |
| mTORC2 signaling regulates generation/function of tissue-resident macrophages | 21 | |
| Rictor/mTORC2 regulates macrophage proliferation and survival | 20 | |
| Chronic mTORC2 activity impairs lysosome degradation in lupus-prone mice | 59 | |
| Dendritic cells | Absence of Rictor does not affect Langerhans cell homeostasis | 22 |
| Rictor deficiency enhances conventional DC maturation and T cell (allo) stimulatory function in vitro and in vivo | 24 | |
| Intratumoral delivery of Rictor−/− DC enhances CD8+ T cell-mediated anti-tumor immunity | 25 | |
| Rictor deficiency in skin CD11c+ DC enhances cutaneous CD8+ T cell inflammatory responses and minor histocompatibility Ag-mismatched skin graft rejection | 26 | |
| NK cells | mTORC2 represses TORC1-modulated NK cell effector functions | 28 |
| mTORC2 deficiency impairs terminal NK cell maturation, proliferation and migration | 27 |
4.1. Macrophage activation and function
Phosphorylation of the cytoplasmic protein NDRG1 (N-Myc Downstream Regulated 1), which is a downstream target of mTORC2, is lost in Rictor−/− macrophages, but increased in Raptor−/− macrophages, suggesting that mTORC1 may restrict mTORC2 activation in these cells19. Macrophage colony-stimulating factor synergizes with IL-4 to facilitate elevated glycolysis during activation of M2 macrophages (that are associated with wound healing/tissue repair) by enhancing mTORC2 signaling involved in the PI3K/mTORC2/Akt pathways19. Furthermore, the mTORC2 pathway enhances interferon regulatory factor (IRF)-4 expression that is essential for metabolic reprogramming to support M2 macrophage activation19.
In response to lipopolysaccharide stimulation, Rictor−/− macrophages exhibit enhanced levels of proinflammatory gene expression and lower levels of IL-10 expression than control cells20. Other evidence21 shows that mTORC2 negatively regulates the generation of tissue-resident peritoneal macrophages, but not monocyte-derived peritoneal macrophages, through inhibiting GATA6 transcription factor expression by negatively controlling forkhead box protein O1 (FoxO1) activation. Rictor deficiency in peritoneal tissue-resident macrophages results in enhanced phagocytosis and proliferation in the resolution phase of zymosan-induced peritonitis21. Additionally, Rictor-deleted peritoneal tissue-resident macrophages have increased mitochondrial mass compared with wild-type (wt) counterparts, suggesting a critical role for mTORC2 in metabolic reprogramming21. Therapeutically, mTORC2 deletion in macrophages can suppress B16 melanoma tumor growth and loss of resistance to helminth parasites19.
4.2. Dendritic cell (DC) function
In mice, epidermal Langerhans cell homeostasis appears to depend strictly on mTORC1 and not mTORC2 function22. On the other hand, several studies have documented enhanced pro-inflammatory and T cell stimulatory activity of DC lacking mTORC2 activity. Thus, Rictor−/− conventional DC that lack mTORC2 display decreased programed death-ligand 1 (PD-L1) expression, suggesting that mTORC2 positively regulates this T cell co-regulatory molecule23. Enhanced PD-L1 expression induced by the dual mTORC1/2 inhibitor Torin1 occurs through a signal transducer and activator of transcription 3 (STAT3)-dependent but IRF-1-independent pathway23. Torin1-treated DC can promote regulatory T cell (Treg) induction through PD-L1 and IL-1β cooperative activation23. Conditional or CD11c-specific Rictor deletion in DC results in stronger alloreactive T helper (Th)1 and Th17 cell stimulatory ability after Toll-like receptor 4 ligation compared to control DC24. This enhanced pro-inflammatory profile of Rictor-deficient DC is partially dependent on glycogen synthase kinase 3 (GSK-3) regulation24. Therapeutically, intra-tumoral Rictor−/− DC injection inhibits B16 melanoma growth in wt B6 mice by promoting interferon (IFN)-γ and granzyme-B+ cytotoxic CD8+ T cell infiltration into the tumor25. In agreement with these findings, CD8+ T effector cell responses are augmented in skin grafts transplanted from major histocompatibility complex (MHC)- or minor histocompatibility antigen (Ag) (HY)-mismatched CD11c Rictor−/− donors26, with the latter undergoing accelerated rejection. In the absence of Rictor specifically in cutaneous DC, CD8+ T cell responses are also enhanced in a mouse cutaneous delayed-type hypersensitivity model26.
4.3. NK cells
mTORC1 and 2 play distinct roles and are essential for NK cell development and function. Conditional deletion of mTORC2 in mice blocks the transition of double positive (CD27+CD11b+) cells to terminally mature CD11b single positive NK cells27. This defect is associated with impaired induction of the T-box transcription factor T-bet through a mechanism involving the mTORC2-Akt S473-FoxO1 signaling axis. Other recent data28 provide evidence of positive and negative cross-talk between mTORC1 and mTORC2 via cytokine signaling that variegates the extent of NK cell maturation and effector function.
5. Role of mTORC2 in T cell subsets (Table 2)
TABLE 2.
Functions of Rictor/mTORC2 in adaptive immune cells
| Cell type | Observation | Reference(s) |
|---|---|---|
| T cell subsets | ||
| Tfh cells | mTORC2 is essential for Tfh cell differentiation and germinal center formation under steady-state, immunization and viral infection conditions | 31, 32 |
| Th9 cells | mTORC2 controls Th9 cell polarization | 34 |
| Th1/Th2 cells | mTORC2 regulates Th1/Th2 cell differentiation via distinct signaling pathways | 60 |
| CD8+ effector T cells | mTORC2 is dispensible for CD8+ T cell differentiation | 36 |
| CD8+ memory T cells | mTORC2 controls CD8+ Tmem differentiation in a FoxO1-dependent manner; Rictor deficiency enhances CD8+ Tmem formation | 36, 37 |
| Treg cells | Loss of mTORC2 increases thymic-derived Treg generation and enhances induced Treg differentiation; excessive mTORC2 activation disrupts Treg stability and function | 38, 40 |
| B cells | Rictor regulates B cell receptor signaling via actin reorganization | 43 |
| Rictor is required for early B cell development in bone marrow and for homeostasis and function of mature B cells | 44, 61 | |
| NKT cells | mTORC2 regulates multiple aspects of invariant NKT cell subset development and function | 41, 42 |
Abbreviation: Tfh cell, T follicular helper cell
Immune cells, including T cells, are auxotrophs for various essential and non-essential amino acids (aa)29. Little is known about how proliferating Th cells integrate signals from limiting aa29. Recent studies demonstrate that mTORC2 rather than mTORC1 plays a critical role in aa sensing that arrests Th cells in G1 when environmental aa are limiting30. Rictor-deficient CD4+ T cells proliferate normally in MLR cultures with limiting arginine or leucine, suggesting that mTORC2 determines the aa-sensitive checkpoint for Th cell proliferation30.
T follicular helper (Tfh) cells are a unique helper CD4+ subset that highly express B cell lymphoma 6 (Bcl6), the chemokine receptor CXCR5, T cell inhibitory receptor programed death 1 (PD1) and T cell inducible co-stimulator (ICOS). They are essential for B cell-mediated humoral immunity. Deletion of Rictor in CD4+ T cells results in less T cell accumulation, germinal center (GC) B cells and Tfh cells in Peyer’s patches31. Mechanistically, mTORC2 is activated by ICOS to further drive glycolysis and lipogenesis and enhance Tfh differentiation after foreign Ag immunization31. In the absence of mTORC2, FoxO1 activity is upregulated. However, ablation or reduction of FoxO1 can largely or partially restore defective Tfh cells in mTORC2 KO T cells, suggesting that mTORC2 promotes Tfh cell responses via FoxO1 inhibition31. Consistently, there is evidence that mTORC2 enhances Tfh differentiation through Akt activation and subsequently T cell factor 1 (TCF1) upregulation32. Other studies also show that mTORC2 kinase activity integrates T cell receptor (TCR) with ICOS to promote late maturation, but not early induction of virus-specific Tfh cells. Moreover, mTORC2 can ensure Tfh cells maintain their phenotype, migratory ability towards B cell follicles and effector function to promote B cell differentiation33.
Th9 cells secrete high levels of IL-9 upon stimulation. There is recent evidence that mTORC2 controls Th9 polarization and allergic airway inflammation. Thus, in an allergic airway inflammation model, Th9 differentiation is diminished in the absence of Rictor34. Interestingly, negative regulation of Th9 polarization is FoxO1-independent, but relies on IRF4 reduction due to decreased Akt or STAT6 activation34. Of note, IL-9 acting on Treg has been implicated in allograft tolerance.35
mTORC2 has been reported as dispensible for CD8+ effector T cell differentiation36. On the other hand, Rictor deficiency in CD8+ T cells results in enhanced memory precursor cell generation and stronger recall responses to viral infection, but reduced short-lived effector cells in a FoxO1-dependent manner37. FoxO1 is increased in Rictor-deleted CD8+ T cell nuclei to enhance expression of the transcription factors Eomes and TCF1 that further enhance CD8+ memory T cell differentiation, highlighting the roles of the mTORC2-Akt-FoxO1 signaling axis in memory T cell development37.
Several studies have demonstrated that over-activation of either TORC1 or TORC2 impairs Treg stability, accompanied by defects in their ability to suppress specific effector T cells38, 39. As with TORC1, an optimal level of mTORC2 activity is required for Treg differentiation, trafficking and function40.
mTORC2 is required for NKT-17 cell development, optimal NKT cell cytotoxicity, expansion and survival41. In the absence of Rictor, the overall frequency and absolute number of invariant NKT (iNKT) cells are decreased dramatically in the thymus41, as well as in the spleen and liver42. Rictor regulates the generation of GATA-3-expressing iNKT cells that affects IL-4-producing cells, however this effect is independent of the promyelocytic leukemia zinc-finger which is a critical transcriptional factor for iNKT cell development42.
6. Role of mTORC2 in B cells (Table 2)
Early Rictor deletion in CD19+ cells leads to reduced B cell receptor (BCR) signaling through decreasing phosphorylated Brutons tyrosine kinase and increasing phosphorylated SH2-containing inositol phosphatase, that are the key positive and negative molecules of upstream BCR signaling43. Mechanistically, absence of Rictor enhances actin polymerization to restrict BCR movement that eventually reduces the humoral immune response43. Inducible deletion of Rictor in Cre-ERT2 transgenic mice impairs Ab production, marginal zone and B1a B lymphocyte generation through regulation of nuclear factor (NF)-κB and NFκB2/p52 generation44, highlighting the vital role of Rictor in mature B cell function44. Genetic ablation of SIN1, a key component of mTORC2, in mouse B cells, impairs their metabolism and proliferation and humoral immunity45.
Rapamycin and the ATP-competitive dual mTORC inhibitor PP242 can attenuate BAFF (B cell-activating factor from the tumor necrosis family)-mediated normal and neoplastic B cell proliferation and survival by inhibiting mTORC1/2 signaling46.
7. Regulation of ischemia/reperfusion (I/R) injury
Observations of the regulatory functions of mTORC2 in I/R injury are summarized in Table 3. During hepatic ischemia/reperfusion (I/R) injury, Rictor expression is up-regulated, whereas Rictor deficiency aggravates I/R injury by increasing macrophage/neutrophil accumulation, proinflammatory cytokine production and apoptosis induction, with enhanced activation of MAPK signaling47. In an acute kidney I/R injury model in mice, Rictor deficiency specifically in CD11c+ DC worsens renal tissue damage, with enhanced neutrophil infiltration and increased proinflammatory cytokine production compared to littermate controls48. Ablation of Rictor in DC results in enhanced T cell costimulatory, but reduced coinhibitory molecule expression, with enhanced migration of the DC to the injured kidney48. Tubule-specific ablation of Rictor exacerbates cisplatin-induced acute kidney injury with reduced tubular cell autophagy, but increased apoptosis compared to littermate counterparts49. In a model of chronic kidney injury after I/R or unilateral ureteric obstruction, Rictor is upregulated in macrophages from the fibrotic kidneys. Conversely, deletion of Rictor in macrophages attenuates kidney fibrosis, inflammatory cell infiltration, macrophage proliferation and M2 polarization50. Collectively, these findings highlight the potential therapeutic value of Rictor in both acute and chronic tissue injury.
TABLE 3.
Regulatory functions of Rictor/mTORC2 in I/R injury and transplant models
| Disease model | Observation | Reference(s) |
|---|---|---|
| I/R injury (kidney) | Rictor deficiency specifically in DCs augments inflammatory response and tissue injury | 48 |
| Ablation of Rictor in macrophages reduces kidney fibrosis and inflammatory cell accumulation after I/R injury | 50 | |
| I/R injury (liver) | Rictor deficiency enhances liver injury by increasing macrophage and neutrophil infiltration, cytokine and chemokine release | 47 |
| Skin graft rejection | DC-specific Rictor deletion in donor skin augments CD8+ T effector cell responses and accelerates minor HA-mismatched graft rejection | 26 |
| Heart allograft rejection | Dual mTORC1/2 targeting using ATP-competitive inhibitors AZD8055 or AZD2014 suppresses rejection (mouse) | 52, 53 |
| Co-inhibition of mTORC1/2 and RhoA/ROCK pathways prevent chronic rejection (rat) | 54 |
Abbreviations: ATP, adenosine triphosphate; DC, dendritic cell; HA, histocompatibility Ag; I/R, ischemia-reperfusion; RhoA, Ras homolog gene family, member A; ROCK, rho-associated, coiled-coil-containing protein kinase
8. Pharmacologic targeting of mTORC2 and transplant outcome
Novel insights into the roles of mTORC1 and mTORC2 in regulation of immune cell homeostasis and function are improving our understanding of the complex effects of mTOR targeting on immune responses, including those that impact transplant outcomes51. Since there is no specific pharmacologic inhibitor that selectively targets mTORC2, most investigators have used dual mTORC1 and mTORC2 inhibitors to examine the potential roles of mTORC2 in transplantation (Table 3). AZD8055 is an ATP competitive dual mTORC1/2 inhibitor that suppresses both CD4+ and CD8+ T cell proliferation and decreases inflammatory cytokine production52. A short course of AZD8055 prolongs heart allograft survival, increases Treg within the grafts and decreases IFN-γ production, with no impairment of wound healing52. Another dual mTORC1/2 inhibitor (AZD2014), with a more favorable pharmacokinetic profile, decreases DC generation and T cell proliferation in vitro, as well as T cell and B cell responses in vivo53. In addition, a nine-day course of AZD2014 prolongs heart allograft mean survival time by preventing mononuclear cell infiltration into the grafts and elevating the ratio of Treg to T effector memory cells in the spleen53. However, the immunoregulatory effects AZD2014 are not maintained after drug withdrawal and thus differ from the longer-lasting effects of Rapamycin. This may possibly be due to mTORC2 inhibition resulting in a pro-inflammatory effect that would be consistent with mTORC2 deletion in DC promoting an inflammatory phenotype24 or/and to limited bioavailability. Recently, Chen et al54 reported that coinhibition of the mTORC1/2 (with everolimus) and RhoA/ROCK pathways in rat heart allograft recipients prevented chronic rejection. There is also evidence that the rapalogue everolimus is more effective than sirolimus at antagonizing both mTORC1 and mTORC2, the latter of which is critical in endothelial cell functional changes leading to transplant vasculopathy in human organ transplantation after HLA I crosslinking55.
The dual mTORC1/2 inhibitor CC214–1 decreases Th1/Th2 cytokine production and T cell activation in vitro56, while in allogeneic hematopoietic stem cell transplantation, injection of CC214–2 prolongs mouse survival from graft-versus-host disease56.
9. Conclusions and future prospects
The past few years have seen important advances in our understanding of mTORC2 biology, including its intracellular localization and role in aa sensing and mitochondrial fitness. mTORC2 also regulates cytoskeletal modeling and cell migration. Various strategies, that include genetic deletion of Rictor, transient or stable knockdown/targeting of Rictor (using siRNA or shRNA respectively), or/and use of new generation dual mTORC inhibitors, have helped define multiple diverse roles of mTORC2 in innate and adaptive immune cells. These include roles of mTORC2 signaling in the generation, differentiation, metabolism, survival, activation and function of APC, T cell subsets, B lymphocytes and NKT cells. mTORC2 also affects endothelial cell proliferation/angiogenesis and tumor growth57, 58. Of relevance to transplantation, studies in mice suggest a potential therapeutic value of the Rictor/mTORC2 axis in alleviation of hepatic or renal I/R injury. There may also be potential therapeutic benefits of targeting/antagonizing both mTORC1 and 2, rather than mTORC1 alone, to inhibit chronic allograft rejection. More work is needed to ascertain how these inhibitors compare with rapamycin and its analogues in suppressing acute and chronic allograft rejection, including their use in combination with other anti-rejection agents. Although a lack of selective small molecule inhibitors of mTORC2 has limited testing of the role of this complex in vivo, -selective in vivo targeting is feasible in pre-clinical models using nanobiologic approaches. Much remains to be understood regarding the role of mTORC2 and the impact of its antagonism in transplantation.
ACKNOWLEDGMENTS
The authors work is supported by National Institutes of Health grants R01 AI118777, U19 AI131453 and U01 AI137799 (to AWT). HD was supported by the National Science Foundation of China (81800664). We thank Dr. Alicia R. Watson for helpful discussion.
Abbreviations:
- 4EBP1
eukaryotic initiation factor 4E binding protein 1
- Deptor
Dep-domain-containing partner of mTOR
- FKBP12
FK506 binding protein of 12kDa
- mLST8
mammalian lethal with SEC13 protein 8
- mSIN1
mammalian stress-activated MAP kinase-interacting protein 1
- PI3K
phosphatidylinositol 3-kinase
- PRAS40
proline-rich Akt sustrate 40kDa
- Protor
protein observed with Rictor
- Rheb
Ras homologue-enriched in brain
- Rictor
rapamycin-insensitive comparison of mTOR
- S6K1
ribosomal protein S6 kinase
- SGK1
serum and glucocorticoid-regulated kinase 1
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017;168(6):960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 1991;253(5022):905–909. [DOI] [PubMed] [Google Scholar]
- 3.Kunz J, Henriquez R, Schneider U, Deuter-Reinhard M, Movva NR, Hall MN. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell 1993;73(3):585–596. [DOI] [PubMed] [Google Scholar]
- 4.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci 2009;122(Pt 20):3589–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhaskar PT, Hay N. The two TORCs and Akt. Developmental cell 2007;12(4):487–502. [DOI] [PubMed] [Google Scholar]
- 6.Gan X, Wang J, Su B, Wu D. Evidence for direct activation of mTORC2 kinase activity by phosphatidylinositol 3,4,5-trisphosphate. The Journal of biological chemistry 2011;286(13):10998–11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berchtold D, Piccolis M, Chiaruttini N, Riezman I, Riezman H, Roux A et al. Plasma membrane stress induces relocalization of Slm proteins and activation of TORC2 to promote sphingolipid synthesis. Nature cell biology 2012;14(5):542–547. [DOI] [PubMed] [Google Scholar]
- 8.Diz-Munoz A, Thurley K, Chintamen S, Altschuler SJ, Wu LF, Fletcher DA et al. Membrane Tension Acts Through PLD2 and mTORC2 to Limit Actin Network Assembly During Neutrophil Migration. PLoS Biol 2016;14(6):e1002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaubitz C, Prouteau M, Kusmider B, Loewith R. TORC2 Structure and Function. Trends Biochem Sci 2016;41(6):532–545. [DOI] [PubMed] [Google Scholar]
- 10.Ebner M, Sinkovics B, Szczygiel M, Ribeiro DW, Yudushkin I. Localization of mTORC2 activity inside cells. J Cell Biol 2017;216(2):343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Current biology : CB 2004;14(14):1296–1302. [DOI] [PubMed] [Google Scholar]
- 12.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Molecular cell 2006;22(2):159–168. [DOI] [PubMed] [Google Scholar]
- 13.Schreiber KH, Ortiz D, Academia EC, Anies AC, Liao CY, Kennedy BK. Rapamycin-mediated mTORC2 inhibition is determined by the relative expression of FK506-binding proteins. Aging cell 2015;14(2):265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science 2011;332(6035):1317–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalim KW, Zhang S, Chen X, Li Y, Yang JQ, Zheng Y et al. mTOR has a developmental stage-specific role in mitochondrial fitness independent of conventional mTORC1 and mTORC2 and the kinase activity. PLoS One 2017;12(8):e0183266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linke M, Fritsch SD, Sukhbaatar N, Hengstschlager M, Weichhart T. mTORC1 and mTORC2 as regulators of cell metabolism in immunity. FEBS Lett 2017;591(19):3089–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005;307(5712):1098–1101. [DOI] [PubMed] [Google Scholar]
- 18.Hagiwara A, Cornu M, Cybulski N, Polak P, Betz C, Trapani F et al. Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell metabolism 2012;15(5):725–738. [DOI] [PubMed] [Google Scholar]
- 19.Huang SC, Smith AM, Everts B, Colonna M, Pearce EL, Schilling JD et al. Metabolic Reprogramming Mediated by the mTORC2-IRF4 Signaling Axis Is Essential for Macrophage Alternative Activation. Immunity 2016;45(4):817–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babaev VR, Huang J, Ding L, Zhang Y, May JM, Linton MF. Loss of Rictor in Monocyte/Macrophages Suppresses Their Proliferation and Viability Reducing Atherosclerosis in LDLR Null Mice. Front Immunol 2018;9:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh MH, Collins SL, Sun IH, Tam AJ, Patel CH, Arwood ML et al. mTORC2 Signaling Selectively Regulates the Generation and Function of Tissue-Resident Peritoneal Macrophages. Cell Rep 2017;20(10):2439–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kellersch B, Brocker T. Langerhans cell homeostasis in mice is dependent on mTORC1 but not mTORC2 function. Blood 2013;121(2):298–307. [DOI] [PubMed] [Google Scholar]
- 23.Rosborough BR, Raich-Regue D, Matta BM, Lee K, Gan B, DePinho RA et al. Murine dendritic cell rapamycin-resistant and rictor-independent mTOR controls IL-10, B7-H1, and regulatory T-cell induction. Blood 2013;121(18):3619–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raich-Regue D, Rosborough BR, Watson AR, McGeachy MJ, Turnquist HR, Thomson AW. mTORC2 Deficiency in Myeloid Dendritic Cells Enhances Their Allogeneic Th1 and Th17 Stimulatory Ability after TLR4 Ligation In Vitro and In Vivo. J Immunol 2015;194(10):4767–4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raich-Regue D, Fabian KP, Watson AR, Fecek RJ, Storkus WJ, Thomson AW. Intratumoral delivery of mTORC2-deficient dendritic cells inhibits B16 melanoma growth by promoting CD8(+) effector T cell responses. Oncoimmunology 2016;5(6):e1146841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson AR, Dai H, Diaz-Perez JA, Killeen ME, Mathers AR, Thomson AW. mTORC2 deficiency in cutaneous dendritic cells potentiates CD8(+) effector T cell responses and accelerates skin graft rejection. Am J Transplant 2018;In press. [DOI] [PMC free article] [PubMed]
- 27.Yang C, Tsaih SW, Lemke A, Flister MJ, Thakar MS, Malarkannan S. mTORC1 and mTORC2 differentially promote natural killer cell development. Elife 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang F, Meng M, Mo B, Yang Y, Ji Y, Huang P et al. Crosstalks between mTORC1 and mTORC2 variagate cytokine signaling to control NK maturation and effector function. Nature communications 2018;9(1):4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray PJ. Amino acid auxotrophy as a system of immunological control nodes. Nat Immunol 2016;17(2):132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van de Velde LA, Murray PJ. Proliferating Helper T Cells Require Rictor/mTORC2 Complex to Integrate Signals from Limiting Environmental Amino Acids. The Journal of biological chemistry 2016;291(50):25815–25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng H, Cohen S, Guy C, Shrestha S, Neale G, Brown SA et al. mTORC1 and mTORC2 Kinase Signaling and Glucose Metabolism Drive Follicular Helper T Cell Differentiation. Immunity 2016;45(3):540–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J, Lin X, Pan Y, Wang J, Chen P, Huang H et al. Critical roles of mTOR Complex 1 and 2 for T follicular helper cell differentiation and germinal center responses. Elife 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao Y, Wang Y, Liu X, Yang X, Wang P, Tian Q et al. The Kinase Complex mTOR Complex 2 Promotes the Follicular Migration and Functional Maturation of Differentiated Follicular Helper CD4(+) T Cells During Viral Infection. Front Immunol 2018;9:1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H, Zhang L, Wang P, Su H, Wang W, Chu Z et al. mTORC2 controls Th9 polarization and allergic airway inflammation. Allergy 2017;72(10):1510–1520. [DOI] [PubMed] [Google Scholar]
- 35.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature 2006;442(7106):997–1002. [DOI] [PubMed] [Google Scholar]
- 36.Pollizzi KN, Patel CH, Sun IH, Oh MH, Waickman AT, Wen J et al. mTORC1 and mTORC2 selectively regulate CD8(+) T cell differentiation. The Journal of clinical investigation 2015;125(5):2090–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Tschumi BO, Lopez-Mejia IC, Oberle SG, Meyer M, Samson G et al. Mammalian Target of Rapamycin Complex 2 Controls CD8 T Cell Memory Differentiation in a Foxo1-Dependent Manner. Cell Rep 2016;14(5):1206–1217. [DOI] [PubMed] [Google Scholar]
- 38.Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol 2015;16(2):178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM et al. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol 2015;16(2):188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng H, Chi H. mTOR signaling in the differentiation and function of regulatory and effector T cells. Current opinion in immunology 2017;46:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sklarz T, Guan P, Gohil M, Cotton RM, Ge MQ, Haczku A et al. mTORC2 regulates multiple aspects of NKT-cell development and function. Eur J Immunol 2017;47(3):516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prevot N, Pyaram K, Bischoff E, Sen JM, Powell JD, Chang CH. Mammalian target of rapamycin complex 2 regulates invariant NKT cell development and function independent of promyelocytic leukemia zinc-finger. J Immunol 2015;194(1):223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang L, Zhang Y, Xu C, Gu X, Niu L, Wang J et al. Rictor positively regulates B cell receptor signaling by modulating actin reorganization via ezrin. PLoS Biol 2017;15(8):e2001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee K, Heffington L, Jellusova J, Nam KT, Raybuck A, Cho SH et al. Requirement for Rictor in homeostasis and function of mature B lymphoid cells. Blood 2013;122(14):2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M, Lazorchak AS, Ouyang X, Zhang H, Liu H, Arojo OA et al. Sin1/mTORC2 regulate B cell growth and metabolism by activating mTORC1 and Myc. Cellular & molecular immunology 2019;Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 46.Zeng Q, Qin S, Zhang H, Liu B, Qin J, Wang X et al. Rapamycin attenuates BAFF-extended proliferation and survival via disruption of mTORC1/2 signaling in normal and neoplastic B-lymphoid cells. J Cell Physiol 2018;233(1):516–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu D, Zhu J, Jeong S, Li D, Hua X, Huang L et al. Rictor Deficiency Aggravates Hepatic Ischemia/Reperfusion Injury in Mice by Suppressing Autophagy and Regulating MAPK Signaling. Cell Physiol Biochem 2018;45(6):2199–2212. [DOI] [PubMed] [Google Scholar]
- 48.Dai H, Watson AR, Fantus D, Peng L, Thomson AW, Rogers NM. Rictor deficiency in dendritic cells exacerbates acute kidney injury. Kidney Int 2018;94(5):951–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J, Xu Z, Jiang L, Mao J, Zeng Z, Fang L et al. Rictor/mTORC2 protects against cisplatin-induced tubular cell death and acute kidney injury. Kidney Int 2014;86(1):86–102. [DOI] [PubMed] [Google Scholar]
- 50.Ren J, Li J, Feng Y, Shu B, Gui Y, Wei W et al. Rictor/mammalian target of rapamycin complex 2 promotes macrophage activation and kidney fibrosis. J Pathol 2017;242(4):488–499. [DOI] [PubMed] [Google Scholar]
- 51.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol 2009;9(5):324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosborough BR, Raich-Regue D, Liu Q, Venkataramanan R, Turnquist HR, Thomson AW. Adenosine triphosphate-competitive mTOR inhibitors: a new class of immunosuppressive agents that inhibit allograft rejection. Am J Transplant 2014;14(9):2173–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fantus D, Dai H, Ono Y, Watson A, Yokota S, Mohib K et al. Influence of the Novel ATP-Competitive Dual mTORC1/2 Inhibitor AZD2014 on Immune Cell Populations and Heart Allograft Rejection. Transplantation 2017;101(12):2830–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen W, Chen W, Li XC, Ghobrial RM, Kloc M. Coinhibition of mTORC1/mTORC2 and RhoA/ROCK pathways prevents chronic rejection of rat cardiac allografts. Transplantation Reports 2018;3:21–28. [Google Scholar]
- 55.Jin YP, Valenzuela NM, Ziegler ME, Rozengurt E, Reed EF. Everolimus inhibits anti-HLA I antibody-mediated endothelial cell signaling, migration and proliferation more potently than sirolimus. Am J Transplant 2014;14(4):806–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herrero-Sanchez MC, Rodriguez-Serrano C, Almeida J, San-Segundo L, Inoges S, Santos-Briz A et al. Effect of mTORC1/mTORC2 inhibition on T cell function: potential role in graft-versus-host disease control. Br J Haematol 2016;173(5):754–768. [DOI] [PubMed] [Google Scholar]
- 57.Guri Y, Colombi M, Dazert E, Hindupur SK, Roszik J, Moes S et al. mTORC2 Promotes Tumorigenesis via Lipid Synthesis. Cancer cell 2017;32(6):807–823 e812. [DOI] [PubMed] [Google Scholar]
- 58.Gu Y, Albuquerque CP, Braas D, Zhang W, Villa GR, Bi J et al. mTORC2 Regulates Amino Acid Metabolism in Cancer by Phosphorylation of the Cystine-Glutamate Antiporter xCT. Molecular cell 2017;67(1):128–138 e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monteith AJ, Vincent HA, Kang S, Li P, Claiborne TM, Rajfur Z et al. mTORC2 Activity Disrupts Lysosome Acidification in Systemic Lupus Erythematosus by Impairing Caspase-1 Cleavage of Rab39a. J Immunol 2018;201(2):371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity 2010;32(6):743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y, Hu T, Hua C, Gu J, Zhang L, Hao S et al. Rictor is required for early B cell development in bone marrow. PLoS One 2014;9(8):e103970. [DOI] [PMC free article] [PubMed] [Google Scholar]


